Abstract
Dyslipidemia is a hallmark of chronic kidney disease (CKD). The severity of dyslipidemia not only correlates with CKD stages but is also associated with cardiovascular disease and mortality. Understanding how lipids are dysregulated, however, is challenging because of the incredible diversity in lipid structures. The dyslipidemia and its association with CKD stages and progression encompass complex interactions between genetic, environmental, and kidney specific factors that require an integrated understanding of perturbations in the network of genes, proteins, and lipids. Modern lipidomic technologies attempt to systematically identify and quantitate lipids from biological systems. The recent rapid development of a variety of analytical platforms based on mass spectrometry (MS) have enabled identification of complex lipids at great precision and depth. From current lipidomics studies of CKD patients it is apparent that overall architecture of free fatty acid partitioning between fatty acid oxidation and complex lipid fatty acid composition is an important driver of CKD progression.
Introduction
According to the Centers for Disease Control and Prevention, an estimated 37 million adults (15% of the population) in the US suffer from chronic kidney disease (CKD)1. Although various etiologies exist, diabetes mellitus (DM) alone is responsible for approximately 40% of cases of kidney failure 2. Patients with CKD have a high prevalence of cardiovascular disease3 and it is therefore of no surprise that the majority of patients with CKD present with DM and cardiovascular disease (CVD) as comorbid conditions2.
CKD and its comorbidities lead to significant multi-organ metabolic derangements, including dyslipidemia that occurs in CKD is thought to contribute to the development of CVD4, the leading cause of deaths in CKD5. Therefore, of particular interest is lipidomics, a subset of metabolomics that specifically focuses on the identification and quantification of lipids. Population-based studies over the past five decades have been limited to the measurement of traditional lipid panel which include total cholesterol, lipoproteins and triglycerides which does not account for the chemical diversity and complexity of lipids. Recent advances in analytical mass spectrometry in the past decade have enabled broader profiling of the plasma and tissue lipidome allowing identification of lipid species by class, subclass, chain length, degree of unsaturation, chain hydroxylation, amongst other chemical characteristics6–19. Lipid phenotype alterations associated with CKD progression involve significant alterations in a large number of intra-class lipids due to differential elongation, desaturation, synthesis, and lipolysis that basis the alterations at class level. Hence, a system biology level analysis as opposed to individual lipid analysis is required to unravel pertinent lipid alterations. Comprehensive lipidomics studies allow for a systems-view of CKD-associated changes in lipid levels and regulation, as opposed analysis driven by a few highly altered lipid species. Although lipidomics in CKD is still a burgeoning field and therefore requires further experimental validation of its findings, lipidomic studies have established that fatty-acid oxidation and lipogenesis, thought previously to be independent processes occurring in separate cellular compartments are interrelated, perturbed concurrently in CKD, and predict future clinical progression.
In this review, we aim to introduce the reader to lipidomics studies, including a technical overview of various methods. We will discuss statistical and bioinformatics strategies that can be employed to analyze complex lipidomic data. Finally, we will then discuss the most recent lipidomics biomarker studies and mechanistic studies investigating the role of these clinically relevant lipids in CKD.
Lipidomics: an overview
Lipidomics is the comprehensive analysis of individual lipids in a biological system. Lipidomics is most often conducted with mass-spectrometers, which provide accurate mass determination with mass/charge (m/z) ratio and subsequent unequivocal structural identification by fragmentation which generates a characteristic mass spectral fingerprint. MS1 is the unfragmented mass spectra that generates “precursor” ions and MS2 (MS/MS) is tandem mass spectra that generates fragmented “product ions” from the precursor ions. These acquired MS1 and MS2 spectra can be searched against databases such as METLIN-XCMS20,21 and LIPID MAPS22 for compound identification. Lipids are unique in that each lipid class is associated with characteristic “diagnostic fragments”, usually arising from the lipid head group, and serve as specific signatures for the lipid class. Examples of lipid head groups and associated diagnostic ions are listed in Table 1 for different lipid classes. Figure 1 demonstrates MS1 and MS2 spectra obtained for phosphatidylethanolamine 36:1.
Table 1:
Table of lipid classes, their head groups, most prevalent precursor ion, and class-specific diagnostic fragments generated from the lipid head group.
| Lipid Class | Chemical Structure | Precursor Ion | Diagnostic Fragments |
|---|---|---|---|
| Fatty acid (FA) |
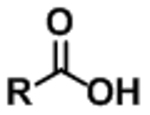
|
[M−H]− | (see note)** |
| Phosphatidylcholine (PC) |
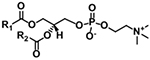
|
[M+H]+ |
m/z 184.0733 NL 183.0661 |
| [M+CH3COO]− | NL 74.0368 | ||
| Phosphatidyl-ethanolamine (PE) |
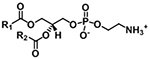
|
[M+H]+ | NL 141.0191 |
| [M−H]− |
m/z 140.0118 m/z 196.0380 |
||
| Phosphatidylserine (PS) |
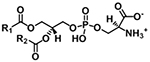
|
[M+H]+ | NL 185.0089 |
| [M−H]− |
m/z 152.9958 NL 87.0320 |
||
| Phosphatidylglycerol (PG) |
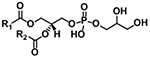
|
[M+NH4]+ | NL 189.0402 |
| [M−H]− |
m/z 152.9958 m/z 171.0063 |
||
| Phosphatidic acid (PA) |
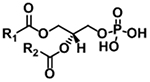
|
[M+NH4]+ | NL 115.0035 |
| [M−H]− | m/z 152.9958 | ||
| Phosphatidylinositol (PI) |
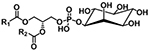
|
[M+NH4]+ | NL 277.0563 |
| [M−H]− |
m/z 241.0119 m/z 223.0013 m/z 152.9958 m/z 259.0224 |
||
| Ceramide with d18:1 backbone |
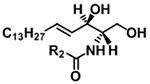
|
[M+H]+ | m/z 264.2685 |
| Diacylglycerol (DAG) |
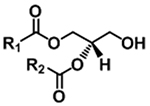
|
[M+NH4]+ | NL (FA+NH3) |
| Triacylglycerol (TAG) |
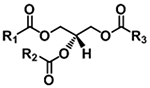
|
[M+NH4]+ | NL (FA+NH3) |
| Sphingomyeline with d18:1 backbone (SM) |
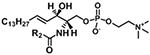
|
[M+H]+ | m/z 184.0733 |
| [M+CH3COO]− |
m/z 168.0431 NL 74.0368 |
||
| Cardiolipin |
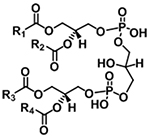
|
[M−H]− | m/z 152.9958 |
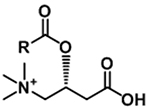
| Acylcarnitine |
|
[M+H]+ |
m/z 85.0284 NL 59.0735 |
| Bile Acid |
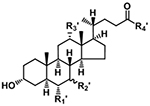
|
[M−H]− | R1′, R2′, R3′ = H or OH If R4′ = OH, fragment varies If R4′ = NHCH2CO2H, m/z 74.0248 If R4′ = NH(CH2)2SO3H, m/z 124.0074 m/z 79.9574 |
NL = neutral loss.
Functional group R is saturated or unsaturated acyl chain.
Free fatty acids are generally not fragmented in common collision-induced dissociation (CID) experiments. For the convenience of multiple reaction monitoring detection, precursor ion mass is also used as fragment ion mass, i.e. for palmitate, FA 16:0, use transition: 255.2 → 255.2. Table is organized from resources: 206–208
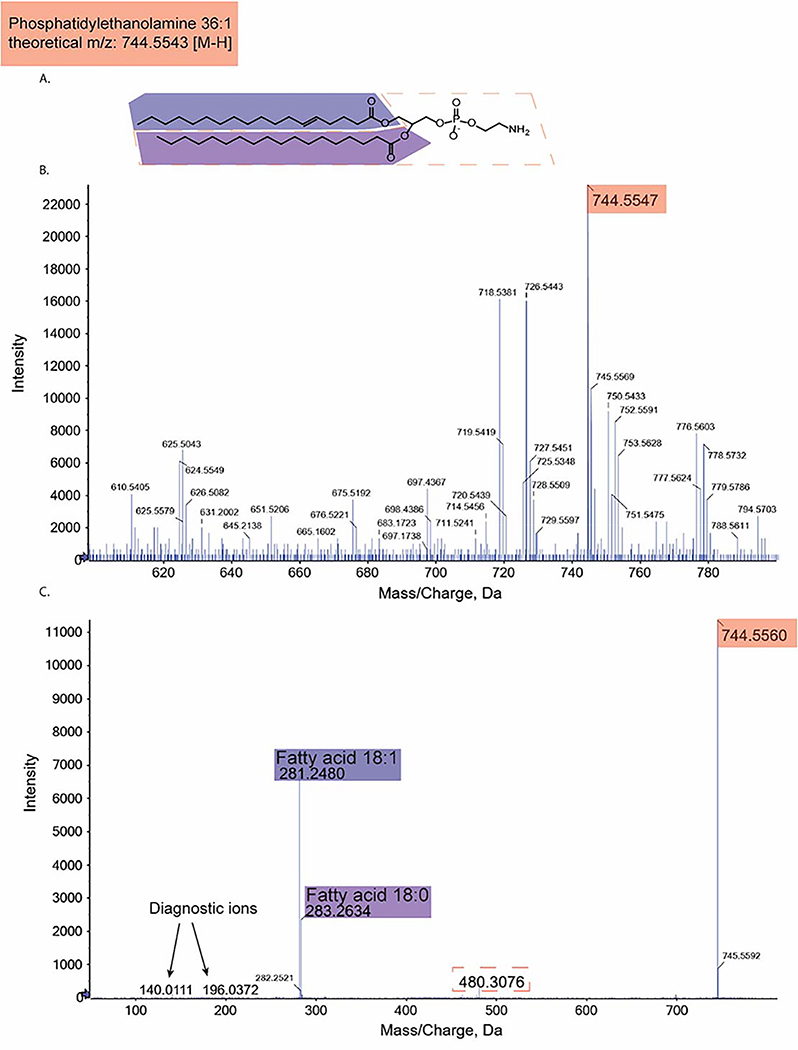
Figure 1:
Diagram of the MS and MS/MS spectra acquired from a triple-TOF for phosphatidylethanolamine (PE) 36:1 with theoretical m/z 744.5543 for its [M-H] precursor ion. A) Chemical structure of PE 36:1. Fragments identified in the following spectra are highlighted. B) MS spectra identifying the [M-H] precursor ion with its m/z highlighted. C) MS/MS spectra identifying fragments of PE 36:1. Each m/z is highlighted with colors that correspond to its fragments highlighted in A). Diagnostic ions for the PE class are labeled.
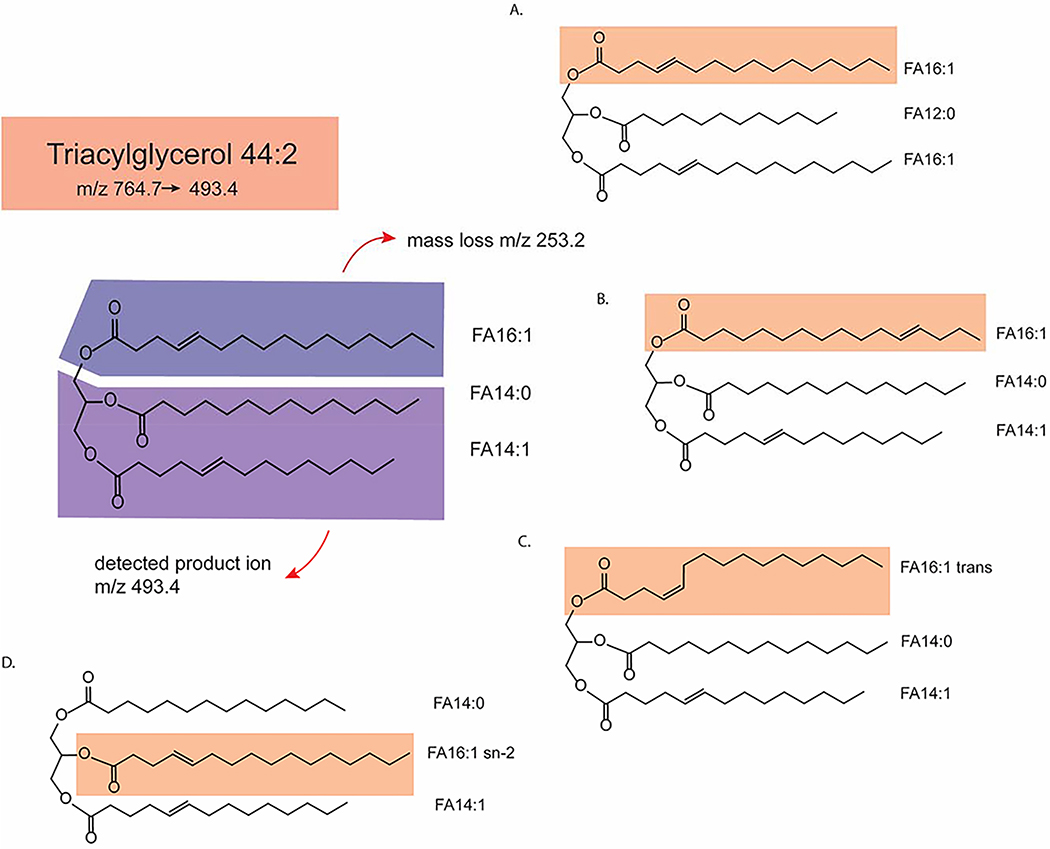
There are numerous isomeric combinations possible for a single identified lipid feature. Figure 2 demonstrates the structural diversity of a triglyceride with a specific precursor and product m/z transition: carbon number isomers, cis/trans double bond isomer, location of double bond isomer, glycerol-chain linkage position (sn-1/sn-2/sn-3) side chain isomer, as well as stereoisomers (not depicted in fig. 2). A large drawback to many lipidomics studies published to-date is lack of such isomer separation and identification; most untargeted lipidomics studies provide information only on lipid class, total number of carbons in the side chains, degree on unsaturation, and presence or absence of carbon backbone hydroxyl groups. Lipid isomers can have distinct biological roles. For instance, phospholipase A1, phospholipase A2, and phospholipase C hydrolyze phospholipids at sn-1, sn-2, and sn-3 sites, respectively and release different lipid products that serve as different cellular messengers23. Therefore, additional biological insights may be gained from lipidomics studies that can deconvolute lipid isomers.
