Abstract
In recent years, plant-nanomaterial interactions have been studied, highlighting their effects at physiological and molecular levels. Transcriptomics and proteomics studies have shown pathways and targets of nanomaterial exposure and plant response, with particular regard to abiotic stress and oxidative stress. Only little information has been reported on engineered nanomaterial (ENMs) interactions with plant genetic material, both at a genomic and organellar DNAs level. Plants can be useful experimental material, considering they both contain chloroplast and mitochondrial DNAs and several plant genomes have been completely sequenced (e.g., Arabidopsis thaliana, Solanum lycoperiscum, Allium cepa, Zea mays, etc.). In this mini review, the methods and the evidence reported in the present literature concerning the level of genotoxicity induced by ENMs exposure have been considered. Consolidated and potential strategies, which can be applied to assess the nanomaterial genotoxicity in plants, are reviewed.
Keywords: nanomaterials, plant genotoxicity, methods, biomarkers, organelles
1. ENM Genotoxicity in Plant: The Current State
The global market for nanotechnology might grow from USD 5.2 bln in 2021 to USD 23.6 bln by 2026, with annual growth rate (CAGR) of 35.5%, respectively for the years 2021–2026. The North American market for nanotechnology is estimated to grow from USD 1.6 bln in 2021 to USD 7.2 bln by 2026, at a CAGR of 34.5% for the period 2021–2026, while the Asia–Pacific market for nanotechnology is estimated to grow from USD 1.2 to USD 6.0 bln, at a CAGR of 37.6%, respectively, for the same time period, as reported by Nanotechnology Services Global Market Report 2022.
Nanotechnology has captured the attention of a wide range of industries in many sectors, gaining in a short period large attraction and significant public investments in research and development, in addition to increasing private-sector investments. Many governments are implementing the application of nanotechnology notwithstanding the associated risks and uncertainties [1]. Nanotechnology allows the development and improvement of completely new products, processes, and services [2].
However, engineered nanomaterials (ENMs) are in the process of being dispersed into the environment, coming into contact with non-mammal organisms and plants [3,4]. So far, scientists have just started to investigate the impact of nanomaterials on plants, which has contrasting outcomes depending on the type of nanomaterial and on the plant species [5]. The field of nanotoxicology has been extended from microorganisms to plants and animals, even if the idea of ENM genotoxicology for plants is not so widespread. In fact, a search in Scopus [6] for publications with the word “Nanotoxicology” since 2013 produced 625 results. Research in the same timeframe, from 2013 to 2022, using the word “nanomaterial genotoxicology” produced only four outcomes. A more extensive database research has been conducted by Ghosh et al. [7], who found that there are few papers dealing with the genotoxicity of the nanoparticles in respect to other effects that nanoparticles exert on plants.
In the field of toxicology, the term genotoxicity generally refers to any kind of damage to the genetic material, the genome, as cytotoxicity indicates injury to the cell instead. Toxic effects to the genetic material have attracted great attention for many reasons, including in particular that the genome of germ cells, the reproductive cells, determine all heritable characteristics of organisms [8]. Investigation of injury to the genome has led to the definition of a specific kind of toxicity, genotoxicity, and to the development of the subspecialty of genetic toxicology [9].
Several plant species have the intrinsic capability of being used as multiple genetic assay systems. These plant genetic systems have played important roles in detecting new mutagens and developing techniques later used in other systems for advancing mutagenesis knowledge. Some of the mainly used higher plant species are: Allium cepa L. (2n = 16), Arabidopsis thaliana L. (2n = 10), Crepis capsularis (L.) Wallr (2n = 6), Glycine max L. (2n = 40), Hordeum vulgare L. (2n = 14), Solanum lycopersocum L. (2n = 16), Nicotiana tabacum L. (2n = 48), Pisum sativum L. (2n = 14), Tradescantia Ruppius ex L. (2n = 24), Vicia faba L. (2n = 12) and Zea mays L. (2n = 20) [10].
2. Mechanisms of ENM-Induced Genotoxicity
In vitro and in vivo characterization of the response to ENM exposure in both growth media and biological matrices have been extensively discussed in recent years [11]: uptake, pathways, biotransformation, and the mechanisms of ENM genotoxicity. In vitro and in vivo characterization of the response to ENM exposure in both growth media and biological matrices have been extensively discussed in recent years [11]: uptake, pathways, biotransformation, and the mechanisms of ENM genotoxicity. Different mechanisms can be exploited depending on the different ENM physico-chemical properties: (i) ENMs simply able to pass through the cellular membrane lipid bilayer, depending on several factors such as size, charge, hydrophobicity, composition and shape; (ii) endocytosis processes by which ENMs are taken up and accumulated in plant tissues, as well as Trojan horse mechanism and possible biotransformation processes (including corona protein interactions), lead to ENMs accumulation in plant cells; (iii) the utilization of membrane transporters which can mediate the translocation of ENMs into the plant cell, due to their affinity to the transporter itself [12,13]. As a result, ENMs response can be explicated by two different mechanisms: effects directly ascribed to the ENMs interaction with the cellular components, or its biotransformed physico-chemical forms (including ions released, depending on the ENM stability) [14] and indirectly, due to ROS production, increase mediated by mitochondrion and chloroplast functionality alteration, leading to a general cellular oxidative stress increase by triggering ENM-induced cytotoxicity and genotoxicity mechanisms [12]. The response observed is an effect of the activation defense mechanisms, including antioxidant defense mechanisms, apoptosis and secondary metabolite (e.g., phytohormone) production and antioxidant enzymes [11].
