Abstract
This cross-sectional study aimed to investigate the prevalence and risk factors of Hepatitis B virus infection among Japanese immigrants and their descendants from São Paulo (SP), and to verify the occurrence of occult hepatitis B and coinfection with HCV, Delta, and HTLV. All samples (n = 2.127) were tested for HBV serological markers by electrochemiluminescence. HBsAg and/or total anti-HBc positive samples were tested for HBV DNA by real-time PCR, and genotyped by sequencing using the Sanger methodology. The prevalence rate of HBV exposure was 13.4% (CI 95%: 11.9–14.9%), and 22 (1.1%) were HBsAg positive. A high rate of susceptibility to HBV infection was found (67.4%; CI 95%: 65.4–69.4%). In contrast, only 19.2% (CI 95%: 17.6–20.9%) presented a serological profile analogous to that elicited by Hepatitis B vaccination. HBV isolates (n = 8) were classified as genotypes HBV/B1 (62.5%), HBV/C2 (12.5%), HBV/F1b (12.5%), and HBV/A1 (12.5%). Hepatitis B vaccination strategies and educational measures to control this infection should be considered.
Keywords: Hepatitis B, epidemiology, seroprevalence, genotype
1. Introduction
Hepatitis B virus (HBV) is an important public health problem. The World Health Organization (WHO) estimates that 1/3 of the global population has serological evidence of HBV infection. Of these, 296 million are chronic carriers, with 820.000 deaths from liver complications related to chronic infection per year [1]. Among individuals chronically infected with HBV, 75% live in Asia, and 25% die from liver complications resulting from infection [2].
Currently, Brazil and Japan are considered countries of low endemicity for HBV infection, with a prevalence of HBsAg less than 2% and 1%, respectively [3,4]. However, the control and elimination of this viral infection has been a challenging task for both countries, given certain regions and populations experience a high prevalence of HBV infection [5].
In Japan, decreases in HBsAg carrier rates nationally have been observed as a product of medical and public health precautions, such as improvement of sanitary conditions and administration of Hepatitis B immunoglobulin and/or Hepatitis B vaccination to neonates of HBsAg-positive mothers. By contrast, the rate of HBsAg carriers in the Okinawa prefecture was found to be substantially higher, such that the region was declared endemic for HBV in a survey conducted by Hayashi et al. from 1968 through the 1980s [6].
In Brazil, the vaccination against Hepatitis B has been applied since the end of the 90s; this measure has been part of the vaccination schedule for children and also some specific population groups [7]. Nowadays, this vaccine is available in all health facilities to the whole population regardless of age and/or vulnerability conditions [8]. The coverage of vaccination in Brazil varies in different population groups. In a systematic review of the epidemiology of HBV in the 21st century conducted by Souto et al., the prevalence of vaccine-induced protection profiles (anti-HBs positive only) varies from 18% in the state of Pará (North), to 92.7% in the state of Bahia (Northeast) [5].
The immigration contingent coming from Japan to Brazil from 1908, as a result of a bilateral contract between Brazil and Japan, today reflects approximately 1.5 million Japanese descendants living in Brazil, considered the country with the largest amount of Japanese people outside of Japan. The majority of this group resides in the state of São Paulo, which received the largest contingent of Japanese immigrants, followed by Paraná, Mato Grosso do Sul, and Pará [9]. After their arrival in Brazil, one of the most serious problems that the first Japanese immigrants faced was the difficulty of accessing medical and health care [10,11].
Considering the high number of Japanese descendants in Brazil, the prevalence of HBV and HDV infections found in Asia and in some regions of Japan, the population exchange between these two countries, and the scarcity of studies in this population, this study aimed to investigate seroepidemiological and molecular aspects of HBV infection; its associated factors; and HBV co-infection with HDV, HCV, and HTLV among Japanese immigrants and their descendants residing in the Metropolitan region of São Paulo, SP.
2. Materials and Methods
This cross-sectional study was conducted among Japanese immigrants and their descendants living in São Paulo, Brazil. Between July 2017 and December 2017, participants were recruited from the five main Okinawan associations in the state of São Paulo (named A to E) from a study investigating HTLV (Human T-cell Lymphotropic Virus) prevalence [12]. The study population included male and female subjects who were Japanese immigrants, descendants of Japanese immigrants, or have a family relationship with these populations. The individuals were informed in detail about the research objectives and the confidentiality of the data.
Informed written consent was obtained, after a detailed explanation of the study, at the time of sampling from all participants or their legal guardians, in case of individuals under age 18. The study protocol was approved by the Ethics Committee of Federal University of Mato Grosso do Sul (3.415.536 CAAE 15432319.3.0000.0021), and all participants gave a written informed consent (IC).
The participants were interviewed to obtain information on sociodemographic characteristics, HBV vaccination, and work-related and other risk behaviors [10]. Of the original 2.139 participants from the previous HTLV study, 2.127 who authorized future research in IC forms were included in the present investigation.
Serum samples from 2.127 participants were screened for serological markers of HBV (HBsAg, total anti-HBc anti-HBs), HCV (anti-HCV), and HTLV (anti-HTLV-1/2) infections by electrochemiluminescence assay (ECLIA) following the manufacturer’s guidelines (Elecsys-cobas-Roche®). HBsAg-positive samples were tested for HBeAg, anti-HBe, and anti-HBc IgM by electroimmunoassay (ECLIA) using the Cobas1 e 601 Analyzer (Roche Diagnostics, Mannheim, Germany) following the manufacturer’s guidelines; anti-HCV-positive samples were confirmed by “line immunoassay” (INNO-LIA III HCV Ab, Innogenetics, Belgic).
Current HBV infection was defined as a positive HBsAg test result. HBV exposure was defined as a positive total anti-HBc and/or HBsAg test result. The HBV-vaccination-like profile included all participants with the anti-HBs positive result (≥10 mIU/mL) combined with negative total anti-HBc and HBsAg results. Participants lacking total anti-HBc, anti-HBs, and HBsAg were considered serologically susceptible to HBV infection. Twelve samples from twenty-two positive HBsAg individuals were tested for the qualitative detection of total antibodies to hepatitis D virus, using a commercial chemiluminescence immunoassay (CLIA), according to the manufacturer’s instructions (LIAISON® XL, Murex, DiaSorin, Saluggia, Italy).
All HBsAg and/or total anti-HBc positive samples were retested, and subjected to HBV DNA detection and quantification by real-time PCR (qPCR). HBV DNA was extracted from serum samples using an Abbott mSample Preparation System DNA kit in an automated platform. After extraction, we proceeded with a real-time PCR in the Abbott m2000rt® System, targeting the S-gene (surface antigen), given it is a highly conserved and specific HBV region that allows the detection of genotypes A to H. The limit of detection of the Abbott RealTime HBV is 10 IU/mL, the upper limit of quantification of the Abbott RealTime HBV is 1 billion IU/mL, and the lower limit corresponds to the detection limit.
Samples with detectable HBV DNA were submitted to HBV genotype/subgenotype identification. To achieve this, a fragment (1270 bp) of the overlapped S/Pol genes was amplified and sequenced as previously described [13]. HBV sequences characterized in this study were aligned by the ClustalW program implemented in BioEdit software [14], with 306 sequences available in the GenBank database of different HBV genotypes/subgenotypes originating from several countries. The phylogenetic tree was inferred using the Bayesian Markov Chain Monte Carlo (MCMC) method implemented in the BEAST package v.1.8.3 under a relaxed molecular clock, and GTR + G + I as the nucleotide substitution model; MCMC was run for 20 million generations, and trees were sampled every 2000 generations. The maximum clade credibility tree was summarized using TreeAnnotator v.1.8.3 [15], and the tree was visualized in FigTree v1.4.2. software (available at: http://tree.bio.ed.ac.uk/software/figtree, accessed on: 5 August 2021).
To compose the database, the questionnaires from 2127 participants included were analyzed to obtain sociodemographic, clinical, and behavioral information, and factors associated with HBV transmission, including: age; sex; history of residence in Japan; blood transfusion; surgery; unprotected sex; and whether the patient had ever been diagnosed with any sexually transmitted infection.
