Abstract
Objective: to identify factors associated with COVID19 vaccine hesitancy, including sources of information among residents of Maine. Methods: 148 study participants, recruited through community partners and primary care offices in Maine, completed an anonymous 15 item online survey. Recruitment and data collection occurred from May to September, 2021. Hesitancy was determined through a single question, “Will you get one of the COVID vaccines when it is offered to you?” Results: vaccine hesitant respondents were younger than not hesitant respondents (p = 0.01). Hesitant individuals were significantly more likely to report concerns regarding the speed of COVID-19 vaccine production, vaccine efficacy, and potential vaccine side effects (p < 0.05 for each). Hesitant individuals were also significantly more likely to have discussed vaccination with their primary physician (p = 0.04). Conclusions: overall, hesitant individuals are more likely to be younger and had less trust in information from government sources, but they sought input from primary care. They were also more concerned about efficacy, side effects, and the rapid development of COVID-19 vaccines. Primary care physicians are in key positions to address these concerns due to contact with individuals who need accurate information.
Keywords: vaccine hesitancy, vaccine misinformation, COVID-19 vaccine hesitancy
1. Introduction
The coronavirus pandemic remains a threat to public health worldwide. Vaccination remains the most effective measure for reducing hospitalizations and deaths and for mitigating the global impact of COVID-19. Vaccinations have been available in the United States since December of 2020, and FDA approvals continue to increase public access to the vaccine. Despite efforts to increase vaccine uptake and distribution, the percentage of people fully vaccinated in the United States was 59.7% with a seven day case rate of 203/100 k, as of 3 December 2021 [1]. Targeted efforts to increase vaccine uptake are, therefore, critical. Vaccination against COVID-19 and achieving normality has proven to be challenging politically and logistically [2,3]. In past studies, public concerns regarding the safety and side effects of other vaccines, as well as perceptions of vaccine safety and efficacy, were the strongest predictors of overall uptake [4,5]. Large scale cross-sectional studies have reported factors associated with the intention to get the COVID-19 vaccine, including efficacy, protection duration, perceived COVID-19 risk, short and long-term side effects, speed of the vaccine approval process, origin of the vaccine, and sources of information and endorsement [6,7,8]. COVID-19 vaccine hesitancy is also associated with exposure to misinformation and political affiliation [9].
Rural Americans may have different beliefs about contracting COVID-19, as well as unique risks for adverse outcomes, due to poverty and lack of access to health care [10,11]. Prior work in rural communities found higher COVID-19 vaccine hesitancy, lower vaccination rates, and lower trust in the government as a source of information [6,12,13,14,15].
Several studies have identified other demographic variables associated with a lower likelihood of getting the vaccine, including having no college degree and being female and white [6,16,17]. Acceptance of the COVID-19 vaccine varies substantially across different sectors of the American population and is changing over time. Surveys among Americans showed the highest intended acceptance was in early April of 2020, dropping significantly by October of 2020, and rising again in February of 2021 [1]. In September of 2021, the Kaiser Family Foundation reported the Delta variant and full FDA approval of the vaccines to be key motivators for individuals to get vaccinated. Vaccine mandates, however, showed minor influence on vaccine intention [18].
Additional factors associated with increased confidence in getting vaccinated against COVID-19 include receiving encouragement from a personal physician and opinions of families and friends supporting vaccination [7,19]. Verger and Dube [20] found an association between confidence in the vaccine with trust in professionals and science. The influence of politics has played an unprecedented role in COVID-19 vaccine hesitancy, with marked differences between Democrats and Republicans [21], possibly due to misinformation such as conspiracy theories and lack of trust in government and healthcare professionals [8].
A “3C” model of influenza vaccine hesitancy, described by Larson et al. [22], was recently expanded to a “5C” construct by Razai et al. [23] The model defines five factors that influence vaccine hesitancy: (1) confidence, (2) complacency, (3) convenience, (4) communications or sources of information, and (5) context or sociodemographic status [23]. Confidence focuses on attitudes, beliefs, and concerns about vaccine effectiveness, efficacy, and safety [22,24]. Kreps et al. [7] further explained confidence as whether a vaccine received full US Food and Drug Administration (FDA) approval and whether the source of vaccine endorsement was trusted. Dube et al. [25] suggested that confidence in vaccines depends on trust in science and professionals in a socio-political context. Complacency describes individuals who do not perceive a value or need for getting a vaccine. Convenience is associated with ease of access to vaccines [22,24]. Communications focuses on sources of rapidly changing guidance and the spread of false information about COVID-19. Context reflects sociodemographic constructs such as age, sex, and education.
