Abstract
Tuberculosis (TB), caused by the human pathogen Mycobacterium tuberculosis (Mtb), is an infectious disease that presents a major threat to human health. Bacillus Calmette-Guérin (BCG), the only licensed TB vaccine, is ineffective against latent TB infection, necessitating the development of further TB drugs or therapeutic vaccines. Herein, we evaluated the therapeutic effect of a novel subunit vaccine AEC/BC02 after chemotherapy in a spontaneous Mtb relapse model. Immunotherapy followed 4 weeks of treatment with isoniazid and rifapentine, and bacterial loads in organs, pathological changes, and adaptive immune characteristics were investigated. The results showed slowly increased bacterial loads in the spleen and lungs of mice inoculated with AEC/BC02 with significantly lower loads than those of the control groups. Pathological scores for the liver, spleen, and lungs decreased accordingly. Moreover, AEC/BC02 induced antigen-specific IFN-γ-secreting or IL-2-secreting cellular immune responses, which decreased with the number of immunizations and times. Obvious Ag85b- and EC-specific IgG were observed in mice following the treatment with AEC/BC02, indicating a significant Th1-biased response. Taken together, these data suggest that AEC/BC02 immunotherapy post-chemotherapy may shorten future TB treatment.
Keywords: tuberculosis, AEC/BC02 vaccine, latent tuberculosis infection, immunotherapy, relapse
1. Introduction
Tuberculosis is a chronic infectious disease caused by Mycobacterium tuberculosis that is transmitted through the respiratory tract and remains one of the top 10 causes of mortality worldwide [1]. The World Health Organization has reported that the incidence of TB in 2019 was 10 million, including 1.2 million deaths among HIV-negative individuals and an additional 208,000 deaths among HIV-positive individuals [2]. China currently accounts for 8.4% of global TB patients, ranking third in the world. Particularly in light of the disruption to medical services caused by the coronavirus disease-2019 (COVID-19) pandemic, it may also have contributed to the neglect of known diseases such as tuberculosis, the knowledge of which was already less than ideal [3]. Further, it is also estimated that a quarter of the population in the world have latent tuberculosis infection (LTBI), and most cases of tuberculosis resulted from reactivation of LTBI [4]. Therefore, the prevention and control of LTBI has major implications for the approach to tuberculosis elimination [5].
Currently, Bacillus Calmette-Guérin (BCG) is the only available vaccine to prevent life-threatening TB disease in infants and children [6,7]. However, it has demonstrated limited and variable effectiveness (0% to 80%) in preventing pulmonary TB and in curbing the transmission of Mtb in adults and adolescents [8,9,10]. In addition, BCG is ineffective in containing reactivation of latent TB infection (LTBI) [11]. Chemotherapy of TB has a cure rate of approximately 85%, with shortcomings such as long treatment cycles and side effects [12,13]. Currently, immunotherapy as an adjunct to chemotherapy has been used in many experimental models to eliminate proliferation of latent infection Mtb by non-specific or antigen-specific immunological agents [14]. Relapse of Mtb in the lungs can be reduced with an immunotherapy regimen consisting of recombinant IFN-γ + IgA + anti-IL-4 or muramyl dipeptide followed by short-term (incomplete) chemotherapy [14,15]. Studies have found that vaccines can effectively reduce the post-chemotherapy relapse of Mtb-infected mice when using hsp65 or Ag85A DNA vaccine, or fragmented Mtb cells detoxified with liposomed (RUTI) [12,16,17,18]. On the basis of the efficacy of vaccines after chemotherapy, new therapeutic vaccines are a potential preventive or therapeutic method, as an adjunct to chemotherapy.
It is reported that Ag85b, ESAT6, and CFP10 are promising vaccine candidate molecules given that they are strongly recognized by T-cell antigens in the first phase of infection [19,20]. Previous studies demonstrated that Ag85b immunization can provide protection against TB infection, which is associated with increased IFN-γ production [21]. ESAT6 induces a strong cellular immune response that may provide protection in individuals with prior TB exposure [22,23], and CFP10 activates CD8+ T cells in vivo, which have persistent cytolytic activity after Mtb infection [24]. We have constructed a candidate TB vaccine, designated AEC/BC02, using the Ag85b antigen and ESAT6-CFP10 (EC) fusion protein, along with the new adjuvant BC02 from the BCG-derived unmethylated cytosine–phosphate–guanine (CpG) DNA fragment and aluminum salt. It has been demonstrated that BC02 was safe and increased both antigen-specific IL-12 and IFN-γ levels [25], and also induced innate immunity [26]. Interestingly, we found that AEC/BC02 cannot provide effective protection as a pre-exposure prophylaxis vaccine, possibly because the recombinant protein vaccine contained only a few antigens, which was not sufficient to elicit the equal protective immune effect to BCG, but AEC/BC02 can be used as an immunotherapeutic against progressive disease in a guinea pig LTBI model after chemotherapy [27]. However, existing studies are limited to the analysis of a single immunological index induced by AEC/BC02, while other immune effectors induced by the vaccine, the effects on the humoral immune response, and the immune response induced in cases of Mtb latent infection remain unclear. Therefore, in this study, we investigated the therapeutic effect and immunological properties in terms of humoral and cellular immunity of the candidate AEC/BC02 vaccine of post-chemotherapy against latent Mtb infection in a mouse model.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
The Mtb strain (CMCC 95052) used in this study was grown on Lowenstein Jensen medium at 37 °C for approximately 4 weeks. The turbidity of the bacterial suspensions was measured using a McNamara turbidimetric tube assay, and then bacteria were diluted to 1 mg/mL. The samples were stored at −70 °C in aliquots.
2.2. Mice
Specific-pathogen-free C57BL/6 mice (females, 6–8-weeks-old) were purchased from the Institute for Laboratory Animal Resources, National Institutes for Food and Drug Control (NIFDC). Mice were housed under barrier conditions at an Animal Biosafety Level III laboratory. All animals used in this study were treated according to the standards of animal welfare and procedures were reviewed by the Animal Care and Welfare Committee of the NIFDC.
2.3. Vaccine Preparation
To generate the recombinant tuberculosis vaccine AEC/BC02, Ag85b and fusion protein ESAT6-CFP10 were combined with Bacillus Calmette-Guérin CpG and the aluminum (InvivoGen, Cayla, France) adjuvant system. The Mtb antigen proteins and CpG were provided by Anhui Zhifei Longcom Biologic Pharmacy Company (Hefei, China).
