Abstract
Purpose
To investigate separate and combined effects of vitamin D supplementation during the extended winter and increased dairy protein intake on muscle strength and physical function in children, and furthermore to explore potential sex differences.
Methods
In a 2 × 2-factorial, randomized winter trial, 183 healthy, 6–8-year-old children received blinded tablets with 20 µg/day vitamin D3 or placebo, and substituted 260 g/day dairy with yogurts with high (HP, 10 g protein/100 g) or normal protein content (NP, 3.5 g protein/100 g) for 24 weeks during winter at 55° N. We measured maximal isometric handgrip and leg press strength, and physical function by jump tests and a 30 s sit-to-stand test. Physical activity was measured by 7-day accelerometry.
Results
Baseline (mean ± SD) serum 25-hydroxyvitamin D was 80.8 ± 17.2 nmol/L, which increased to 88.7 ± 17.6 nmol/L with vitamin D supplementation and decreased to 48.4 ± 19.2 nmol/L with placebo. Baseline protein intake was 15.5 ± 2.4 E%, which increased to 18.4 ± 3.4 E% with HP and was unchanged with NP. We found no separate or combined effects of vitamin D supplementation and/or increased dairy protein intake on muscle strength or physical function (all P > 0.20). There was an interaction on the sit-to-stand test (Pvitamin×yogurt = 0.02), which however disappeared after adjusting for physical activity (P = 0.16). Further, vitamin D supplementation increased leg press strength relatively more in girls compared to boys (mean [95% CI] 158 [17, 299] N; Pvitamin×sex = 0.047).
Conclusion
Overall, vitamin D and dairy protein supplementation during the extended winter did not affect muscle strength or physical function in healthy children. Potential sex differences of vitamin D supplementation should be investigated further.
Registered at clinicaltrials.gov
NCT0395673.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-022-02912-0.
Keywords: Pediatric, Muscle function, Dietary supplements, Physical performance, Diet, Milk protein
Introduction
Muscle strength and physical function in childhood are increasingly emphasized as independent predictors of health status [1, 2]. Further, emerging evidence shows that lean mass (LM), i.e. primarily skeletal muscle mass, and muscle strength are positively associated with bone mineralization during growth [3, 4]. Thus, it is crucial to uncover potential strategies to support the development of muscle mass, muscle strength, and physical function in growing children to support and improve their physical condition and preference for movement long term.
In adults, primarily of older age, vitamin D status has been positively associated with muscle strength and physical function, and vitamin D supplementation has been shown to increase muscle strength, especially in elderly with vitamin D deficiency (serum 25-hydroxyvitamin D (25(OH)D) < 30 nmol/L) [5, 6]. Mechanistically, vitamin D deficiency has been suggested to down-regulate vitamin D receptors in muscle cells, which may slow force development and lead to muscle fiber atrophy [7]. Additionally, in rats, vitamin D supplementation increased myotube differentiation and, together with insulin, positively stimulated the mTOR pathway and protein synthesis [8], which are important for muscle growth. During extended winter from October to March dermal vitamin D synthesis is negligible at northern latitudes [9] which increases the risk of vitamin D deficiency in children [10], who thus may benefit from vitamin D supplementation. Large-scale cross-sectional studies have shown positive associations between serum 25(OH)D and handgrip strength in children [11, 12]. However, previous trials examining effects of vitamin D supplementation on muscle strength and physical function in children and adolescents have shown mixed results. Notably, most were of relatively short duration (i.e. 6–12 weeks) or only assessed handgrip strength. Yet, a meta-analysis in elderly primarily showed positive effects of vitamin D supplementation on lower limb strength [5]. This underlines the importance of evaluating the effect of vitamin D supplementation on children’s lower limb strength.
Dairy products are a significant source of protein, calcium, and vitamins and contain all essential amino acids. Protein has been shown to stimulate growth and LM accretion in undernourished children [13]. Moreover, positive associations between intake of dairy protein and LM [14] as well as total protein intake and whole body net protein balance [15, 16] have been observed in healthy children. Since LM is a prerequisite for muscle strength and physical function, enhanced dairy protein intake may positively influence these factors in growing children. However, to our knowledge, no studies have yet examined effects of a prolonged nutritional intervention with dairy protein supplementation on muscle strength or physical function in healthy children.
Importantly, since vitamin D and dairy protein may both affect musculoskeletal parameters yet through different mechanisms, there is a potential for an added effect when combining them. A trial in elderly showed that LM and muscle strength increased with a combined vitamin D and whey protein supplementation compared to an isocaloric control of maltodextrin [17]. However, the trial also included physical training and other nutrients, such as n-3 fatty acids and calcium, potentially influencing their findings. Studies investigating the concomitant effects of vitamin D and dairy protein on muscle strength and physical function in children are lacking.
Therefore, we aimed to assess the combined and separate effects of vitamin D and dairy protein supplementation on various measures of muscle strength and physical function in 6–8-year-old children, based on secondary outcomes from the 24-week extended winter trial, D-pro. Secondarily, we aimed to explore whether potential effects were sex-specific and whether adjusting for physical activity (PA) influenced the results. We hypothesized that vitamin D and high dairy protein intake would increase children’s muscle strength and physical function compared to placebo and normal dairy protein, respectively.
Methods
Study design and ethics
The study design of the D-pro trial has been described in detail elsewhere [18]. The overall aim of the D-pro study was to investigate effects of vitamin D and dairy protein on children’s growth and health. Effects on the primary outcome, bone mineral density [18], and the secondary outcomes fat mass index, fat-free mass index (FFMI), and cardiometabolic markers have been published previously [19]. Noteworthy, we found no effects of the intervention on FFMI [19]. Here, we report results on muscle strength and physical function along with LM as a supportive outcome linked to the former two outcomes. LM is highly comparable with FFMI as previously reported, but LM will be used in the present context, because it is a more accurate measure of muscle mass compared to FFMI, since bone mineral content is subtracted [20].
In brief, D-pro was a 2 × 2-factorial, randomized, controlled trial where 200 healthy, 6–8-year-old white, Danish children were included. They were randomly allocated to (a) ingest blinded tablets with either 20 µg/day vitamin D3 or placebo, and (b) substitute 260 g/day dairy in their diet with high protein (HP) or normal protein (NP) yogurt. The intervention lasted 24 weeks and was conducted at latitude 55°N during extended winter to avoid cutaneous synthesis of vitamin D. The study was conducted at Department of Nutrition, Exercise and Sports, University of Copenhagen, Frederiksberg, Denmark. Baseline and endpoint measurements were performed during August–October 2019 and February–April 2020, respectively [18]. The study was conducted following the Declaration of Helsinki and approved by the Committees on Biomedical Research Ethics for the Capital Region of Denmark (no. H-19008199) and registered at clinicaltrials.gov with the ID: NCT03956732 before recruitment was initiated.
