Abstract
Simple Summary
Spider mite is major pest in agriculture and have developed resistance to commonly used pesticides. Therefore, it is urgent to discover new pesticides to control the pest. In order to provide alternatives for its management, we evaluated the effectiveness of a new agent GC16 against the spider mite Tetranychus pueraricola. Then, we preliminarily revealed the its acaricidal mechanism of action based on the damage of cuticle and organelles of mites. We confirmed that GC16 has a good controlling effect on T. pueraricola and it is not harmful to Picromerus lewisi and Harmonia axyridis. Our research provides not only an alternative pesticide for the management of spider mites, but also guidance for the application of GC16 in sustainable agriculture.
Abstract
Chemical control plays a crucial role in pest management but has to face challenges due to insect resistance. It is important to discover alternatives to traditional pesticides. The spider mite Tetranychus pueraricola (Ehara & Gotoh) (Acari: Tetranychidae) is a major agricultural pest that causes severe damage to many crops. GC16 is a new agent that consists of a mixture of Calcium chloride (CaCl2) and lecithin. To explore the acaricidal effects and mode of action of GC16 against T. pueraricola, bioassays, cryogenic scanning electron microscopy (cryo-SEM) and transmission electron microscopy (TEM) were performed. GC16 had lethal effects on the eggs, larvae, nymphs, and adults of T. pueraricola, caused the mites to dehydrate and inactivate, and inhibited the development of eggs. GC16 displayed contact toxicity rather than stomach toxicity through the synergistic effects of CaCl2 with lecithin. Cryo-SEM analysis revealed that GC16 damaged T. pueraricola by disordering the array of the cuticle layer crest. Mitochondrial abnormalities were detected by TEM in mites treated by GC16. Overall, GC16 had the controlling efficacy on T. pueraricola by cuticle penetration and mitochondria dysfunction and had no effects on Picromerus lewisi and Harmonia axyridis, indicating that GC16 is likely a new eco-friendly acaricide.
Keywords: GC16, insecticidal activity, action mechanism, Tetranychus pueraricola
1. Introduction
Sustainable agriculture focuses on producing long-term crops with minimal effects on the environment and is being watched around the world [1,2,3]. Biodiversity is an important part of sustainable agriculture [3,4]. Considering the damage to biodiversity caused by conventional pesticides, there is a need to develop new alternative and eco-friendly pesticides to protect biodiversity [5].
Spider mites are major pests worldwide that cause serious damage to many crops and vegetables and great economic losses in agriculture and horticulture [6,7,8,9,10]. For a long time, people have mainly and widely relied on chemical pesticides to control mites. However, because of the overuse and misuse of pesticides, an increasing number of reports from different countries have revealed that spider mites have developed different degrees of resistance or cross-resistance to a variety of pesticides, including the global top-selling acaricide abamectin [8,9,11,12,13,14]. Unfortunately, the development of resistance has led to the increased use of chemicals. Therefore, pesticide residue and pest resurgence have appeared in ecosystems and are threatening agriculture and human health [15,16]. Ecological (eco-) pesticides are good alternatives to synthetic chemical pesticides in sustainable agriculture. GC16 (a mixture of natural botanical extracts and other inorganic nutrients) is promising to be developed as an ecopesticide.
GC16, composed of Calcium chloride (CaCl2, 45%) and lecithin (55%, extracted from soybeans), was recently found to have the ability of killing pests. GC16 has not been registered and is still being tested in a field trial across 15 provinces in China, including Beijing, Heilongjiang, Hainan, and Yunnan [17]. CaCl2, an inorganic salt, can be used as a plant nutrient or as an additive or firming agent in general [18,19,20]. Lecithin is a food-grade substance that is safe for human health and has been evaluated as a food additive by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1477 (accessed on 1 April 2022)). As an edible and digestible surfactant, antioxidant, and emulsifier of natural origin, lecithin has been widely used in the food, cosmetics, and pharmaceutical industries, especially for margarines and in chocolate [21,22,23,24,25]. Studies have shown that lecithin enhances the thermal tolerance of fish and protects them against cellular stress after exposure to organochlorine pesticides [26]. Additionally, lecithin can be used in carriers for drug delivery [27]. Moreover, lecithin was reported to decrease fungicide residue [28] and this compound along with its derivatives could be applied for post-harvest treatment because they can protect fruits and vegetables from stress-related damage and improve their quality during storage and shelf life [29]. Lecithin has been mainly used as a food additive, surface-active agent, or emulsifier of pesticides, but its insecticidal potential as a major component has not been evaluated, and the mechanism of action of CaCl2 + lecithin coapplication to control pests has not yet been revealed.
Tetranychus pueraricola (Ehara & Gotoh) (Acari: Tetranychidae) is a newly reported spider mite that is often found in China [30]. It has become the second most frequently sampled Tetranychus spider mite in China with 21.2% frequency, compared with T. truncatus (48.5%) and T. urticae (red) (5.7%) based on a long-term survey from 2008–2017 [10]. However, T. pueraricola is not a recent invasive pest but rather a long-standing species in China [30]. This species was first described as a new species by Ehara and Gotoh (1996) [31], is close to the two-spotted spider mite T. urticae Koch (red form) [7,32], and has long been misidentified as T. urticae (red form), T. cinnabarinus, or even T. truncatus [30].
This work is the first to evaluate the insecticidal efficacy and explore the mechanism of action of GC16 by using the spider mite T. pueraricola as a model species through bioassays, cryogenic scanning electron microscopy (cryo-SEM), and transmission electron microscopy (TEM) as the technological means.
2. Materials and Methods
2.1. Insects and Pesticide
The T. pueraricola population was collected in 2021 from Polygonatum sibiricum Delar. ex Redoute in a forest of Pinus kesiya Royle ex Gordon var. langbianensis (A.Chev.) Gaussen in Lancang, Yunnan Province, China (altitude: 1492 m, 22°48′15″ N, 99°47′22″ E). The population was collected by random sampling. The collection points of the eight subsamples were at least 500 m away from each other, and each subsample consisted of at least 500 adult mites. The field population of mites from this area was obtained by mixing the subsamples together. Then, the collected mites were maintained on pesticide-free and insect-free kidney bean plants (Phaseolus vulgaris Linn) “Bifeng” in the greenhouse of Yunnan Agricultural University, Kunming, Yunnan Province, China. After three generations of indoor breeding, these mites were used for the following experiments. The mite species was identified by Professor Xiaoyue Hong, a mite classification expert working at Nanjing Agricultural University in China. Based on specimen morphological identification and mitochondrial examination, the mite was confirmed to be T. pueraricola.
The common natural enemy insects Picromerus lewisi Scott (Hemiptera: Pentatomidae) and Harmonia axyridis Pallas (Coleoptera: Coccinellidae) were selected as non-target organisms. Non-target organism P. lewisi was fed on yellow mealworm and kept in the greenhouse of Yunnan Agricultural University, and H. axyridis was bought from Zhongnong Yaxing (Beijing Zhongnong Yaxing Biotechnology Co., Ltd., Beijing, China).
The tested agent GC16 is composed of a mixture of CaCl2 (45%) and lecithin (55%). Detailed information is as follows: CaCl2 (CAS: 10043-52-4, AR, 96%, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China); lecithin (CAS: 8002-43-5, from soybeans, >98%, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China). Water was used as the solvent when preparing GC16 solutions.
2.2. Bioassays
2.2.1. Bioassay of GC16 against Different Stages of T. pueraricola
Egg Bioassay
The ovicidal toxicity of GC16 to T. pueraricola eggs was assessed by the petri dish-spraying method according to a previous study with some modifications [33,34,35,36,37]. In total, 15 adult females were placed on a 20 mm diameter bean leaf disc placed on 0.3% agar in a petri dish for egg deposition. After 12 h, the females were removed, and the leaf discs were evenly and fully sprayed with a power sprayer. After the discs had dried, the eggs were counted, and the petri dishes with the treated leaf discs were placed in an incubator at 25 ± 2 °C with 65 ± 10% RH on a 16:8 (L/D) photoperiod. Five concentrations of GC16 were used to generate a regression equation (0, 1.25, 5, 10, 20 g/L). Each treatment was represented by three replicate leaf discs. The number of emerged larvae was counted daily until all the eggs for control had hatched except died eggs with physiological causes.
Larva and Nymph Bioassay
For the bioassays with larvae and nymphs, 20 adult female T. pueraricola were placed on leaf discs on 0.3% agar to lay eggs for 12 h [14,35]. Once the larvae/nymphs had emerged, their numbers were counted using a microscope, and the dead larvae/nymphs and other instars were removed. Then, the discs were sprayed with GC16 solutions (six concentrations of GC16; 0, 0.83, 1.67, 3.33, 6.67, 13.33 g/L) according to the same method described above. The leaf discs with larvae and nymphs were dried and stored in the incubator described previously. After 24 h, the living larvae and nymphs were counted.
Adult Bioassay
According to Ay (2005) [34], mortality tests were performed before each experiment to determine the range of concentrations that would produce 10~95% mortality. Adult female mites (30~50) were transferred to each bean leaf disc (2 cm diameter) placed in a petri dish, and each dish was covered with a lid containing small holes to avoid condensation of water vapor [14]. All experiments were conducted using three replicates of each concentration of GC16 (0, 0.83, 1.67, 3.33, 6.67, 13.33 g/L). The leaf discs with mites were examined under a microscope to remove the dead and inactive mites and then the discs were sprayed with a power sprayer as described above. After air-drying, the leaf discs were placed back into the plastic petri dishes (3.5 cm diameter) with 0.3% agar to preserve moisture, a method modified according to Xu et al. (2018) [14]. The petri dishes with the treated mites were placed in an incubator described earlier. After 24 h, the numbers of live and dead mites were counted. Mites were recorded as dead if they failed to move when touched with a soft brush. The results were not used if the mortality in the control exceeded 15%.