As a key metabolite, ROS are necessary in plants for many important signaling reactions, however they also constitute by-products in aerobic metabolism that can induce oxidative damage in plants [15]. It has been demonstrated that nanoparticles and ROS can directly enter the nucleus of the plant cell and, by binding chromatin and/or interacting with DNA, induce damages [16], showing potential mutagenic effect.
For nanoparticles (NPs) such as Ag NPs (coated and uncoated), carbon nanotubes, ZnO NPs, Al2O3 NPs, Fe2O3 NPs, Co3O4 NPs, and NiO NPs, the main features that determine genotoxicity have been found to be ions release, dimension, and zeta potentials [7]. As a fact, these features contribute to the penetration of the nanoparticle into the cell nucleus and the consequent damage to DNA [17]. Several assays have been developed that use higher plants to measure the mutagenic effects of chemicals in general as indicators of carcinogenicity. These assays using plants require less complex equipment and materials than many other genotoxicity tests, which is a potential advantage, particularly when research resources are limited [18]. Standard genotoxicity tests have been reviewed by the Gene-Tox program of the U.S. Environmental Protection Agency (EPA) concerning gene mutation, chromosomal effects and DNA damage repair on the following plants: A. thaliana, G. max, H. vulgare, Tradescantia, Z. mays [18,19]. Early studies on plants progressed to more sophisticated and complex assays on many other plants, and to many more materials including ENMs [7,17].
In this minireview, the most important genotoxicity assays applied on plants are explained, with a focus on how they can be utilized to determine the genotoxic effects for nanoparticles, which include standard techniques available and new tools and instruments. DNA damage may cause epigenetic changes, through covalent DNA modification, histones modification, and regulation of non-coding RNAs (miRNAs, lncRNAs, piRNAs). Modifications at the level of DNA methylation (global or gene-specific) may have a profound impact on chromatin remodeling and on locus-specific gene expression, respectively [20].
3. Current Methods and Functional Applied Strategies
3.1. Standard Techniques
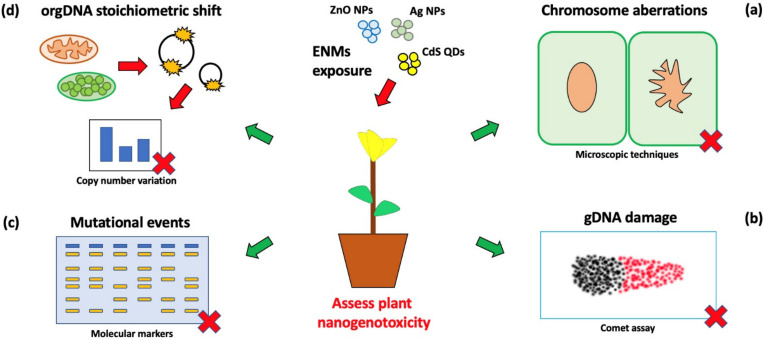
From an operational point of view, different approaches can be utilized to pinpoint the genotoxic effects of ENMs on plant DNA [7]. All these approaches are able to assess ENM genotoxicity from different points of view, showing potential advantages and disadvantages in terms of sensitivity and resolution, respectively. Methods described and relevant examples are schematized in Figure 1.
Figure 1.
Schematic representation of the methodologies utilized to highlight plant ENM genotoxicity: (a) microscopic techniques to highlight chromosomal aberrations, (b) electrophoresis-based methods (e.g., comet assay) to highlight genomic DNA (gDNA) damage, (c) molecular markers (e.g., RAPD) to show mutational events and (d) Real time PCR based methods to highlight copy number variation (stoichiometric or sub-stoichiometric shift) in plastid (ptDNA) or mitochondrial (mtDNA) genomes. These techniques can be utilized as Alternative Testing Strategies (ATS), in assessing and/or characterizing the risk associated with ENMs exposure/effects, not only in experimental controlled conditions, but also in monitoring of realistic scenarios, at early exposure stages.
