Abstract
In this study, plant-root-associated Bacillus species were evaluated as antifungal biocontrol agents by analyzing the production of surface bioactive molecules known as lipopeptide biosurfactants. This study aimed to isolate and characterize antifungal biosurfactant-producing Bacillus bacterium. Bacillus velezensis PW192 was isolated from the rhizosphere of Lagerstroemia macrocarpa var macrocarpa and identified based on phylogenetic analysis of the 16S rRNA gene. The biosurfactant was excreted to cultured supernatant and exhibited emulsification power up to 60% and a decrease in surface tension from 72 in distilled water to 21 mN/m. The surface tension properties were stable in a broad range of pH from 6 to 10, in high temperatures up to 100 °C, and in salinities with a NaCl concentration up to 12% (w/v). Starting from 0.5 mg of acid, precipitated crude biosurfactant exhibited antifungal activity toward Anthracnose, caused by the phytopathogens Colletotrichum gloeosporioides and C. musae. The chemical structures of the biosurfactant were structurally characterized as lipopeptides fengycin A and fengycin B. The stability of the biosurfactant, as well as the antifungal properties of B. velezensis PW192, can potentially make them useful as agricultural biocontrol agents, as well as in other biotechnological applications.
Keywords: Bacillus velezensis, biosurfactant, lipopeptides, antifungal, Colletotrichum spp.
1. Introduction
Bacillus are spore-forming, ubiquitous microorganisms found in nature, including in soil, water, and extreme terrestrial environments [1]. Bacteria from the Bacillus genus are known as factories for the production of biologically-active compounds. They produce a variety of potential enzymes, insecticides, polymers, antibiotics, and surfactants [2]. Some of their products are regarded as harmless and are listed as Generally Recognized as Safe (GRAS).
Biosurfactants are surface-active compounds derived from various microbial sources, including bacteria and fungi [3,4]. They are amphiphilic molecules that comprise both hydrophilic and hydrophobic moieties. The remarkable properties of biosurfactants, such as their biodegradability, biocompatibility, low toxicity, high surface activity, and stability under extreme conditions (temperature, pH, and salinity), have attracted researchers [5]. Cyclic lipopeptides (CLPs) are a class of surfactants produced from Bacillus. CLPs are biosynthesized by non-ribosomal peptide synthetases (NRPS), which install fatty acid chains (hydrophobic) to cyclic peptide moieties (hydrophilic) [6]. B. subtilis, B. amyloliquefaciens, B. pumilus, and B. licheniformis have been reported to produce CLPs such as surfactin, iturin, licenysin, and fengycin [7]. Due to their surface-active properties, CLPs increase the bioavailability of hydrophobic substrates around the plant rhizosphere, leading to the indirect promotion of plant growth due to improvements in agriculture soil properties [8]. Bacillus-derived surfactants also have antimicrobial and antifungal abilities that enable the elimination of several plant pathogens. In terms of commercial development, biosurfactants have therefore emerged as biological control agents on a sustainable agricultural basis.
Colletotrichum-caused Anthracnose is a common post-harvest disease in various tropical and subtropical fruits and vegetables that causes more than 50% of loss of the agricultural produce [9]. C. gloeosporioides causes severe losses of tropical fruits including papaya, mango, and avocado. C. musae is the causal agent of anthracnose in banana fruits [10]. The application of potential biological fungicide agents may be a safe and environmentally-compatible method of disease management. The aims of the present study were to (i) isolate and identify the biosurfactant produced by Bacillus species, (ii) elucidate the biosurfactant stability in different conditions including temperature, pH, and salinity, and (iii) determine the antifungal activity of the biosurfactant against the phytopathogens Colletotrichum gloeosporioides and C. musae. In addition, the chemical structure of the biosurfactant was characterized.
2. Materials and Methods
2.1. Screening for Bacillus Producing Biosurfactant
Soils from the surrounding rhizosphere were collected and preserved at 4 °C until bacteria were isolated. Ten grams of soil was serially diluted with 0.85% of NaCl. The diluents were heated at 80 °C for 10 min to eliminate the vegetative cells. The heat-resistant bacteria were screened on Trypticase soy agar medium (TSA, BD Bacto). After 24 h of incubation, the bacterial isolates were Gram-stained and the cell morphologies were observed under a microscope. Gram-positive, rod-shaped bacterium was further screened to examine biosurfactant production.