Figure 2:
Schematic demonstrating the structural diversity of triglyceride 44:2: which has a precursor ion m/z of 764.7 and product ion of 493.4 and mass loss of 253.2 that corresponds to fatty acid (FA) 16:1. Four isomers of the original structure (FA 16:1, FA 14:0, FA14:1) are proposed (A-D). FA 16:1 is highlighted in every structure. A) TAG 44:2 with 16:1 and sn-2 and sn-3 acyl-chain carbon isomers. B) TAG 44:2 with FA 16:1 double-bond position isomer. C) TAG 44:2 with FA 16:1 trans double-bond isomer. D). TAG 44:2 with16:1 sn-2 positional isomer.
Analytical techniques in lipidomics
Lipidomics can be conducted using a variety of techniques. Liquid chromatography-mass spectrometry is however the most used analytical method in lipidomics. LC-MS requires comparatively little processing for samples and provide high reproducibility when combined with quality-control (QC) strategies. The high sensitivity of LC-MS requires minimal amounts of sample, making it ideal for clinical studies of patient samples. All our own lipidomic studies referenced in this literature have been conducted with LC-MS. However, similar quality control and data analysis strategies can be applied to other lipidomic analytical techniques. Some of the studies discussed in the review use alternative analytical methods, each of which provide unique advantages and information that cannot be acquired with LC-MS mass spectrometry. The relative advantages and disadvantages of other analytical techniques have been briefly outlined in table 2. For further information on the details of analytical strategies, including information on general structural and biological characteristics of different lipid classes, we direct readers to these excellent reviews: 24–28.
Table 2:
Relative advantages and disadvantages of alternative lipidomic analytical methods to LC-MS.
| Method | Advantages | Disadvantages |
|---|---|---|
| Nuclear magnetic resonance | Uniquely suited to be able to identify the chemical locations of isotopically labeled nuclei in isotopic labeling studies (fluxomics) | Low sensitivity |
| Matrix-assisted laser desorption ionization-time of flight mass spectrometry | Localize measured metabolites to sub-tissue structures; it has been used to provide glomerular and tubulointerstitial metabolite information in the kidney | Can be poorly reproducible; results variable depending on prepared matrix |
| Direction-infusion (shotgun) lipidomics | Average more mass scans to achieve better signal-to-noise ratio and accomplish more sophisticated structural analysis of lipids | Lack of chromatographic separation means loss of retention time information and matrix effects, which occurs in complex biological samples |
| Gas-chromatography tandem mass spectrometry (GC-MS) | High-reproducibility | Extensive sample preparation and derivatization, which is time-consuming and potentially results in irreproducibility. |
| Ion-mobility spectrometry (IMS) | Orthogonal separation method to GC and LC, can be used to separate lipid isomers Can be added to other separation workflows as an additional separation method | Depending on the IMS method and design, low sensitivity due to the ion-mobility compartment |
| Liquid-chromatography tandem mass spectrometry (LC-MS) | Relatively minimal sample preparation, high sensitivity | Reproducibility can depend on chromatography methods |
Study design: targeted and untargeted lipidomics
Researchers first must determine whether to conduct an untargeted or targeted lipidomics study. Untargeted lipidomic studies serve as the hypothesis generating portions of the study; the aim is to discover any “features” that are differentially altered between study groups. Targeted analysis aims to detect and measure levels of pre-determined lipids of interest. Therefore, initial lipidomics study may begin with untargeted analysis, aimed at discovering as many different features between study groups, and significant findings may be validated with targeted analysis. The decision to pursue an untargeted or targeted study will inform all downstream steps: sample preparation, separation strategies, data acquisition, quality control, and data processing and analysis. The distinction between the two types of studies is perhaps especially important in lipidomics as lipids are structurally diverse molecules with varying polarities and sizes, and methods may need to be highly customized for particular species of interest.
Quality control (QC)
One of the challenges in metabolomics is to ensure as little biases have occurred during the entire experiment. Studies must be properly designed to incorporate rigorous quality controls. Errors may be introduced at any point from experimental design, sample storage, sample preparation (e.g human error in variability in pipetting) to instrumental issues (e.g batch effect, instrumental drift) to data analysis (e.g missing data, normalization). The reader is referred to previous reviews which provide in-depth discussion on these topics29–31.
Internal standards are usually heavy-labeled isotopologue of analytes of interest that are introduced at the beginning of sample preparation to aid in QC. For lipidomics, internal standards simply do not exist for every specific lipid species of interest; instead, an internal standard representative of each class is incorporated into the study. Internal standards also serve to control for any differences in metabolite extraction, as the ratio of lipid analyte to internal standard will remain unchanged. They also help in the identification of metabolites of interest, as they co-elute on LC and generate fragmentation patterns with a known mass-shift.
Another QC method is application of “pooled samples” made by pooling aliquots of study samples or reference samples and analyzed intermittently during an experimental queue for intra-assay variations, and between analytical experiments for inter-assay variations. Upon achieving a high coefficient of variation, the potential causes including non-random sources of bias need to be investigated. Generally, a coefficient of variation of 10–20% is considered acceptable for targeted metabolomics, although ranges up to 20–30% have been reported in previous studies31,32.
Bioinformatics and systems integration for lipidomics:
Lipidomics has evolved tremendously in the past decade. Despite this rapid growth, significant barriers remain to incorporate this data into metabolic pathways that will allow for a precise and comprehensive evaluation of the importance of the lipidome. Lipidomic data are characterized by (i) high dimensionality in the form of more features than available samples, (ii) heterogeneity due to the collection of diverse cellular compartments, tissue types or from different experimental conditions and (iii) availability of important metadata information about the nature of the biomolecules being profiles. The goal is to use statistical methods for gaining deeper insights into lipids function, lipid-lipid interactions across different biological phenotypes in vivo and mapping them to functional pathways.
As a large proportion of lipids are not mapped to a particular pathway, most lipids in lipidomics datasets fall outside of our current capabilities of bioinformatics tools and databases, such as the Kyoto Encyclopedia of Genes and Genomes (KEGG)33 and Human Metabolome Database (HMDB)34. One reason for this discrepancy is that pathway databases provide a genome-centric view, that is pathways defined by genes/enzymes that regulate the lipidome. In contrast, experimental lipidomics provides a chemistry-centric sampling defined by a chosen analytical method, on molecules whose diversities to a great extent are driven by modifications of simpler fatty acids as their building blocks. The overlap of these two views typically includes primary metabolic pathways (e.g phospholipase A2 clevage of sn-2 fatty acid in phospholipids to yield free fatty acid and lysophosphatidic acid), while coverage of secondary pathways and lipid metabolism (e.g sn-1 versus sn-2 fatty acid distribution amongst complex lipids such as triacylglycerols) is scarce. Alternatively, the LIPID MAPS Gene/Proteome Database22 provides a tabular list of reported interactions between genes, proteins, and individual lipids. However, this does not place these interactions in a pathway map and relies on precise lipid identification, whereas most lipidomics experiments do not provide precise identification (for example, lipids are mostly identified by lipid class and the number of carbons and double bonds present, not by the precise position of double bonds). Therefore, the current pathway databases either describe lipid metabolism at the class level or at the individual, uniquely identified level, providing coverage that is too broad or too precise for current lipidomic detection methods. The user is then left to decide how to extrapolate this information. Here, we will outline statistical, and systems-biology approaches we have utilized to analyze lipidomics data.
Structure and composition based lipidomic analysis
Selecting lipids or lipid classes of interest from an untargeted study for further investigation can be accomplished through many different strategies. The endogenous elongation and desaturation processes of fatty acids induce generation of longer and more polyunsaturated products from simpler shorter fatty acids, so that the quantity of endogenous lipids within each lipid class becomes the net effect of the efficacy of these processes and a function of synthesis versus lipolysis, which in turn determines the total concentration of the corresponding lipid class at any given time. As a result, the intra-class members of each lipid sub-class are highly correlated, and hence no single lipid may adequately represent the intra-class disease specific alterations. On the other hand, lipid phenotype alterations associated with CKD progression, involves significant alterations in a large number of intra-class lipids due to differential elongation, desaturation, synthesis, and lipolysis that basis the alterations at class level. Hence, a system biology level analysis as opposed to individual lipid analysis is required to unravel pertinent lipid alterations.
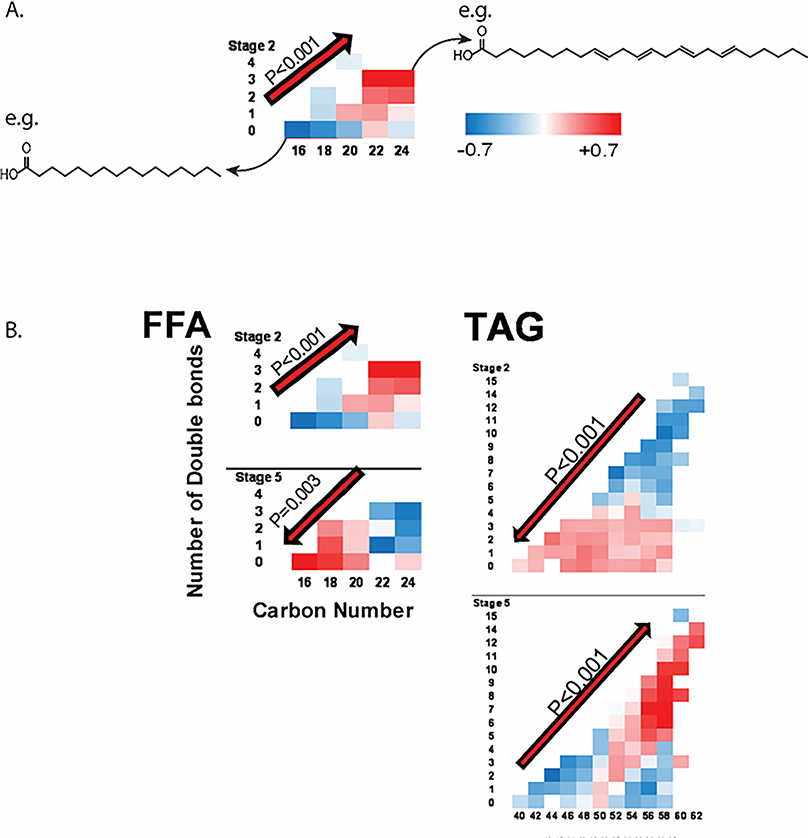
Due to the numerous potential acyl-carbon backbones combinations that can occur in lipids, grouping the identified lipid species within a class by secondary characteristics of carbon chain length and/or degree of unsaturation in order to reduce the analytical complexity could be a powerful step in data analysis. The groupings can reflect the inherent biological differences associated with degree of unsaturation and carbon bond number. For example, acylcarnitine metabolism differs for short-chain (C2-C6), medium-chain (C8-C14), and long-chain acylcarnitines (>C14); short-chain acylcarnitines can be derived from glucose, amino acids, and fatty acids whereas medium and long-chain acylcarntines are derived usually exclusively from fatty acids. Fatty acid degradation is carried out by acyl-CoA dehydratases of different degree of acyl-carbon length preference35. However, researchers can also determine how many groups per lipid class and which lipid species can be aggregated to a specific group empirically. In our studies, we have used principal component analysis to generate lipid class subgroups36–38. In the study of the Clinical Phenotyping and Resource (CPROBE) cohort37, we compared the interaction term between carbon number and number of double bonds for each lipid class (Figure 3), and found significant enrichment for long-unsaturated plasma free fatty acids (FFAs) in stage 2 CKD patients and in shorter (C16-C18)-unsaturated FFAs in stage 5 CKD patients, and the opposite in complex lipids such as TAGs, diacylglycerols (DAGs), and phosphatidylethanolamines (PEs). Overall, these findings show incremental shift toward higher abundance of circulating unsaturated C16-C18 free fatty acids and longer polyunsaturated complex lipids by worsening CKD from stage 2 to 5.
Figure 3:
Analysis of lipid classes by their secondary characteristics in the CPROBE cohort. Lipid species for specific class are plotted by their carbon number (x-axis) and double-bond number (y-axis), and color coded (blue – low, red – high) to represent standardized measured abundance for CKD stages 2 through 5. A) Example of secondary characteristic plot for free-fatty acids (FFA) in CPROBE CKD stage 2 patients. Each box represents mean standardized abundance for FFA species with the denoted carbon and double-bond number; structures for saturated 16-carbon fatty acid and 24-carbon fatty acid with four double bonds are drawn as representatives of their respective boxes. The interaction term between carbon and double bond number are noted with its p-value and the red arrow denotes the directionality of lipid accumulation with regards to carbon and double bond number. B) CPROBE FFA and triacylglycerol (TAG) secondary characteristic plots demonstrate significant interaction terms for CKD stage 2 and 5 with opposite directionality of lipid accumulation with regards to carbon and double bond number for each stage: at stage 2, FFA demonstrate increased levels of high carbon number and double bond number, whereas tags demonstrate increased level of low carbon number and double bond number; this directionality is reversed for stage 5. Figure adapted with permission from Journal of American Society of Nephrology.
