Abstract
“Dehalococcoides ethenogenes” 195 can reductively dechlorinate tetrachloroethene (PCE) completely to ethene (ETH). When PCE-grown strain 195 was transferred (2% [vol/vol] inoculum) into growth medium amended with trichloroethene (TCE), cis-dichloroethene (DCE), 1,1-DCE, or 1,2-dichloroethane (DCA) as an electron acceptor, these chlorinated compounds were consumed at increasing rates over time, which indicated that growth occurred. Moreover, the number of cells increased when TCE, 1,1-DCE, or DCA was present. PCE, TCE, 1,1-DCE, and cis-DCE were converted mainly to vinyl chloride (VC) and then to ETH, while DCA was converted to ca. 99% ETH and 1% VC. cis-DCE was used at lower rates than PCE, TCE, 1,1-DCE, or DCA was used. When PCE-grown cultures were transferred to media containing VC or trans-DCE, products accumulated slowly, and there was no increase in the rate, which indicated that these two compounds did not support growth. When the intermediates in PCE dechlorination by strain 195 were monitored, TCE was detected first, followed by cis-DCE. After a lag, VC, 1,1-DCE, and trans-DCE accumulated, which is consistent with the hypothesis that cis-DCE is the precursor of these compounds. Both cis-DCE and 1,1-DCE were eventually consumed, and both of these compounds could be considered intermediates in PCE dechlorination, whereas the small amount of trans-DCE that was produced persisted. Cultures grown on TCE, 1,1-DCE, or DCA could immediately dechlorinate PCE, which indicated that PCE reductive dehalogenase activity was constitutive when these electron acceptors were used.
The solvents tetrachloroethene (PCE) and trichloroethene (TCE) are among the most pervasive pollutants at contaminated groundwater sites. Under aerobic conditions, PCE is considered nonbiodegradable, while TCE can be broken down to mainly nontoxic products by certain nonspecific oxygenases, such as methane monooxygenase or toluene dioxygenase (5, 14). Under anaerobic conditions, PCE and TCE have been reductively dechlorinated by mixed cultures to less-chlorinated ethenes (30) and, under certain conditions, to the nontoxic products ethene (ETH) (4, 10, 19, 26) and ethane (2).
Pure cultures of various anaerobes have been shown to reductively dechlorinate PCE and TCE. Methanogens, acetogens, and sulfate-reducing bacteria contain reduced transition metal cofactors, such as corrinoids, hemes, and cofactor F430, which can reductively dechlorinate chloroethenes in an essentially cometabolic process (1, 6, 7, 11, 15). Workers have described several anaerobes which can use PCE as a respiratory electron acceptor and reduce it as far as cis-dichloroethene (cis-DCE) (12, 13, 17, 24, 27, 28).
We have studied an anaerobic enrichment culture which can reductively dechlorinate PCE to ETH (3, 4, 8, 10, 23). More recently, we isolated an organism from this culture, tentatively named “Dehalococcoides ethenogenes” 195, which can completely dechlorinate PCE to ETH (21). Based on its 16S ribosomal DNA sequence, strain 195 clustered phylogenetically with the eubacteria but did not fall in any of the previously described eubacterial branches. This organism had a complex nutrition, requiring extracts of mixed dechlorinating cultures for growth. It used a restricted range of substrates; only H2 was used as an electron donor, and certain chloroethenes and 1,2-dichloroethane (DCA) were used as electron acceptors (21).
Growth of strain 195 on PCE was examined in detail in a previous study (21). The doubling time during growth on PCE was approximately 19.2 h, the growth yield was 4.8 ± 0.3 g of protein per mol of chloride released, and the specific activity was 69.0 ± 10.5 nmol of chloride released per min per mg of protein. Cultures that received H2 but not PCE did not exhibit significant growth. A time course for utilization of a dose of PCE by a culture which had received several previous doses of PCE showed that PCE was converted stoichiometrically to vinyl chloride (VC) with essentially zero-order kinetics and with little accumulation of TCE or DCE isomers as intermediates. VC conversion to ETH began only when PCE was depleted, and VC disappearance could be described by first-order kinetics. This substrate utilization pattern resembled that of the original enrichment culture (29) except that VC utilization was slower relative to PCE consumption in the pure culture.
