Abstract
Our study aims to develop an effective integrated machine learning (ML) scheme to predict vascular events and bleeding in patients with nonvalvular atrial fibrillation taking dabigatran and identify important risk factors. This study is a post-hoc analysis from the Randomized Evaluation of Long-Term Anticoagulant Therapy trial database. One traditional prediction method, logistic regression (LGR), and four ML techniques—naive Bayes, random forest (RF), classification and regression tree, and extreme gradient boosting (XGBoost)—were combined to construct our scheme. Area under the receiver operating characteristic curve (AUC) of RF (0.780) and XGBoost (0.717) was higher than that of LGR (0.674) in predicting vascular events. In predicting bleeding, AUC of RF (0.684) and XGBoost (0.618) showed higher values than those generated by LGR (0.605). Our integrated ML feature selection scheme based on the two convincing prediction techniques identified age, history of congestive heart failure and myocardial infarction, smoking, kidney function, and body mass index as major variables of vascular events; age, kidney function, smoking, bleeding history, concomitant use of specific drugs, and dabigatran dosage as major variables of bleeding. ML is an effective data analysis algorithm for solving complex medical data. Our results may provide preliminary direction for precision medicine.
Keywords: arrhythmia, cardioembolic stroke, non-vitamin K antagonist oral anticoagulants, dabigatran, machine learning
1. Introduction
Stroke is the leading cause of death and disability worldwide [1]. Cardioembolic stroke is a primary subtype, and nonvalvular atrial fibrillation (NVAF) is one of the most common risk factors for cardioembolic stroke, with a global prevalence of 1–2% [2]. In recent decades, this event has been treated by shifting from the traditional vitamin K antagonist warfarin to nonvitamin K antagonists (NOACs) [3,4,5]. Because of the significant increase in the clinical demands for NOACs, off-label use, especially the dosage selection regimen, has become an important issue in recent years. In real-world studies, off-label low-dose NOACs were prescribed to approximately 9–31% of patients with NVAF [6,7]. Adverse effects including a higher risk of ischemic stroke and systemic embolism have been observed in these patients [8,9].
Dabigatran etexilate, the only direct thrombin inhibitor, is an NOAC with two approved doses based on the Randomized Evaluation of Long-Term Anticoagulant Therapy (RE-LY) trial [10]. In this trial, low-dose dabigatran (110 mg twice daily) had similar vascular prevention effects as those of warfarin with lower rates of major hemorrhage. High-dose dabigatran (150 mg twice daily) was associated with lower rates of vascular events but similar rates of major hemorrhage. The dosage adjustment plan of dabigatran was based on previous studies and expert opinions (European label) [11], which suggested that clinicians could decrease the dosage of dabigatran among patients aged >80 years, those aged 75–80 years with a high risk of bleeding, or those with concomitant use of verapamil. Physicians must balance the risk of recurrent stroke and bleeding tendency in clinical practice. Currently, the congestive heart failure, hypertension, age ≥ 75 years [doubled], diabetes mellitus, prior stroke, transient ischemic attack or thromboembolism [doubled], vascular disease, age 65–74 years, and sex category (CHA2DS2-VASc) [12] and hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly [age ≥ 65 years], drugs/alcohol concomitantly (HAS-BLED) [13] scores are used to calculate the risk of recurrent ischemic stroke and assess bleeding risk, respectively. However, these tools share the same grading factors: old age, hypertension, and stroke history. This may lead to a clinical dilemma, i.e., one patient could score high in both scoring systems. Although the CHA2DS2-VASc score has been widely used for years with convenience and reliability [14,15], insufficient prediction performance (C statistic of 0.679) has remained a concern [16]. Machine learning (ML) methods have been recently used as well-constructed analytical, classification and prediction tools for medical problems [17,18,19,20,21,22]. Their advantage and performance in demonstrating complex relationships between risk factors and outcomes and analyzing important information hidden in the vast amount of medical data have made them an emerging research topic. Kamel et al. [23] and Chun et al. [24] have confirmed the feasibility of predicting vascular events. Unlike prediction models that use only one ML technique that might be insufficient to provide complete, adequate and stable feature selection results, our study developed an integrated ML feature selection scheme with the benefits of stable and balanced performance. Our method may reveal important variables influencing the efficacy and safety of dabigatran to provide a precision medical suggestion regarding dose selection and risk control for patients with different characteristics.
2. Materials and Methods
2.1. Study Population
This study is a post-hoc analysis based on RE-LY trial dataset. This study was reviewed and approved by the Research Ethics Review Committee of the Fu Jen Catholic University Hospital. The requirement for informed consent was waived, since the data contain only de-identified information.
In the RE-LY trial, >18,000 patients with newly diagnosed arrhythmia and indications of secondary prevention with an anticoagulant were randomized to receive dabigatran 110 or 150 mg twice daily or an adjusted dose of warfarin with a median follow-up period of approximately 2 years. Exclusion criteria included a history of severe heart valve disorders, a recent stroke, and renal insufficiency. The primary outcome was stroke or systemic embolism and the primary safety outcome was major hemorrhage. The definitions and results of other secondary outcomes have been described in detail and published. We collected the data of patients taking dabigatran with complete follow-up in the RE-LY trial for the present analysis.
2.2. Proposed Integrated Machine Learning Scheme
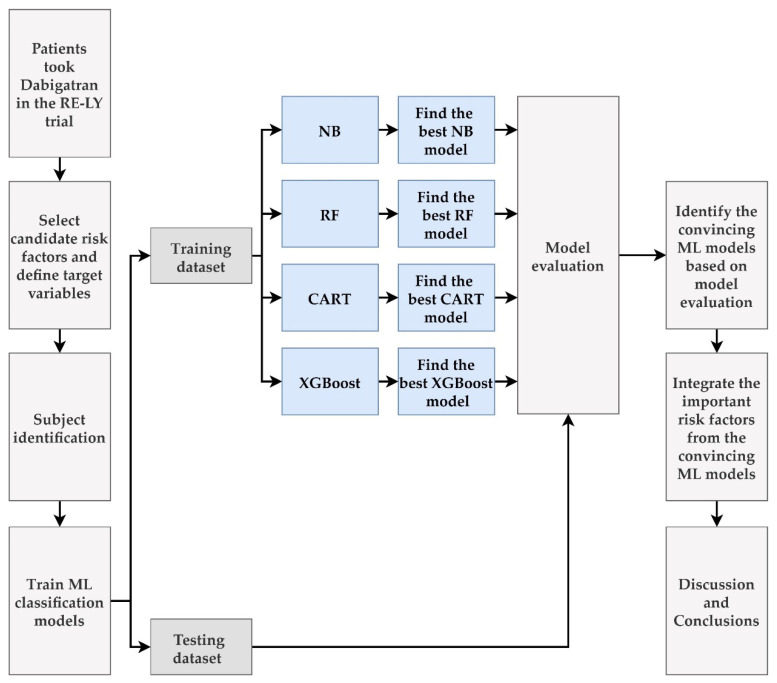
We proposed an integrated ML feature selection scheme for predicting vascular events and bleeding in patients with NVAF taking dabigatran and for identifying important risk factors. Figure 1 shows the process of establishing the proposed scheme.