One dose of AEC/BC02 vaccine comprised 10 μg of Ag85b and 10 μg of ESAT6-CFP10 (EC) in 0.2 mL of adjuvant BC02. Per mouse, the BC02 contained 0.2 mg of aluminum hydroxide and 75 μg of CpG in phosphate-buffered saline (PBS).
2.4. Study Design
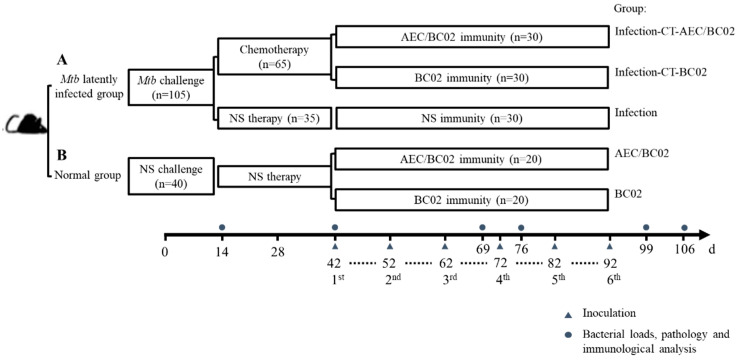
One hundred and forty-five mice were randomly allocated into group A (Mtb latently infected group, n = 105) and group B (normal control group, n = 40). On day 0, mice in group A (Figure 1A) were intraperitoneally challenged with 0.5 mL of Mtb (5 × 105 CFU). On day 14, mice were administered chemotherapy (CT) or normal saline (NS) therapy twice a week for four weeks by oral gavage. The administration volume for each mouse was 0.2 mL of isoniazid and rifapentine suspension, containing 0.5 mg and 0.2 mg, respectively. Mice in group B (Figure 1B) were injected intraperitoneally and treated orally with an equal volume of NS to that of group A. On day 42, mice subjected to the infection-CT-AEC/BC02 (n = 30), AEC/BC02 (n = 20) schedule, were administered intramuscularly (i.m.) with a dose of AEC/BC02 vaccine; mice subjected to the infection-CT-BC02 (n = 30), BC02 (n = 20) schedule, were administered i.m. with a dose of BC02; and mice subjected to the infection (n = 30) schedule were administered i.m. with 0.2 mL of NS. All mice were inoculated six times at 10-day intervals with a dosage volume of 0.2 mL. Finally, all animals were euthanized at pre-treatment (day 14), pre-immune (day 42), and 1 and 2 weeks after immunization 3 (day 66 and day 76) and 6 (day 99 and day 106), and were tested for immunogenicity, organ bacterial loads, and pathological analysis.
Figure 1.
Immunotherapy schedule for evaluation of a potential vaccine candidate in a Mtb latently infected mouse model. Mice were randomly divided into groups A and B. (A) For Mtb latently infected group: mice were intraperitoneally challenged with 5 × 105 CFU of Mtb (t = 0 day), followed by chemotherapeutic treatment twice a week for 4 weeks (2 weeks after challenge) or NS as an infection control. (B) Normal group: mice were injected and treated with NS on days 0 and 14. Except for the infection control group of group A that was not immunized, the other mice were separately inoculated with one dose of AEC/BC02 and BC02 six times at 10-day intervals. Mice in all groups were euthanized at 14, 42, 69, 76, 99, or 106 days for immunological, bacterial load, and pathological analysis.
2.5. Determination of Bacterial Loads and Pathology
After the mice had been euthanized, the liver, spleen, and lungs were excised. Half of the harvested spleens and lungs were diluted with 0.5 mL of ground homogenate and 4.5 mL of normal saline and were plated on modified Lowenstein Jenson medium. The CFUs were determined after 4 weeks of incubation at 37 °C.
The other half of the lungs, spleens, and livers were examined for pathology. The tissues were embedded in paraffin blocks, sectioned at a 5 μm thickness, and stained with a hematoxylin and eosin (H&E) staining solution. The sections were then observed for lesions under an optical microscope. Pathological scores for the livers, spleens, and lungs were evaluated separately. The pathological changes of granuloma, edema, monocyte infiltration, and macrophage aggregation were semi-quantitatively scored. No lesions were scored as 0 points, very mild as 1 point, mild as 2 points, moderate as 3 points, and severe as 4 points. All pathological results in this experiment were scored by the same professional pathologist for blind analysis.
2.6. IFN-γ/IL-2 Enzyme-Linked Immunospot (ELISPOT) Assay
To quantify the number of cells secreting antigen-specific IFN-γ/IL-2 in the spleen, an ELISPOT assay was performed according to the manufacturer’s instructions (Mabtech AB, Nacka Strand, Sweden). In brief, freshly harvested splenocytes (2.5 × 105/well) at 1 and 2 weeks after the third and sixth immunizations were stimulated with the synthesized Ag85b, ESAT6, and CFP10 peptide pools (15 amino acids, overlapping 10 amino acids, 2 μg/mL); concanavalin A (positive control, 1 μg/mL); or medium alone (negative control) for 48 h in a 37 humidified incubator with 5% CO2. At the end of the culture period, plates were washed five times with PBS (200 μL/well) and incubated for 2 h with the related biotinylated detection antibody. Streptavidin–horseradish peroxidase was then added for 1 h, followed by the substrate solution. The ELISPOT plates were incubated in the dark at room temperature until spots were visible, and dried after the reaction was terminated by washing with deionized water. The number of spot-forming cells (SFCs) was counted by an automated ELISPOT reader (Cellular Technology Ltd., Cleveland, OH, USA). The negative control was subtracted from the antigen-specific response at each time point, and the results were reported as the number of SFCs per 2.5 × 105 splenocytes.
2.7. Determination of Antibody Titers
Serum samples were collected 1 and 2 weeks after the third and sixth immunizations to evaluate antibody levels. Antigen-specific IgG, IgG1, and IgG2a levels in serum were measured via an enzyme-linked immunosorbent assay (ELISA). The 96-well plates were coated with 0.1 μg of Ag85b or EC proteins per well and incubated overnight at 4 °C, then blocked with PBS containing 1% BSA at 37 °C for 2 h and washed with 0.05% Tween-20 in PBS. Sera were serially diluted twofold and incubated at 37 °C for 1 h. Then, the plates were washed, and the bound antibodies were reacted with HRP-conjugated goat anti-mouse IgG (1:5000) (Abcam), IgG1 (1:5000) (Alpha Diagnostic International), and IgG2a (1:10,000) (Alpha Diagnostic International) at 37 °C for 1 h, followed by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution. The reaction was stopped by the addition of 2 M H2SO4, and the optical density (OD) of the samples was measured at 450 nm, with 620 nm as the reference wavelength. The endpoint titers were calculated as the reciprocal of the last dilution reaching a cut-off value set to 2.1-fold above the mean optical density of a negative control.
