Abstract
Plant-based medicines have received a lot of attention in recent years. Such medicines have been employed to treat medical conditions since ancient times, and in those times only the observed symptoms were used to determine dose accuracy, dose efficacy, and therapy. Rather than novel formulations, the current research work on plant-based medicines has mostly concentrated on medicinal active phytoconstituents. In the past recent decades, however, researchers have made significant progress in developing “new drug delivery systems” (NDDS) to enhance therapeutic efficacy and reduce unwanted effects of bioactive compounds. Nanocapsules, polymer micelles, liposomes, nanogels, phytosomes, nano-emulsions, transferosomes, microspheres, ethosomes, injectable hydrogels, polymeric nanoparticles, dendrimers, and other innovative therapeutic formulations have all been created using bioactive compounds and plant extracts. The novel formulations can improve solubility, therapeutic efficacy, bioavailability, stability, tissue distribution, protection from physical and chemical damage, and prolonged and targeted administration, to name a few. The current study summarizes existing research and the development of new formulations, with a focus on herbal bioactive components.
Keywords: phytoconstituents, nano-formulations, liposomes, cubosomes, phytosomes, nanomedicines
1. Introduction
For this advanced and developed world afflicted with numerous health issues and ailments, nature has all the solutions. Nature offered various naturally existing bioactive plants for the treatment of various diseases, and such plants have been extensively used during the past several centuries all over the world due to their lesser side effects and extensive health benefits [1]. From ancient times, plants have been used for medicine and food [2]. The use of herbal medicine for basic health care in poor nations has undeniably grabbed the attention of the modern world [3]. Despite numerous advantages, pharmaceutical industries hesitate to finance natural product-based drug discovery and explore the synthetic compounds library for novel drug discovery. However, natural products and phytoconstituents have been extracted and screened for their benefits in primary health linked issues such as diabetes, cancer, microbial diseases, cardiovascular diseases, and inflammatory conditions because of their exclusive benefits, including lowered toxicity, low price, side effects, and outstanding health benefits [4].
Plant-based therapies have some limitations, such as poor lipid solubility, poor stability, and requiring a well-validated process for isolation and purification of constituent [5]. Indeed, this is the prime responsibility of the manufacturer to overcome these limitations and provide sufficient stability to the product and safer consumption by the patients. Typically, in traditional medicines, a limited amount of the drug has been reached at the target site. Most of the amount was dispersed all over the body based on the physicochemical properties of the medicine, resulting in lower therapeutic potency [6,7]. Herbal plants have a number of phytoconstituents that result in the instability of herbal formulation [8]. Delivery of the herbal formulations at the targeted site is a big challenge for most of the plant species that have medicinal significance. For instance, flavonoids, tannins, and terpenoids have water solubility, however they cannot cross the biological membrane, resulting in lesser absorption. Additionally, they possess a larger molecular size, resulting in diminished bioavailability and effectiveness [9].
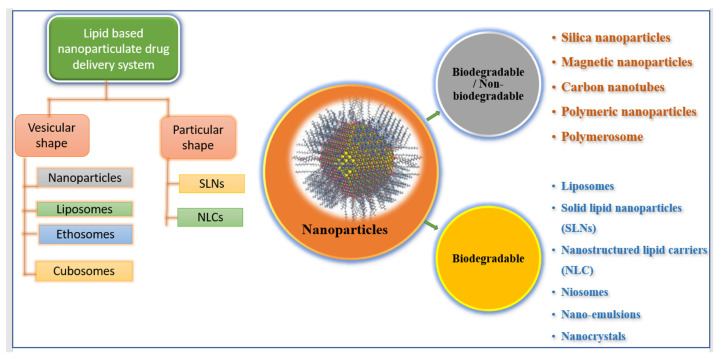
To conquer these limitations, newer advanced drug delivery systems (DDS) have been developed for plant-based medicines. These include liposomes, phytosomes, ethosomes, transferosomes, nanostructured lipid carriers (NLCs), cubosomes, solid lipid nanoparticles (SLNs), hexosomes, microspheres, nanoparticles, and nano-emulsions [10]. These innovative DDSs have been associated with numerous improvements regarding targeted drug delivery, enhancement of solubility, stability, bioavailability, depletion of toxicity, and sustaining as well as controlling the release of the drug molecule [10,11].
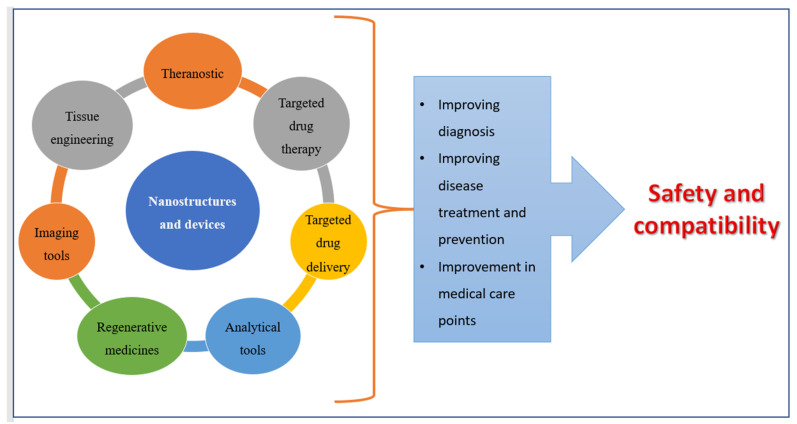
Nanomedicine is an evolving area, using the application of nanoscience information and technology in remedial biology for treatment as well as disease prevention. It includes the use of nano-dimensional building resources such as nano-robots, diagnostic nano-sensors, sensory targets, and materials in living cells. An example includes the development of nanoparticle-based methods employed collectively for cancer diagnosis as well as cancer treatment [4]. In recent years, medicinal regulators authorized the first nanoparticle-based formulations that included lipid systems like liposomes and micelles [12]. Both of these formulations contained inorganic nanoparticles such as magnetic and gold nanoparticles [13]. The application of inorganic nanoparticles mainly emphasizes imaging, drug delivery, and medical activities. Additionally, nanostructures are reported to prevent drug deprivation in the intestinal tract and to facilitate the distribution of hydrophilic drugs over the targeted site. Additionally, nano-drugs resulted in improved drug bioavailability through the oral route, probably due to absorptive endocytosis mechanisms adopted by them. These nanostructures remained in the blood stream for a longer time and enabled drug release at a specified rate, resulting in reduced plasma fluctuation with reduced side effects. Because of their nano-size, these structures easily entered the cell membrane and facilitated drug uptake by cells, resulting in efficient target delivery. Additionally, the uptake of the nanosized structure by the cell is higher than larger particles, having sizes between 1 and 10 nm [14,15]. Therefore, these worked specifically for treating infected cells, resulting into higher efficacy and almost no adverse effects [4].
Concerning the nanomaterials utilizing drug delivery at a particular site, the choice of nano-based formulations depends on the physicochemical characteristics of drug molecules. Incorporating natural bioactives into nanoparticles using nanoscience is popular and rapidly growing in recent times. Most of the materials used are eco-friendly, biodegradable, bioadhesive, and of natural origin, which provides extensive benefits as well as distinctive size to the nano-formulations [16]. It offers various benefits for the treatment of cancer and many other ailments. Numerous extraordinary properties of natural compounds, including “tumor-suppressing autophagy” and antimicrobial properties, make them suitable for study in various life-threatening diseases. For instance, curcumin and caffeine exhibited autophagy [17], while antimicrobial and antibacterial effects have been demonstrated with cinnamaldehyde, curcumin, carvacrol, and eugenol [18,19]. The integration of drug molecules in nano-formulations resulted in enhanced oral bioavailability, identification, and control release of the drug. Considering an example, thymoquinone incorporated with nanocarriers showed a six-fold increase in oral bioavailability compared with free thymoquinone [20]. In addition, these nano-formulations also improved the pharmacokinetic properties of the natural bioactive, resulting in enhanced therapeutic effects [20]. Several reviews have been published on this tremendous technology, explaining its benefits to society. The current review is a compilation of the application of herbal-based nanoformulations developed through nanotechnology, which is one of the key novel drug delivery methods under investigation in recent years. These nanoformulations are thought to have a wide variety of benefits in comparison with conventional preparations of plant constituents, which include enhanced permeability, solubility, bioavailability, therapeutic activity, stability, improved distribution within tissues, and sustained delivery.
Nanotechnology is a fast-evolving branch of science due to its widespread application in other disciplines, making it more advanced and user-friendly. Nanotechnology is now used in nearly every major field of science, including agriculture, pharmaceutical sciences, medical sciences, computer sciences, food technologies, polymer sciences, textile technologies, chemical and biological sciences [21]. The pharmaceutical industry is experiencing a dilemma in drug research since they have strong therapeutic molecules, but their poorer water solubilities, low distribution, protein interaction, and short half-life lead to limited clinical usage. These nanotechnologies offer extensive benefits in this regard [22].
According to a report, the global market for nanotechnology-based products is expected to reach USD 91.8 billion by 2028. The Indian and Australian governments have committed around $20 million to create the “Australia-India Science Research Funding Program”. According to research released by BCC Research, the global value of the nanomedicines market in 2010 was 63.8 billion and 72.8 billion in 2011 [23].
Nanotechnology has numerous applications in many aspects of life, and it contributes significantly to the advancement of many scientific and industrial sectors, including information technology, energy, medical, national security, environmental science, food safety, and many more. Improved manufacturing methods, water purification systems, energy systems, physical enhancement, nanomedicine, better food production methods, nutrition, and large-scale infrastructure auto-fabrication are all major advantages of nanotechnology.
Changing the major properties of nanocarriers such as their constituents, sizes, shapes, and surface properties resulted in altered physio-chemical features of nano-formulations. The foremost aim of introducing nano-preparation is only to treat unwellness with supreme therapeutic potential and the least adverse effects. The use of an appropriate drug and nano-DDS-has been determined primarily by the biochemical and biophysical properties of the target drug [24]. Nevertheless, some hindrances such as toxicity could not be ignored while seeing their benefits. The lack of information about the toxicity and harmfulness of nanostructures is the main concern and undeniably needs more detailed studies to explore their maximum safety performance [25]. In view of the above facts, the current review article aimed to report various natural products based on nano-DDSs, significant use of natural nanomedicines in various ailments, along with various methods of preparations and their applications.
2. What Is a Nano?
Advances in technology over the last two decades have resulted in the creation of nanoscale materials, which have resulted in a decrease in particle size and overall increases in surface area. Nanoparticles are particles with a size ranging from 1 nm to 1000 nm. The term “nano” is easily defined, but it encompasses a wide range of applications (Figure 1), including multiple nano-based systems made up of various types of materials used as nanocarriers (Figure 2) [26,27].
Figure 1.
Applications of nanomedicines.
Figure 2.
Illustrating various types of nano-formulations.
3. Nanotechnology-Based Drug Delivery Systems
3.1. Solid Lipid Nanoparticles (SLNs)
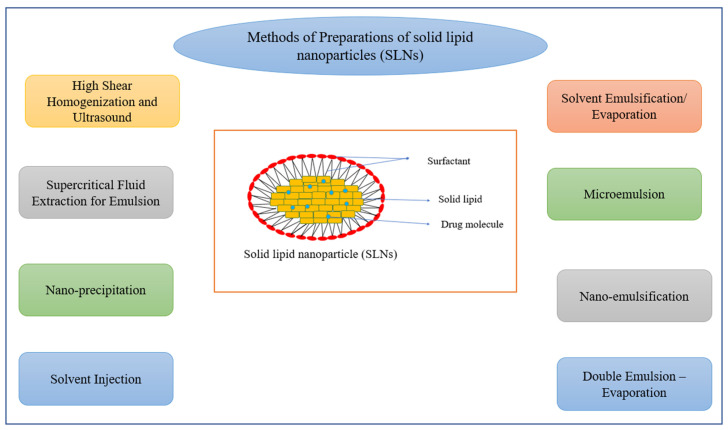
The SLN colloidal drug system was created in the early 1990s and has particle sizes ranging from 50 to 1000 nm. These are made of emulsifiers that help to keep a melted solid lipid dispersion water-stable [28]. For the preparation of SLNs (Figure 3), many procedures have been devised, the most prevalent of which are high-pressure homogenization (HPH) and micro-emulsification [29]. The main advantages of SLN include a lipophilic lipid matrix that allows pharmaceuticals to disperse, encapsulation of drug molecules such as medications, antigens, proteins, and nucleotides, and drug delivery to specified tissues and cells. Improved in vivo and in vitro drug stability, as well as reduced adverse effects, are among the unique characteristics of SLN [30]. SLN and nano-emulsions are quite similar, with the exception that SLN uses both solid and liquid lipids in their formulation, whereas nano-emulsions solely employ liquid lipids. In rats, the most often used SLN is puerarin-loaded SLN, which is characterized by quick absorption, increased bioavailability, and increased drug concentrations in targeted organs such as the brain and heart [31,32]. According to another study, “triptolide-loaded SLN” showed a significant reduction in myeloperoxidase (MPO) and glutathione (GSH) activities and acted as an anti-inflammatory and antioxidant product, resulting in improved solubility, reduced toxicity, and reduced irritation to the gastrointestinal tract (GIT), as well as avoiding higher local drug concentration and gradual drug release [33]. Table 1 has further examples.