Data analysis was performed in the statistical software Stata SE, version 13 (StataCorp LP, College Station, TX, USA). This study used the chi-squared test (χ2) or the Fisher’s exact test (for categorical variables) to assess differences between proportions, and determine p values (two-tailed). The prevalence rate of HBV exposure (any HBV marker: HBsAg positive; HBsAg/total anti-HBc positive; anti-HBc alone positive; anti-HBc/anti-HBs positive) and a 95% confidence interval (CI) were calculated. Odds ratios and 95% confidence intervals (CI) were used to verify potential predictors of HBV infection and/or exposure (presence of anti-HBc and/or HBsAg markers). Variables with a p-value of less of 0.20 were included in multiple linear regression analysis. The selection of variables for the final model was performed stepwise, according to the number of events per variable (EPV). A Hosmer–Lemeshow test was used to assess goodness-of-fit, choosing the best regression equation [16]. A p-value less than 0.05 was considered statistically significant. Individuals with anti-HBs alone (≥10 mIU/mL) were excluded from the HBV associated factors analysis, since it probably indicates an HBV-vaccination-like profile (n = 409).
3. Results
A total of 2.127 individuals, Japanese immigrants, descendants of immigrants, and individuals with a family relationship with these populations residing in the metropolitan region of São Paulo were enrolled in this study. Most of this population (59.8% female and 40.2% male) was over 60 years old (41.9%), and reported more than twelve years of formal education (44.9%). The majority of participants were either married or divorced/widowed (74.5%), and reported receiving more than US$500 (65.1%) as monthly income. Among this cohort, 47.3% were sons or daughters of Japanese people, of which 87.5% were of Okinawan descendant with no history of living in Japan (61.4%). In addition, 93.2% reported no blood transfusion; 65.5% had never had surgery; 90.1% had never been diagnosed with a sexually transmitted infection; 96.9% had never had any accidental contact with the blood of others; 59.4% reported they had never shared sharp objects; 83.7% reported no regular use of condoms; and 95.2% were born in São Paulo. Socio-demographic characteristics, sexual behavior, using or sharing objects, and origin are shown in Table 1.
Table 1.
Characteristics of Japanese immigrants and their descendants living in São Paulo (n = 2.127).
| Characteristics | N a | % |
|---|---|---|
| Age (years) | ||
| ≤45 | 710 | 33.4 |
| 46–60 | 524 | 24.6 |
| ≥60 | 893 | 41.9 |
| Gender | ||
| Male | 856 | 40.2 |
| Female | 1.271 | 59.8 |
| Formal education (years) a | ||
| Illiterate | 18 | 0.85 |
| 1–9 | 559 | 26.4 |
| 10–12 | 588 | 27.8 |
| >12 | 949 | 44.9 |
| Monthly household income * a | ||
| <1 | 121 | 6.5 |
| 1–3 | 565 | 28.7 |
| >3 | 1.281 | 65.1 |
| Marital status | ||
| Single | 544 | 25.6 |
| Married, divorced, or widowed | 1.583 | 74.4 |
| Recruitment site | ||
| Association A | 709 | 33.3 |
| Association B | 302 | 14.2 |
| Association C | 440 | 20.7 |
| Association D | 344 | 16.2 |
| Association E | 332 | 15.6 |
| Family heritage | ||
| Japanese immigrant | 316 | 14.9 |
| Japanese son/daughter | 1.007 | 47.3 |
| Grandson and great-grandson of Japanese immigrant | 750 | 35.3 |
| Non-Japanese descendant | 54 | 2.5 |
| Okinawan Descendant | ||
| No | 265 | 12.5 |
| Yes | 1.862 | 87.5 |
| History of residence in Japan | ||
| No | 1.305 | 61.4 |
| Yes | 822 | 38.7 |
| Blood transfusion | ||
| No | 1.982 | 93.2 |
| Yes | 145 | 6.8 |
| Surgery | ||
| No | 734 | 34.5 |
| Yes | 1.393 | 65.5 |
| History of Sexually transmitted infection a | ||
| No | 1.917 | 90.1 |
| Yes | 144 | 7.0 |
| Shared sharp objects | ||
| No | 1.263 | 59.4 |
| Yes | 864 | 40.6 |
| Accidental contact with blood of others | ||
| No | 2.061 | 96.9 |
| Yes | 66 | 3.1 |
| Regular use of condoms a | ||
| No | 1.576 | 83.7 |
| Yes | 308 | 16.3 |
| Born in São Paulo | ||
| No | 1.153 | 54.2 |
| Yes | 974 | 45.8 |
* National minimum wage: during the study period, one minimum wage was approximately R$937.00 BRL (U$285.00 USD). a The total represents the number of individuals who answered the question.
A low frequency was found for several variables among the studied population, including: tattooing (7.2%); piercing (4.0%); having ever had a homosexual relationship (0.7%); previous incarceration (0.33%); injecting drug usage (0.1%); and non-injectable drug use (2.2%).
Of 2.127 Japanese immigrants, their descendants, and non-Japanese family members, the prevalence rate of HBV exposure was 13.4% (284; 95% CI: 11.97–14.86). Among them, six (0.3%) were positive for HBsAg only, and sixteen (0.8%) were HBsAg- and total anti-HBc-positive, resulting in an HBsAg-positive “overt” HBV infection rate of 1.1%. All of them were anti-HBc IgM-negative.
Two hundred and five (9.6%) had been infected with HBV, and had developed natural immunity (total anti-HBc-positive and anti-HBs-positive), and 2.7% (57/2.127) were positive only for total anti-HBc. Most of them (67.4%) were susceptible to HBV infection (had no HBV serological marker), and only 19.2% (409/2.127; 95% CI: 17.6–20.9) had serological evidence of previous Hepatitis B vaccination (isolated anti-HBs ≥ 10 mIU/mL) (Table 2). Among them (409/2.127), the majority were women (265/409); the highest prevalence rate of an HBV-vaccination-like profile occurred in the age group of less than 45 years (63.1% 258/409); and with increasing age, there was a decrease of vaccinated individuals.
Table 2.
Prevalence of Hepatitis B virus serological markers among 2.127 Japanese immigrants residing in the Metropolitan region of São Paulo–SP.
| Markers | n | % | 95% CI 1 |
|---|---|---|---|
| Infected | |||
| HBsAg-positive | 22 | 1.1 | 0.68–1.56 |
| HBsAg only | 6 | 0.3 | 0.13–0.62 |
| HBsAg/anti-HBc | 16 | 0.8 | 0.46–1.22 |
| Anti-HBc positive | 262 | 12.3 | 11.10–13.93 |
| Anti-HBc only | 57 | 2.7 | 2.07–3.46 |
| Total anti-HBc/anti-HBs | 205 | 9.6 | 8.45–10.96 |
| Any HBV infection marker | 284 | 13.4 | 11.97–14.86 |
| Not susceptible, possibly vaccinated | |||
| Anti-HBs only | 409 | 19.2 | 17.61–20.96 |
| Not exposed, susceptible | 1434 | 67.4 | 65.40–69.40 |
1 Confidence Interval.
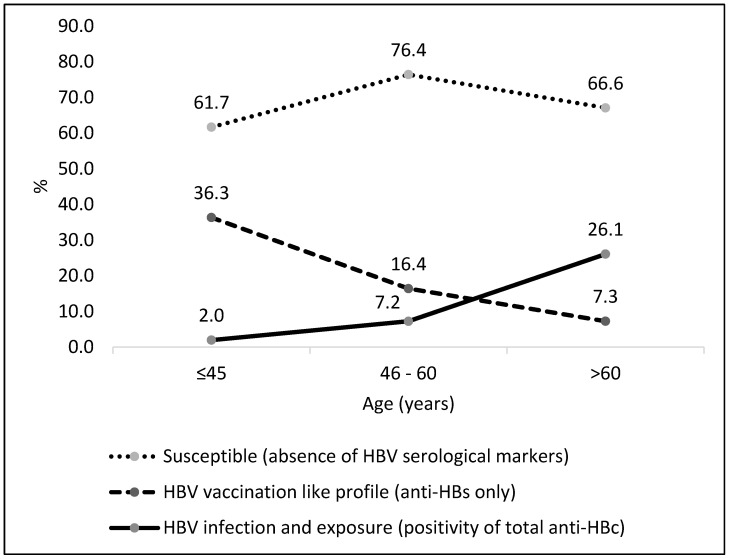
As shown in Figure 1, the group aged ≥60 years had the highest prevalence of HBV exposure (26.1%), and the group aged ≤45 years presented the highest prevalence of anti-HBs alone (36.3%). On the other hand, the number of susceptible individuals was high in all age groups.
Figure 1.
HBV serological profile among Japanese immigrants and their descendants according to age (years), Brazil (n = 2.127).
Considering individuals with a vaccination serological profile (409/2.127), those whose grandparents were Japanese immigrants represent the generation with the highest rate of HBV vaccination (43.8%; 179/409), followed by those who were a son/daughter (36.9%; 151/409) of immigrants, and individuals who were a great-grandson/granddaughter (12.2%, 50/409) of immigrants. The lowest HBV vaccination rate was observed among first-generation Japanese immigrants (7.1%; 29/409).
