Abstract
During recent decades, a tick-borne rickettsial syndrome, characterized by eschar and painful lymphadenopathy after Dermacentor marginatus-bite, has been described as an emerging rickettsiosis in Europe. Our group named it DEBONEL (Dermacentor-borne-necrosis-erythema-lymphadenopathy), regarding the vector and the main infection signs. Other groups called it TIBOLA (tick-borne-lymphadenophathy) and, later, SENLAT (scalp-eschar-and-neck-lymphadenopathy-after-tick-bite), expanding, in the latter, the etiological spectrum to other pathogens. Objective: To investigate the etiology of DEBONEL agents in our area, and to compare their epidemiological/clinical/microbiological characteristics. During 2001–2020, 216 patients clinically diagnosed of DEBONEL (the largest series from one center) in La Rioja (northern Spain) were examined. Rickettsia spp. were amplified in 14/104 (13.46%) blood samples, 69/142 (48.59%) eschar swabs, 7/7 (100%) biopsies, and 71/71 (100%) D. marginatus from patients. For samples in which Rickettsia was undetected, no other microorganisms were found. ‘Candidatus Rickettsia rioja’, Rickettsia slovaca, Rickettsia raoultii, and Rickettsia DmS1 genotype were detected in 91, 66, 4, and 3 patients, respectively. DEBONEL should be considered in patients with clinical manifestations herein described in areas associated to Dermacentor. The most frequently involved agent in our environment is ‘Ca. R. rioja’. The finding of Rickettsia sp. DmS1 in ticks attached to DEBONEL patients suggests the implication of other rickettsia genotypes.
Keywords: DEBONEL, Dermacentor-borne-necrosis-erythema-lymphadenopathy, Dermacentor marginatus, ‘Candidatus Rickettsia rioja’, Rickettsia slovaca, Rickettsia raoultii, Rickettsia sp. DmS1, Spain
1. Introduction
During the past two decades, new tick-borne rickettsial diseases have been described in Europe [1]. One of these is known as DEBONEL/TIBOLA, acronyms of ‘Dermacentor-borne-necrosis-erythema-lymphadenopathy’ and ‘tick-borne lymphadenopathy’, respectively. DEBONEL/TIBOLA was described from several points of view; thus, compatible clinical cases were notified by Lakos et al. in Hungary [2], the microbiological approach was made by Raoult et al. in France [3], and the complete epidemiological, clinical and microbiological description was achieved in Spain and France [4,5,6,7]. Afterwards, several cases have been described in Bulgaria, Italy, Germany, Poland, Portugal, United Kingdom, France, and Spain [1,8,9,10,11,12,13,14,15,16,17,18,19,20]. Dermacentor marginatus is the main vector, although Dermacentor reticulatus has been also involved in France. After being bitten by a Dermacentor sp. tick, a high percentage of patients develop an inoculation eschar (point of necrosis) at the site of the tick-bite surrounded by an erythema and regional enlarged and painful lymphadenopathies. For these reasons, we proposed and defended the acronym DEBONEL since it makes reference to the main clinical features and to the involved tick