Our aim was to use the “5C” model to describe factors contributing to vaccine hesitancy and uptake intention from a sample of residents in Maine, one of the most rural states in the country. This was a descriptive study, and no hypothesis was developed or tested.
2. Methods
We developed a 15-item survey in Qualtrics, designed according to the “5C” construct, to explain vaccine hesitancy [23]. To assess confidence among Maine residents, we measured participants’ trusted sources of information, trust in science and professionals, concerns about side effects, how well the vaccine works, and trust in the vaccine approval process. We measured complacency via examining beliefs about protecting one’s self and others, personal experience with COVID-19 infection, and political affiliation. To assess convenience, we measured levels of concern about where vaccines were being administered among survey respondents. Lastly, we queried respondents about trusted sources of information about the virus and vaccines. Demographic data (context) were collected for age, sex, race, ethnicity, education, and county of residence. The survey combined questions from different entities and provided discrete response categories. Four questions assessed vaccine concerns using a four point Likert scale. Comment sections were added to allow elaboration regarding trusted sources of information, concerns, vaccine preference, motivations, and plans for getting the vaccine. Survey data were de-identified to ensure anonymity and confidentiality during data collection. The survey instrument was not validated prior to implementation.
Three community partners were eager to support participant recruitment and provide input on all aspects of the research. The efforts of the Northern New England Clinical and Translational Research network Rural Core established our partners, including Healthy Community Coalition (HCC) of Greater Franklin County, Pen Bay Community Health and Wellness (CHW), and Healthy Oxford Hills (HOH) located in Norway, Maine. Providers from three primary care practices volunteered to participate in the recruitment of survey participants with interest in results reflecting their populations. Study flyers were used to support in-person, email, and social media recruitment and communication. Instructions included a web link for access to a survey administered via REDCap. A contact number was provided for completing the survey via telephone with a research coordinator; however, all respondents used the web address. The study design did not include interviews, and there were no incentives or gifts for participation.
Recruitment and data collection, through the methods described for this descriptive study design, used a convenience sample of people in Maine and occurred over four months from 27 May 2021 to 21 September 2021. We categorized people as “hesitant” or “not hesitant” by their response to the question “Will you get one of the COVID-19 vaccines when it is offered to you?” Hesitant respondents were categorized by a response of “I do not plan to get the vaccine”, “I am not sure what I will do”, or “I will wait and see what happens.” Not hesitant respondents were those with responses including “I will definitely get a vaccine”, “I will probably get a vaccine”, “I got the vaccine”, or “I already got the vaccine”. Rurality was defined using the 2013 USDA Urban Influence Codes to distinguish metro from non-metro counties in the State of Maine [26].
We used Fisher’s exact test to analyze demographic data, and the Wilcoxon rank sum test was used to compare differences between groups in concerns over vaccine effectiveness, side effects, and speed of production, as well as concerns about traveling to be vaccinated. Metro counties were defined as Androscoggin, Cumberland, Penobscot, Sagadahoc, and York using the 2013 USDA Urban Influence Codes [26]. Analysis was conducted in R version 3.2.4 for Apple x86-64. Survey entries were manually reviewed and cleaned for a total of 148 respondents in the analytic file, removing two redundant entries with identical responses and similar time-stamps.
3. Results
The majority of respondents reported being female, white, aged 35 years or older, from metropolitan areas, educated at the college level or higher, affiliation with Democrats, and were vaccinated or planning to be vaccinated (Table 1). Fourteen of 148 (9.5%) were hesitant. The hesitant and not hesitant groups were significantly different in age (p-value = 0.01) (Table 1), with a greater proportion of the hesitant group between 35 and 54 years of age (57% vs. 25%) and a greater proportion of the not hesitant group were 65 years of age and older (31% vs. 0%). A significant difference in political affiliation was also found when comparing hesitant vs. not hesitant respondents (p-value < 0.001). There was no significant difference in reported vaccine hesitancy between male and female respondents (p-value = 0.07), metro and non-metro county of residence (p-value = 0.09), education level (p-value = 0.12), or by self-reported race and ethnicity (p-value = 1).