In the previous study (21), no quantitative data on chloroethene or DCA utilization by strain 195 were presented. In this study, we demonstrated that strain 195 is able to use certain chloroethenes and DCA as electron acceptors. We also examined the accumulation of intermediates during PCE and TCE dechlorination and paid particular attention to DCE isomers whose fates were not resolved in the previous analyses. Finally, we examined the ability of cells grown on less-chlorinated substrates to use PCE and other chloroethenes.
MATERIALS AND METHODS
Chemicals and analyses of chloroethenes.
PCE, other chlorinated ethenes, ETH, H2, and other chemicals were purchased and utilized as described previously (23). DCA was purchased from Aldrich Chemical Co. (Milwaukee, Wis.).
For quantitative analysis of chloroethenes, DCA, and ETH, headspace gas samples (100 μl) were analyzed by using a temperature-programmed Perkin-Elmer model 8500 gas chromatograph (GC) equipped with a flame ionization detector. The GC contained a type RTX-502.2 capillary column (60 m by 0.53 mm) that was operated in the splitless injection mode (film thickness, 3 μm; Restek Corp., Bellefonte, Pa.). The carrier gas utilized was helium at a flow rate of 10 ml/min. Peak areas were calculated by using the software supplied with the GC and were compared to standard curves.
Growth medium and culture conditions.
Cultures were incubated in anaerobic culture tubes (total volume, 27 ml) containing 10 ml (final volume) of growth medium and sealed with Teflon-coated butyl rubber stoppers as previously described (23). The basal salts medium utilized for growth of strain 195 was amended with 2 mM acetate, a vitamin solution containing 0.05 mg of vitamin B12 per liter, and 25% (vol/vol) filter-sterilized anaerobic digestor sludge supernatant, as described previously (23).
The cultures also received 5% (vol/vol) of an extract from a mixed culture grown on butyrate-PCE (8, 9), which replaced the extract from the mixed H2-PCE containing culture described previously (21). To prepare the extract, the culture was centrifuged for 20 min at 14,460 × g. The pellet was resuspended in distilled water so that the cells were concentrated 50-fold, and the suspension was frozen at −20°C until it was used. After the suspension was thawed at room temperature, it was passed through a French pressure cell at 20,000 lb/in2 and centrifuged at 34,800 × g for 20 min. The pellet was discarded, and the supernatant was then purged with N2 for 10 min, filter sterilized inside an anaerobic glove box by using a 25-mm-diameter Acrodisc combined filter (pore sizes, 0.8 and 0.2 μm; Gelman Sciences, Ann Arbor, Mich.), and transferred into an autoclaved vial with a 70%N2–30%CO2 atmosphere. NaHCO3 (final concentration, 1 g/liter) and Na2S · 7H2O (final concentration, 0.5 g/liter) were then added to buffer and reduce the extract before it was frozen and stored at −20°C until it was used. After thawing, the extract was kept at 4°C and then discarded after 4 weeks.
Unless otherwise stated, the inoculum size was 2% (vol/vol), all incubations were done in duplicate, and each experiment was performed at least twice and produced similar results. Culture tubes were incubated upside down in the dark in a model R76 incubator-shaker (New Brunswick Scientific, Edison, N.J.) operated at 35°C and 150 rpm. H2 was added to the headspace as overpressure (67 kPa; ca. 47.5 mmol/liter) immediately after inoculation. After several doses of PCE were consumed, NaHCO3 was added to neutralize the HCl produced by the dechlorination process (29), and H2 was added to replenish the headspace gas. The quantity of PCE added to a culture tube was estimated directly from the syringe volume delivered because a few hours was required for PCE to dissolve and equilibrate with the headspace (23).
Cells were counted by using a Petroff-Hauser chamber after they were stained with 4 mg of acridine orange per liter and viewed with an epifluorescence microscope as described previously (18).
RESULTS
Use of chloroethenes and DCA as electron acceptors.