Participants and recruitment
Children, aged 6–8 years, who lived in the Capital Region of Denmark were identified through the Danish Civil Registration System. Children were recruited by postal invitation letters to the parents. Families who responded to the invitation letter were prescreened by telephone and were subsequently invited for an information meeting together with the child. Written informed consent was obtained from all custody holders [18].
Eligible children had to be of Danish or European origin and have white skin since randomization of children with dark skin to placebo tablets would conflict with the national vitamin D recommendations at the time of the study. Children should also like yogurt and high protein yogurt, “skyr”, and have a habitual intake of dairy products of at least 250 mL/day. Moreover, families should not be planning a stay below a latitude of 50° N during the intervention, and at least one parent had to read and speak Danish to be properly informed about the study procedures. Exclusion criteria were (1) known or suspected allergy or intolerance to dairy products, (2) diseases or medication that could interfere with the study outcomes, and (3) concomitant participation in other studies involving dietary interventions or blood sampling. Children could not use vitamin D-containing supplements > 3 days/week the last two months and any in the month immediately preceding the start of the intervention. Finally, only one child from each household could participate in the study [18].
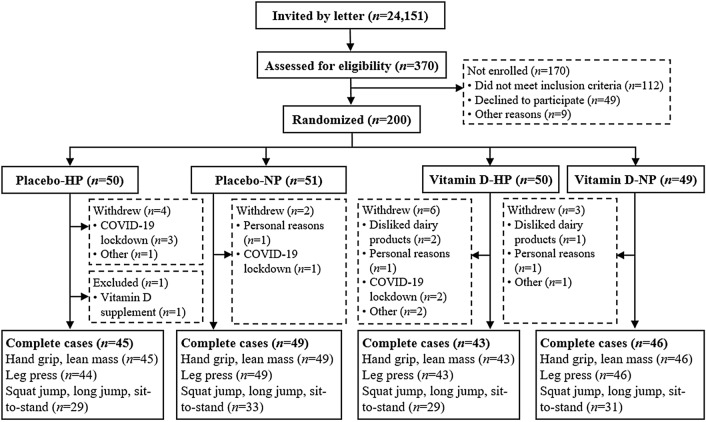
A flowchart of the children from recruitment to completion is shown in Fig. 1. Children with test data from both baseline and endpoint visits were included in the present study, resulting in a sample of n = 183 for handgrip, n = 182 for leg press, and n = 122 for squat jump, long jump, and the 30 s sit-to-stand test. Handgrip and leg press data were lost for respectively one and two children, due to an electronic disconnection at the baseline visit. During the last three weeks of the endpoint examinations, the protocol was changed as a result of restrictions due to the COVID-19 pandemic. Consequently, performance in squat jump, long jump, and the 30 s sit-to-stand test were missing from 60 children at endpoint (Fig. 1).
Fig. 1.
Flow chart. Complete cases refers to children with both baseline and endpoint measurement. HP high protein, NP normal protein
Randomization and blinding
Block randomization with 12 children in each block was used to allocate children equally to each of the four intervention groups throughout the autumn. An impartial staff member produced a computer-generated randomization list, from which sealed, sequentially numbered envelopes containing the corresponding group allocation were produced, as previously described [18]. Children were randomly assigned at the end of the baseline visit. The tablet intervention was blinded to all involved in the study, but due to the taste and nature of the yogurt products, blinding the yogurt intervention was not possible. However, the investigators were blinded for data analysis by recoding the allocation.
Intervention
Vitamin D (Minisun®) and placebo tablets were provided by Oy Verman Ab, Finland. Tablets were chewable, identical in taste and appearance, and packaged in identical bottles. Parents were supplied with one bottle of tablets (containing 200 tablets) and a 7-day tablet dispenser and were instructed to provide one tablet per day to the child in connection with a meal as described previously [18, 19]. Tablet vitamin D content was analyzed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) after completion of the trial (National Food Institute, Søborg, Denmark). The vitamin D content was 25 ± 2 µg/tablet and non-detectable in the vitamin D and placebo tablets, respectively. Tablet compliance was assessed by count of leftover tablets [18].
The HP and NP yogurts that were provided to the children were commercially available in Denmark and contained 9–11 g protein/100 g and 3–4 g protein/100 g, respectively [18]. The HP yogurts were low-fat “skyr”, which is strained yogurt, similar to Greek yogurt, with a similar 20:80 whey:casein ratio as in normal yogurt (NP). All yogurt products were fermented with Streptococcus Thermophilus and Lactobacillus Bulgarius. Yogurts were provided free of charge from Arla Foods amba (Denmark) and came in both unflavored and fruit-flavored variants in original packaging. The target intake was 300 g/day, six days per week, and was to be substituted with the same amount of milk or yogurt in the child’s habitual diet to maintain the child’s usual dietary pattern [18]. Therefore, the protein intake was expected to be around 17 g/day higher in HP compared to NP, corresponding to a total protein intake increase of around 25% [21]. Parents were instructed to record the child's daily yogurt intake in pre-coded recording sheets, which were used to assess compliance and macronutrient intake from the yogurts [18].
The placebo tablets and NP yogurts were regarded as controls since vitamin D supplementation was not recommended in Denmark at the time the project was conducted [18], and Danish children typically have a dairy protein intake similar to the targeted intake in the NP groups. Apart from the intervention, parents were encouraged to maintain their child’s habitual dietary habits and participation in physical leisure activities during the study period.
Muscle strength and physical function
A battery of muscle strength and physical function tests were selected to obtain measures of both upper and lower body maximal strength, as well as physical function. This comprised: maximal isometric handgrip strength, isometric leg press, squat jump, long jump, and a 30 s sit-to-stand test. The test procedures have been described elsewhere [22]. We previously found the reliability of these tests to be good-to-excellent for handgrip, leg press, and long jump tests [intraclass correlation coefficient (ICC) = 0.86–0.90], and moderate for the squat jump test (ICC = 0.71), even without familiarization [22], in Danish 6–9-year-olds. Retest reliability for the sit-to-stand-test was low (ICC = 0.63) without familiarization, yet moderate after familiarization (ICC = 0.78) [22]. Therefore, the children of the present study were familiarized with the sit-to-stand test at the information meeting, approximately 1–2 weeks before the baseline test. All physical tests were led by one of two investigators according to standard operating procedures, and for 95% of the children, the examinations were performed by the same investigator at baseline and endpoint.