Table 1.
Characteristics of Survey Participants and Comparison Hesitant and Not Hesitant Group Characteristics (N = 148).
| Total N (%) | Hesitant N (%) | Not Hesitant (N%) | Fisher Exact | |
|---|---|---|---|---|
| Total N | 148 | 14 | 134 | |
| Age Range | ||||
| 18 to 34 years | 26 (18%) | 3(21%) | 23(17%) | 0.01 |
| 35 to 54 years | 42 (28%) | 8(57%) | 34(25%) | |
| 55 to 64 years | 38 (26%) | 3(21%) | 35(26%) | |
| 65 and older years | 41 (28%) | 41(31%) | ||
| Unknown | 1(1%) | |||
| Sex | ||||
| Female | 124 (84%) | 9(64%) | 115(86%) | 0.07 |
| Male | 19 (13%) | 4(29%) | 15(11%) | |
| Other or prefer not to answer | 5 (3%) | 1(7%) | 4(3%) | |
| Race/Ethnicity | 1.0 | |||
| White | 136 (92%) | 13(93%) | 123(92%) | |
| Hispanic or Latinx | 3 (2%) | 3(2%) | ||
| Rurality (17 Counties) | ||||
| Metro | 76 (51%) | 4(29%) | 72(54%) | 0.09 |
| Non-Metro | 65 (44%) | 9(64%) | 56(42%) | |
| Education | 0.12 | |||
| High School Graduate or Less | 9 (6%) | 3(21%) | 6(4%) | |
| Some College | 19 (13%) | 2(14%) | 17(13%) | |
| Four Year Degree | 42 (28%) | 3(21%) | 39(29%) | |
| Post Graduate | 78 (53%) | 6(43%) | 72(54%) | |
| Political Affiliation | ||||
| Democrat | 88 (59%) | 88(66%) | <0.001 | |
| Republican | 13 (9%) | 2(14%) | 11(8%) | |
| Independent | 25 (17%) | 6(43%) | 19(14%) | |
| No Affiliation | 22 (15%) | 6(43%) | 16(12%) | |
| Vaccination Status | ||||
| Vaccinated or Plan to Be | 134 (84%) | |||
| Vaccine hesitant | 14 (9%) |
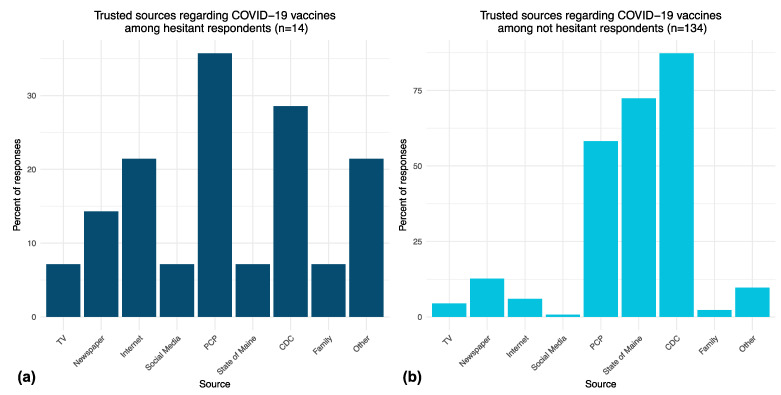
The top trusted sources of information on COVID-19 vaccines differed somewhat between hesitant and not hesitant respondents (Figure 1). When asked to pick three trusted sources on COVID-19 vaccines, hesitant respondents most frequently reported trust in their PCP (36% of respondents), the CDC (29% of respondents), and “other” sources (21% of respondents) (Figure 1a). Not hesitant respondents most frequently reported trust in the CDC (87% of respondents), the State of Maine website (72% of respondents), and their PCP (58% of respondents) for information on COVID-19 vaccines (Figure 1b).
Figure 1.
(a) Percentage of responses related to trusted sources of information about COVID-19 vaccines among hesitant respondents. (b) Trusted sources of information among not hesitant respondents.