Biosurfactant production was screened by cultivating the bacterium in modified biosurfactant production medium from Khondee et al. [11] (1 L consisted of 1 g of glucose, 0.5 g of beef extract, 3.3 g of K2HPO4, 0.14 g of KH2PO4, 20 g of glycerol, 2.2 g of NH4Cl, 0.2 g of NaNO3, 0.1 g of FeSO4.7H2O, and 0.6 g of MgSO4). Single colonies were inoculated into 10 mL of the medium. After 5 days of incubation at 30 °C on a 180 rpm orbital shaking incubator, the supernatant was collected by centrifuging the cultivation medium at 8000 rpm for 5 min. The supernatant was filtered through a 0.45-micron sterile filter. The biosurfactant activity of the cell-free supernatants was examined.
2.2. Determination of Biosurfactant Activity
Biosurfactant activity was determined using surface tension assay and emulsification assay. Surface tension was measured with the pendant drop method using a DM-CE1 instrument (Kyowa Interface Science, Saitama, Japan). Deionized water was used as the control. To determine the emulsification index (E24), 3 mL of cultured broth was mixed with an equal volume of hexadecane. After being vortexed for 2 min, the mixture was settled for 24 h at 30 °C and the height of the emulsion layer and the total mixture were measured [12]. The emulsification index (E24) was calculated as the percentage of the height of the emulsified layer (mm) divided by the total height of the liquid column (mm). The biosurfactant screening experiments were performed in triplicate.
2.3. 16S rRNA Phylogenetic Construction
Genomic DNA of the biosurfactant isolate was extracted using a Wizard genomic DNA purification kit (Promega, Madison, WI, USA). The genomic DNA was used as a DNA template for the amplification of the 16S rRNA gene. Full-length 16S rRNA was amplified using 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3’) as the forward and reverse primers, respectively. DNA sequencing was conducted using 785F (5′-GGATTAGATACCCTGGTA-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′), which are the inter-primers, to identify bacteria. Full-length 16S rRNA was used for phylogenetic construction. The phylogenetic tree was analyzed.
The values of sequence similarities between the biosurfactant isolate (PW192) and available reference strains were computed with the EzBioCloud server (http://eztaxon-e.ezbiocloud.net, accessed on 30 November 2021) [13]. An almost full-length size of the isolate PW192 16S rRNA gene (1497 bp) was aligned with multiple sequences of available Bacillus-type strains using CLUSTAL_X software [14]. Evolutionary trees of the taxa were constructed with the neighbor-joining method [15] using the MEGA 7 software [16]. Bootstrap analysis with 1000 replicates was performed to determine the robustness of the tree topologies [17]; only the bootstrap values of 50% or above are shown.
2.4. Biosurfactant Production and Extraction
Bacillus isolates were cultivated in biosurfactant production medium as mentioned above. The cell-free supernatant was collected after 5 days of incubation via centrifugation at 8000 rpm for 10 min. Biosurfactant was precipitated by adjusting the pH of the supernatant to pH 2 using 1 M hydrochloric acid. The precipitate was washed twice with sterile water and neutralized with 2 N NaOH [18]. The precipitate was then lyophilized to obtain crude biosurfactant in the form of pale-yellow powder.
2.5. Stability Analysis of the Biosurfactant
The stability of the biosurfactant in different conditions, including different salinity concentrations, temperatures, and pH, was elucidated. The salinity of the supernatant was adjusted by adding NaCl in concentrations of 4%, 8%, 12% 16%, and 20% (w/v). The pH of the supernatant was adjusted from 2 to 12 using HCl or NaOH. The effect of temperature stability was determined by incubating the supernatant 4, 25, 40, 60, 80, and 100 °C. The supernatant was incubated for 1 h for the temperature tests and 24 h for the salinity and pH stability tests. The stability of the biosurfactant was determined using surface tension and E24 measurements. All of the experiments were performed in triplicate. All of the triplicate results were expressed as the mean ± standard deviation.
2.6. Antagonistic Activity Testing against Plant Pathogens
The antifungal activity of the crude extract was determined using the disc diffusion test. The tested fungi which caused anthracnose disease were C. gloeosporioides DOA c1060 and C. musae BCC 13080. The plant pathogenic fungi were obtained from the Department of Agriculture Culture Collection, Thailand. They were cultured on potato dextrose agar (PDA; HiMedia Laboratories, Maharashtra, India) at room temperature for 7 days. After that, the fungal agar blocks were prepared using a sterile cork borer and were transferred to the center of a PDA plate using a sterile needle. The freeze-dried crude biosurfactant sample was dissolved in dimethyl sulfoxide (DMSO) to the concentration of 50 mg/mL, passed through a 0.45-micron filter, and two-fold serial diluted. For each filtered sample, ten microliters was loaded in a sterile paper disc. After the discs were dried in a biosafety cabinet, they were transferred to the PDA plate about 2.8 cm away from the fungal cork. The plate was incubated at room temperature for 7 days, and the size of the inhibition zone was recorded by measuring the distance between the edges of the disc to the edge of fungal colony. As the negative control, 100% DMSO was used. The test was performed in two replicates, and the average size of the inhibition zones was recorded.