Physical activity
As previously described [19], children's PA was objectively measured by waterproof tri-axis AX3 accelerometers with a temperature sensor (Axivity Ltd., Newcastle upon Tyne, United Kingdom) during seven consecutive days and eight nights before the baseline and endpoint visits. The software OmGUI (v. 1.0.0.37) was used in the set-up and data download. The sensitivity was set to ± 8 g and sampling frequency to 100 Hz. Parents were instructed to attach the accelerometer to the children's non-dominant thigh with Fixomull tape (BSN Medical), as described elsewhere [23]. All acceleration data was processed in Matlab (Version 9.9.0 R2020b, Mathworks Inc., Natick, Massachusetts, US), which included resampling into 30 Hz, generating ActiGraph counts [24], identification of non-wear periods, and summarizing the time spent in different intensity domains. Non-wear periods were identified from both acceleration and temperature [25]. All periods identified as not worn were marked as missing data. Time spent with sedentary, light, moderate, and vigorous PA was estimated using 10-s epochs accounting for the elevated post oxygen consumption during intermittent PA, using the second-by-second epoch data to improve the assessment of vigorous activity [26, 27]. The cut-points defining the intensity domains were determined using an internal calibration study conducted in 5–13-year-old children [27]. Moderate intensity was determined as the average counts for walking at self-selected speed. The energy expenditure during walking at self-selected speed was set by stature and the intensity corresponds to 30–35% of the children’s maximal aerobic capacity [28]. The vigorous intensity threshold was determined as the count level for which 95% of the intensities measured during running were included. Running was considered a vigorous activity performed at > 60% of the children's aerobic capacity. The thigh cut points were 100 counts for sedentary behavior, 4822 for moderate behavior, and 9143 for vigorous behavior. Only subjects with at least two valid (≥ 90% wear time) weekdays and one valid weekend day [19] was included in the analysis. All PA outcomes were estimated as an average and adjusted using a 5/7 and 2/7 rule to account for the number of weekdays and weekend-days. Moderate-vigorous physical activity (MVPA) was calculated by adding time spent at moderate- and vigorous intensity. Participation in organized sport, i.e. type of sport, weekly frequency, and duration, was evaluated from an electronic questionnaire filled out by the parents at both visits.
Dietary intake
Prior to the baseline and endpoint visit, parents completed a weighed, four-day dietary record in the web-based software Madlog (Madlog Aps, Kolding, Denmark) [29] from which the children’s energy and macronutrient intake was assessed, as previously described [18]. A study investigator looked through the dietary registrations, and if any abnormalities were present, clarification was obtained by dialogue with the parents at the following examination visit. Vitamin D and calcium intake the preceding month was obtained from an electronic, previously validated food frequency questionnaire [30] at both visits [18].
Anthropometry and body composition
Bodyweight, height, BMI z-score, weight categories, and pubertal stage were obtained as previously described [18]. LM was obtained from dual-energy X-ray absorptiometry (DXA) scans using a GE Lunar Prodigy (GM Healthcare, Chicago, Illinois, USA) with GE Healthcare software version 17, SP1) [18, 19], and was the result of total mass minus fat mass and bone mass. The scans were performed after a standardized breakfast and emptying of the bladder with the child wearing light clothes without metal. The same investigator checked the lines defining regions of interest on both baseline and endpoint scans and corrected these when necessary. Daily and weekly quality assurance (QA) was conducted with a QA-phantom and a Spine Phantom no. 17466, respectively, and coefficients of variation (CV) for LM was 1.5%.
Blood sampling
To assess vitamin D status [18], an overnight fasting venous blood sample was drawn from the forearm of the child at both baseline and endpoint, after a 10 min supine rest. Local anesthetic patches (EMLA, AstraZeneca, Sweden) were provided beforehand to relieve the discomfort of blood sampling. Serum 25(OH)D2 and 25(OH)D3 were analyzed by LC–MS/MS at University College Cork, Ireland [31], and vitamin D deficiency and insufficiency were defined as total serum 25(OH)D < 30 nmol/L and < 50 nmol/L, respectively [32]. The intra- and interassay CV were < 6% and < 5%.
Power calculation
The original sample size calculation of the D-pro trial was based on the primary outcome, bone mineral density. Assuming a power of 0.80 and a significance level of 0.05, n = 49 completers were required in each group to detect an accretion difference of 0.0085 g/cm2 or 0.8 SD between any two groups during the intervention [18].
Statistical analyses
Data is presented as mean ± SD, median [25th–75th percentile], or n (%), as appropriate. All analyses were conducted using Stata/IC 17 and included children with available data from both baseline and endpoint for the specific outcome (complete cases). Statistical significance and trends towards significance were established at P values below 0.05 and 0.1, respectively. Normal distribution of variables and model assumptions were evaluated visually by residual histograms and QQ-plots. All models met the model assumptions.
Differences in baseline characteristics between dropouts and completers were tested with Pearson's Chi-squared, Wilcoxon rank-sum, or two-sample t test, as appropriate. Within-group changes from baseline to endpoint were tested with paired t test. Differences in dietary intake and PA changes between the four study groups were analyzed with one-way ANCOVA models with study group as fixed factor, adjusted for baseline. Combined effects of the vitamin D and yogurt interventions on the outcomes were evaluated as between-group change differences adjusted for baseline with two-way ANCOVA with the interaction term vitamin D × yogurt, and main effects of vitamin D and yogurts were tested in an additive model. If any interaction was found, subsequent Bonferroni-corrected pairwise comparisons were performed.
To account for biological variation, secondary models were adjusted for age and sex. Further, we investigated whether the observed effects were mediated by change in energy intake or MVPA. In subsequent analyses, we also adjusted for calcium intake due to the interplay of vitamin D and calcium [33], and the potential association between calcium and muscle function [14]. To check for consistency, per-protocol analyses were conducted with exclusion of those who reported breach of the protocol, either by commencement of non-study-related vitamin D supplementation (n = 4) or reporting that they had traveled south of 50° N during the intervention (n = 4). Finally, to assess whether sex influenced potential effects, the interaction terms vitamin D × sex and yogurt × sex were included in the additive models. We assessed the Bonferroni-adjusted pairwise associations between LM and muscle strength and physical function, respectively, at baseline and endpoint as well as for change from baseline to endpoint.