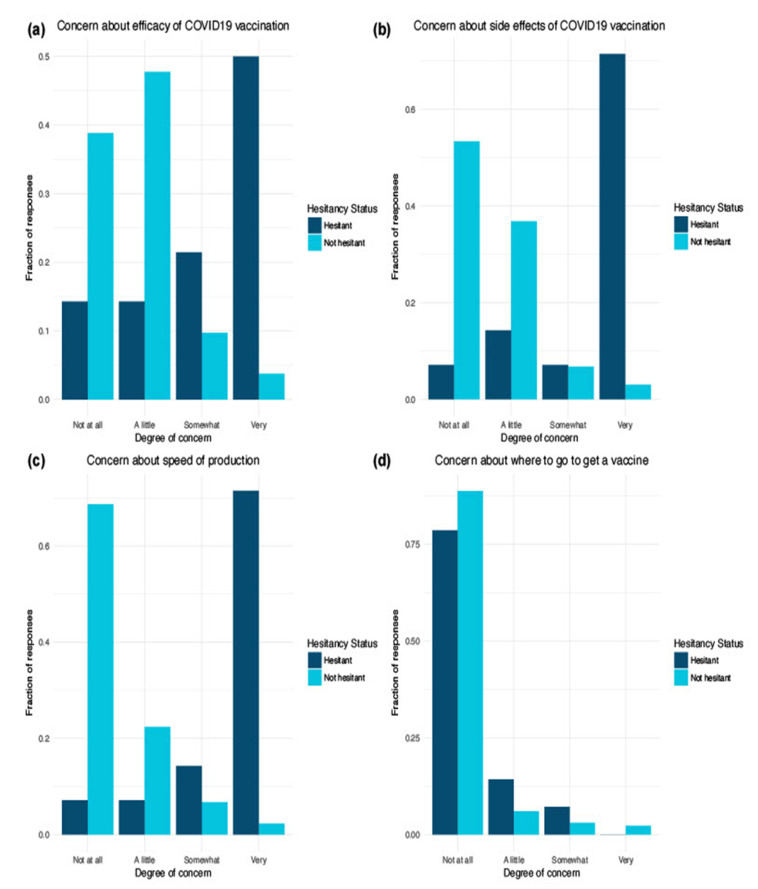
We identified several differences between hesitant and not hesitant respondents related to concerns about COVID-19 vaccines (Figure 2). Hesitant respondents were more likely to be concerned about the efficacy of COVID-19 vaccines than the not hesitant group (p-value < 0.05) (Figure 2a). Hesitant respondents were more likely to be concerned about potential COVID-19 vaccine side effects (p-value < 0.05) (Figure 2b).
Figure 2.
(a) Degree of concern between hesitant and not hesitant respondents about efficacy of COVID19 vaccination. (b) Degree of concern about side effects of COVID19 vaccination. (c) Degree of concern about COVID19 vaccine speed of production. (d) Degree of concern about where to get a COVID19 vaccine.
The comments by women of child bearing age revealed misinformation about side effects (Table 2). For example, “I have heard a lot of women saying their cycles have been irregular after the vaccine, although the CDC says it doesn’t impact fertility, I am skeptical because of this.” Multiple comments specified worries about the vaccine causing medical conditions to worsen. A vaccinated respondent said, “I am worried it has increased my already present health conditions into something more quickly progressive (still, better than a ventilator and death).” Hesitant respondents were more likely to be concerned about the speed of production of the COVID-19 vaccine (p-value < 0.05). A respondent shared concerns about a rushed process, possibly influenced by misinformation, stating, “I’m scared of COVID but I am also scared of the vaccine. I have researched each one extensively and I still believe we have somewhat rushed the process.” Another respondent believed “it should take 20 years plus to test” a vaccine. Respondents were highly vocal regarding the construct of confidence, with over 200 comments providing a deeper understanding of concerns driving hesitancy to inform strategies to address misinformation.
Table 2.