Figure 3.
Methods of preparation of solid lipid nanoparticles (SLNs).
Table 1.
SLN encapsulating natural bioactive.
| SLNs Loaded with Natural Bioactive | Plant Source | Limitations of Free Drugs |
Advantages of Loaded Drug Molecules | References |
|---|---|---|---|---|
| Triptolide incorporated SLN | Tripterygium wilfordii Hook F | Poor water solubility and high toxicity, | Improved solubility, hyperemia, reduced toxicity, irritation to GIT, etc. | [33] |
| Puerarin-loaded SLN | Pueraria lobata (wild) Howe | Poor water solubility and low oral bioavailability | 3-folds increase in absorption and bioavailability improved tissue concentration in targeted organs (heart and brain) | [31,32] |
| Noscapine PEG conjugated SLN | Papaveraceae family | Shorter half-life, less efficacy to glioblastoma cells | Improved biological half-life, and anticancer efficacy in glioblastoma in vitro and in Swiss male albino mice induced with brain cancer. | [34] |
| Tetrandrine-loaded SLN | Stephania tetrandra S. Moore | Lesser bioavailability and drug release | Improved bioavailability, in vitro drug release, cellular uptake into human lens epithelial cell line (SRA 01/04) | [35] |
| Cantharidin-loaded SLN | Mylabris phalerata pallas or mylabris cicchorii linnaeus | Lesser bioavailability and drug release | Sustained drug release without a burst effect, improved bioavailability when administered orally in rats induced with gastric mucus membrane irritation. | [36] |
| Hydroxycitric acid-loaded SLN | Garcinia cambogia | Low bioavailability | Increased bioavailability tested on Wistar rat, anti-obesity medication | [37] |
| Ginkgo biloba leaf extract-loaded SLN | Ginkgo biloba | Low bioavailability | Improved oral bioavailability at a 5 mg/kg dose, causing blood coagulation at higher doses i.e., 50 mg/kg. | [38] |
| Aloe vera-loaded SLNs | Aloe vera | Cause irritation to the skin on multiple uses in some cases | Incorporated into sunscreen cream, SPF was found to be as per the marketed formulation. | [39] |
| Zataria multiflora essential oil (ZMEO) containing SLN | Zataria multiflora | Mosquito repellant properties at higher doses | Improved mosquito repellent activities, three times increase in protection time of nano-formulation compared to non-formulated essential oil | [40] |
| Witepsol-loaded SLNs | Cocoa butter | Lower stability | Suitable vehicle for herbal extracts, higher stability, and proper release profile in the intestine. | [41] |
3.2. Nanostructured Lipid Carriers (NLCs)
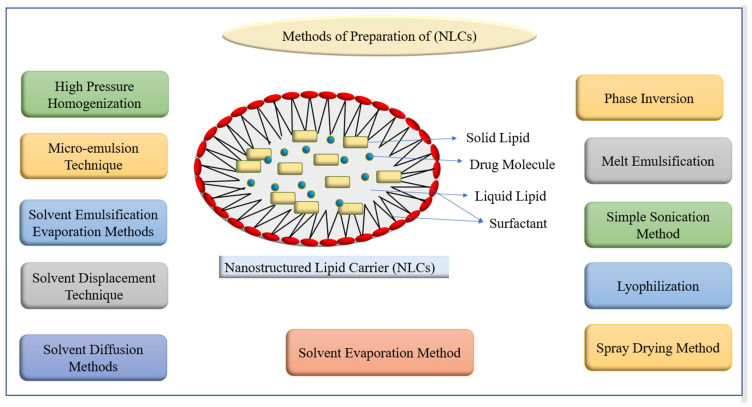
These are referred to as “second-generation lipid nanoparticles” because they are made up of a mixture of solid and liquid lipids and were produced from SLN with various lipid matrix flaws [42]. Solid lipids such as hydrogenated palm oil, glyceryl monostearate, stearic acid, and cetyl alcohol have been utilized in large quantities, while liquid lipids such as olive oil, mustard oil, castor oil, and cod liver oil have been employed. In this system, thiomersal has been utilized as a stabilizer [43]. NLCs outperform SLN because of their superior regulated drug release, enhanced drug loading ability, stability, and little drug loss during encapsulation [44]. Various studies focused on the entrapment of bioactives into NLC by altering water solubilities, controlling drug release, lengthening circulation time, co-delivery, routes of drug delivery, and enhancing gastrointestinal absorption and oral bioavailability. Different methods of NLCs preparation have been listed in Figure 4. NLCs carriers were found to be a better carrier for oral drug delivery, encapsulating various natural and synthetic bioactives. For example, tripterine, triptolide, and curcumin-loaded NLCs showed enhanced absorption that may be because of lipid components, smaller particle size, and surface contents. Silymarin-loaded NLC showed best examples, used clinically to overcome various liver diseases. Additionally, NLCs loaded with cardamom essential oil (CEO) have been developed successfully using food grade lipids olive oil and cocoa butter, showed small size and enhanced loading capacity (>25%), providing physical and chemical stability [45]. More examples of compound-loaded NLCs have been given in Table 2.
Figure 4.
Methods of preparation of nanostructured lipid carriers (NLCs).
Table 2.
NLCs incorporated bioactives.
| Drug-Loaded NLCs | Plant Source | Limitations of Free Drugs | Advantages of Loaded Drug Molecules over Conventional Systems | Reference |
|---|---|---|---|---|
| Cardamom essential oil-loaded NLCs | Elettaria cardamom | Low antimicrobial activities | Protect antimicrobial activity of the plant extract, used as food supplement | [46] |
| Thymoquinone-loaded NLCs | Nigella sativa | Low bioavailability | Enhanced bioavailability and oral drug delivery, antioxidant potential, improved liver biomarkers affected with PCM induced hepatotoxicity | [47] |
| Citral-loaded NLCs | Cymbopogon citratus | Low solubility | Improved water solubility and sustained drug release | [48] |
| β-Elemene incorporated NLCs | Nigella damascena L. | Low bioavailability and anticancer efficacy | Improved bioavailability in male wistar rats and anti-tumor efficacy in H22 hepatoma induced in Kunming mice, reduced venous irritation after i.v., injection in New Zealand white rabbits. | [49] |
| Zerumbone-loaded NLCs | Zingiber zerumbet L. Smith | Low solubility | Improved water solubility, bioavailability, and sustained drug release with enhanced anticancer activities both in vitro and in vivo. | [50,51] |
| Baicalin-loaded NLCs | Scutellaria baicalensis | Low solubility and bioavailability | Improved sustained drug release and antidiabetic effect of baicalin | [52] |
| Berberine incorporated NLCs | Coptis chinensis | Low bioavailability | Enhanced anti-inflammatory potential of the berberine, improved ulcerative colitis symptoms. | [53] |
| Curcumin-loaded NLCs | Curcuma longa | Low solubility and bioavailability | Improving impressions of DR5 proteins, enhanced caspase 8 and caspase 3 activities, enhanced apoptosis in hepatocellular carcinoma | [54] |
| Hesperidin and clarithromycin-loaded NLCs | Flavanone glycoside | Low bioavailability | Improved sustained and controlled drug release that can be used to increase the rate of H. pylori eradication. | [55] |
| Diosgenin and Glycyrrhiza glabra extract-loaded NLCs | Dioscorea deltoidea Glycyrrhiza glabra | Possessed lessened anti-inflammatory properties | Inhibition of pro-inflammatory cytokines, TNF-α, IL, and enhanced anti-inflammatory properties | [56] |
| Cinnamaldehyde-loaded (NLC) | Cinnamomum ceylanicum | Low bioavailability and shelf life | Total bacteria and fungi count in the treated CA-loaded NLC samples was about 3.5 log CFU/g less than the control. CA-loaded NLC can extend the shelf life of date fruit without any undesirable impacts on sensory attributes. | [57] |
| Ursolic acid-loaded NLCs | Pentacyclic terpene acid | Low solubility | Animals infected with Leishmania (Leishmania) infantum and treated with UA-NLC showed lower parasitism than the infected controls, Increased protective immune response, spleen and liver preservation, and the normalization of hepatic and renal functions. | [58] |
| Naringenin (NGN) incorporated NLCs | Citrus fruits and tomato | Poor water solubility | Elevated drug release rate in simulated intestinal solutions in vitro, improved transepithelial transport in MDCK cells, improved oral absorption in mice, enhanced inhibitory effects of NGN on MCD diet-induced mouse NAFLD. | [59] |
3.3. Nanocrystals
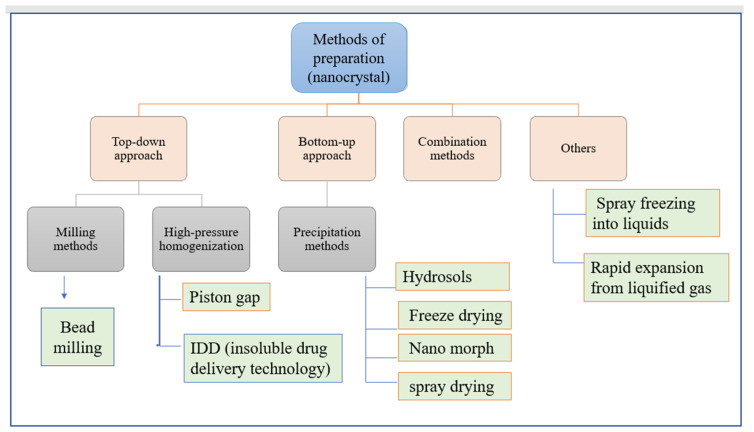
These are pure solid drug particle with sizes up to 1000 nm, primarily composed of 100% drug substance that is stabilized by stabilizer(s) or surfactant(s). Water, liquid polyethylene glycol (600), oil, or any “aqueous or non-aqueous” media has been used as a dispersing medium [60,61]. The noteworthy characteristics of nanocrystals enabled them to overwhelm difficulties such as increased “dissolution velocity, saturation solubility, and thickness to surface and cell membranes”. For the production of nanocrystals, two methodologies have been developed: a top-down approach and a bottom-up one. The top-down approach has defined approaches such as precipitation, high gravity-controlled precipitation technology, sono-crystallization, restricted impinging liquid jet precipitation technique, and multi-inlet vortex mixing techniques (Figure 5) [60]. In this process, the use of organic solvent, and its removal at the end, makes it moderately expensive. However, the bottom-up approach includes the application of high-pressure homogenization in grinding procedures [60]. Amongst all the methods, milling, precipitation, and high-pressure homogenization have been used most commonly for production. In nanocrystals, the mechanism followed by the drug for absorption includes solubility enhancement, suspension rate, and intestinal wall holding capacity [60]. These are associated with enormous advantages like enhanced solubility, disintegration, dissolution, bioavailability, and safer dosage form, and provide a higher level of safety because of their molecular size and surface properties [62]. Ni et al. developed a method by implanting “cinaciguat nano-crystals” into chitosan-based micro-particles, applied for hydrophobic drug delivery to the lungs. The polymer’s abilities of swelling and mucoadhesion enabled the continuous release of the drug, resulting in enhanced inhalation efficacy under diseased conditions [63]. More examples have been given in Table 3.
Figure 5.
Methods of preparation of nanocrystal.
Table 3.