Network analysis
Lipids, in particular, are capable of extensive inter-class and intra-class conversion, and network analysis may identify targets of dysfunctional lipid metabolism in CKD. However, such networks tend to be very strongly connected, since correlations do not delineate direct from indirect associations. To that end, the focus has shifted to partial correlation networks that identify direct associations, but require more sophisticated algorithms to construct especially when the number of metabolites/lipids exceeds that of available samples39,40. Once a data driven network is obtained, one can identify strongly interconnected subnetworks of metabolites/lipids by network clustering algorithms (e.g. see 41) that can act as input to a topology-based pathway enrichment algorithm.
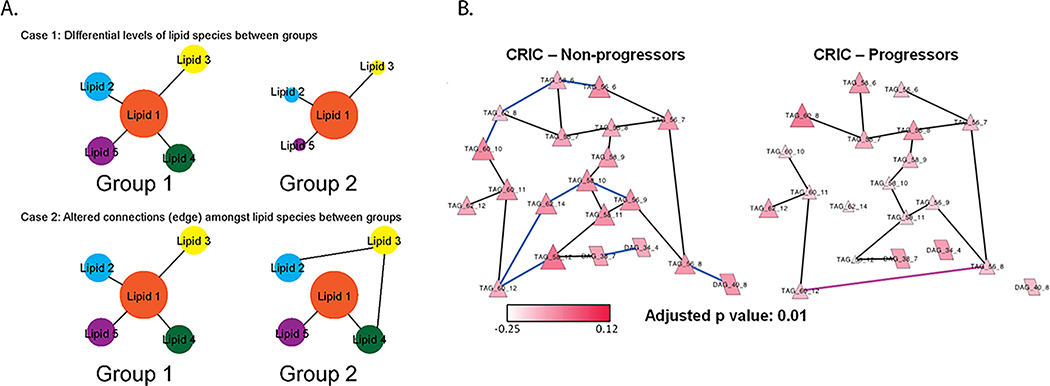
An example of the latter strategy is in a study of lipid interactions in the Chronic Renal Insufficiency Cohort (CRIC)42 and CPROBE cohorts37, wherein we constructed a partial correlation interaction network based on plasma lipidomics data and then identified through network clustering algorithms strongly interconnected subnetworks. The latter were tested for enrichment based on the topology-based pathway enrichment algorithm Network-based Gene Set Analysis (NetGSA), which assesses differential abundance and differential correlations simultaneously amongst various different lipids within a dataset (figure 4). Networks were built separately for non-progressors or progressors for CRIC patients and stage 2–3 (early-stage) or stage 4–5 (late-stage) for CPROBE patients. Comparisons of these lipid networks between the CRIC non-progressors and progressors and CPROBE early-stage and late-stage patients found decreased interconnectedness between lipid species in the progressor and late-stage CKD patients compared to the non-progressor and early-stage CKD patients. The decrease in number of these connections or “edges” were especially evident in the clusters of long-chain TAGs and clusters of cardiolipins (CL) and PE. We interpreted the loss of edges between these lipid species in the worse CKD groups to represent reduction in desaturation-elongation mechanisms between TAGs sub-network, and mitochondrial CL-PE subnetwork. These findings highlight that mitochondrial lipidome changes are associated with neutral lipid changes and this process may play a causal role in mitochondrial dysfunction.
Figure 4:
Differential network enrichment analysis (DNEA) for lipidomics data between CKD groups. A) DNEA is equipped to differentiate networks that are differentiated by differences in lipid abundance (Group 2 vs Group 1 in Case 1) or altered correlations or edges (Group 2 vs Group 1 in Case 2) or both. B) DNEA for non-progressors and progressors for CRIC patients for triacylglycerols (TAGs) and diacylglycerols (DAGs) in CPROBE. Nodes represent specific lipid species. Black edges represent correlations present in both progressors and non-progressors, blue edges represent correlations more likely to be present in non-progressors and early-stage CKD, and pink edges represent correlations more likely to be present in progressors and late-stage CKD. Higher abundance of longer polyunsaturated TAGs in CKD stages 4 and 5 with new edges in neighboring lipids specific to progressors aligns with upregulation of elongation and desaturation of longer chain fatty acids and their incorporation in synthesis of longer chain polyunsaturated TAGs in advanced CKD. Figure adapted with permission from Bioinformatics.
Lipidomics and other -omics integration
Lipidomics integration with other datasets, particularly tissue transcriptomics and proteomics data can lend to more meaningful interpretations of how changes in plasma or serum lipidome in CKD reflect and/or impact kidney disease pathogenesis. Integration of data may occur without any preselection for genes or metabolites of interest using network analysis tools, or analysis can be conducted on relevant genes/proteins associated with metabolites of interest. There are many strategies available in the literature (see review 43). One simple strategy is to examine Pearson correlation coefficients, corrected for multiple testing to select significant associations, and network visualization tools such as MetScape, which provides a framework for visual integration of metabolomic and transcriptomic profiles enabling visualizing connections between datasets44.
The initial experimental design is perhaps the most salient factor in multi-omic data integration. In the study of Pima American Indian DKD cohorts36, we were able to take advantage of transcriptomics data acquired from kidney biopsies taken close in time to the serum collection. Of note, kidney biopsies were micro-dissected into glomerular and tubulointerstitial compartments before gene-expression analysis. We sought to identify lipid-regulating transcripts that correlated significantly with the levels of lipid classes that were found to discriminate between progressors and non-progressors amongst the cohort. In particular, we discovered that the transcript levels for Acetyl-CoA Carboxylase Alpha (ACACA), the rate limiting enzyme of fatty-acid biosynthesis, correlated positively with serum long-chained saturated diacylglycerol levels in the glomerular compartment (nominal P = 0.0062 and FDR = 0.060) and with serum intermediate chain low-double-bond TAGs (nominal P = 0.0042, FDR = 0.009) in the tubulointerstitial compartment. While it is still unclear whether kidney ACACA contributes to the observed serum lipid changes associated with DKD progression, renal upregulation of ACACA may poses its detrimental effect on kidneys by upregulation of local de novo lipogenesis and impairing mitochondrial β-oxidation. These findings underscore how integrating lipidomic findings with other available -omics datasets could identify tissue-specific genes relevant to disease pathogenesis in CKD.
Lipid metabolism in chronic kidney disease
Kidney energy metabolism in CKD
The kidney is an energy intensive organ. The substrate and energy generating pathways are cell-specific in the kidney, and depend on nutritional challenges or availabilities. In the glomeruli, podocytes harbor a strong preference for anaerobic glycolysis, and rely little on mitochondrial oxidative phosphorylation for energy generation, although they are capable of fatty acid oxidation (FAO)45,46. Significant heterogeneity of substrate preferences exists amongst the different kidney tubules, depending on their location along the nephron and the kidney47. On the other hand proximal tubules, which have a large mitochondrial content, prefer to use free fatty-acids, along with glutamine as their main source of energy and display a severely limited capacity to use glucose as fuel under normal nutritional condition47–50. This inflexibility is thought to occur due to low activities of glycolytic enzymes on proximal tubules, and proximal tubular role in gluconeogenesis51. Under normal conditions, kidney uptake of fatty-acids is thought to outpace FAO oxidation; early studies with labeled lipid substrates in vivo and kidney sections from various animals demonstrated that most of labeled fatty-acid are stored in the form of diglycerides (DAG), TAGs, phospholipids and cholesterol esters51. Lipid transport, particularly long-chain lipids, into the kidney is thought to be mediated mainly by the cluster of differentiation (CD) 36 receptors. However, other transporters are implicated in fatty-acid import into the kidney, as fasted CD36 knock-out mice do not exhibit decreased levels of injected labeled oleate in the kidney compared to the wildtype mice52. Fatty-acid transporter-2 (FATP2), which is expressed mainly in proximal tubules, has been demonstrated to be responsible for fatty-acid uptake in polarized proximal tubule culture and micro-dissected proximal tubules53.
In CKD, dysregulation of lipid oxidation, lipid uptake, and lipogenesis are thought to contribute to disease. Reduced and inefficient FAO has been long thought to be a major mechanism of tubular injury and fibrosis. A landmark study demonstrated that FAO transcripts and its regulatory genes are down-regulated in the tubulointerstitium of CKD patients, and mouse models of tubulointerstitial fibrosis54. For cultured podocytes, palmitate toxicity, which is cytotoxic stress and cell death caused by palmitate in vitro, is worsened by etomoxir blockage of FAO55. Peroxisome proliferator-activated receptor-gamma coactivator (PGC1α) upregulation by drugs or transgenic overexpression is associated with disease amelioration in multiple murine models of kidney disease: aldosterone induced podocyte damage56, acute kidney injury (AKI)57, and DKD58 and the improvement in disease is thought in part to be upregulation in FAO by these regulators, amongst many other mechanisms. A recent study of renal tubular specific conditional carnitine palmitoyltransferase 1A (CPT1a) over-expression demonstrated reduced fibrosis and disease in UUO, folic-acid induce nephropathy, and adenine-induced nephropathy59. CPT1a overexpression improved mitochondrial morphology and improved FAO, supporting the hypothesis that FAO downregulation is a key driver of kidney fibrosis.
Decreased FAO, in addition to causing insufficient ATP production, is also thought to contribute to increased kidney lipid accumulation in CKD. However, increased transport of fatty-acids has also been attributed to increasing lipid uptake by the kidneys. CD36 expression has been shown to be increased in patients with nephrotic syndrome60 and DKD61,62. In addition, CD36 deficiency or blockade of CD36 has been demonstrated to ameliorate disease in models of kidney disease and fibrosis with reduced intracellular lipid deposition 63–66. Of note, CD36 has been demonstrated to mediate CKD not only via increased uptake of lipids also activation of the NLRP3 inflammasome67–69, and interactions with partners such as thrombospondin 170 and discoidin domain receptor 171 in podocytes and Na/K-ATPase72 in proximal tubules to induce lipotoxicity. CD36 deficient macrophage models of UUO and IR models of kidney disease also demonstrate CD36 role in regulating immune cells in CKD73. CD36 binds to numerous ligands, including oxidized lipids, for its activation (see ref 74) and therefore serves as a relay between circulating lipid profiles and cellular function.
Apical uptake of fatty-acid albumin complexes by proximal tubular FATP253,53 and megalin75 in the setting of CKD are also thought to contribute to lipotoxicity in CKD. A recent study76 of high-fat diet and streptozotocin and Leprdb/db eNOS–/– FATP2 deficient mouse models of DKD demonstrated a reduction in kidney fibrosis and loss of eGFR, along with reduction in intracellular lipid deposition in the FATP2 deficient DKD mice compared to its controls. However, the FATP2 deficient DKD mice also displayed reduced hyperglycemia and somewhat preserved insulin production compared to FATP2 control DKD mice; further work is necessary to clarify the role of FATP2 in DKD, although evidence suggests it a therapeutic target for CKD.
Increased neutral lipid accumulation in the kidney, particularly TAGs, occurs after many types of hypoxic, ATP-depleting, and inflammatory insults77–79. Histological studies of patients with DKD have also found lipid deposits in the glomeruli and tubules61. TAGs are widely accepted as biologically inert storage form of lipids, particularly of lipotoxic saturated fatty acids. It is thought that accumulations of bioactive diacylglycerol (DAGs), ceramides, and mitochondrial overloading with fatty-acids leads to cellular toxicity80. Upregulation in sterol-regulatory binding element (SREBP) transcripts, which induce increased expression of lipogenic enzymes such as fatty-acid synthase and acetyl-coa carboxylase, is thought to be a maladaptive response in several models of CKD. This increase in SREBP transcripts leads to increased TAG synthesis, but also cholesterol and intracellular fatty-acid synthesis, which likely confer the harmful effects of increased lipid accumulation in CKD81–83.
In summary, the kidney is a highly energetically demanding organ that relies heavily on FAO for fuel. CKD has been associated with aberrations in FAO, lipid uptake and lipogenesis that lead to intracellular lipid deposition and eventually loss of function and fibrosis.
Lipid biomarkers of CKD
Plasma and serum lipid profiles are highly dysregulated in CKD. In this next section, we will discuss recent lipidomics findings on specific lipid classes, and studies that investigate their mechanism of disease pathogenesis. For information on lipoprotein associated dyslipidemia in CKD as well as cardiovascular disease risk in CKD, we direct readers to a recent review in Nature Reviews Nephrology that provides in-depth discussion on this topic: (10.1038/s41581–021-00423–5).
Free fatty acids in CKD
Fatty acids serve as the backbone to all complex lipid synthesis and serve as the substrate for lipid oxidation. Therefore, the circulating free fatty acids or non-esterified fatty acids (NEFAs) may drive the kidney intracellular lipid profile and metabolism in CKD. Plasma and serum profiles of NEFAs in CKD are elevated usually only with advanced CKD (stage 4–5)84, although the NEFA profile is altered even in early-stage CKD. In many studies, decreased polyunsaturated fatty acid (PUFA) levels in the plasma are associated with CKD, particularly omega 3 and omega 6 fatty acids85–88, whereas increased monounsaturated fatty acid (MUFA) levels are associated with CKD and worsening CKD87–89. Saturated fatty acid (SFA) levels, especially of intermediate and long chain fatty acids are elevated in CKD85,87. Analysis of plasma free fatty acids in the CPROBE cohort demonstrated significantly higher abundance of “shorter” chain saturated fatty acids (≤C20) and lower abundance of “longer” chain unsaturated fatty acids (>C20) by worsening CKD stage from stage 2 to 537. In the Pima American Indian diabetic cohort, a cox regression model adjusted for ACR, GFR found that one standard deviation increase in unsaturated free fatty acids was associated with 0.54 fold (95% CI: 0.36 to 0.79, p=0.002) lower risk of DKD progression36. In summary, CKD patients develop altered NEFA profiles that become progressively more enriched in shorter saturated fatty acids (SFAs) and reduced in polyunsaturated fatty acids (PUFAs).