genus. This fact has epidemiological implications because this tick genus is more active in the coldest months, when most cases appear. Regarding the etiological agents, Rickettsia slovaca was detected by polymerase chain reaction (PCR) in 1997 from a French patient with a scalp eschar and lymphadenopathy after a tick-bite in the Pyrenees mountains (France) [21]. Six years later, in 2003, the culture and isolation of R. slovaca from another French patient was reported, showing that R. slovaca is a human pathogen and an etiological agent of at least some patients affected with this syndrome [5]. In La Rioja (northern Spain), R. slovaca was also detected by PCR in ticks removed from DEBONEL patients [4,6,7,22,23]. In 2001, we achieved to amplify DNA corresponding to a new rickettsial genotype that we named ’Candidatus Rickettsia rioja’ (GenBank accession no. EF028201) in human blood and ticks removed from DEBONEL patients [24]. Moreover, Rickettsia raoultii [25], initially named Rickettsia spp. RpA4, DnS14 and DnS28 [26,27], had been detected in ticks from DEBONEL patients [6,7,11]. In 2010, since the tick-bite is more frequently found on the scalp, Angelakis et al. proposed the name SENLAT (scalp-eschar-and-neck-lymphadenopathy-after-tick-bite) to describe this syndrome [28]. Nevertheless, this acronym is only useful when the tick-bite is on the scalp, and the term is also used in association with other infectious agents without referring to the arthropod vector that may belong to other tick genera, such as Ixodes sp. or Rhipicephalus sp. Apart from R. slovaca, R. raoultii, and ‘Ca. R. rioja’, the infectious agents include other Rickettsia spp. (Rickettsia sibirica subsp. mongolitimonae or Rickettsia massiliae) and non-Rickettsia microorganisms, like Borrelia burgdorferi sensu lato (s.l.), Bartonella henselae, Francisella tularensis, or Coxiella burnetii [28,29,30,31,32,33,34]. In this manuscript we will use our original term, DEBONEL, for naming the clinical picture of our patients since all of them fulfilled the criteria shown in the Section 4 and there is no consensual name. Herein, we describe the clinical and epidemiological characteristics of patients with DEBONEL caused by R. slovaca, ‘Ca. R. rioja’, and R. raoultii attended in the Department of Infectious Diseases at San Pedro University Hospital in La Rioja (SPUH) (Spain). The possible existence of epidemiological or clinical distinguishing features among DEBONEL patients infected with ‘Ca. R. rioja’, R. slovaca, or R. raoultii was also investigated. Moreover, we reported the first implication of the uncultured Rickettsia sp. DmS1 in three DEBONEL patients.
2. Results
Two hundred and sixteen out of 232 patients that met clinical and epidemiological inclusion’s criteria could be completely studied by microbiological methods and followed up. Seventy-one patients (32.87%) were attended with the tick attached or brought the tick removed by them or by sanitary personal. All the ticks were adults of D. marginatus, 63 females (88.73%) and 8 males (11.27%). The remaining patients 145 (67.12%) remembered being bitten by a large tick during the months in which D. marginatus is active, but they did not keep the arthropod.