Results
Subject characteristics and compliance
Of the 200 randomized children, a total of 183 were included in the present analysis. The 17 non-included children were mainly boys (n = 15) and therefore spend more time with MVPA (P = 0.004), but did otherwise not differ from the included children with regard to anthropometry, age, or parental education (P > 0.11, data not shown).
Table 1 shows baseline characteristics for the included children (n = 183) in the present study. As previously reported [18], median [25th–75th percentile] age was 7.7 [7.0–8.2] years, few children (4%) had entered puberty, and parental educational level was generally high. Most children had normal weight (79%) and participated in organized sports (91%). Time spent on MVPA was 0.7 [0.4–1.0] h/day. Seven children (4%) had serum 25(OH)D < 50 nmol/L, and mean ± SD serum 25(OH)D was 79.6 ± 17.6 nmol/L. Dietary protein intake was 15.5 ± 2.4 E% and did not differ between the sexes (P = 0.93). Boys spent more time with MVPA and had greater total LM than girls (P < 0.001), but there was no sex-difference in maximal strength measures (P > 0.42). However, boys jumped longer than girls (P = 0.008).
Table 1.
Baseline characteristics of included children (n = 183)
| Placebo tablets | Vitamin D tablets | |||
|---|---|---|---|---|
| HP yogurt | NP yogurt | HP yogurt | NP yogurt | |
| n | 45 | 49 | 43 | 46 |
| Sex, boys, n (%) | 21 (47%) | 22 (45%) | 22 (51%) | 17 (37%) |
| Age (y) | 7.6 [6.9–8.3] | 7.6 [7.0–8.2] | 7.8 [7.3–8.3] | 7.6 [7.0–8.2] |
| Puberty, have entered, n (%) | 2 (4%) | 0 (0%) | 1 (2%) | 4 (9%) |
| Parental education, n (%) | ||||
| ≤ Vocational or short academic | 6 (13%) | 10 (20%) | 6 (14%) | 5 (11%) |
| ≥ Bachelors’s degree | 39 (87%) | 39 (80%) | 37 (86%) | 41 (89%) |
| Height (cm) | 128.4 ± 6.8 | 129.3 ± 7.5 | 130.6 ± 7.3 | 130.5 ± 6.5 |
| Weight (kg) | 26.2 ± 4.9 | 26.7 ± 5.4 | 26.1 ± 4.3 | 28.3 ± 5.3 |
| BMI-for-age z-score | − 0.06 ± 1.06 | − 0.02 ± 1.09 | − 0.28 ± 0.77 | 0.36 ± 1.07 |
| Weight category, n (%)a | ||||
| Underweight | 5 (11%) | 5 (10%) | 1 (2%) | 4 (9%) |
| Normal weight | 34 (76%) | 37 (76%) | 40 (93%) | 34 (74%) |
| Overweight or obesity | 6 (13%) | 7 (14%) | 2 (5%) | 8 (17%) |
| Total LM, kg | 19.0 ± 3.1 | 18.7 ± 2.9 | 19.3 ± 2.6 | 19.7 ± 2.7 |
| Physical activity | ||||
| CPMc | 1254 ± 286 | 1301 ± 281 | 1311 ± 340 | 1275 ± 276 |
| MVPA, min/dayc | 46.1 ± 22.1 | 45.9 ± 19.9 | 48.1 ± 23.6 | 46.7 ± 19.3 |
| Organized sport, yes n (%) | 41 (91%) | 44 (90%) | 40 (93%) | 41 (89%) |
| Organized sport, h/w | 1.5 (0.7–2.5) | 1.3 (0.6–2.8) | 1.5 (0.6–3.0) | 2.0 (1.0–3.0) |
| Dietary intake | ||||
| Energy intake (kJ/day) | 6289 ± 1431 | 6523 ± 1128 | 7248 ± 1508 | 6738 ± 1252 |
| Protein intake (E%) | 15.5 ± 2.5 | 15.2 ± 2.3 | 15.6 ± 2.3 | 15.7 ± 2.5 |
| Carbohydrate intake (E%) | 52.6 ± 4.7 | 54.5 ± 5.0 | 52.2 ± 4.6 | 54.6 ± 4.2 |
| Fat intake (E%) | 31.9 ± 4.7 | 30.4 ± 4.5 | 32.0 ± 4.4 | 29.7 ± 4.3 |
| Calcium intake (mg/day) | 784 [529–1053] | 906 [674–1291] | 955 [708–1238] | 917 [683–1272] |
| Vitamin D intake (µg/day) | 2.6 [1.5–3.8] | 2.3 [1.4–3.7] | 2.2 [1.3–4.6] | 2.6 [1.7–3.6] |
| Serum 25(OH)D (nmol/L)b | 80.2 ± 19.7 | 78.0 ± 17.6 | 80.5 ± 14.3 | 81.4 ± 18.3 |
Values are mean ± SD, median [25th–75th percentile], percentage ratios or number and percentages. Data, except for MVPA and organized sports, have been reported previously [19]
BMI body mass index, CPM counts per minute, E% energy percentage, HP high protein, LM lean mass, MVPA moderate-vigorous physical activity, NP normal protein, 25(OH)D 25-hydroxyvitamin D
aMeasured by accelerometry; n = 172 (n = 44, n = 43, n = 43 and n = 42 in the HP-placebo, NP-placebo, HP-vitamin D, and NP-vitamin D groups, respectively)
bFrom fasting blood sample; missing data due to unsuccessful or insufficient blood sampling; n = 178 (n = 41, n = 48, n = 43 and n = 46 in the HP-placebo, NP-placebo, HP-vitamin D, and NP-vitamin D groups, respectively)
As previously reported [18], tablet compliance was 95% [90–98%] with no difference between vitamin D and placebo groups (P = 0.37). But as expected, the total vitamin D intake (including the vitamin D supplement) differed between groups (P < 0.001).Serum 25(OH)D increased in the vitamin D groups and decreased in the placebo groups (6.6 ± 14.9 nmol/L versus − 29.6 ± 21.7 nmol/L, P < 0.001 between groups). At endpoint, all children in the vitamin D groups had serum 25(OH)D ≥ 50 nmol/L, whereas respectively 14% and 45% of children in the placebo groups were vitamin D deficient or insufficient. Dairy protein intake from the yogurt intervention was 22.8 [18.9–24.7] g/day in the HP and 9.2 [8.5–10.0] g/day in the NP groups (P < 0.001). Thus, the protein intake increased to 18.3 ± 3.4 E% during the intervention in the HP groups and was unchanged in the NP groups (P < 0.001 between groups). Overall, total PA (counts per minute (CPM)) decreased from baseline to endpoint by 9.6 ± 24.3% (P < 0.001), as previously described [19]. This was due to a decrease in light (26.8 ± 62.1 min/day), moderate (5.4 ± 15.9 min/day), and vigorous (1.8 ± 7.5 min/day) intensity and an increase in sedentary time (30.4 ± 63.3 min/day) from baseline to endpoint (all P < 0.001) (Table 2), as expected when transitioning from summer to winter [34]. Change in PA did not differ between groups (P > 0.05).