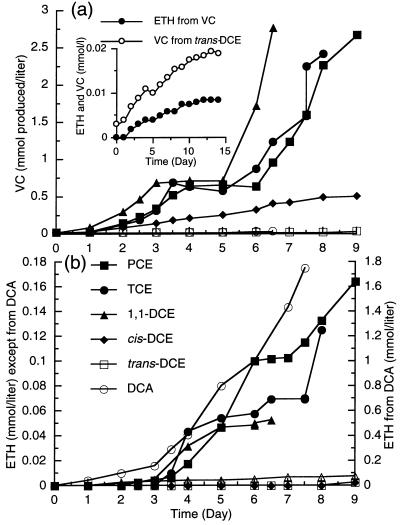
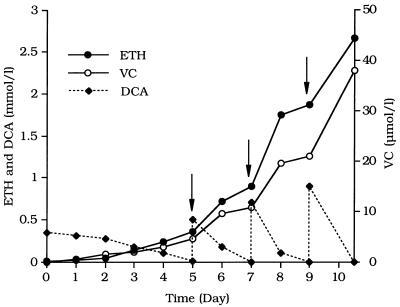
PCE-grown cultures of strain 195 were transferred into fresh medium containing H2 as the electron donor and one of the chlorinated ethenes or DCA (0.3 mmol per liter of growth medium) as an electron acceptor, and the products were monitored during incubation (Fig. 1). Cultures fed PCE, TCE, or 1,1-DCE consumed these compounds within 2.5 days, converting them mainly to VC at increasing rates. After the first doses consisting of 0.3 mmol of electron acceptor per liter were consumed, the cultures received second doses consisting of 0.5 mmol per liter, which were consumed within 3 to 4 days. After consuming the second doses, the cultures were not given electron acceptors for 2 days in order to examine whether there was an increase in the rate of VC conversion to ETH in the absence of substrates. It is known that high PCE concentrations depress ETH formation from VC by strain 195 (21). With PCE, TCE, and 1,1-DCE, the amount of ETH increased during the 2-day period (Fig. 1b), which is consistent with the finding that all three substrates inhibit VC conversion to ETH. The cultures then consumed two more doses (0.7 and 0.9 mmol per liter) of electron acceptors (Fig. 1). DCA was also utilized at increasing rates, and the primary product was ETH.
FIG. 1.
(a) VC formation by cultures that were inoculated with PCE-grown strain 195 and received H2 and one of the chloroethenes or DCA. Substrates were added in increasing incremental doses beginning with 0.3 mmol per liter. The total amounts of the substrates utilized by the cultures were as follows: PCE, 2.5 mmol/liter; TCE, 2.5 mmol/liter; 2.5 cis-DCE, 0.67 mmol/liter; trans-DCE, 0.07 mmol/liter; 1,1-DCE, 2.85 mmol/liter; VC, 0.17 mmol/liter; and DCA, 1.8 mmol/liter. (Inset) Plot with an expanded scale, showing VC formation from trans-DCE and ETH formation from VC. (b) ETH production by the cultures shown in panel a. Note the expanded scale for ETH formation from DCA.
Cultures grown with PCE, TCE, 1,1-DCE, or DCA could be transferred at least two additional times into fresh medium containing the same electron acceptor, and these cultures also exhibited increasing rates of substrate utilization, which indicated that growth occurred (data not shown). Most cultures were transferred several more times and used in subsequent experiments. Cultures which received H2 and 0.3-, 0.5-, and 0.7-mmol/liter doses of TCE, 1,1-DCE, and DCA contained 7.0 × 106 ± 0.1 × 106, 5.4 × 106 ± 0.3 × 106, and 5.8 × 106 ± 0.6 × 106 cells of strain 195 per ml (mean ± standard deviation), respectively, while cultures that received H2 and no electron acceptor contained only 0.1 × 106 ± 0.07 × 106 cells per ml, a value similar to the inoculum value, which demonstrated that strain 195 grew on these substrates. Because uncoupling of substrate utilization and growth can occur in strain 195 (21), these single-point growth yields should not be directly compared.
Cultures transferred to medium containing cis-DCE (0.3 mmol per liter) used this compound much more slowly than cultures used the electron acceptors described above, and cis-DCE was converted mainly to VC (Fig. 1a). After an initial increase in the rate, VC production became linear during utilization of the first dose, and a second dose consisting of 0.5 mmol of cis-DCE per liter was not completely consumed by the cultures. Further studies indicated that the cis-DCE preparation used was toxic, a phenomenon which is being investigated (22).
Cultures transferred to medium amended with trans-DCE or VC metabolized these compounds at negligible rates compared to the rates of metabolism of the other electron acceptors and never consumed the first dose of electron acceptor. The inset in Fig. 1a shows the data for trans-DCE and VC with an expanded scale and demonstrates that these substrates were reductively dechlorinated at detectable, albeit low, rates and that the rates never increased, which indicated that the cultures using these two potential electron acceptors did not grow. Uninoculated controls did not produce detectable amounts of dechlorination products in this and other experiments (data not shown). Thus, the extracts of cultures provided as nutrients in the culture medium did not dechlorinate at significant rates.