2.2.2. Bioassays of GC16 by Different Methods
Slide-Dip Assay
As recommended by the FAO, 2–3 cm long pieces of double-sided tape were adhered to glass slides [35,38]. The backs (rather than feet or mouthparts) of uniform adult females were gently stuck to the tape using a small brush. There were 45 mites on each slide, and the experiment was repeated three times. The slides were kept in an incubator for 4 h, and the mites were examined with a microscope (SZ51, Olympus, Tokyo, Japan). Dead and inactive individuals were removed. Slides containing mites were dipped into 6.67 g/L GC16 and shaken gently for 5 s. The excess liquid was quickly removed with absorbent paper. The control group was treated in the same way with water. The slides were then placed in a glass petri dish with soaked gauze on the bottom and plastic wrap with a small hole on the top and placed in an incubator as mentioned earlier. After 24 h, the dead mites and live mites were counted. A mite was considered dead if it did not respond to light touch with a small brush. If the mortality for the control exceeded 15%, the trial was repeated.
Leaf-Dip Assay
Following the methods of Xu et al. (2018) [14] and Wang et al. (2015, 2016) [13,39], bean leaf discs (2 cm in diameter) were dipped into a 6.67 g/L GC16 solution for 10 s, while leaf discs dipped into water were set as control. The leaf discs were then placed on filter paper to dry. After drying, the leaf discs were backed up and attached to 0.3% agar in a 3.5 cm diameter plastic petri dish. Approximately 25 ~ 30 adult female mites were transferred to each disc, and each petri dish was covered by a cover with small holes to avoid water vapor condensation. The experiment was repeated three times. Petri dishes containing the mites being tested were kept in incubators under the same conditions as those described previously. After 24 h, the numbers of live and dead mites were counted in each petri dish. Mites that did not move after being touched by a soft brush were recorded as dead. The results were not used if the mortality rate for the control treatment exceeded 15%.
Spraying Assay
The spraying assay was conducted in a similar manner as the assay in the section Adult Bioassay [2]. Approximately 35 healthy adult female mites were transferred to bean leaves, and three replicates were performed. Dead and inactive mites were removed under a microscope. Then, a solution containing 6.67 g/L GC16 was sprayed equally on the bean leaves with the mites with a power sprayer. Then, the plants with the mites were kept in incubators at the conditions described previously. Twenty-four hours later, the numbers of live and dead mites were counted. Mites that did not move after being touched by a soft brush were recorded as dead. Sprayed water was used as a control under the same conditions. If the mortality rate for the control exceeded 15%, the experiments were repeated.
2.2.3. Bioassays for the Different Components of GC16
Bioassays for the different components of GC16 in adult female T. pueraricola were performed by the spraying method as described in the previous paragraph with a GC16 concentration of 6.67 g/L. Water was used as a blank control, and the CaCl2 and lecithin treatments were administered at concentrations of 3.03 g/L and 3.64 g/L, respectively.
2.3. Observation of Poisoning Symptoms for T. pueraricola
To observe poisoning symptoms [40,41,42], 25 mites were carefully transferred to bean leaves. T. pueraricola were treated with GC16 at concentrations of 6.67 g/L and 2.00 g/L by the spraying method, and water was used as the control. Observations were made under a microscope (SZ51, Olympus, Tokyo, Japan) at 20 min, 40 min, and then every 2 h from 2 to 24 h; each observation was made with three replicates.
2.4. Effects of GC16 on the Morphology of Female Adult T. pueraricola
By the slide-dip method (details described above), 6.67 g/L GC16 was used to evaluate the effects on the morphology of female adult T. pueraricola. Treatment with water under the same condition was set as the control. After treatment for 24 h and 48 h, mites were photographed by microscopy (LEICA M205 FA, Wetzlar, Germany), and the body lengths and widths of mites were measured [43]. The relative shrinkage rate (Rst) of length = (body length of control for n h—body length of GC16 for n h)/body length of control for n h (n is the number of hours after treatment; h means hour); the Rst of width was calculated the same.
2.5. Effects of GC16 on the Egg Hatching Rate and Developmental Duration of T. pueraricola
Fresh, healthy, and uniform female adult mites were selected and placed on common bean leaves. After 12 h of laying eggs, the adult mites were removed with a brush, and approximately 20 ~ 40 eggs were kept on each leaf [13,35]. The mite eggs were treated with 10 g/L GC16 by the petri dish-spraying method described above, with water as a control, and each treatment consisted of three biological replicates. The eggs were observed and photographed under a microscope (LEICA M205 FA, Wetzlar, Germany) every day, and the hatching rate and development time (time after egg lay) were calculated.
2.6. Cryo-SEM (Scanning Electron Microscopy)
The tested mites were treated with water (control), GC16, CaCl2, or lecithin. The concentration of GC16 was set to its LC50 (2.00 g/L), and the concentrations of CaCl2 (0.90 g/L) or lecithin (1.10 g/L) corresponded to the ratio in GC16. After treatment for 24 h, live mites were used for scanning/transmission electron microscopy (SEM/TEM) analysis.
According to Walther (2001) [44], Yu et al. (2011) [45], and Yan et al. (2021a) [46], to avoid chemical fixation and drying artifacts and obtain the most direct and real images of mites in a defined physiological state, a fast frozen technique was used. For cryo-SEM, fresh mites were directly and gently glued to the sample table and frozen in supercooled liquid nitrogen for 2 min. The samples of each replicate were then transferred to a preparation chamber at −140 °C. Next, sublimation was performed at −90 °C for 10 min, followed by coating twice for 60 s each time. The samples were observed and photographed with a ZEISS Sigma 300 scanning electron microscope.
2.7. TEM (Transmission Electron Microscopy)
Sample preparation was the same as that described above. A previous method was modified as appropriate [47,48,49,50]. Samples were fixed overnight at 4 °C using 2.5% glutaraldehyde in 0.1 M PB (pH 7.4). Samples were then washed with 0.1 M PB (pH 7.4) three times for 15 min each time. Afterward, the samples were post-fixed with 1% OsO4 for 2 h at 4 °C, washed with 0.1 M PB (pH 7.4) three times for 15 min each time, followed by serial ethanol dehydration and acetone transition for 5 min, embedded in Epon 812 resin, and polymerized at 60 °C for 48 h. Serial ultrathin sections with a uniform thickness (60 nm) were made using a Leica EM UC7 ultramicrotome. The ultrathin sections were then loaded onto 50-mesh Cu grids and double-stained with 2% uranyl acetate and lead citrate before observation with a JEM 1400 Plus transmission electron microscope at 120 kV.
2.8. The Effects of GC16 on Non-Target Organisms
The target pest mite T. pueraricola and non-target organisms P. lewisi and H. axyridis were treated with 6.67 g/L GC16 using the spraying method mentioned above in the section Spraying Assay. The P. lewisi and H. axyridis were placed in insect-rearing cages (120 mesh, 30 cm × 30 cm × 30 cm) that provided yellow mealworm and aphids, respectively. In total, 20~40 insects were tested each repeat (three repeats each treatment), and insects treated with water in the same way were set as control. Twenty-four hours later, the dead insects were counted and the mortality rate was calculated.
2.9. Statistical Analysis
For the developmental stage bioassay data, the slope ± SE, LC50 values, 95% fiducial limits, chi-square values, and degrees of freedom (df) were calculated by probit analysis using Polo Plus 2.0 software (LeOra software, Berkeley, CA, USA).
The other data were analyzed using SPSS software, v. 25.0 (SPSS Inc., Chicago, IL, USA). The graphs were created by Sigmaplot 14.0 (Systat Software Inc., San Jose, CA, USA) and grouped by Adobe Illustrator 2021 (Adobe Systems Inc., San Jose, CA, USA). After normality test, the data were in accordance with normal distribution or approximate normal distribution. Differences between the effects of different bioassay methods or different components were analyzed using one-way analysis of variance (ANOVA). Significant differences between treatments were based on Tukey’s honestly significant difference (HSD) test. Differences in body length/width among the different treatments were analyzed by one-way ANOVA with Tukey’s honestly significant difference (HSD) test. Differences in the egg hatching rate and development duration between the two groups were compared using independent sample t tests. One-way ANOVA with Tukey’s honestly significant difference (HSD) test was used to analyze the differences of effects of GC16 on non-target organisms across three organisms, and an independent sample t test was used to compare differences of pesticides treatments (GC16 vs. Control) for the same organism. Statistical significance was set at p < 0.05.
3. Results
3.1. Bioassays
3.1.1. Bioassays of GC16 against Different Stages of T. pueraricola
To evaluate the effects of GC16 against T. pueraricola, the LC50 values were determined by spraying method (Table 1). The LC50 values of GC16 against eggs, larvae, nymphs, and adults of T. pueraricola were in the range of 1.266~2.239 g/L, and the LC90 values ranged from 5.951 to 26.888 g/L. Results indicated that GC16 had clear insecticidal effects on all instars and stages of T. pueraricola, and their mortality increased with increasing concentrations of GC16; however, the same concentration of GC16 had different lethal effects on mites in different stages. At a concentration of 6.67 g/L, the mortality of female adults reached over 80% (Table S1).