Table 2.
Changes in physical activity from baseline to endpoint
| Placebo tablets | Vitamin D tablets | P | |||
|---|---|---|---|---|---|
| HP yogurt | NP yogurt | HP yogurt | NP yogurt | ||
| n | 38 | 39 | 38 | 39 | |
| Average CPM (Δ) | − 173 (292) | − 243 (260) | − 72 (348) | − 123 (327) | 0.056 |
| Sedentary (Δ min/day) | 24.7 (69.1) | 46.4 (70.8) | 13.8 (59.6) | 34.1 (53.2) | 0.29 |
| Light (Δ min/day) | − 26.4 (70.3) | − 41.1 (59.3) | − 9.8 (60.0) | − 27.8 (60.4) | 0.43 |
| Moderate (Δ min/day) | − 7.9 (14.3) | − 8.2 (15.0) | − 2.0 (18.8) | − 4.0 (15.7) | 0.094 |
| Vigorous (Δ min/day) | − 3.0 (6.3) | − 2.9 (5.1) | − 0.1 (10.7) | − 1.3 (8.1) | 0.14 |
| MVPA (Δ min/day) | − 10.9 (18.5) | − 11.0 (18.9) | − 2.1 (25.6) | − 5.2 (22.5) | 0.075 |
| Steps (Δ) | − 1445 (3261) | − 2426 (2587) | − 840 (2729) | − 1273 (3302) | 0.18 |
Data are presented as mean (SD) change from baseline to endpoint. P values are from one-way ANCOVA-models testing group differences with study group as fixed factor
CPM counts per minute, HP high protein, NP normal protein, MVPA moderate-vigorous physical activity, Δ change from baseline to endpoint
Muscle strength and physical function
A vitamin D × yogurt interaction was found on 30 s sit-to-stand performance (P = 0.02), apparently due to slightly smaller increases in the placebo-NP and vitamin D-HP groups than in the two other groups (Table 3). However, the interaction disappeared after adjusting for change in MVPA (P = 0.16). There were no other combined nor separate effects of vitamin D supplementation and/or increased protein intake on maximal muscle strength or physical function (Table 3). Covariate-adjustments and per-protocol analyses did not change the results (data not shown). Sex-specific effects of vitamin D was observed on maximal lower body strength (Pvitamin×sex = 0.047), with greater changes observed in girls compared to boys within the vitamin D supplemented groups (158 [17, 299] N; mean [95% CI]) and with vitamin D compared to placebo in girls (125 [− 6, 256] N) (Supplemental Fig. 1). We found no other sex-specific effects (data not shown).
Table 3.
Muscle strength and physical function in the study groups
| Placebo tablets | Vitamin D tablets | Vitamin D × yogurt | Vitamin D | Yogurt | Pb | Pb | |
|---|---|---|---|---|---|---|---|
| HP yogurt | NP yogurt | HP yogurt | NP yogurt | Pa | |||
| Nc | 45 | 49 | 43 | 46 | |||
| Handgrip (kg) | |||||||
| Baseline | 11.2 ± 3.0 | 11.1 ± 3.2 | 11.9 ± 2.7 | 11.8 ± 2.5 | |||
| Endpoint | 12.9 ± 2.9 | 12.6 ± 3.2 | 13.6 ± 3.1 | 13.4 ± 2.2 | 0.1 [-0.3, 0.6] | 0.2 [-0.3, 0.6] | |
| Change | 1.7 ± 1.6* | 1.5 ± 1.6* | 1.7 ± 1.6* | 1.6 ± 1.4* | P = 0.53 | P = 0.64 | P = 0.41 |
| Leg press (N) | |||||||
| Baseline | 1106 ± 395 | 1144 ± 403 | 1188 ± 304 | 1117 ± 359 | |||
| Endpoint | 1642 ± 411 | 1654 ± 443 | 1677 ± 407 | 1705 ± 383 | 38 [− 61, 136] | − 29 [− 128, 69] | |
| Change | 536 ± 368* | 510 ± 385* | 489 ± 337* | 588 ± 348* | P = 0.27 | P = 0.58 | P = 0.55 |
| Squat jump (cm) | |||||||
| Baseline | 19.9 ± 4.4 | 18.9 ± 4.5 | 18.5 ± 4.0 | 19.7 ± 3.6 | |||
| Endpoint | 20.0 ± 4.1 | 19.2 ± 5.2 | 19.8 ± 3.5 | 20.1 ± 3.2 | 0.5 [− 0.4, 1.4] | 0.5 [− 0.4, 1.4] | |
| Change | 0.1 ± 3.1 | 0.2 ± 2.5 | 1.3 ± 2.6* | 0.4 ± 2.5 | P = 0.70 | P = 0.29 | P = 0.29 |
| Long jump (cm) | |||||||
| Baseline | 122 ± 16 | 115 ± 24 | 120 ± 17 | 121 ± 17 | |||
| Endpoint | 128 ± 18 | 122 ± 19 | 124 ± 19 | 124 ± 14 | − 2.0 [− 5.0, 1.1] | 0.4 [− 2.6, 3.5] | |
| Change | 5 ± 9* | 7 ± 11* | 4 ± 7* | 3 ± 10* | P = 0.63 | P = 0.20 | P = 0.78 |
| 30 s sit-to-stand (repetitions) | |||||||
| Baseline | 33 ± 5 | 31 ± 5 | 32 ± 5 | 32 ± 4 | |||
| Endpoint | 37 ± 4 | 34 ± 5 | 35 ± 4 | 36 ± 4 | 0.6 [− 0,8, 2.0] | 0.5 [− 0.9, 1.8] | |
| Change | 4 ± 4* | 3 ± 5* | 3 ± 3* | 4 ± 5* | P = 0.023 | P = 0.38 | P = 0.51 |
Values are presented as mean ± SD. All complete cases are included. *Significant within-group changes (P < 0.05), tested by paired t test
HP high protein, LM lean mass, NP normal protein
aP values are from the treatment interaction term in two-way ANCOVA models adjusted for baseline of the outcome
bP values are from main effects of vitamin D and dairy protein, respectively, obtained from additive two-way ANCOVA models adjusted for baseline of the outcome
cn represent maximal number. For leg press, n = 44, n = 49, n = 43, and n = 46; for squat jump, long jump, and 30 s sit-to-stand, n = 29, n = 33, n = 29, and n = 31 in the placebo-HP, placebo-NP, vitamin D-HP, and vitamin D-NP group, respectively
Across all groups, maximal muscle strength and physical function were improved during the study period. Moreover, LM increased by 0.86 ± 0.60 kg (P < 0.001) with no between-group differences (P = 0.42) which corresponds to our previous finding of no effect on FFMI in this trial [19]. At baseline, LM was positively associated with handgrip strength (r = 0.71), leg press strength (r = 0.37), long jump (r = 0.28), and squat jump (r = 0.27) (all P < 0.01), but not with 30 s sit-to-stand performance.