Nanocrystals encapsulating herbal medicines.
| Nanocrystals of Herbal Compounds | Plant Source | Limitations of Free Drugs | Results and Outcomes of Loaded Formulations | References |
|---|---|---|---|---|
| Rutin incorporated nanocrystals (RNs) | Buckwheat, eucalyptus | Poor water solubility | Improved water solubility and bioavailability, RNs showed 100 times more cytotoxic effect on HN5 cells, decreased expressions of Bcl-2 mRNA | [64] |
| Cellulose nanocrystals isolated from Amla pomace | Phyllanthus emblica | Free drugs do not possess this property | Cellulose nanocrystals help in converting food industry waste into valuable products, and act as a low-cost precursor for various nanoformulations | [65] |
| Curcumin (CUR) and beclomethasone dipropionate (BDP) nanocrystals | Curcuma longa | Poor water solubility and bioavailability | Improved water solubility and bioavailability, therapeutic efficacy, improved lung delivery of active molecule, improved asthmatic conditions | [66] |
| Silymarin nanocrystals | Silybum Marianum | Low solubility | Improved drug dissolution profile, sustained drug release | [67] |
| Ethanol extract from Ficus glomerata nanocrystals | Ficus glomerata | Lesser biological properties | Showed comparable activities against Aedes aegypti, Culex quinquefasciatus, and Anopheles stephensi to the conventional neem oil-based nano-emulsion and repellent properties are more effective than commercial formulation. | [68] |
| Puerarin | Pueraria lobata | Low bioavailability | Enhanced oral bioavailability and upgraded brain accumulation for the treatment of Parkinson’s disease (PD) | [69] |
| Resveratrol nanocrystals | Natural polyphenol | Low water solubility | Improved water solubility and dermal patches preparation for treatments of acne and skin diseases | [70] |
3.4. Nano-Emulsions
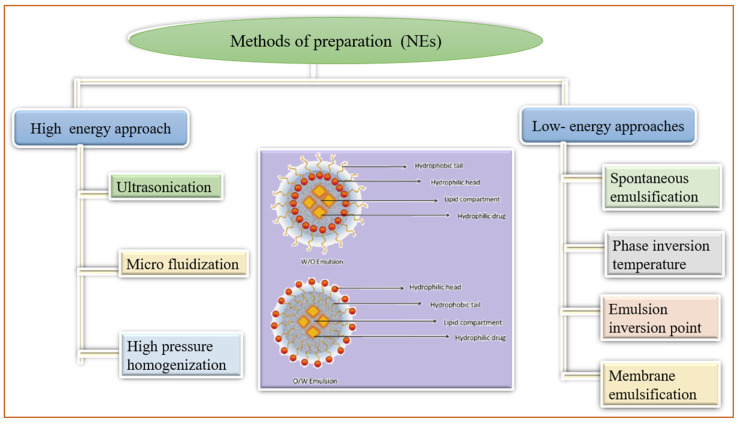
Nano-emulsions (NE) are non-homogeneous, transparent colloidal dispersion systems of 100 nm size that are optically isotropic and thermodynamically stable. These are comprised of water and oil followed by the addition of co-surfactant and surfactant [71]. The lipophilic drug has been entrapped into the oil droplets, both in o/w and w/o suspensions. These oil droplets were engulfed by the macrophages and found in higher amounts in the liver, spleen, and kidneys. However, hydrophilic drugs were in the aqueous phase of w/o or w/o/w nano-emulsions. Figure 6 demonstrated different methods of NE preparation and the structure of nano-emulsions. Owing to their higher internal membrane permeability, these condensed to the lymphatic system, administered through intramuscular and subcutaneous routes [49]. The absorption of NE through the intestine has been attributed to lymphatic transport processes, resulting in amended oral bioavailability of the entrapped drugs [72]. The main characteristics of NE involve the stability of entrapped components, targeted sustained release, enhancing membrane permeability through the skin and mucous membranes, solubilizing components of different lipophilicities, improving drug absorption, lessening pain and allergy conditions, lowering viscosities, simple methods of production, and fewer chances of contamination [71,73]. The attractive properties of NE enabled their use as a vehicle for the distribution of essential oils, nucleic acid, drugs antimicrobial agents, repellents, and as an imaging agent [71,73]. In the past few eras, nano-emulsions have been amended for transdermal remedial use like phospholipids, Transcutol®P, fatty alcohol, alkyl poly-glycosides, and PEGylated fatty acid ester [74]. Numerous nano-emulsion formulations incorporating herbal drugs like camptothecin, genistein, rutin, oils of Brucea javanica, resveratrol, coixenolide, etc., with plenty of health benefits have been listed in the literature [75,76]. With great application scenarios of NE, more examples have been presented in Table 4.
Figure 6.
Methods of preparation of nano-emulsions (NEs).
Table 4.
Nano-emulsions containing herbal bioactive.
| Herbal Nano-Emulsion | Plant Source | Limitations of Free Drugs |
Results and Outcomes of Loaded Bioactive |
References |
|---|---|---|---|---|
| Hydroxy-safflor yellow A NE | Carthamus tinctorius | Low absorption and bioavailability | Enhanced systemic absorption and improved bioavailability. | [77] |
| Oregano oil NE | Origanum vulgare | Limited spectrum antibiotics | Reduced and controlled growth of food-borne bacteria (L. monocytogenes, S. Typhimurium, and E. coli) on fresh lettuce. | [71] |
| Elemene oil NE | Curcuma species | Low stability and bioavailability | Improved stability and oral bioavailability in Sprague Dawley rats than a commercial elemene emulsion. | [78] |
| Quercetin NE | Many plant parts like nuts | Low skin penetration cause skin irritation | Increased cutaneous permeability reached the systemic circulation with lower skin retention. | [79] |
| Basil oil NE | Ocimum basilicum | Have lesser antibacterial activity | Antibacterial activity against pure E. coli culture | [80] |
| Nigella sativa L. NE | Nigella sativa L. | Limited free radicle scavenging activity | Enhanced and dose-dependent radical scavenging capacity in the DPPH assay (IC50 of about 47 µg/mL), reduced bioavailability of A2780 cancerous cells, NE showed pro-apoptotic, antioxidant, and anticancer effects. | [81] |
| Linseed oil NE | Linum usitatissimum seed | Poorer stability and penetration through the skin membrane | Improved stability and physicochemical properties for topical applications, suitable for atopic dermatitis evaluated through in vitro and in silico studies. | [82] |
| Cumin tincture-loaded NE | Cuminum cyminum L. | Limited free radicle scavenging activity and antibacterial properties | Good and dose-dependent radical scavenging capacity, antioxidant, anti-angiogenic effect, antibacterial activity against S. aureus and K. pneumonia. | [83] |
| Essential oil NE | Alhagi maurorum | Limited bioavailability | Enhanced antibacterial and antibiofilm activity, identified as antimicrobial agents against antibiotic-resistant bacteria. | [84] |
| Nelumbo nucifera crude extracts. | Nelumbo nucifera | Poorer stability | Enhanced stability and antimicrobial activities act as an alternative active ingredient for skin bacterial infection. | [85] |
| Peppermint and rosemary essential oils NE | Mentha piperita, Mint family Lamiaceae | Dermal irritation and toxicity | Reduced osteoarthritis pain via increasing antioxidant capacity and improving the histopathological features of the rats’ knee joint. | [86] |
| Essential oil NE | Thymus vulgaris | Limited antifungal properties | Obtained as promising alternatives for the treatment of cutaneous mycoses, especially when the etiological agents are resistant to conventional antifungal drugs. | [87] |
| Essential oil NE | Myristica fragrans or Lavandula dentata | Poorer stability | Improved physical and chemical stability in different temperature and storage conditions | [88] |
3.5. Liposomes
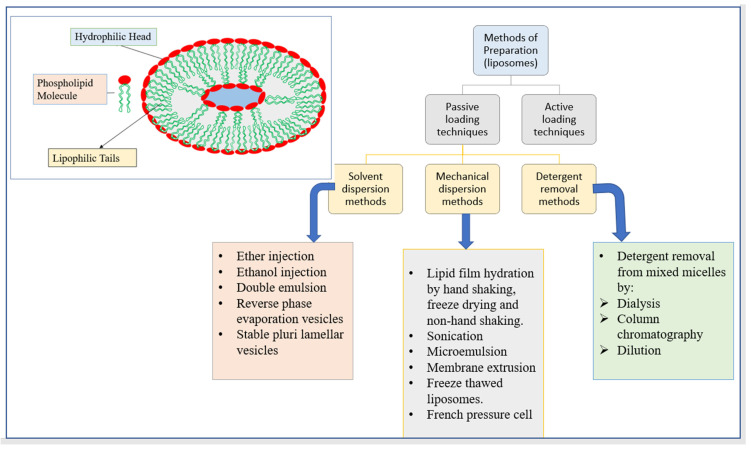
Alec Bangham developed liposomes in 1960. These polar lipid nanoparticles are spherical in form and range in size from 50 to 450 nanometers. These can encase an “aqueous core” in “single or multiple lipid bilayers of natural or synthetic origin” into which it freely diffuses [89]. They have a membrane structure that is similar to that of cells. Liposomes are composed of materials that have both lipophilic and hydrophilic groups, allowing them to encapsulate both types of pharmacological molecules in the same structure [90]. Liposomes can increase drug solubility, drug delivery, the bioavailability of the entrapped drug, absorption of the drug within a cell, and drug distribution throughout the body both in vivo and in vitro due to their unique property of possessing phospholipid bilayers [91,92]. Figure 7 depicts the structure of liposomes as well as several liposome manufacturing methods.
Figure 7.
The structure of liposomes and different methods of preparation of liposomes.
The ADME profiles of drugs such as herbal, enzymes, and proteins can be modified accordingly, which is needed for preparing vaccines, nutraceuticals, and cosmetics. Additionally, some exclusive features like environmental protection of the entrapped drug molecule, devastating primary destruction of loaded bioactives, cost-effective, and quick treatment with least systemic morbidness, exaggerated their use in bio-medicine preparations [93].
Antibodies or ligands, on the other hand, can be added to liposomes to improve target specificity. Thangapazham et al., for example, developed curcumin-loaded liposomes coated with PSMA antibodies to treat the human prostate cancer cell lines LNCaP and C4-2B. As a consequence, improved targeted administration, 70–80% suppression of cell growth, and a 10-fold dosage advantage have been achieved [94]. Table 5 contains further instances of herbal compounds encapsulated in liposomes.
Table 5.
Liposomes containing herbal bioactives.