Table 1.
The overall lethal effect of GC16 on Tetranychus pueraricola in the different developmental stages.
| Stage | N a | Slope ± SE | LC50 (95% FL b(g/L)) | LC90 (95% FL b(g/L)) | χ2(df) c |
|---|---|---|---|---|---|
| egg | 425 | 1.187 ± 0.182 | 2.239 (1.349 ~ 3.131) | 26.888 (16.980 ~ 58.918) | 2.615 (10) |
| larva | 885 | 2.652 ± 0.202 | 1.963 (1.655 ~ 2.300) | 5.973 (4.759 ~ 8.245) | 21.988 (13) |
| nymph | 686 | 1.907 ± 0.199 | 1.266 (0.782 ~ 1.705) | 5.951 (4.044 ~ 12.833) | 39.524 (13) |
| adult | 625 | 2.107 ± 0.193 | 1.996 (1.624 ~ 2.396) | 8.099 (6.143 ~ 12.155) | 17.138 (13) |
a Number of adult mites used in the bioassay, including controls. b FL = fiducial limit. c Chi-square value and degrees of freedom.
3.1.2. Bioassays of GC16 by Different Bioassay Method
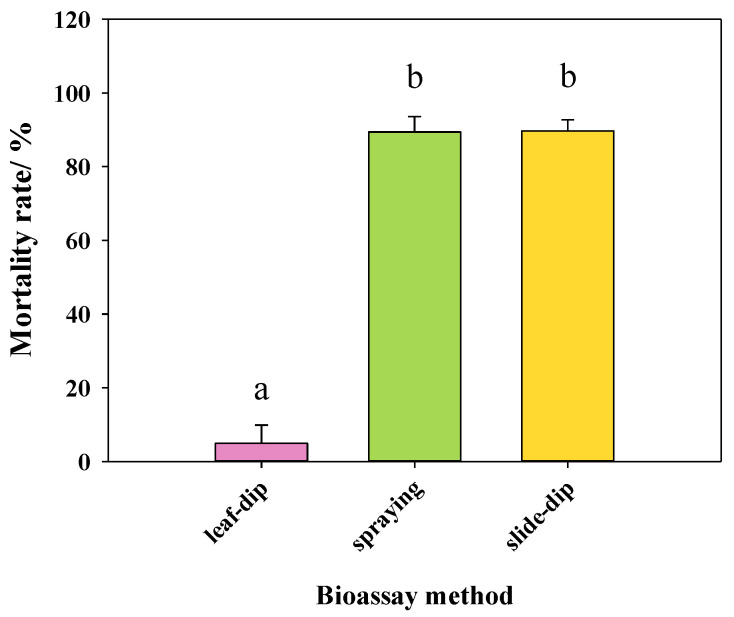
The spray method and slide-dip method are commonly used techniques to determine the contact toxicity of pesticides, while the leaf-dip method can better test the stomach toxicity. To determine the mode of action of GC16, we compared its toxicity by different bioassay methods. The results showed that the mortality rate of T. pueraricola treated with 6.67 g/L GC16 by the leaf-dip method was 4.94%, while the mortality rates of the spraying method and slide-dip method were both greater than 80%, which were significantly higher than that of the leaf-dipping method (Figure 1). There was no significant difference between the spray method and the slide-dip method. The results of these experiments indicated that GC16 mainly acted on mites through contact.
Figure 1.
The mortality rates after 24 h of Tetranychus pueraricola under 6.67 g/L GC16 with different bioassay methods. Data are expressed as mean ± SE. Different lowercase letters represent significant differences at p < 0.05 based on a one-way ANOVA with Tukey’s HSD test.
3.1.3. Bioassays for the Different Components of GC16
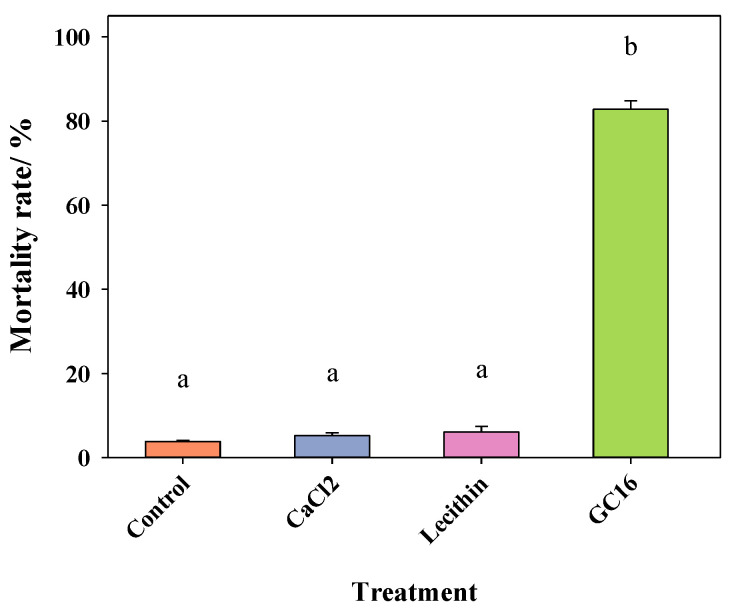
GC16 is composed of a mixture of CaCl2 and lecithin. To determine which component is the main active component and/or how the two components synergize, the lethal efficacy of each component was tested and the results were compared. The mortality rates of mites were less than 10% when CaCl2 or lecithin acted alone, and there were no significant differences between control and the CaCl2/lecithin alone groups. However, the mortality of mites treated for 24 h with GC16 reached 80%, and the coapplication of lecithin + CaCl2 significantly increased mortality compared with mites treated with lecithin or CaCl2 alone (Figure 2). These data indicated that CaCl2 or lecithin alone were not lethal to mites, but the combination of lecithin + CaCl2 (GC16) produced very different results.
Figure 2.
The mortality rates for 24 h of T. pueraricola under GC16 and its components. Component 1 is Calcium chloride (CaCl2), and Component 2 is Lecithin. Data are expressed as mean ± SE. Different lowercase letters represent significant differences among different treatments at p < 0.05 based on one-way ANOVA in Tukey’s HSD test.
3.2. Observation of Poisoning Symptoms
To investigate the mechanism by which GC16 influenced T. pueraricola, the poisoning and death symptoms of T. pueraricola were observed. After GC16 treatment, the mites first entered the quiescent stage (Table 2). Then, they died and later shriveled at high concentrations. At a low concentration, some mites gradually resumed movement, while the others moved slightly and then became sluggish until death.
Table 2.
Poisoning symptoms after the GC16 treatment.
| Treatment (g/L) | 20 min | 40 min | 2 h | 4 h | 6 h | 8–18 h | 18–24 h |
|---|---|---|---|---|---|---|---|
| Control (0) | feed and oviposit actively | feed and oviposit actively | feed and oviposit actively | feed and oviposit actively | feed and oviposit actively | feed and oviposit actively | feed and oviposit actively |
| GC16 (2.00) | stationary | stationary | moved slightly | moved slightly | 1. crawled 2. moved slightly 3. stationary/died |
1. crawled 2. moved slightly 3. died |
1. crawled 2. wiggled when tapped 3. died |
| GC16 (6.67) | stationary | stationary | stationary | stationary/died | stationary/died | died, started shrinking | shriveled |
Note: 2.00 g/L (LC50) is the concentration of GC16 lethal to 50% of adult mites, while 6.67 g/L, the recommended concentration for field use, is the concentration of GC16 lethal to approximately 85% of the adult mites.
3.3. Effects of GC16 on the Morphology of Female Adult T. pueraricola
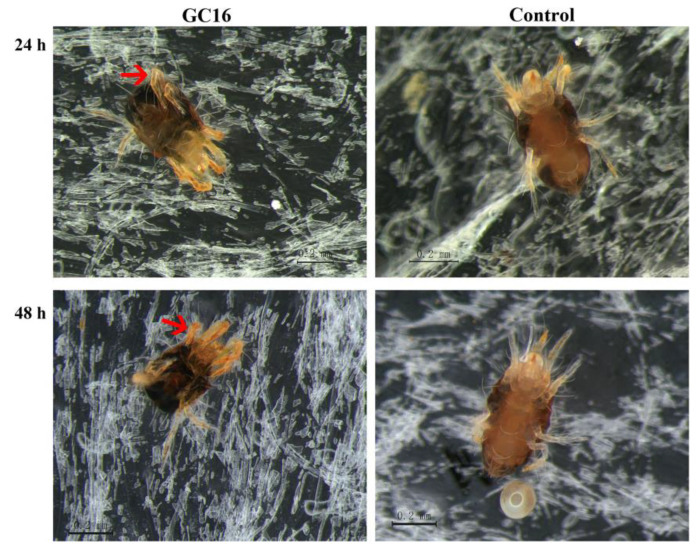
After GC16 treatment, most of the tested mites died 24 h later, but the control mites treated with water were still alive and active enough to walk, forage, and oviposit. Under a microscope, it was clearly seen that the mite bodies became small, crumpled, and shriveled and the legs became bent and curled up after treatment with GC16 (Figure 3). Compared with the water control, the body lengths of the mites treated with GC16 were significantly shortened at 24 h and 48 h, and the relative shrinkage rates were 14% and 25%, respectively (Table 3). In addition, the body widths of the mites treated with GC16 for 48 h were significantly smaller than those of the control, and the relative shrinkage rate reached 14%.