In a univariate analysis, seroprevalence of HBV was associated with: individuals who were >46 years old; originally from Association C, D, and E; married or single; Japanese immigrants or sons of Japanese immigrants; of Okinawan descendant; had lived in Japan; had less than 9 years of education; received one to three minimum wages; had at least one b(OR = 3.46; p = 0.001); being originally from Association C (OR = 2.63; p = 0.000), Association D (OR = 2.96; p = 0.000), or Association E (OR = 2.74; p = 0.000); being a son of Japanese immigrants (OR = 2.39; p = 0.002); and being a first-generation Japanese immigrant (OR = 6.79; p = 0.000) were independently associated with positivity for Hepatitis B exposure (Table 3).
Table 3.
Factors associated with Hepatitis B virus infection among Japanese immigrants residing in the metropolitan region of São Paulo, Brazil (n = 1.718).
| Factors/Variables | HBV Exposure a N (%) |
OR (95% CI) |
p-Value | Adjusted OR c (95%CI) | p-Value b | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤45 | 14/452 | 3.1 | 1 | - | 1 | - |
| 46–60 | 37/438 | 8.4 | 2.88 (1.53–5.42) | 0.001 | 1.26 (0.62–2.57) | 0.526 |
| >60 | 233/828 | 28.1 | 12.25 (7.04–21.30) | 0.000 | 3.46 (1.70–7.04) | 0.001 |
| Gender | ||||||
| Female | 160/1006 | 15.9 | 1 | |||
| Male | 124/712 | 17.4 | 1.11 (0.86–1.44) | 0.406 | - | - |
| Marital status | ||||||
| Separated/divorced | 61/246 | 24.8 | 1 | 1 | 1 | - |
| Married | 202/1122 | 18.0 | 0.67 (0.48–0.92) | 0.015 | 1.01 (0.68–1.51) | 0.929 |
| Single | 21/350 | 6.0 | 0.15 (0.11–0.32) | 0.000 | 1.17 (0.56–2.44) | 0.674 |
| Recruitment site | ||||||
| Association A | 40/366 | 10.9 | 1 | - | 1 | - |
| Association B | 69/563 | 12.4 | 1.14 (0.75–1.72) | 0.539 | 1.40 (0.88–2.25) | 0.150 |
| Association C | 41/237 | 17.3 | 1.70 (1.06–2.73) | 0.026 | 2.63 (1.53–4.51) | 0.000 |
| Association D | 74/269 | 27.5 | 3.10 (2.02–4.72) | 0.000 | 2.96 (1.82–4.81) | 0.000 |
| Association E | 60/283 | 21.2 | 2.19 (1.42–3.39) | 0.000 | 2.74 (1.66–4.51) | 0.000 |
| Family heritage b | ||||||
| Grandchild/great-grandchild of Japanese | 21/537 | 3.91 | 1 | 1 | 1 | - |
| Japanese son/daughter | 135/856 | 15.7 | 4.60 (2.86–7.38) | 0.000 | 2.39 (1.38–4.12) | 0.002 |
| Japanese immigrant | 127/287 | 44.2 | 19.50 (11.89–31.98) | 0.000 | 6.79 (3.45–13.38) | 0.000 |
| Okinawan descendant | ||||||
| No | 22/210 | 10.48 | 1 | - | ||
| Yes | 262/1508 | 17.3 | 1.79 (1.13–2.85) | 0.013 | 1.07 (0.62–1.85) | 0.802 |
| History of residence in Japan | ||||||
| No | 109/1017 | 10.7 | 1 | 1 | ||
| Yes | 175/701 | 24.9 | 2.77 (2.13–3.60) | 0.000 | 1.21 (0.81–1.80) | 0.339 |
| Education (years) b | ||||||
| >9 | 152/1172 | 12.9 | 1 | 1 | ||
| <9 | 130/534 | 24.3 | 2.16 (1.66–2.80) | 0.000 | 1.06 (0.74–1.53) | 0.742 |
| Income b | ||||||
| >3 minimum wage (0) | 138/998 | 13.8 | 1 | 1 | ||
| 1 to 3 minimum wages (2) | 104/483 | 21.5 | 1.71 (1.29–2.27) | 0.000 | 1.08 (0.76–1.54) | 0.660 |
| <1 minimum wage (3) | 22/109 | 20.2 | 1.57 (0.95–2.60) | 0.075 | 0.91 (0.51–1.62) | 0.760 |
| Piercing | ||||||
| No | 281/1663 | 16.90 | 1 | |||
| Yes | 3/55 | 5.45 | 0.28 (0.87–0.91) | 0.025 d | 0.50 (0.62–4.12) | 0.524 |
| History of surgery | ||||||
| No | 78/551 | 14.16 | 1 | |||
| Yes | 206/1167 | 17.65 | 1.29 (0.98–1.72) | 0.069 | 0.85 (0.59–1.19) | 0.347 |
| Tattooing | ||||||
| No | 271/1609 | 16.84 | 1 | |||
| Yes | 13/109 | 11.93 | 0.67 (0.37–1.21) | 0.184 | 0.78 (0.39–1.56) | 0.484 |
| Shared personal sharp objects | ||||||
| No | 187/1037 | 18.03 | 1 | 1 | ||
| Yes | 97/681 | 14.24 | 0.75 (0.57–0.98) | 0.039 | 1.02 (0.73–1.41) | 0.906 |
| Non-injectable drug usage | ||||||
| No | 281/1679 | 16.7 | 1 | |||
| Yes | 3/39 | 7.7 | 0.41 (0.13–1.35) | 0.145 | 0.39 (0.11–1.40) | 0.150 |
| Sexually transmitted infection (STI) b | ||||||
| No | 255/1546 | 16.49 | 1 | 1 | ||
| Yes | 29/124 | 23.39 | 1.54 (0.99–2.39) | 0.051 | 1.20 (0.71–2.04) | 0.495 |
| Condom use b | ||||||
| Always | 29/217 | 13.36 | 1 | |||
| Sometimes/never | 248/1332 | 18.62 | 0.67 (0.44–1.02) | 0.062 | 1.26 (0.75–2.11) | 0.382 |
| Anti-HTLV positivity | ||||||
| No | 252/1626 | 15.50 | ||||
| Yes | 32/92 | 34.78 | 2.90 (1.80–4.56) | 0.000 | 1.32 (0.78–2.24) | 0.297 |
95% CI: 95% confidence interval; OR: odds ratio; a HBV exposure was defined as a positive total anti-HBc and/or anti-HBs test result; b the total represents the number of individuals who answered the question; c adjusted for age and gender; d Fisher’s test.
Among all HBsAg-positive samples, anti-HTLV-1 was detected in three (13.0%) of the subjects, and no samples tested positive for anti-HCV. Out of the 16 HBsAg-positive and 4 total anti-HBc-positive tested samples, no one was positive for total anti-HD antibodies (Table 4).
Table 4.
Demographic, serological, and virological characteristics of HBsAg- and/or HBV-DNA-positive Japanese immigrants and their descendants in São Paulo, Brazil (n = 22).
| ID | Age (Years) | Recruitment Site | Gender | Serological Markers | HBV Viral Load (log) | HBV Subgenotype | ||
|---|---|---|---|---|---|---|---|---|
| HBeAg | Anti-HBe | Anti-HTLV | ||||||
| 49 a | 63 | A | M | Negative | Positive | Negative | <1.00 | NP |
| 481 a | 82 | B | M | Negative | Positive | Negative | 2.79 | B1 |
| 533 a | 73 | B | M | Negative | Positive | Positive | 2.07 | C2 |
| 815 b | 44 | B | M | Negative | Positive | Negative | 1.4 | NP |
| 836 a | 60 | B | F | Negative | Positive | Negative | 2.25 | B1 |
| 851 a | 66 | B | M | Negative | Positive | Negative | 1.98 | B1 |
| 1156 b | 69 | B | M | Negative | Positive | Negative | 1.57 | A1 |
| 1189 a | 58 | C | M | Negative | Positive | Negative | 3.32 | B1 |
| 1338 b | 77 | C | F | Negative | Positive | Positive | ND | NP |
| 1367 a | 56 | C | F | Negative | Positive | Negative | ND | NP |
| 1576 b | 71 | D | M | Negative | Negative | Negative | ND | NP |
| 1592 a | 73 | D | F | Negative | Negative | Negative | ND | NP |
| 1596 b | 68 | D | M | Negative | Negative | Negative | ND | NP |
| 1621 a | 67 | D | M | NP | Positive | Positive | NP | NP |
| 1646 c | 47 | D | F | Negative | Negative | Negative | NP | NP |
| 1677 a | 67 | D | F | Negative | Positive | Negative | ND | NP |
| 1751 b | 64 | D | M | Negative | Negative | Negative | ND | NP |
| 1916 c | 39 | E | M | Negative | Negative | Negative | ND | NP |
| 1922 a | 64 | E | F | Negative | Positive | Negative | 2.53 | NP |
| 1960 b | 52 | E | F | Negative | Positive | Negative | 2.69 | F1b |
| 1974 a | 66 | E | F | Negative | Positive | Negative | 1.6 | NP |
| 2052 b | 75 | E | M | Negative | Positive | Negative | 2.12 | B1 |
a Japanese immigrant; b Japanese son/daughter; c Japanese grandchild/great-grandchild; M, male; F, female; HBeAg, Hepatitis B e antigen; anti-HBe, antibodies against HBeAg; ND, not detected; NP, not performed (insufficient specimen volume for testing).