PCE and TCE metabolism to ETH: intermediate formation.
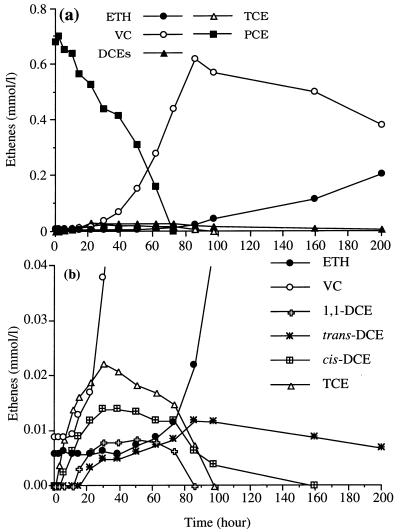
Figure 2a shows the dechlorination intermediates found in cultures which received a high initial dose of PCE (0.7 mmol per liter instead of 0.3 mmol per liter) to encourage intermediate formation. As shown previously (21, 29), PCE was converted mainly to VC, and VC conversion to ETH occurred after the PCE was depleted. Figure 2b, which has an expanded scale and includes all DCE isomers, shows the sequence and levels at which these intermediates and ETH accumulated. TCE was present within 2 h of inoculation and was produced before any DCE isomer was produced. cis-DCE appeared within 5.5 h; the 1,1-DCE and VC concentrations increased compared with the concentrations initially present in the inoculum within 14.5 h; and trans-DCE appeared at 21.5 h. Once PCE had been consumed (after about 72 h), most of the intermediates rapidly disappeared; the only exception was trans-DCE, which persisted for the duration of the experiment. The ETH concentration began to increase from the initial level by 40 h, and the rate of appearance increased once PCE was consumed.
FIG. 2.
(a) Product formation by PCE-grown strain 195 inoculated into medium to which one dose of PCE (0.7 mmol/liter) was added. (b) Plot with an expanded scale, showing intermediates in PCE metabolism, including individual DCE isomers.
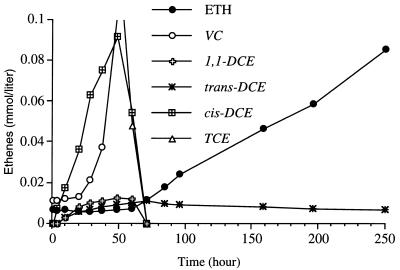
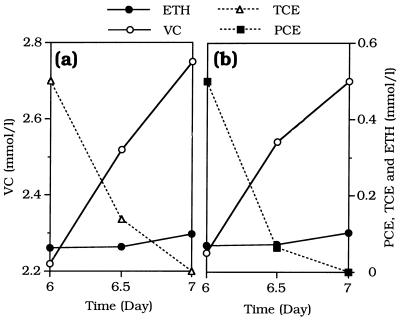
Figure 3 shows the sequence of formation and levels of the intermediates formed after an initial dose of TCE (0.35 mmol per liter) was added to a culture of strain 195. There was considerably greater accumulation of cis-DCE than there was in the PCE-grown culture, whereas the amounts of the other DCE isomers that accumulated were similar to the amounts that accumulated in PCE-grown cultures. The order of formation of intermediates was the same as it was for PCE, and trans-DCE persisted after TCE and the other DCE isomers were consumed.
FIG. 3.
Intermediate formation by TCE-grown strain 195 inoculated into medium to which a single dose consisting of 0.35 mmol of TCE per liter was added.
trans-DCE utilization.
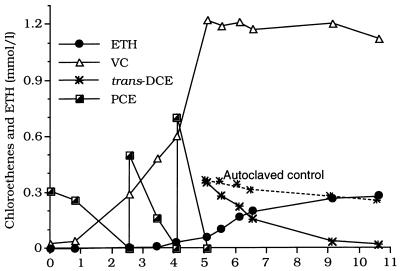
We examined whether a culture fed three doses of PCE could metabolize a relatively large dose of trans-DCE (0.35 mmol per liter) (Fig. 4). In this culture, trans-DCE disappeared with first-order kinetics for which a semilogarithmic plot (data not shown) predicted a half-life of 28.8 h with an r2 value of 0.996. During this time, ETH accumulated at nearly the same rate as the rate at which trans-DCE was utilized, and the VC concentration remained nearly constant at the steady-state concentration until DCE was depleted, after which it decreased. Thus, trans-DCE dechlorination and VC dechlorination occurred simultaneously, and therefore trans-DCE did not appear to inhibit VC dechlorination, in contrast to dechlorination of the other chloroethenes. In cultures of strain 195 that were autoclaved immediately before they received a trans-DCE dose, there was only a small decrease in the trans-DCE concentration (Fig. 4), which indicated that the losses detected in nonautoclaved cultures were not due to absorption to the stopper or to abiological reactions. No products of trans-DCE dechlorination were detected in autoclaved cultures.