Figure 1.
Flow chart of the proposed integrated ML feature selection scheme.
First step: Identify risk factors as predictor variables and define target variables. For risk factors we referred to the recommendations in the guidelines of the American Heart Association and the European Society of Cardiology [11,25], which included sex; age; body mass index (BMI); body weight; ethnicity; kidney function abnormality; concomitant use of specific drugs; history of hypertension, stroke, previous bleeding, myocardial infarction (MI), diabetes mellitus (DM), congestive heart failure (CHF), or systemic embolism; smoking; and liver function abnormality. Boundaries of subgroups in most variables followed the definition of CHA2DS2-VASc and HAS-BLED scores. BMI was classified according to the definition of the World Health Organization [26]. Moderate and severe kidney function abnormality was labeled according to the United States Food and Drug Administration (USFDA) [27].
For analyzing the influence of these factors on efficacy and safety, we selected two target variables including vascular events (P1: stroke, MI, systemic embolism, and vascular death) and major bleeding (P2: major bleeding defined as blood loss with a decrease in hemoglobin level of ≥2 g/dL (1.2 mmol/L), transfusion of ≥2 packed red blood cells, or symptomatic bleeding in a critical area or organ). Our presumed important variables and prognostic outcomes were individually categorized according to the definition shown in Table 1.
Table 1.
Description of predictor and target variables in this study.
| Variables | Description | Units | |
|---|---|---|---|
| V1 | Sex | 0: Male; 1: Female | - |
| V2 | Age | 1: <65; 2: ≥65 and <75; 3: ≥75 | years |
| V3 | BMI | 1: <18.5; 2: ≥18.5 and <30; 3: ≥30 | kg/m2 |
| V4 | Body weight | 0: <60; 1: ≥60 | kg |
| V5 | Ethnicity | 0: Arab/others; 1: European | - |
| V6 | Hypertension history | 0: Record of hypertension that required medical treatment); 1: No | - |
| V7 | Kidney function (GFR) | 1: <30; 2: ≥30 and <50; 3: ≥50 | mL/min/1.73 m2 |
| V8 | Previous stroke history | 0: History of stroke or TIA; 1: No | - |
| V9 | Previous bleeding history | 0: History of bleeding; 1: No | - |
| V10 | Concomitant use of drugs | 0: Concomitant use of verapamil, diltiazem, antithrombotic agent, NSAID, or COX inhibitor; 1: No | - |
| V11 | History of MI | 0: History of MI; 1: No | - |
| V12 | History of DM | 0: History of DM; 1: No | - |
| V13 | History of CHF | 0: Medical history of CHF or heart echo revealed ejection fraction <40%; 1: No | - |
| V14 | Smoking | 1: Never; 2: Current smoker; 3: Former smoker | - |
| V15 | History of systemic embolism | 0: History of systemic embolism; 1: No | - |
| V16 | Liver function abnormality # |
0: Presence of liver function abnormality 1: No |
- |
| V17 | Anemia | 0: Hemoglobin ≥10; 1: <10 | g/dL |
| V18 | Medicine dosage (dabigatran) |
0: 110 mg twice per day 1: 150 mg twice per day |
- |
| P1 | Vascular events † |
0: No vascular event happened within the first year of follow-up 1: Yes |
- |
| P2 | Bleeding events * |
0: No bleeding event happened within the first year of follow-up 1: Yes |
- |
Abbr.: BMI, body mass index; GFR, glomerular filtration rate; TIA, transient ischemic attack; NSAID, nonsteroidal anti-inflammatory drug; COX, cyclooxygenase; MI, myocardial infarction; DM, diabetes mellitus; CHF, congestive heart failure. # Liver function abnormality defined as a medical history of cirrhosis or abnormal biochemical data when the patients were enrolled (bilirubin level more than two times the upper limit of normal, plus one or more of aspartate transaminase, alanine transaminase, or alkaline phosphatase level more than three times the upper limit of normal). † Vascular events defined as stroke, myocardial infarction, systemic embolism, and vascular death. * Major bleeding was defined as blood loss with a decrease in hemoglobin level of ≥2 g/dL (1.2 mmol/L), transfusion of ≥2 packed red blood cells, or symptomatic bleeding in a critical area or organ. Critical areas were intraocular, intracranial (including hemorrhagic stroke), intraspinal, intramuscular with compartment syndrome, retroperitoneal, intraarticular, or pericardial.
Subjects were identified according to participants’ characteristics and laboratory data collected during their enrolment in the RE-LY trial. Only patients with available complete information were included in our analysis. Two independent investigators confirmed prognostic outcomes according to the criteria mentioned in the trial.
The study protocol included one traditional prediction method, logistic regression (LGR), and four ML techniques, viz., naive Bayes (NB), random forest (RF), classification and regression tree (CART), and extreme gradient boosting (XGBoost). NB is a popular ML model used for classification tasks. This algorithm can sort objects according to specific characteristics and variables based on the Bayes theorem. It calculates the probability of hypotheses on presumed groups [28]. RF is an ensemble learning method developed by constructing several decision trees. It collects numerous random samples of variables as the training dataset to alleviate the overfitting feature of decision trees. Each tree in the RF outputs its prediction result, and the class with the most votes sums up the best performance model [29]. CART is a classification ML algorithm that constructs a decision tree based on Gini’s impurity index. The decision tree structure comprises root, internal, and leaf nodes, which may represent training data and decision-making points. The CART prediction model is constructed by picking variables and evaluating split ends until an appropriate tree is produced [30]. XGBoost is an optimized distributed gradient boosting system that implements ML algorithms based on the gradient boosting framework. It uses the regularization term to control model complexity and simultaneously uses first- and second-order derivatives to perform a second-order Taylor expansion of the loss function [31]. These ML methods, which share characteristics of interpretable tools for prediction and classification with good performance in vast unprocessed data, have been widely applied in solving cerebrovascular and cardiovascular disease problems [32,33,34,35]. Meanwhile, the logistic regression, which is a widely accepted analytic method in medical research, was defined as the benchmark in our study.
Second step: Train NB, RF, CART, and XGBoost models and evaluate their predictive performance. The models are trained using two combinations of predictor and target variables. One combination involves using 18 variables (V1–V18) as predictors and vascular events (P1) as the target variable; the other combination involves using V1–V18 as predictors but bleeding (P2) as the target variable. In training these models, the data of recruited patients were randomly separated into 90% training and 10% testing datasets according to the 10-fold cross-validation (CV) method. Our scheme applied the 10-fold nested CV method for enhancing stability to estimate the best performance of each model [36]. This process consisted of 10-fold inner CV for tuning and then determining the best hyperparameter set of each method for model selection and 10-fold outer CV for evaluating the predictive performance of the best model of each method for model evaluation.