| Liposomes of Herbal Compounds | Plant Source | Limitations of Free Drugs | Results | Reference |
|---|---|---|---|---|
| Baicalin-loaded liposomes | Root of Scutellaria baicalensis Georgi) | Low water solubility and drug release | Improved solubility, sustained release, enhanced drug concentration in brain tissue after i.v. administration in rats | [95] |
| Polydatin-loaded liposomes | Root and rhizome of Polygonum cuspidatum Sieb | Poorer solubility and bioavailability | Enhanced oral bioavailability, improved solubility, and sustained release in vitro. | [96] |
| Paclitaxel-loaded/PEGylated/saturated PC-based liposomes | The bark of Taxus brevifolia or pacific yew | Low solubility and bioavailability | Improved bioavailability, solubility, biodistribution, and intracellular uptake. | [97,98] |
| Naringenin-loaded liposomes | Immature orange fruit and the peels of grapefruits) | Poorer solubility and bioavailability | Improved stability, solubility, bioavailability, and tissue distribution the sustained release both in vivo and in vitro after oral administration. | [99] |
| Sterols-loaded liposomes | Flammulina velutipes | Limited solubility and bioavailability | Improved water solubility, oral bioavailability, and tissue distribution in liver tumor-bearing Kunming mice. | [100] |
| Quercetin-loaded liposomes | Flavonoids | Reduced solubility and bioavailability | Improved water solubility, and oral bioavailability, used in wound healing | [101] |
| Curcumin-loaded liposomes | Curcuma longa | Low anticancer properties | Anticancer and anti-inflammatory potential | [102] |
| Curcumin-loaded thiolated polymer-coated liposomes | Curcuma longa | Low bioavailability | The improved therapeutic index of curcumin, Aphthous ulcer | [103] |
| Colchicine-loaded liposomes | Colchicum autumnal, gloriosa superba extract | Poorer drug release | The anti-gout drug, improved drug transport | [104] |
| Liposomal neem gel | Azadirachta indica leaves | Limited antibacterial spectrum | Enhanced anti-bacterial activities | [105] |
| Capsaicin liposomes | Genus capsicum | Low bioavailability | Enhanced bioavailability, treating neuropathic pain | [106] |
| Brucine liposomes | Nux vomica | Low bioavailability and showed side effects | Reduced side effects of brucine like violent seizures | [107] |
| Guggul liposomes | Commiphora Mukul. | Low bioavailability | Improved anti-inflammatory properties. | [108] |
| Asparagus racemosus liposomes | Asparagus racemosus | Low bioavailability | Improved anti-inflammatory properties. | [109] |
| Polygonum aviculare L. herba (PAH) extract entrapped liposomes quercetin-entrapped liposomes | Polygonum aviculare L.Quercetin | Low cell viability | Moderately efficient on cell viability while quercetin-loaded liposomes showed increased cell viability and provide better endothelial protection compared to free quercetin and PAH-loaded liposomes | [110] |
3.6. Phytosomes
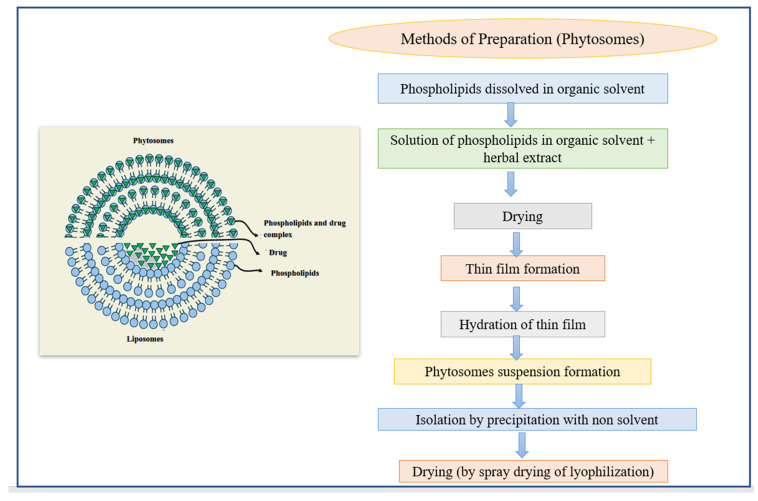
Phytosomes are lipid-compatible molecular complexes that encapsulate pharmacological bioactive and water-soluble phytochemicals in phospholipids, resulting in increased absorption and bioavailability [111]. Hydrophilic phytochemicals, such as polyphenols and flavonoids, have lower absorption in the body due to their high molecular size, which made absorption across biological membranes difficult. These constraints have been overcome thanks to phytosome [71]. The uniqueness associated with them includes their molecular complex and chemical bond formation between plant material and phosphatidylcholine at a ratio of either 1:1 or 1:2 [112]. Structurally, phytosomes resemble liposomes, except for the entrapment of the material. In liposomes, the active material is dissolved in the medium present in the membrane layers, while in phytosomes the active material is a vital part of the membrane (Figure 8). The phytosomes created a better transition of the enterocyte cell membrane from a water-soluble to a lipid-soluble state, then inside the cell, reaching the bloodstream, and protecting entrapped herbal medications from stomach fluids and gut microorganisms. A large number of studies have been carried out to determine their use and qualities in comparison to other traditional delivery techniques. Recently, a group of researchers combined several flavonoids, including quercetin, kaempferol, and apigenin, into a single phytosome called flavonosome, which proved to be an effective antioxidant, hepatoprotective agent, and heat supplement [113]. In Table 6 below, we have included some more instances.
Figure 8.
The structure of phytosomes and different methods of the preparation of liposomes.
Table 6.
Phytosomes containing herbal medicines.
| Phytosome | Plant Source | Limitations of Free Drugs | Results and Outcomes | Reference |
|---|---|---|---|---|
| Epigallocatechin gallate-loaded phytosome | Camellia sinensis | Low stability and bioavailability | Improved solubility and bioavailability. Physicochemical stability through organoleptic, water content, and physicochemical properties at various temperatures | [114] |
| Rutin-loaded phytosome | Citrus fruits | Low stability and poor drug release | Improved solubility, stability, releasing dynamics and bioavailability in vitro, good antioxidant agent | [115] |
| Soybean seed Phytosome-based thermogel | Glycine max L. | Low drug absorption and solubility | Improved absorption, instability, insolubility, and fast releasing. A clear reduction in body weight, adipose tissue weight, studied in vivo. | [116] |
| Gingerol-loaded phytosome | Zingiber officinale | Poor stability and drug absorption | Improved stability, oral absorption, bioavailability, sustained release, showing potent antioxidant, antibacterial (against Staphylococcus aureus and E. coli), and anti-inflammatory activities in vitro. | [117] |
| Butea monosperma flower extract-loaded phytosome | Butea monosperma | Poor water solubility and bioavailability | Improved solubility, bioavailability, stability, and release dissolution pattern and showed significant free radical scavenging activity in vitro using the DPPH model. | [118] |
| Swertia perennis L.-loaded phytosome | Swertia perennis L. | Poor drug release profile. | Improved entrapment efficiency and in vitro drug release of embedded phytomedicine. | [119] |
| Aloe Vera extract-loaded phytosome | Aloe Vera | Limited anticancer activity | Inhibitory effect on the growth of the MCF-7 cancer cell line, enhanced oral delivery of aloe vera, making its use in cancer therapy. | [120] |
| Morinda lucida extract-loaded phytosome | Morinda lucida | Limited antimicrobial activities | In vivo, anti-plasmodium studies confirmed a higher anti-malarial effect comparable/similar to the standard drug (artesunate). | [121] |
| Aqueous extract of stem bark and lecithin of Tecomella undulata-loaded phytosome | Tecomella undulata | Poor drug release profile and bioavailability | Good entrapment efficiency and drug release in nano sizes (up to 90%), improved bioavailability without resorting to any pharmacological adjuvant or structural modification of the ingredients. | [122] |
3.7. Ethosomes
Ethosomes are soft, non-invasive lipid-based elastic vehicles comprised of water, phospholipids such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylglycerol, and about 30–45% ethanol and isopropyl alcohol [123,124]. The proportion composition of ethosomes improves their entrapment efficiency, topical drug delivery, and transdermal transport efficiency for both hydrophilic and lipophilic drugs. They provide delivery of ingredients into deeper tissue as well in blood circulation. Improved physical stability of ethosomes compared to liposomes is due to flexible lecithin bilayers [123]. On the contrary, ethosomes have some limitations like poorer stability, growing size from nanometer to micrometer caused by alcohol evaporation, and leakage of entrapped material after a while. Combining alcohol with trehalose and propylene glycol can help to overcome this weakness. To test their capacity as a transporter for delivering entrapped molecules to the skin in a rat model, “curcumin-encapsulated PEGlycated and conventional liposomes and ethosomes” were developed and tested. As a consequence, PEGlycated liposomes were shown to be the most promising ex vivo transdermal drug delivery technology, suppressing paw edema in the rat model to a greater extent [124]. More instances may be found in Table 7 (below).
Table 7.
Ethosomes incorporated with herbal medicines.
| Herbal Drug-Loaded Ethosomes | Plant Source | Limitations of Free Drugs | Results and Outcomes | References |
|---|---|---|---|---|
| Apigenin-loaded ethosomes | From many fruits and vegetables such as chamomile | Low bioavailability | The strong anti-inflammatory activity caused by ultraviolet B light exposure after topical application | [125] |
| Berberis aristata extract-loaded ethosomal gel | Berberis aristata | Lesser drug penetration and bioavailability | Enhanced permeation profile and transdermal delivery of the extract provide a better approach for dermatological disorders | [126] |
| Cryptotanshinone-loaded ethosomal gel | Salvia miltiorrhiza | Lesser drug penetration and bioavailability | Enhanced transdermal flux, skin permeation, and deposition on pigskin in vitro. Improved anti-acne activity with reduced skin irritation in the ear of rabbit model associated with ethosomal gel. | [127] |
| Colchicine trans ethosomal gel | From dried corns and seeds of plants of the genus Colchicum | Poor stability, solubility drug release bioavailability | Improved stability, solubility, sustained release, bioavailability, and skin diffusion in vitro.Enhanced drug accretion, tissue biodistribution, and skin permeation in an ex vivo using Sprague Dawley rats’ back skin | [123] |
| Piperine-loaded ethosomes | Piper nigrum | Lesser drug penetration and bioavailability | Ethosomal cream showed higher deposition in skin layers, non-toxic to HaCat cell lines, and novel drug carrier for management of atopic dermatitis. | [128] |
| Achillea millefolium L.-loaded ethosomes | Achillea millefolium L. | Limited free radical scavenging activities and drug release | Enhanced free radical scavenging activities by about 88%, improved drug release by about 79.8% | [129] |
| Sambucus nigra L. Extract-loaded ethosomes | Sambucus nigra L. | Cause skin irritation | Possessed collagenase inhibition activity, excellent skin compatibility, recognized as a potent cosmeceutical ingredient | [130] |
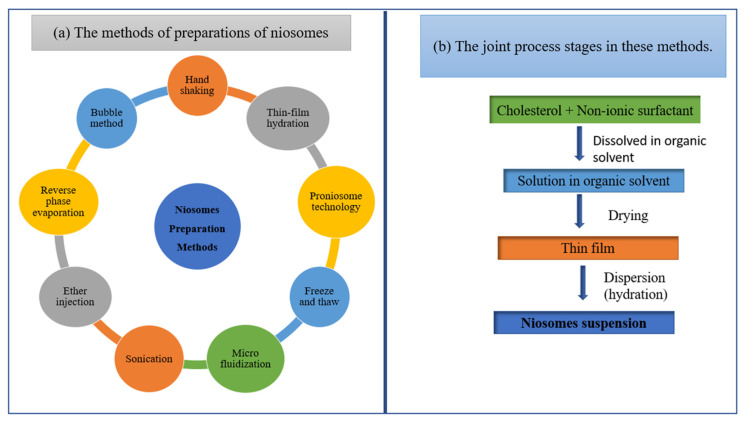
3.8. Niosomes
These are nano-sphere vesicles of diameter ranging from 100 nm to 2 µm. These are non-ionic with a watery center, surrounded by non-ionic amphiphilic lipids in the lamellar phase [131]. Different methods of preparation include sonication, thin-film hydration, micro fluidization, multiple-membrane extrusion, remote loading, reverse-phase evaporation technique, and bubble method, as shown in Figure 9 [132]. The structure of niosome almost resembled the liposome, showing more stability, penetrating capability, and beneficial efficacy of the drug along with reduced toxicity [133]. The main advantages of niosomes are flexibility, cost-effectiveness, higher drug solubility and controlled release of the encapsulated bioactive, making them an effective peptide carrier and hemoglobin carrier, targeting vehicle for neoplasia, providing transdermal drug delivery of entrapped molecules. Niosomes, on the other hand, demonstrated extended drug circulation, skin retention, and penetration, as well as sustained drug release at the target location [134]. These are more stable nanocarriers than liposomes, with no notable toxicity, especially for topical usage in the treatment of skin problems such as skin cancer [135]. Table 8 shows several instances of niosomes that include natural remedies.
Figure 9.
Schematic diagram of (a) the methods of preparations of niosomes and (b) the joint process stages in these methods.
Table 8.