2.7. Biosurfactant Fractionation
The crude powder was extracted with methanol. The soluble portion was decanted and then evaporated in vacuo to obtain the honey-brown-colored crude biosurfactant. The crude biosurfactant was re-dissolved in 5.0 mL of 1:1 isopropanol/water. The reconstituted crude biosurfactant was then fractionated with preparative reversed phase HPLC using a PFP HPLC column (Luna 5 um Phenomenex PFP column (250 mm × 21.2 mm) connected with an Agilent 1260 Technology HPLC system. The mobile phases consisted of water (solvent A) and isopropanol (solvent B). The gradient of the mobile phases with the flow rate of 10 mL/min was programmed as follows: the isocratic elution of 1%B for 5 min; the gradient elution of 1%B to 100%B for 100 min; the isocratic elution of 100%B for 10 min (column washing); the isocratic elution of 1%B for 15 min (column equilibration for the next fractionation). The eluted fractions were manually collected and divided into five fractions. Each fraction was concentrated under vacuum and then subjected to mass spectrometric analysis, which is described below.
2.8. Structural Characterization Using LC–MS/MS
Each collected fraction was subjected to LC–MS/MS analysis using a Dionex HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) coupled with the Q-Exactive Focus mass spectrometer (Thermo Fisher Scientific). Each sample (20 uL) was injected into a C18 analytical column (Acclaim PolarAdvantageII, 3 µm 120Å, 3 × 250 mm, ThermoScientific) and eluted using 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The solvent system was set up with the flow rate of 0.300 mL/min and programmed as follows: the isocratic elution of 1%B for 5 min; the linear gradient of 1%B to 55%B for 50 min; the isocratic elution of 100%B for 10 min (column washing); the isocratic elution of 1%B for 15 min (column equilibration for the next analysis). The eluted peaks from each sample were ionized with an electrospray ionization (ESI) source, which underwent MS/MS fragmentation via higher-energy collision dissociation (HCD) under the positive ion mode of data-dependent acquisition (DDA) using these parameters: sheath gas 25, auxiliary gas 10, spray voltage 3.25 kV, capillary temperature 325 °C, S-lens RF 50, and auxiliary gas temperature 200 °C. The parent and fragmented ions were monitored using the Orbitrap mass analyzer with resolutions of 35,000 and 17,000 for the parent and fragmented ions, respectively; the precursor mass range (m/z) was 400–2000. The post-acquisition analysis was performed using Compound Discoverer 3.1 (Thermo Fisher Scientific).
3. Results
3.1. Biosurfactant-Producing Bacillus Isolation and Identification
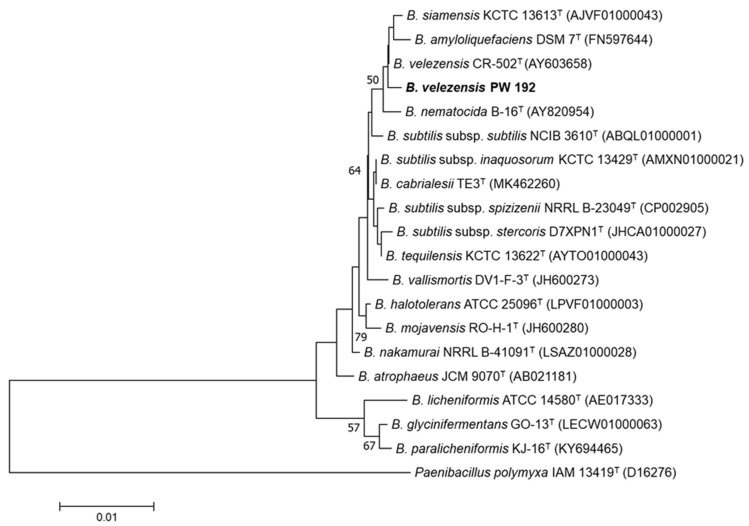
Bacillus velezensis PW192 was isolated from the soil sample taken from the rhizosphere of Lagerstroemia macrocarpa var macrocarpa in the Nakornsawan province of Thailand. PW192 is a Gram-positive, motile, and rod-shaped bacterium. The strain was identified via 16S rRNA gene sequence analysis. Based on genetic data and phylogenetic tree construction, the PW192 was designated as Bacillus velezensis, of which, the sequence showed the highest similarity to that of strain Bacillus velezensis CR-502T (99.9%). Furthermore, phylogenetic analysis showed that isolate PW192 clustered with B. velezensis CR-502T, B. siamensis KCTC 13613T (99.8%), and B. amyloliquefaciens DSM 7T (99.5%) (Figure 1). Based on genetic data and phylogenetic tree construction (Figure 1), PW192 was designated as Bacillus velezensis.