Among the major effects observed from the exogenous genotoxic effects on plant genomes, the chromosomal aberrations, which are the result of structural and numerical chromosome changes, preferentially within heterochromatic regions, are composed mainly of repetitive DNA sequences [21]. Optical, fluorescence and confocal laser scanning microscopy techniques are able to highlight aberrations at the level of the chromosome structure, including chromosomal breaks, sticky, multipolar, and laggard chromosomes, as well as micronucleus formation [22,23,24].
Chromosomal aberrations have been observed by Pakrashi et al. [25], studying the effect of titania nanoparticles (TiO2 NPs) on Allium cepa L. root tips, in the range 0–100 mg L−1. Optical and fluorescence microscopic analyses showed a dose-dependent frequency of the aberration appearance, which includes chromosomal breaks, chromosome stickiness during metaphase, multiple micronucleus formation, as well as the occurrence of binucleate cells. Confocal microscopic images highlighted the formation of chromosomal bridges, in addition to a distorted and notched nucleus.
Similarly, Panda et al. [26] observed micronucleus mitotic aberrations formations in Allium cepa L. cells exposed to 0–80 mg L−1 of different forms of silver ionic colloidal nanoparticles (Ag NPs). Additionally, in this case, the percentage of increase in aberrations was concentration dependent.
Silva and Monteiro [27] investigated the genotoxic and phytotoxic impacts of silica-based nanomaterials (SiO2 NPs, in a range between 0.54–1.82 g L−1) using root tip cells of Allium cepa L., highlighting chromosomal aberrations and delays in mitosis due to disturbed metaphase. Sun et al. [28] studied the cytotoxic and genotoxic effects of ZnO NPs (5–50 mg L−1) in root meristems of Allium cepa L. cells by cell membrane integrity, metabolic activity, reactive oxygen species (ROS) accumulation, DNA damage and chromosomal aberration, highlighting how ZnO NP accumulation within cell nucleuses affected cell mitosis, inducing chromosome breaks, bridges, stickiness, and micronuclei formation. As often reported, the utilization of Allium cepa L. is considered an efficient bioindicator in genotoxicity testing, due to its reduced number of chromosomes and rapid root growth rate [29]. Abdelsalam et al. [30] investigated the effects of foliar application of (nitrogen-phosphorus-potassium) NPK nanoparticles (2.5 to 5 kg ha−1) for two harvest seasons on Triticum aestivum L. as an alternative to conventional fertilizers, assessing yield and genotoxic effects. Although fertilization with NPK nanoparticles produced an increase in yield, root-tip cells showed various types of chromosomal aberrations such as multinuclei, micronuclei, chromosome deletion, lagging chromosome and cell membrane damage, and the NPK nanoparticles treatment at 5 kg ha−1 produced 35.7–38.9% of abnormal cells. With a similar approach, Abdelsalam et al. [31] tested on Triticum aestivum L. seeds the utilization of (amino-zinc) AZ nanoparticles (50–150 mg L−1) on in vitro medium for 8, 16, or 24 h. Genotoxicity was evaluated in root meristems, revealing mitotic activity variations, chromosomal aberrations, and micronuclei formation and a growth inhibit of the normal cellular function.
3.2. Gel Electrophoresis-Based Methods
DNA damage in individual plant cells can be highlighted by gel electrophoresis-based methods [32]: cells embedded in agarose on a microscope slide are lysed with detergent and high salt concentrations to form nucleoids containing supercoiled loops of DNA linked to the nuclear matrix; subsequent electrophoresis conducted at high pH produces structures resembling comets, which can be observed by fluorescence microscopy. The intensity of the “comet tail” reflects the breaks in DNA sequences. Comet assay is able to detect DNA single-strand breaks, DNA double-strand breaks, and the formation of apoptotic nuclei [33]. This assay is often utilized as a confirmation method for microscopic evidence [26,34,35].
Several examples can be found in recent literature related to ENM genotoxicity in plants: Panda et al. [26], through comet assay, observed a significant DNA damage rate determined by dose-dependent Ag NPs exposure and correlated to ROS formation. Faisal et al. [36] utilized the comet assay to assess the genotoxic effects in Solanum lycopersicum L. seedlings exposed to NiO NPs (0–2 g L−1). Analyses showed a significant increase in genomic DNA damage, and an increase in the number of apoptotic (21.8%) and necrotic (24.0%) cells. Ciğerci et al. [34] studied Indium tin oxide (ITO, In2O3/SnO2, ration 90/10%) particles (1–100 mg L−1), observing a significant increase in DNA damages in A. cepa root meristematic cells, highlighting potential alterations in the cell cycle, as demonstrated by the higher number of cells able to enter into mitosis, as compared with the untreated controls. Thiruvengadam et al. [37] studied physiological, metabolic, and transcriptional effects of Ag NPs (1–10 mg L−1) Brassica rapa spp. observing a dose dependent DNA damage effects in turnip cells. Sun et al. [28] confirmed by comet assay the chromosomal aberration generated in A. cepa, highlighting a significant increase in DNA fragmentation after ZnO NPs exposure.