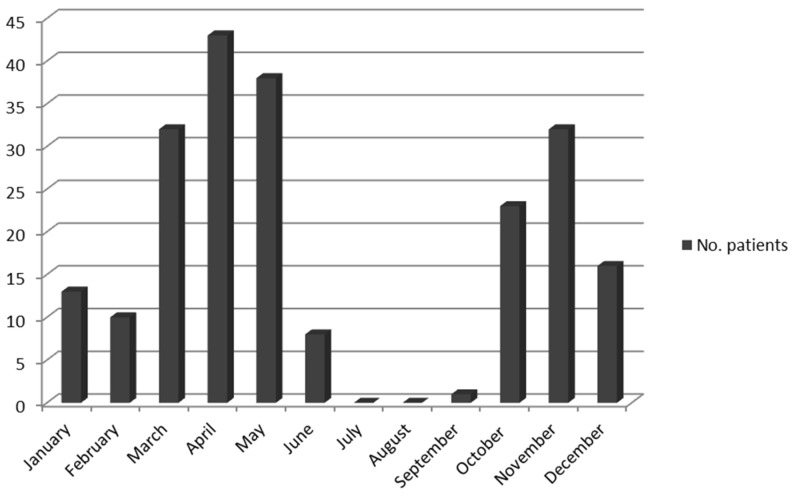
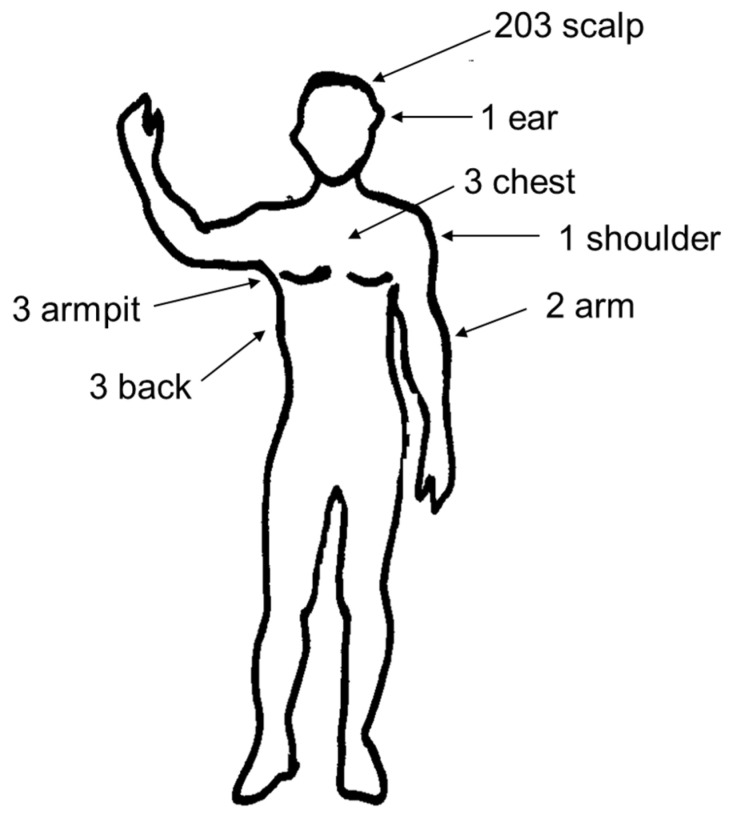
Clinical and epidemiological data of the series are shown in Table 1. One hundred and forty-one patients (65.28%) were women and 75 (34.72%) were men. The mean age was 37.7 years (range 3–83) and the median age was 40. A total of 61 patients (28.24%) were younger than 15 years old. All patients were bitten mainly during the coldest months (from October to May), with a peak in November and in April–May (113 out of 216 cases), Figure 1. The incubation period varied from 1 to 15 days (mean: 5.61; median: 5). The tick-bite was located on the scalp in 203 patients (93.98%), and in 13 patients (6.02%) not on the scalp, including the back (3), armpits (3), arms (2), chest (3), ear (1), and shoulder (1), Figure 2. All patients with the inoculation lesion on the scalp shown local headache and multiple large and painful cervical lymphadenopathies. Facial local swelling was observed in 4 of them (1.97%). Furthermore, 76 patients (37.44%) developed alopecia at tick-bite site (0.5–2.0 cm diameter) that persisted after 3 months. Low grade fever (<38 °C axillary) was present in 71 patients (32.87%), and 8 patients (3.70%) had fever ≥38 °C. Diffuse macular rash was only observed in one patient (three macules in legs). All patients were treated with antibiotics. Doxycycline (100 mg/bid 14 days) was administered to 155 patients (71.76%), whereas 61 (28.24%) (59 children <15-year-old, a pregnant woman and a woman allergic to doxycycline) were treated with azithromycin (10 mg/Kg qd 5 days or 500 mg/qd 5 days). Improvement of the signs and symptoms were observed in all but one cases. This was a 15-year-old woman that worsened after 5-day-treatment with azithromycin. Later, she received a course of doxycycline and recovered. Fever disappeared 48 h after starting the treatment in all patients, and the painful lymphadenopathy improved in 1 week (5 to 15 days), although it was present during at least 3 or 4 weeks in most patients.
Figure 1.
Monthly distribution of DEBONEL cases.
Figure 2.
Location of skin lesions in DEBONEL patients.
2.1. Microbiological Tests
2.1.1. Serological Assays
Evidence of recent infection (seroconversion or fourfold rise in titer) by a Spotted Fever Group (SFG) Rickettsia, was observed in 91 out of 109 patients with available paired sera (83.49%). It was detected during the first month in most cases, but seroconversion was delayed (in the second month) in 39 cases (24.84%).
2.1.2. Molecular Methods (PCR)
PCR was performed in all 216 patients. A total of 14/104 blood samples (13.46%), 69/142 eschar swabs (48.59%), 7/7 biopsies (100%), and 71/71 D. marginatus (100%) were positive for Rickettsia. For those samples that yielded PCR negative results for Rickettsia, no other microorganisms were detected.