FIG. 4.
Product formation from a 0.35-mmol/liter dose of trans-DCE after cultures of strain 195 were fed three consecutive doses of PCE (0.3, 0.5, and 0.7 mmol/liter). DCE utilization by a culture that was autoclaved after day 5 is also shown (dashed line).
DCA utilization.
Strain 195 was able to dechlorinate DCA as a sole electron acceptor (Fig. 1) and could be transferred on the same substrate without any acclimation phase. As shown in Fig. 5, DCA was dechlorinated at an increasing rate by a culture of strain 195 that had been transferred twice previously with DCA as the electron acceptor. ETH was the primary product of DCA utilization, and VC production from DCA was approximately 1% of the ETH production, with the relative proportion remaining constant throughout the incubation period. No monochloroethane was detected in cultures utilizing DCA.
FIG. 5.
Product formation by strain 195 growing on DCA. Note the differences in the scales for VC and ETH.
Abilities of cultures grown on TCE, 1,1-DCE, and DCA to use PCE and other chloroethenes.
Whereas a culture grown on PCE would be expected to be able to use TCE, since the latter is a likely intermediate in the PCE dechlorination pathway, a TCE-grown culture may not necessarily be able to use PCE, since PCE is not an intermediate in TCE reductive dechlorination. Indeed, the recent discovery of separate PCE and TCE reductive dehalogenases in the enrichment culture from which strain 195 was derived (20) makes this possibility feasible. Similarly, it is possible that DCA-grown cells may not use chloroethenes at all if DCA is dechlorinated by an enzyme that is different from the enzymes used for chloroethene dechlorination. We examined the abilities of cultures derived from cultures grown with TCE and 1,1-DCE to use substrates with equal or greater numbers of chlorines and cultures grown on DCA to use various rapidly utilized chloroethenes. Cultures were given three increasing doses of an electron acceptor (0.3, 0.5, and 0.7 mmol per liter) and then either a fourth dose consisting of 0.5 mmol of the electron acceptor per liter or the same concentration of another electron acceptor. Figure 6 shows the results of an experiment in which we examined the ability of TCE-grown cells to use PCE and TCE. PCE was used slightly more rapidly than TCE was used, and both electron acceptors were converted mainly to VC.
FIG. 6.
TCE (a) and PCE (b) utilization and VC formation by TCE-grown cultures of strain 195. The cultures received three previous doses of TCE, which was converted mainly to VC (note the different scale for VC).
Table 1 shows the rates determined from the initial linear portions of graphs similar to the graphs in Fig. 6 for TCE, 1,1-DCE, and DCA. The cultures were able to use substrates other than the growth substrates at rates that were at least 62% of the rates of utilization of the growth substrates and were often much closer to 100%. Thus, the ability to use PCE, for example, appeared to be constitutive under the conditions used.
TABLE 1.
Relative rates of reductive dechlorination of growth substrates or alternative substrates, expressed as percentages of the rates obtained with the growth substratesa
| Growth substrate | Relative rates of substrate utilization (%)
|
||||
|---|---|---|---|---|---|
| PCE | TCE | cis-DCE | 1,1-DCE | DCA | |
| TCE | 133 (12)b | 100 (7) | NDc | ND | ND |
| 1,1-DCE | 79 (9) | 89 (8) | 105 (5) | 100 (11) | ND |
| DCA | 78 (10) | 108 (6) | 62 (4) | 62 (6) | 100 (8) |
The cultures received three doses of a growth substrate, followed by 0.5 mmol of the same growth substrate or another growth substrate per liter.
The values in parentheses are standard deviations.
ND, not determined.
DISCUSSION
A PCE-grown inoculum of strain 195 readily carried out reductive dechlorination of TCE, cis-DCE, 1,1-DCE, and DCA in liquid cultures. The rates of utilization of these compounds increased over time, which indicated that growth occurred. Moreover, cultures could be transferred at least three times with any of these compounds as the sole electron acceptor in a manner similar to cultures amended with PCE, which clearly has been shown to support growth as an electron acceptor, as measured by microscopic cell counting and increases in cellular protein (21). We detected increases in numbers of cells in cultures receiving TCE, 1,1-DCE, and DCA, which demonstrated that these compounds can serve as electron acceptors for growth.