Discussion
The present study showed an effect of combining vitamin D supplementation and increased intake of dairy protein on 30 s sit-to-stand performance in healthy, mainly normal-weight, 6–8-year-old children, which, however, disappeared after adjustment for PA. No other combined or separate effects of the interventions were observed on muscle strength or physical function. However, vitamin D supplementation increased maximal leg press strength relatively more in girls compared to boys.
In our trial, vitamin D supplementation prevented the profound winter decline in vitamin D status observed with placebo [18]. Nevertheless, no overall effect of vitamin D supplementation compared to placebo was detected on muscle strength or physical function, measured by a comprehensive panel of tests. This is in accordance with our previous finding of no effect on FFMI in this trial [19]. Moreover, our results correspond to the findings of other vitamin D interventions in children with both vitamin D sufficiency [35–37] and insufficiency [38, 39] at baseline. In adults, some [5, 40–42], but not all [6, 43–45] meta-analyses have shown that vitamin D supplementation increases muscle strength, at least if vitamin D status is insufficient at baseline. Moreover, a single mega-dose of vitamin D3 improved squat jump performance in 8–15-year-old soccer players with vitamin D insufficiency, when compared to placebo [46]. Yet, that study did not show consistent effects on other physical function measures. Taken together with the results from our study and other trials, it may be questioned if vitamin D supplementation has clinical relevance for improving muscle strength and physical function in children with sufficient vitamin D levels for at least some parts of the year. Nevertheless, we cannot rule out the possibility that the duration of our intervention (24 weeks) and those of others (~ 6–20 weeks) may have been too short to influence the outcome parameters.
Interestingly, we did observe that vitamin D supplementation resulted in a greater gain in maximal lower body strength in girls compared to boys, but with no sex-specific differences in upper body (handgrip) strength. Sex-specific responses to vitamin D supplementation have rarely been investigated in children. A previous trial from our group in healthy, 4–8 year-olds [35] showed no sex-specific effects of 20-week vitamin D supplementation during winter on handgrip strength; however, their sample size was smaller (n = 117). It has been suggested that vitamin D deficiency preferentially affects muscle strength of the lower limbs [47], and a meta-analysis found a positive effect of vitamin D supplementation on lower limb muscle strength but not on handgrip strength in healthy individuals across the lifespan [5], which may explain the different observations we made in lower and upper body strength in girls. We can only speculate on the potential underlying mechanisms responsible for the sex-specific response in lower body strength. Yet differences in sex hormones have been shown in mice [48], which may be linked to earlier growth and development in girls. Also, since vitamin D status tended to be lower in girls than boys at baseline, the girls may have had greater potential for effects of the supplementation.
A vitamin D × protein interaction was observed for the 30 s sit-to-stand test, which disappeared when adjusting for PA. This test only showed moderate test–retest reliability (ICC = 0.78), and since we did not find consistent combined or separate intervention effects on the other maximal physical function measures, we speculate whether changes in PA may have confounded the result of this submaximal physical function test.
The dairy intervention, which resulted in a daily protein intake of 2.5 ± 0.8 g/kg BW in HP compared to 2.1 ± 0.6 g/kg BW in NP did not influence gains in muscle strength or physical function. It is well established that development of muscle strength is stimulated by PA, especially of moderate-to-vigorous intensity [49]. Further, in physically active adults, a protein intake of about ~ 1.6 g/kg BW/day, which is higher than the 0.83 g/kg BW/day recommended by the WHO, leads to superior muscle growth when combined with strength training [50]. The potential interaction between physical activity and protein intake is less studied in children, but recent studies have shown that protein intake after physical exercise can stimulate muscle protein synthesis in healthy children [16, 51]. The children's daily protein intake in both the HP and NP groups was well above the WHO recommendation of 0.9 g protein/kg BW for children [52]. The lack of intervention effect may be because the children’s protein intake was already sufficient for maximal accretion of LM along with muscle strength and physical function. Hence, we speculate that a ceiling effect of protein ingestion exists in growing children in energy balance, comparable to what has been observed in adults [50] or that concomitant physical training is needed to evoke an effect.
Some methodological considerations of the present study deserve mention. Conducting the trial during winter and avoiding sunny holidays minimized confounding by dermal vitamin D synthesis. Several physical tests were included to comprehensively assess muscle strength and physical function, and all tests were found to be valid and reliable in a comparable child population [22]. Additional strengths were the use of accelerometry to objectively measure PA and DXA to measure LM. Further, the children showed good compliance with both the yogurt and vitamin D intervention, and there was low drop-out (5%), which was evenly distributed among groups. We were not able to blind the yogurt intervention due to different flavors and textures, but the investigators were re-blinded before data analysis. Apart from protein intake, the relative fat intake (E%) also differed between HP and NP groups. However, adjusting for fat intake (E%) did not change the findings. Importantly, total energy intake did not differ between groups [18]. Since our study participants were all white children with normal weight and sufficient protein intake, other studies are needed to investigate this in populations of children with dark skin, obesity, or undernourishment. Further, more studies are needed to confirm the results in a population of vitamin D-deficient children and to evaluate possible long-term effects of repeated winter vitamin D supplementation on muscle strength and physical function.
Conclusions
We found no overall effect of vitamin D supplementation, in a dose that prevented winter deficiency, or of increased dairy protein intake on muscle strength or physical function in healthy, white, 6–8-year-old children. Also, there were no consistent combined effects of vitamin D supplementation and increased intake of dairy protein on the outcomes. However, we observed a larger improvement in leg strength with vitamin D supplementation in girls compared to boys, which should be investigated further.