2.8. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software), and the statistical significance of the difference between two groups was performed using the t-test or Mann–Whitney U test, with one-way ANOVA or Kruskal–Wallis test for the analysis of differences between multiple groups. The results are expressed as mean ± SEM. Significant differences were established if p < 0.05. */#p < 0.05, or **/##p < 0.01.
3. Results
3.1. Therapeutic Effect of the AEC/BC02 Vaccine on the Bacterial Loads in Latently-Infected Mice
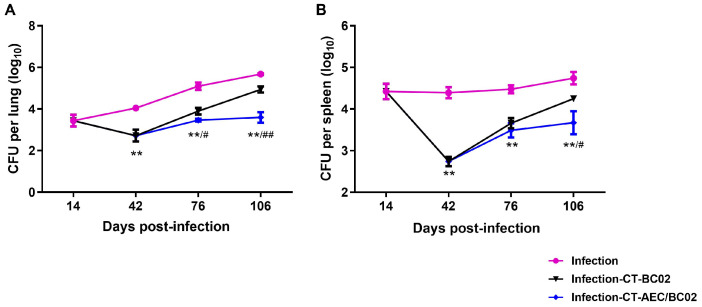
To evaluate the therapeutic effect of AEC/BC02 vaccine after chemotherapy on mice with Mtb latent infection, the bacterial loads in the lungs and spleens of mice on different time points were analyzed. As shown in Figure 2, 2 weeks after Mtb infection of mice, the bacterial counts in the lungs and spleens were 3.44 ± 0.28 log10 CFU and 4.42 ± 0.18 log10 CFU, respectively. The counts of bacteria in the lungs and spleens of the infected group without chemotherapy or immunotherapy, increased gradually with time, while the number of bacteria in the infected mice after 4 weeks of chemotherapy (day 42) declined significantly.
Figure 2.
Evaluation of the immunotherapeutic effects of AEC/BC02 vaccine after chemotherapy in the Mtb latent infection mouse model. Bacterial loads in the lungs (A) and spleens (B) were measured in mice. Data are shown as the mean ± SEM. ** The infection-CT-AEC/BC02 group was compared with the infection group. #/## The infection-CT-AEC/BC02 group was compared with the infection-CT-BC02 group. Statistical significance was set at # p < 0.05 or **/## p < 0.01 (n = 5).
In contrast, the bacterial burden in the organs of each group gradually increased after chemotherapy, but this burden was lower in the infection-CT-AEC/BC02 group than in the infection-CT-BC02 group and differed significantly from the infection group (p < 0.01). Two weeks after the third dose of immunization (76 days), mice in the infection-CT-AEC/BC02 group had a significantly lower bacterial load in the lungs and lower bacterial load in the spleen compared with the infection-CT-BC02 group (0.43 ± 0.17 log10 CFU, p < 0.05 and 0.17 ± 0.13 log10 CFU reduction, respectively). Similar but lower reductions were observed in the lungs and spleen two weeks after the sixth immunization (106 days), with the bacterial load being significantly lower in the infection-CT-AEC/BC02 group than the infection-CT-BC02 group (1.34 ± 0.30 log10 CFU reduction, p < 0.01 and 0.58 ± 0.29 log10 CFU reduction, p < 0.05). These results indicated that AEC/BC02 immunotherapy can effectively inhibit the proliferation of Mtb in mice with latent Mtb infection and provide effective immune protection against Mtb.
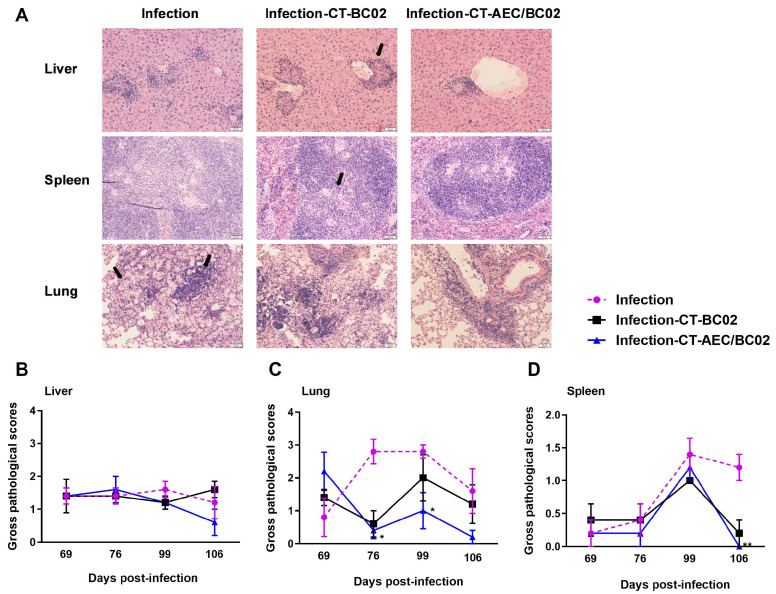
3.2. Histological Changes and Lesion Scoring in Latently Infected Mice following the Treatment with AEC/BC02
Histological findings revealed that the livers and spleens of mice mainly developed granulomatous lesions, and the lungs mainly showed pathological changes involving monocyte infiltration, macrophage aggregation, and edema (Figure 3A). Semi-quantitative scoring was performed for the above lesions, and the detailed results are described in Figure 3B–D. From 69 days to 106 days after infection, the pathological scores for the livers, lungs, and spleens in the infection-CT-AEC/BC02 group gradually decreased below those of the infection and infection-CT-BC02 groups. Statistically significant differences were found in the pathological scores for lungs between the infection-CT-AEC/BC02 and infection groups at 76 and 99 days after infection (p < 0.05). Furthermore, the pathological scores for spleens of the infection-CT-AEC/BC02 and infection groups also showed a significant difference on day 106 after infection (p < 0.01). Taken together, these data indicated that the AEC/BC02 vaccine could reduce tissue lesions and thus provided a degree of protection in mice latently infected with Mtb.