Comments by Construct.
| Construct (5Cs) | Survey Category |
Comments |
|---|---|---|
| Communication Source of Information |
Info about Virus or Vaccine |
|
| Confidence Vaccine Plans |
Vaccine Plans Self |
|
| Confidence | Family and Friend Vaccine Plans |
|
| Confidence Convenience |
Concerns |
|
| Confidence | Type of Vaccine Matters |
|
| Complacency | Motivation |
|
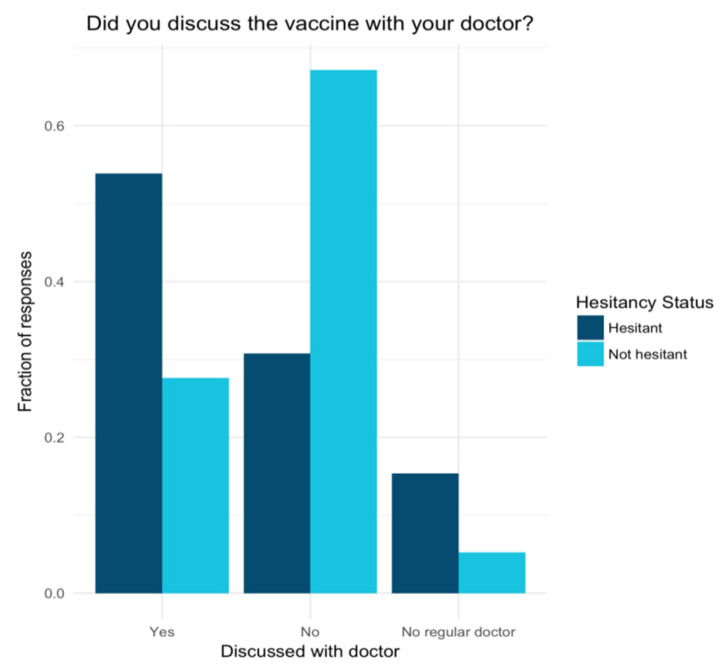
No significant difference was found between the two groups regarding knowledge of where to obtain a vaccine (p-value = 0.3), with 79% of hesitant and 89% of not hesitant respondents “not at all” concerned about where to go to get a vaccine. Finally, hesitant respondents were significantly more likely to have discussed the vaccine with their doctor than not hesitant respondents (p-value = 0.04) (Figure 3).
Figure 3.
Hesitant and not hesitant group comparisons of whether they discussed the vaccine with a doctor.
4. Discussion
We found several differences between hesitant and not hesitant residents of a rural state, during a period of evolving disease prevalence and vaccine access, including trusted sources of information. Hesitant respondents were significantly more likely to endorse concerns about the efficacy of COVID-19 vaccination, potential side effects of COVID-19 vaccination, and the speed with which COVID-19 vaccines were produced.
Overall, we find increased hesitancy among younger individuals, consistent with previous studies, showing that older adults tend to prioritize the benefit of reducing serious illness over the cost of side effects [21]. This may reflect an initial prioritization of adults 65 years and older as eligible for vaccination. Furthermore, age is frequently cited in the scientific literature and lay press as contributing to the morbidity and mortality of COVID-19 infection, which may have an influence on decision making [27]. We also found a significant difference in political affiliation between hesitant and not hesitant respondents, with hesitant respondents more frequently reporting being independents or unaffiliated versus not hesitant respondents reporting affiliation with the Democratic Party.
Notably, we did not find a significant difference in hesitancy when comparing metro and non-metro respondents, suggesting that strategies employed to increase uptake in urban areas could be employed in a rural setting as well. Additionally, we found that family and friends are trusted sources of information. These networks could be leveraged more effectively to address concerns related to COVID-19 vaccination efficacy, side effects, and speed of production. However, vaccine misinformation may also spread in these networks. As identified in prior work, the effects of misinformation and political polarization should inform all strategies to increase vaccination rates [9].
Assumptions about the finding that hesitant respondents were more likely to discuss vaccination with their primary care provider include: (a) not hesitant respondents had already made up their minds regarding the vaccine and, therefore, did not solicit more information from their physicians, or (b) respondents in the hesitant group may be eager to pursue information on vaccinations, but overall, they are more skeptical of conventional or government sources, such as physicians, the CDC, and the State of Maine. Moreover, primary care providers were the most frequently reported trusted source of information on COVID-19 vaccines in the hesitant group and greater than media sources, the CDC, and the State of Maine. These data suggest health care providers may be in a key position to address misinformation about vaccines and address patient concerns about getting the vaccine. A national survey of 9000 adults, ages 18–64, reported that two-thirds had a personal doctor, and three in four of these adults trusted health care providers for information. However, only one in five had obtained such information from their personal doctor. These findings suggest a greater need for advancing the role and training of health care professionals to address lingering concerns of COVID-19 vaccine hesitant individuals [28].