3.3. Molecular Markers and Biomarker Assays
Not only electrophoresis-based methods and chromosomal aberration analyses are utilized to detect potential genotoxic effects. Molecular markers can be also implemented as tools to detect the ENMs effect on genetic materials [38]. Molecular markers are defined as fragments or amplicons of DNA associated with a certain location within the genome. Molecular markers can be used as a biotechnological tool to identify and characterize a particular sequence of DNA when there is a limited knowledge of the sequence itself. This is the case, for example, of Random Amplified Polymorphic DNA (RAPD), markers based on PCR amplification of DNA fragments from random segments of genomic DNA, with a single primer of an arbitrary nucleotide sequence [39]. RAPDs do not require specific knowledge of the DNA sequence of the target organism. The occurrence of mutation at the level of DNA, particularly at the site that was previously complementary to the primer, will not allow amplicon production, resulting in a different pattern of amplified DNA fragments, which results in a molecular marker that is mainly dominant [40]. Since the early 1990s, several molecular marker tools have been developed in order to increase the detail of the physical genomic mapping and QTL analysis, with pros and cons related to the intrinsic properties of each molecular marker, respectively [41].
Molecular markers can be also utilized as tools to determine potential mutations at the level of the DNA sequence [42], which can support or validate data previously obtained, but also to isolate potential targets functional to biomarker characterization and development [43,44].
Hosseinpour et al. [45] studied the effects of the application of ZnO NPs (0–40 mg L−1) and plant growth promoting bacteria on S. lycopersicum L. under salt stress, with particular regard to DNA damage and cytosine methylation changes. RAPD analysis has been performed to determine the effects of co-exposure to bacteria and ZnO NPs on tomato genomic DNA. The rate of polymorphism observed in case of salinity stress treatment (42.2%) was a decrease in case of exposure to ZnO NPs and/or plant growth promoting bacteria from 32.4% to 25.3%, respectively. The results obtained through the application of different bacteria and ZnO NPs concentrations suggest the inverse relationship between the level of cytosine methylation and salinity stress tolerance. Mosa et al. [46] studied the genotoxic effects and genomic alterations in Cucumis sativus L. of copper-based nanoparticles (Cu NPs) using the RAPD technique. Cu NPs (0–200 mg L−1) showed a concentration-dependent increase rate of polymorphism occurrence, highlighting the Cu NPs genotoxic effect. Kokina et al. [47] studied the impact of iron oxide nanoparticles (Fe3O4 NPs, 0–4 mg L−1) on Medicago falcata L. The utilization, in this case, of the RAPD technique highlighted the genotoxic effect of Fe3O4 NPs, which induced genomic DNA modifications. This type of PCR-based molecular marker for its randomic amplification nature may be subject to experimental or technical variability, and thus requires procedures of validation [38,39]. Several other type of molecular markers and biomarkers can be utilized as more reliable tools to assess genomic variations, either at the level of genomic DNA (gDNA) and plastid and mitochondrial DNA (ptDNA, mtDNA). Pagano et al. [44] highlighted a modulation of the organellar functionality in Arabidopsis thaliana L. Heynh in direct comparison to a modulated organelle genome replication level, upon exposure to CeO2 NPs, FeOx NPs, ZnS QDs, CdS QDs (80–500 mg L−1). In this case, multiple target genes at the level of ptDNA and mtDNA were utilized as structural markers to assess the potential variations at the level of DNA replication by real time qPCR. In particular, CdS QD exposure triggered potential variations at the sub-stoichiometric level of the two organellar genomes, while nanoscale FeOx NPs and ZnS QDs exposure triggered an increase in organellar DNA copy numbers. These findings suggested how modification in organellar genomes stoichiometry may result from a potential morpho-functional adaptive response to ENM exposure, which led to decreased rates of photosynthesis and cellular respiration.
3.4. Other Approaches
Other approaches, which included A. thaliana transgenic lines for homologous recombination and transcriptional gene silencing, were adopted to assess the genotoxicity of ZnO NPs [48]. The results showed, at the level of roots, how exposure to ZnO NPs (0–20 mg L−1) resulted in an increase in homologous recombination (in particular the gene atRad54-GFP-GUS expression) and a reduction in transcriptional gene silencing in leaves (which contained the multicopy construct P35S::GUS), which can be ascribed to genotoxic effects triggered by ZnO NPs dissolution to free Zn ions. Methods described and relevant examples are reported in Table 1.
Table 1.