Niosomes loaded with herbal bioactive.
| Herbal Medicine-Loaded Niosomes | Plant Source | Limitations of Free Drugs | Results and Outcomes | References |
|---|---|---|---|---|
| Permacoce hispida-loaded niosome | Permacoce hispida- | Poor stability and bioavailability | Improved stability, bioavailability, sustained release, and permeability in vitro. Enhanced anti-tuberculosis in vitro. | [136] |
| Embelin-loaded niosome | Embelia ribes Burm. | Poor stability and bioavailability | Improved stability, bioavailability, sustained release, and biocompatibility in vitro. Upgraded streptozotocin-induced diabetes in Albino Wistar rats with potential antioxidant activity. | [137] |
| Lawsone-loaded niosome | Persian Henna, Lawsonia inermis | Poor stability and bioavailability | Improved stability, bioavailability, sustained release, and in vitro permeability. Significantly improved the antitumor activity in MCF-7 cells in vitro. | [138] |
| Rosemarinic acid-loaded niosome | Rosmarinus officinalis | Limited drug release and drug stability | Improved sustained delivery of Niosomal gel of rosmarinic acid to bacteria (Propionibacterium acne and Staphylococcus aureus) infected cells in vitro (anti-acne vulgaris). Improved delivery of naturally occurring antimicrobial and anti-inflammatory agents, in deeper tissues of skin in vivo using Swiss albino mice. | [139] |
| Nerium oleander -loaded niosome | Nerium oleander | Limited antioxidant activity and bioavailability | Improved cell effectiveness and tolerability of active substances. Improved in vitro cytotoxicity toward cervical and alveolar cancer cells (HeLa and A549) using MTT assay. Displayed potential antioxidant activity in vitro using DPPH radical scavenging assay. | [140] |
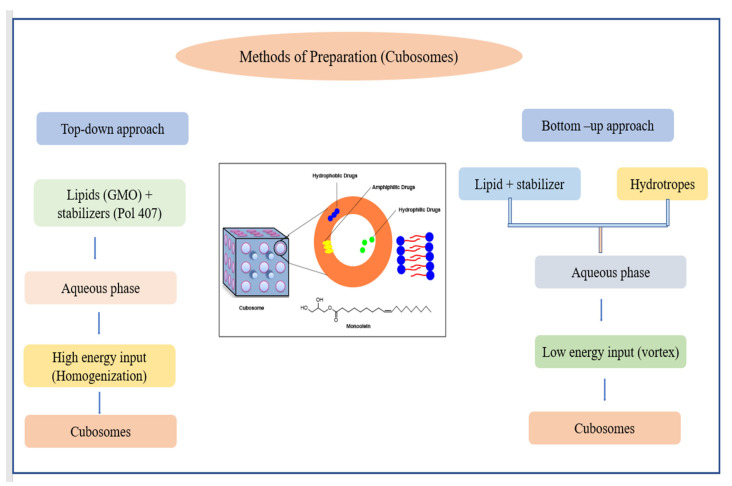
3.9. Cubosomes
These are viscous isotropic vesicles made up of mainly amphiphilic lipids (unsaturated monoglycerides) and thermodynamically stable surfactants such as poloxamers [141,142]. Due to properties like a large interior surface area per unit volume (approximately 400 m2/g) and a 3D structure with hydrophilic and hydrophobic domains, they easily entrap water-soluble and non-soluble, as well as amphiphilic, compounds. Its large interfacial surface can offer a variety of diffusion channels for the long-term release of entrapped drug molecules, and its lipid components are biodegradable, bio-adhesive, and digestible [143]. They are frequently created by dispersing or fragmenting the cubic phases of gel in the liquid phase.
Two approaches, the top-down and bottom-up approaches, have been developed for cubosomes production (Figure 10). Somatostatin, indomethacin, insulin, rifampicin, etc., have been successfully encapsulated within the cubosomes. Moreover, peptides, anti-muscarinic effects, enzymatic effects, antibiotics, and analgesic administration are just a couple of small pharmacological uses of cubosomes that have been studied [144]. Because cubosomes have a structure that is almost identical to that of the stratum corneum, they may readily release the entrapped bioactive into the epidermis. Additionally, cubosomes’ features of adhesion and penetration increase imply their potential value in skin cancer (melanoma) treatment. Recently, a study was conducted to develop polymer-free cubosomes, for photodynamic treatment of the skin as well as bio-imaging of skin malignant tumors with extremely minimal cytotoxicity to the cutaneous system [145]. In Table 9, examples of cubosomes incorporating herbal bioactive have been listed.
Figure 10.
Top-down and bottom-up approaches for preparing cubosomes.
Table 9.
List of herbal bioactive-loaded cubosomes.
| Herbal Medicine-Loaded Cubosomes | Plant Sources | Limitations of Free Drugs | Results and Outcomes | References |
|---|---|---|---|---|
| Piperine-loaded cubosomes | Fruits of the piperaceae family | Low stability | Improved stability, hydrophobicity, the enhanced and cognitive effect of piperine, displayed anti-inflammatory, anti-apoptotic, and antioxidant effects. | [146] |
| Curcumin-loaded cubosomes | Curcuma longa L. | Low stability | Upgraded stability, production of nanosized vesicles, and enhanced anti-bacterial properties in topical drug delivery. | [147] |
| Achyranthes bidentata-loaded cubosomes | Polysaccharides | Low stability and immunomodulatory effect | Improved stability, immunomodulatory effect, and displayed fewer toxicities to splenic lymphocytes in vitro. | [148] |
| Capsaicin incorporated cubosomes | All plants of the capsicum family | Cause skin irritation | Lowered skin irritation, enhanced stability under light and heat, sustained delivery for transdermal administration of capsaicin. | [149] |
| Essential oil of Citrus trifoliata L. incorporated cubosomes | Citrus trifoliata L. | Limited insecticidal activities | Enhanced insecticidal and fungicidal activities against Fusarium oxysporum, Spodoptera littoralis, and Fusarium solani. | [150] |
4. Discussion
4.1. Nanotechnologies Applications
Nanotechnology has not only changed medicine but has also provided accuracy and precision for the treatment of many diseases. It has been considered an excellent technology for drug delivery as well as drug release at the target site. Nanotechnologies have been used for the applications such as fluorescent biological labels, detection of pathogens, drug and gene delivery, detection of proteins, probing of DNA structure and tissue engineering, etc. Moreover, tumor destruction through heating (hyperthermia), separation and purification of biological molecules and cells, MR imaging contrast enhancement, and phagokinetic studies are some others in the area of medicine for diagnosis and treatment of cancer. The nanotechnology provides advanced therapies with a reduced degree of invasiveness, and faster, smaller, and highly sensitive diagnostic tools which provide cost-effectiveness.
4.2. Drawbacks of Nanotechnology
The biocompatibility of nanoformulations is the major issue of concern. The ease with which nanotechnology-based treatment has been provided all over the world at basic levels (primary health care, government hospitals, etc.) and the cost of such treatments are the most crucial aspects. Moreover, the lack of knowledge about its toxicity and its impact on the biochemical pathways, human body, and environment needs to be studied very closely. Society’s ethical use of nanomedicine and the concerned safety issues pose a serious question to the researchers [151,152,153,154,155,156,157].
4.3. Ethical Concern
Nanoscience and nanotechnology, like any new scientific approach, are involved in a dispute regarding the degree of usefulness. Research on the ethical, legal, social, and environmental aspects of nanoscience and nanotechnology has been recognized as a viable subject of investigation in Western countries. Because nanomedicine is a relatively new field of science and nano-technology-based drug treatments differ significantly from existing treatments, there may be considerable uncertainty and difficulty in regulating the nanotechnology-based treatment and its applications. As a result, it may be difficult to regulate nanotechnology-based treatment and applications [158].
5. Conclusions
For the last few decades, nanocarrier-based DDSs have been investigated as a new drug transporter because of the benefits offered by the active ingredients. Naturally occurring medicines contained a wide range of therapeutic characteristics that should be investigated using advanced drug delivery methods. Poorer water solubilities and bioavailability are some limiting issues associated with these methods. Researchers developed new methods either by entrapping into a drug carrier or by modulating drug structure by adding some stable groups. The primary element to consider while developing any formulation is the necessity of developed formulation to cross biological membranes. The main criteria for this are lipid solubilities and molecular size of the drug. Recent studies have predicted their applicability in the treatment of diseases such as diabetes, cancer, anemia, hypertension, and a variety of others, and current research has attempted to address current challenges by applying nanocarriers methodologies. Nanocarriers created a low drug level in the blood, resulting in reduced toxicity, which is advantageous for patients who required medications on a daily basis. The developments in nanomedicine achieved to date have changed the techniques for the administration of drugs in our body, even though the underlying mechanism, safety, and toxicity profile of nanomedicine is still being developed. The technology developed to identify illnesses and even combined therapy and diagnosis a reality is realistic thanks to current developments in nanomedicine.
In conclusion, pharmaceutical nanotechnology is an emerging field of science in every aspect of maintaining the drug stability, solubility, absorption, and bioavailability of poorly water-soluble and less bioavailable drugs. Additionally, nanotechnology-based systems enhance the targeted delivery and sustained delivery of the entrapped material, leading to efficient therapeutic potency with reduced side effects. In numerous laboratories in India, pharmaceutical development of nanotechnology-based DDSs is being undertaken with zeal. These are being studied in vitro for release patterns and in vivo in animals for pharmacokinetics, but not often for efficacy. There is a lack of information on clinical research and the development of nanotechnology-based DDSs utility in patients. It is required to involve any pharmacologists in the investigation of pharmacokinetics and pharmacodynamics of DDS to know if the products have reached their meaningful outcome—clinical use.
Acknowledgments
The authors are thankful to Chitkara College of Pharmacy, Chitkara University, Patiala, India, and the Faculty of Pharmacy, Sivas Cumhuriyet University, Sivas, Turkey for institutional facilities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article.
Conflicts of Interest
The author confirmed that article content has no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Atmakuri L.R., Dathi S. Current trends in herbal medicines. J. Pharm. Res. 2010;3:109–113. [Google Scholar]
- 2.Bhokare S.G., Dongaonkar C.C., Lahane S.V., Salunke P.B., Sawale V.S., Thombare M.S. Herbal novel drug delivery: A review. World J. Pharm. Pharm. Sci. 2016;5:593–611. [Google Scholar]
- 3.Goldberg B. lternative Medicine, the Definitive Guide. Volume 257 Future Medicine Publications; Puyallup, WA, USA: 1994. [Google Scholar]
- 4.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni G.T. Herbal drug delivery systems: An emerging area in herbal drug research. J. Chronother. Drug Deliv. 2011;2:113–119. [Google Scholar]
- 6.Sharma R., Hazra J., Prajapati P. Nanophytomedicines: A Novel Approach to Improve Drug Delivery and Pharmacokinetics of Herbal Medicine. Biol. Bullettin. 2017;3:132–135. [Google Scholar]
- 7.Kumar K., Rai A. Miraculous therapeutic effects of herbal drugs using novel drug delivery systems. Int. Res. J. Pharm. 2012;3:27–30. [Google Scholar]
- 8.Shah S.M.A., Nisar Z., Nisar J., Akram M., Ghotekar S., Oza R. Nanobiomedicine: A New Approach of Medicinal Plants and Their Therapeutic Modalities. J. Mater. Environ. 2021;12:1–14. [Google Scholar]
- 9.Da Silva P.B., dos Santos Ramos M.A., Bonifacio B.V., Negri K.M.S., Sato M.R., Bauab T.M., Chorilli M. Nanotechnological strategies for vaginal administration of drugs—A review. J. Biomed. Nanotechnol. 2014;10:2218–2243. doi: 10.1166/jbn.2014.1890. [DOI] [PubMed] [Google Scholar]
- 10.Nune S.K., Chanda N., Shukla R., Katti K., Kulkarni R.R., Thilakavathy S., Mekapothula S., Kannan R., Katti K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009;19:2912–2920. doi: 10.1039/b822015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapadiya G.G. Impact of nanotechnology on global trade of herbal drugs: An overview. Int. J. Green Pharm. (IJGP) 2017;11:S171. [Google Scholar]