The ompA and ompB genes sequences obtained from 8 blood samples, 38 eschar swabs, 4 biopsies, and 41 D. marginatus shown the highest similarity (99.4–100%) to ‘Ca. R. rioja’ (GenBank accession no. EF028201 and GQ404431, respectively). In three of them, positive PCR for gltA gene was obtained, and the nucleotide sequences shown the highest similarity (99.4%) with partial gltA gene from Rickettsia sp. DmS1 (GenBank accession no. AY129300). The ompA, ompB and gltA fragment genes amplified from 6 blood, 31 eschar swabs, 3 biopsies, and 26 D. marginatus were 100% identical to R. slovaca (GenBank accession no. CP002428.1). The ompA and ompB nucleotide sequences corresponding to the remaining four D. marginatus shown the highest similarity (99.3–99.8%) to R. raoultii strain Khabarovsk (GenBank accession no. CP010969).
In summary, diagnoses of infection by ‘Ca. R. rioja’ (91 cases), R. slovaca (66 cases), or R. raoultii (4 cases) were made in 161 out of 216 enrolled patients, Figure 3.
Figure 3.
Etiological agents detected in DEBONEL cases.
2.2. Epidemiological and Clinical Comparison of ‘Ca. R. rioja’, R. slovaca and R. raoultii Infections
2.2.1. ‘Ca. R. rioja’ Infection
Ninety-one patients had evidence of ‘Ca. R. rioja’ infection. More women (53.85%) than men were affected. Their mean and median age was 32.98 and 32.5 years (range 5–83), respectively; 38 of them (41.9%) were younger than 15 years old. All these patients were bitten during the coldest months (17 in March, 19 in April and May, 15 in November, 11 in October, 4 in February, 2 in December, 2 in January, and 2 in September). The incubation period ranged from 2 to 12 days (mean 5.43 and median 6 days). In 89 patients, the inoculation lesion was located on the scalp and two patients were bitten on the back. Two patients had fever that disappeared within 48h. after starting the antibiotic treatment. Fifty-eight patients received doxycycline (63.64%) and 33 azithromycin (36.26%). The painful lymphadenopathy improved during the first week for 39 out of 43 patients and for the remaining patients, during the following 15 days. Twenty-six out of 89 patients with the lesion on the scalp region (29.21%) developed persistent alopecia at the site of the tick-bite.
Thirty-nine patients (81.25%) had evidence of recent infection by SFG Rickettsia based on indirect immunofluorescence assay (IFA) using Rickettsia conorii and R. slovaca antigens. Nine patients shown seroconversion during the second month.
2.2.2. R. slovaca Infection
Sixty-six patients had evidence of R. slovaca infection. Forty-five were women (one was pregnant) and 21 were men. The mean and median age was 37.62 and 40.5 years (range 3–79), respectively; twenty-three of them (34.85%) were younger than 15 years old. Fifteen patients were bitten in April; 13 in November; 11 in May; 9 in March; 6 in January and October; and 2 in February, June, and December. The incubation period ranged from 2 to 12 days (mean and median 4.68 and 5 days). In 64 patients, the bite was located on the scalp, one patient was bitten on the arm and another one was bitten on the armpit. Only three patients had fever. All patients were treated with antibiotics, 43 with doxycycline (65.15%) and 24 with azithromycin (36.36%). In all cases, improvement of the signs and symptoms were observed. Fever disappeared 48h after the beginning of the treatment. The painful lymphadenopathy improved in one week for 27 out of 31 patients, and in 15 days for the remaining ones. Forty patients (62.50%) developed persistent alopecia at the site of the tick-bite (0.5–2 cm in diameter).
Evidence of recent infection by SFG Rickettsia-IFA, was observed in 28 patients (82.35%). The number of patients with reactivity against the two rickettsial antigens was similar. In all cases seroconversion was detected during the first month. In four cases, IgG antibody titers against R. slovaca were two serial dilutions higher than against R. conorii.
2.2.3. R. raoultii Infection
Four patients had evidence of R. raoultii infection. Two cases occurred in April, and one in May and in June. The incubation period was 5 days. In three patients, the bite was located on the scalp, while one patient was bitten on the chest. No patient had fever. In four cases, the painful lymphadenopathy improved in one week.
Evidence of recent infection with SFG Rickettsia was demonstrated by IFA in one patient.