HBV DNA was detected in 10/22 (45.4%) HBsAg-positive samples, and in 13/263 (4.9%) total anti-HBc positive samples without HBsAg characterizing HBsAg-negative “occult” Hepatitis B infection (OBI). Demographic, serological and virological characteristics of these cases are detailed in Table 5. Of the 13 occult HBV infections, 5 (36.5%) were positive for anti-HBc only, and 8 (61.5%) were positive for total anti-HBc and anti-HBs. Considering all participants with HBV DNA detectable, the prevalence of HBV active/persistence infection was 1.6%. No one was positive for anti-HCV, and only one (OKW487) was positive for anti-HTLV-1/2.
Table 5.
Demographic, serological, and virological characteristics of HBV-DNA-positive/HBsAg-negative of Japanese immigrants and their descendants in São Paulo, Brazil (n = 13).
| ID | Age (Years) |
Recruitment Site | Gender | Serological Markers | HBV Viral Load (log) | Subgenotype | |
|---|---|---|---|---|---|---|---|
| HBeAg | Anti-HBe | ||||||
| 49 a | 63 | A | M | Negative | Positive | <1.00 | NP |
| 168 | 42 | A | M | NP | NP | <1.00 | NP |
| 317 | 70 | A | F | NP | NP | 1.66 | NP |
| 487 | 87 | B | F | NP | NP | <1.00 | NP |
| 815 b | 44 | B | M | Negative | Positive | 1.4 | NP |
| 935 | 72 | B | M | NP | NP | 1.02 | NP |
| 976 | 66 | B | M | NP | NP | <1.00 | NP |
| 1313 | 34 | C | F | NP | NP | <1.00 | NP |
| 1604 | 64 | D | F | NP | NP | 1.67 | NP |
| 1922 a | 64 | E | F | Negative | Positive | 2.53 | NP |
| 1974 a | 66 | E | F | Negative | Positive | 1.6 | NP |
| 2087 | 75 | E | F | NP | NP | <1.00 | NP |
| 2103 | 65 | E | M | NP | NP | <1.00 | NP |
a Japanese immigrant; b Japanese son/daughter; M: male; F: female; HBeAg: Hepatitis B e antigen; anti-HBe: antibodies against HBeAg; NP: not performed; ND: not detected.
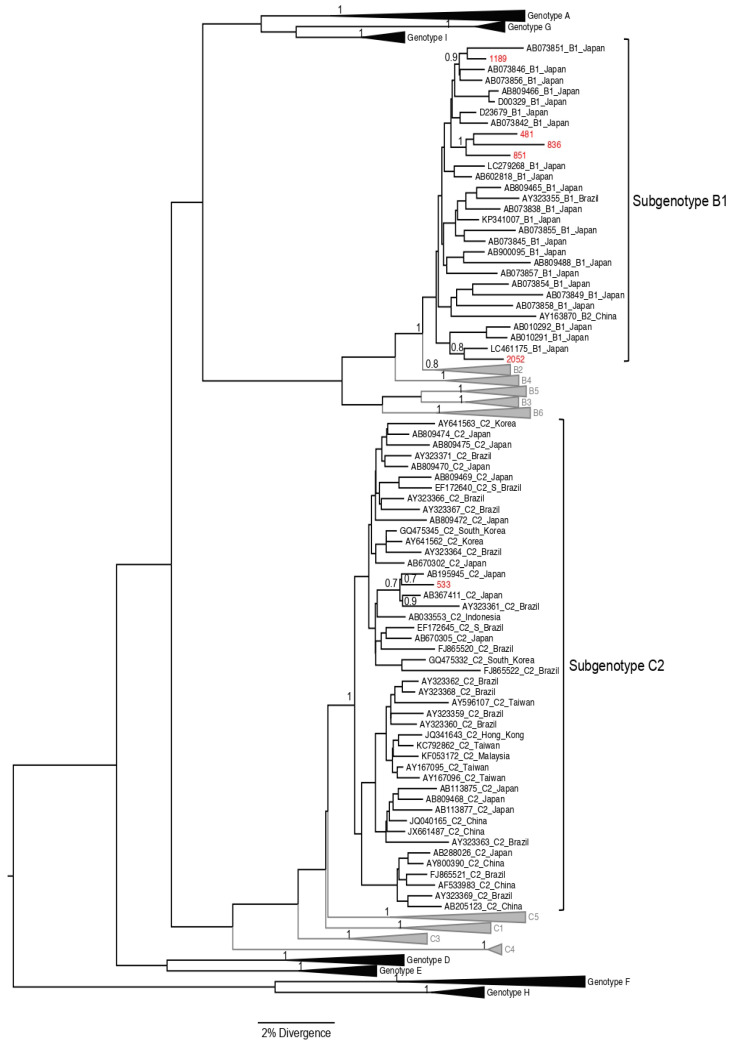
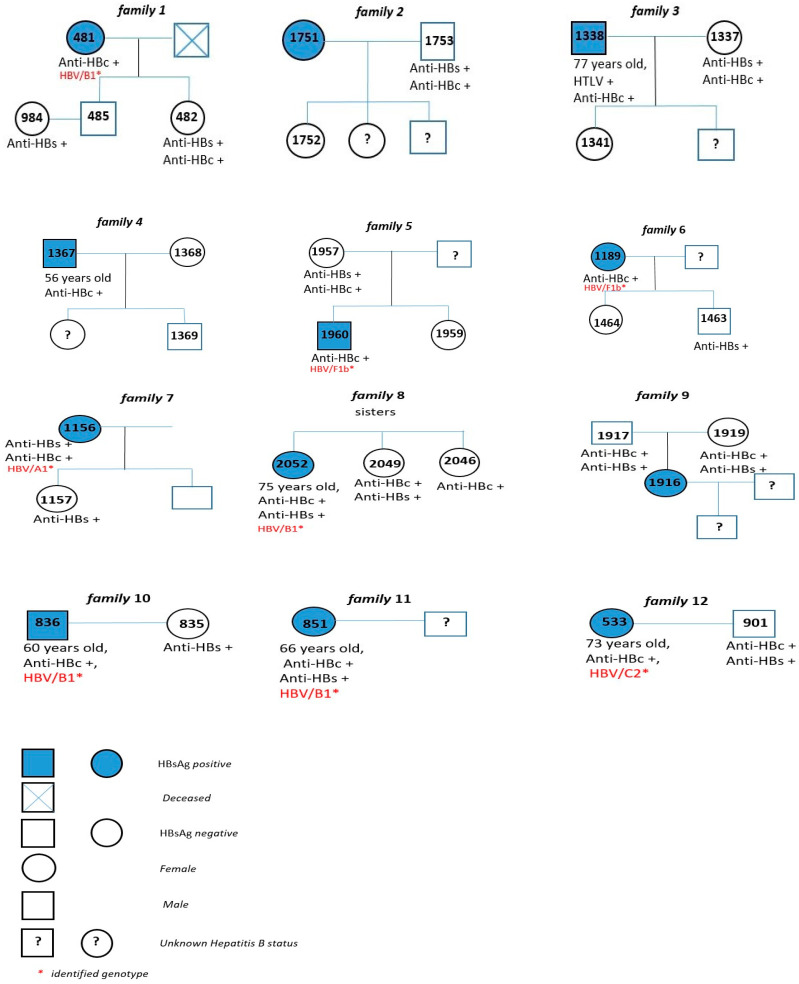
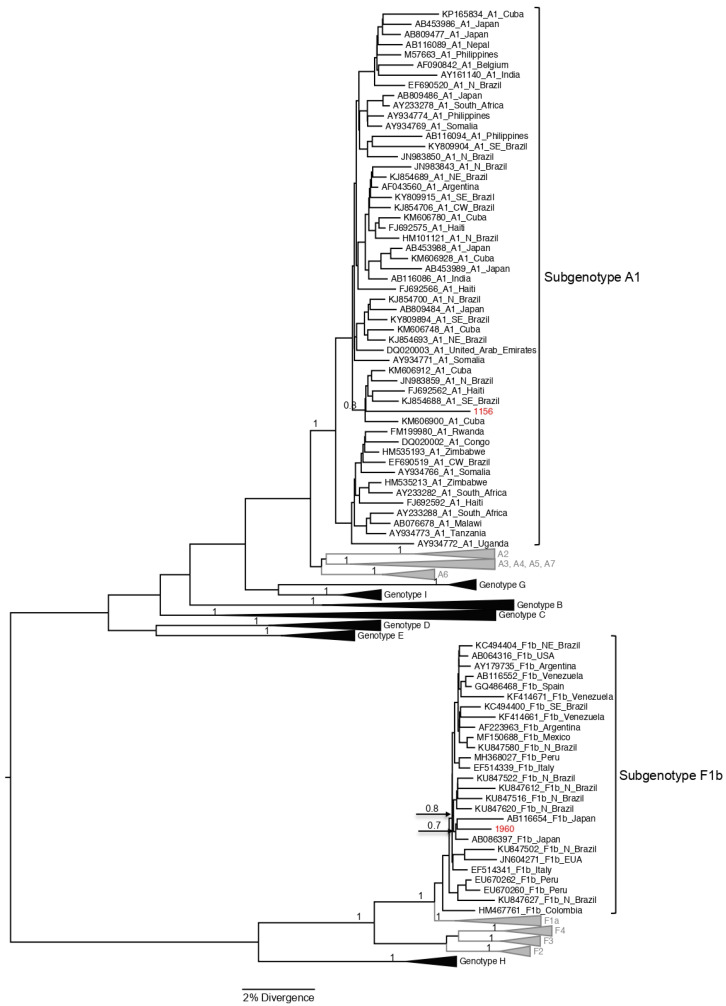
The S/Pol region was amplified and sequenced from 8 of the 26 samples with HBV DNA detectable, and all of them were from cases with positivity to HBsAg. Subgenotype B1 was the most frequent (62.5%; 5/8) among the study population; four of these cases were Japanese immigrants, and one was a Japanese son. Three HBV/B1 sequences were closely related, but there was not any familiar relationship among these HBV carriers (Figure 2 and Figure 3). As reported by Carrilho et al., families represent an excellent model to study transmission mechanisms through people exposed to environmental characteristics [17]. Subgenotypes A1, C2, and F1b (one case each) were also found (Figure 2 and Figure 3). HBV/C2 was isolated from a Japanese immigrant and in a phylogenetic tree grouped together with HBV/C2 sequences isolated in Japan. HBV/A1 was also isolated from a Japanese son, and grouped closely with HBV/A1 sequences isolated in Brazil previously (Figure 4).
Figure 2.
The maximum clade credibility (MCC) tree estimated by Bayesian analysis of S/POL sequences with 1270 bp of HBV strains characterized from Japanese immigrants and descendants living in São Paulo, Brazil. For better visualization, only clades of HBV/B1 and C2 are shown in this figure. Sequences characterized in this study are in red; sequences obtained from GenBank (n = 306) are indicated by their corresponding accession number, genotype, and geographic origin. The values of posterior probability are shown for key nodes.
Figure 3.
Pedigree of 12 families of index cases (HBsAg-positive) under study, São Paulo—SP.
Figure 4.
The maximum clade credibility (MCC) tree estimated by Bayesian analysis of S/POL sequences with 1270 bp of HBV strains characterized from Japanese immigrants and descendants living in São Paulo, Brazil. For better visualization, only clades of HBV/A1 and F1b are shown in this figure. Sequences characterized in this study are in red; sequences obtained from GenBank (n = 306) are indicated by their corresponding accession number, genotype, and geographic origin. The values of posterior probability are shown for key nodes.
Subgenotype F1b (Figure 4) also was isolated from a Japanese son and the sequence grouped with two sequences isolated from Japanese patients in Okinawa (GenBank AB086397 and AB116654) [18]. The cluster of these three HBV/F1b sequences was closely related with a cluster with four F1b sequences isolated in North/Northwest regions of Brazil.
4. Discussion
To our knowledge, this is the largest HBV epidemiological study involving this population ever performed in Brazil considering the number of Japanese immigrants. The prevalence of HBV serological markers describes the endemicity of Hepatitis B in this population group, and may provide necessary information to guide prevention and control policies improving public health.