These models’ efficacy were evaluated based on their mean and standard deviation of accuracy, sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC) [37]. Sensitivity is the proportion of true positives tests of all patients with predicted events. Specificity is the proportion of true negative tests out of all patients who have not predicted events. Accuracy is the proportion of correct predictions (both true positives and true negatives tests) among the total number of patients examined. ROC curve is a graphic performance measurement of a classification model at various classification thresholds. AUC is the Area under the ROC curve, which provides an aggregate performance measure across all possible classification thresholds. The best hyperparameters with leading validation performance based on the AUC value for each model can be chosen to construct tuned NB, RF, CART, and XGBoost best models. The results of the best performance model with AUC values exceeding those of LGR were the cornerstone of our predicting models of vascular events and bleeding.
Third step: Importance ranking of risk factors. The “caret” R package version 6.0-90 [38] was applied for each of the four methods to generate each variable’s importance value. We defined the priority demonstrated in each model ranking 1 as the most critical factor and 18 as the least critical factor. Each model would perform 10 times due to the use of 10-fold outer CV to gain the average ranking of each variable for more confident results. Individual ML methods may produce different importance rankings owing to distinct characteristics. Ensemble machine learning method based on a combination of multiple models’ outputs is widely accepted and has produced good results in recent years [39]. An integrated ML feature selection scheme might assemble the prediction powers of these methods. We summarized the major important variables from the average ranking of each risk factor based on the identified convincing ML models to enhance stability and integrity.
According to the individual priority of the variables presented in the predictive models of vascular events and bleeding, we may establish an instruction concept for patients with NVAF taking dabigatran. In the final stage, we summarized our significant findings and discussed them in light of previous concepts.
All analyses were conducted using R software version 4.1.2 (R core team, Vienna, Austria) and RStudio version 1.1.453 (http://www.R-project.org; accessed on 2 March 2022; https://www.rstudio.com/products/rstudio/; accessed on 2 March 2022). The methods were applied using the R software with the required installed packages: “randomForest” package version 4.6-14 for RF [40]; “rpart” R package version 4.1-15 [41] for CART; and “XGBoost” package version 1.5.0.2 for XGBoost [42]. To estimate the best parameter set for developing effective RF, CART, and XGBoost methods, the “caret” package version 6.0-90 [38] was used for tuning the relevant hyperparameters. NB was implemented using the “klaR” package version 0.6-15 [43] with the default setting of hyperparameters.
3. Results
There were 12,091 patients randomized to take dabigatran in the RE-LY trial. After excluding 289 patients with missing data, 11802 patients were enrolled in our study. Subjects’ demographic data are outlined in Table 2. There were 318 (2.69%) patients with vascular events, and 2238 (18.96%) patients had bleeding within the first year of follow-up when taking dabigatran, while others were event-free.
Table 2.
Subjects’ demographics.
| Characteristics | Metrics |
|---|---|
| V1 Sex | N (%) |
| 0: Male | 7519 (63.70) |
| 1: Female | 4284 (36.30) |
| V2 Age (years) | N (%) |
| 1: <65 | 1982 (16.79) |
| 2: ≥65 and <75 | 5123 (43.41) |
| 3: ≥75 | 4697 (39.80) |
| V3 BMI (kg/m2) | N (%) |
| 1: <18.5 | 123 (1.04) |
| 2: ≥18.5 and <30 | 7589 (64.30) |
| 3: ≥30 | 4091 (34.66) |
| V4 Body weight | N (%) |
| 0: <60 | 1098 (9.30) |
| 1: ≥60 | 10,705 (90.70) |
| V5 Ethnicity | N (%) |
| 0: Arab/others | 3594 (30.45) |
| 1: European | 8209 (69.55) |
| V6 Hypertension history | N (%) |
| 0: Record of hypertension that required medical treatment | 9301 (78.80) |
| 1: No | 2502 (21.20) |
| V7 Kidney function (GFR) | N (%) |
| 1: <30 | 45 (0.38) |
| 2: ≥30 and <50 | 2245 (19.02) |
| 3: ≥50 | 9513 (80.60) |
| V8 Previous stroke history | N (%) |
| 0: Yes | 2366 (20.05) |
| 1: No | 9437 (79.95) |
| V9 Previous bleeding history | N (%) |
| 0: Yes | 774 (6.56) |
| 1: No | 11,029 (93.44) |
| V10 Concomitant use of drugs | N (%) |
| 0: Yes | 2845 (24.10) |
| 1: No | 8958 (75.90) |
| V11 History of myocardial infarction | N (%) |
| 0: Yes | 1982 (16.79) |
| 1: No | 9821 (83.21) |
| V12 History of diabetes mellitus | N (%) |
| 0: Yes | 2739 (23.21) |
| 1: No | 9064 (76.79) |
| V13 History of congestive heart failure | N (%) |
| 0: Yes | 4125 (34.95) |
| 1: No | 7678 (65.05) |
| V14 Smoking | N (%) |
| 1: Never | 5781 (48.98) |
| 2: Current | 867 (7.35) |
| 3: Former | 5155 (43.68) |
| V15 History of systemic embolism | N (%) |
| 0: Yes | 306 (2.59) |
| 1: No | 11,497 (97.41) |
| V16 Liver function abnormality | N (%) |
| 0: Presence of liver function abnormality | 84 (0.71) |
| 1: No | 11,719 (99.29) |
| V17 Anemia | N (%) |
| 0: Hemoglobin ≥10 g/dL | 11,773 (99.75) |
| 1: Hemoglobin <10 g/dL | 30 (0.25) |
| V18 Medicine dosage (dabigatran) | N (%) |
| 1: 110 mg | 5870 (49.73) |
| 2: 150 mg | 5933 (50.27) |
| P1 Vascular events | N (%) |
| 0: No | 11,485 (97.31) |
| 1: Yes | 318 (2.69) |
| P2 Bleeding events | N (%) |
| 0: No | 9565 (81.04) |
| 1: Yes | 2238 (18.96) |
Abbr.: BMI, body mass index; GFR, glomerular filtration rate.
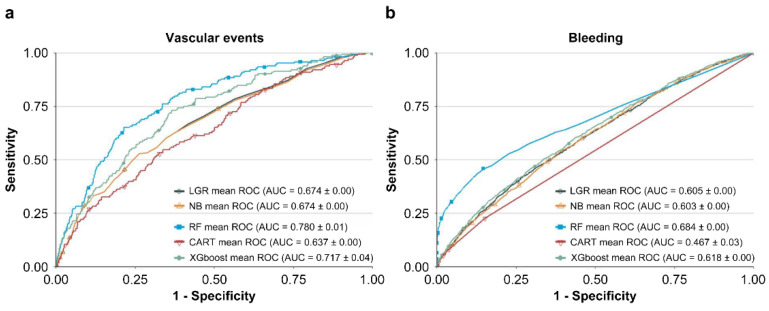
Table 3 shows the values of hyperparameters which train best NB, RF, CART, and XGBoost models with leading AUC values. The performances of LGR, NB, RF, CART, and XGBoost methods in predicting vascular events and bleeding are listed in Table 4. The ROC curve of each model is presented in Figure 2. In predicting vascular events, RF (AUC = 0.780) and XGBoost (AUC = 0.717) showed higher AUC values than LGR (AUC = 0.674). In predicting bleeding, RF (0.684) and XGBoost (0.618) showed higher AUC values than LGR (0.605). In contrast, NB and CART showed inferior performance to LGR in predicting vascular events and bleeding. Therefore, we selected RF and XGBoost as the basis of our integrated ML feature selection model.