Figure 3.
The morphology of female adults for T. pueraricola after GC16 treatment by slide-dip method for 24 h and 48 h. The red arrow indicates that the legs of mite were bent and curled up after treatment with GC16.
Table 3.
Mean (± SE) body length and width of female adult T. pueraricola under GC16 treatment.
| Treatment | Body Length (mm) | Rst for Body Length | Body Width (mm) | Rst for Body Width |
|---|---|---|---|---|
| Control-24 h | 0.57 ± 0.01 a | 24 h Rst = 14.36% | 0.28 ± 0.01 a,b | 24 h Rst = −0.16% |
| GC16-24 h | 0.49 ± 0.01 b | 0.28 ± 0.01 a,b | ||
| Control-48 h | 0.56 ± 0.01 a | 48 h Rst = 24.69% | 0.30 ± 0.01 a | 48 h Rst = 14.53% |
| GC16-48 h | 0.42 ± 0.01 c | 0.26 ± 0.01 b |
Note: Rst indicates the relative shrinkage rate. Control/GC16-24 h/48 h indicates the mites were treated with water/GC16 for 24 h/48 h, respectively. Data are expressed as mean ± SE. Different lowercase letters in the same column indicate significant differences between treatments (p < 0.05). One-way ANOVA and Tukey’s honestly significant difference (HSD) test were adopted. The F and p values for HSD test of body length and body width are F(3,20) = 18.431, p < 0.001 and F(3,20) = 2.972, p = 0.056, respectively.
3.4. Effects of GC16 on the Hatching Rate and Developmental Duration of T. pueraricola Egg
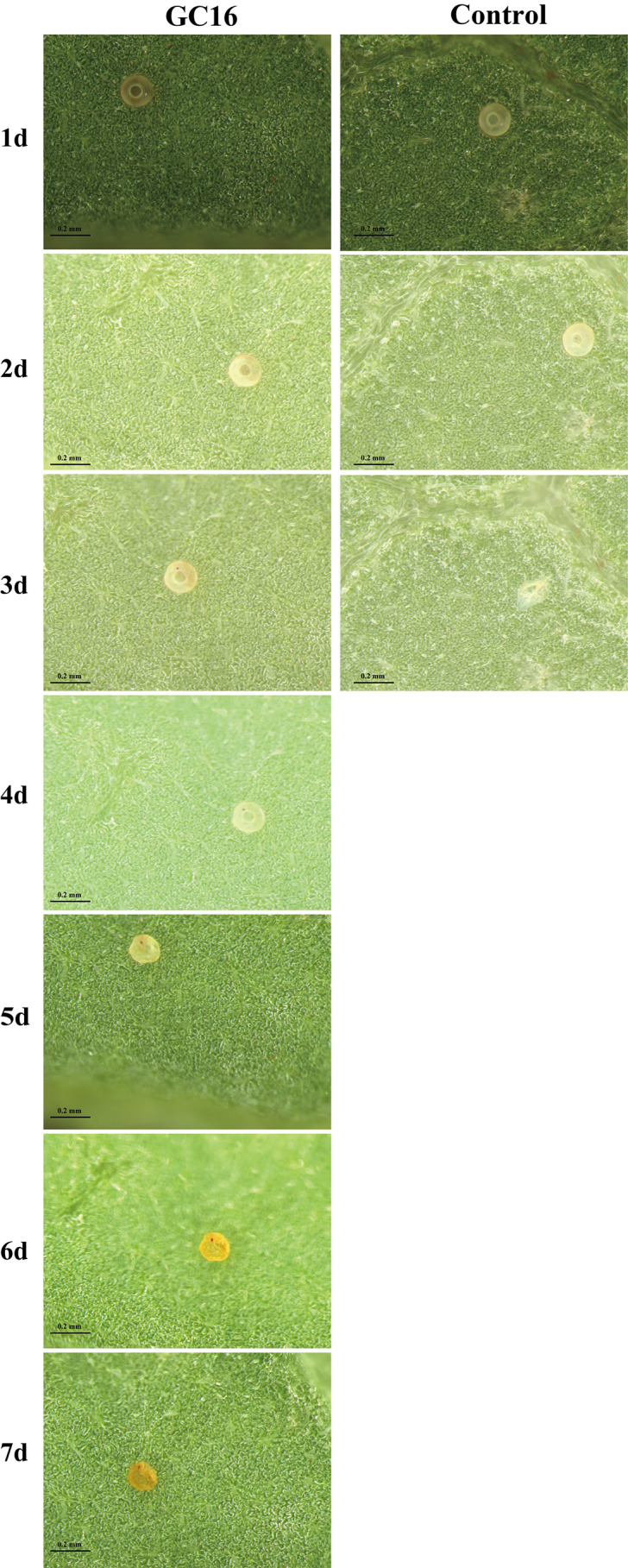
After treatment with GC16, it was found that the mite eggs gradually shriveled, became withered and deformed, and could not hatch successfully. However, under the same conditions, the eggs treated with water could molt and hatch normally (Figure 4). The hatching rates of eggs treated with GC16 and control were 14.30% and 94.38%, respectively, and there was a significant difference between them (t = 4.576, df = 4, p < 0.001 Table S2). Moreover, the development duration of the control eggs in water was 4.07 days, while their development duration after GC16 treatment was 5.10 days, a significant difference of 1.03 days longer than that in water (t = 1.480, df = 4, p = 0.005), indicating that GC16 could significantly reduce the hatching rate of eggs and prolong the egg development duration.
Figure 4.
The egg morphology of T. pueraricola after GC16 treatment (10 g/L) vs. Control (water).
3.5. Cryo-SEM Analysis
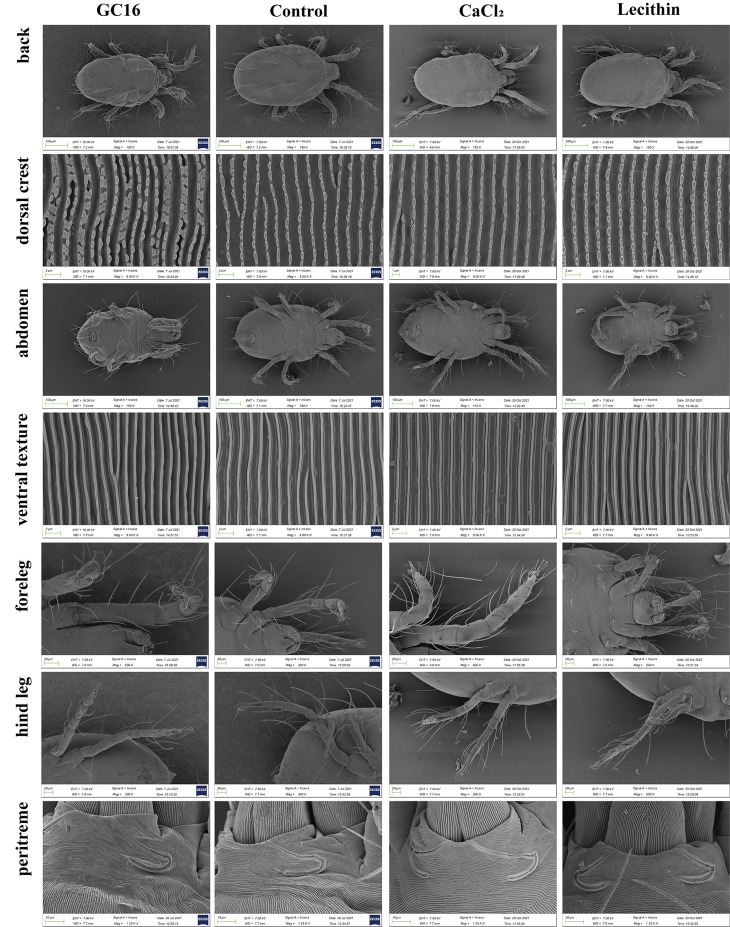
According to the above results, the application of GC16 caused the insect bodies to dehydrate and atrophy; however, is this related to cuticle damage? Considering that cuticle penetration plays an important role as an insecticide mechanism, to determine the cuticle integrity of mites treated with GC16, cryo-SEM was performed. After GC16 treatment, the crest lines on the dorsal surfaces of T. pueraricola showed disordered and irregular arrangements, and the cuticle ridges snuggled close each other (Figure 5). In contrast, the dorsal dermatoglyphs of control (water-treated) T. pueraricola were arranged in an orderly and regular manner, and the cuticle ridge was evenly and regularly distributed. Additionally, there were no obvious abnormalities in the dorsal crest with CaCl2 or lecithin treatment alone. Furthermore, no obvious differences were seen in the forelegs, hind legs, abdomens, or peritremes between the mites receiving different treatments.
Figure 5.
Scanning electron microscopy (SEM) observation of T. pueraricola after treated for 24 h. The treatments include GC16, Control (water), CaCl2, and lecithin. The parts that were photographed included the back, dorsal crest, abdomen, ventral texture, foreleg, hind leg, and peritreme.