Increased levels of SFA, in particular palmitate, are associated with lipotoxicity, whereas unsaturated lipids are known to be protective against SFA toxicity. Addition of monounsaturated fatty acid (MUFA) oleate in addition to palmitate, is known to protect cultured proximal tubules and other cell cultures from palmitate lipotoxicity90–94. In addition, overexpression of stearoyl-coa desaturase (SCD) in cultured podocytes and proximal tubules is protective against palmitate toxicity95,96. This protection by MUFAs is thought to be conferred by increased palmitate partitioning to TAGs synthesis, instead of being metabolized into bioactive lipids such as ceramides, and also from increased competition for these cytotoxic pathways. The exact mechanism for why unsaturated fatty acids can induce fatty acid remodeling of TAGs is not fully understood, but some putative mechanisms may include insulin sensitivity improvement97 and favorable alteration of fasting appetite hormones98. Protein activity assays of diacylglycerol O-acyltransferase 1 and 2 (DGAT1 and DGAT2), which catalyze diacylglycerols and fatty-acyl CoAs to TAGS, demonstrate that oleyl-CoAs are preferred substrates to palmitoyl-CoAs99. In addition, oleic acid and oleoyl-CoA, in addition to other MUFAs are stronger ligands and conformational activators of PPARα in comparison to palmitate and palmitoyl-CoA100. Studies have also demonstrated that triglyceride “loading” with oleate before cellular injury can protect against cytotoxic stress that leads to increases in saturated fatty acids. An early study with HK-2 cells demonstrated that oleic acid supplementation prior to iron-mediated oxidative stress or addition of calcium inonophore with antimycin A and 2-deoxyglucose resulted in decreased cell death that was dependent on the concentration of oleate added to the media77. A recent study of clear renal cell carcinoma (ccRCC) demonstrated that triglyceride loading with oleate was protective against hypoxia likely due to hormone-sensitive lipase mediated excretion of oleate from triglyceride pool protected against SFA partitioning into forming harmful lipids such as ceramides and acylcarnitines101.
PUFAs also serve to protect against SFA toxicity like MUFAs, by serving as ligand activators of PPARα and PPARγ102 and also mediating conversion of DAGs to TAGs90,103. Additionally, PUFAs are known regulators of blood pressure and inflammation, and higher levels omega-3 fatty acids in particular are associated with decreased risk for CVD104. Recently, a study has proposed a role for PUFA in regulating redox balance. Kim et. al., demonstrated that PUFA can serve as substrates to generate highly unsaturated fatty acids by delta-desaturase 5 and 6 (D5D, D6D) with consumption of NADH to generate NAD+. This mechanism seems to be driven by decreased NAD+/NADH ratio. In vivo treatment of mice with rotenone to block aerobic respiration increased TAG production, of increased highly-unsaturated fatty acid (HUFA) backbone in multiple organs. Interestingly, kidney production of HUFA incorporated TAGs outmatched those of skeletal muscle, liver, and heart in rotenone treated mice105.
Dietary FFA profiles have been demonstrated to impact mitochondrial membrane profiles in the liver106–113. The relative abundance of saturated or unsaturated dietary FA has been associated with levels of such fatty-acyl residues in mitochondrial phospholipids, which in turn is thought to control cardiolipin acyl-chain profiles114. Excessive dietary saturated FA has been associated with increased mitochondrial oxidative stress and reduced mitochondrial oxygen consumption capacity106,109. On the other hand, increased PUFA content, particularly of long chain omega 3 and omega 6 fatty acids has been demonstrated to be able to replace linoleic acid content of membrane phospholipids110, and is associated with delayed Ca2+ induced opening of the mitochondrial permeability transition pore for apoptosis115, improve mitochondrial sensitivity to ADP levels111, and has been demonstrated to be beneficial in the context of heart-failure108,112. However, how dietary FFA affect renal mitochondrial phospholipid composition in the context of CKD remains to be investigated.
Of note, a Cochrane review of PUFA intake clinical studies concluded that there is slight reduction in cardiovascular events with increased dietary PUFA, but does not significantly impact cardiac mortality116, although this conclusion is controversial117. Other beneficiary effects of PUFA diet include improvement of insulin resistance97, decreased synthesis of cholesterol and lipoproteins118, and favorable alteration of fasting appetite hormones98.
In summary, fatty acids serve as the precursor to all complex lipids. Circulating lipid profiles become progressively more saturated and shorter in length as eGFR declines in CKD patients. While circulating FFA itself can mediate damage and have recently been implicated in regulating redox balance, FFAs also serve as the basic building blocks for all complex lipids, including mitochondrial membranes. Enrichment for saturated short FFA may dictate the level and lipid profile of complex lipids that contribute to CKD pathogenesis; we will discuss some of those complex lipids in the following sections.
Plasma acylcarnitines
Acylcarnitines are metabolites of interest in CKD because they represent fatty acids or branch-chain amino acids designated for mitochondrial oxidation and mobilization across cellular membranes. Numerous metabolomic studies of CKD patient plasma acylcarnitines have shown that circulating acylcarnitines accumulate to higher levels in CKD119–121 or hemodialysis122,123 patients compared to healthy controls, and that levels of acylcarnitines increase with worsening CKD status. Studies of diabetic have also found higher levels of plasma/serum acylcarnitines in DKD patients compared to diabetic patients without kidney disease119,120,124, and predicted DKD progression119,124–126. Large scale metabolomic studies of the general population have also established that circulating levels of acylcarnitines are significantly associated with eGFR: study of KORA F4 and Twins UK population found acylcarnitine, in particular glutarylcarnitine, inversely correlated with eGFR127 and another study of Chinese adults with GFR ≥60 found acylcarnitines of various lengths also inversely correlate with decrease in eGFR128.
Incomplete FAO leads to acylcarnitines accumulation129 in tissues that become eventually reflected in the plasma. Apart from measuring the absolute levels of acylcarnitines, another marker of mitochondrial inefficiency is the relative abundance of various acylcarnitines by chain length, including short, medium, and long chain acylcarnitines. In the CPROBE cohort, relatively lower levels of long chain acylcarnitines is associated with advanced stages of CKD and (C16-C20)/(C5-C14) ratio, which is the ratio of the levels of long-chain to medium-chain acylcarnitines, were associated with worsening CKD stage42. Of note, the CPROBE cohort exhibited a graded increase in relative abundance of short and medium chain saturated FFA with increasing CKD stage, consistent with similar alteration in FFAs with the same carbon number, suggesting that FFA profiles may contribute to acylcarnitine profiles as FFA serve as the substrate for medium to long acylcarnitine synthesis and FAO. This association of relatively low levels of long-chain acylcarnitine and high levels of medium and short-chain acylcarnitine and CKD status was also found to be associated with DKD progression (defined as decrease in iGFR by at least 40%) in the American Pima Indian cohort at baseline before onset of iGFR reduction31. Lower levels of long-chain acylcarnitines were associated with decreased expression of FAO genes in the glomerular compartment and genes regulating FAO in the tubulointerstitial compartment36, suggesting inefficient β-oxidation of longer chain fatty acids with CKD progression and in ESKD.
Acylcarnitines are filtered by the kidney and is excreted into the urine and therefore decreasing eGFR levels likely contribute to increased short- to medium-chain plasma acylcarnitine levels130. With progression to ESKD and reliance on dialysis, the abundance of longer chain acylcarnitines increase123,131, which is primarily attributed to the inefficient of filtration of acylcarnitines greater than 8 acyl-chain carbons through dialyzer membranes130. Increased levels of long-chain acylcarnitines in ESKD patients has been demonstrated to predict increased cardiovascular mortality123 and reduced physical function132. A study of 111 hemodialysis patients found that C2/(C16+C18:1) serum acylcarnitine ratio, which can also represent FAO efficiency, predicted mortality; patients with higher ratio and therefore better predicted mitochondrial FAO efficiency had lower morality rates133. To explain these results, researchers have suggested that the accumulation of plasma acylcarnitine is likely mitochondrial overload and dysfunction that occurs in CKD due to increased free-fatty acid levels and insulin resistance from CKD and its comorbidities, and therefore serve as a biomarker of overall patient health in CKD.
Researchers have proposed the idea that increased acylcarnitines export into plasma and elimination in urine may be an adaptive method of dealing with mitochondrial overload, preventing fatty acid mediated cellular damage, and freeing CoA from acyl-CoAs to participate in other energy generating capacities especially in early CKD. Studies of carnitine-acetyltransferase (CrAT) demonstrates the important role of acylcarnitine, especially of acetylcarnitine, efflux out of the mitochondria. Muscle-specific deletion of CrAT, which serves to remove short-chain acetyl-CoAs out of the mitochondria by conjugation to carnitines, leads to decreased glucose tolerance and increased insulin resistance, attributed due to the increased inhibition of pyruvate dehydrogenase (PDH) by accumulating acetyl-CoA134. A recent study of proximal tubular cell-specific CrAT deficient mice demonstrated that CrAT function is indispensable to the normal function of proximal tubules. CrAT deficient mice develop tubulointerstitial fibrosis and secondary glomerulosclerosis, which is worsened by a high-fat diet challenge. CrAT deficient kidneys accumulated long-chain acylcarnitines and decreased mitochondrial oxygen-consumption rate. Interestingly, these CrAT deficient mice secreted less short chain and medium chain acylcarnitines in the urine, supporting the notion that efflux of short and medium chain efflux into plasma and urine aid in mitochondrial homeostasis with FAO135. Of note, carnitine supplementation, especially in ESKD where patients are prone to dialysis related free-carnitine loss, has not yielded clear improvements in disease parameters such as serum lipid levels, hemoglobin levels, and skeletal muscle weakness136–138. However, there is lack of research assessing for whether carnitine supplementation improves insulin resistance or improves mitochondrial function in CKD.
Plasma acylcarnitines are thought to be mainly derived from skeletal muscle139, as it is the largest reservoir of carnitine and acylcarnitine in the body, although the liver also contributes to circulating acylcarntines140. In the models of muscle specific and proximal tubule specific CrAT mice134,135, there was no effect on plasma acylcarnitine levels despite dysregulated tissue acylcarnitine levels, suggesting that multi-organ changes to acylcarnitine metabolism may be necessary to affect plasma acylcarnitine levels. In summary, increase in plasma acylcarnitines in CKD patients likely represents the run-off of systemic mitochondrial FAO overload, decreased excretion by the kidney, and may serve as an adaptive response to combat mitochondrial stress in CKD.
Triacylglycerols
It is well established that CKD is associated with increased levels of circulatory triglycerides (TAG) thought to be due to decreased catabolism and increased hepatic production of TAG lipoproteins141. A higher TAG/HDL ratio, which has been proposed as a measure of increased insulin resistance, have been found to be associated with increased CKD risk142,143 and predictive of CKD development and progression144,145. Hypertriglyceridemia is also associated with increased risk of atherosclerotic disease and events. A recent secondary analysis of the Study of Heart and Renal Protection trial found increased risk of atherosclerotic events with increase in triglyceride, TAG/HDL ratio, and triglyceride associated lipoproteins: apo-B and triglyceride-rich lipoprotein cholesterol146. In ESKD patients treated with hemodialysis, however, the total TAG levels have been reported to be lower than in healthy patients147 and a study found elevated TAG/HDL associated with positive cardiovascular and overall mortality148, although the finding has not been recapitulated with peritoneal dialysis patients149.
Recent studies have improved our understanding of the compositions of TAGs in CKD. Analysis of triglycerides fatty-acyl chain compositions in the CPROBE cohort found enrichment of TAG species with longer acyl chain length and higher degree of saturation with increasing CKD severity37. Another cross-sectional study of 44 hemodialysis patients found TAGs of medium chain length (52–56) to be relatively increased while shorter TAGs were relative decreased compared to TAG profiles of health controls147. In the Pima Native America cohort, clinical progressors had significantly higher levels of polyunsaturated TAGs, and Cox regression included main effect term of short and lower double-bond TAG species levels to predict DKD progression36. A mechanism for the relative enrichment of TAGs with high double-bond and length in CKD, is likely due to the up-regulation of elongation and desaturation of shorter more toxic fatty acids to their less toxic polyunsaturated counterparts. The incorporation of the relatively less toxic polyunsaturated long into TAGs may be a compensatory mechanism due to the alteration of mitochondrial fuel preference for shorter-chain fatty acids (≤C10), which do not depend on the carnitine for mitochondrial transmembrane transport150, in the setting of mitochondrial dysfunction and carnitine deficiency in ESKD. Analysis of the (C16-C20)/(C5-C14) ratio, aforementioned in the acylcarnitine section, found an inverse association with this ratio with high double-bond longer-chain TAGs in the CPROBE and Pima cohorts36,37, suggesting a relationship between mitochondrial FAO fuel profiles and TAGs profiles. In summary, TAG profiles in late-stage CKD and ESKD are enriched for longer and saturated fatty acids and may be due to an increased mitochondrial demand for shorter and saturated fatty acid for fuel.
Of note, fibrates, which reduce circulating cholesterol and triglyceride levels, have not been strongly indicated for lipid management in CKD. Clinical trials and meta-analyses have demonstrated that fenofibrates can reduce cardiovascular disease events and death151,152, decrease eGFR reductions and microalbuminuria in DKD patients152,153, but seem to confer no reduction in CKD incidence and progression to ESKD152. In summary, further studies are required to fully understand how fatty acyl profiles of TAGs are altered in CKD at different stages of disease, and how the TAG profiles affect TAG function and role in CKD.
Phosphatidylethanolamines
Phosphatidylethanolamines have been identified in multiple clinical studies to be associated with CKD. A secondary analysis of serum samples from two clinical studies: African American Study of Kidney Disease and Hypertension (AASK) and the Modification of Diet in Renal Disease (MDRD) found six PE species that correlated significantly with proteinuria in both AASK and MDRD cohorts154. Pathway enrichment analysis of all metabolites associated significantly with proteinuria found PEs overrepresented in the analysis. In the CRIC cohort, intra-class mean of PEs were higher in the progressors as compared to the non-progressors42. In the Pima Native American cohort, higher polyunsaturated PEs were independently associated with higher risk of DKD progression in type 2 diabetes; a Cox regression model, which included unsaturated PEs as a main effector term, found that each 1 SD higher unsaturated fatty acids was associated with 2.57 times higher risk of DKD progression36. Lipidomic analysis of CPROBE patients found higher levels of PCs and PEs in CKD patients who experienced stroke compared to CKD patients who did not155.