Table 3.
Summary of the values of the hyperparameters which train the best NB, RF, CART, and XGBoost models.
| Methods | Hyperparameters | Best Value | Meanings |
|---|---|---|---|
| CART | minispilt | 20 | The minimum number of observations that must exist in a node for a split to be attempted. |
| minibucket | 20 | The minimum number of observations in any terminal node. | |
| maxdepth | 10 | The maximum depth of any node of the final tree. | |
| xval | 10 | Number of cross-validations. | |
| cp | 0.0013 | Complexity parameter: The minimum improvement in the model needed at each node. | |
| RF | ntree | 500 | The number of trees in forest. |
| mtry | 2 | The number of predictors sampled for splitting at each node. | |
| NB | fL | 1 | Adjustment of Laplace smoother. |
| usekernel | FALSE | Using kernel density estimate for continuous variable versus a Gaussian density estimate. | |
| adjust | 1 | Adjust the bandwidth of the kernel density. | |
| XGBoost | nrounds | 100 | The number of boosted trees. |
| maximum depth | 2 | The maximum depth of a tree. | |
| learning rate | 0.4 | Shrinkage coefficient of tree. | |
| gamma | 0 | The minimum loss reduction. | |
| subsample | 1 | Subsample ratio of columns when building each tree. | |
| colsample_bytree | 0.8 | Subsample ratio of columns when constructing each tree. | |
| rate_drop | 0.01 | Rate of trees dropped. | |
| skip_drop | 0.95 | Probability of skipping the dropout procedure during a boosting iteration. | |
| min_child_weight | 1 | The minimum sum of instance weight. |
Abbr: CART, classification and regression tree; RF, random forest; NB, naive Bayes; XGBoost, eXtreme gradient boosting.
Table 4.
Performance of the four machine learning methods in predicting (a) vascular events and (b) bleeding.
| Methods | Accuracy Mean (SD) |
Sensitivity Mean (SD) |
Specificity Mean (SD) |
AUC Mean (SD) |
|---|---|---|---|---|
| (a) Vascular events | ||||
| LGR | 0.574 (0.03) | 0.571 (0.04) | 0.707 (0.03) | 0.674 (0.00) |
| NB | 0.569 (0.03) | 0.565 (0.03) | 0.711 (0.04) | 0.674 (0.00) |
| RF | 0.890 (0.03) | 0.898 (0.03) | 0.599 (0.04) | 0.780 (0.01) |
| CART | 0.599 (0.09) | 0.598 (0.10) | 0.621 (0.09) | 0.637 (0.00) |
| XGBoost | 0.646 (0.09) | 0.645 (0.09) | 0.693 (0.04) | 0.717 (0.04) |
| (b) Bleeding | ||||
| LGR | 0.604 (0.03) | 0.622 (0.05) | 0.527 (0.05) | 0.605 (0.00) |
| NB | 0.599 (0.01) | 0.613 (0.02) | 0.537 (0.02) | 0.603 (0.00) |
| RF | 0.757 (0.01) | 0.822 (0.02) | 0.479 (0.02) | 0.684 (0.00) |
| CART | 0.787 (0.07) | 0.959 (0.12) | 0.052 (0.16) | 0.467 (0.03) |
| XGBoost | 0.625 (0.03) | 0.650 (0.05) | 0.517 (0.05) | 0.618 (0.00) |
Abbr.: SD, standard deviation; AUC, area under the receiver operating characteristic curve; LGR, logistic regression; NB, naive Bayes; RF, random forest; CART, classification and regression tree; XGBoost, eXtreme gradient boosting. In predicting both vascular events and bleeding, RF and XGBoost demonstrated higher AUC values (indicated in bold) than LGR.
Figure 2.
Receiver operating characteristic (ROC) curves of the five methods in predicting (a) vascular events and (b) bleeding.
Table 5 shows the overall importance ranking of each risk factor in predicting vascular events based on RF and XGBoost. The average rankings with 10-fold cross-validation of the two models were demonstrated as “Average Ranking of 10 Times RF” and “Average Ranking of 10 Times XGBoost”. The different methods generated individual importance ranking according to their analyzing rules. For a more comprehensive view, we summarized the findings of the two models equally in our integrated ML feature selection scheme. We obtained the “Average ranking of the 2 Models” with simple averaging the average ranking values from the RF and XGBoost models. To clarify the ranking, we ranked the result from 1 and showed that the “Final ranking in predicting vascular events” was listed using the “Average ranking of the 2 Models” value. Age; history of CHF, MI, DM, and stroke; smoking; kidney function; BMI; ethnicity, and dabigatran dosage were the major predictor variables of vascular events.
Table 5.
Importance ranking of risk factors in predicting vascular events based on RF and XGBoost.
| Risk Factors | Average Ranking of 10 Times RF | Average Ranking of 10 Times XGBoost | Average Ranking of the 2 Models | Final Ranking in Predicting Vascular Events |
|---|---|---|---|---|
| Age | 1 | 5.2 | 3.1 | 1 |
| History of congestive heart failure | 4.6 | 2.1 | 3.35 | 2 |
| History of myocardial infarction | 4 | 2.8 | 3.4 | 3 |
| Smoking | 2.2 | 5.6 | 3.9 | 4 |
| Kidney function | 5.9 | 6.1 | 6 | 5 |
| BMI | 3.5 | 10.5 | 7 | 6 |
| Ethnicity | 7.8 | 7.3 | 7.55 | 7 |
| History of diabetes mellitus | 8.6 | 7 | 7.8 | 8 |
| Medicine dosage (dabigatran) | 8.5 | 7.5 | 8 | 9 |
| Previous stroke history | 9.4 | 9.6 | 9.5 | 10 |
| Body weight | 12.2 | 8.9 | 11.05 | 11 |
| Concomitant use of drugs | 14.3 | 9.8 | 12.05 | 12 |
| Hypertension history | 11.8 | 13.2 | 12.5 | 13 |
| Sex | 11.7 | 14.3 | 13 | 14 |
| Previous bleeding history | 14.5 | 14 | 14.25 | 15 |
| History of systemic embolism | 16.3 | 14.8 | 15.55 | 16 |
| Liver function abnormality | 16.7 | 15 | 15.85 | 17 |
| Anemia | 18 | 18 | 18 | 18 |
Abbr.: RF, random forest; XGBoost, eXtreme gradient boosting; BMI, body mass index.
Table 6 presents the overall importance ranking of each risk factor in predicting bleeding. By averaging the rank values of RF and XGBoost methods, we concluded that age, kidney function, smoking, bleeding history, concomitant use of specific drugs, dabigatran dosage, BMI, MI and CHF history, and ethnicity were the major predictor variables of bleeding.