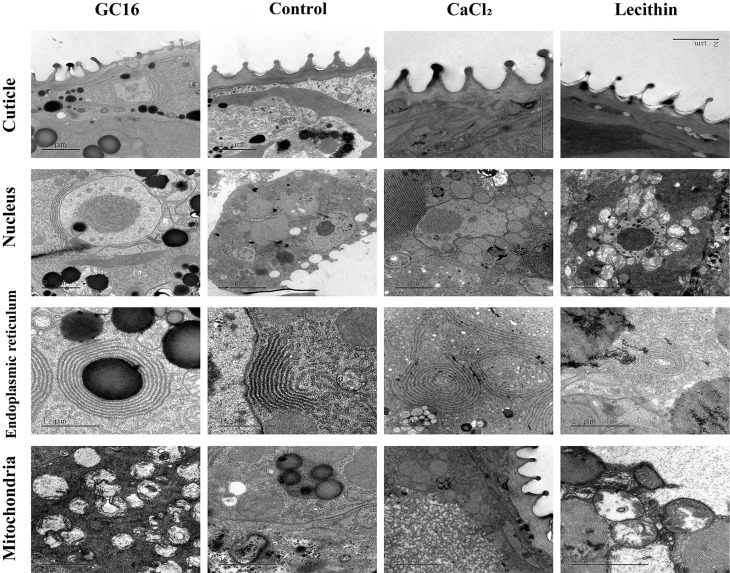
3.6. TEM Analysis
From previous poisoning symptom observations, mites displayed inactive and motionless states after GC16 treatment, and it is unclear whether this state is related to energy metabolism. To determine the cause behind these symptoms, the ultramicrostructure of the inner tissue was observed. TEM observations showed that the endoplasmic reticulum, mitochondria, and nuclear membrane system were not damaged, and the cuticle was compact and had regular protuberances in the control (water group) (Figure 6). The submicroscopic structure of the GC16 group was also clearly visible, with no abnormalities in the nucleus or endoplasmic reticulum but obvious abnormalities in the mitochondria were found. Specifically, the mitochondria were swollen and malformed, the intercristae matrix was vacuolated, and a flocculent amorphous substance appeared in the mitochondrial lumen. Compared with the water control, the cuticles of the mites treated with GC16 were dissolved, protrusion was seriously damaged, and the arrangement of the crest was irregular. Notably, there was no clear cuticle damage or organelle damage in the CaCl2 treatment group. In the lecithin treatment group, except for mitochondrial abnormalities (the mitochondria swelled irregularly and their cristae became fractured and fuzzy), additional changes were not noted.
Figure 6.
Transmission electron microscopy (TEM) observation of T. pueraricola after treated with GC16, Control (water), CaCl2, and lecithin for 24 h. The cuticle, nucleus, endoplasmic reticulum, and mitochondria of mites were photographed.
3.7. The Effects of GC16 on the Non-Target Organisms
Safety assessment for non-target organisms is an important part of pesticide environmental toxicology, which guides agricultural production. Here, the mortality rate for mites treated with GC16 was more than 80%, while the mortality rates for non-target organisms P. lewisi and H. axyridis under GC16 treatment were less than 10% and there were no significant differences on mortality rates for them between GC16 treatment and the blank control, respectively (Table 4). This indicates that GC16 has a good control efficacy on the spider mite T. pueraricola, while it has no lethal effects on non-target organisms, and GC16 may have the potential to be developed as an eco-friendly acaricide.
Table 4.
The mortality rates (Mean ± SE) of target organism (Tetranychus pueraricola) and non-target organisms (Picromerus lewisi, Harmonia axyridis) after treating with GC16 vs. Control (water).
| Organism | Mortality Rate (%) | t, p | |
|---|---|---|---|
| GC16 | Control | ||
| Tetranychus pueraricola | 86.21 ± 2.02 A,a | 2.38 ± 1.19 A,b | t = −35.693, df = 4, p < 0.001 |
| Picromerus lewisi | 4.15 ± 0.23 B,a | 4.35 ± 0.37 A,a | t = 0.447, df = 4, p = 0.678 |
| Harmonia axyridis | 8.33 ± 1.67 B,a | 5.83 ± 0.83 A,a | t = −1.342, df = 4, p = 0.251 |
Note: Values are mean ± SE. The same uppercase letter in a column expresses no significant difference among three organisms according to the one-way ANOVA and Tukey’s honestly significant difference (HSD) test, and the same lowercase letter in a row indicates no significant difference between GC16 treatment (6.67 g/L) and control (water) based on independent sample t test at p < 0.05. The F and p values for HSD test of GC16 and Control across the three organisms are F(2,6) = 925.992, p < 0.001 and F(2,6) = 3.973, p = 0.080, respectively.
4. Discussion
Exploring novel pesticides has been an important part of integrated pest management (IPM) in the current situation of agricultural development. In this study, to evaluate the performance of a new agent, GC16, on T. pueraricola, we carried out bioassays with mites at different developmental stages with different treatment methods; we also examined the bioassays for different components of GC16 in addition to their combination. The results showed that GC16 had effects on the eggs, larvae, nymphs, and adults of T. pueraricola by contact with the synergistic reaction mechanism of lecithin and CaCl2. Subsequently, ultrastructures of the mites were observed. The combined results demonstrated that GC16 killed mites by damaging their cuticles to first dehydrate and then destroying the mitochondria to disrupt metabolism, making the mites inactive.
In general, the median lethal concentration (LC50) is the elemental parameter to analyze the acaricidal activity of acaricides. The LC50 value is affected by the pest species, type of pesticide, bioassay method, bioassay time, and treatment environment [51,52,53]. Previous studies have reported that the LC50 values of avermectin, bifenazate, etoxazole, and spirodiclofen against T. cinnabarinus over 24 h by the slide-dip method were 3.2 × 10−6, 14.932 × 10−3, 4.4 × 10−4, and 0.356 g/L, respectively [52]. Additionally, the LC50 values of the botanical pesticide scoparone against T. cinnabarinus and T. urticae by the slide-dip method were found to be 0.279 and 0.906 g/L, respectively [53]. The LC50 value at 24 h of osthole to T. urticae was 0.332 g/L by the spraying method [2]. The LC50 of the crude acetone extract from Aloe vera L. against female adult T. cinnabarinus was 6.165 g/L by the slide-dip method after 24 h [51]. Herein, we found that the LC50 value of GC16 against female adult T. pueraricola was 2.00 g/L. In contrast to commercial pesticides, the LC50 value of GC16 was greater but at an intermediate level when compared with the plant-derived extract. Combined with the above studies, different pesticides have different acaricidal activities against different spider mite species, and GC16 has the potential to become a new acaricide compared with the plant-derived extracts.
Poison symptom investigation is the first step in understanding the mechanism of action of pesticides. To investigate the mechanism of GC16, we observed poisoning and death symptoms of T. pueraricola. The findings revealed that mites became stationary after GC16 treatment and then died with curly legs and shrunken and wizened bodies, which is somewhat similar to the paralyzing effects of nerve agents but without the excitement [54]. In addition, after treatment with abamectin, pyridazin, curcumin, and scopolamine, T. cinnabarinus showed symptoms of excitement, coma, stasis, and death [43]. There have also been other previous studies on insect poisoning symptoms. Essential oils and monoterpenes had knockdown effects on Musca domestica [40]. Distinct poisoning symptoms, such as extended proboscis, expanded wings, unhooked wings, extended legs, and twisted bodies, were also observed in Apis mellifera mellifera [42].
Naturally, insecticidal agents control insects through a variety of mechanisms, including contact, stomach, repellent, fumigant, and systemic methods or through food intake prevention or oviposition inhibition, etc. [40,49,51]. Zhang et al. (2013) found that the A. vera L. leaf acetone extract had contact acaricidal, repellent, fumigant, and oviposition inhibitory activities against T. cinnabarinus [51]. Ma et al. (2021a) reported that 1,3,4-oxadiazoles possessed excellent contact activity and weak systemic activity against E. lanigerum [49]. Here, a study of the mode of action demonstrated that GC16 had contact activity against T. pueraricola, similar to the mite contact activity of botanical extracts from A. vera and Artemisia annua [51,55]. The egg hatching inhibition and ovicidal activity of GC16 against T. pueraricola is consistent with azadirachtins against the maize stem borer Chilo partellus [56]. Moreover, egg hatching inhibition and the delay in egg hatching of C. partellus were supposedly due to the overall detrimental effects of azadirachtins on the reproductive systems of C. partellus [56]. Furthermore, scoparone was found to bind to the Vg protein and lower Vg gene expression to inhibit egg development in T. cinnabarinus [57]. Whether the decrease in egg hatching rate and prolongation of the developmental duration observed in this work are related to the reproductive system needs further exploration.
Undoubtedly, investigating the action mechanisms of pesticides against pests is an important strategy to develop new prospective pesticides. To explore the action mechanism, SEM, TEM, cuticle permeability, enzyme activity, gene expression profile, and RNAi are generally analyzed [49,52,53,58,59].
Cryo-SEM is an important method to study the surfaces of biological samples rich in water. Compared with traditional SEM, there is no sample pretreatment processes required for cryo-SEM, which would inevitably be related to sample distortion, shrinkage, or a loss of the inner cellular soluble components; therefore, we can obtain the most realistic images of sample shape and structure [46,60]. In this work, through cryo-SEM, we found that the cuticle layer of T. pueraricola was destroyed and its arrangement was disordered by GC16, similar to another finding, i.e., graphene oxide can absorb and impair the structure of the cuticle layer of mites [52]. The insect cuticle is its primary protective barrier against the penetration of pesticides. Previous studies have shown that pesticides more easily penetrate weaker and damaged cuticles, and cuticle damage is positively correlated with insecticide permeability and insect mortality [61,62]. Moreover, cuticle permeability is regarded to be associated with insecticide sensitivity, and damaged cuticles are often accompanied by dehydration and shriveling [52,58]. Therefore, the reason for death of T. pueraricola in this study might be that the impaired cuticle layer lost its protective function against the penetration of GC16.