- 12.Shi X., Sun K., Baker J.R., Jr. Spontaneous formation of functionalized dendrimer-stabilized gold nanoparticles. J. Phys. Chem. C. 2008;112:8251–8258. doi: 10.1021/jp801293a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S.-H., Oh S.-G., Mun J.-Y., Han S.-S. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surf. B Biointerfaces. 2006;48:112–118. doi: 10.1016/j.colsurfb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Kabanov A.V., Lemieux P., Vinogradov S., Alakhov V. Pluronic® block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002;54:223–233. doi: 10.1016/S0169-409X(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 15.Mirza A.Z., Siddiqui F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014;4:94. doi: 10.1007/s40089-014-0094-7. [DOI] [Google Scholar]
- 16.Bairwa N.K., Sethiya N.K., Mishra S. Protective effect of stem bark of Ceiba pentandra linn. against paracetamol-induced hepatotoxicity in rats. Pharmacogn. Res. 2010;2:26. doi: 10.4103/0974-8490.60584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N., Feng Y. Elaborating the role of natural products-induced autophagy in cancer treatment: Achievements and artifacts in the state of the art. BioMed Res. Int. 2015;2015:934207. doi: 10.1155/2015/934207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouattara B., Simard R.E., Holley R.A., Piette G.J.-P., Bégin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997;37:155–162. doi: 10.1016/S0168-1605(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 19.Sharma G., Raturi K., Dang S., Gupta S., Gabrani R. Combinatorial antimicrobial effect of curcumin with selected phytochemicals on Staphylococcus epidermidis. J. Asian Nat. Prod. Res. 2014;16:535–541. doi: 10.1080/10286020.2014.911289. [DOI] [PubMed] [Google Scholar]
- 20.Abdelwahab S.I., Sheikh B.Y., Taha M.M.E., How C.W., Abdullah R., Yagoub U., El-Sunousi R., Eid E.E. Thymoquinone-loaded nanostructured lipid carriers: Preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013;8:2163. doi: 10.2147/IJN.S44108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paroha S., Chandel A.K.S., Dubey R.D. Nanosystems for drug delivery of coenzyme Q10. Environ. Chem. Lett. 2018;16:71–77. doi: 10.1007/s10311-017-0664-9. [DOI] [Google Scholar]
- 22.Chandel A.K.S., Bhingradiya N. Enhancing the Therapeutic Efficacy of Herbal Formulations. IGI Global; Hershey, PA, USA: 2021. Therapeutic Efficacy of Herbal Formulations Through Novel Drug Delivery Systems; pp. 1–42. [Google Scholar]
- 23.Sahu A.N. Nanotechnology in herbal medicines and cosmetics. Int. J. Res. Ayurveda Pharm. (IJRAP) 2013;4:472–474. doi: 10.7897/2277-4343.04334. [DOI] [Google Scholar]
- 24.ud Din F., Aman W., Ullah I., Qureshi O.S., Mustapha O., Shafique S., Zeb A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017;12:7291. doi: 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam P.-L., Wong W.-Y., Bian Z., Chui C.-H., Gambari R. Recent advances in green nanoparticulate systems for drug delivery: Efficient delivery and safety concern. Nanomedicine. 2017;12:357–385. doi: 10.2217/nnm-2016-0305. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Feng N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM) Adv. Colloid Interface Sci. 2015;221:60–76. doi: 10.1016/j.cis.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Nagalingam A. Drug delivery aspects of herbal medicines. Jpn. Kampo Med. Treat. Common. Dis. Focus Inflamm. 2017;17:143. [Google Scholar]
- 28.Lin C.-H., Chen C.-H., Lin Z.-C., Fang J.-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017;25:219–234. doi: 10.1016/j.jfda.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yingchoncharoen P., Kalinowski D.S., Richardson D.R. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol. Rev. 2016;68:701–787. doi: 10.1124/pr.115.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostami E., Kashanian S., Azandaryani A.H., Faramarzi H., Dolatabadi J.E.N., Omidfar K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids. 2014;181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Luo C.-F., Yuan M., Chen M.-S., Liu S.-M., Zhu L., Huang B.-Y., Liu X.-W., Xiong W. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int. J. Pharm. 2011;410:138–144. doi: 10.1016/j.ijpharm.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 32.Luo C.-F., Hou N., Tian J., Yuan M., Liu S.-M., Xiong L.-G., Luo J.-D., Chen M.-S. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int. J. Nanomed. 2013;8:933. doi: 10.2147/IJN.S39349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C., Gu C., Peng F., Liu W., Wan J., Xu H., Lam C.W., Yang X. Preparation and optimization of triptolide-loaded solid lipid nanoparticles for oral delivery with reduced gastric irritation. Molecules. 2013;18:13340–13356. doi: 10.3390/molecules181113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madan J., Pandey R.S., Jain V., Katare O.P., Chandra R., Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomed. Nanotechnol. Biol. Med. 2013;9:492–503. doi: 10.1016/j.nano.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Guo X., Liu Z., Okeke C.I., Li N., Zhao H., Aggrey M.O., Pan W., Wu T. Preparation and evaluation of charged solid lipid nanoparticles of tetrandrine for ocular drug delivery system: Pharmacokinetics, cytotoxicity and cellular uptake studies. Drug Dev. Ind. Pharm. 2014;40:980–987. doi: 10.3109/03639045.2013.795582. [DOI] [PubMed] [Google Scholar]
- 36.Rajput S.B., Shinde R.B., Routh M.M., Karuppayil S.M. Anti-Candida properties of asaronaldehyde of Acorus gramineus rhizome and three structural isomers. Chin. Med. 2013;8:18. doi: 10.1186/1749-8546-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shende P., Narvenker R. Herbal nanotherapy: A new paradigm over conventional obesity treatment. J. Drug Deliv. Sci. Technol. 2020;61:102291. doi: 10.1016/j.jddst.2020.102291. [DOI] [Google Scholar]
- 38.Jammanesh A., Arbabi Bidgoli S., Ghaffari S., Avadi M.R. Formulation, Characterization and Toxicity Assessment of Ginkgo Biloba Extract Solid Lipid Nanoparticle in female mice. Nanomed. Res. J. 2021;6:28–40. [Google Scholar]
- 39.Rodrigues L.R., Jose J. Exploring the photo protective potential of solid lipid nanoparticle-based sunscreen cream containing Aloe vera. Environ. Sci. Pollut. Res. 2020;27:20876–20888. doi: 10.1007/s11356-020-08543-4. [DOI] [PubMed] [Google Scholar]
- 40.Kelidari H.R., Moemenbellah-Fard M.D., Morteza-Semnani K., Amoozegar F., Shahriari-Namadi M., Saeedi M., Osanloo M. Solid-lipid nanoparticles (SLN) s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. J. Parasit. Dis. 2021;45:101–108. doi: 10.1007/s12639-020-01281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos D.A., Madureira A.R., Sarmento B., Gomes A.M., Pintado M.M. Stability of bioactive solid lipid nanoparticles loaded with herbal extracts when exposed to simulated gastrointestinal tract conditions. Food Res. Int. 2015;78:131–140. doi: 10.1016/j.foodres.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Motawea A., Ahmed D.A.M., El-Mansy A.A., Saleh N.M. Crucial Role of PLGA Nanoparticles in Mitigating the Amiodarone-Induced Pulmonary Toxicity. Int. J. Nanomed. 2021;16:4713. doi: 10.2147/IJN.S314074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das S., Ng W.K., Tan R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012;47:139–151. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Muhammad H.S.R. Anti-Leukemic Effects of Zerumbone Nanoparticle on Human Jurkat T Lymphoblastoid Cell Lines In Vitro and Murine Leukemic WEHI-3B Model In Vivo. Universiti Putra Malaysia Seri Kembangan; Selangor, Malaysia: 2014. [Google Scholar]
- 45.Shangguan M., Lu Y., Qi J., Han J., Tian Z., Xie Y., Hu F., Yuan H., Wu W. Binary lipids-based nanostructured lipid carriers for improved oral bioavailability of silymarin. J. Biomater. Appl. 2014;28:887–896. doi: 10.1177/0885328213485141. [DOI] [PubMed] [Google Scholar]
- 46.Nahr F.K., Ghanbarzadeh B., Hamishehkar H., Kafil H.S. Food grade nanostructured lipid carrier for cardamom essential oil: Preparation, characterization and antimicrobial activity. J. Funct. Foods. 2018;40:1–8. doi: 10.1016/j.jff.2017.09.028. [DOI] [Google Scholar]
- 47.Ong Y.S., Saiful Yazan L., Ng W.K., Abdullah R., Mustapha N.M., Sapuan S., Foo J.B., Tor Y.S., How C.W., Abd Rahman N. Thymoquinone loaded in nanostructured lipid carrier showed enhanced anticancer activity in 4T1 tumor-bearing mice. Nanomedicine. 2018;13:1567–1582. doi: 10.2217/nnm-2017-0322. [DOI] [PubMed] [Google Scholar]
- 48.Nordin N., Yeap S.K., Zamberi N.R., Abu N., Mohamad N.E., Rahman H.S., How C.W., Masarudin M.J., Abdullah R., Alitheen N.B. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. PeerJournals. 2018;6:e3916. doi: 10.7717/peerj.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal M., Dudhe R., Sharma P. Nanoemulsion: An advanced mode of drug delivery system. Biotechnology. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohamad N.E., Abu N., Rahman H.S., Ky H., Ho W.Y., Lim K.L., How C.W., Rasedee A., Alitheen N.B., Yeap S.K. Nanostructured lipid carrier improved in vivo anti-tumor and immunomodulatory effect of Zerumbone in 4T1 challenged mice. RSC Adv. 2015;5:22066–22074. doi: 10.1039/C5RA00144G. [DOI] [Google Scholar]
- 51.Rahman H.S., Rasedee A., Chartrand M.S., Othman H.H., Yeap S.K., Namvar F. Zerumbone induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in Jurkat cell line. Nat. Prod. Commun. 2014;9:1934578X1400900904. doi: 10.1177/1934578X1400900904. [DOI] [PubMed] [Google Scholar]
- 52.Shi F., Wei Z., Zhao Y., Xu X. Nanostructured lipid carriers loaded with baicalin: An efficient carrier for enhanced antidiabetic effects. Pharmacogn. Mag. 2016;12:198. doi: 10.4103/0973-1296.186347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng J., Wu Z., Zhao Z., Wu C., Yuan M., Su Z., Wang Y., Wang Z. Berberine-loaded nanostructured lipid carriers enhance the treatment of ulcerative colitis. Int. J. Nanomed. 2020;15:3937. doi: 10.2147/IJN.S247406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F., Ye X., Zhai D., Dai W., Wu Y., Chen J., Chen W. Curcumin-loaded nanostructured lipid carrier induced apoptosis in human HepG2 cells through activation of DR5/caspases-mediated extrinsic apoptosis pathway. Acta Pharm. 2020;70:227–237. doi: 10.2478/acph-2020-0003. [DOI] [PubMed] [Google Scholar]
- 55.Sharaf M., Arif M., Khan S., Abdalla M., Shabana S., Chi Z., Liu C. Co-delivery of hesperidin and clarithromycin in a nanostructured lipid carrier for the eradication of Helicobacter pylori in vitro. Bioorg. Chem. 2021;112:104896. doi: 10.1016/j.bioorg.2021.104896. [DOI] [PubMed] [Google Scholar]
- 56.Lacatusu I., Iordache T.A., Mihaila M., Mihaiescu D.E., Pop A.L., Badea N. Multifaced Role of Dual Herbal Principles Loaded-Lipid Nanocarriers in Providing High Therapeutic Efficacity. Pharmaceutics. 2021;13:1511. doi: 10.3390/pharmaceutics13091511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akhavan H.-R., Hosseini F.-S., Amiri S., Radi M. Cinnamaldehyde-Loaded Nanostructured Lipid Carriers Extend the Shelf Life of Date Palm Fruit. Food Bioprocess Technol. 2021;14:1478–1489. doi: 10.1007/s11947-021-02645-8. [DOI] [Google Scholar]
- 58.Jesus J.A., Sousa I.M.O., da Silva T.N.F., Ferreira A.F., Laurenti M.D., Antonangelo L., Faria C.S., da Costa P.C., de Carvalho Ferreira D., Passero L.F.D. Preclinical Assessment of Ursolic Acid Loaded into Nanostructured Lipid Carriers in Experimental Visceral Leishmaniasis. Pharmaceutics. 2021;13:908. doi: 10.3390/pharmaceutics13060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu R., Liu S., Shen W., Chen C., Cao Y., Su Z., Sun M., Qi R. Study on the Inhibitory Effects of Naringenin-Loaded Nanostructured Lipid Carriers Against Nonalcoholic Fatty Liver Disease. J. Biomed. Nanotechnol. 2021;17:942–951. doi: 10.1166/jbn.2021.3077. [DOI] [PubMed] [Google Scholar]