In this study, a high prevalence rate of HBV exposure (13.5%: 95% CI: 12.1–14.9%) was found, which was high when compared to the prevalence found in blood prime donors (3.0%) from the same region [19]; this information reinforces the idea that Brazil has a heterogeneous distribution of HBV exposure despite the country’s low endemicity [5,20]. This rate is as high as another survey performed in Okinawa in the 1980s in which the prevalence of exposure varied from 3.0% in people aged 0–9 years old to 91.2% in people aged 30–39 years old [21]. This supports the hypothesis that immigrants of first generation were infected in their childhood in Japan.
HBV can persist in an “overt” or in a long term “occult” state, depending on the detection of viral markers. The overt state, known as HBV chronic infection, is defined as persistence of HBsAg for six months or more after acute infection with HBV. The occult HBV infection is defined as the presence of replication-competent HBV DNA in the liver, in the absence of detectable serum HBsAg. Though the prevalence of HBV exposure was high, the prevalence of chronic HBV infection (1.1%), even when we consider the prevalence of HBV persistence (1.8%), was considered low, similar to other countries with low endemicity [5,22]. As reported by Rossi et al., in a study involving immigrants and refugees, chronic HBV seroprevalence was found to be high for migrants from East Asia (11.3% (95% CI: 10.3–12.4%)) when compared to migrants from Central Asia and South Asia (5.8% (95% CI: 4.3–7.9%)), and migrants from Latin America and the Caribbean (1.7% (95% CI 1.1–2.7%)) [23]. The CDC in the United States and the Canadian Collaboration for Immigrants and Refugee Health strongly recommends that all immigrants originating from countries with a seroprevalence of HBV higher than 2% should be screened for chronic HBV infection and prior immunity to HBV, and vaccinated if found to be susceptible [24,25]. The role of chronic HBV infection in the development of hepatocellular carcinoma (HCC), a global public health issue, is undisputed. Globally, chronic infection with HBV is the most common type of liver cancer, accounting for 85–90% of the cases. Globally, HBV chronic infection is the most prominent risk factor for HCC development, and it was responsible for 33% of liver cancer mortality, followed by alcohol (30%), HCV (21%), and other causes (16%), with substantial variation among countries in the underlying etiologies. HCC cases associated with chronic Hepatitis B infection account for 60% of cases in Asia and Africa, and 20% of cases in the West [26,27]. In Brazil, in a study conducted among 884 compensated cirrhotic patients under a surveillance program in the largest referral center for hepatology in Brazil, it was observed that 50.0% (8/16) of patients with HBV infection and HCC were Brazilian East-Asian descendants with a family history of HCC [28,29].
About 5% of people who have chronic infection with HBV are affected by hepatitis D virus (HDV), which cannot occur in the absence of HBV. Co-infection of HDV and HBV is considered the most severe form of chronic viral hepatitis due to rapid progression towards hepatocarcinoma [30]. Epidemiological studies have revealed that the incidence of HDV infection is low in Japan, varying from 0.6 to 3%, and it is considered lower when compared to the rates reported in Africa, North America, Central America, South America, and Europe [31,32]. For hepatitis D, 12 HBsAg-positive samples were analyzed, and none were positive for total anti-HD antibodies. This information reinforces that HDV infection is uncommon among HBsAg carriers in Japan [31,33] and in Brazil, the latter of which was found by Lago et al. (2018) to have a 3.2% national prevalence, and a 1.7% prevalence in the Southeast region [34].
HBV/HTLV-1 co-infection was found in 13% of HBsAg-positive samples. This result is in concordance with the high prevalence of HTLV-1 infection observed among this population in a study conducted by Bandeira et al. (2021) [12]. Interestingly, despite HBV and HCV sharing overlapping routes of transmission, no cases of HCV infection were found in the present study.
The association between HBV exposure and older age (>60 years old) found in this study has been reported previously [20,35,36]. It is noteworthy that most of the Japanese immigrants infected were over 60 years old, given Japanese immigration to Brazil has largely ceased since the mid-20th century. This association suggests that, with increasing age, the risk of acquiring HBV by exposure increases mainly because of the elevated HBV prevalence among the inhabitants of Okinawa, when compared to Japan’s national average observed two decades before contracted migration to Brazil.