Figure 3.
Evaluation of the immunotherapeutic effects of AEC/BC02 vaccine on tissue pathology in the livers, lungs, and spleens in Mtb latently infected mice. (A) Pathological changes in the livers, lungs, and spleens of mice. Black arrows indicate granulomatous lesions of the livers and spleens, as well as the infiltration of monocytes and the accumulation of macrophages in the lungs. Pathological scores are shown for the livers (B), lungs (C), and spleens (D) of mice. The infection-CT-AEC/BC02 group was compared with the infection group, and statistical significance was set at * p < 0.05 or ** p < 0.01 (n = 5).
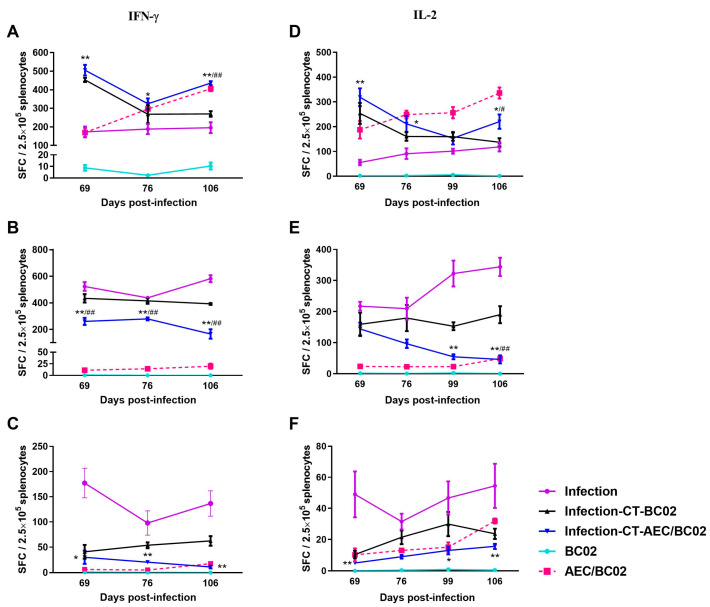
3.3. Cellular Immune Response in Mouse Models
To further investigate the cell-mediated response induced by AEC/BC02, the number of antigen-specific IFN-γ/IL-2-secreting cells was determined in the splenocytes of mice re-stimulated with the Ag85b/ESAT6/CFP-10 peptide by ELISPOT. As shown in Figure 4, in the normal mouse model, specific IFN-γ/IL-2-secreting cells were almost undetectable in the BC02 group, while the number of specific IFN-γ/IL-2 SFCs was significantly higher in the AEC/BC02 group and increased with the increase in immunization dose and time.
Figure 4.
The frequency of spot-forming cells expressing IFN-γ or IL-2 was determined by ELISPOT. The magnitude of the IFN-γ-secretion response produced by stimulation of splenic lymphocytes in normal mice and Mtb latently infected mice with Ag85b (A), ESAT6 (B), and CFP10 (C) peptides. The secretion of IL-2 cells stimulated by Ag85b (D), ESAT6 (E), and CFP10 (F) peptides was detected in the splenocytes of the two mouse models. */** The infection-CT-AEC/BC02 group was compared with the infection group. #/## The infection-CT-AEC/BC02 group was compared with the infection-CT-BC02 group. Statistical significance was set at */# p < 0.05 or **/## p < 0.01 (n = 5).
In the Mtb latently infected mouse model, the number of Ag85b/ESAT6-specific IFN-γ/IL-2 cells and the number of CFP10-specific IFN-γ cells decreased with increased immunization dose and time, while the number of CFP10-specific IL-2 cells increased in the infection-CT-AEC/BC02 group. Furthermore, the number of Ag85b-specific IFN-γ/IL-2 cells was higher than that in the infection-CT-BC02 and infection groups, and the number of ESAT6/CFP10-specific IFN-γ/IL-2 cells showed the opposite trend. In addition, the Ag85b/ESAT6/CFP10-specific IFN-γ cell immune response and the ESAT6-specific IL-2 cell immune response induced by AEC/BC02 in the Mtb latent infection mice were both higher than those in normal mice, while Ag85b/CFP10-specific IL-2 cell immunity was lower than that in normal mice. Taken together, a long-term specific cellular immune response could be induced in all groups of Mtb latently infected mice and the AEC/BC02 group of normal mice.
3.4. Humoral Immune Response in Mouse Models
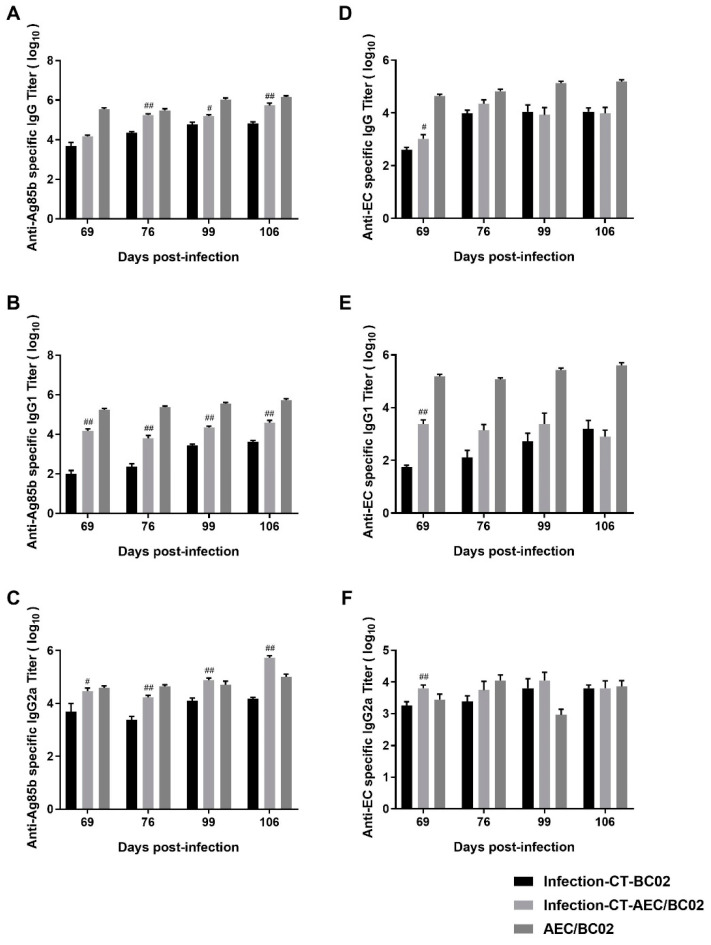
To evaluate the level of humoral immunity induced by the AEC/BC02 vaccine, antigen-specific IgG, IgG1, and IgG2a antibody levels were determined in the serum of test mice 1 and 2 weeks after the third and sixth immunizations with latently-infected Mtb and normal mice. As shown in Figure 5, the Ag85b- and EC-specific IgG antibodies and subtypes were detectable in the sera of all groups, and high antibody titers were only detected in the infection-CT-AEC/BC02, infection-CT-BC02, and AEC/BC02 groups, and no positive antibody was detected in the other groups. For specific anti-Ag85b antibodies (Figure 5A–C), the antibody titers of the three groups increased along with the increase in immunization dose and time, and the antibody levels of IgG and IgG1 were highest in the AEC/BC02 group, followed by the infection-CT-AEC/BC02 group, and lastly the infection-CT-BC02 group. However, the titer of IgG2a increased in the infection-CT-AEC/BC02 group, eventually exceeding that of the AEC/BC02 group over time. With the exception of the anti-Ag85b IgG titer at 69 days, the IgG titers in the vaccine groups were significantly higher than in the adjuvant group (p < 0.05 or p < 0.01). For specific anti-EC antibodies (Figure 5D–F), the titers and antibody intensities were similar to Ag85b, except that the infection-CT-AEC/BC02 group antibodies showed a slight downward trend over time. The infection-CT-AEC/BC02 group showed a slight increase in specific anti-EC antibodies compared with the infection-CT-BC02 group, but no statistical significance was detected, with the exception of the anti-EC antibody titers at 69 d. In conclusion, the AEC/BC02 vaccine could induce a high level of humoral immune response in normal mice and significantly enhance antibody levels in Mtb latently infected mice compared with the adjuvant group.