Comparison of data from human ‘Ca. R. rioja’, R. slovaca, and R. raoultii infections are shown in Table 1. Significant differences were detected in relation to the sex of the patients between those with DEBONEL caused by an unknown agent or by ‘Ca. R. rioja’. Besides, in terms of developing persistent alopecia significant differences were found between patients with ‘Ca. R. rioja’ infection and R. slovaca infection, and between those with R. slovaca infection and those with DEBONEL due to an unknown agent.
Table 1.
Comparison of clinical and epidemiological data from human ‘Candidatus Rickettsia rioja’, Rickettsia slovaca and Rickettsia raoultii infection.
| Clinical and Epidemiological Data |
‘Ca. R. rioja’ Infection (n:91) |
R. slovaca Infection (n:66) |
R. raoultii Infection (n:4) |
Patients with DEBONEL by Unknown Agent (n:55) | p Value | Total Patients (n:216) |
|---|---|---|---|---|---|---|
| Sex (female) | 49/91 (53.85%) | 45/66 (68.18%) | 3/4 (75.00%) | 44/55 (80.00%) ** | 0.008 | 141 |
| Mean age (years) | 32.98 ± 2.42 | 37.62 ± 3.09 | 27.00 ± 12.27 | 39.13 ± 2.80 | 0.325 | 37.7 |
| IP (days) | 5.43 ± 0.31 | 4.68 ± 0.28 | 5.00 ± 0.41 | 5.78 ± 0.40 | 0.161 | 5.61 |
| Low grade fever 1 | 34/91 (37.36%) | 19/66 (28.79%) | 2/4 (50.00%) | 16/55 (29.09%) | 0.505 | 71 (32.87%) |
| Fever 2 | 2/91 (2.20%) | 3/66 (4.55%) | 0/4 (0.00%) | 3/55 (5.45%) | 0.653 | 8 (3.70%) |
| Persistent alopecia 3 | 26/89 (29.21%) | 40/64 (62.50%) *** | 1/3 (33.33%) | 9/47 (19.15%) ### | <0.001 | 76/203 (37.44%) |
| Evidence of recent infection by IFA (seroconversion or fourfold rise in titer) | 39/48 (81.25%) | 28/34 (82.35%) | 1/1 (100.00%) | 23/26 (88.46%) | 0.798 | 91/109 (83.49%) |
Qualitative variables are represented in percentage while quantitative variables are represented as mean ± standard error mean. The p value refers to the comparison between four groups. Asterisks indicate statistically significant differences with respect to ‘Ca. R. rioja’ group, while hashtags indicate statistically significant differences with respect to R. slovaca (** p < 0.01 vs. ‘Ca. R. rioja’, *** p < 0.001 vs. ‘Ca. R. rioja’ and ### p < 0.001 vs. R. slovaca). ‘Ca. R. Rioja’: ‘Candidatus Rickettsia rioja’; n: number; R.: Rickettsia; DEBONEL: Dermacentor- borne-necrosis-erythema-lymphadenopathy; IP: Incubation period; 1 Low grade fever: <38 °C; 2 Fever > 38 °C; 3 Persistent alopecia: The patient developed persistent alopecia at the site of the tick-bite (0.5–2 cm in diameter); IFA: Immunofluorescence assay.