Figure 1.
Neighbor-joining phylogenetic tree of B. velezensis PW192; the closely-related Bacillus spp. Paenibacillus polymyxa IAM 13419T (D16276) was used as the outgroup. Bootstrap values above 50% or higher are shown at branch points based on 1000 resamplings. The scale bar represents 0.01 substitutions per nucleotide position.
3.2. Property of Biosurfactant
To determine the level of biosurfactant production, PW192 was grown as described in the Materials and Methods section. After 5 days of incubation, the cell-free supernatant was collected and its biosurfactant activity was examined. The supernatant could reduce the surface tension from 74 (deionized water) to 21 mN/m, and its emulsification index was determined to be 60.17%.
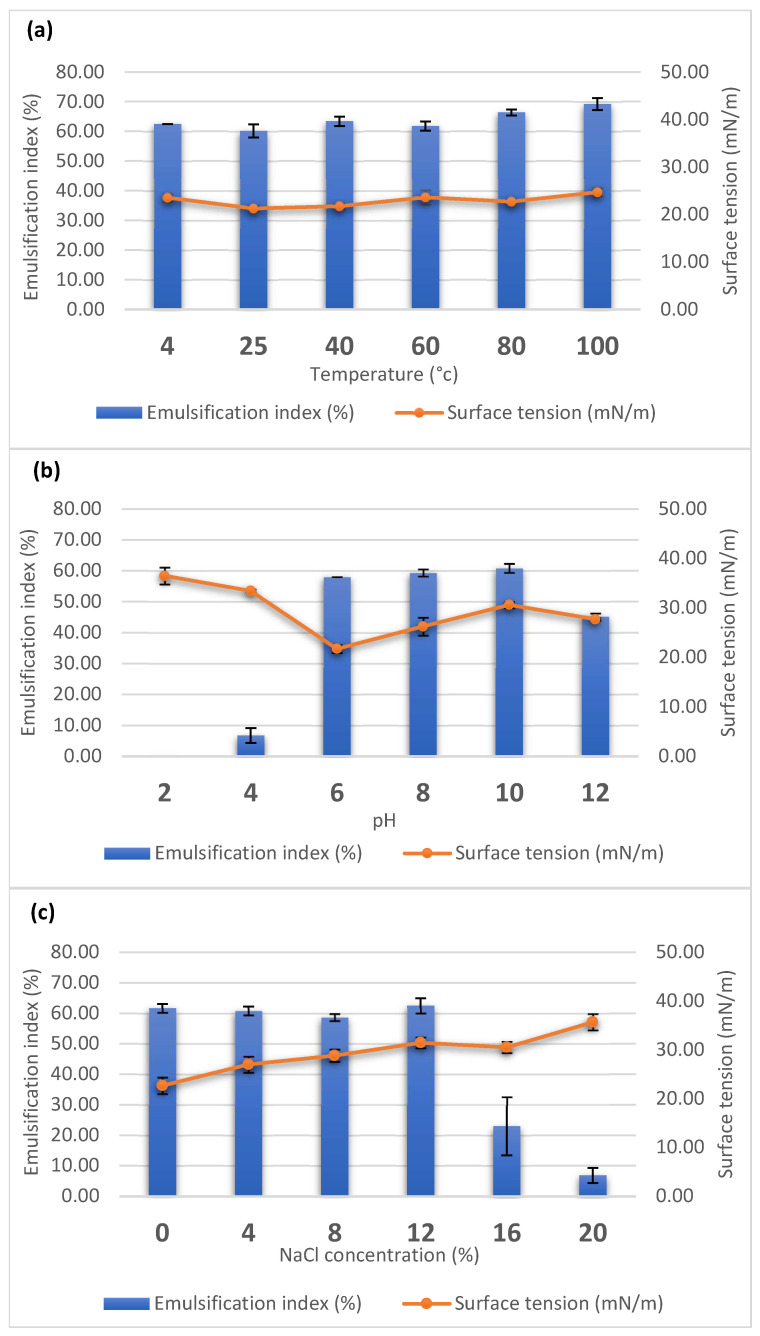
The stability of the biosurfactant was also tested under different temperatures, pH, and salt concentrations. The surface tension activities and E24 of the supernatant were measured after the supernatant was treated with different conditions. The supernatant was incubated at 4 to 100 °C for 1 h before measurement. Overall, the surface tension activities and E24 of the supernatant were maintained in all of the tested temperatures (Figure 2a). The pH of the supernatant was adjusted to the range of 2–12 and maintained for 24 h. The surface tension activities and E24 were stabilized at a pH ranging from 6–10, of which pH 6 showed the highest surface tension activity of 21.70 mN/m (Figure 2b). The salt resistance of the biosurfactant was determined by adding 0–20% (w/v) NaCl into the supernatant and maintaining this condition at room temperature for 24 h. The supernatant-containing biosurfactant was stable in 0–12% (w/v) NaCl concentrations (Figure 2c).