Figure 5.
Characteristics of the humoral immune responses in Mtb latently infected and normal mice immunized with AEC/BC02. Ag85b-specific IgG (A), IgG1 (B), and IgG2a (C) in the infection-CT-BC02, infection-CT-AEC/BC02, and AEC/BC02 immunized mice were determined by ELISAs. EC-specific IgG (D), IgG1 (E), and IgG2a (F) in the infection-CT-BC02, infection-CT-AEC/BC02, and AEC/BC02 immunized mice were determined by ELISAs. Data from the infection-CT-AEC/BC02 group were compared with the infection-CT-BC02 group, and statistical significance was set at # p < 0.05, ## p < 0.01 (n = 5).
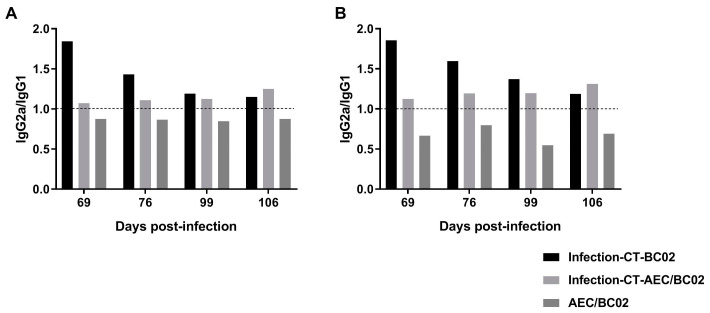
Furthermore, we analyzed the ratio of IgG2a/IgG1 in each group, as shown in Figure 6, and the immune response induced by AEC/BC02 in normal mice was biased towards Th2, while the immune response induced in Mtb latently infected mice was biased towards Th1. Furthermore, the Th1 immune response was increased with prolonged immunization time. BC02 could also induce a Th1-biased immune response in mice latently infected with Mtb, but this type of response gradually decreased with time.
Figure 6.
The immunological types of Mtb latently infected and normal mice induced by AEC/BC02. (A) The ratio of Ag85b-specific IgG2a/IgG1. (B) The ratio of EC-specific IgG2a/IgG1. IgG2a/IgG1 > 1 indicates Th1-type bias, whereas IgG2a/IgG1 < 1 indicates Th2-type bias.
4. Discussion
TB remains the most common cause of death from a single infectious pathogen [2], highlighting the urgent need for safer and more effective vaccines to control the spread of TB, especially because the commercially available vaccine BCG is ineffective in adults and LTBI. Due to the presence of latent Mtb in lesions, chemotherapy is less efficacious [28]. Previous studies have shown the AEC/BC02 vaccine to not be used as a pre-exposure preventive vaccine. However, the vaccine can inhibit Mtb infection in guinea pigs latently infected after chemotherapy, suggesting the potential of AEC/BC02 as an adjuvant therapy after chemotherapy [27]. Analyzing the immunological characteristics of this type of vaccine may help our understanding of the immune mechanism involved in Mtb infection and potential immunological indicators related to protective efficacy.
The intraperitoneal infection model has similar characteristics to low-dose aerosol infection, such as the establishment of stable CFU levels in organs after a period of challenge that does not change significantly over time [29]. Dhillon et al. reported that after 4 weeks of intraperitoneal challenge in mice, bacteria isolated from organs had some similarities to dormant Mtb obtained in vitro, suggesting the formation of a dormant (latent) bacterial population in this model [30]. In addition, intraperitoneal challenge also has the advantages of low levels of cross-contamination between animals and low experimental costs. Therefore, in this study, we used the less commonly used intraperitoneal challenge. The effect of AEC/BC02 immunotherapy was evaluated by organ load and pathological score. The results showed that the bacterial load of the spleen and lungs of the AEC/BC02 vaccine group was significantly decreased compared with the BC02 control group in a mouse model of latent infection after chemotherapy. Bacterial load was significantly lower than that of the infection control group, indicating that the immune response induced by AEC/BC02 may elicit a protective response to inhibit or delay the recurrence of Mtb in mice. Alternatively, the effectiveness of the vaccine may be related to the previous chemotherapy, on the basis of the observations of our previous study that immunization with the AEC/BC02 vaccine alone could not effectively reduce the number of bacteria in the organs [27]. This may be linked to the reduction of the immune response by chemotherapy, enabling the AEC/BC02 vaccine induced immune response to be effective [31]. AEC/BC02 vaccine may enhance previous immune responses or induce new immune responses against Mtb antigens. The increase in bacterial load in the chemotherapy + adjuvant group was higher than that in the vaccine group due to lack of sufficient immune response. Furthermore, the pathological scores for the liver, spleen, and lungs were also decreased in the AEC/BC02 vaccine group. It is worth noting that the pathological score of the BC02 group was also reduced, but higher than that of the vaccine group, which may have been related to the innate immune response induced by the BC02 adjuvant [26].