In addition, this age group had not been vaccinated against Hepatitis B when Brazil started vaccination campaigns against HBV infection among the youngest citizens, and consequently, this decreased the number of new cases of HBV infection in this younger cohort.
A high susceptibility rate to HBV infection (67.3%) and a low positivity index for the serological marker of vaccine immunity (19.2%) were found in this group population, probably because it is predominantly older and has not received the HBV vaccine, highlighting that vaccination strategies and educational measures to control this infection should be considered in this population group. Similar to findings reported in other studies, increasing age was associated with HBV exposure, suggesting that people who were born before the initiation of vaccination programs were more likely exposed to the risk of infection [20,36].
The prevalence found for OBI was 6.1%; this is considered high in comparison to another study conducted among blood donors performed in São Paulo in which the prevalence was 0.6% [37]. It has been shown in many studies that rates for OBI vary in different populations according to the endemicity of HBV infection. In a survey conducted in a population from the Brazilian Amazon region, the rate of OBI among anti-HBc-positive patients was 14.36% [38]; in another study of patients on hemodialysis in the Northeast of Brazil, the prevalence found was 2.3% [39].
As shown in Table 3, being a Japanese immigrant (OR = 6.56; p = 0.000) or son/daughter (OR = 2.31; p = 0.000) were found to be independent factors associated with HBV exposure. As previously described, Brazil is home to the largest Japanese population outside of Japan, and about half of these immigrants came from southern Okinawa after World Wars I and II. This may be inferred because, as reported by Sakugawa et al. in 1991 [40], the prevalence of HBV exposure in Okinawa was the highest in Japan until the 1960s, decreasing from 12.3% in 1968 to 7.5% in 1979–1981 [6], and 4.7% in 1983, as reported by Kashiwagi et al. [41], and to 1.7% in 1985, as reported by Kashiwagi et al. (1988) [42], among children (1–5 years old). This high prevalence, mainly among the elderly (over 60 years old), likely reflects the geographical origin of infected individuals mainly between 1917 and 1940, when 164.000 Japanese people migrated to Brazil; 75% of these immigrants settled in São Paulo, where most of the coffee plantations were located. HBV exposure decreased among participants with higher educational levels (>9 years of education), which might be due to awareness of prevention; this finding is supported by similar observations reported by Rezende et al., 2020 [43].
After multivariate analysis, being originally from Association C (OR = 2.47; p = 0.001), D (OR = 2.94; p = 0.000), or E (OR = 2.67; p = 0.000) was associated with positivity for anti-HBc. This finding could be inferred given the majority of people from these associations were >60 years old when compared with Association A and B (p = 0.000).
The distribution of HBV genotype may demonstrate different patterns of migration to the Americas, and reflect human migration from different areas into the studied region [44]. In this study, subgenotypes A1, B1, C2, and F1b were found.
Genotype A is distributed globally, and is the main genotype found in sub-Saharan Africa, North America, India, Northern Europe, and Western Africa [45,46]. In Brazil, the most common genotype is A, followed by genotypes D and F [45]. Among the population of Japanese immigrants and their descendants living in São Paulo studied, HBV subgenotype A1, B1, C2, and F1b were found.
HBV/A1 was isolated from a Japanese son, and the sequence was related with HBV/A1 sequences isolated previously in Brazil, where this subgenotype is widespread. Genotypes B (classified into B1, B2, B4–B6, and quasi-subgenotype B3) and C (divided into C1, quasi-subgenotype C2, C3–C16) are found predominantly in East and Southeast Asia, Indonesia, and Oceania [46,47]. Subgenotype B1 is predominantly found in Japan; and C2 is found in Japan, Taiwan, China, and Southeast Asia [48]. All samples classified as subgenotype HBV/B1 (n = 5) were isolated from Japanese immigrants, except one (OKW2052), who was the child of a Japanese immigrant. This finding is in agreement with studies conducted in Okinawa and Tohoku that found a high rate of HBV/B, and another survey performed among HBV chronic patients in Okinawa that found a positivity of 86.9% of HBV/B [49,50]. It is interesting that three of these HBV/B1 sequences grouped in a well-supported monophyletic clade, suggesting a common origin of the HBV strains (Figure 2). Notably, among the five isolates that were classified as HBV/B1, three were from the same Okinawa association, emphasizing the importance of screening families of chronic Hepatitis B carriers to increase the chances of effective treatment, and reduce the spread of this infectious disease [51].
The singular sample classified as HBV/C2 was from a 75-year-old male Japanese immigrant. This genotype has been previously found in Japanese and Chinese people from South and Southeast Brazil, as reported by Clemente et al. [52]. Most reports of HBV/B and HBV/C are linked to Asian communities due to behavioral factors and/or route of exposure, as reported by Lago et al. [34]. As described by Sunbul (2014), geographic distribution of HBV genotypes may be related to the route of exposure. For example, HBV/B and HBV/C are more common in high-endemic regions, where they are spread by perinatal or vertical exposure, both considered important transmission routes of HBV [46].
In a study conducted by Lago et al. [53], genotype HBV/F, subdivided into four subgenotypes (F1–F4), is more frequent in Amerindian populations of Central and South America. Subgenotype F1 includes two clusters, 1a (with strains isolated in Central America) and 1b (with strains isolated in Argentina) [48,54]. In this study, the HBV/F1b was identified in one sample from a 52-year-old female descendant of a Japanese immigrant. This sequence was closely related (p value = 0.7) with F1b sequences isolated from two patients in a hospital in Okinawa, Japan (AB086397 and AB116654), one of whom was a 43-year-old male chronic HBV carrier [18]. In Brazil, subgenotype F1b shows a low prevalence, and was described in few cases [55]; however, a recent study found this subgenotype in a large number of cases in patients from Rio Branco city, Acre state, and the Northern region of Brazil [56]. There is neither enough information about the ancestral origins of the patients from the Northern region of Brazil [56] infected by HBV/F1b, nor epidemiological information about the possible infection of Japanese patients with HBV/F1b described by Kato et al. [18], and we cannot make conclusions about the origin of this subgenotype among the Japanese population.
This study has some limitations. First, as a cross-sectional study, the exposure and outcome were simultaneously assessed. For this reason, it is impossible to draw any conclusions on causality. Although this study included the most populous Associations of Japanese immigrants and their descendants, this group might not represent the population as a whole, and might be biased by volunteers. Self-reporting and recall-bias are also limitations of the present study. The lack of HBV vaccination records likely overestimated the frequency of susceptibility, given some HBV vaccinated individuals lose detectable levels of anti-HBs over time.
Despite these limitations, this work highlights the importance of promoting further investigation surrounding HBV and other infectious diseases in immigrant populations. More important than preventive methods are to plan health interventions and policies to prioritize allocation of resources for the improvement of health surveillance. The high susceptibility rate to HBV infection and the low positivity index for the serological marker of vaccine immunity found in this study highlight the urgent need for Hepatitis B vaccination strategies, educational measures, and screening strategies to control this infection.
Author Contributions
Conceived and designed the experiments: L.H.F.D., L.M.B., A.R.C.M.-C. and M.S.G.-G.; Performed the experiments: L.H.F.D., D.L.T., M.C.S.U.Z., M.L.I., M.S.G.-G., A.R.C.M.-C., G.A.C., R.B.L., S.N.d.O.U., G.R.d.R., T.S.O.T. and M.A.M.P.; Analyzed the data: L.H.F.D., L.M.B., D.L.T., L.D.C.d.A., C.C.M.G., S.M.d.S.W.-T., A.R.C.M.-C., A.O.P.E., M.S.G.-G. and T.S.O.T.; Contributed reagents/materials/analysis tools: L.H.F.D., L.M.B., D.L.T., L.D.C.d.A., C.C.M.G., S.M.d.S.W.-T., J.R.R.P., M.S.G.-G., A.R.C.M.-C. and R.V.d.C.; Wrote the paper: L.H.F.D., D.L.T., J.R.R.P., M.S.G.-G., A.R.C.M.-C. and F.J.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Federal University of Mato Grosso do Sul (protocol code 3.415.536 CAAE 15432319.3.0000.0021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was financially supported by Fundação para Desenvolvimento Científico e Tecnologico em Saúde (FIOTEC-FIOCRUZ-RJ), funding number VPPLR-002-FIO-15.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Hepatitis B. Key Facts. [(accessed on 22 August 2021)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b.