Table 6.
Overall importance ranking of each risk factor in predicting bleeding based on RF and XGBoost.
| Risk Factors | Average Ranking of 10 Times RF | Average Ranking of 10 Times XGBoost | Average Ranking of the 2 Models | Final Ranking in Predicting Bleeding |
|---|---|---|---|---|
| Age | 1 | 1.3 | 1.15 | 1 |
| Kidney function | 3.2 | 3.5 | 3.35 | 2 |
| Smoking | 2.1 | 4.7 | 3.4 | 3 |
| Previous bleeding history | 4.7 | 2.4 | 3.55 | 4 |
| Concomitant use of drugs | 4.8 | 5 | 4.9 | 5 |
| Medicine dosage (dabigatran) | 7 | 6.7 | 6.85 | 6 |
| BMI | 5.2 | 9.6 | 7.4 | 7 |
| History of myocardial infarction | 9.2 | 6.1 | 7.65 | 8 |
| History of congestive heart failure | 10.1 | 10.3 | 10.2 | 9 |
| Ethnicity | 9.1 | 12.2 | 10.65 | 10 |
| Sex | 10.8 | 11.3 | 10.55 | 11 |
| History of diabetes mellitus | 12.8 | 11.5 | 12.15 | 12 |
| Previous stroke history | 11 | 14.2 | 12.6 | 13 |
| Hypertension history | 14 | 12.6 | 13.3 | 14 |
| Body weight | 15 | 12.3 | 13.65 | 15 |
| History of systemic embolism | 16 | 13 | 14.5 | 16 |
| Liver function abnormality | 17 | 17 | 17 | 17 |
| Anemia | 18 | 17.4 | 17.7 | 18 |
Abbr.: RF, random forest; XGBoost, eXtreme gradient boosting; BMI, body mass index.
4. Discussion
To our knowledge, this is the first study attempting to analyze risk factors in patients with NVAF taking dabigatran using integrated ML feature selection methods. RF and XGBoost demonstrated prominent prediction values exceeding those of LGR. We could conclude the ranking of essential risk factors in these patients after averaging the results of these two methods. In order to balance simplicity and practicality against precision, we selected the top nine important variables to discuss according to physicians’ decision. (Table 7).
Table 7.
Major nine important variables in predicting vascular events and bleeding.
| Average Ranking of Variables | Variable of Prediction of Vascular Events | Variable of Prediction of Bleeding |
|---|---|---|
| 1 | Age | Age |
| 2 | History of CHF | Kidney function |
| 3 | History of MI | Smoking |
| 4 | Smoking | Previous bleeding history |
| 5 | Kidney function | Concomitant use of drugs |
| 6 | BMI | Medicine dosage (dabigatran) |
| 7 | Ethnicity | BMI |
| 8 | History of diabetes mellitus | History of MI |
| 9 | Medicine dosage (dabigatran) | History of CHF |
Abbr.: CHF, congestive heart failure; MI, myocardial infarction; BMI, body mass index.
In most predictive models, an age of >65 years is a standard variable that predicts ischemic stroke and bleeding. As expected, age was the leading predictor of vascular events and bleeding in our study.
Smoking induces atherosclerosis and endothelial dysfunction, simultaneously resulting in more ischemic insults and hemorrhage [44,45,46]. Smoking also contributes to an increased probability of developing arrhythmia via several metabolic factors and underlying diseases [47]. Smoking cessation is a well-documented strategy to prevent vascular disease either with or without arrhythmia. Regarding the medical management of patients with atrial fibrillation, smoking has received insufficient attention. In a consensus, smoking was reported to increase warfarin clearance, influencing the drug effects [48]. There was no similar concern when the anticoagulant was shifted to NOACs. However, in our study, smoking appeared to be a more important variable than other common systemic diseases in patients taking dabigatran.
In the CHA2DS2-VASc score, ischemic stroke history played a more critical role than MI after adding the two scores when patients ever had a stroke. However, MI was a prevalent risk factor for vascular events rather than stroke in our study. CHF that might result from ischemic heart disease or hypertension complications also has a significant impact on most evaluating tools [49]. Cardiomegaly caused by these underlying diseases leads to left ventricular hypokinesia, the major cause of thrombus formation [50,51,52]. However, endothelial dysfunction and cerebral autoregulation disturbance are also the consequences of CHF [53]. In ML models, we may comprehensively analyze several variables with different interactions; hence, CHF and MI show higher grades in the prediction of vascular events among all underlying diseases.
Kidney dysfunction was infrequently mentioned as a major risk factor for ischemic stroke. Severe kidney impairment (estimated creatinine clearance <30 mL/min/1.73 m2) was an exclusion criterion for most NOACs [10,54,55,56]. Delayed drug clearance may increase the possibility of bleeding [57]. In a study on Danish population, kidney function impairment was found to contribute to a high tendency of developing vascular events and bleeding [58]. The levels of inflammatory and procoagulant factors including C-reactive protein, fibrinogen, factor VIIc, and factor VIIIc were high [59]. Furthermore, hemostatic dysfunction including decreased glycoprotein IIb and IIIa levels, reduced von Willebrand factor activity, and altered arachidonic acid metabolism were detected in older individuals with renal insufficiency [60]. These double-sided adverse effects may be due to these physiological alterations, and kidney dysfunction is the end-organ damage result of hypertension and diabetes. Our scheme selects it as a significant representative variable of vascular events and bleeding.
In general, a high BMI may be associated with metabolic syndrome and hypertension [61]. High BMI increases the prevalence of cerebrovascular and heart diseases [62]. However, this trend is controversial in patients with arrhythmia. Meta-analysis and real-world cohort studies have revealed less ischemic stroke and bleeding prevalence in patients with high BMI [63,64]. The all-cause death rate was higher in underweight patients. BMI was critical for predicting vascular events and bleeding in our study.
For a competitive relationship in the CYP3A4 and P-glycoprotein inhibition pathway [65], the recommended dabigatran dose in the European label is 110 mg if a patient is on verapamil. In the United States, the USFDA recommended that clinicians use dabigatran with caution when patients are on long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs), antithrombotic agents, or medicines that may elevate the blood levels and effects of dabigatran, such as dronedarone or ketoconazole [66]. Observational studies conducted in the US and Taiwan have indicated that concomitant use of these drugs enhances bleeding risk in patients taking dabigatran [67,68]. Combining antithrombotic and antiplatelet agents is a well-known therapy limited in certain conditions owing to high bleeding probability [69]. Our results confirm that these drug interactions have an important effect on bleeding risk.