In terms of TEM, ultrastructural changes were detected in T. pueraricola, indicating that GC16 might exert its acaricidal activity by destroying the mitochondria and perturbing the cuticle layer array. It has been previously reported that the steroid PSNW targets the midgut cells of Mythimnazus separata Walker by destroying the cell membrane and mitochondria [63]. In addition, the target site of the steroid 1,3,4-oxadiazole was demonstrated to be the mitochondria and nucleus in the midgut tissues of Eriosoma lanigerum [49]. Mitochondria are the powerhouses of the cell and mitochondrial dysfunction is related to oxidative homeostasis and lipid and energy metabolism [50,64]. For example, zebrafish exposed to triazoles had impaired mitochondrial oxidative phosphorylation and oxidative stress as well as dysregulation of lipid metabolism, which resulted in developmental disorders and movement disorders [64]. In this study, we found mitochondrial dysfunction in mites treated with GC16 accompanied by motionless poisoning symptoms, which were inferred to be related to lipid or energy dysmetabolism. In addition, the mitochondrial dysfunction phenomenon of mites after GC16 treatment was similar to treatment with cyflumetofen, which was demonstrated to be an inhibitor of complex II in the mitochondrial electron transport chain [65]. Mitochondrial abnormalities were also seen in mites in the lecithin group; however, the corresponding mortality rate of the mites after this treatment was low, which might be because lecithin acted alone with a sublethal effect rather than a lethal effect. It was also previously reported that lecithin could induce mitochondrial membrane alterations in mammals but lecithin effectively protected certain sperm quality characteristics against freezing-induced damage [66]. Lecithin is structurally similar to the cell membrane (both contain phospholipids). Moreover, the epicuticle is the outermost layer of the insect integument and mainly composed of lipids and proteins. According to the principle of “like dissolves like”, lecithin can dissolve cell membranes and the cuticles of insects in theory. In addition, calcium chloride has the property of water absorption and may influence Ca2+ balance of the mite. Therefore, we speculate that it is the symmetrical structure of GC16 [inorganic ions + organic substance (dissolve membrane)] that caused the cuticle of mite to be adsorbed, dissolved, and lose water and to lead to ionic imbalance.
Overviewing the action mechanism of pesticides, the Ca2+ homeostasis disruption and cuticle permeability increase hypotheses were mentioned. For example, Zhou et al. (2021a) reported that curcumin might activate and overexpress the CaM gene and disrupt Ca2+ homeostasis in T. cinnabarinus to achieve the control effect [67]. Scopoletin acts by regulating the calcium signaling pathway and disrupting intracellular Ca2+ homeostasis [68]. Further results showed that the acaricidal mechanism of scopoletin on T. cinnabarinus may be related to the calcium channel gene TcT-VDCC [69]. In addition, the mechanism of action of scoparone against T. cinnabarinus is by targeting the interface between CaM1 and L-VGCC to activate the CaM binding site located in the IQ motif at the L-VGCC C-terminus [53]. Moreover, scopoletin could act on mites by inhibiting chitinase (CHIT) gene expression [70], and graphene oxide could inhibit the expression of the cuticle protein (CPR) gene to disturb the construction of the cuticle layer and increase cuticle permeability and acaricide sensibility [52]. Additionally, in Blattella germanica, low expression of CYP4G19 disordered the array of the lipid layer, enhanced cuticle permeability, and compromised insecticide tolerance [58]. Because CaCl2 is an important component of GC16, whether the application of GC16 affects the calcium homeostasis in mites needs further exploration. However, the destruction of the cuticles of the mites in this study supports the cuticle penetration hypothesis.
Putting the above together, GC16 exhibited a stronger lethal effect on T. pueraricola than lecithin or CaCl2 alone. In addition, GC16 destroyed and disordered the cuticles of the mites, while those treated with lecithin or CaCl2 alone had intact and regular cuticles. In contrast to the normal mitochondria of the control and CaCl2 group, there were mitochondrial abnormalities, such as inner ridge fracture and degradation, in the GC16 group and lecithin group. The combined results suggest that GC16 broke the cuticles of the mites by the coapplication of lecithin + CaCl2 and lecithin was the main source of T. pueraricola mitochondria damage, indicating that cuticle damage was more important than mitochondrial dysfunction for the lethal effects of GC16 against mites.
5. Conclusions
In conclusion, we found that GC16 caused insecticidal efficacy against T. pueraricola through contact by disordering the arrangement of the crest in the cuticular layer and destroying the mitochondria. Considering that 6.67 g/L GC16 (recommended concentration for the control of T. pueraricola) has no lethal effects on natural enemy insects P. lewisi and H. axyridis, it may possess the potential to be developed as an ecological agent. However, the effects of GC16 on the overall ecosystem need to be further evaluated in field applications. Our findings may accelerate the development of novel ecological pesticides to control destructive spider mites worldwide. Furthermore, this work will provide alternative pesticide support for pest control for sustainable agriculture; for example, planting of Chinese medicinal herbs. Moreover, how GC16 acts on the cuticle and mitochondria and which genes are involved in this process remain to be studied.
Acknowledgments
This work and the article processing charges (APC) were supported by the Major Science and Technology Project of the Yunnan Provincial Science and Technology Department (NO.202102AE090042-02-06), and the scientific research project (Study on the insecticidal mechansim of GC16) from academician office of academician Zhu Youyong.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13050433/s1, Table S1: The raw data of bioassays of GC16 on mites, Table S2: The egg hatchability and developmental duration of T. pueraricola under GC16 treatment.
Author Contributions
Conceptualization, B.C., Y.Z., Y.H., G.D., X.H., S.Z. and Y.L.; methodology, Y.H., S.X., X.L. and G.S.; software, Y.H.; validation, Y.H. and G.D.; formal analysis, Y.H. and S.X.; investigation, Y.H., S.X., G.S. and X.L.; resources, Y.H., G.D. and X.L.; data curation, Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, B.C., X.H., G.D., Y.L., S.X., G.S., X.L. and S.Z.; visualization, Y.H.; supervision, B.C., G.D., Y.L., X.H., S.Z. and Y.Z.; project administration, B.C.; funding acquisition, B.C., X.H. and S.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumawat S., Asiwal R., Nitharwal M. Agricultural sustainability: Various projects and programmes. Agric. Food E-Newsl. 2021;3:84–86. [Google Scholar]
- 2.Yan S., Hu Q., Jiang Q.H., Chen H.T., Wei J., Yin M.Z., Du X.G., Shen J. Simple osthole/nanocarrier pesticide efficiently controls both pests and diseases fulfilling the need of green production of strawberry. ACS Appl. Mater. Interfaces. 2021;13:36350–36360. doi: 10.1021/acsami.1c09887. [DOI] [PubMed] [Google Scholar]
- 3.Reganold J.P., Wachter J.M. Organic agriculture in the twenty-first century. Nat. Plants. 2016;2:15221. doi: 10.1038/nplants.2015.221. [DOI] [PubMed] [Google Scholar]
- 4.Pauline L. Sustainable agriculture for biodiversity, biodiversity for sustainable agriculture. Biodiversity. 2017;18:124–125. doi: 10.1080/14888386.2017.1366873. [DOI] [Google Scholar]
- 5.Kumar J.K., Monica S.S., Bojan V., Suganthi A., Paramasivam M. Impact of pesticide exposure on environment and biodiversity: A review. Agric. Rev. 2021 doi: 10.18805/ag.R-2325. in press. [DOI] [Google Scholar]
- 6.Gotoh T., Suwa A., Kitashima Y., Rezk H. Developmental and reproductive performance of Tetranychus pueraricola Ehara and Gotoh (Acari: Tetranychidae) at four constant temperatures. Appl. Entomol. Zool. 2004;39:675–682. doi: 10.1303/aez.2004.675. [DOI] [Google Scholar]