- 2.Sato T., Do S.H., Asao T., Akita T., Katayama K., Tatara K., Miyakawa Y., Tanaka J. Estimating numbers of persons with persistent hepatitis B virus infection transmitted vertically and horizontally in the birth cohort during 1950–1985 in Japan. Hepatol. Res. 2014;44:E181–E188. doi: 10.1111/hepr.12288. [DOI] [PubMed] [Google Scholar]
- 3.Rani M., Yang B., Nesbit R. Hepatitis B control by 2012 in the WHO Western Pacific Region: Rationale and implications. Bull. World Health Organ. 2009;87:707–713. doi: 10.2471/BLT.08.059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol. Clin. N. Am. 2020;49:179–189. doi: 10.1016/j.gtc.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Souto F.J.D. Distribution of hepatitis B infection in Brazil: The epidemiological situation at the beginning of the 21st century. Rev. Soc. Bras. Med. Trop. 2016;49:11–23. doi: 10.1590/0037-8682-0176-2015. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi J., Kajiyama W., Noguchi A., Ikematsu H., Nomura H., Nakashima K., Morofuji M., Kashiwagi S. Marked Decrease of Hepatitis B Virus Infection among Children in Okinawa, Japan. Int. J. Epidemiol. 1990;19:1083–1085. doi: 10.1093/ije/19.4.1083. [DOI] [PubMed] [Google Scholar]
- 7.Magalhães R.L.B., Teles S.A., Reis R.K., Galvão M.T.G., Gir E. Low completion rate of hepatitis B vaccination in female sex workers. Rev. Bras. Enferm. 2017;70:514–519. doi: 10.1590/0034-7167-2016-0567. [DOI] [PubMed] [Google Scholar]
- 8.Brasil, Ministério Da Saúde Secretaria de Vigilância em Saúde. Departamento de Condições Crônicas e Infecções Sexualmente Transmissíveis. Hepatite B. [(accessed on 9 October 2020)]; Available online: http://www.aids.gov.br/pt-br/publico-geral/hv/o-que-sao-hepatites/hepatite-b.
- 9.Consulado Geral do Japão em São Paulo Dados Gerais. [(accessed on 16 August 2021)]. Available online: emb-japan.go.jp.
- 10.Osaki M.M. A Evolução da Assistência à saúde dos Imigrantes Japoneses no Brasil. Rev. Adm. Saúde. 2017;17 doi: 10.23973/ras.67.27. [DOI] [Google Scholar]
- 11.Prutsch U. Migrantes na periferia: Indígenas, europeus e japoneses no Paraná durante as primeiras décadas do século XX. Hist. Ciênc. Saúde-Manguinhos. 2014;21:218–236. doi: 10.1590/S0104-59702014005000005. [DOI] [PubMed] [Google Scholar]
- 12.Bandeira L.M., Puga M.A.M., Weis-Torres S.M.S., Rezende G.R., Domingos J.A., Tanaka T.S.O., Cesar G.A., Nukui Y., Vicente A.C.P., Casseb J., et al. Human T-cell leucemia virus type 1 infection among Japanese immigrants and their descendants living in Southeast Brazil: A call for preventive and control responses. PLoS Negl. Trop. Dis. 2021;15:e0009066. doi: 10.1371/journal.pntd.0009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes-Gouvêa M.S., Ferreira A.C., Teixeira R., Andrade J.R., Ferreira A.S., Barros L.M., Rezende R.E., Nastri A.C., Leite A.G., Piccoli L.Z., et al. HBV carrying drug-resistence mutations in chronically infected treatment-naive patients. Antivir. Ther. 2015;20:387–395. doi: 10.3851/IMP2938. [DOI] [PubMed] [Google Scholar]
- 14.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 15.Suchard M.A., Lemey P., Baele G., Ayres D.L., Drummond A.J., Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer D.W., Jr., Lemeshow S., Sturdivant R.X. Applied Logistic Regression. 3rd ed. John Wiley & Sons; Hoboken, NJ, USA: 2013. [Google Scholar]
- 17.Carrilho F.J., Ono-Nita S.K., Cardoso R.A., Cancado E.L.R., Pinho J.R.R., Alves V.A.F., Silva L.C. A prospective study of hepatitis B virus markers in patients with chronic HBV infection from Brazilian families of Western and Asian origin. Braz. J. Med. Biol. Res. 2005;38:1399–1408. doi: 10.1590/S0100-879X2005000900015. [DOI] [PubMed] [Google Scholar]
- 18.Kato H., Fujiwara K., Gish R.G., Sakugawa H., Yoshizawa H., Sugauchi F., Orito E., Ueda R., Tanaka Y., Kato T., et al. Classifying genotype F of hepatitis B virus into F1 and F2 subtypes. World J. Gastroenterol. 2005;11:6295–6304. doi: 10.3748/wjg.v11.i40.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida-Neto C., Sabino E.C., Liu J., Blatyta P.F., Mendrone-Junior A., Salles N.A., Leão S.C., Wright D.J., Basques F.V., Ferreira J.E., et al. Prevalence of serologic markers for hepatitis B and C viruses in Brazilian blood donors and incidence and residual risk of transfusion transmission of hepatitis C virus. Transfusion. 2013;53:827–834. doi: 10.1111/j.1537-2995.2012.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima L.A., Lago B.V., Weis-Torres S.M.S., Martins R.M.B., Cesar G.A., Bandeira L.M., Rezende G.R., Lindenberg A.S.C., Gomes S.A., Motta-Castro A.R.C. Hepatitis B: Changes in epidemiological features of Afro-descendant communities in Central Brazil. Sci. Rep. 2020;10:6708. doi: 10.1038/s41598-020-63094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguchi A., Hayashi J., Nakashima K., Ikematsu H., Hirata M., Kashiwagi S. Decrease of Hepatitis A and B virus infection in the population of Okinawa, Japan. J. Infect. 1991;23:255–262. doi: 10.1016/0163-4453(91)92828-S. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) Guidelines Approved by the Guidelines Review Committee. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 23.Rossi C., Shrier I., Marshall L., Cnossen S., Schwartzman K., Klein M.B., Schwarzer G., Greenaway C. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: A systematic review and meta-analysis. PLoS ONE. 2012;7:e0044611. doi: 10.1371/journal.pone.0044611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pottie K., Greenaway C., Feightner J., Welch V., Swinkels H., Rashid M., Narasiah L., Kirmayer L.J., Ueffing E., MacDonald N.E., et al. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824–E925. doi: 10.1503/cmaj.090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinbaum C.M., Mast E.E., Ward J.W. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology. 2009;49:35–44. doi: 10.1002/hep.22882. [DOI] [PubMed] [Google Scholar]
- 26.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 27.Global Burden of Disease Liver Cancer Collaboration The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes G.D.S., Campos D., Ballalai A., Palhares R., da Silva M.R.A., Palhares D.M.F., Neto B.F., Barros F.M.D.R., Gil R.A., Chagas A., et al. Epidemiological and Clinical Patterns of Newly Diagnosed Hepatocellular Carcinoma in Brazil: The Need for Liver Disease Screening Programs Based on Real-World Data. J. Gastrointest. Cancer. 2021;52:952–958. doi: 10.1007/s12029-020-00508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paranaguá-Vezozzo D.C., Ono S.K., Alvarado-Mora M.V., Farias A.Q., Cunha-Silva M., França J.I., Alves V.A., Sherman M., Carrilho F.J. Epidemiology of HCC in Brazil: Incidence and Risk Factors in a Ten-Year Cohort. Ann. Hepatol. 2014;13:386–393. doi: 10.1016/S1665-2681(19)30845-2. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO) Hepatitis D. Key Facts. [(accessed on 1 June 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-d.