PEs are also complex lipids, like TAGs, whose acyl-chain profiles become progressively longer and unsaturated with increasing CKD severity in the CPROBE cohort. Analysis of the (C16-C20)/(C5-C14) ratio in the CPROBE and Pima cohorts found an inverse association with this ratio with high double-bond longer-chain PEs36,37; similar to TAGs, it is possible that mitochondrial fuel demands for shorter and saturated fatty acids lead to PE profiles with high abundance of high double-bond longer-chain PEs and consumption of PEs with low double-bond and shorter-chain PEs.
PEs are phospholipids involved in numerous cellular functions. For mammalian cells, major de novo synthesis of PEs occurs in the ER (Kennedy pathway) and in the mitochondrial inner membrane by the action of phosphatidylserine (PSD1)156. PEs can further be processed via the Kennedy pathway to generate phosphatidylcholines (PCs) or via the action of PEMT specifically in the liver157. Maintaining normal levels and production of PEs in mitochondria is indispensable to mitochondrial morphology, fission and fusion, and respiration158–160. Clinical studies have associated increased PC:PE ratio with decreased insulin sensitivity, and exercise has been demonstrated to increased PC and PE levels in skeletal muscles161 and PC:PE ratio in the context of long-term exercise162. Deficiency of phosphatidylethanolamine N-methyltransferase (PEMT), a liver specific enzyme that converts PE to PC, resulted in increased levels of mitochondrial PE and therefore lower PC:PE ratio, and was associated with increased hepatocyte mitochondrial respiration163. Of interest, PEMT −/− mice exhibited reduced DKD severity when injected with STZ despite unchanged PC:PE ratio in the kidney, suggesting hepatic, and therefore the systematic, PC and PE levels may influence kidney disease164. The authors proposed that reduction in ER stress with downregulation of PEMT contributes to DKD amelioration.
Ceramides
Sphingolipids are of large interest not only in CKD, but many other diseases such as diabetes, and cardiovascular disease because they are a large group of lipids with important regulatory functions. Incorporation of fatty acids into generating these signaling and regulatory molecules may therefore impact CKD pathogenesis. At the center of sphingolipid synthesis are ceramides, which can be further modified to generate sphingomyelin, glycosphingolipids, or broken down to generate sphingosine-1-phosphate165. Previous studies of sphingolipid levels in plasma of CKD patients report on few specific sphingolipids of specific length and saturation, which makes understanding the dynamics of sphingolipid metabolism difficult. Different acyl-chain lengths, saturation and lipid species of sphingolipids can have different and sometimes opposing impacts on biology. Of note, sphingolipids are highly associated with DKD because substrates for sphingolipid and glycosphingolipid synthesis such as fatty acids and glucose derived substrates (lactose, galactose, etc) are increased in diabetes166.
Of particular interest amongst sphingolipids in CKD are ceramides. Ceramides are second messengers involved in promotion of apoptosis, amongst many of its function. Studies have demonstrated that the ceramide carbon number and degree of unsaturation can alter this activity. Ceramide synthases 1–6 (CerS) catalyze ceramides of differential carbon lengths. In HeLa cells, overexpression of CerS 5, which preferentially uses palmitoyl-CoA to generate C16:0 containing ceramides, increased irradiation (IR) induced apoptosis, whereas overexpression of CerS2, which preferentially uses long-chain fatty acids to generate C22:0-C24:0 containing ceramides, protected cells from IR apoptosis167. A recent study using a method of in situ selective ceramide synthesis called “traceless ceramide ligation”, found that synthesis of ceramide containing saturated fatty acid backbone (16:0, 18:0) reduced viability in HeLa cells but not when synthesis occurred with monounsaturated fatty acids (24:1, 18:1)168.
CKD patients generally exhibit an increase in plasma/serum levels of ceramides. In a recent study of 415 patients who were evaluated for ischemic heart disease, researchers found increased levels of Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20:0), Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/24:1). The levels of these ceramides also correlated with CKD stage, increasing with CKD progression. Adjustment for risk factors for CKD, such as diabetes, body mass index, and hypertension did not alter the significance of association of these ceramides and CKD status169. These results are corroborated by other studies: ceramide levels of these species were increased in a study of juvenile patients with CKD compared to healthy controls170, and in a study of patients with T2DM DKD compared to patients with T2DM without DKD for Cer(18:1/16:0) and Cer(18:1/16:1)120. On the other hand, in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort, researchers found that very long chain (C20 – 26) ceramides were significantly decreased in T1DM DKD patients with micro and macroalbuminuria as compared to T1DM without albuminuria. Baseline plasma ceramide levels from the DCCT/EDIC patients collected before patients progressed to micro or macroalbuminuria, demonstrated that increase in very-long ceramides were associated with decreased odds of developing macroalbuminuria171.
It is unclear how circulating levels of ceramides levels reflect kidney tissue levels; a metabolomics study of ceramides in the BKS-db/db mice demonstrated elevated plasma but decreased kidney levels of ceramides and glucosylceramides, which suggest disparity in plasma and kidney ceramide regulation172. Although kidney levels of sphingolipid levels in CKD are relatively unknown, transcriptomics data and inhibition of sphingolipid enzymes through drug or genetic engineering suggest that sphingolipid metabolism is dysregulated in CKD, and accumulation of kidney ceramides seem to mediate damage. Blockage of ceramide synthesis by de novo synthesis with myrocin or conversion from sphingomyelin to by amitriptyline has been demonstrated to improve high-fat induced murine nephropathy173,174 and cisplatin induced AKI175.
Studies of CerS deficient obese mice have demonstrated tissue-specific CerS subtype and ceramide subspecies relevance. For example, in HFD fed mice, skeletal muscles were found to accumulate C18:0 fatty acid backbones preferentially, mediated by the action of CerS1, and its absence in the skeletal muscle leads to reduction in C18:0 accumulation and improvement in glucose handling in obese mice, whereas CerS5 and CerS6 skeletal muscle deficiency does not result in significant alterations to the C16:0 ceramide pool and glucose metabolism176. Study of CerS2 haplo-insufficient mice demonstrated insulin signaling impairment in the liver associated with the accumulation of C16:0 through the action of CerS6177. While it is unclear whether ceramides and which ceramide species mediate insulin resistance in CKD, recent studies of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b) lipid raft enzyme have uncovered ceramide-1-phosphate (C1P) as a regulatory sphingolipid of podocyte insulin signaling. SMPDL3b is a lipid-raft enzyme that binds to and dephosphorylates C1P to ceramide, in addition to binding ceramide kinase and modulating its activity178,179. SMPDL3b expression is increased the glomeruli patients with DKD and db/db mice178,180, and overexpression of SMPDL3b in podocytes hinders insulin receptor B association with caveolin-1 and downstream Akt phosphorylation178. Proper insulin signaling is restored in SMPDL3b OE podocytes and db/db mice when supplemented with exogenous C1P, suggesting a lipid driven regulatory role in podocyte insulin signaling in diabetes180. Of note, SMPDL3b has been shown to also play a role in focal segmental glomerulosclerosis (FSGS); levels of SMPDL3b is decreased in FSGS patients in contrast to DKD patients, and this decrease leads potentially to increased suPAR-mediated β3 integrin activation180. It is interesting that differential SMPDL3b expression is associated with different CKD etiologies, and further work is necessary to uncover SMPDL3b and ceramide role in CKD.
While the roles of specific CerS proteins and ceramide species in CKD have not yet been fully elucidated, increase in short and saturated NEFA plasma levels may affect intracellular ceramide profiles to generated shorter and unsaturated C16:0 and C18:0 ceramides that enhance apoptosis, insulin resistance, and decreased mitochondrial FAO, amongst many other cytotoxic mechanisms. In the kidney, C1P deficiency has emerged as a potential underlying mechanism for insulin resistance in the podocytes. For future studies, further comprehensive lipidomics analysis of the sphingolipid pathway may find additional sites of dysregulation important to CKD.
Bile acids
Bile acids (BA) are synthesized from hepatocytes and play numerous roles in lipid emulsification and digestion, in addition to serving as signaling molecules. Although clinical lipidomics of BAs in the CKD population is very limited, current studies concur in that serum BA levels in CKD patients are elevated, due to decreased eGFR, and its compositions are significantly altered CKD181–185. Secondary BAs are primary BAs modified through the action of gut microbiome. A study reported increased share of deoxycholic acid (DCA), an unconjugated second, as a percent of total bile acids in CKD patients compared to the control group181. In another study of ESKD patients, researchers found an increase in the proportion of conjugated BAs and a decrease in the proportion of unconjugated BAs in ESKD patients182. A study of microbiome-derived metabolites in ESKD patients demonstrated significantly elevated levels of conjugated secondary BAs relative to conjugated primary BAs in CKD patients, and microbiome analysis demonstrated an enrichment for bacterial species generating secondary bile acids in the disease population185. A study of CKD stage 3b to 4 patients demonstrated a positive correlation with the levels of DCA with vascular calcification183. Of note, while serum BA levels can be reduced through dialysis in ESKD patients, the composition of BAs is not normalized181.
The strongest evidence for the importance of BAs in CKD comes from studies of bile-acid receptors farnesoid X receptor (FXR) and membrane-bound G protein coupled receptor TGR5. FXR deficient STZ type 1 diabetic mice exhibit significant kidney fibrosis, albuminuria, lipid-deposition, as compared to the WT STZ mice186. FXR activation with various agonists (INT-747, GW4064, cholic acid) in db/db, STZ type 1 or HFD-induced murine models of kidney dysfunction demonstrated improvement in disease parameters through modulation of SREBP-1 expression which leads to reduction in lipogenesis genes, reduction in fibrosis and proinflammatory cytokine signaling pathways, and increase in oxidative stress handling mechanisms187–190. TGR5 activation with agonist INT-777 in db/db mice improve disease by upregulating lipid-oxidation and mitochondrial biogenesis genes186,187. Of note, activation of these receptors leads to an improvement in circulating lipid panels: lower serum total cholesterol, mostly due to LDL reduction, and serum triglyceride contents, and this improvement likely contributes to amelioration of DKD186,188,189. However, FXR and TGR5 agonism on cultured mesangial cells and podocytes, respectively, also activate or deactivate same set of pathways as in vivo, suggesting direct impact of FXR and TGR5 action on kidney tissues186,189.
Studies of patient kidney biopsies have demonstrated decreased FXR transcript and protein expression in glomeruli and tubules of patients with DKD and obesity188. TGR5 transcripts were reduced in DN patients and its levels correlated with degree of proteinuria, glomerular sclerosis, and eGFR measurements186. These clinical data suggest FXR and TGR5 relevance in disease. Of note, FXR and TGR5 agonist Nidufexor is currently under investigation as a potential therapeutic for DKD191 (NCT03804879).
From a lipidomics standpoint, it is unclear whether the alterations in serum BAs in CKD affect FXR and TRG5 signaling. Primary BA chenodeoxycholic acid (CDCA) is the strongest activator of FXR, whereas secondary BA lithocholic acid (LCA) is the strongest activator of TRG5192. It is possible that a shift in the levels and composition in BAs in CKD could lead to changes in FXR and TRG5 function. More work is necessary to uncover the regulatory function of CKD BAs in disease.
Discussion and future perspectives
Lipids are a structurally diverse group of molecules that, despite belonging to the same class, have major differences and sometimes opposing biological functions depending on its chemical composition. Technological improvements in chromatography and mass spectrometry have generated new insights into the altered lipid profiles in CKD, with the most recent comprehensive lipidomics studies reporting on lipid subclass information on carbon number, degree of unsaturation, and hydroxylation. Further structural (e.g. isomer) lipid deconvolution, which is an ongoing area of research, will grant further insights into how lipid profiles are altered in CKD.
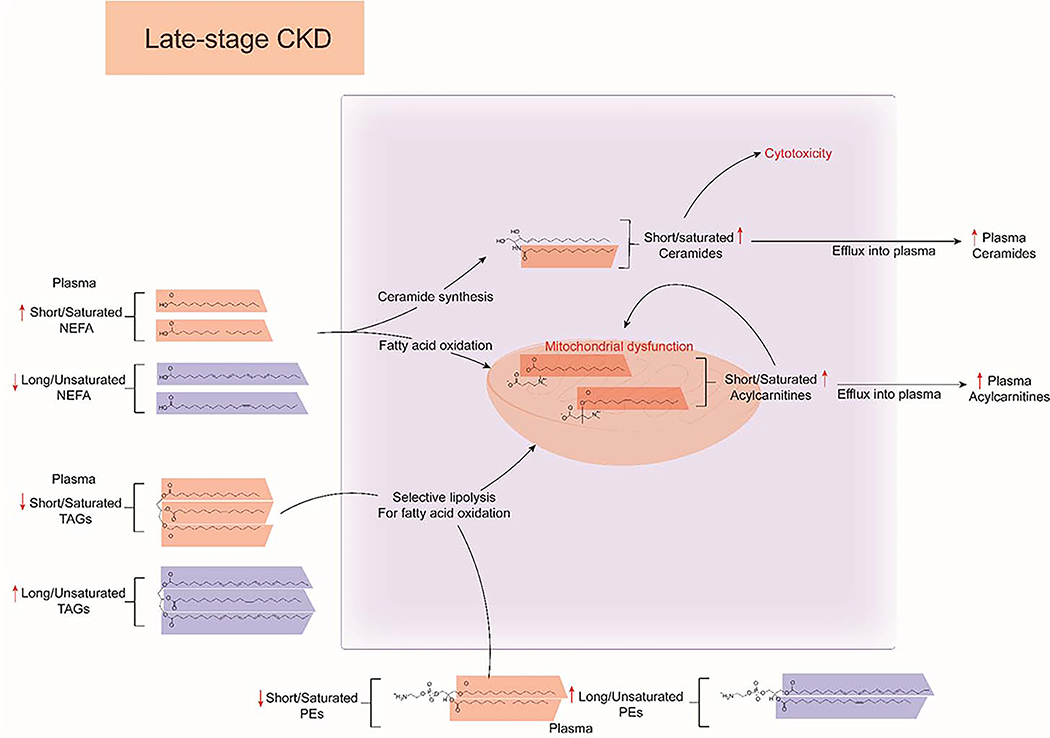
Lipidomics of plasma and serum from CKD patients have demonstrated both an increase in the overall concentration and alterations of the structural lipid species in numerous lipid classes. NEFA levels of short and saturated fatty acids has been associated with late-stage CKD and has also been predictive of disease progression, whereas decreased PUFA levels have been associated with early-stage CKD and have been demonstrated to have cardioprotective effects in dietary supplementation studies. Short and saturated fatty acids have been demonstrated to be weak PPAR activating ligands, and their shunting into acylcarnitines and ceramides is thought to be cytotoxic, whereas long and unsaturated fatty acids are protective against the actions of short and saturated NEFAs. In turn, complex lipids such as TAGs and PEs become enriched for their long-chained and saturated counterparts, potentially due to mitochondrial substrate preference for shorter chain-fatty acids due to mitochondrial stress and dysfunction in CKD (Figure 5).