- 7.Gotoh T., Suwa A., Kitashima Y. Development and oviposition of Tetranychus pueraricola ehara and gotoh (acari: Tetranychidae) on various plants. J. Acarol. Soc. Jpn. 2004;13:135–140. doi: 10.2300/acari.13.135. [DOI] [Google Scholar]
- 8.Ilias A., Vontas J., Tsagkarakou A. Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014;48:17–28. doi: 10.1016/j.ibmb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Ilias A., Vassiliou V.A., Vontas J., Tsagkarakou A. Molecular diagnostics for detecting pyrethroid and abamectin resistance mutations in Tetranychus urticae. Pestic. Biochem. Physiol. 2017;135:9–14. doi: 10.1016/j.pestbp.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Jin P.Y., Tian L., Chen L., Hong X.Y. Spider mites of agricultural importance in China, with focus on species composition during the last decade (2008–2017) Syst. Appl. Acarol. 2018;23:2087–2098. doi: 10.11158/saa.23.11.1. [DOI] [Google Scholar]
- 11.Van N.P., Van L.T., Khajehali J., Vanholme B., Tirry L. Mutations in the mitochondrial cytochrome b of Tetranychus urticae Koch (Acari:Tetranychidae) confer cross-resistance between bifenazate and acequinocyl. Pest Manag. Sci. 2009;65:404–412. doi: 10.1002/ps.1705. [DOI] [PubMed] [Google Scholar]
- 12.Vassiliou V.A., Kitsis P. Acaricide resistance in Tetranychus urticae (Acari:Tetranychidae) populations from Cyprus. J. Econ. Entomol. 2013;106:1848–1854. doi: 10.1603/EC12369. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Zhang Y.J., Xie W., Wu Q.J., Wang S.L. Sublethal effects of spinetoram on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae) Pestic. Biochem. Physiol. 2016;132:102–107. doi: 10.1016/j.pestbp.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Xu D.D., He Y.Y., Zhang Y.J., Xie W., Wu Q.J., Wang S.L. Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic. Biochem. Physiol. 2018;150:89–96. doi: 10.1016/j.pestbp.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Ionel I.L., Mara G., Stefania R., Margarita G.O., Corina P. A hazard to human health-pesticide residues in some vegetal and animal foodstuff. J. Biotechnol. 2019;305:S22–S23. doi: 10.1016/j.jbiotec.2019.05.089. [DOI] [Google Scholar]
- 16.Shahid E., Khan D.J., Qaisrani M., Noman M., Rani A., Ali S. Effect of pesticide residues on agriculture crops. J. Toxicol. Pharmaceut. Sci. 2021;5:18–23. [Google Scholar]
- 17.(USTCA) Ulanqab Science and Technology Commissioners Association The scientific and technological achievements of GC16 ecological preparations have taken root in Hainan. China Rural Sci. Technol. 2021;5:60–61. [Google Scholar]
- 18.Hou Q.L., Zhang Y.Q., Li C.X., Ding W., Liu X.J., Luo J.X. Acaricidal toxicity of scopoletin combined with Ca2+ and its influence on Ca2+-ATPase activity in Tetranychus cinnabarinus (Boisduval) Chin. J. Pestic. Sci. 2015;17:475–479. doi: 10.3969/j.issn.1008-7303.2015.04.14. [DOI] [Google Scholar]
- 19.Chen J., Ma H.W., Zhang P., Liu M.T. Preparation of potassium chloride from K-feldspar with calcium chloride as additive agent: A review. Chem. Ind. Eng. Prog. 2016;35:3954–3963. doi: 10.16085/j.issn.1000-6613.2016.12.031. [DOI] [Google Scholar]
- 20.Ding X.T., Jiang J.G., Li D.A., Li T.R., Wang J.M. Immobilizing effects of calcium-based agents on soil contaminated by vanadium ore. J. Agro-Environ. Sci. 2016;35:274–280. doi: 10.11654/jaes.2016.02.010. [DOI] [Google Scholar]
- 21.Alhajj M., Montero N., Yarce C., Salamanca C. Lecithins from vegetable, land, and marine animal sources and their potential applications for cosmetic, food, and pharmaceutical sectors. Cosmetics. 2020;7:87. doi: 10.3390/cosmetics7040087. [DOI] [Google Scholar]
- 22.Szuhaj B., Yeo J.D., Shahidi F. Lecithins. In: Shahidi F., editor. Bailey’s Industrial Oil and Fat Products. Wiley; Hoboken, NJ, USA: 2020. pp. 1–86. [DOI] [Google Scholar]
- 23.Younes M., Aquilina G., Castle L., Engel K.H., Fowler P., Frutos M.J., Furst P., Rainer G., Gundert-Remy U., Trine H., et al. Safety of use of oat lecithin as a food additive. EFSA J. 2020;18:5969. doi: 10.2903/j.efsa.2020.5969. [DOI] [Google Scholar]
- 24.Wang M.Z., Yan W.Q., Zhou Y.L., Fan L.P., Liu Y.F., Li J.W. Progress in the application of lecithins in water-in-oil emulsions. Trends Food Sci. Technol. 2021;118:388–398. doi: 10.1016/j.tifs.2021.10.019. [DOI] [Google Scholar]
- 25.Velasco L., Rascón M., Calvo M.V., Montalvo R., Fontecha J., Garcia H.K. Krill lecithin as surfactant for preparation of oil/water nanoemulsions as curcumin carriers. Eur. J. Lipid Sci. Technol. 2021;123:2000238. doi: 10.1002/ejlt.202000238. [DOI] [Google Scholar]
- 26.Kumar N., Minhas P.S., Ambasankar K., Krishnani K.K., Rana R.S. Dietary lecithin potentiates thermal tolerance and cellular stress protection of milk fish (Chanos Chanos) reared under low dose endosulfan-induced stress. J. Therm. Biol. 2014;46:40–46. doi: 10.1016/j.jtherbio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Djekic L., Krajisnik D., Martinovic M., Djordjevic D., Primorac M. Characterization of gelation process and drug release profile of thermosensitive liquid lecithin/poloxamer 407 based gels as carriers for percutaneous delivery of ibuprofen. Int. J. Pharm. 2015;490:180–189. doi: 10.1016/j.ijpharm.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Schirra M., D’Aquino S., Migheli Q., Pirisi F.M., Angioni A. Influence of post-harvest treatments with fludioxonil and soy lecithin co-application in controlling blue and grey mould and fludioxonil residues in coscia pears. Food Addit. Contam. Part A. 2009;26:68–72. doi: 10.1080/02652030802348080. [DOI] [PubMed] [Google Scholar]
- 29.Ozgen M., Palta J.P. A natural lipid, lysophosphatidylethanolamine (lpe), can mitigate adverse effect of fungicide, chlorothalonil, on fruit set and yield in cranberries. Acta Hortic. 2003;628:747–752. doi: 10.17660/ActaHortic.2003.628.94. [DOI] [Google Scholar]
- 30.Jin P.Y., Tian L., Chen L., Hong X.Y. High genetic diversity in a ‘recent outbreak’ spider mite, Tetranychus pueraricola, in mainland China. Exp. Appl. Acarol. 2019;78:15–27. doi: 10.1007/s10493-019-00377-1. [DOI] [PubMed] [Google Scholar]
- 31.Ehara S., Gotoh T. Two new species of spider mites occurring in Japan (Acari: Tetranychidae) J. Acarol. Soc. Jpn. 1996;5:17–25. doi: 10.2300/acari.5.17. [DOI] [Google Scholar]
- 32.Suwa A., Gotoh T. Geographic variation in diapause induction and mode of diapause inheritance in Tetranychus pueraricola. J. Appl. Entomol. 2006;130:329–335. doi: 10.1111/j.1439-0418.2006.01050.x. [DOI] [Google Scholar]
- 33.Kabir K.H., Chapman R.B. Operational and biological factors influencing responses of spider mites (Acari: Tetranychidae) to propargite by using the Petri dish-Potter tower method. J. Econ. Entomol. 1997;90:272–277. doi: 10.1093/jee/90.2.272. [DOI] [Google Scholar]
- 34.Ay R. Determination of susceptibility and resistance of some greenhouse populations of Tetranychus urticae Koch to chlorpyrifos (Dursban 4) by the petri dish-Potter tower method. J. Pest Sci. 2005;78:139–143. doi: 10.1007/s10340-005-0084-7. [DOI] [Google Scholar]
- 35.Tang X.F., Zhang Y.J., Wu Q.J., Xie W., Wang S.L. Stage-specific expression of resistance to different acaricides in four field populations of Tetranychus urticae (Acari: Tetranychidae) J. Econ. Entomol. 2014;107:1900–1907. doi: 10.1603/EC14064. [DOI] [PubMed] [Google Scholar]
- 36.Amer M., Mekky H., Fedawy H. Effect of combined plant essential oils on Dermanyssus gallinae: In vitro and in vivo study. J. World’s Poult. Res. 2020;10:199–206. doi: 10.36380/scil.2020.wvj26. [DOI] [Google Scholar]
- 37.Cheng S., Lin R., You Y., Lin T., Zeng Z., Yu C. Comparative sensitivity of Neoseiulus cucumeris and its prey Tetranychus cinnabarinus, after exposed to nineteen pesticides. Ecotoxicol. Environ. Saf. 2021;217:112234. doi: 10.1016/j.ecoenv.2021.112234. [DOI] [PubMed] [Google Scholar]
- 38.Dittrich V., Cranham J.E., Jepson L.R., Helle W. Revised method for spider mites and their eggs (e.g., Tetranychus spp. and Panonychus ulmi Koch), FAO method No. 10a. FAO Plant Prod. Prot. Pap. 1980;21:49–53. [Google Scholar]
- 39.Wang L., Zhang Y.J., Xie W., Wu Q.J., Wang S.L. A bioassay for evaluation of the resistance of Tetranychus urticae (Acari: Tetranychidae) to selected acaricides. Syst. Appl. Acarol. 2015;20:579–590. doi: 10.11158/saa.20.6.1. [DOI] [Google Scholar]