Figure 2.
Effect of temperature (a), pH (b), and NaCl concentration (c) on the emulsifying index (%) and surface tension (mN/m) of PW192 biosurfactant. The bar and line plots represent emulsification index and surface tension, respectively. Data presented are the average of triplicate experiments, and error bars indicate standard deviation.
3.3. Antagonistic Activity against Anthracnose-Causing Pathogen
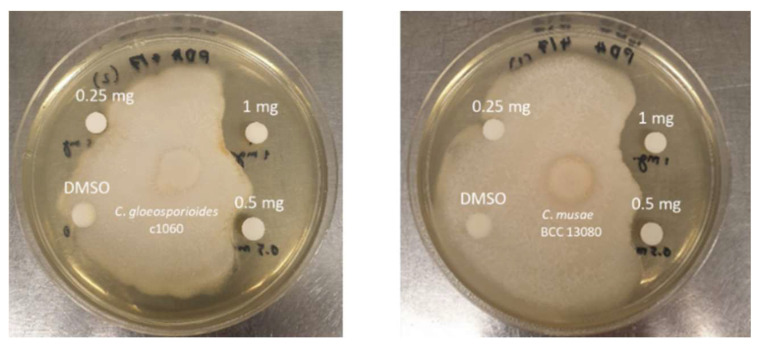
To evaluate the potential bioactivity of the biosurfactant samples, the antifungal activity against the causative agents of anthracnose disease in mango and chili, C. gloeosporioides c1060 and C. musae BCC 13080, was determined using the disc diffusion susceptibility test [19]. The result showed that an initial amount of 500 micrograms of sample could inhibit both fungi (C. gloeosporioides c1060 and C. musae BCC 13080), as shown in Table 1 and Figure 3. The antifungal activity was confirmed from the absence of activity from DMSO.
Table 1.
Inhibition zone of the biosurfactant sample against Colletotrichum sp. in milliliters.
| C. gloeosporioides c1060 | C. musae BCC 13080 | |
|---|---|---|
| 1 mg | 7.5 | 6.5 |
| 0.5 mg | 3 | 3 |
| 0.25 mg | 0 | 0 |
| 100% DMSO | 0 | 0 |
Note: The size of inhibition zone was the average of duplicates.
Figure 3.
Inhibition zone of the biosurfactant sample against Colletotrichum sp.