The primary dechlorination product obtained from PCE, TCE, cis-DCE, and 1,1-DCE was VC. When TCE or 1,1-DCE was withheld after the initial consumption (Fig. 1), ETH began to accumulate more rapidly, as was observed for PCE (21). The appearance of VC was initially most rapid in tubes amended with 1,1-DCE; however, 1,1-DCE reduction to VC requires only two electrons, while reduction of TCE and reduction of PCE require four and six electrons, respectively. Dechlorination of cis-DCE was slow compared to dechlorination of the other substrates utilized, apparently due to toxicity of the cis-DCE preparation. Studies are under way to investigate this phenomenon further.
In contrast to the compounds discussed above, trans-DCE and VC were not rapidly utilized by cultures inoculated with strain 195, and the rates of utilization of these compounds did not increase over time. These two substrates were used with first-order kinetics in short-term incubations by active methanol-PCE-grown enrichment cultures, as described by Tandoi et al. (29). VC was used with first-order kinetics in our previous study of strain 195 (21), and trans-DCE was used with first-order kinetics by strain 195 in this study (Fig. 5). First-order kinetics are typical of cometabolic reactions in which catalysis is carried out by chemical reactions involving constituents in the cell or by enzymes poorly adapted to the substrate such that the apparent Ks value is greater than the substrate concentration. Indeed, reductive dechlorination by reduced transition metal cofactors, such as vitamin B12, heme, and cofactor F430, follows first-order kinetics (11). In contrast, the other chloroethenes and DCA disappeared with zero-order kinetics (linearly) in the study of the methanol-PCE enrichment culture, which indicated that there was catalysis by enzymes with low apparent Ks values relative to the concentrations.
We concluded, therefore, that neither trans-DCE nor VC could serve as an electron acceptor for growth of strain 195 and that utilization of these compounds is apparently cometabolic. It is interesting that trans-DCE and VC are the only chloroethenes which lack adjacent chlorines. An examination of the structures of these compounds with molecular modeling software (Chem 3D; CambridgeSoft Corp., Cambridge, Mass.) showed that there is considerable steric interaction between adjacent chlorines either on the same carbon, as in 1,1-DCE, or on different carbons, as in cis-DCE.
The sequence of the appearance of intermediates during PCE dechlorination to ETH (Fig. 2) is consistent with a pathway that includes TCE, cis-DCE, and VC. 1,1-DCE and trans-DCE accumulated only after cis-DCE appeared, which suggests that cis-DCE is the precursor of 1,1-DCE and trans-DCE or perhaps must be present for the other DCE forms to appear. 1,1-DCE disappeared at the same time that PCE, TCE, and cis-DCE disappeared, which indicated that 1,1-DCE was turned over. Therefore, 1,1-DCE may be a quantitatively significant intermediate in reductive dechlorination of PCE by strain 195. It has been shown that 1,1-DCE is formed chemically from TCE (16) and from 1,1,1-trichloroethane (31). In contrast, trans-DCE persisted after other compounds containing more than one chlorine were consumed, which indicated that it was not turned over and was not a significant intermediate. The persistence of trans-DCE may explain why it, rather than cis-DCE, was detected in previous studies (10) of the mixed dechlorinating culture from which strain 195 was derived.
Considerably more cis-DCE accumulated as an intermediate in cultures grown on TCE (Fig. 4) than in cultures grown on PCE, whereas other dichloroethenes accumulated to similar levels. There was also greater accumulation of DCE from TCE than from PCE in the enrichment culture from which strain 195 was derived (29), but DCE isomers were not resolved by the GC column used in that study. As was the case for PCE, cis-DCE and 1,1-DCE disappeared when TCE was depleted, while trans-DCE persisted.
The greater accumulation of cis-DCE from TCE than from PCE may be explained the finding that TCE and cis-DCE compete for an enzymatic binding site that PCE does not compete for. In a recent study Magnuson et al. (20) examined dechlorinating enzymes purified from the methanol-PCE enrichment culture from which strain 195 was derived and in which it is a major constituent (29). They found that there was a PCE reductive dehalogenase which reduced PCE only as far as TCE and a TCE reductive dehalogenase which reduced TCE, cis-DCE, and 1,1-DCE to VC and slowly reduced trans-DCE to VC and VC to ETH. The TCE reductive dehalogenase did not utilize PCE. Thus, both TCE and cis-DCE could compete for the same site in the TCE reductive dehalogenase.