Supplementary Information
Below is the link to the electronic supplementary material.
SUPPLEMENTAL FIGURE 1: Sex-specific changes in isometric leg press strength after supplementation with vitamin D or placebo. Between-group differences were tested by one-way ANCOVA adjusted for baseline and protein intervention. a, p<0.05 between groups; b, p<0.10 between groups; vit-D, vitamin D; N, newton; pla, placebo (PNG 40 kb)
Acknowledgements
We thank all the participating children and their parents. We also thank Julia W. Clerico, Rikke R. Carlsson, Josefine M. Terp, Camilla F. B. Nielsen, Siv Rohde, Zahraa Yaqub, Malene Nygaard, Astrid D. Jensen, and Jannie W. Jensen for assistance in the conduct of the study as well as Vivian Anker and Søren Andresen for blood sampling and analysis.
Abbreviations
- 25(OH)D
25-Hydroxyvitamin D
- BW
Body weight
- CPM
Counts per minute
- CV
Coefficient of variation
- D-pro
Effects of milk protein and vitamin D on children’s growth and health
- DXA
Dual-energy X-ray absorptiometry
- E%
Percentage of total energy intake
- FFMI
Fat-free mass index
- HP
High protein
- ICC
Intraclass correlation coefficient
- LC–MS/MS
Liquid chromatography–tandem mass
- LM
Lean mass
- MVPA
Moderate-vigorous physical activity
- NP
Normal protein
- PA
Physical activity
- QA
Quality assurance
Author contributions
CM, MH, CTD, and LGH designed research; LT and NGS conducted research; JCB analyzed and interpreted physical activity data; LT analyzed data, wrote the paper, and had primary responsibility for final content. All the authors reviewed, provided input to, and approved the final manuscript.
Funding
This work was supported by the consortium Arla Food for Health (2018), The Augustinus Foundation (19-1300), and Hede Nielsens Foundation (32880). Vitamin D3 (Minisun®) and placebo tablets were kindly provided by Oy Verman Ab, Finland. The supporters had no role in the study design, data collection, data analyses, data interpretation, or writing of the article.
Availability of data and material
Data described in the manuscript will not be made available because data is not anonymized and therefore considered as “personal data”.
Code availability
Not applicable.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
This study involving humans was approved by the Committees on Biomedical Research Ethics for the Capital Region of Denmark (no. H-19008199), and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The trial was registered at clinicaltrials.gov as NCT03956732.
Consent to participate
We obtained written consent from legal guardians.
Consent for publication
We affirm that all legal guardians provided informed consent for publication of the data.
References
- 1.World Health Organization . Global recommendations on physical activity for health. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 2.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsey KB, Thornton JC, Heymsfield SB, Gallagher D. Greater lean tissue and skeletal muscle mass are associated with higher bone mineral content in children. Nutr Metab (Lond) 2010;7:41. doi: 10.1186/1743-7075-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, Jung HW, Hong H, et al. The role of overweight and obesity on bone health in Korean adolescents with a focus on lean and fat mass. J Korean Med Sci. 2017;32:1633–1641. doi: 10.3346/jkms.2017.32.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–4345. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 6.Stockton KA, Mengersen K, Paratz JD, et al. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–871. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 7.Dzik KP, Kaczor JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol. 2019;119:825–839. doi: 10.1007/s00421-019-04104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romeu Montenegro K, Carlessi R, Cruzat V, Newsholme P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J Steroid Biochem Mol Biol. 2019;193:105423. doi: 10.1016/j.jsbmb.2019.105423. [DOI] [PubMed] [Google Scholar]
- 9.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 10.Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurson KR, Thomas JN, Barnes JL. Vitamin D status is associated with muscular strength in a nationally representative sample of US youth. Acta Paediatr. 2020;109:2755–2761. doi: 10.1111/apa.15253. [DOI] [PubMed] [Google Scholar]
- 12.Al-Jwadi RF, Jespersen E, Dalgård C, et al. S-25OHD is associated with hand grip strength and myopathy at 5 years in girls: an odense child cohort study. J Clin Endocrinol Metab. 2018;103:2630–2639. doi: 10.1210/jc.2018-00281. [DOI] [PubMed] [Google Scholar]
- 13.Grenov B, Michaelsen KF. Growth components of cow’s milk: emphasis on effects in undernourished children. Food Nutr Bull. 2018;39:S45–S53. doi: 10.1177/0379572118772766. [DOI] [PubMed] [Google Scholar]
- 14.Magnussen CG, Fraser BJ, Smith KJ. Dietary calcium and dairy intake and muscular fitness phenotypes in Australian children. J Sports Sci. 2020;38:717–718. doi: 10.1080/02640414.2020.1727606. [DOI] [PubMed] [Google Scholar]
- 15.Karagounis LG, Volterman KA, Breuillé D, et al. Protein intake at breakfast promotes a positive whole-body protein balance in a dose-response manner in healthy children: a randomized trial. J Nutr. 2018;148:729–737. doi: 10.1093/jn/nxy026. [DOI] [PubMed] [Google Scholar]
- 16.Moore DR, Volterman KA, Obeid J, et al. (2014) Postexercise protein ingestion increases whole body net protein balance in healthy children. J Appl Physiol. 1985;117:1493–1501. doi: 10.1152/japplphysiol.00224.2014. [DOI] [PubMed] [Google Scholar]
- 17.Bell KE, Snijders T, Zulyniak M, et al. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One. 2017;12:e0181387. doi: 10.1371/journal.pone.0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stounbjerg NG, Thams L, Hansen M, et al. Effects of vitamin D and high dairy protein intake on bone mineralization and linear growth in 6- to 8-year-old children: the D-pro randomized trial. Am J Clin Nutr. 2021 doi: 10.1093/ajcn/nqab286. [DOI] [PubMed] [Google Scholar]
- 19.Thams L, Stounbjerg NG, Hvid LG, et al. Effects of high dairy protein intake and vitamin D supplementation on body composition and cardiometabolic markers in 6–8-y-old children-the D-pro trial. Am J Clin Nutr. 2022 doi: 10.1093/ajcn/nqab424. [DOI] [PubMed] [Google Scholar]
- 20.Ogle GD, Allen JR, Humphries IR, et al. Body-composition assessment by dual-energy X-ray absorptiometry in subjects aged 4–26 y. Am J Clin Nutr. 1995;61:746–753. doi: 10.1093/ajcn/61.4.746. [DOI] [PubMed] [Google Scholar]