3.4. LC–MS/MS Analysis of the Fractionated Biosurfactants
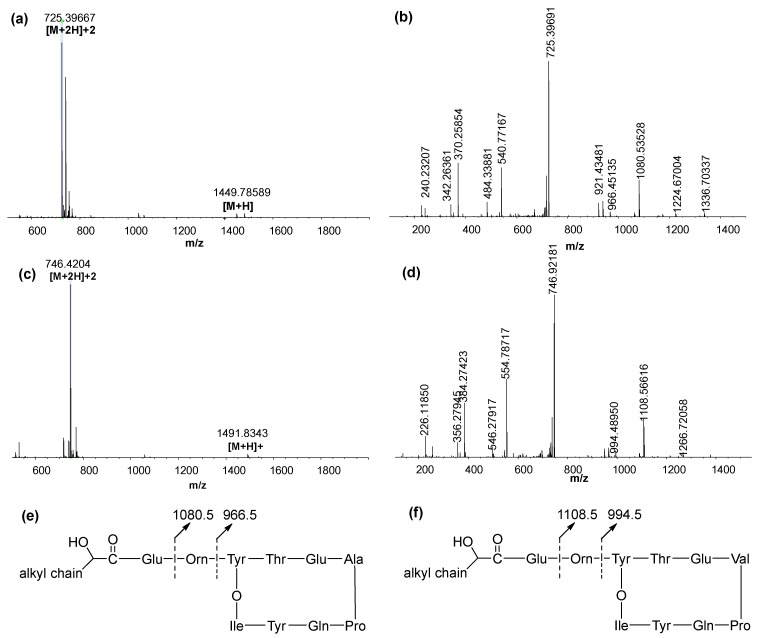
To identify the unknown structures of lipopeptides biosynthesized by B. velezensis, data-dependent acquisition (DDA) LC–MS/MS, a top-down approach commonly used for peptide characterization, was applied to analyze the structures of lipopeptides fractionated from the crude biosurfactant using preparative HPLC. Illustrated in Figure 4a and Figure S1, the mass spectra of fengycin A predominantly showed doubly charged species [M + 2H]2+ of all the precursors, including m/z 725.3967, 732.4052, 739.4124, 746.4210, 724.4075, and 731.4156. Due to much higher intensity than their corresponding [M + H]+, these doubly-charged ions were used for MS/MS analysis. Depicted in Figure 4b,e and Figure S1, the HCD fragmentation revealed the identical product ions at m/z 1080.5 and 966.5, which were derived from Glu-Orn-Tyr-Thr-Glu-Ala-Pro-Gln-Tyr-Ile and Orn-Tyr-Thr-Glu-Ala-Pro-Gln-Tyr-Ile, respectively. Due to the mass difference by 14.0156 (-CH2 group), the compounds with [M + H]+ 1449.7859, 1463.8040, 1477.8178, and 1491.8354 were considered as a series of homologous molecules.
Figure 4.
(a,b) The MS and MS/MS spectra of a fengycin A derivative from [M + H]+ 1449.7859. (c,d) The MS and MS/MS spectra of a fengycin B derivative from [M + H]+ 1419.8343. (e,f) Illustration of the identical product ions derived from fengycin A and fengycin B, respectively.
According to the MS spectra shown in Figure 4c and Figure S2, six derivatives of fengycin B were doubly protonated, giving rise to [M + 2H]2+ of 739.4119, 746.4205, 753.4281, 760.4357, and 738.4226. After being fragmented by HCD, all six of the compounds exhibited the characteristic pattern of fengycin B fragmentation by displaying the identical daughter ions of m/z 1108.5 and 994.5 (Figures S1 and S2). The m/z values of 1108.5 and 994.5 were matched with Glu-Orn-Tyr-Thr-Glu-Val-Pro-Gln-Tyr-Ile and Orn-Tyr-Thr-Glu-Val-Pro-Gln-Tyr-Ile, respectively. Again, molecules with homologous series were detected, as evidenced by the mass difference of 14.0156 Da between [M + H]+ of 1477.8164, 1491.8343, 1505.8499 and 1519.8665. To determine the chain length of fatty acids linked to fengycins, the m/z value of [M + H – 1108.566 – Glu]+ could be employed for calculating of the number of carbons in the fatty acid chain. The numbers of carbons in fatty acids are shown in Table 2.
Table 2.
Precursor ions, identical product ions, fatty acid chain length, and fengycin types.
| [M + H]+ | [M + 2H]2+ | Identical Product Ions | Fatty Acid | Surfactin |
|---|---|---|---|---|
| 1449.7859 | 725.3967 | 1080.5, 966.5 | 15:0 | Fengycin A |
| 1463.8040 | 732.4052 | 1080.5, 966.5 | 16:0 | |
| 1477.8178 | 739.4124 | 1080.5, 966.5 | 17:0 | |
| 1491.8354 | 746.4210 | 1080.5, 966.5 | 18:0 | |
| 1447.8081 | 724.4075 | 1080.5, 966.5 | 15:1 | |
| 1461.8243 | 731.4156 | 1080.5, 966.5 | 16:1 | |
| 1477.8164 | 739.4119 | 1108.5, 994.5 | 15:0 | Fengycin B |
| 1491.8343 | 746.4205 | 1108.5, 994.5 | 16:0 | |
| 1505.8499 | 753.4281 | 1108.5, 994.5 | 17:0 | |
| 1519.8665 | 760.4357 | 1108.5, 994.5 | 18:0 | |
| 1461.8242 | 731.4160 | 1108.5, 994.5 | 14:1 | |
| 1475.8339 | 738.4226 | 1108.5, 994.5 | 15:1 |
4. Discussion
Bacillus velezensis PW192, a Gram-positive, endospore-forming bacterium, was isolated from the Lagerstroemia macrocarpa var macrocarpa rhizosphere. This strain was able to produce a biosurfactant with 61% emulsification activity and could reduce the surface tension from 74 to 21 mN/m. The 16S rRNA phylogenetic analysis showed that this strain is a member of the genus Bacillus and is related to the species Bacillus velezensis.