Reference list of relevant experiments performed with different tools to identify ENM genotoxic effects in plants.
| ENM | Treatment (*) | Plant | Analyses | Reference |
|---|---|---|---|---|
| TiO2 NPs | Conc.: 0–100 mg L−1 (hydroponic), 4 h treatment | Allium cepa L. | Chromosome aberration | Pakrashi et al. [25] |
| Ag NPs | Conc.: 0–80 mg L−1 (hydroponic), 1 h treatment | Allium cepa L. | Chromosome aberration, Comet assay | Panda et al. [26] |
| SiO2 NPs | Conc.: 0.54–1.82 g L−1 (hydroponic), 24 h treatment | Allium cepa L. | Chromosome aberration | Silva and Monteiro [27] |
| ZnO NPs | Conc.: 5–50 mg L−1 (hydroponic), 36 h treatment | Allium cepa L. | Chromosome aberration, Comet assay | Sun et al. [28] |
| NPK particles | Conc: 2.5–5.0 kg ha−1 (in soil, foliar spray), two harvest seasons | Triticum aestivum L. | Chromosome aberration | Abdelsalam et al. [30] |
| AZ particles | Conc: 50–150 mg L−1 (in vitro), 8, 16, 24 h treatment | Triticum aestivum L. | Chromosome aberration | Abdelsalam et al. [31] |
| NiO NPs | Conc.: 0–2 g L−1 (in vitro), 12 d treatment | Solanum lycopersicum L. | Comet assay | Faisal et al. [36] |
| In2O3/SnO2 particles | Conc.: 1–100 mg L−1 (hydroponic), 4 h treatment | Allium cepa L. | Comet assay | Ciğerci et al. [34] |
| Ag NPs | Conc.: 1–10 mg L−1 (in vitro), 14 d treatment | Brassica rapa spp. | Comet assay | Thiruvengadam et al. [37] |
| ZnO NPs | Conc.: 0–40 mg L−1 (in vitro), 14 d treatment | Solanum lycopersicum L. | RAPD | Hosseinpour et al. [45] |
| Cu NPs | Conc.: 0–200 mg L−1 (in vitro), 21 d treatment | Cucumis sativus L. | RAPD | Mosa et al. [46] |
| Fe3O4 NPs | Conc.: 0–4 mg L−1 (hydroponic), 35 d treatment | Medicago falcata L. | RAPD | Kokina et al. [47] |
| CeO2 NPs, FeOx NPs, ZnS QDs, CdS QDs | Conc.: 80 mg L−1 (CdS QDs), 500 mg L−1 (CeO2 NPs, FeOx NPs, ZnS QDs), (in vitro) 21 d treatment | Arabidopsis thaliana L. | mtDNA, ptDNA copy number variation | Pagano et al. [44] |
| ZnO NPs | Conc.: 0–20 mg L−1 (hydroponic), 20 d treatment | Arabidopsis thaliana L. | Gene silencing | Yang et al. [48] |
*, treatment conditions information includes concentration, experimental setup, and time of exposure utilized. Reference list order in the table reflects the order of appearance in the text, depending on the type of analyses performed.
4. Conclusions
In conclusion, in recent years, different techniques, previously exploited for animal cells, have been developed and applied to plants to assess the genotoxic effects related to ENM exposure. These approaches, considering their properties, and the relative pros and cons, which include high/low resolution vs. high/low target specificity, may be implemented for cross-validation of the results obtained. This may also include potential applications related to the utilization of novel methods of mutagenesis (e.g., CRISPR-Cas9) [49].
In this context, plants and microorganisms can be utilized as model organisms instead of animal models for Alternative Testing Strategies (ATS) to assess and characterize the risk, with particular regard to genotoxicity, related to ENMs exposure/effects [44,50]. Adoption of ATS for new organisms, endpoints, and span of variations in experimental scale and complexity have been increasingly functional in nanotoxicological literature through iterative processes able to combine results from physiological and molecular approaches [51]. Moreover, the monitoring of ENMs dispersal in the environment, especially at very early exposure stages and in realistic scenarios, can be further implemented [52] in accordance with the recently published EFSA guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain, human and animal health, which considers in vitro/in vivo toxicological testing (e.g., in vitro degradation, toxicokinetics, genotoxicity, local and systemic toxicity), and the European Registration, Evaluation Authorization and Restriction of Chemicals (REACH) protocols for chemical safety assessment [1,53].
Acknowledgments
All authors acknowledge the support of FIL (“Fondi Locali per la Ricerca”).