This study attempted to solve the dilemma of dose selection of dabigatran to obtain the maximum benefit of prevention and avoidance of side effects in patients with various physiological conditions and comorbidities. Dabigatran dose was also defined as a variable in our model. Although it had a noticeable influence on both vascular events and bleeding, it was not a major factor in either result. This issue remains a complex problem that our study could not solve completely because of three of the top five risk factors of either vascular event or bleeding being the same (Table 7). Nevertheless, we could identify essential factors to provide good suggestions using this model. First, smoking cessation and maintaining an appropriate body shape are vital for patients prescribed dabigatran. CHF and MI imply a high risk for thrombotic events with secondary prevention with dabigatran, and intensive medical control and prescribing a standard dabigatran dose are essential. In contrast, a previous bleeding history and concomitant use of antithrombotic agents, NSAIDs, and medicines with effects on CYP3A4 have adverse effects on bleeding when we select a low dabigatran dose. However, older age and kidney function impairment have double-sided adverse effects causing more vascular events and bleeding simultaneously. Other methods are indicated to determine the dividing line of each factor if it exists.
5. Limitations
Our study has several limitations. First, our findings must be applied with caution in clinical practice considering the inclusion and exclusion criteria of the RE-LY trial. The trial population comprised subjects with relatively low CHA2DS2-VASc scores (2.1 ± 1.1), and patients with certain comorbidities were excluded. Second, the trial participants were regularly followed up for two years with good compliance that might be infrequent in our outpatients. Specific effects of other systemic diseases might be alleviated. Third, we intended to establish a prediction model for patients with NVAF taking dabigatran; vascular events including stroke, MI, systemic embolism, and vascular death were defined as the primary outcome. Given that we selected only one NOAC instead of an antiplatelet agent or combined therapy, and though an antiplatelet agent could prevent atherosclerosis, this issue might be affected by risk factors including dyslipidemia, lifestyle, and genetics, which were not included in our study. Nevertheless, our study design was suitable for clinical practice when considering the secondary prevention of cardioembolic stroke.
6. Conclusions
NOACs could replace warfarin owing to their similar protective effects and better safety quality in real-world studies. Appropriate dose selection and intensive risk factor control are necessary to achieve high-quality secondary prevention. In our research, RF and XGBoost generated higher accuracies and AUC values than LGR in simultaneously predicting vascular events and bleeding even with the disproportionate prevalence. Furthermore, these methods remained relatively stable between their sensitivities and specificities in the imbalanced data. This integrated ML feature selection scheme showed a great opportunity to solve complex medical data. Although further evaluation is indicated, our study might provide a preliminary direction of precision medicine for secondary prevention in patients with arrhythmia.
Acknowledgments
We submitted the research proposal of the current study at https://vivli.org/ accessed on 28 July 2021 (A global clinical research data sharing platform). This publication is based on research using data from data contributor Boehringer-Ingelheim that has been made available through Vivli, Inc. in Cambridge, MA, USA. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. This work is partially supported by Ministry of Science and Technology, Taiwan (110-2221-E-030 -010) and Fu Jen Catholic University (A0110181).
Author Contributions
Conception and design, Y.-C.H., M.C. and C.-J.L.; project administration, Y.-C.H., Y.-C.C., M.-J.J., C.-J.L.; methodology, Y.-C.C., M.-J.J. and C.-J.L.; formal analysis, Y.-C.H., M.-J.J., M.C. and C.-J.L.; writing—original draft: Y.-C.H. and M.-J.J.: writing—review & editing, C.-J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was reviewed and approved by the Research Ethics Review Committee of the Fu Jen Catholic University Hospital (IRB No. FJUH111180).
Informed Consent Statement
The requirement for informed consent was waived, since the data contain only de-identified information.
Data Availability Statement
The data are available through application to Boehringer-Ingelheim on the research data sharing platform (https://vivli.org/; accessed on 28 July 2021). Restrictions apply to the availability of these data, which were used under license for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin V.L., Stark B.A., Johnson C.O., Roth G.A., Bisignano C., Abady G.G., Abbasifard M., Abbasi-Kangevari M., Abd-Allah F., Abdi V., et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V., Singer D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The An Ticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.-C., Heidenbuchel H., Hendriks J., et al. ESC Scientific Document Group 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Chan Y.-H., See L.-C., Tu H.-T., Yeh Y.-H., Chang S.-H., Wu L.-S., Lee H.-F., Wang C.-L., Kuo C.-F., Kuo C.-T. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians with Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2018;7:e008150. doi: 10.1161/JAHA.117.008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao T.-F., Chiang C.-E., Lin Y.-J., Chang S.-L., Lo L.-W., Hu Y.-F., Tuan T.-C., Liao J.-N., Chung F.-P., Chen T.-J., et al. Evolving changes of the use of oral anticoagulants and outcomes in patients with newly diagnosed atrial fibrillation in Taiwan. Circulation. 2018;138:1485–1487. doi: 10.1161/CIRCULATIONAHA.118.036046. [DOI] [PubMed] [Google Scholar]
- 6.Chan Y.-H., Chao T.-F., Chen S.-W., Lee H.-F., Yeh Y.-H., Huang Y.-C., Chang S.-H., Kuo C.-T., Lip G.Y.H., Chen S.-A. Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. 2020;17:2102–2110. doi: 10.1016/j.hrthm.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg B.A., Shrader P., Thomas L., Ansell J., Fonarow G.C., Gersh B.J., Kowey P.R., Mahaffey K.W., Naccarelli G., Reiffel J., et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes: The ORBIT-AF II Registry. J. Am. Coll. Cardiol. 2016;68:2597–2604. doi: 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]
- 8.Yu H.T., Yang P.-S., Jang E., Kim T.-H., Uhm J.-S., Kim J.-Y., Pak H.-N., Lee M.-H., Lip G.Y.H., Joung B. Label Adherence of Direct Oral Anticoagulants Dosing and Clinical Outcomes in Patients with Atrial Fibrillation. J. Am. Heart Assoc. 2020;9:e014177. doi: 10.1161/JAHA.119.014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X., Hu L., Liu J., Gu Q. Off-Label Underdosing or Overdosing of Non-vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation: A Meta-Analysis. Front. Cardiovasc. Med. 2021;8:724301. doi: 10.3389/fcvm.2021.724301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 11.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomstrom-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 12.Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 14.Chao T.-F., Lip G.Y., Liu C.-J., Tuan T.-C., Chen S.-J., Wang K.-L., Lin Y.-J., Chang S.-L., Lo L.-W., Hu Y.-F., et al. Validation of a Modified CHA2DS2-VASc Score for Stroke Risk Stratification in Asian Patients with Atrial Fibrillation: A Nationwide Cohort Study. Stroke. 2016;47:2462–2469. doi: 10.1161/STROKEAHA.116.013880. [DOI] [PubMed] [Google Scholar]