Biosurfactants can be applied in different industrial processes under extreme conditions of temperature, pH, and salinity. The stability of the cell-free supernatant was investigated under such conditions. We found that both the surface tension and emulsification activity were stable in temperature treatments ranging from 0 to 100 °C with surface tension remaining (21.2 to 24.7 mN/m) the same as the control (21.2 mN/m). The emulsification activity was also maintained at the tested temperatures (E24 = 64%). These results agree with other reports that proved that heat treatment does not alter the interfacial properties of biosurfactants from Bacillus species [20]. The biosurfactant activity remained stable from pH 6 to 10; however, the activity was decreased at pH 2 and 4. The loss of surface activity was observed in acidic pH because of the precipitation of the lipopeptides. The biosurfactants also remained stable at different ionic strengths or salt concentrations ranging from 0 to 12% (w/v) NaCl. The stability of the biosurfactant in various conditions strongly suggests that it has potential to be applied in industries.
Many strains of B. velezensis have been isolated. Some studies have shown that B. velezensis can produce a variety of metabolites that are capable of stimulating plant growth and inhibiting plant pathogens, including antibacterial proteins, lipopeptide antibiotics, polyketides, siderophores, and ammonium [21]. Previous reports demonstrated the antifungal activity of Bacillus velezensis strains against several phytopathogenic fungi, including Fusarium graminearum [22,23] and Colletotrichum gloeosporioides Penz [24]. The mechanisms of the antifungal activity of Bacillus species are expected to involve the presence of hydrolytic enzymes, e.g., cellulase and protease [25], as well as other antifungal compounds, e.g., bacillomycin-D [24] and lipopeptides [26]. Examples of secondary metabolites produced by B. velezensis include amylocyclicin, bacilysin, bacillomycin-D, bacillibactin, bacillaene, difficidin, fengycin, macrolactin, plantazolicin, and surfactin [27]. Lipopeptide compounds, including surfactin, fengycin, and bacillomycin-D, demonstrate antifungal properties [21,28]. Bacillomycin-D and fengycin exhibit synergistic antifungal activity that inhibits the growth of Fusarium oxysporum [29].
In this study, the antagonistic activity of acid-precipitated lipopeptide compounds from B. velezensis PW192 against Antracnose disease were studied. The antifungal activity shown by the lipopeptide extract of PW192 suggests it could potentially be used as a biocontrol agent. The potential of a particular lipopeptide to exhibit antimicrobial properties largely depends on its molecular structure. Based on structural characterization using LC–MS/MS, it was clear that B. velezensis PW192 produces fengycin A and fengycin B biosurfactants. However, the underlying mechanism for this Bacillus velezensis PW192 needs to be investigated further.
5. Conclusions
The present study indicated that B. velezensis PW192 generates potent biocontrol agents, namely fengycin A and B, that serve as fungicides of Colletotrichum gloeosporioides and Colletotrichum musae. Moreover, the biosurfactants produced by B. velezensis PW192 have high potential for industrial applications, mainly due to their stability when subjected to different environmental conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10051017/s1, Figure S1. The MS spectra showing doubly- and singly-charged species of molecular ions of fengycin A derivatives along with the MS/MS spectra of the corresponding molecular ions (inset). Figure S2. The MS spectra showing doubly- and singly-charged species of molecular ions of fengycin B derivatives along with the MS/MS spectra of the corresponding molecular ions (inset).