Author Contributions
Conceptualization, M.M., N.M. and L.P.; writing—original draft preparation, M.M., N.M. and L.P.; writing—review and editing, M.M., N.M. and L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
Authors declare no competing financial interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nielsen M.B., Baun A., Mackevica A., Thit A., Odnevall Wallinder I., Gallego J.A., Westergaard Clausen L.P., Rissler J., Skjolding L., Nilsson A., et al. Nanomaterials in the European chemicals legislation—Methodological challenges for registration and environmental safety assessment. Environ. Sci. Nano. 2021;8:731–747. doi: 10.1039/D0EN01123A. [DOI] [Google Scholar]
- 2.Aithal P.S., Aithal S. Business Strategy for Nanotechnology Based Products and Services. Int. J. Bus. Manag. Sci. 2016;5:139–149. [Google Scholar]
- 3.Keller A.A., McFerran S., Lazareva A., Suh S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013;169:6. doi: 10.1007/s11051-013-1692-4. [DOI] [Google Scholar]
- 4.Marmiroli N., White J.C., Song J., editors. Exposure to Engineered Nanomaterials in the Environment. Elsevier; Amsterdam, The Netherlands: 2019. (Micro&Nano Technologies Series). [Google Scholar]
- 5.Holden P.A., Gardea-Torresdey J.L., Klaessig F., Turco R.F., Mortimer M., Hund-Rinke K., Cohen Hubal E.A., Avery D., Barceló D., Behra R., et al. Considerations of Environmentally Relevant Test Conditions for Improved Evaluation of Ecological Hazards of Engineered Nanomaterials. Environ. Sci. Technol. 2015;50:6124–6145. doi: 10.1021/acs.est.6b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scopus Search for “Nanotoxicology” by Title, Abstract and Keywords. [(accessed on 10 April 2022)]. Available online: https://www.scopus.com/search/form.uri?display=basic&zone=TopNavBar&origin=sbrowse#basic.
- 7.Ghosh M., Ghosh I., Godderis L., Hoet P., Mukherjee A. Genotoxicity of engineered nanoparticles in higher plants. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis. 2019;842:132–145. doi: 10.1016/j.mrgentox.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Sugden K.D., Campo C.K., Martin D.D. Direct Oxidation of Guanine and 7,8-Dihydro-8-oxoguanine in DNA by a High-Valent Chromium Complex: A Possible Mechanism for Chromate Genotoxicity. Chem. Res. Toxicol. 2001;14:1315–1322. doi: 10.1021/tx010088+. [DOI] [PubMed] [Google Scholar]
- 9.Williams G.M. Methods for evaluating chemical genotoxicity. Annu. Rev. Pharmacol. Toxicol. 1989;29:189–211. doi: 10.1146/annurev.pa.29.040189.001201. [DOI] [PubMed] [Google Scholar]
- 10.Grant W.F. The present status of higher plant bioassays for the detection of environmental mutagens. Mut. Res. 1994;310:175–185. doi: 10.1016/0027-5107(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 11.Ma C., White J.C., Zhao J., Zhao Q., Xing B. Uptake of Engineered Nanoparticles by Food Crops: Characterization, Mechanisms, and Implications. Annu. Rev. Food Sci. Technol. 2018;9:129–153. doi: 10.1146/annurev-food-030117-012657. [DOI] [PubMed] [Google Scholar]
- 12.Mehrian S.K., De Lima R. Nanoparticles cyto and genotoxicity in plants: Mechanisms and abnormalities. Environ. Nanotechnol. Monit. Manag. 2016;6:184–193. doi: 10.1016/j.enmm.2016.08.003. [DOI] [Google Scholar]
- 13.Pagano L., Maestri E., Caldara M., White J.C., Marmiroli N., Marmiroli M. Engineered Nanomaterial Activity at the Organelle Level: Impacts on the Chloroplasts and Mitochondria. ACS Sustain. Chem. Eng. 2018;6:12562–12579. doi: 10.1021/acssuschemeng.8b02046. [DOI] [Google Scholar]
- 14.Marmiroli M., Lepore G.O., Pagano L., d’Acapito F., Gianoncelli A., Villani M., Lazzarini L., White J.C., Marmiroli N. The fate of CdS Quantum Dots in plants as revealed by Extended X-ray Absorption Fine Structure (EXAFS) analysis. Environ. Sci. Nano. 2020;7:1150–1162. doi: 10.1039/C9EN01433K. [DOI] [Google Scholar]
- 15.Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Degtyareva N.P., Heyburn L., Sterling J., Resnick M.A., Gordenin D.A., Doetsch P.W. Oxidative stress-induced mutagenesis in single-strand DNA occurs primarily at cytosines and is DNA polymerase zeta-dependent only for adenines and guanines. Nucleic Acids Res. 2013;41:8995–9005. doi: 10.1093/nar/gkt671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Wu F., Wei Meng W., White J.C., Holden P., Xing B. Engineered Nanoparticles May Induce Genotoxicity. Environ. Sci. Technol. 2013;47:13212–13214. doi: 10.1021/es404527d. [DOI] [PubMed] [Google Scholar]