- 15.Chang G., Xie Q., Ma L., Hu K., Zhang Z., Mu G., Cui Y. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J. Thromb. Haemost. 2020;18:791–801. doi: 10.1111/jth.14692. [DOI] [PubMed] [Google Scholar]
- 16.Chen L.Y., Norby F.L., Chamberlain A.M., MacLehose R.F., Bengtson L.G.S., Lutsey P.L., Alonso A. CHA2DS2-VASc Score and Stroke Prediction in Atrial Fibrillation in Whites, Blacks, and Hispanics. Stroke. 2019;50:28–33. doi: 10.1161/STROKEAHA.118.021453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Chen P.-H.C., Krause J., Peng L. How to Read Articles That Use Machine Learning: Users’ Guides to the Medical Literature. JAMA. 2019;322:1806–1816. doi: 10.1001/jama.2019.16489. [DOI] [PubMed] [Google Scholar]
- 18.Wu C.-W., Shen H.-L., Lu C.-J., Chen S.-H., Chen H.-Y. Comparison of Different Machine Learning Classifiers for Glaucoma Diagnosis Based on Spectralis OCT. Diagnostics. 2021;11:1718. doi: 10.3390/diagnostics11091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertini F., Allevi D., Lutero G., Montesi D., Calzà L. Automatic speech classifier for mild cognitive impairment and early dementia. ACM Trans. Comput. Healthc. 2022;3:1–11. doi: 10.1145/3469089. [DOI] [Google Scholar]
- 20.Li J., Tobore I., Liu Y., Kandwal A., Wang L., Nie Z. Non-invasive monitoring of three glucose ranges based on ECG by using DBSCAN-CNN. IEEE J. Biomed. Health Inform. 2021;25:3340–3350. doi: 10.1109/JBHI.2021.3072628. [DOI] [PubMed] [Google Scholar]
- 21.Enayati M., Farahani N.Z., Skubic M. Machine Learning Approach for motion artifact detection in Ballistocardiogram signals; Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare; Atlanta, GA, USA. 18–20 May 2020; pp. 406–410. [Google Scholar]
- 22.Bertini F., Bergami G., Montesi D., Veronese G., Marchesini G., Pandolfi P. Predicting frailty condition in elderly using multidimensional socioclinical databases. Proc. IEEE. 2018;106:723–737. doi: 10.1109/JPROC.2018.2791463. [DOI] [Google Scholar]
- 23.Kamel H., Navi B.B., Parikh N.S., Merkler A.E., Okin P.M., Devereux R.B., Weinsaft J.W., Kim J., Cheung J.W., Kim L.K., et al. Machine Learning Prediction of Stroke Mechanism in Embolic Strokes of Undetermined Source. Stroke. 2020;51:e203–e210. doi: 10.1161/STROKEAHA.120.029305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun M., Clarke R., Cairns B.J., Clifton D., Bennett D., Chen Y., Guo Y., Pei P., Lv J., Yu C., et al. Stroke risk prediction using machine learning: A prospective cohort study of 0.5 million Chinese adults. J. Am. Med. Inform. Assoc. 2021;28:1719–1727. doi: 10.1093/jamia/ocab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Jr., Ellinor P.T., Ezekowitz M.D., Field M.E., Furie K.L., et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 26.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 27.Center for Drug Evaluation and Research (CDER), Guidance, Compliance, & Regulatory Information. [(accessed on 2 March 2022)]; Available online: https://www.fda.gov/drugs/guidance-compliance-regulatory-information.
- 28.Lewis D.D. Machine Learning: ECML-98. Springer; Chemnitz, Germany: 1998. Naive (Bayes) at forty: The independence assumption in information retrieval; pp. 4–15. [DOI] [Google Scholar]
- 29.Breiman L. Random Forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 30.Breiman L., Friedman J.H., Olshen R.A., Stone C.J. Classification and Regression Trees. Biometrics. 1984;40:874. doi: 10.2307/2530946. [DOI] [Google Scholar]
- 31.Chen T., Guestrin C. XGBoost: A Scalable Tree Boosting System; Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; San Francisco, CA, USA. 13–17 August 2016; pp. 785–794. [Google Scholar]
- 32.Quesada J.A., Lopez-Pineda A., Gil-Guillén V.F., Durazo-Arvizu R., Orozco-Beltrán D., López-Domenech A., Carratalá-Munuera C. Machine learning to predict cardiovascular risk. Int. J. Clin. Pract. 2019;73:e13389. doi: 10.1111/ijcp.13389. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Lozano C., Hervella P., Mato-Abad V., Rodríguez-Yáñez M., Suárez-Garaboa S., López-Dequidt I., Estany-Gestal A., Sobrino T., Campos F., Castillo J., et al. Random forest-based prediction of stroke outcome. Sci. Rep. 2021;11:10071. doi: 10.1038/s41598-021-89434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonarow G.C. Risk stratification for in-hospital mortality in acutely decompensated heart failure classification and regression tree analysis. JAMA. 2005;293:572. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y., Yang X., Huang H., Peng C., Ge Y., Wu H., Wang J., Xiong G., Yi Y. Extreme Gradient Boosting Model Has a Better Performance in Predicting the Risk of 90-Day Readmissions in Patients with Ischaemic Stroke. J. Stroke Cerebrovasc. Dis. 2019;28:104441. doi: 10.1016/j.jstrokecerebrovasdis.2019.104441. [DOI] [PubMed] [Google Scholar]
- 36.Cui M., Gang X., Gao F., Wang G., Xiao X., Li Z., Wang G. Risk assessment of sarcopenia in patients with type 2 diabetes mellitus using data mining methods. Front. Endocrinol. 2020;3:123. doi: 10.3389/fendo.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajian-Tilaki K. Receiver Operator Characteristic Analysis of Biomarkers Evaluation in Diagnostic Research. J. Clin. Diagn. Res. 2018;12:LE01–LE08. doi: 10.7860/JCDR/2018/32856.11609. [DOI] [Google Scholar]
- 38.Kuhn M. Caret: Classification and Regression Training. 2022. [(accessed on 2 March 2022)]. R Package Version, 6.0-91. Available online: https://CRAN.R-project.org/package=caret.
- 39.Bolón-Canedo V., Alonso-Betanzos A. Ensembles for Feature Selection: A Review and Future Trends. Inf. Fusion. 2019;52:1–12. doi: 10.1016/j.inffus.2018.11.008. [DOI] [Google Scholar]
- 40.Breiman L., Cutler A., Liaw A., Wiener M. randomForest: Breiman and Cutler’s Random Forests for Classification and Regression. 2022. [(accessed on 2 March 2022)]. R Package Version, 4.7-1. Available online: https://CRAN.R-project.org/package=randomForest.
- 41.Therneau T., Atkinson B. Rpart: Recursive Partitioning and Regression Trees. 2022. [(accessed on 2 March 2022)]. R Package Version, 4.1.16. Available online: https://CRAN.R-project.org/package=rpart.
- 42.Chen T., He T., Benesty M., Khotilovich V., Tang Y., Cho H., Chen K., Mitchell R., Cano I., Zhou T., et al. Xgboost: Extreme Gradient Boosting. 2022. [(accessed on 2 March 2022)]. R Package Version, 1.5.2.1. Available online: https://CRAN.R-project.org/package=xgboost.
- 43.Roever C., Raabe N., Luebke K., Ligges U., Szepannek G., Zentgraf M., Meyer D. klaR: Classification and Visualization. 2020. [(accessed on 2 March 2022)]. R Package Version, 0.6-15. Available online: https://CRAN.R-project.org/package=klaR.