Author Contributions
Conceptualization, W.J. and P.W.; methodology, W.J. and P.W.; software, B.I. and W.J.; validation, P.W., W.J. and J.E.; formal analysis, B.I.; investigation, W.J., J.E., B.I. and P.W.; resources, P.W.; data curation, W.J. and P.W.; writing—original draft preparation, W.J., J.E., B.I. and P.W.; writing—review and editing, P.W.; visualization, W.J. and P.W.; supervision, P.W.; project administration, B.I.; funding acquisition, P.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research project is supported by Mahidol University.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fritze D. Taxonomy of the genus bacillus and related genera: The aerobic endospore-forming bacteria. Phytopathology. 2004;94:1245–1248. doi: 10.1094/PHYTO.2004.94.11.1245. [DOI] [PubMed] [Google Scholar]
- 2.Su Y., Liu C., Fang H., Zhang D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories. 2020;19:173. doi: 10.1186/s12934-020-01436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naughton P.J., Marchant R., Naughton V., Banat I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019;127:12–28. doi: 10.1111/jam.14243. [DOI] [PubMed] [Google Scholar]
- 4.Marchant R., Banat I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012;34:1597–1605. doi: 10.1007/s10529-012-0956-x. [DOI] [PubMed] [Google Scholar]
- 5.Banat I.M., Franzetti A., Gandolfi I., Bestetti G., Martinotti M.G., Fracchia L., Smyth T., Marchant R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010;87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 6.Gudiña E.J., Rangarajan V., Sen R., Rodrigues L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013;34:667–675. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Kim P.I., Ryu J., Kim Y.H., Chi Y.T. Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010;20:138–145. doi: 10.4014/jmb.0905.05007. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y.C., Leveau J., McSpadden Gardener B.B., Pierson E.A., Pierson L.S., III, Ryu C.M. The multifactorial basis for plant health promotion by plant-associated bacteria. Appl. Environ. Microbiol. 2011;77:1548–1555. doi: 10.1128/AEM.01867-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakaria L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture. 2021;11:297. doi: 10.3390/agriculture11040297. [DOI] [Google Scholar]
- 10.Udayanga D., Manamgoda D.S., Liu X., Chukeatirote E., Hyde K.D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013;61:165–179. doi: 10.1007/s13225-013-0257-2. [DOI] [Google Scholar]
- 11.Khondee N., Tathong S., Pinyakong O., Müller R., Soonglerdsongpha S., Ruangchainikom C., Tongcumpou C., Luepromchai E. Lipopeptide biosurfactant production by chitosan-immobilized Bacillus sp. GY19 and their recovery by foam fractionation. Biochem. Eng. J. 2015;93:47–54. doi: 10.1016/j.bej.2014.09.001. [DOI] [Google Scholar]
- 12.Cooper D.G., Goldenberg B.G. Surface-active agents from two bacillus species. Appl. Environ. Microbiol. 1987;53:224–229. doi: 10.1128/aem.53.2.224-229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon S.-H., Ha S.-M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 18.Yakimov M.M., Timmis K.N., Wray V., Fredrickson H.L. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 1995;61:1706–1713. doi: 10.1128/aem.61.5.1706-1713.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha S.S., Joshi S.J., Geetha S.J. Lipopeptide production by Bacillus subtilis R1 and its possible applications. Braz. J. Microbiol. 2016;47:955–964. doi: 10.1016/j.bjm.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabbee M.F., Ali M.S., Choi J., Hwang B.S., Jeong S.C., Baek K.-H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24:1046. doi: 10.3390/molecules24061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palazzini J.M., Dunlap C.A., Bowman M.J., Chulze S.N. Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: Genome sequencing and secondary metabolite cluster profiles. Microbiol. Res. 2016;192:30–36. doi: 10.1016/j.micres.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang S., Sun L., Zhang W., Chi F., Hao X., Bian J., Li Y. Bacillus velezensis BM21, a potential and efficient biocontrol agent in control of corn stalk rot caused by Fusarium graminearum. Egypt. J. Biol. Pest Control. 2020;30:9. doi: 10.1186/s41938-020-0209-6. [DOI] [Google Scholar]
- 24.Jin P., Wang H., Tan Z., Xuan Z., Dahar G.Y., Li Q.X., Miao W., Liu W. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic. Biochem. Physiol. 2020;163:102–107. doi: 10.1016/j.pestbp.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Khan N., Martínez-Hidalgo P., Ice T.A., Maymon M., Humm E.A., Nejat N., Sanders E.R., Kaplan D., Hirsch A.M. Antifungal activity of bacillus species against fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol. 2018;9:2363. doi: 10.3389/fmicb.2018.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan F., Li C., Ye X., Lian Y., Wu Y., Wang X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Control. 2020;146:104281. doi: 10.1016/j.biocontrol.2020.104281. [DOI] [Google Scholar]
- 27.Chen X.H., Koumoutsi A., Scholz R., Schneider K., Vater J., Süssmuth R., Piel J., Borriss R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009;140:27–37. doi: 10.1016/j.jbiotec.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury S.P., Uhl J., Grosch R., Alquéres S., Pittroff S., Dietel K., Schmitt-Kopplin P., Borriss R., Hartmann A. Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol. Plant Microbe Interact. 2015;28:984–995. doi: 10.1094/MPMI-03-15-0066-R. [DOI] [PubMed] [Google Scholar]
- 29.Koumoutsi A., Chen X.-H., Henne A., Liesegang H., Hitzeroth G., Franke P., Vater J., Borriss R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004;186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.