- 60.Junyaprasert V.B., Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015;10:13–23. doi: 10.1016/j.ajps.2014.08.005. [DOI] [Google Scholar]
- 61.Du J., Li X., Zhao H., Zhou Y., Wang L., Tian S., Wang Y. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int. J. Pharm. 2015;495:738–749. doi: 10.1016/j.ijpharm.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Pardhi V.P., Verma T., Flora S., Chandasana H., Shukla R. Nanocrystals: An overview of fabrication, characterization and therapeutic applications in drug delivery. Curr. Pharm. Des. 2018;24:5129–5146. doi: 10.2174/1381612825666190215121148. [DOI] [PubMed] [Google Scholar]
- 63.Ni R., Zhao J., Liu Q., Liang Z., Muenster U., Mao S. Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur. J. Pharm. Sci. 2017;99:137–146. doi: 10.1016/j.ejps.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Bohlouli S., Jafarmadar Gharehbagh F., Dalir Abdolahinia E., Kouhsoltani M., Ebrahimi G., Roshangar L., Imani A., Sharifi S., Maleki Dizaj S. Preparation, Characterization, and Evaluation of Rutin Nanocrystals as an Anticancer Agent against Head and Neck Squamous Cell Carcinoma Cell Line. J. Nanomater. 2021;2021:9980451. doi: 10.1155/2021/9980451. [DOI] [Google Scholar]
- 65.Gupta V., Ramakanth D., Verma C., Maji P.K., Gaikwad K.K. Isolation and characterization of cellulose nanocrystals from amla (Phyllanthus emblica) pomace. Biomass Convers. Biorefinery. 2021:1–12. doi: 10.1007/s13399-021-01852-9. [DOI] [Google Scholar]
- 66.Casula L., Lai F., Pini E., Valenti D., Sinico C., Cardia M.C., Marceddu S., Ailuno G., Fadda A.M. Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma. Pharmaceutics. 2021;13:1300. doi: 10.3390/pharmaceutics13081300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Batal A.I., Elmenshawi S.F., Ali A.M.A., Goodha E. Preparation and characterization of silymarin nanocrystals and phytosomes with investigation of their stability using gamma irradiation. Indian J. Pharm. Educ. Res. 2018;52:174–183. doi: 10.5530/ijper.52.4s.96. [DOI] [Google Scholar]
- 68.Nazeer A.A., Rajan H.V., Vijaykumar S.D., Saravanan M. Evaluation of larvicidal and repellent activity of nanocrystal emulsion synthesized from F. glomerata and neem oil against mosquitoes. J. Clust. Sci. 2019;30:1649–1661. doi: 10.1007/s10876-019-01611-x. [DOI] [Google Scholar]
- 69.Xiong S., Liu W., Li D., Chen X., Liu F., Yuan D., Pan H., Wang Q., Fang S., Chen T. Oral delivery of puerarin nanocrystals to improve brain accumulation and anti-parkinsonian efficacy. Mol. Pharm. 2019;16:1444–1455. doi: 10.1021/acs.molpharmaceut.8b01012. [DOI] [PubMed] [Google Scholar]
- 70.Karakucuk A., Tort S. Preparation, characterization and antimicrobial activity evaluation of electrospun PCL nanofiber composites of resveratrol nanocrystals. Pharm. Dev. Technol. 2020;25:1216–1225. doi: 10.1080/10837450.2020.1805761. [DOI] [PubMed] [Google Scholar]
- 71.Vickers N.J. Animal communication: When i’m calling you, will you answer too? Curr. Piology. 2017;27:R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 72.Lovelyn C., Attama A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011;2:626. doi: 10.4236/jbnb.2011.225075. [DOI] [Google Scholar]
- 73.Mahato R. Nanoemulsion as targeted drug delivery system for cancer therapeutics. J. Pharm. Sci. Pharmacol. 2017;3:83–97. doi: 10.1166/jpsp.2017.1082. [DOI] [Google Scholar]
- 74.Kotta S., Khan A.W., Pramod K., Ansari S.H., Sharma R.K., Ali J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2012;9:585–598. doi: 10.1517/17425247.2012.668523. [DOI] [PubMed] [Google Scholar]
- 75.Khani S., Keyhanfar F., Amani A. Design and evaluation of oral nanoemulsion drug delivery system of mebudipine. Drug Deliv. 2016;23:2035–2043. doi: 10.3109/10717544.2015.1088597. [DOI] [PubMed] [Google Scholar]
- 76.Patel R.P., Joshi J.R. An overview on nanoemulsion: A novel approach. Int. J. Pharm. Sci. Res. 2012;3:4640. [Google Scholar]
- 77.Qi J., Zhuang J., Wu W., Lu Y., Song Y., Zhang Z., Jia J., Ping Q. Enhanced effect and mechanism of water-in-oil microemulsion as an oral delivery system of hydroxysafflor yellow A. Int. J. Nanomed. 2011;6:985. doi: 10.2147/IJN.S18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Z., Zhou G., Wang X., Huang E.Z., Zhan X., Liu J., Wang S., Wang A., Li H., Pei X. Preparation, characterization and relative bioavailability of oral elemene o/w microemulsion. Int. J. Nanomed. 2010;5:567. doi: 10.2147/IJN.S12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonifácio B.V., da Silva P.B., dos Santos Ramos M.A., Negri K.M.S., Bauab T.M., Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014;9:1. doi: 10.2147/IJN.S52634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gosh V., Mukherjee A., Chandrasekaran N. Ultrasonic emulsifivation of food-grade nanoemulsion formulation and evulation of its bactericial activity. Ultrason. Sono-Chem. 2013;20:338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Arazmjoo S., Es-haghi A., Mahmoodzadeh H. Evaluation of anti-cancer and antioxidant properties of nanoemulsions synthesized by Nigella sativa L. tincture. Nanomed. J. 2021;8:57–64. [Google Scholar]
- 82.Kildaci L., Budama-Kilinc Y., Kecel-Gunduz S., Altuntas E. Linseed Oil Nanoemulsions for Treatment of Atopic Dermatitis Disease: Formulation, Characterization, In Vitro and In Silico Evaluations. J. Drug Deliv. Sci. Technol. 2021;64:102652. doi: 10.1016/j.jddst.2021.102652. [DOI] [Google Scholar]
- 83.Asgari H.T., Es-haghi A., Karimi E. Anti-angiogenic, antibacterial, and antioxidant activities of nanoemulsions synthesized by Cuminum cyminum L. tinctures. J. Food Meas. Charact. 2021;15:3649–3659. doi: 10.1007/s11694-021-00947-1. [DOI] [Google Scholar]
- 84.Hassanshahian M., Saadatfar A., Masoumipour F. Formulation and characterization of nanoemulsion from Alhagi maurorum essential oil and study of its antimicrobial, antibiofilm, and plasmid curing activity against antibiotic-resistant pathogenic bacteria. J. Environ. Health Sci. Eng. 2020;18:1015–1027. doi: 10.1007/s40201-020-00523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pengon S., Chinatangkul N., Limmatvapirat C., Limmatvapirat S. Development of Antimicrobial Nanoemulsions Containing Nelumbo nucifera Extract. Key Eng. Mater. 2020;859:226–231. doi: 10.4028/www.scientific.net/KEM.859.226. [DOI] [Google Scholar]
- 86.Mohammadifar M., Aarabi M.H., Aghighi F., Kazemi M., Vakili Z., Memarzadeh M.R., Talaei S.A. Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: Behavioral, biochemical, and histopathological evidence. BMC Complementary Med. Ther. 2021;21:57. doi: 10.1186/s12906-021-03236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moazeni M., Davari A., Shabanzadeh S., Akhtari J., Saeedi M., Mortyeza-Semnani K., Abastabar M., Nabili M., Moghadam F.H., Roohi B. In vitro antifungal activity of Thymus vulgaris essential oil nanoemulsion. J. Herb. Med. 2021;28:100452. doi: 10.1016/j.hermed.2021.100452. [DOI] [Google Scholar]
- 88.Cossetin L.F., Garlet Q.I., Velho M.C., Gündel S., Ourique A.F., Heinzmann B.M., Monteiro S.G. Development of nanoemulsions containing Lavandula dentata or Myristica fragrans essential oils: Influence of temperature and storage period on physical-chemical properties and chemical stability. Ind. Crops Prod. 2021;160:113115. doi: 10.1016/j.indcrop.2020.113115. [DOI] [Google Scholar]
- 89.Ganesan P., Narayanasamy D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017;6:37–56. doi: 10.1016/j.scp.2017.07.002. [DOI] [Google Scholar]
- 90.Robson A.-L., Dastoor P.C., Flynn J., Palmer W., Martin A., Smith D.W., Woldu A., Hua S. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front. Pharmacol. 2018;9:80. doi: 10.3389/fphar.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song Z., Lin Y., Zhang X., Feng C., Lu Y., Gao Y., Dong C. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: Enhanced cellular uptake and improved therapeutic effects. Int. J. Nanomed. 2017;12:1941. doi: 10.2147/IJN.S125573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong S.-S., Kim S.H., Lim S.-J. Effects of triglycerides on the hydrophobic drug loading capacity of saturated phosphatidylcholine-based liposomes. Int. J. Pharm. 2015;483:142–150. doi: 10.1016/j.ijpharm.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 93.Aqil F., Munagala R., Jeyabalan J., Vadhanam M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133–141. doi: 10.1016/j.canlet.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thangapazham R.L., Puri A., Tele S., Blumenthal R., Maheshwari R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008;32:1119–1123. doi: 10.3892/ijo.32.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li N., Feng L., Tan Y., Xiang Y., Zhang R., Yang M. Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after iv administration in rats. Molecules. 2018;23:1747. doi: 10.3390/molecules23071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X., Guan Q., Chen W., Hu X., Li L. Novel nanoliposomal delivery system for polydatin: Preparation, characterization, and in vivo evaluation. Drug Des. Dev. Ther. 2015;9:1805. doi: 10.2147/DDDT.S77615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hong S.-S., Choi J.Y., Kim J.O., Lee M.-K., Kim S.H., Lim S.-J. Development of paclitaxel-loaded liposomal nanocarrier stabilized by triglyceride incorporation. Int. J. Nanomed. 2016;11:4465. doi: 10.2147/IJN.S113723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kannan V., Balabathula P., Divi M.K., Thoma L.A., Wood G.C. Optimization of drug loading to improve physical stability of paclitaxel-loaded long-circulating liposomes. J. Liposome Res. 2015;25:308–315. doi: 10.3109/08982104.2014.995671. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., Wang S., Firempong C.K., Zhang H., Wang M., Zhang Y., Zhu Y., Yu J., Xu X. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: Preparation and in vitro and in vivo evaluations. Aaps Pharmscitech. 2017;18:586–594. doi: 10.1208/s12249-016-0537-8. [DOI] [PubMed] [Google Scholar]
- 100.Yi C., Fu M., Cao X., Tong S., Zheng Q., Firempong C.K., Jiang X., Xu X., Yu J. Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. J. Agric. Food Chem. 2013;61:5961–5971. doi: 10.1021/jf3055278. [DOI] [PubMed] [Google Scholar]
- 101.Jangde R., Singh D. Preparation and optimization of quercetin-loaded liposomes for wound healing, using response surface methodology. Artif. Cells Nanomed. 2016;44:635–641. doi: 10.3109/21691401.2014.975238. [DOI] [PubMed] [Google Scholar]
- 102.Jadia R., Kydd J., Piel B., Rai P. Liposomes aid curcumin’s combat with cancer in a breast tumor model. Oncomedicine. 2018;3:94–109. doi: 10.7150/oncm.27938. [DOI] [Google Scholar]
- 103.Kongkaneramit L., Aiemsum-ang P., Kewsuwan P. Development of curcumin liposome formulations using polyol dilution method. Songklanakarin J. Sci. Technol. 2016;38:605–610. [Google Scholar]
- 104.Singh H.P., Utreja P., Tiwary A.K., Jain S. Elastic liposomal formulation for sustained delivery of colchicine: In vitro characterization and in vivo evaluation of anti-gout activity. AAPS J. 2009;11:54–64. doi: 10.1208/s12248-008-9078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh A., Vengurlekar P., Rathod S. Design, development and characterization of liposomal neem gel. Int. J. Pharm. Sci. Res. 2014;5:140–148. [Google Scholar]
- 106.Zhu Y., Wang M., Zhang J., Peng W., Firempong C.K., Deng W., Wang Q., Wang S., Shi F., Yu J. Improved oral bioavailability of capsaicin via liposomal nanoformulation: Preparation, in vitro drug release and pharmacokinetics in rats. Arch. Pharmacal Res. 2015;38:512–521. doi: 10.1007/s12272-014-0481-7. [DOI] [PubMed] [Google Scholar]
- 107.Qin X.-q., Yuan Y., Liu C.-s., Wang Q.-y., Shen X., Yang B.-C. Preparation of liposomal brucine and its pharmaceutical/pharmacodynamic characterization. Acta Pharmacol. Sin. 2007;28:1851–1858. doi: 10.1111/j.1745-7254.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- 108.Paliwal S., Sharma J., Tilak A., Sohgaura A., Gupta C., Dave V. Synergistic effect of guggul liposomes lipid nano-vesicles for the treatment of inflammation. Int. J. Life Sci. Bioengg. 2011;6:1–13. [Google Scholar]