DCA is the only compound that is not a chloroethene that was used as an electron acceptor by strain 195 (21). As observed in studies of the enrichment culture from which strain 195 was derived, DCA was dechlorinated mainly to ETH, a reductive dihalo elimination reaction (25). Small amounts of VC were also produced from DCA, which indicated that there was a dehydrochlorination reaction that led to these products (25).
The ability of cells grown with one electron acceptor to use another electron acceptor with the same number or a greater number of chlorines was examined (Table 1). If it is assumed that strain 195 has separate PCE and TCE reductive dehalogenases (20), then TCE-grown cells might not possess a PCE reductive dehalogenase. However, in the cases tested, even for the nonchloroethene compound DCA, PCE utilization was constitutive, and there were only minor variations in the other activities measured. It is interesting that the rates were all rather similar, since PCE dechlorination to VC, the primary product in these studies (Fig. 2), requires six electrons, while TCE reduction to VC requires four electrons and DCE dechlorination to VC or DCA dechlorination to ETH requires two electrons. This result suggests that the supply of electrons from H2 to the enzymes is not rate limiting.
ACKNOWLEDGMENTS
This work was supported by the U. S. Air Force Armstrong Laboratory, Environmental Quality Directorate, Tyndall Air Force Base, Fla., by the Cornell Center for Advanced Technology in Biotechnology, and by the “La Caixa” Foundation, Catalonia, Spain (X. Maymó-Gatell).
We thank James Gossett and Donna Fennell for advice and for providing the butyrate-grown enrichment culture used as a nutrient supplement in this study and Eugene Madsen for the use of his gas chromatograph.
REFERENCES
- 1.Bagley D M, Gossett J M. Tetrachloroethene transformation to trichloroethene and cis-1,2-dichloroethene by sulfate-reducing enrichment cultures. Appl Environ Microbiol. 1990;56:2511–2516. doi: 10.1128/aem.56.8.2511-2516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bruin W P, Kotterman M J J, Posthumus M A, Schraa G, Zehnder A J B. Complete biological reductive transformation of tetrachloroethylene to ethane. Appl Environ Microbiol. 1992;58:1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiStefano T D, Gossett J M, Zinder S H. Hydrogen as an electron donor for the dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl Environ Microbiol. 1992;58:3622–3629. doi: 10.1128/aem.58.11.3622-3629.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiStefano T D, Gossett J M, Zinder S H. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol. 1991;57:2287–2292. doi: 10.1128/aem.57.8.2287-2292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ensley B D. Biochemical diversity of trichloroethylene metabolism. Annu Rev Microbiol. 1991;45:283–299. doi: 10.1146/annurev.mi.45.100191.001435. [DOI] [PubMed] [Google Scholar]
- 6.Fathepure B Z, Boyd S A. Dependence of tetrachloroethylene dechlorination on methanogenic substrate consumption by Methanosarcina sp. strain DCM. Appl Environ Microbiol. 1988;54:2976–2980. doi: 10.1128/aem.54.12.2976-2980.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathepure B Z, Boyd S A. Reductive dechlorination of perchloroethylene and the role of methanogens. FEMS Microbiol Lett. 1988;49:149–156. [Google Scholar]
- 8.Fennell D E, Gossett J M, Zinder S H. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ Sci Technol. 1997;31:918–926. [Google Scholar]
- 9.Fennell D E, Stover M A, Zinder S H, Gossett J M. Comparison of alternative electron donors to sustain PCE anaerobic reductive dechlorination. In: Hinchee R E, Leeson A, Semprini L, editors. Bioremediation of chlorinated solvents. Columbus, Ohio: Battelle Press; 1995. pp. 9–16. [Google Scholar]
- 10.Freedman D L, Gossett J M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989;55:2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gantzer C J, Wackett L P. Reductive dechlorination catalyzed by bacterial transition-metal coenzymes. Environ Sci Technol. 1991;25:715–722. [Google Scholar]
- 12.Gerritse J, Renard V, Pedro-Gomes T M, Lawson P A, Collins M D, Gottschal J C. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol. 1996;165:132–140. doi: 10.1007/s002030050308. [DOI] [PubMed] [Google Scholar]