Figure 5:
Overview of dyslipidemia in late-stage CKD. Short/saturated non-esterified fatty acids (NEFAs) are highlighted in orange and and long/unsaturated NEFAs are highlighted in blue. Increased levels of circulating short/saturated NEFA in CKD serve as substrates for fatty-acid oxidation and ceramide synthesis. Increase in short/saturated acylcarnitines contribute to mitochondrial overload and dysfunction, while increased production of short/saturated ceramides contribute to cellular cytotoxicity. Acylcarnitine and ceramides are transported outside the cell and their levels and profiles reflected in the plasma. In turn, mitochondrial overload leads to selective lipolysis of complex lipids such as triacylglycerols (TAGs) and phosphatidylethanolamines (PEs) with short/saturated fatty acid side-chains as they can bypass the carnitine shuttle. This leads to consumption of plasma level short/saturated complex lipids and relative increase in long/unsaturated TAGs
Clinical lipidomics studies have been limited to measurement of circulating lipid profiles as markers of CKD, but the extent of the effect of CKD on peripheral lipid profiles and levels require further investigation. To our knowledge, the only study that systematically compared plasma and kidney (also liver, retina, and nerve) lipid profiles across different lipid classes in a model of kidney disease found that liver lipid composition most closely reflected plasma lipid composition193. Interestingly, this was much more pronounced in nondiabetic control mice than in mice with diabetes. Furthermore, the majority of lipid subclasses that were similarly coregulated between diabetic plasma and diabetic liver tissue were also similar between control plasma and control liver tissue. This association was not as pronounced between plasma and the other tissues (kidney, nerve, and retina), where there were fewer similarities across the control and diabetic conditions for each plasma/tissue comparison. This was particularly true between plasma and kidney, as only one chain-length lipid subclass and two saturation lipid subclasses were commonly regulated in both control and diabetic conditions. To examine how lipid levels were associated within and across classes, we performed correlation analyses in control and diabetic plasma, kidney, nerve, and retina using the 364 lipid features identified in all samples. In this analysis, lipids in plasma and kidney displayed much more correlation than did nerve and retina. Other murine studies examining the relationship between serum and kidney lipid compositions demonstrate highly class-specific responses between the two compartments. A lipidomics study of aging mice demonstrated concurrent changes in TAG and DAG levels, but disparate changes in many phospholipids (many of which are not abundant in the serum)194. In the aforementioned proximal tubular specific CrAT knock-out mice135, which developed tubulointerstitial fibrosis, circulating acylcarnitine levels did not significantly change. Therefore, changes in circulating lipids in CKD likely represent systematic effects and multi-organ dysfunction as a result of CKD.
The relationship between circulating lipid profiles and mitochondrial dysfunction, and furthermore, exactly which processes contribute to CKD requires further investigation. Exogenous lipids likely affect mitochondrial function either by serving as substrates for oxidation or altering mitochondrial lipid composition. Excessive fatty acid load, much like excess glucose load is thought to also contribute to increased reactive oxygen species production in mitochondria. Cardiolipin peroxidation due to such reactive oxygen species production is thought to alter cardiolipin association with oxidative phosphorylation machinery and cue the cell for apoptosis195. In murine models of diabetic kidney disease (DKD), reduction in poly-unsaturated cardiolipin and increases in saturated and mono-unsaturated cardiolipin species occur and is attributed to the increased peroxidation of cardiolipins196,197. On the other hand, pharmacological approaches that target cardiolipin peroxidation such as Elamipretide195,198–201 or transgenic upregulation of FAO with CPT1202 have been successful in ameliorating disease in various murine models of CKD, suggesting that targeting mitochondrial dysfunction, regardless of dyslipidemia status, is sufficient for disease prevention. In conclusion, it is likely that circulating dyslipidemia and mitochondrial dysfunction are two inter-linked processes that form a vicious cycle that contributes to CKD progression.
More research is necessary in understanding how unfavorable circulating lipid profiles directly contribute to tissue dysfunction in CKD of various etiologies, and recent efforts in integrating lipidomics and tissue transcriptomics have been able to identify FAO and de novo lipogenesis as dysregulated pathways in CKD. Many of the studies discussed in this review address CKD of metabolic etiologies, but transcriptomic profiling studies of kidney biopsy in patients from the Nephrotic Syndrome Study Network (NEPTUNE) cohort demonstrated that genes regulating cholesterol transport and metabolism are upregulated203 and are potentially dysregulated in the presence of apolipoprotein L1 allele variants G1 and G2204, suggesting that lipid dysregulation also contributes to CKD of non-metabolic etiologies. The various CKD cohorts such as NEPTUNE and Kidney Precision Medicine Program (KPMP)205 provide the opportunity to integrate, multi-omics approaches (transcriptomics, lipidomics, proteomics) since the kidney biopsy, blood and urine samples used to generate these different types of molecular data are frequently obtained during routine clinical care. The ultimate goal is to develop an integrated molecular classification which will confer insight into individual renal disease specific targets of lipid dysregulation.
Major points:
Lipidomic analyses remain a challenge due to the numerous species that compose each class; techniques that reduce analytical complexity while retaining important structural information about lipid structure is useful.
A key challenge with profiling technologies including lipidomics is the ability to integrate data to gain insights into biological systems, as well as disease onset and progression mechanisms. We discuss framework for systems integration to identify critical nodes for mechanistic and therapeutic interrogation.
Fatty acid profiles enriched in shorter and more saturated species are associated with later stage of CKD and predictive of CKD progression.
Due to the immense structural diversity of lipids, lipidomics analyses are guided by choice of untargeted and targeted analysis. Sample preparation, extraction and separation methods may require optimization for specific classes of interest.
Acylcarnitine profiles are a marker of mitochondrial function. A lower long-chain to medium-chain acylcarnitine ratio represents increased mitochondrial inefficiency that is associated with worsening CKD.
Complex lipids such as triacylglycerols (TAGs) and phosphatidylethanolamines (PEs) become enriched in longer and more unsaturated fatty acyl side-chains in late-stage CKD and end-stage kidney disease (ESKD). Inverse association of mitochondrial efficiency and high double-bond longer-chain TAGs and PEs suggest a mechanistic link between fatty-acid oxidation and TAG and PE fatty acid profiles.
Acknowledgments
Support: Supported by the NIH grants 5F30DK121463, T32GM007863, T32GM008322, 5T32DK101357, K08DK106523, R03DK121941, R56DK126647, R24 DK082841, P30DK089503, P30DK081943, P30DK020572 and 1R01DK110541-01A1, 5U01CA235487-03, 5R01GM114029-05 and JDRF Center for Excellence (5-COE-2019-861-S-B).
References
- 1.Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 2.United States Renal Data System. 2019 Annual Data Report. Bethesda, Maryland, USA: NIH and National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 3.Sarnak Mark J et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease. Circulation 108, 2154–2169 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Tsimihodimos V, Dounousi E & Siamopoulos KC Dyslipidemia in Chronic Kidney Disease: An Approach to Pathogenesis and Treatment. Am. J. Nephrol. 28, 958–973 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Thompson S et al. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 26, 2504–2511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y-Y, Wu S-P, Liu S, Zhang Y & Lin R-C Ultra-performance liquid chromatography–mass spectrometry as a sensitive and powerful technology in lipidomic applications. Chem. Biol. Interact. 220, 181–192 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Sun T, Wang X, Cong P, Xu J & Xue C Mass spectrometry-based lipidomics in food science and nutritional health: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 19, 2530–2558 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-Y et al. Microbiome–metabolome reveals the contribution of gut–kidney axis on kidney disease. J. Transl. Med. 17, 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y-Y, Vaziri ND & Lin R-C Chapter Six - Lipidomics: New Insight Into Kidney Disease. in Advances in Clinical Chemistry (ed. Makowski GS) vol. 68 153–175 (Elsevier, 2015). [DOI] [PubMed] [Google Scholar]
- 10.Aldana J, Romero-Otero A & Cala MP Exploring the Lipidome: Current Lipid Extraction Techniques for Mass Spectrometry Analysis. Metabolites 10, 231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y-Y, Cheng X & Lin R-C Chapter One - Lipidomics Applications for Discovering Biomarkers of Diseases in Clinical Chemistry. in International Review of Cell and Molecular Biology (ed. Jeon KW) vol. 313 1–26 (Academic Press, 2014). [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y-Y, Cheng X-L, Lin R-C & Wei F Lipidomics applications for disease biomarker discovery in mammal models. Biomark. Med. 9, 153–168 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Rund KM et al. Development of an LC-ESI(−)-MS/MS method for the simultaneous quantification of 35 isoprostanes and isofurans derived from the major n3- and n6-PUFAs. Anal. Chim. Acta 1037, 63–74 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y-Y, Miao H, Cheng X-L & Wei F Lipidomics: Novel insight into the biochemical mechanism of lipid metabolism and dysregulation-associated disease. Chem. Biol. Interact. 240, 220–238 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Wang Y-N et al. The Dysregulation of Eicosanoids and Bile Acids Correlates with Impaired Kidney Function and Renal Fibrosis in Chronic Renal Failure. Metabolites 11, 127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren J et al. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan Baiyao formulation. Phytomedicine 77, 153266 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Feng Y-L et al. Activated NF-κB/Nrf2 and Wnt/β-catenin pathways are associated with lipid metabolism in CKD patients with microalbuminuria and macroalbuminuria. Biochim. Biophys. Acta BBA - Mol. Basis Dis 1865, 2317–2332 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Hou B et al. Comprehensive Lipidome Profiling of the Kidney in Early-Stage Diabetic Nephropathy. Front. Endocrinol. 11, 359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dou F et al. An Integrated Lipidomics and Phenotype Study Reveals Protective Effect and Biochemical Mechanism of Traditionally Used Alisma orientale Juzepzuk in Chronic Kidney Disease. Front. Pharmacol. 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CA, Want EJ, O’Maille G, Abagyan R & Siuzdak G XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 78, 779–787 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Guijas C et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 90, 3156–3164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotter D, Maer A, Guda C, Saunders B & Subramaniam S LMPD: LIPID MAPS proteome database. Nucleic Acids Res. 34, D507–D510 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richmond GS & Smith TK Phospholipases A1. Int. J. Mol. Sci. 12, 588–612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldana J, Romero-Otero A & Cala MP Exploring the Lipidome: Current Lipid Extraction Techniques for Mass Spectrometry Analysis. Metabolites 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaženović I, Kind T, Ji J & Fiehn O Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cajka T & Fiehn O Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 88, 524–545 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Han X Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 12, 668–679 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Züllig T, Trötzmüller M & Köfeler HC Lipidomics from sample preparation to data analysis: a primer. Anal. Bioanal. Chem. 412, 2191–2209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn WB, Wilson ID, Nicholls AW & Broadhurst D The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. 10.4155/bio.12.204 (2012) doi: 10.4155/bio.12.204. [DOI] [PubMed] [Google Scholar]
- 30.Sas KM, Karnovsky A, Michailidis G & Pennathur S Metabolomics and Diabetes: Analytical and Computational Approaches. Diabetes 64, 718–732 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afshinnia F et al. Lipidomics and Biomarker Discovery in Kidney Disease. Semin. Nephrol. 38, 127–141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee EP et al. Variability of Two Metabolomic Platforms in CKD. Clin. J. Am. Soc. Nephrol. 14, 40–48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M & Goto S KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wishart DS et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608–D617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Gharbawy A & Vockley J Defects of Fatty Acid Oxidation and the Carnitine Shuttle System. Pediatr. Clin. North Am. 65, 317–335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afshinnia F et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight (2019) doi: 10.1172/jci.insight.130317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afshinnia F et al. Impaired β -Oxidation and Altered Complex Lipid Fatty Acid Partitioning with Advancing CKD. J. Am. Soc. Nephrol. ASN.2017030350 (2017) doi: 10.1681/ASN.2017030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afshinnia F et al. Elevated lipoxygenase and cytochrome P450 products predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 35, 303–312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu S et al. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 33, 1545–1553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krumsiek J, Bartel J & Theis FJ Computational approaches for systems metabolomics. Curr. Opin. Biotechnol. 39, 198–206 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Ma J et al. Differential network enrichment analysis reveals novel lipid pathways in chronic kidney disease. Bioinformatics (2019) doi: 10.1093/bioinformatics/btz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afshinnia F et al. Lipidomic Signature of Progression of Chronic Kidney Disease in the Chronic Renal Insufficiency Cohort. Kidney Int. Rep. 1, 256–268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian I, Verma S, Kumar S, Jere A & Anamika K Multi-omics Data Integration, Interpretation, and Its Application. Bioinforma. Biol. Insights 14, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karnovsky A et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics 28, 373–380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S-Y et al. Increasing the level of peroxisome proliferator-activated receptor γ coactivator-1α in podocytes results in collapsing glomerulopathy. JCI Insight 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brinkkoetter PT et al. Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics. Cell Rep. 27, 1551–1566.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forbes JM & Thorburn DR Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 14, 291–312 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Klein KL, Wang M-S, Torikai S, Davidson WD & Kurokawa K Substrate oxidation by isolated single nephron segments of the rat. Kidney Int. 20, 29–35 (1981). [DOI] [PubMed] [Google Scholar]