- 109.Plangsombat N., Rungsardthong K., Kongkaneramit L., Waranuch N., Sarisuta N. Anti-inflammatory activity of liposomes of Asparagus racemosus root extracts prepared by various methods. Exp. Ther. Med. 2016;12:2790–2796. doi: 10.3892/etm.2016.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mureşan M., Olteanu D., Filip G.A., Clichici S., Baldea I., Jurca T., Pallag A., Marian E., Frum A., Gligor F.G. Comparative Study of the Pharmacological Properties and Biological Effects of Polygonum aviculare L. herba Extract-Entrapped Liposomes versus Quercetin-Entrapped Liposomes on Doxorubicin-Induced Toxicity on HUVECs. Pharmaceutics. 2021;13:1418. doi: 10.3390/pharmaceutics13091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suryawanshi J.S. Phytosome: An emerging trend in herbal drug treatment. J. Med. Genet. Genom. 2011;3:109–114. [Google Scholar]
- 112.Neelam K., Vijay S., Lalit S. Various Techniques For The Modification Of Starch And The Applications Of Its Derivatives. Int. Research. J. Pharm. 2012;3:25–31. [Google Scholar]
- 113.Karthivashan G., Masarudin M.J., Kura A.U., Abas F., Fakurazi S. Optimization, formulation, and characterization of multiflavonoids-loaded flavanosome by bulk or sequential technique. Int. J. Nanomed. 2016;11:3417. doi: 10.2147/IJN.S112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anwar E., Farhana N. Formulation and Evaluation of Phytosome-Loaded Maltodextrin-Gum Arabic Microsphere System for Delivery of Camellia sinensis Extract. J. Young Pharm. 2018;10:S56–S62. doi: 10.5530/jyp.2018.2s.11. [DOI] [Google Scholar]
- 115.Hooresfand Z., Ghanbarzadeh S., Hamishehkar H. Preparation and characterization of rutin-loaded nanophytosomes. Pharm. Sci. 2015;21:145–151. doi: 10.15171/PS.2015.29. [DOI] [Google Scholar]
- 116.El-Menshawe S.F., Ali A.A., Rabeh M.A., Khalil N.M. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: An approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomed. 2018;13:307. doi: 10.2147/IJN.S153429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh R.P., Gangadharappa H., Mruthunjaya K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2018;122:214–229. doi: 10.1016/j.ejps.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 118.Gahandule M., Jadhav S., Gadhave M., Gaikwad D. Formulation and development of hepato-protective butea monosperma-phytosome. Int. J. Res. Pharm. Pharm. Sci. 2016;1:21–27. [Google Scholar]
- 119.Chouhan A., Shah S.K., Tyagi C., Budholiya P., Wazid A., Khan F. Formulation, development and evaluation of phytosomes of swertia perennis. Asian J. Pharm. Educ. Res. 2021;10:55–56. doi: 10.38164/AJPER/10.2.2021.55-66. [DOI] [Google Scholar]
- 120.Murugesan M.P., Ratnam M.V., Mengitsu Y., Kandasamy K. Evaluation of anti-cancer activity of phytosomes formulated from aloe vera extract. Mater. Today Proc. 2021;42:631–636. doi: 10.1016/j.matpr.2020.11.047. [DOI] [Google Scholar]
- 121.Calister U., Lydia U., Edith D., Chioma N., Ben A., Andrew E., Nwokedi U. Formulation and evaluation of Morinda lucida based-phytosome complexes for malaria treatment. Technology. 2020;6:9. [Google Scholar]
- 122.Nagpal N., Arora M., Swami G., Kapoor R. Designing of a phytosome dosage form with Tecomella undulata as a novel drug delivery for better utilization. Pak. Pharm. Sci. 2016;29:1231–1236. [PubMed] [Google Scholar]
- 123.Abdulbaqi I.M., Darwis Y., Abou Assi R., Khan N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Dev. Ther. 2018;12:795. doi: 10.2147/DDDT.S158018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Y.-Z., Lu C.-T., Zhang Y., Xiao J., Zhao Y.-P., Tian J.-L., Xu Y.-Y., Feng Z.-G., Xu C.-Y. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int. J. Pharm. 2013;454:302–309. doi: 10.1016/j.ijpharm.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 125.Khaled S.A., Burley J.C., Alexander M.R., Roberts C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014;461:105–111. doi: 10.1016/j.ijpharm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 126.Fatima Z. Formulation and Performance evaluation of Berberis aristata extract loaded ethosomal gel. Asian J. Pharm. 2017;11:1401. [Google Scholar]
- 127.Yu Z., Lv H., Han G., Ma K. Ethosomes loaded with cryptotanshinone for acne treatment through topical gel formulation. PLoS ONE. 2016;11:e0159967. doi: 10.1371/journal.pone.0159967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumar P., Sharma D.K., Ashawat M.S. Topical creams of piperine loaded lipid nanocarriers for management of atopic dermatitis: Development, characterization, and in vivo investigation using BALB/c mice model. J. Liposome Res. 2021;32:62–73. doi: 10.1080/08982104.2021.1880436. [DOI] [PubMed] [Google Scholar]
- 129.Andleeb M., Shoaib Khan H.M., Daniyal M. Development, Characterization and Stability Evaluation of Topical Gel Loaded With Ethosomes Containing Achillea millefolium L. Extract. Front. Inppharmacol. 2021;12:336. doi: 10.3389/fphar.2021.603227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mota A.H., Prazeres I., Mestre H., Bento-Silva A., Rodrigues M.J., Duarte N., Serra A.T., Bronze M.R., Rijo P., Gaspar M.M. A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract. Pharmaceuticals. 2021;14:467. doi: 10.3390/ph14050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagalakshmi S., Krishnaraj K., Jothy A.M., Chaudhari P.S., Pushpalatha H., Shanmuganthan S. Fabrication and characterization of herbal drug-loaded nonionic surfactant based niosomal topical gel. J. Pharm. Sci. Res. 2016;8:1271. [Google Scholar]
- 132.Seleci D.A., Seleci M., Demir B., Timur S. Phyto-Niosomes: In Vitro Assessment of the Novel Nanovesicles Containing Marigold Extract. Int. J. Polym. Mater. Polym. Biomater. 2015;64:927–937. [Google Scholar]
- 133.Rohilla R., Garg T., Goyal A.K., Rath G. Herbal and polymeric approaches for liver-targeting drug delivery: Novel strategies and their significance. Drug Deliv. 2016;23:1645–1661. doi: 10.3109/10717544.2014.945018. [DOI] [PubMed] [Google Scholar]
- 134.Ambwani S., Tandon R., Ambwani T.K., Malik Y.S. Current knowledge on nanodelivery systems and their beneficial applications in enhancing the efficacy of herbal drugs. J. Exp. Biol. Agric. Sci. 2018;6:87–107. doi: 10.18006/2018.6(1).87.107. [DOI] [Google Scholar]
- 135.Rameshk M., Sharififar F., Mehrabani M., Pardakhty A., Farsinejad A., Mehrabani M. Proliferation and in vitro wound healing effects of the Microniosomes containing Narcissus tazetta L. bulb extract on primary human fibroblasts (HDFs) DARU J. Pharm. Sci. 2018;26:31–42. doi: 10.1007/s40199-018-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anghore D., Kulkarni G.T. Development of novel nano niosomes as drug delivery system of spermacoce hispida extract and in vitro antituberculosis activity. Curr. Nanomater. 2017;2:17–23. doi: 10.2174/2405461502666170314151949. [DOI] [Google Scholar]
- 137.Scognamiglio I., De Stefano D., Campani V., Mayol L., Carnuccio R., Fabbrocini G., Ayala F., La Rotonda M.I., De Rosa G. Nanocarriers for topical administration of resveratrol: A comparative study. Int. J. Pharm. 2013;440:179–187. doi: 10.1016/j.ijpharm.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 138.Barani M., Mirzaei M., Torkzadeh-Mahani M., Nematollahi M.H. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: A Nano-herbal treatment for Cancer. DARU J. Pharm. Sci. 2018;26:11–17. doi: 10.1007/s40199-018-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Budhiraja A., Dhingra G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015;22:723–730. doi: 10.3109/10717544.2014.903010. [DOI] [PubMed] [Google Scholar]
- 140.Kaur H., Mohanta G.C., Gupta V., Kukkar D., Tyagi S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017;41:106–112. doi: 10.1016/j.jddst.2017.07.004. [DOI] [Google Scholar]
- 141.Rizwan S.B., Boyd B.J. Subunit Vaccine Delivery. Springer; Berlin/Heidelberg, Germany: 2015. Cubosomes: Structure, preparation and use as an antigen delivery system; pp. 125–140. [Google Scholar]
- 142.Garg M., Goyal A., Kumari S. An Update on the Recent Advances in Cubosome: A Novel Drug Delivery System. Curr. Drug Metab. 2021;22:441–450. doi: 10.2174/1389200221666210105121532. [DOI] [PubMed] [Google Scholar]
- 143.Tian Y., Li J.-c., Zhu J.-x., Zhu N., Zhang H.-m., Liang L., Sun L. Folic acid-targeted etoposide cubosomes for theranostic application of cancer cell imaging and therapy. Med. Sci. Int. Med. J. Exp. Clin. Res. 2017;23:2426. doi: 10.12659/MSM.904683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Azmi I.D., Moghimi S.M., Yaghmur A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015;6:1347–1364. doi: 10.4155/tde.15.81. [DOI] [PubMed] [Google Scholar]
- 145.Bazylińska U., Kulbacka J., Schmidt J., Talmon Y., Murgia S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid Sci. 2018;522:163–173. doi: 10.1016/j.jcis.2018.03.063. [DOI] [PubMed] [Google Scholar]
- 146.Elnaggar Y.S., Etman S.M., Abdelmonsif D.A., Abdallah O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015;10:5459. doi: 10.2147/IJN.S87336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Archana A., Vijayasri K., Madhurim M., Kumar C. Curcumin loaded nano cubosomal hydrogel: Preparation, in vitro characterization and antibacterial activity. Chem. Sci. Trans. 2015;4:75–80. [Google Scholar]
- 148.Ou N., Sun Y., Zhou S., Gu P., Liu Z., Bo R., Hu Y., Liu J., Wang D. Evaluation of optimum conditions for Achyranthes bidentata polysaccharides encapsulated in cubosomes and immunological activity in vitro. Int. J. Biol. Macromol. 2018;109:748–760. doi: 10.1016/j.ijbiomac.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 149.Peng X., Zhou Y., Han K., Qin L., Dian L., Li G., Pan X., Wu C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Dev. Ther. 2015;9:4209. doi: 10.2147/DDDT.S86370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abdel-Kawy M.A., Michel C.G., Kirollos F.N., Hussien R.A., Al-Mahallawi A.M., Sedeek M.S. Chemical composition and potentiation of insecticidal and fungicidal activities of Citrus trifoliata L. fruits essential oil against Spodoptera littoralis, Fusarium oxysporum and Fusarium solani via nano-cubosomes. Nat. Prod. Res. 2021;35:2438–2443. doi: 10.1080/14786419.2019.1675063. [DOI] [PubMed] [Google Scholar]
- 151.Mahtab R., Rogers J.P., Murphy C.J. Protein-sized quantum dot luminescence can distinguish between “straight”, “bent”, and “kinked” oligonucleotides. J. Am. Chem. Soc. 1995;117:9099–9100. doi: 10.1021/ja00140a040. [DOI] [Google Scholar]
- 152.Ma J., Wong H., Kong L.B., Peng K.W. Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology. 2003;14:619–623. doi: 10.1088/0957-4484/14/6/310. [DOI] [Google Scholar]
- 153.Yoshida J., Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes. J. Magn. Magn. Mater. 1999;194:176–184. doi: 10.1016/S0304-8853(98)00586-1. [DOI] [Google Scholar]
- 154.Molday R.S., MacKenzie D. Immunospecific ferromagnetic irondextran reagents for the labeling and magnetic separation of cells. J. Immunol. Methods. 1982;52:353–367. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- 155.Weissleder R., Elizondo G., Wittenberg J., Rabito C.A., Bengele H.H., Josephson L. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 156.Parak W.J., Boudreau R., Le Gros M., Gerion D., Zanchet D., Micheel C., Williams S., Alivisatos A., Larabell C. Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Adv. Mater. 2002;14:882–885. doi: 10.1002/1521-4095(20020618)14:12<882::AID-ADMA882>3.0.CO;2-Y. [DOI] [Google Scholar]
- 157.Salata O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnology. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alok A., Sunil P., Aggarwal A., Upadhyay N., Agarwal N., Kishore. M. Nanotechnology: A boon in oral cancer diagnosis and therapeutics. SRM J. Res. Dent. Sci. 2013;4:154–160. doi: 10.4103/0976-433X.125591. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available within the article.