- 21.Nadelmann Pedersen A, Fødevareinstituttet, Afdeling for Ernæring (2015) Danskernes kostvaner 2011–2013: hovedresultater. DTU Fødevareinstituttet, Afdeling for Ernæring: [Eksp.] www.schultzboghandel.dk, Søborg
- 22.Thams L, Hvid LG, Damsgaard CT, Hansen M. Test–retest reliability of muscle strength and physical function tests in 6–9-year-old children. MPEES. 2021 doi: 10.1080/1091367X.2021.1943400. [DOI] [Google Scholar]
- 23.Schneller MB, Bentsen P, Nielsen G, et al. Measuring children’s physical activity: compliance using skin-taped accelerometers. Med Sci Sports Exerc. 2017;49:1261–1269. doi: 10.1249/MSS.0000000000001222. [DOI] [PubMed] [Google Scholar]
- 24.Brønd JC, Andersen LB, Arvidsson D. Generating ActiGraph counts from raw acceleration recorded by an alternative monitor. Med Sci Sports Exerc. 2017;49:2351–2360. doi: 10.1249/MSS.0000000000001344. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen MGB, Pedersen J, Olesen LG, et al. Short-term efficacy of reducing screen media use on physical activity, sleep, and physiological stress in families with children aged 4–14: study protocol for the SCREENS randomized controlled trial. BMC Public Health. 2020;20:380. doi: 10.1186/s12889-020-8458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen TL, Brønd JC, Kristensen PL, et al. Resemblance in accelerometer-assessed physical activity in families with children: the Lolland–Falster Health Study. Int J Behav Nutr Phys Act. 2020;17:161. doi: 10.1186/s12966-020-01067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brønd JC, Aadland E, Andersen LB, et al. The ActiGraph counts processing and the assessment of vigorous activity. Clin Physiol Funct Imaging. 2019;39:276–283. doi: 10.1111/cpf.12571. [DOI] [PubMed] [Google Scholar]
- 28.Weyand PG, Smith BR, Puyau MR, Butte NF. The mass-specific energy cost of human walking is set by stature. J Exp Biol. 2010;213:3972–3979. doi: 10.1242/jeb.048199. [DOI] [PubMed] [Google Scholar]
- 29.MADLOG—Kalorietæller og kalorieberegner online—Tilmeld dig her. https://www.madlog.dk/da/. Accessed 7 Sep 2020
- 30.Kiely M, Collins A, Lucey AJ, et al. Development, validation and implementation of a quantitative food frequency questionnaire to assess habitual vitamin D intake. J Hum Nutr Diet. 2016;29:495–504. doi: 10.1111/jhn.12348. [DOI] [PubMed] [Google Scholar]
- 31.Cashman KD, Kiely M, Kinsella M, et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr. 2013;97:1235–1242. doi: 10.3945/ajcn.112.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol. 2017;453:36–45. doi: 10.1016/j.mce.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkin AJ, Sharp SJ, Harrison F, et al. Seasonal variation in children’s physical activity and sedentary time. Med Sci Sports Exerc. 2016;48:449–456. doi: 10.1249/MSS.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortensen C, Mølgaard C, Hauger H, et al. Winter vitamin D(3) supplementation does not increase muscle strength, but modulates the IGF-axis in young children. Eur J Nutr. 2019;58:1183–1192. doi: 10.1007/s00394-018-1637-x. [DOI] [PubMed] [Google Scholar]
- 36.Wright CS, Laing EM, Pollock NK, et al. Serum 25-hydroxyvitamin D and intact parathyroid hormone influence muscle outcomes in children and adolescents. J Bone Miner Res. 2018;33:1940–1947. doi: 10.1002/jbmr.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubnov-Raz G, Livne N, Raz R, et al. Vitamin D supplementation and physical performance in adolescent swimmers. Int J Sport Nutr Exerc Metab. 2015;25:317–325. doi: 10.1123/ijsnem.2014-0180. [DOI] [PubMed] [Google Scholar]
- 38.El-Hajj Fuleihan G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 39.Ward KA, Das G, Roberts SA, et al. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010;95:4643–4651. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 40.Rejnmark L. Effects of vitamin d on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis. 2011;2:25–37. doi: 10.1177/2040622310381934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59:2291–2300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Quan M, Cao ZB. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: a meta-analysis. PLoS One. 2019;14:e0215826. doi: 10.1371/journal.pone.0215826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosendahl-Riise H, Spielau U, Ranhoff AH, et al. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. J Hum Nutr Diet. 2017;30:3–15. doi: 10.1111/jhn.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51:1219–1226. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 45.Annweiler C, Bridenbaugh S, Schott AM, et al. Vitamin D and muscle function: new prospects? BioFactors. 2009;35:3–4. doi: 10.1002/biof.4. [DOI] [PubMed] [Google Scholar]
- 46.Bezrati I, Ben Fradj MK, Hammami R, et al. A single mega dose of vitamin D(3) improves selected physical variables in vitamin D-deficient young amateur soccer players: a randomized controlled trial. Appl Physiol Nutr Metab. 2020;45:478–485. doi: 10.1139/apnm-2019-0525. [DOI] [PubMed] [Google Scholar]
- 47.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–424. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Fleet JC. 1,25 dihydroxycholecalciferol-mediated calcium absorption and gene expression are higher in female than in male mice. J Nutr. 2004;134:1857–1861. doi: 10.1093/jn/134.8.1857. [DOI] [PubMed] [Google Scholar]
- 49.Moore DR. Protein metabolism in active youth: not just little adults. Exerc Sport Sci Rev. 2019;47:29–36. doi: 10.1249/JES.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 50.Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volterman KA, Moore DR, Breithaupt P, et al. Timing and pattern of postexercise protein ingestion affects whole-body protein balance in healthy children: a randomized trial. Appl Physiol Nutr Metab. 2017;42:1142–1148. doi: 10.1139/apnm-2017-0185. [DOI] [PubMed] [Google Scholar]
- 52.WHO (2007) Protein and amino acid requirements in human nutrition: report of a joint WHO/FAO/UNU expert consultation. http://ebookcentral.proquest.com/lib/kbdk/detail.action?docID=305231. Accessed 28 Jan 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1: Sex-specific changes in isometric leg press strength after supplementation with vitamin D or placebo. Between-group differences were tested by one-way ANCOVA adjusted for baseline and protein intervention. a, p<0.05 between groups; b, p<0.10 between groups; vit-D, vitamin D; N, newton; pla, placebo (PNG 40 kb)
Data Availability Statement
Data described in the manuscript will not be made available because data is not anonymized and therefore considered as “personal data”.
Not applicable.