- 49.Uchida S & Endou H Substrate specificity to maintain cellular ATP along the mouse nephron. Am. J. Physiol.-Ren. Physiol 255, F977–F983 (1988). [DOI] [PubMed] [Google Scholar]
- 50.Kang HM et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandel LJ Metabolic Substrates, Cellular Energy Production, and the Regulation of Proximal Tubular Transport. Annu. Rev. Physiol. 47, 85–101 (1985). [DOI] [PubMed] [Google Scholar]
- 52.Scerbo D et al. Kidney triglyceride accumulation in the fasted mouse is dependent upon serum free fatty acids. J. Lipid Res. 58, 1132–1142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan S et al. Kidney Proximal Tubule Lipoapoptosis Is Regulated by Fatty Acid Transporter-2 (FATP2). J. Am. Soc. Nephrol. 29, 81–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang HM et al. Defective fatty acid oxidation in renal tubular epithelial cells plays a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kampe K, Sieber J, Orellana JM, Mundel P & Jehle AW Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am. J. Physiol. - Ren. Physiol 306, F401–F409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan Y et al. Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 82, 771–789 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Tran M et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 121, 4003–4014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H et al. Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am. J. Physiol. - Ren. Physiol 295, F1071–F1081 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miguel V et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. (2021) doi: 10.1172/JCI140695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baines RJ et al. CD36 mediates proximal tubular binding and uptake of albumin and is upregulated in proteinuric nephropathies. Am. J. Physiol.-Ren. Physiol 303, F1006–F1014 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Herman-Edelstein M, Scherzer P, Tobar A, Levi M & Gafter U Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 55, 561–572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hua W et al. CD36 Mediated Fatty Acid-Induced Podocyte Apoptosis via Oxidative Stress. PLoS ONE 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okamura DM et al. CD36 Regulates Oxidative Stress and Inflammation in Hypercholesterolemic CKD. J. Am. Soc. Nephrol. 20, 495–505 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Souza ACP et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int. 89, 809–822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang P et al. Inflammatory stress promotes the development of obesity-related chronic kidney disease via CD36 in mice. J. Lipid Res. 58, 1417–1427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y-L et al. CD36 is a novel and potential anti-fibrogenic target in albumin-induced renal proximal tubule fibrosis. J. Cell. Biochem. 101, 735–744 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Zhao J et al. CD36-Mediated Lipid Accumulation and Activation of NLRP3 Inflammasome Lead to Podocyte Injury in Obesity-Related Glomerulopathy. Mediators Inflamm. 2019, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L-C et al. Palmitate aggravates proteinuria-induced cell death and inflammation via CD36-inflammasome axis in the proximal tubular cells of obese mice. Am. J. Physiol.-Ren. Physiol 315, F1720–F1731 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Sheedy FJ et al. CD36 coordinates NLRP3 inflammasome activation by facilitating the intracellular nucleation from soluble to particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui W et al. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim. Biophys. Acta 1852, 1323–1333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J-J et al. Discoidin domain receptor 1 activation links extracellular matrix to podocyte lipotoxicity in Alport syndrome. EBioMedicine 63, 103162 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy DJ et al. CD36 and Na/K-ATPase-α1 Form a Pro-inflammatory Signaling Loop in Kidney. Hypertension 61, 216–224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pennathur S et al. The Macrophage Phagocytic Receptor CD36 Promotes Fibrogenic Pathways on Removal of Apoptotic Cells during Chronic Kidney Injury. Am. J. Pathol. 185, 2232–2245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang X et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 13, 769–781 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Kuwahara S et al. Megalin-Mediated Tubuloglomerular Alterations in High-Fat Diet–Induced Kidney Disease. J. Am. Soc. Nephrol. JASN 27, 1996–2008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan S et al. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zager RA, Johnson ACM & Hanson SY Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 67, 111–121 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Johnson ALICM, Stahl A & Zager RA Triglyceride accumulation in injured renal tubular cells: Alterations in both synthetic and catabolic pathways. Kidney Int. 67, 2196–2209 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Li S et al. Reduced kidney lipoprotein lipase and renal tubule triglyceride accumulation in cisplatin-mediated acute kidney injury. Am. J. Physiol.-Ren. Physiol 303, F437–F448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinberg JM Lipotoxicity. Kidney Int. 70, 1560–1566 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Wang Z et al. Regulation of Renal Lipid Metabolism, Lipid Accumulation, and Glomerulosclerosis in FVBdb/db Mice With Type 2 Diabetes. Diabetes 54, 2328–2335 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Jiang T et al. Diet-induced Obesity in C57BL/6J Mice Causes Increased Renal Lipid Accumulation and Glomerulosclerosis via a Sterol Regulatory Element-binding Protein-1c-dependent Pathway. J. Biol. Chem. 280, 32317–32325 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Lin Y-C et al. Nifedipine Exacerbates Lipogenesis in the Kidney via KIM-1, CD36, and SREBP Upregulation: Implications from an Animal Model for Human Study. Int. J. Mol. Sci. 21, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen H et al. Combined Clinical Phenotype and Lipidomic Analysis Reveals the Impact of Chronic Kidney Disease on Lipid Metabolism. J. Proteome Res. 16, 1566–1578 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Szczuko M et al. Comparison of Fatty Acid Profiles in a Group of Female Patients with Chronic Kidney Diseases (CKD) and Metabolic Syndrome (MetS)–Similar Trends of Changes, Different Pathophysiology. Int. J. Mol. Sci. 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gc S, Jj C, O H, P B & P S Plasma fatty acids in chronic kidney disease: nervonic acid predicts mortality. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 22, 277–283 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Szczuko M et al. The C18:3n6/C22:4n6 ratio is a good lipid marker of chronic kidney disease (CKD) progression. Lipids Health Dis. 19, 77 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czumaj A et al. Alterations of Fatty Acid Profile May Contribute to Dyslipidemia in Chronic Kidney Disease by Influencing Hepatocyte Metabolism. Int. J. Mol. Sci. 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L et al. Plasma lipidomics investigation of hemodialysis effects by using liquid chromatography-mass spectrometry. J Proteome Res 15, 1986 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Soumura M et al. Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem. Biophys. Res. Commun. 402, 265–271 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Hardy S et al. Saturated Fatty Acid-induced Apoptosis in MDA-MB-231 Breast Cancer Cells A ROLE FOR CARDIOLIPIN. J. Biol. Chem. 278, 31861–31870 (2003). [DOI] [PubMed] [Google Scholar]
- 92.Listenberger LL et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. 100, 3077–3082 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuzefovych L, Wilson G & Rachek L Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am. J. Physiol.-Endocrinol. Metab 299, E1096–E1105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ackerman D et al. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 24, 2596–2605.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sieber J et al. Susceptibility of Podocytes to Palmitic Acid Is Regulated by Stearoyl-CoA Desaturases 1 and 2. Am. J. Pathol. 183, 735–744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iwai T et al. Stearoyl-CoA Desaturase-1 Protects Cells against Lipotoxicity-Mediated Apoptosis in Proximal Tubular Cells. Int. J. Mol. Sci. 17, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Luis DA, Izaola O, Aller R, de La Fuente B & Pacheco D Effects of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) on weight loss, adipocytokines levels, and insulin resistance after a high polyunsaturated fat diet in obese patients. J. Endocrinol. Invest. 36, 965–969 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Stevenson JL, Paton CM & Cooper JA Hunger and satiety responses to high-fat meals after a high-polyunsaturated fat diet: A randomized trial. Nutrition 41, 14–23 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Cases S et al. Cloning of DGAT2, a Second Mammalian Diacylglycerol Acyltransferase, and Related Family Members. Journal of Biological Chemistry http://www.jbc.org.proxy.lib.umich.edu doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 100.Hostetler HA, Petrescu AD, Kier AB & Schroeder F Peroxisome Proliferator-activated Receptor α Interacts with High Affinity and Is Conformationally Responsive to Endogenous Ligands. J. Biol. Chem. 280, 18667–18682 (2005). [DOI] [PubMed] [Google Scholar]
- 101.Ackerman D et al. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 24, 2596–2605.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kliewer SA et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. 94, 4318–4323 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas ME, Morrison AR & Schreiner GF Metabolic effects of fatty acid-bearing albumin on a proximal tubule cell line. Am. J. Physiol.-Ren. Physiol 268, F1177–F1184 (1995). [DOI] [PubMed] [Google Scholar]
- 104.Kris-Etherton Penny M, Harris William S, & Appel Lawrence J Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 106, 2747–2757 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Kim W et al. Polyunsaturated Fatty Acid Desaturation Is a Mechanism for Glycolytic NAD+ Recycling. Cell Metab. 29, 856–870.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crescenzo R et al. Fat Quality Influences the Obesogenic Effect of High Fat Diets. Nutrients 7, 9475–9491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruiz-Ramírez A, Barrios-Maya M-A, López-Acosta O, Molina-Ortiz D & El-Hafidi M Cytochrome c release from rat liver mitochondria is compromised by increased saturated cardiolipin species induced by sucrose feeding. Am. J. Physiol.-Endocrinol. Metab. 309, E777–E786 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Shah KB et al. The Cardioprotective Effects of Fish Oil During Pressure Overload Are Blocked by High Fat Intake. Hypertension 54, 605–611 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cavaliere G et al. Polyunsaturated Fatty Acids Attenuate Diet Induced Obesity and Insulin Resistance, Modulating Mitochondrial Respiratory Uncoupling in Rat Skeletal Muscle. PLOS ONE 11, e0149033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cortie CH & Else PL Dietary Docosahexaenoic Acid (22:6) Incorporates into Cardiolipin at the Expense of Linoleic Acid (18:2): Analysis and Potential Implications. Int. J. Mol. Sci. 13, 15447–15463 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herbst E a. F. et al. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 592, 1341–1352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mulligan CM et al. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc. Res. 94, 460–468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pepe S, Tsuchiya N, Lakatta EG & Hansford RG PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca 2+ activation of PDH. Am. J. Physiol.-Heart Circ. Physiol 276, H149–H158 (1999). [DOI] [PubMed] [Google Scholar]
- 114.Oemer G et al. Phospholipid Acyl Chain Diversity Controls the Tissue-Specific Assembly of Mitochondrial Cardiolipins. Cell Rep. 30, 4281–4291.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Khairallah RJ et al. Improved Mitochondrial Function with Diet-Induced Increase in Either Docosahexaenoic Acid or Arachidonic Acid in Membrane Phospholipids. PLOS ONE 7, e34402 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abdelhamid AS et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clifton PM & Keogh JB A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 27, 1060–1080 (2017). [DOI] [PubMed] [Google Scholar]
- 118.St-Onge M-P, Zhang S, Darnell B & Allison DB Baseline Serum C-Reactive Protein Is Associated with Lipid Responses to Low-Fat and High-Polyunsaturated Fat Diets. J. Nutr. 139, 680–683 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ibarra-González I et al. Optimization of kidney dysfunction prediction in diabetic kidney disease using targeted metabolomics. Acta Diabetol 55, 1151–1161 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Liu J-J et al. Profiling of Plasma Metabolites Suggests Altered Mitochondrial Fuel Usage and Remodeling of Sphingolipid Metabolism in Individuals With Type 2 Diabetes and Kidney Disease. Kidney Int. Rep. 2, 470–480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kordalewska M et al. Multiplatform metabolomics provides insight into the molecular basis of chronic kidney disease. J. Chromatogr. B 1117, 49–57 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Impact of haemodialysis on individual endogenous plasma acylcarnitine concentrations in end-stage renal disease. Ann. Clin. Biochem. 42, 387–393 (2005). [DOI] [PubMed] [Google Scholar]
- 123.Kalim S et al. A Plasma Long-Chain Acylcarnitine Predicts Cardiovascular Mortality in Incident Dialysis Patients. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Looker HC et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 88, 888–896 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Niewczas MA et al. Uremic solutes and risk of end stage renal disease in type 2 diabetes. Kidney Int. 85, 1214–1224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van der Kloet FM et al. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study). Metabolomics 8, 109–119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Serum Metabolite Concentrations and Decreased GFR in the General Population- ClinicalKey. https://www-clinicalkey-com.proxy.lib.umich.edu/#!/content/playContent/1-s2.0-S0272638612004647?scrollTo=%23hl0001019. [DOI] [PubMed]
- 128.Wang F et al. Associations of Plasma Amino Acid and Acylcarnitine Profiles with Incident Reduced Glomerular Filtration Rate. Clin. J. Am. Soc. Nephrol. CJASN 13, 560–568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koves TR et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 7, 45–56 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Fouque D et al. Relationship Between Serum Carnitine, Acylcarnitines, and Renal Function in Patients With Chronic Renal Disease. J. Ren. Nutr. 16, 125–131 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Impact of haemodialysis on individual endogenous plasma acylcarnitine concentrations in end-stage renal disease. Ann. Clin. Biochem. 42, 387–393 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Murphy WJA et al. Altered carnitine metabolism in dialysis patients with reduced physical function may be due to dysfunctional fatty acid oxidation. Nephrol. Dial. Transplant. 27, 304–310 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Kamei Y, Kamei D, Tsuchiya K, Mineshima M & Nitta K Association between 4-year all-cause mortality and carnitine profile in maintenance hemodialysis patients. PLOS ONE 13, e0201591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muoio DM et al. Muscle-specific Deletion of Carnitine Acetyltransferase Compromises Glucose Tolerance and Metabolic Flexibility. Cell Metab. 15, 764–777 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kruger C et al. Proximal Tubular Cell–Specific Ablation of Carnitine Acetyltransferase Causes Tubular Disease and Secondary Glomerulosclerosis. Diabetes 68, 819 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Y et al. l-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: a systematic review and meta-analysis. Am. J. Clin. Nutr. 99, 408–422 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Huang H et al. Influence of L-Carnitine Supplementation on Serum Lipid Profile in Hemodialysis Patients: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 38, 31–41 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Emami Naini A et al. Effects of Oral L-Carnitine Supplementation on Lipid Profile, Anemia, and Quality of Life in Chronic Renal Disease Patients under Hemodialysis: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Nutr. Metab. 2012, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brass EP Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin. Ther. 17, 176–185 (1995). [DOI] [PubMed] [Google Scholar]