Study limitations include convenience sampling, a small sample size for the not hesitant group, lack of racial and ethnic diversity, high levels of participant education, and increases in availability of the vaccine during the study period. Data were collected before the Delta and Omicron virus surges, which may have positively impacted vaccine intention, as recently reported by Kaiser Family Foundation.18 Low and unequal representation of Republican Party affiliation (9%) could be a limitation of hesitancy findings associated with politicization. Comparison of metro and non-metro groups was limited by similar demographics across groups, potentially impacting significance of the analysis.
5. Public Health Implications
Findings from this study, regarding vaccine hesitancy and sources of information, are important for developing public health strategies to increase vaccination rates for successful mitigation of the COVID-19 pandemic. Vaccine hesitant individuals comprise one end of a continuum, ranging from total acceptors to complete refusers. Understanding the concerns of individuals, as assessed in this study, provides opportunities to address misinformation, target messaging, and influence the ‘movable middle’ toward getting the vaccine [25,29]. Our study reveals that vaccine hesitant individuals have specific concerns about COVID-19 vaccinations, such as worries about existing conditions and side effects. Trained and trusted health professionals, as well as family and friends who need accurate information as trusted sources, could effectively address such concerns and misinformation. Women of child bearing age were particularly vocal about a need for information from trusted sources. The findings from this study emphasize the role of health care providers as sought out and trusted resources for guidance. Providers are uniquely positioned to influence the hesitant middle of the continuum, particularly by addressing worries about the effect of vaccines on existing medical conditions. The findings from this study in Maine communities will inform improvements in outreach and messaging strategies. Leveraging the role of health professionals to encourage vaccine acceptance should continue to focus on training to address misinformation and on updating materials for health professionals to share during visits.
Author Contributions
Study conceptualization: A.M.R.H., K.M.F., E.A.J.; survey methodology: A.M.R.H., K.L.B., K.M.F., E.A.J.; data analysis: C.T.W.; manuscript development and critical edits: all authors, funding acquisition: K.M.F., E.A.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Maine Medical Center Human Subjects Committee. #1701214.
Informed Consent Statement
Written consent was waived as implied by response to the survey.
Data Availability Statement
Interested investigators should contact Hess for information on data sharing.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by a pilot grant from the Northern New England CTR project, 3U54GM115516-04.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Center for Disease Control and Prevention COVID Data Tracker: United States COVID-19 Cases, Deaths, and Laboratory Testing (NAATs) by State, Territory, and Jurisdiction. U.S. Department of Health & Human Services. [(accessed on 15 December 2021)]; Available online: https://covid.cdc.gov/covid-data-tracker/#trends_dailydeaths.
- 2.Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021;591:520–522. doi: 10.1038/d41586-021-00728-2. [DOI] [PubMed] [Google Scholar]
- 3.Barker P., Hartley D., Beck A.F., Oliver G.H., Sampath B., Roderick T., Miff S. Rethinking Herd Immunity: Managing the Covid-19 Pandemic in a Dynamic Biological and Behavioral Environment. NEJM Catal. Innov. Care Deliv. 2021;2 [Google Scholar]
- 4.Kwon Y., Cho H.Y., Lee Y.K., Bae G.R., Lee S.G. Relationship between intention of novel influenza A (H1N1) vaccination and vaccination coverage rate. Vaccine. 2010;29:161–165. doi: 10.1016/j.vaccine.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 5.Myers L.B., Goodwin R. Determinants of adults’ intention to vaccinate against pandemic swine flu. BMC Public Health. 2011;11:15. doi: 10.1186/1471-2458-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khubchandani J., Sharma S., Price J.H., Wiblishauser M.J., Sharma M., Webb F.J. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J. Community Health. 2021;46:270–277. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreps S., Prasad S., Brownstein J.S., Hswen Y., Garibaldi B.T., Zhang B., Kriner D.L. Factors Associated with US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open. 2020;3:1–13. doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C., Tu P., Beitsch L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines. 2020;9:16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neely S.R., Eldredge C., Ersing R., Remington C. Vaccine Hesitancy and Exposure to Misinformation: A Survey Analysis. J. Gen. Intern. Med. 2022;37:179–187. doi: 10.1007/s11606-021-07171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark E., Fredricks K., Woc-Colburn L., Bottazzi M.E., Weatherhead J. Disproportionate impact of the COVID-19 pandemic on immigrant communities in the United States. PLoS Negl. Trop. Dis. 2020;14:e0008484. doi: 10.1371/journal.pntd.0008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurencin C.T., McClinton A. The COVID-19 Pandemic: A Call to Action to Identify and Address Racial and Ethnic Disparities. J. Racial Ethn. Health Disparities. 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcendor D.J. Targeting COVID Vaccine Hesitancy in Rural Communities in Tennessee: Implications for Extending the COVID-19 Pandemic in the South. Vaccines. 2021;9:1279. doi: 10.3390/vaccines9111279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett K.J., Pumkam C., Probst J.C. Rural-urban differences in the location of influenza vaccine administration. Vaccine. 2011;29:5970–5977. doi: 10.1016/j.vaccine.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Lu P., Bridges C.B., Euler G.L., Singleton J.A. Influenza vaccination of recommended adult populations, U.S.; 1989–2005. Vaccine. 2008;26:1786–1793. doi: 10.1016/j.vaccine.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Purnell M., Maxwell T., Hill S., Patel R., Trower J., Wangui L., Truong H.A. Exploring COVID-19 vaccine hesitancy at a rural historically black college and university. J. Am. Pharm. Assoc. 2022;62:340–344. doi: 10.1016/j.japh.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:2–8. doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogue K., Jensen J.L., Stancil C.K., Ferguson D.G., Hughes S.J., Mello E.J., Burgess R., Berges B.K., Quaye A., Poole B.D. Influences on Attitudes Regarding Potential COVID-19 Vaccination in the United States. Vaccines. 2020;8:582. doi: 10.3390/vaccines8040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser Family Foundation KFF COVID-19 Vaccine Monitor. 2021. [(accessed on 17 November 2021)]. Available online: https://files.kff.org/attachment/TOPLINE-KFF-COVID-19-Vaccine-Monitor-September-2021.pdf.
- 19.Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine. 2020;38:6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verger P., Dube E. Restoring confidence in vaccines in the COVID-19 era. Expert Rev. Vaccines. 2020;19:991–993. doi: 10.1080/14760584.2020.1825945. [DOI] [PubMed] [Google Scholar]
- 21.Funk C., Tyson A. Growing Share of Americans Say They Plan to Get a COVID-19 Vaccine—Or Already Have. Pew Research Center. 2021. [(accessed on 17 November 2021)]. Available online: https://www.pewresearch.org/science/2021/03/05/growing-share-of-americans-say-they-plan-to-get-a-covid-19-vaccine-or-already-have/
- 22.Larson H.J., Jarrett C., Eckersberger E., Smith D.M., Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine. 2014;32:2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 23.Razai M.S., Oakeshott P., Esmail A., Wiysonge C.S., Viswanath K., Mills M.C. COVID-19 vaccine hesitancy: The five Cs to tackle behavioural and sociodemographic factors. J. R. Soc. Med. 2021;114:295–298. doi: 10.1177/01410768211018951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn S.C., Jamison A.M., An J., Hancock G.R., Freimuth V.S. Measuring vaccine hesitancy, confidence, trust and flu vaccine uptake: Results of a national survey of White and African American adults. Vaccine. 2019;37:1168–1173. doi: 10.1016/j.vaccine.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Dube E., Laberge C., Guay M., Bramadat P., Roy R., Bettinger J. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013;9:1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Agriculture: Economic Research Service. Urban Influence Codes. [(accessed on 15 September 2021)];2013 Available online: https://www.ers.usda.gov/data-products/urban-influence-codes.
- 27.Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and Inflammaging: Risk Factors of Severe COVID-19 in Older People. Front. Immunol. 2020;11:1–18. doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpman M., Zuckerman S. Few Unvaccinated Adults Have Talked to Their Doctors about the COVID-19 Vaccines. Urban Institute; Washington, DC, USA: 2021. [Google Scholar]
- 29.MacDonald N.E., The SAGE Working Group on Vaccine Hesitancy Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Interested investigators should contact Hess for information on data sharing.