- 13.Holliger C, Hahn D, Harmsen H, Ludwig W, Schumacher W, Tindall B, Vazquez F, Weiss N, Zehnder A J B. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in an anaerobic respiration. Arch Microbiol. 1998;169:313–321. doi: 10.1007/s002030050577. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins G D, Semprini L, McCarty P L. Microcosm and in situ field studies of enhanced biotransformation of trichloroethylene by phenol-utilizing organisms. Appl Environ Microbiol. 1993;59:2277–2285. doi: 10.1128/aem.59.7.2277-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonski P E, Ferry J G. Reductive dechlorination of trichloroethylene by CO-reduced CO dehydrogenase enzyme complex from Methanosarcina thermophila. FEMS Microbiol Lett. 1992;96:55–60. doi: 10.1016/0378-1097(92)90456-x. [DOI] [PubMed] [Google Scholar]
- 16.Kästner M. Reductive dechlorination of tri- and tetrachloroethylenes depends on transition from aerobic to anaerobic conditions. Appl Environ Microbiol. 1991;57:2039–2046. doi: 10.1128/aem.57.7.2039-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumholz L R. Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int J Syst Bacteriol. 1997;47:1262–1263. [Google Scholar]
- 18.Krumholz L R, Sharp R, Fishbain S S. A freshwater anaerobe coupling acetate oxidation to tetrachloroethylene dehalogenation. Appl Environ Microbiol. 1996;62:4108–4113. doi: 10.1128/aem.62.11.4108-4113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löffler F E, Ritalahti K M, Tiedje J M. Dechlorination of chloroethenes is inhibited by 2-bromoethanesulfonate in the absence of methanogens. Appl Environ Microbiol. 1997;63:4982–4985. doi: 10.1128/aem.63.12.4982-4985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnuson J K, Stern R V, Gossett J M, Zinder S H, Burrs D R. Reductive dechlorination of tetrachloroethene to ethene by a two-component enzyme pathway. Appl Environ Microbiol. 1998;64:1270–1275. doi: 10.1128/aem.64.4.1270-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maymó-Gatell X, Chien Y T, Gossett J M, Zinder S H. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- 22.Maymó-Gatell, X., I. Nijenhuis, and S. H. Zinder. Unpublished data.
- 23.Maymó-Gatell X, Tandoi V, Gossett J M, Zinder S H. Characterization of an H2-utilizing anaerobic enrichment culture that reductively dechlorinates tetrachloroethene to vinyl chloride and ethene in the complete absence of methanogenesis and acetogenesis. Appl Environ Microbiol. 1995;61:3928–3933. doi: 10.1128/aem.61.11.3928-3933.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller E, Wohlfarth G, Diekert G. Comparative studies on tetrachloroethene reductive dechlorination mediated by Desulfitobacterium sp. strain PCE-S. Arch Microbiol. 1997;168:513–519. doi: 10.1007/s002030050529. [DOI] [PubMed] [Google Scholar]
- 25.Mohn W W, Tiedje J M. Microbial reductive dehalogenation. Microbiol Rev. 1992;56:482–507. doi: 10.1128/mr.56.3.482-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner B M, McCarty P L, Spormann A M. In vitro studies on reductive vinyl chloride dehalogenation by an anaerobic mixed culture. Appl Environ Microbiol. 1997;63:4139–4144. doi: 10.1128/aem.63.11.4139-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholz-Muramatsu H, Neumann A, Messmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 28.Sharma P K, McCarty P L. Isolation and characterization of a facultatively aerobic bacterium that reductively dehalogenates tetrachloroethene to cis-1,2-dichloroethene. Appl Environ Microbiol. 1996;62:761–765. doi: 10.1128/aem.62.3.761-765.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandoi V, DiStefano T D, Bowser P A, Gossett J M, Zinder S H. Reductive dehalogenation of chlorinated ethenes and halogenated ethanes by a high-rate anaerobic enrichment culture. Environ Sci Technol. 1994;28:973–979. doi: 10.1021/es00054a033. [DOI] [PubMed] [Google Scholar]
- 30.Vogel T M, Criddle C S, McCarty P L. Transformations of halogenated aliphatic compounds. Environ Sci Technol. 1987;21:722–736. doi: 10.1021/es00162a001. [DOI] [PubMed] [Google Scholar]
- 31.Vogel T M, McCarty P L. Abiotic and biotic transformations of 1,1,1-trichloroethane under methanogenic conditions. Environ Sci Technol. 1987;21:1208–1213. [Google Scholar]