- 140.Xu G et al. Liver and Muscle Contribute Differently to the Plasma Acylcarnitine Pool During Fasting and Exercise in Humans. J. Clin. Endocrinol. Metab. 101, 5044–5052 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Mikolasevic I, Žutelija M, Mavrinac V & Orlic L Dyslipidemia in patients with chronic kidney disease: etiology and management. Int. J. Nephrol. Renov. Dis. 10, 35–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsuruya K et al. Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: Analysis in a large Japanese population. Atherosclerosis 233, 260–267 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Ho C-I et al. Relationship between TG/HDL-C ratio and metabolic syndrome risk factors with chronic kidney disease in healthy adult population. Clin. Nutr. 34, 874–880 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Tsuruya K et al. Impact of the Triglycerides to High-Density Lipoprotein Cholesterol Ratio on the Incidence and Progression of CKD: A Longitudinal Study in a Large Japanese Population. Am. J. Kidney Dis. 66, 972–983 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Zoppini G et al. Triglyceride–high-density lipoprotein cholesterol is associated with microvascular complications in type 2 diabetes mellitus. Metabolism 61, 22–29 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Lamprea-Montealegre JA et al. Apolipoprotein B, Triglyceride-Rich Lipoproteins, and Risk of Cardiovascular Events in Persons with CKD. Clin. J. Am. Soc. Nephrol. 15, 47–60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rhee EP et al. Metabolite Profiling Identifies Markers of Uremia. J. Am. Soc. Nephrol. 21, 1041–2051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang TI et al. Association of Serum Triglyceride to HDL Cholesterol Ratio with All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. CJASN 12, 591–602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhan X et al. Triglyceride to high-density lipoprotein cholesterol ratio is associated with increased mortality in older patients on peritoneal dialysis. Lipids Health Dis. 18, 199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Longo N, Frigeni M & Pasquali M CARNITINE TRANSPORT AND FATTY ACID OXIDATION. Biochim. Biophys. Acta 1863, 2422–2435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davis TME et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 54, 280–290 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Effects of Fibrates in Kidney Disease: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 60, 2061–2071 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Frazier R et al. Associations of Fenofibrate Therapy With Incidence and Progression of CKD in Patients With Type 2 Diabetes. Kidney Int. Rep. 4, 94–102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luo S et al. Serum Metabolomic Alterations Associated with Proteinuria in CKD. Clin. J. Am. Soc. Nephrol. CJASN 14, 342–353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Afshinnia F et al. Plasma lipidomic profiling identifies a novel complex lipid signature associated with ischemic stroke in chronic kidney disease. J. Transl. Sci. 6, (2020). [PMC free article] [PubMed] [Google Scholar]
- 156.van der Veen JN et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta BBA - Biomembr. 1859, 1558–1572 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Vance JE & Tasseva G Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1831, 543–554 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Chan EYL & McQuibban GA Phosphatidylserine Decarboxylase 1 (Psd1) Promotes Mitochondrial Fusion by Regulating the Biophysical Properties of the Mitochondrial Membrane and Alternative Topogenesis of Mitochondrial Genome Maintenance Protein 1 (Mgm1). J. Biol. Chem. 287, 40131–40139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Friedman JR et al. Lipid Homeostasis Is Maintained by Dual Targeting of the Mitochondrial PE Biosynthesis Enzyme to the ER. Dev. Cell 44, 261–270.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tasseva G et al. Phosphatidylethanolamine Deficiency in Mammalian Mitochondria Impairs Oxidative Phosphorylation and Alters Mitochondrial Morphology. J. Biol. Chem. 288, 4158–4173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Newsom SA et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J. Appl. Physiol. 120, 1355–1363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee S et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine respond to exercise and influence insulin sensitivity in men. Sci. Rep. 8, 6531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Veen J. N. van der, Lingrell S, Silva R. P. da, Jacobs RL & Vance DE The Concentration of Phosphatidylethanolamine in Mitochondria Can Modulate ATP Production and Glucose Metabolism in Mice. Diabetes 63, 2620–2630 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Watanabe M et al. Pemt Deficiency Ameliorates Endoplasmic Reticulum Stress in Diabetic Nephropathy. PLOS ONE 9, e92647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhat OM, Yuan X, Li G, Lee R & Li P-L Sphingolipids and Redox Signaling in Renal Regulation and Chronic Kidney Diseases. Antioxid. Redox Signal. 28, 1008–1026 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shayman JA Targeting Glucosylceramide Synthesis in the Treatment of Rare and Common Renal Disease. Semin. Nephrol. 38, 183–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mesicek J et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell. Signal. 22, 1300–1307 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rudd AK & Devaraj NK Traceless synthesis of ceramides in living cells reveals saturation-dependent apoptotic effects. Proc. Natl. Acad. Sci. 115, 7485–7490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mantovani A et al. Association between increased plasma ceramides and chronic kidney disease in patients with and without ischemic heart disease. Diabetes Metab. (2020) doi: 10.1016/j.diabet.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 170.Magagnotti C et al. Identification of nephropathy predictors in urine from children with a recent diagnosis of type 1 diabetes. J. Proteomics 193, 205–216 (2019). [DOI] [PubMed] [Google Scholar]
- 171.Klein RL et al. Decreased plasma levels of select very long chain ceramide species Are associated with the development of nephropathy in type 1 diabetes. Metabolism. 63, 1287–1295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sas KM et al. Targeted Lipidomic and Transcriptomic Analysis Identifies Dysregulated Renal Ceramide Metabolism in a Mouse Model of Diabetic Kidney Disease. J. Proteomics Bioinform Suppl 14, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Woo C-Y et al. Inhibition of Ceramide Accumulation in Podocytes by Myriocin Prevents Diabetic Nephropathy. Diabetes Metab. J. 43, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boini KM, Zhang C, Xia M, Poklis JL & Li P-L Role of Sphingolipid Mediator Ceramide in Obesity and Renal Injury in Mice Fed a High-Fat Diet. J. Pharmacol. Exp. Ther. 334, 839–846 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dupre TV et al. Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. J. Lipid Res. 58, 1439–1452 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Turpin-Nolan SM et al. CerS1-Derived C18:0 Ceramide in Skeletal Muscle Promotes Obesity-Induced Insulin Resistance. Cell Rep. 26, 1–10.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 177.Raichur S et al. CerS2 Haploinsufficiency Inhibits β-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metab. 20, 687–695 (2014). [DOI] [PubMed] [Google Scholar]
- 178.Mitrofanova A et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 10, 2692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mallela SK, Mitrofanova A, Merscher S & Fornoni A Regulation of the amount of ceramide-1-phosphate synthesized in differentiated human podocytes. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1864, 158517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yoo T-H et al. Sphingomyelinase-Like Phosphodiesterase 3b Expression Levels Determine Podocyte Injury Phenotypes in Glomerular Disease. J. Am. Soc. Nephrol. 26, 133–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jiménez F, Monté M, El-Mir MY, Pascual MJ & Marin J Chronic Renal Failure-Induced Changes in Serum and Urine Bile Acid Profiles. Dig. Dis. Sci. (2004) doi: 10.1023/A:1020575001944. [DOI] [PubMed] [Google Scholar]
- 182.Li R et al. Targeted metabolomics study of serum bile acid profile in patients with end-stage renal disease undergoing hemodialysis. PeerJ 7, e7145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jovanovich A et al. Deoxycholic Acid, a Metabolite of Circulating Bile Acids, and Coronary Artery Vascular Calcification in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found 71, 27–34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chu L, Zhang K, Zhang Y, Jin X & Jiang H Mechanism underlying an elevated serum bile acid level in chronic renal failure patients. Int. Urol. Nephrol. 47, 345–351 (2015). [DOI] [PubMed] [Google Scholar]
- 185.Wang X et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut gutjnl-2019–319766 (2020) doi: 10.1136/gutjnl-2019-319766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang XX et al. G Protein-Coupled Bile Acid Receptor TGR5 Activation Inhibits Kidney Disease in Obesity and Diabetes. J. Am. Soc. Nephrol. JASN 27, 1362–1378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang XX et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 29, 118–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang XX et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. - Ren. Physiol 297, F1587–F1596 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jiang T et al. Farnesoid X Receptor Modulates Renal Lipid Metabolism, Fibrosis, and Diabetic Nephropathy. Diabetes 56, 2485–2493 (2007). [DOI] [PubMed] [Google Scholar]
- 190.Wang XX et al. Diabetic Nephropathy Is Accelerated by Farnesoid X Receptor Deficiency and Inhibited by Farnesoid X Receptor Activation in a Type 1 Diabetes Model. Diabetes 59, 2916–2927 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chianelli D et al. Nidufexor (LMB763), a Novel FXR Modulator for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 63, 3868–3880 (2020). [DOI] [PubMed] [Google Scholar]
- 192.Malhi H & Camilleri M Modulating Bile Acid Pathways and TGR5 Receptors for Treating Liver and GI Diseases. Curr. Opin. Pharmacol. 37, 80–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sas KM et al. Shared and distinct lipid-lipid interactions in plasma and affected tissues in a diabetic mouse model. J. Lipid Res. 59, 173–183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eum JY et al. Aging-related lipidomic changes in mouse serum, kidney, and heart by nanoflow ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1618, 460849 (2020). [DOI] [PubMed] [Google Scholar]
- 195.Szeto HH Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J. Am. Soc. Nephrol. 28, 2856–2865 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tan SM et al. Complement C5a Induces Renal Injury in Diabetic Kidney Disease by Disrupting Mitochondrial Metabolic Agility. Diabetes 69, 83–98 (2020). [DOI] [PubMed] [Google Scholar]
- 197.Zhang G et al. DESI-MSI and METASPACE indicates lipid abnormalities and altered mitochondrial membrane components in diabetic renal proximal tubules. Metabolomics 16, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mizuguchi Y et al. A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am. J. Physiol.-Ren. Physiol 295, F1545–F1553 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hou Y et al. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol.-Ren. Physiol 310, F547–F559 (2015). [DOI] [PubMed] [Google Scholar]
- 200.Szeto HH et al. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD. J. Am. Soc. Nephrol. JASN 28, 1437–1449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Szeto HH et al. Protection of mitochondria prevents high-fat diet–induced glomerulopathy and proximal tubular injury. Kidney Int. 90, 997–1011 (2016). [DOI] [PubMed] [Google Scholar]
- 202.Miguel V et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. (2021) doi: 10.1172/JCI140695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mitrofanova A et al. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 94, 1151–1159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ryu J-H et al. APOL1 renal risk variants promote cholesterol accumulation in tissues and cultured macrophages from APOL1 transgenic mice. PLOS ONE 14, e0211559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.de Boer IH et al. Rationale and design of the Kidney Precision Medicine Project. Kidney Int. 99, 498–510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Isaac G Electrospray Ionization Tandem Mass Spectrometry (ESI-MS/MS)-Based Shotgun Lipidomics. in Metabolic Profiling: Methods and Protocols (ed. Metz TO) 259–275 (Humana Press, 2011). doi: 10.1007/978-1-61737-985-7_16. [DOI] [PubMed] [Google Scholar]
- 207.Qiao X et al. A tandem mass spectrometric study of bile acids: Interpretation of fragmentation pathways and differentiation of steroid isomers. Steroids 77, 204–211 (2012). [DOI] [PubMed] [Google Scholar]
- 208.van der Hooft JJJ, Ridder L, Barrett MP & Burgess KEV Enhanced Acylcarnitine Annotation in High-Resolution Mass Spectrometry Data: Fragmentation Analysis for the Classification and Annotation of Acylcarnitines. Front. Bioeng. Biotechnol 3, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hocher B & Adamski J Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 13, 269–284 (2017). [DOI] [PubMed] [Google Scholar]
- 210.Emwas A-H et al. NMR Spectroscopy for Metabolomics Research. Metabolites 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ren J-L, Zhang A-H, Kong L & Wang X-J Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Adv 8, 22335–22350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grove KJ et al. Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J. Lipid Res. 55, 1375–1385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miyamoto S et al. Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator. EBioMedicine 7, 121–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhou W, Yang S & Wang PG Matrix effects and application of matrix effect factor. Bioanalysis 9, 1839–1844 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Abbas I et al. Kidney Lipidomics by Mass Spectrometry Imaging: A Focus on the Glomerulus. Int. J. Mol. Sci. 20, 1623 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wernisch S & Pennathur S Application of differential mobility-mass spectrometry for untargeted human plasma metabolomic analysis. Anal. Bioanal. Chem. 411, 6297–6308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wernisch S, Afshinnia F, Rajendiran T & Pennathur S Probing the application range and selectivity of a differential mobility spectrometry – mass spectrometry platform for metabolomics. Anal. Bioanal. Chem. 410, 2865–2877 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]