- 31.Sakugawa H., Nakasone H., Shokita H., Kawakami Y., Nakachi N., Adaniya H., Mizushima T., Nakayoshi T., Kinjo F., Saito A., et al. Seroepidemiological study on hepatitis delta virus infection in the Irabu Islands, Okinawa, Japan. J. Gastroenterol. Hepatol. 1997;12:299–304. doi: 10.1111/j.1440-1746.1997.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 32.Arakawa Y., Moriyama M., Taira M., Hayashi N., Tanaka N., Okubo H., Sugitani M., Arakawa Y. Molecular analysis of hepatitis D virus infection in Miyako Island, a small Japanese island. J. Viral Hepat. 2000;7:375–381. doi: 10.1046/j.1365-2893.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H., Nagayama K., Enomoto N., Chinzei R., Yamashiro T., Izumi N., Yatsuhashi H., Nakano T., Robertson B., Nakasone H., et al. Chronic hepatitis delta virus infection with genotype IIb variant is correlated with progressive liver disease. J. Gen. Virol. 2003;84:3275–3289. doi: 10.1099/vir.0.19499-0. [DOI] [PubMed] [Google Scholar]
- 34.Lago B.V., Mello F.C.A., Barros T.M., Mello V.M., Villar L.M., Lewis-Ximenez L.L., Pardini M.I.M.C., Lampe E. Hepatitis D infection in Brazil: Prevalence and geographical distribution of anti-Delta antibody. J. Med. Virol. 2018;90:1358–1363. doi: 10.1002/jmv.25196. [DOI] [PubMed] [Google Scholar]
- 35.Ximenes R.A.A., Figueiredo G.M., Cardoso M.R.A., Stein A.T., Moreira R.C., Coral G., Crespo D., Santos A.A., Montarroyos U.R., Braga M.C., et al. Population-Based Multicentric Survey of Hepatitis B Infection and Risk Factors in the North, South, and Southeast Regions of Brazil, 10–20 Years after the Beginning of Vaccination. Am. J. Trop. Med. Hyg. 2015;93:1341–1348. doi: 10.4269/ajtmh.15-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dos Santos Weis-Torres S.M., Fitts S.M.F., Cardoso W.M., Junior M.G.H., Lima L.A., Bandeira L.M., Castro V.O.L., Carneiro F.A., Iglecias L.M.M., Cesar G.A., et al. High level of exposure to hepatitis B virus infection in a vulnerable population of a low endemic area: A challenge for vaccination coverage. Int. J. Infect. Dis. 2020;90:46–52. doi: 10.1016/j.ijid.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Nishiya A.S., Levi J.E., de Almeida-Neto C., Witkin S.S., Ferreira S.C., Bassit L., Sabino E.C., Di-Lorenzo-Oliveira C., Salles N.A., Coutinho A.S., et al. Occult and active hepatitis B virus detection in donated blood in São Paulo, Brazil. Transfusion. 2021;61:1495–1504. doi: 10.1111/trf.16344. [DOI] [PubMed] [Google Scholar]
- 38.De Castro Sant’Annaa C., Almeida M.K.C., Ferreira P., Oliveira R.G., Baraúna A.R.F., Gonçalvez E.C., Silva A.M., Pereira C.S., Martins L.C. Prevalence of Occult Hepatitis B in a Population from the Brazilian Amazon Region. J. Med. Virol. 2018;90:1063–1070. doi: 10.1002/jmv.25051. [DOI] [PubMed] [Google Scholar]
- 39.Fontenele A.M., Gainer J.B., da Silva E., Silva D.V., Cruz Santos M.D., Salgado J.V., Salgado Filho N., Ferreira A.S. Occult hepatites B among patients with chronic renal failure on hemodialysis from a capital city in northeast Brazil. Hemodial. Int. 2015;19:353–359. doi: 10.1111/hdi.12285. [DOI] [PubMed] [Google Scholar]
- 40.Sakugawa H., Ohwan T., Yamashiro A., Oyakawa T., Kadena K., Kinjo F., Saito A. Natural Seroconversion from Hepatitis Be Antigen to Antibody Among Hepatitis B Virus Carriers in Okinawa Islands. J. Med. Virol. 1991;34:122–126. doi: 10.1002/jmv.1890340210. [DOI] [PubMed] [Google Scholar]
- 41.Kashiwagi S., Hayashi J., Ikematsu H., Nomura H., Kusaba T., Shingu T., Hayashida K., Kaji M. An Epidemiologic Study of Hepatitis B virus in Okinawa and Kyushu, Japan. Am. J. Epidemiol. 1983;118:787–794. doi: 10.1093/oxfordjournals.aje.a113696. [DOI] [PubMed] [Google Scholar]
- 42.Kashiwagi S., Hayashi J., Nomura H., Kajiyama W., Ikematsu H., Noguchi A. Changing Pattern of Intrafamilial Transmission of Hepatitis B Virus in Okinawa, Japan. Am. J. Epidemiol. 1988;127:783–787. doi: 10.1093/oxfordjournals.aje.a114859. [DOI] [PubMed] [Google Scholar]
- 43.Rezende G.R., Lago B.V., Puga M.A., Bandeira L.M., Pompilio M.A., Castro V.O.L., Tanaka T.S., Cesar G.A., Oliveira S.M.V.L., Yassuda R.T.S., et al. Prevalence, incidence and associated factors for HBV infection among male and female prisioners in Central Brazil: A multicenter study. Int. J. Infect. Dis. 2020;96:298–307. doi: 10.1016/j.ijid.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Campos R.H., Mbayed V.A., Pineiro Y.L.F.G. Molecular epidemiology of hepatitis B virus in Latin America. J. Clin. Virol. 2005;34:S8–S13. doi: 10.1016/S1386-6532(05)80028-9. [DOI] [PubMed] [Google Scholar]
- 45.Alvarado-Mora M.V., Santana R.A.F., Sitnik R., Ferreira P.R.A., Mangueira C.L.P., Carrilho F.J., Pinho J.R.R. Full-length genomic sequence of hepatitis B virus genotype C2 isolated from a native Brazilian patient. Mem. Inst. Oswaldo Cruz. 2011;106:495–498. doi: 10.1590/S0074-02762011000400017. [DOI] [PubMed] [Google Scholar]
- 46.Sunbul M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014;20:5427–5434. doi: 10.3748/wjg.v20.i18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramvis A. Genotypes and Genetic Variability of Hepatitis B Virus. Intervirology. 2014;57:141–150. doi: 10.1159/000360947. [DOI] [PubMed] [Google Scholar]
- 48.Norder H., Couroucé A.M., Coursaget P., Echevarria J.M., Lee S.D., Mushahwar I.K., Robertson. B. H., Locarnini S., Magnius L.O. Genetic diversity of hepatitis B virus strains derived worldwide: Genotypes, subgenotypes and HBsAg subtypes. Intervirology. 2004;47:289–309. doi: 10.1159/000080872. [DOI] [PubMed] [Google Scholar]
- 49.Ito K., Yotsuyanagi H., Sugiyama M., Yatsuhashi H., Karino Y., Takikawa Y., Saito T., Arase Y., Imazeki F., Kurosaki M., et al. Geographic distribution and characteristics of genotype A hepatitis B virus infection in acute and chronic hepatitis B patients in Japan. J. Gastroenterol. Hepatol. 2016;31:180–189. doi: 10.1111/jgh.13030. [DOI] [PubMed] [Google Scholar]
- 50.Furusyo N., Nakashima H., Kashiwagi K., Kubo N., Hayashida K., Usuda S., Mishiro S., Kashiwagi S., Hayashi J. Clinical Outcomes of Hepatitis B Virus (HBV) Genotypes B and C in Japanese Patients with Chronic HBV Infection. Am. J. Trop. Med. Hyg. 2002;67:151–157. doi: 10.4269/ajtmh.2002.67.151. [DOI] [PubMed] [Google Scholar]
- 51.Ono-Nita S.K., Carrilho F.J., Cardoso R.A., Nita M.E., Silva C. Searching for cjronic hepatitis B patients in a low prevalence área–role of racial origin. BMC Fam. Pract. 2004;5:1–6. doi: 10.1186/1471-2296-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clemente C.M., Carrilho F.J., Pinho J.R.R., Ono-Nita S.K., da Silva L.C., Moreira R.C., Lemos M.F., Mello I.M.V.G.C. A phylogenetic study of hepatitis B virus chronically infected Brazilian patients od Western and Asian descent. J. Gastroenterol. 2009;44:568–576. doi: 10.1007/s00535-009-0044-8. [DOI] [PubMed] [Google Scholar]
- 53.Lago B.V., Espirito-Santo M.P., Costa V.D., Marques V.A., Villar L.M., Lewis-Ximenez L.L., Lampe E., Mello F.C.A. Genetic Diversity of the Hepatitis B Virus Subgenotypes in Brazil. Viruses. 2019;11:860. doi: 10.3390/v11090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panduro A., Maldonado-Gonzalez M., Fierro N.A., Roman S. Distribution of HBV genotypes F and H in Mexico and Central America. Antivir. Ther. 2013;18:475–484. doi: 10.3851/IMP2605. [DOI] [PubMed] [Google Scholar]
- 55.Mello F.C.A., Araujo O.C., Lago B.V., Motta-Castro A.R.C., Moraes M.T.B., Gomes S.A., Bello G., Araujo N.M. Phylogeography and evolutionary history of hepatitis B virus genotype F in Brazil. Virol. J. 2013;10:1–8. doi: 10.1186/1743-422X-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacheco S.R., Santos M.I.M.A., Stocker A., Zarife M.A.S., Schinoni M.I., Parana R., Reis M.G., Silva L.K. Genotyping of HBV and tracking of resistance mutations in treatment-naïve patients with chronic hepatitis B. Infect. Drug. Resist. 2017;10:201–207. doi: 10.2147/IDR.S135420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.