- 40.Tarelli G., Zerba E., Alzogaray R. Toxicity to vapor exposure and topical application of essential oils and monoterpenes on Musca domestica (Diptera: Muscidae) J. Econ. Entomol. 2009;102:1383–1388. doi: 10.1603/029.102.0367. [DOI] [PubMed] [Google Scholar]
- 41.Qiu Y., Song X.H., Huang Y.Z. Behavior response and insecticidal mechanism of β-Asarone against Sitophilus Zeamais Adult. J. Chin. Cereals Oils Assoc. 2014;29:80–85. [Google Scholar]
- 42.Pashte V., Patil S.C. Toxicity and poisoning symptoms of selected insecticides to honey bees (Apis mellifera mellifera L.) Arch. Biol. Sci. 2017;70:5–12. doi: 10.2298/ABS170131020P. [DOI] [Google Scholar]
- 43.Li C.X., Zhang Y.Q., Wang D., Zhang B.C., Luo J.X., Ding W. Effects of curcumin and scopoletinon morphological change and water loss of Tetranychus cinnabarinus Boisduval (Acarina: Tetranychidae) J. Southwest Univ. 2017;39:10–15. doi: 10.13718/j.cnki.xdzk.2017.11.002. [DOI] [Google Scholar]
- 44.Walther P. Cryo-SEM and TEM of high pressure frozen Cells-some technical contributions. Microsc. Microanal. 2001;7:728–729. doi: 10.1017/S1431927600029718. [DOI] [Google Scholar]
- 45.Yu Z.M., Kang B., He X.W., Lv S.L., Bai Y.H., Ding W.N., Chen M., Cho H.T., Wu P. Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011;66:725–734. doi: 10.1111/j.1365-313X.2011.04533.x. [DOI] [PubMed] [Google Scholar]
- 46.Yan H.T., Wang Y., Zhang J.R., Cui X.R., Wu J.S., Zhou J., Lu J., Guo R.Y., Ou M., Lai H.X., et al. Rice root hair phenotypes imaged by Cryo-SEM. Bio-Protocol. 2021;11:e4037. doi: 10.21769/BioProtoc.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parastou T., Cecilia C., Negar K. Sample Preparation for Transmission Electron Microscopy. Methods Mol. Biol. 2019;1897:417–424. doi: 10.1007/978-1-4939-8935-5_33. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Wang X., Li M.J., Yang C.L., Wang X.C. M05B5.4 (Lysosomal phospholipase A2) promotes disintegration of autophagic vesicles to maintain C. elegans development. Autophagy. 2021;18:595–607. doi: 10.1080/15548627.2021.1943178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma S.C., Jiang W.Q., Li Q., Li T., Wu W.J., Bai H.Y., Shi B.J. Design, synthesis, and study of the insecticidal activity of novel steroidal 1,3,4-Oxadiazoles. J. Agric. Food Chem. 2021;69:11572–11581. doi: 10.1021/acs.jafc.1c00088. [DOI] [PubMed] [Google Scholar]
- 50.Ma T.F., Zhao L.Y., Zhang J., Tang R.F., Wang X., Liu N., Zhang Q., Wang F.Y., Li M.J., Shan Q., et al. A pair of transporters controls mitochondrial Zn2+ levels to maintain mitochondrial homeostasis. Protein Cell. 2021;13:180–202. doi: 10.1007/s13238-021-00881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q., Ding L.J., Li M., Cui W.W., Ding W., Luo J.X., Zhang Y.Q. Action modes of Aloe vera L. extracts against Tetranychus cinnabarinus Boisduval (Acarina: Tetranychidae) Agric. Sci. 2013;4:117–122. doi: 10.4236/as.2013.43018. [DOI] [Google Scholar]
- 52.Zhou H., Liu S., Wan F., Jian Y., Guo F., Chen J., Ning Y.S., Ding W. Graphene oxide-acaricide nanocomposites advance acaricidal activity of acaricides against Tetranychus cinnabarinus by directly inhibiting the transcription of a cuticle. Environ. Sci. Nano. 2021;8:3122–3137. doi: 10.1039/D1EN00521A. [DOI] [Google Scholar]
- 53.Zhou H., Wan F., Guo F., Liu J., Ding W. High value-added application of a renewable bioresource as acaricide: Investigation the mechanism of action of scoparone against Tetranychus cinnabarinus. J. Adv. Res. :2021. doi: 10.1016/j.jare.2021.08.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J., Feng G., Ma Z.Q., Feng J.T., Zhang X. Toxicity of sarisan against Mythimna separata Walker and its effects on AChE and ATPases. Acta Entomol. Sin. 2007;50:574–577. doi: 10.16380/j.kcxb.2007.06.005. [DOI] [Google Scholar]
- 55.Zhang Y.Q., Ding W., Zhao Z.M., Wu J., Fan Y.H. Studies on acaricidal bioactivities of Artemisia annua L. extracts against Tetranychus cinnabarinus Bois. (Acari: Tetranychidae) Agric. Sci. China. 2008;7:577–584. doi: 10.1016/S1671-2927(08)60055-3. [DOI] [Google Scholar]
- 56.Subhomay S., Suresh W., Jitendra K., Panwar V.P.S., Parmar B.S. Ovicidal and egg hatching inhibiting activity of azadirachtin A, B, and H against the maize stem borer Chillo partellus (Swinhoe) Pestic. Res. J. 2005;17:6–9. [Google Scholar]
- 57.Zhou H., Liu J.L., Wan F.L., Guo H., Ning Y.S., Liu S.S., Ding W. Insight into the mechanism of action of scoparone inhibiting egg development of Tetranychus cinnabarinus Boisduval. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021;246:109055. doi: 10.1016/j.cbpc.2021.109055. [DOI] [PubMed] [Google Scholar]
- 58.Chen N., Pei X.J., Li S., Fan Y.L., Liu T.X. Involvement of integument-rich CYP4G19 in hydrocarbon biosynthesis and cuticular penetration resistance in Blattella germanica (L.) Pest Manag. Sci. 2020;76:215–226. doi: 10.1002/ps.5499. [DOI] [PubMed] [Google Scholar]
- 59.Su C., Xia X. Sublethal effects of methylthio-diafenthiuron on the life table parameters and enzymatic properties of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) Pestic. Biochem. Physiol. 2020;162:43–51. doi: 10.1016/j.pestbp.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Echlin P. The application of scanning electron microscopy to biological research. Philo. T R. Soc. B. 1971;261:51–59. doi: 10.1098/rstb.1971.0036. [DOI] [Google Scholar]
- 61.Noble-Nesbitt J. Structural aspects of penetration through insect cuticles. Pestic. Sci. 1970;1:204–208. doi: 10.1002/ps.2780010510. [DOI] [Google Scholar]
- 62.Johnson R.A., Kaiser A., Quinlan M., Sharp W. Effect of cuticular abrasion and recovery on water loss rates in queens of the desert harvester ant Messor pergandei. J. Exp. Biol. 2011;214:3495–3506. doi: 10.1242/jeb.054304. [DOI] [PubMed] [Google Scholar]
- 63.Feng M., Shi B., Zhao Y., Hu Z.N., Wu W.J. Histopathological effects and immunolocalization of periplocoside NW from Periploca sepium Bunge on the midgut epithelium of Mythimna separata Walker larvae. Pestic. Biochem. Physiol. 2014;115:67–72. doi: 10.1016/j.pestbp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Huang T., Jiang H., Zhao Y., He J., Cheng H.G., Martyniuket C.J. A comprehensive review of 1,2,4-triazole fungicide toxicity in zebrafish (Danio rerio): A mitochondrial and metabolic perspective. Sci. Total Environ. 2021;809:151177. doi: 10.1016/j.scitotenv.2021.151177. [DOI] [PubMed] [Google Scholar]
- 65.Nobuyoshi T., Hirofumi N., Yasuhiro S., Naoki I. Development of a new acaricide, cyflumetofen. J. Pestic. Sci. 2012;37:263–264. doi: 10.1584/jpestics.J12-03. [DOI] [Google Scholar]
- 66.Del Valle I., Gómez-Durán A., Holt W.V., Muiño-Blanco T., Cebrián-Pérez J.A. Soy lecithin Interferes with mitochondrial function in frozen-thawed ram spermatozoa. J. Androl. 2012;33:717–725. doi: 10.2164/jandrol.111.014944. [DOI] [PubMed] [Google Scholar]
- 67.Zhou H., Guo F., Luo J., Zhang Y., Liu J., Zhang Y.C., Zheng X.Y., Wan F.L., Ding W. Functional analysis of an upregulated calmodulin gene related to the acaricidal activity of curcumin against Tetranychus cinnabarinus (Boisduval) Pest Manag. Sci. 2021;77:719–730. doi: 10.1002/ps.6066. [DOI] [PubMed] [Google Scholar]
- 68.Zhou H., Zhang Y., Lai T., Liu X., Guo F., Guo T., Ding W. Acaricidal mechanism of scopoletin against Tetranychus cinnabarinus. Front. Physiol. 2019;10:164. doi: 10.3389/fphys.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma X.F., Zhang Y.Y., Zhou H., Liu J.L., Guo H., Luo J.X., Ding W., Zhang Y.Q. Silencing T-type voltage-gated calcium channel gene reduces the sensitivity of Tetranychus cinnabarinus (Boisduval) to scopoletin. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019;227:108644. doi: 10.1016/j.cbpc.2019.108644. [DOI] [PubMed] [Google Scholar]
- 70.Zhou H., Zhang Y., Lai T., Wang D., Liu J.L., Guo H., Ding W. Silencing chitinase genes increases susceptibility of Tetranychus cinnabarinus (Boisduval) to Scopoletin. BioMed Res. Int. 2017;7:9579736. doi: 10.1155/2017/9579736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in article or Supplementary Materials.