- 18.Ennever F.K., Andreano G., Rosenkranz H.S. The ability of plant genotoxicity assays to predict carcinogenicity. Mut. Res. 1988;205:99–105. doi: 10.1016/0165-1218(88)90013-4. [DOI] [PubMed] [Google Scholar]
- 19.Auletta A.E., Brown M., Wassom JSCimino M.C. Current Status of the Gene-Tox Program. Environ. Health Perspec. 1991;96:33–36. doi: 10.1289/ehp.919633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gedda M.R., Babele P.K., Zahra K., Madhukar P. Epigenetic Aspects of Engineered Nanomaterials: Is the Collateral Damage Inevitable? Front. Bioeng. Biotechnol. 2019;7:228. doi: 10.3389/fbioe.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert I., Pecinka A., Meister A., Schubert V., Klatte M., Jovtchev G. DNA damage processing and aberration formation in plants. Cytogenet. Genome Res. 2004;104:104–108. doi: 10.1159/000077473. [DOI] [PubMed] [Google Scholar]
- 22.Janicke M.A., Lasko L., Oldenbourg R., LaFountain J.R. Chromosome malorientations after meiosis II arrest cause nondisjunction. Mol. Biol. Cell. 2007;18:1645–1656. doi: 10.1091/mbc.e06-10-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pessim C., Pagliarini M.S., Silva N., Jank L. Chromosome stickiness impairs meiosis and influences reproductive success in Panicum maximum (Poaceae) hybrid plants. Genet. Mol. Res. GMR. 2015;14:4195. doi: 10.4238/2015.April.28.2. [DOI] [PubMed] [Google Scholar]
- 24.Kwasniewska J., Bara A.W. Plant Cytogenetics in the Micronuclei Investigation—The Past, Current Status, and Perspectives. Int. J. Mol. Sci. 2022;23:1306. doi: 10.3390/ijms23031306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakrashi S., Jain N., Dalai S., Jayakumar J., Chandrasekaran P.T., Raichur A.M., Chandrasekaran N., Mukherjee A. In Vivo Genotoxicity Assessment of Titanium Dioxide Nanoparticles by Allium cepa Root Tip Assay at High Exposure Concentrations. PLoS ONE. 2014;9:e87789. doi: 10.1371/journal.pone.0087789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panda K.K., Achary V.M., Krishnaveni R., Padhi B.K., Sarangi S.N., Sahu S.N., Panda B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. Vitr. 2011;25:1097–1105. doi: 10.1016/j.tiv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Silva G.H., Monteiro R.T.R. Toxicity assessment of silica nanoparticles on Allium cepa. Ecotoxicol. Environ. Contam. 2017;12:25–31. doi: 10.5132/eec.2017.01.04. [DOI] [Google Scholar]
- 28.Sun Z., Xiong T., Zhang T., Wang N., Chen D., Li S. Influences of zinc oxide nanoparticles on Allium cepa root cells and the primary cause of phytotoxicity. Ecotoxicology. 2019;28:178–195. doi: 10.1007/s10646-018-2010-9. [DOI] [PubMed] [Google Scholar]
- 29.Leme D.M., Marin-Morales M.A. Allium cepa test in environmental monitoring: A review on its application. Mut. Res. 2009;682:71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Abdelsalam N.R., Kandil E.E., Al-Msari M.A.F., Al-Jaddadi M.A.M., Ali H.M., Salem M.Z.M., Elshikh M.S. Effect of foliar application of NPK nanoparticle fertilization on yield and genotoxicity in wheat (Triticum aestivum L.) Sci. Total Environ. 2019;653:1128–1139. doi: 10.1016/j.scitotenv.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Abdelsalam N.R., Abdel-Megeed A., Ghareeb R.Y., Ali H.M., Salem M.Z.M., Akrami M., Al-Hayalif M.F.A., Desoky E.M. Genotoxicity assessment of amino zinc nanoparticles in wheat (Triticum aestivum L.) as cytogenetical perspective. Saudi J. Biol. Sci. 2022;29:2306–2313. doi: 10.1016/j.sjbs.2021.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 33.Olive P., Banáth J. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 34.Cigerci I., Liman R., Ozguül E., Konuk M. Genotoxicity of indium tin oxide by Allium and Comet tests. Cytotechnology. 2015;67:157–163. doi: 10.1007/s10616-013-9673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos C.L.V., Pourrut B., Ferreira de Oliveira J.M.P. The use of comet assay in plant toxicology: Recent advances. Front. Genet. 2015;6:216. doi: 10.3389/fgene.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faisal M., Saquib Q., Alatar A.A., Al-Khedhairy A.A., Hegazy A.K., Musarrat J. Phytotoxic hazards of NiO-nanoparticles in tomato: A study on mechanism of cell death. J. Hazard. Mater. 2013;250–251:318–332. doi: 10.1016/j.jhazmat.2013.01.063. [DOI] [PubMed] [Google Scholar]