The cellular immune response is involved in protecting humans and animals from Mtb infection [32,33]. IFN-γ is an important effector that stimulates the production of reactive nitrogen intermediates and reactive oxygen intermediates, which further promote the maturation of phagocytes, thereby activating macrophages and clearing Mtb-infected cells [34,35,36]. IL-2 can not only maintain the survival of memory cells, but also plays an important role in the clonal proliferation and differentiation of T cells after antigen recognition, as well as promoting the activation of macrophages [37]. The antigen-specific IFN-γ and IL-2 secreting cells produced by the splenocytes of mice in each group were quantified by ELISPOT. We found that the production of Ag85b-specific IFN-γ/IL-2 in the vaccine group was upregulated compared with the control group and was negatively correlated with the proliferation of bacteria in the organs of latently infected mice. This result indicates that the Ag85b-specific Th1 response may be more important for protection from recurrence of Mtb. Interestingly, we also found that the number of ESAT6/CFP10-specific IFN-γ/IL-2 cells was greater in the infection group than in the BC02 adjuvant group, or the AEC/BC02 vaccine group. One possible hypothesis is the reduction of ESAT6/CFP10 expression by bacteria in response to chemotherapy-induced stress. Studies have shown that direct injection of Th1-polarized ESAT-6-specific CD4+ T cell clones cannot reduce bacterial loads in chemotherapeutic Mtb-infected mice [38]. The level of MHC-II associated ESAT-6 peptide on the surface of infected cells is reduced, thereby limiting the response of these lymphocytes. AEC/BC02 immunization may further reduce the expression of ESAT-6/CFP-10, resulting in downregulation of ESAT6/CFP10-specific IFN-γ/IL-2 cells. Ag85b, contrary to ESAT6/CFP10, may have enhanced expression during the Mtb chronic infection stage. It is of note that the immune response of IFN-γ/IL-2-secreting lymphocytes induced by AEC/BC02 in the latently infected mice decreased gradually with increased immunization dose and time. At present, there are limited reports evaluating the immunogenicity and immune efficacy of vaccines in infected animals. Alyahya et al. [39] reported that the cellular immune response and bacterial loads in tissues were decreased in mice following antibiotic treatment. In conclusion, the vaccine group showed similar changes in cellular immunology and organ bacterial loads to the antibiotic treatment group in the study by Alyahya and colleagues, suggesting that the AEC/BC02 vaccine provided effective anti-TB immune protection against latent Mtb infection in mice.
Normal and Mtb latently infected mice were immunized with AEC/BC02 or BC02, and serum samples were collected 1 and 2 weeks after the third and sixth immunizations to detect antibody levels. The results showed that AEC/BC02 vaccine could induce high titers of Ag85b- and EC-specific IgG, IgG1, and IgG2a antibodies with increased immunization doses and time. Surprisingly, we found high levels of antibodies in the infection-CT-BC02 group, but no antibodies were detected in the infection group. A possible explanation is that the long-term presence of high Mtb loads in infected mice may lead to rapid depletion and impaired function of B cells [40], so that antibody levels do not reach the limit of detection at the later stage of infection. However, the presence of chemotherapy in the infection-CT-BC02 group can reduce the bacterial load, possibly improve the in vivo environment, and restore the function of B cells. The presence of CpG in BC02 adjuvant may also play a role in the production of antibodies, and the specific mechanism remains to be explored. Some studies have reported that high levels of antibodies can prolong the survival of animals infected with Mtb [41,42], and can reduce the bacterial loads in infected tissues during the early stage of Mtb infection [43,44]. Therefore, it is speculated that specific antibodies induced by AEC/BC02 may be beneficial to the control of Mtb in latently infected animals. In addition, the levels of IgG and IgG1 antibodies induced in mice with latent Mtb infection were lower than those in normal mice, with the exception of IgG2a. Furthermore, the immune response induced by AEC/BC02 in Mtb latently infected mice showed a Th1 bias, indicating that the immune response in infected mice was mainly a cellular immune response. In addition, the antibody level was lower than that in normal mice, which may be related to the role of antibodies during the process of anti-Mtb infection. It is known that specific antibodies can recognize the surface antigen of Mtb, and their Fc segments can bind to the Fc receptors on the surface of killer cells, thereby mediating antibody-dependent cell-mediated cytotoxicity. Furthermore, the binding of Mtb to Fc receptors can guide the uptake of Mtb by macrophages and stimulate the activation of antigen-presenting cells, thus activating a series of subsequent specific immune reactions. It can also increase reactive oxygen intermediate products and promote the fusion of phagocytes and lysosomes [45,46,47,48]. Therefore, the antibody response may have a greater role in Mtb infection.
The limitation of this study is that only one route of infection with Mtb were used in the animal model. The candidate therapeutic vaccine AEC/BC02 should be further investigated in more animal models with other infection route in future.
In summary, this study revealed that AEC/BC02 immunotherapy could provide effective protection against Mtb latently infected mice after chemotherapy. It was confirmed that the vaccine induced a Th1-biased immune response in infected organisms, and this antigen-specific cellular immune response decreased with time. Our findings provided reference data for future research on the immunological characteristics of AEC/BC02 with respect to latent Mtb infection.