- 44.Poredos P., Orehek M., Tratnik E. Smoking is associated with dose-related increase of intima-media thickness and endothelial dysfunction. Angiology. 1999;50:201–208. doi: 10.1177/000331979905000304. [DOI] [PubMed] [Google Scholar]
- 45.Albertsen I.E., Rasmussen L.H., Lane D.A., Overvad T.F., Skjøth F., Overvad K., Lip G.Y.H., Larsen T.B. The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation: Insights from the Danish Diet, Cancer, and Health study. Chest. 2014;145:559–566. doi: 10.1378/chest.13-1740. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa K., Hirai T., Ohara K., Fukuda N., Numa S., Taguchi Y., Dougu N., Takashima S., Nozawa T., Tanaka K., et al. Impact of persistent smoking on long-term outcomes in patients with nonvalvular atrial fibrillation. J. Cardiol. 2015;65:429–433. doi: 10.1016/j.jjcc.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Chamberlain A.M., Agarwal S.K., Folsom A.R., Duval S., Soliman E.Z., Ambrose M., Eberly L.E., Alonso A. Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011;8:1160–1166. doi: 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nathisuwan S., Dilokthornsakul P., Chaiyakunapruk N., Morarai T., Yodting T., Piriyachananusorn N. Assessing evidence of interaction between smoking and warfarin: A systematic review and meta-analysis. Chest. 2011;139:1130–1139. doi: 10.1378/chest.10-0777. [DOI] [PubMed] [Google Scholar]
- 49.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart disease and stroke statistics-2018 update a report from the American Heart Association. Circulation. 2018;137:E67–E492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 50.Pullicino P.M., Halperin J.L., Thompson J.L. Stroke in patients with heart failure and reduced left ventricular ejection fraction. Neurology. 2000;54:288–294. doi: 10.1212/WNL.54.2.288. [DOI] [PubMed] [Google Scholar]
- 51.Freudenberger R.S., Hellkamp A.S., Halperin J.L., Poole J., Anderson J., Johnson G., Mark D.B., Lee K.L., Bardy G.H., Investigators S.-H. Risk of thromboembolism in heart failure: An analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2007;115:2637–2641. doi: 10.1161/CIRCULATIONAHA.106.661397. [DOI] [PubMed] [Google Scholar]
- 52.Lip G.Y. Does atrial fibrillation confer a hypercoagulable state? Lancet. 1995;346:313–1314. doi: 10.1016/S0140-6736(95)92339-X. [DOI] [PubMed] [Google Scholar]
- 53.Georgiadis D., Sievert M., Cencetti S., Uhlmann F., Krivokuca M., Zierz S., Werdan K. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur. Heart J. 2000;21:407–413. doi: 10.1053/euhj.1999.1742. [DOI] [PubMed] [Google Scholar]
- 54.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W., Breithardt G., Halperin J.L., Hankey G.J., Piccini J.P., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 55.Granger C.B., Alexander J.H., McMurray J.J.V., Lopes R.D., Hylek E.M., Hanna M., Al-Khalidi H.R., Ansell J., Atar D., Avezum A., et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 56.Giugliano R.P., Ruff C.T., Braunwald E., Murphy S.A., Wiviott S.D., Halperin J.L., Waldo A.L., Ezekowitz M.D., Weitz J.I., Špinar J., et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 57.Qamar A., Bhatt D.L. Stroke Prevention in Atrial Fibrillation in Patients with Chronic Kidney Disease. Circulation. 2016;133:1512–1515. doi: 10.1161/CIRCULATIONAHA.115.018549. [DOI] [PubMed] [Google Scholar]
- 58.Bonde A.N., Lip G.Y.H., Kamper A.-L., Fosbøl E.L., Staerk L., Carlson N., Torp-Pedersen C., Gislason G., Olesen J.B. Renal Function and the Risk of Stroke and Bleeding in Patients with Atrial Fibrillation: An Observational Cohort Study. Stroke. 2016;47:2707–2713. doi: 10.1161/STROKEAHA.116.014422. [DOI] [PubMed] [Google Scholar]
- 59.Shlipak M.G., Fried L.F., Crump C., Bleyer A.J., Manolio T.A., Tracy R.P., Furberg C.D., Psaty B.M. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2002;107:87–92. doi: 10.1161/01.CIR.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 60.Pavord S., Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011;25:271–278. doi: 10.1016/j.blre.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen N.T., Magno C.P., Lane K.T., Hinojosa M.W., Lane J.S. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 2008;207:928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 62.Calle E.E., Thun M.J., Petrelli J.M., Rodriguez C., Heath C.W., Jr. Body-mass index and mortality in a prospective cohort of US adults. N. Engl. J. Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 63.Zhu W., Wan R., Liu F., Hu J., Huang L., Li J., Hong K. Relation of Body Mass Index with Adverse Outcomes among Patients with Atrial Fibrillation: A Meta-Analysis and Systematic Review. J. Am. Heart Assoc. 2016;5:e004006. doi: 10.1161/JAHA.116.004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S.R., Choi E.K., Jung J.H., Park S.H., Han K.D., Oh S., Lip G.Y. Body Mass Index and Clinical Outcomes in Asian Patients with Atrial Fibrillation Receiving Oral Anticoagulation. Stroke. 2021;52:521–530. doi: 10.1161/STROKEAHA.120.030356. [DOI] [PubMed] [Google Scholar]
- 65.Hellwig T., Gulseth M. Pharmacokinetic and pharmacodynamic drug interactions with new oral anticoagulants: What do they mean for patients with atrial fibrillation? Ann. Pharmacother. 2013;47:1478–1487. doi: 10.1177/1060028013504741. [DOI] [PubMed] [Google Scholar]
- 66.U.S. Food and Drug Administration Drugs@FDA: FDA Approved Drug Products. [(accessed on 3 March 2022)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- 67.Chang S.-H., Chou I.-J., Yeh Y.-H., Chiou M.-J., Wen M.-S., Kuo C.-T., See L.-C., Kuo C.-F. Association between Use of Non-Vitamin K Oral Anticoagulants with and without Concurrent Medications and Risk of Major Bleeding in Nonvalvular Atrial Fibrillation. JAMA. 2017;318:1250–1259. doi: 10.1001/jama.2017.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pham P., Schmidt S., Lesko L., Lip G.Y.H., Brown J.D. Association of Oral Anticoagulants and Verapamil or Diltiazem with Adverse Bleeding Events in Patients with Nonvalvular Atrial Fibrillation and Normal Kidney Function. JAMA Netw. Open. 2020;3:e203593. doi: 10.1001/jamanetworkopen.2020.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandiver J.W., Diane Beavers K. Combining oral anticoagulation and antiplatelet therapies: Appropriate patient selection. J. Thromb. Thrombolysis. 2018;45:423–431. doi: 10.1007/s11239-018-1635-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available through application to Boehringer-Ingelheim on the research data sharing platform (https://vivli.org/; accessed on 28 July 2021). Restrictions apply to the availability of these data, which were used under license for this study.