- 37.Thiruvengadam M., Gurunathan S., Chung I.M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.) Protoplasma. 2015;252:1031–1046. doi: 10.1007/s00709-014-0738-5. [DOI] [PubMed] [Google Scholar]
- 38.Hasan N., Choudhary S., Naaz N., Sharma N., Laskar R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021;19:128. doi: 10.1186/s43141-021-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conte C., Mutti I., Puglisi P., Ferrarini A., Regina G., Maestri E., Marmiroli N. DNA fingerprinting analysis by a PCR based method for monitoring the genotoxic effects of heavy metals pollution. Chemosphere. 1998;37:2739–2749. doi: 10.1016/S0045-6535(98)00317-8. [DOI] [PubMed] [Google Scholar]
- 40.Mbwana J., Bölin I., Lyamuya E., Mhalu F., Lagergård T. Molecular characterization of Haemophilus ducreyi isolates from different geographical locations. J. Clin. Microbiol. 2006;44:132–137. doi: 10.1128/JCM.44.1.132-137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhingani R.M., Umrania V.V., Tomar R.S., Parakhia M.V., Golakiya B.A. Introduction to QTL mapping in plants. Ann. Plant Sci. 2015;4:1072–1079. [Google Scholar]
- 42.Monteiro M., Santos C., Mann R.M., Soares A.M.V.M., Lopes T. Evaluation of cadmium genotoxicity in Lactuca sativa L. using nuclear microsatellites. Environ. Exp. Bot. 2007;60:421–427. doi: 10.1016/j.envexpbot.2006.12.018. [DOI] [Google Scholar]
- 43.Li C., Zheng Y., Huang P. Molecular markers from the chloroplast genome of rose provide a complementary tool for variety discrimination and profiling. Sci. Rep. 2020;10:12188. doi: 10.1038/s41598-020-68092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagano L., Marmiroli M., Villani M., Magnani J., Rossi R., Zappettini A., White J.C., Marmiroli N. Engineered nanomaterial exposure affects organelle genetic material replication in Arabidopsis thaliana. ACS Nano. 2022;16:2249–2260. doi: 10.1021/acsnano.1c08367. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinpour A., Haliloglu K., Tolga Cinisli K., Ozkan G., Ozturk H.I., Pour-Aboughadareh A., Poczai P. Application of Zinc Oxide Nanoparticles and Plant Growth Promoting Bacteria Reduces Genetic Impairment under Salt Stress in Tomato (Solanum lycopersicum L. ‘Linda’) Agriculture. 2020;10:521. doi: 10.3390/agriculture10110521. [DOI] [Google Scholar]
- 46.Mosa K.A., El-Naggar M., Ramamoorthy K., Alawadhi H., Elnaggar A., Wartanian S., Ibrahim E., Hani H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018;9:872. doi: 10.3389/fpls.2018.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kokina I., Plaksenkova I., Jermaļonoka M., Petrova A. Impact of iron oxide nanoparticles on yellow medick (Medicago falcata L.) plants. J. Plant. Inter. 2020;15:e1708489. doi: 10.1080/17429145.2019.1708489. [DOI] [Google Scholar]
- 48.Yang A., Wu J., Deng C., Wang T., Bian P. Genotoxicity of Zinc Oxide Nanoparticles in Plants Demonstrated Using Transgenic Arabidopsis thaliana. Bull. Environ. Contam. Toxicol. 2018;101:514–520. doi: 10.1007/s00128-018-2420-7. [DOI] [PubMed] [Google Scholar]
- 49.Demirer G.S., Silva T.N., Jackson C.T., Thomas J.B., Ehrhardt S.W., Rhee S.Y., Mortimer J.C., Landry M.P. Nanotechnology to advance CRISPR—Cas genetic engineering of plants. Nat. Nanotechnol. 2021;16:243–250. doi: 10.1038/s41565-021-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boldrin A., Hansen S.F., Baun A., Hartmann N.I.B., Astrup T.F. Environmental Exposure Assessment Framework for Nanoparticles in Solid Waste. J. Nanopart. Res. 2014;16:2394. doi: 10.1007/s11051-014-2394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hjorth R., Holden P.A., Hansen S.F., Colman B.P., Griegerf K., Hendrene C.O. The role of alternative testing strategies in environmental risk assessment of engineered nanomaterials. Environ. Sci. Nano. 2017;4:292–301. doi: 10.1039/C6EN00443A. [DOI] [Google Scholar]
- 52.Marmiroli M., Pagano L., Rossi R., De La Torre-Roche R., Lepore G.O., Ruotolo R., Gariani G., Bonanni V., Pollastri S., Puri A., et al. Copper Oxide Nanomaterial Fate In Plant Tissue: Nanoscale Impacts On Reproductive Tissues. Environ. Sci. Technol. 2021;55:10769–10783. doi: 10.1021/acs.est.1c01123. [DOI] [PubMed] [Google Scholar]
- 53.EFSA Scientific Committee Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021;19:e06768. doi: 10.2903/j.efsa.2021.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.