Author Contributions
Conceptualization, M.X. and J.L.; methodology, J.L., X.G., C.W., W.D., X.S. and C.S.; formal analysis, J.L., X.G. and C.W.; writing—original draft preparation, X.G.; writing—review and editing, Y.W. and M.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animals used in this study were treated according to the standards of animal welfare. Procedures were reviewed by the Animal Care and Welfare Committee of the NIFDC.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a grant from the National Key R&D Program of China (no. 2021YFC2301703).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khoshnood S., Heidary M., Haeili M., Drancourt M., Darban-Sarokhalil D., Nasiri M.J., Lohrasbi V. Novel vaccine candidates against Mycobacterium tuberculosis. Int. J. Biol. Macromol. 2018;120:180–188. doi: 10.1016/j.ijbiomac.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Chakaya J., Khan M., Ntoumi F., Aklillu E., Razia F., Mwaba P., Kapata N., Mfinanga S., Hasnain S.E., Katoto P.D., et al. Global Tuberculosis Report 2020—Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021;113:S7–S12. doi: 10.1016/j.ijid.2021.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montagna M.T., Napoli C., Tafuri S., Agodi A., Auxilia F., Casini B., Coscia M.F., D’Errico M.M., Ferrante M., Fortunato A., et al. Knowledge about tuberculosis among undergraduate health care students in 15 Italian universities: A cross-sectional study. BMC Public Health. 2014;14:970. doi: 10.1186/1471-2458-14-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horsburgh C.R., Jr., O’Donnell M., Chamblee S., Moreland J.L., Johnson J., Marsh B.J., Narita M., Johnson L.S., von Reyn C.F. Revisiting rates of reactivation tuberculosis: A population-based approach. Am. J. Respir. Crit. Care Med. 2010;182:420–425. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zumla A., Marais B.J., McHugh T.D., Maeurer M., Zumla A., Kapata N., Ntoumi F., Chanda-Kapata P., Mfinanga S., Centis R., et al. COVID-19 and tuberculosis—Threats and opportunities. Int. J. Tuberc. Lung Dis. 2020;24:757–760. doi: 10.5588/ijtld.20.0387. [DOI] [PubMed] [Google Scholar]
- 6.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B., Dockrell H.M., Ottenhoff T.H., Evans T.G., Zhang Y. Tuberculosis vaccines: Opportunities and challenges. Respirology. 2018;23:359–368. doi: 10.1111/resp.13245. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher H.A., Schrager L. TB vaccine development and the End TB Strategy: Importance and current status. Trans. R. Soc. Trop. Med. Hyg. 2016;110:212–218. doi: 10.1093/trstmh/trw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen P., Doherty T.M. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 10.Dockrell H.M., Smith S.G. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front. Immunol. 2017;8:1134. doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E., Rodrigues L.C., Smith P.G., Lipman M., Whiting P.F., et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 12.Ha S.-J., Jeon B.-Y., Kim S.-C., Kim D.-J., Song M.-K., Sung Y.-C., Cho S.-N. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003;10:1592–1599. doi: 10.1038/sj.gt.3302057. [DOI] [PubMed] [Google Scholar]
- 13.Nahid P., Dorman S.E., Alipanah N., Barry P.M., Brozek J.L., Cattamanchi A., Chaisson L.H., Chaisson R.E., Daley C.L., Grzemska M., et al. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016;63:853–867. doi: 10.1093/cid/ciw566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buccheri S., Reljic R., Caccamo N., Meraviglia S., Ivanyi J., Salerno A., Dieli F. Prevention of the post-chemotherapy relapse of tuberculous infection by combined immunotherapy. Tuberculosis. 2009;89:91–94. doi: 10.1016/j.tube.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Venkataprasad N., Ledger P., Ivanyi J. The effect of glucosaminylmuramyl dipeptide injection to mice on the course of tuberculous infection and in vitro superoxide anion production. Int. Arch. Allergy Immunol. 1997;114:23–29. doi: 10.1159/000237638. [DOI] [PubMed] [Google Scholar]
- 16.Lowrie D.B., Silva C.L. Enhancement of immunocompetence in tuberculosis by DNA vaccination. Vaccine. 2000;18:1712–1716. doi: 10.1016/S0264-410X(99)00512-5. [DOI] [PubMed] [Google Scholar]
- 17.Lowrie D.B., Tascon R.E., Bonato V., Lima V., Faccioli L.H., Stavropoulos E., Colston M.J., Hewinson R.G., Moelling K., Silva C. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 18.Cardona P.-J., Amat I., Gordillo S., Arcos V., Guirado E., Díaz J., Vilaplana C., Tapia G., Ausina V. Immunotherapy with fragmented Mycobacterium tuberculosis cells increases the effectiveness of chemotherapy against a chronical infection in a murine model of tuberculosis. Vaccine. 2005;23:1393–1398. doi: 10.1016/j.vaccine.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu W., Xu Y., Yan J., Shen H., Yang E., Wang H. Ag85B synergizes with ESAT-6 to induce efficient and long-term immunity of C57BL/6 mice primed with recombinant Bacille Calmette-Guerin. Exp. Ther. Med. 2016;13:208–214. doi: 10.3892/etm.2016.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farsiani H., Mosavat A., Soleimanpour S., Sadeghian H., Eydgahi M.R.A., Ghazvini K., Sankian M., Aryan E., Jamehdar S.A., Rezaee S.A. Fc-based delivery system enhances immunogenicity of a tuberculosis subunit vaccine candidate consisting of the ESAT-6:CFP-10 complex. Mol. BioSyst. 2016;12:2189–2201. doi: 10.1039/C6MB00174B. [DOI] [PubMed] [Google Scholar]
- 21.Norrby M., Vesikari T., Lindqvist L., Maeurer M., Ahmed R., Mahdavifar S., Bennett S., McClain J.B., Shepherd B.M., Li D., et al. Safety and immunogenicity of the novel H4:IC31 tuberculosis vaccine candidate in BCG-vaccinated adults: Two phase I dose escalation trials. Vaccine. 2017;35:1652–1661. doi: 10.1016/j.vaccine.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Wang C., Lu J., Du W., Wang G., Li X., Shen X., Su C., Yang L., Chen B., Wang J., et al. Ag85b/ESAT6-CFP10 adjuvanted with aluminum/poly-IC effectively protects guinea pigs from latent mycobacterium tuberculosis infection. Vaccine. 2019;37:4477–4484. doi: 10.1016/j.vaccine.2019.06.078. [DOI] [PubMed] [Google Scholar]
- 23.Gupta N., Vedi S., Kunimoto D.Y., Agrawal B., Kumar R. Novel lipopeptides of ESAT-6 induce strong protective immunity against Mycobacterium tuberculosis: Routes of immunization and TLR agonists critically impact vaccine’s efficacy. Vaccine. 2016;34:5677–5688. doi: 10.1016/j.vaccine.2016.08.075. [DOI] [PubMed] [Google Scholar]
- 24.Kamath A.B., Woodworth J., Xiong X., Taylor C., Weng Y., Behar S.M. Cytolytic CD8+ T Cells Recognizing CFP10 Are Recruited to the Lung after Mycobacterium tuberculosis Infection. J. Exp. Med. 2004;200:1479–1489. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L., Xu M., Wang Z.Y., Chen B.W., Du W.X., Su C., Shen X.B., Zhao A.H., Dong N., Wang Y.J., et al. The development and preliminary evaluation of a new Mycobacterium tuberculosis vaccine comprising Ag85b, HspX and CFP-10:ESAT-6 fusion protein with CpG DNA and aluminum hydroxide adjuvants. FEMS Immunol. Med. Microbiol. 2010;59:42–52. doi: 10.1111/j.1574-695X.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Fu L., Wang G., Subbian S., Qin C., Zhao A. Bacillus calmette-guerinUnmethylated CpG motif-containing genomic DNA fragment of promotes macrophage functions through TLR9-mediated activation of NF-B and MAPKs signaling pathways. Innate Immun. 2020;26:183–203. doi: 10.1177/1753425919879997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J.-B., Chen B.-W., Wang G.-Z., Fu L.-L., Shen X.-B., Su C., Du W.-X., Yang L., Xu M. Recombinant tuberculosis vaccine AEC/BC02 induces antigen-specific cellular responses in mice and protects guinea pigs in a model of latent infection. J. Microbiol. Immunol. Infect. 2014;48:597–603. doi: 10.1016/j.jmii.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Scanga C.A., Mohan V.P., Joseph H., Yu K., Chan J., Flynn J.L. Reactivation of latent tuberculosis: Variations on the Cornell murine model. Infect. Immun. 1999;67:4531–4538. doi: 10.1128/IAI.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biketov S., Potapov V., Ganina E., Downing K., Kana B.D., Kaprelyants A. The role of resuscitation promoting factors in pathogenesis and reactivation of Mycobacterium tuberculosis during intra-peritoneal infection in mice. BMC Infect. Dis. 2007;7:146. doi: 10.1186/1471-2334-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhillon J., Lowrie D.B., Mitchison D.A. Mycobacterium tuberculosis from chronic murine infections that grows in liquid but not on solid medium. BMC Infect. Dis. 2004;4:51. doi: 10.1186/1471-2334-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardona P.J., Julián E., Vallès X., Gordillo S., Muñoz M., Luquin M., Ausina V. Production of Antibodies against Glycolipids from the Mycobacterium tuberculosis Cell Wall in Aerosol Murine Models of Tuberculosis. Scand. J. Immunol. 2002;55:639–645. doi: 10.1046/j.1365-3083.2002.01088.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann S.H., Hussey G., Lambert P.-H. New vaccines for tuberculosis. Lancet. 2010;375:2110–2119. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- 33.Darrah P.A., Zeppa J.J., Maiello P., Hackney J.A., Wadsworth M.H., 2nd, Hughes T.K., Pokkali S., Swanson P.A., 2nd, Grant N.L., Rodgers M.A., et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domingo-Gonzalez R., Prince O., Cooper A., Khader S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016;4:4–5. doi: 10.1128/microbiolspec.TBTB2-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braverman J., Stanley S.A. Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB. J. Immunol. 2017;199:ji1700515. doi: 10.4049/jimmunol.1700515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi H.-G., Kwon K.W., Choi S., Back Y.W., Park H.-S., Kang S.M., Choi E., Shin S.J., Kim H.-J. Antigen-Specific IFN-γ/IL-17-Co-Producing CD4+ T-Cells Are the Determinants for Protective Efficacy of Tuberculosis Subunit Vaccine. Vaccines. 2020;8:300. doi: 10.3390/vaccines8020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewinsohn D.A., Lewinsohn D.M., Scriba T.J. Polyfunctional CD4+ T Cells As Targets for Tuberculosis Vaccination. Front. Immunol. 2017;8:1262. doi: 10.3389/fimmu.2017.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa D.L., Amaral E.P., Namasivayam S., Mittereder L.R., Andrade B.B., Sher A. Enhancement of CD4(+) T Cell Function as a Strategy for Improving Antibiotic Therapy Efficacy in Tuberculosis: Does It Work? Front. Cell. Infect. Microbiol. 2021;11:672527. doi: 10.3389/fcimb.2021.672527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alyahya S.A., Nolan S.T., Smith C.M.R., Bishai W.R., Sadoff J., Lamichhane G. Immunogenicity without Efficacy of an Adenoviral Tuberculosis Vaccine in a Stringent Mouse Model for Immunotherapy during Treatment. PLoS ONE. 2015;10:e0127907. doi: 10.1371/journal.pone.0127907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joosten S.A., Van Meijgaarden K.E., Del Nonno F., Baiocchini A., Petrone L., Vanini V., Smits H., Palmieri F., Goletti D., Ottenhoff T.H.M. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016;12:e1005687. doi: 10.1371/journal.ppat.1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamasur B., Haile M., Pawlowski A., Schröder U., Källenius G., Svenson S.B. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′)2 fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prados-Rosales R., Carreño L., Cheng T., Blanc C., Weinrick B., Malek A., Lowary T.L., Baena A., Joe M., Bai Y., et al. Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog. 2017;13:e1006250. doi: 10.1371/journal.ppat.1006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Wang X.-X., Wang B., Fu L., Liu G., Lu Y., Cao M., Huang H., Javid B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2017;114:5023–5028. doi: 10.1073/pnas.1611776114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alastair C., Diogo G.R., Peter H., Shane H., Tran A.C., Paul M.J., Mahavir S., Cutting S.M., Rajko R. Mucosal Delivery of Fusion Proteins with Bacillus subtilis Spores Enhances Protection against Tuberculosis by Bacillus Calmette-Guérin. Front. Immunol. 2018;9:346. doi: 10.3389/fimmu.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons J.D., Stein C.M., Chetan S., Monica C., Galit A., Sarah F., Erwin S., Wallis R.S., Gavin C., Harriet M.K. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018;18:575–589. doi: 10.1038/s41577-018-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S.K., Singh P., Sinha S. Naturally produced opsonizing antibodies restrict the survival of Mycobacterium tuberculosis in human macrophages by augmenting phagosome maturation. Open Biol. 2015;5:150171. doi: 10.1098/rsob.150171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achkar J.M., Prados-Rosales R. Updates on antibody functions in Mycobacterium tuberculosis infection and their relevance for developing a vaccine against tuberculosis. Curr. Opin. Immunol. 2018;53:30–37. doi: 10.1016/j.coi.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs A.J., Mongkolsapaya J., Screaton G.R., Mcshane H., Wilkinson R.J. Antibodies and tuberculosis. Tuberculosis. 2016;101:102–113. doi: 10.1016/j.tube.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.