3. Discussion
In this report, we describe the epidemiological, clinical, and microbiological characteristics of 216 patients with DEBONEL (the largest series from a unique Center) observed over a 20-year period in La Rioja, a small region in the North of Spain, where this entity was first described by clinical and epidemiological observation and afterwards, as other groups, by microbiological techniques. Microbiological assays, and specifically PCR techniques, demonstrate that the etiological agents, when the clinical-epidemiological criteria are fulfilled, are ‘Ca. R. rioja’, followed by R. slovaca and R. raoultii. Furthermore, we have demonstrated that other Rickettsia genotypes, such as Rickettsia sp. DmS1, could be implicated. These facts are important since the empirical treatment made with doxycycline or azithromycin are effective against the involved agents [35,36]. As in other reports, there are clinical and epidemiological bases to diagnose DEBONEL [7,23]. It could be difficult to distinguish DEBONEL from tick-borne tularemia, since Dermacentor is involved in its transmission. In fact, in the 1990s a case of tularemia associated with Dermacentor was reported in our area [37], but the clinical picture was more severe. The studied area, La Rioja, is also endemic for Lyme borreliosis (LB) [38], but the clinical picture and the epidemiology should be enough to distinguish these two tick-borne diseases, although the activity of Ixodes ricinus (vector of LB) may sometimes overlap with D. marginatus. This last tick species typically inhabits steppes, meadows, and open forests. As in Central Europe, adult questing D. marginatus ticks start in late August and can last until May–June of the next year, including the winter months [39]. In addition, eschar inoculation is not present in LB, and the tick is usually unnoticed, or when noticed, it is smaller than the one that bites patients who develop DEBONEL. In our series, 100% of patients were aware of being bitten by a large tick. Searching in the literature, only four cases of tick-borne diseases related to B. henselae as agent of SENLAT have been published [28,34]. Moreover, we have studied a large number of I. ricinus and have not found Bartonella spp. (data not published). The possibility of a tick-borne rickettsiosis transmitted by Rhipicephalus spp. is, in our experience, easy to distinguish, since R. conorii, R. sibirica subsp. mongolitimonae, or R. massiliae and other possible Rickettsia transmitted by these ticks are associated with eschar, fever, malaise and other systemic manifestations. Besides, these ticks are more active in warm months. The same can be applied for the bite and illness caused by Hyalomma spp. Therefore, we recommend investigating the etiology when possible, although, in our environment, no patients with clinical picture of DEBONEL and negative microbiological studies for Rickettsia have shown the presence of Francisella, Bartonella, Coxiella, Borrelia, or other Rickettsia species different from those herein reported. In this series, we documented the etiology in 161 patients (74.53%) using molecular tools. Silva-Pinto et al. published in 2014 a review of 37 articles reporting TIBOLA/DEBONEL cases. The etiological agent was identified only in 149 out of the 537 (27.74%) cases of TIBOLA/DEBONEL, and, in most cases, it was R. slovaca [40]. In our study, ‘Ca. R. rioja’ was detected in 91 patients by PCR, R. slovaca in 66 patients and R. raoultii in four patients. These differences can be due to the geographical distribution of the agents. In addition, in our series, an uncultured rickettsial genotype DmS1 was amplified from three D. marginatus removed from patients. In 2003, Rickettsia sp. DmS1 was first detected in D. marginatus removed from game pigs [41], and, subsequently, it was detected in D. marginatus removed from asymptomatic patients from Eastern Spain [42]. Thus, to our knowledge, we describe here the first implication of this rickettsial genotype as human pathogen. In 55 out of 216 patients with identical clinical manifestations, the molecular methods did not allow us to achieve the identification of any SFG Rickettsia. These patients could either be infected by ‘Ca R. rioja’, R. slovaca, R. raoultii, Rickettsia sp. DmS1, or by another unidentified microorganism. Therefore, in our study we have not found any pathogens other than Rickettsia spp. from those associated with SENLAT. DEBONEL patients exhibit very typical and homogeneous epidemiological and clinical features. These characteristics were similar in patients infected by ‘Ca R. rioja’, R. slovaca, R. raoultii, and Rickettsia genotypes here involved, and in patients in whom etiological diagnosis could not be achieved. In all cases, the symptoms were mild, but 39% of patients presented sequelae as persistent alopecia at the site of the tick-bite. Regarding the most useful sample for the study of the etiological agent, we strongly recommend the use of eschar swabs that have allowed us to detect the etiological agent in half of the patients. It is also interesting to study the tick, since we have been able to identify rickettsial agents in 100% of the studied samples and they also allow the taxonomic identification of the vector [1,43]. The serological test, although sensitive for the diagnosis of rickettsiosis, does not allow an early microbiological diagnosis and it is not specific due to cross reactions demonstrated among Rickettsia spp. [44]. Throughout this study, we have incorporated real time-PCR assays, thus improving the sensitivity of the results. However, to know the etiologic agent, the best tool is the PCR and sequencing of the ompA gene [45]. In conclusion, in La Rioja, at least three different SFG Rickettsia, ‘Ca. R. rioja’, R. slovaca, and R. raoultii, besides the genotype DmS1, are responsible for the same disease that we named DEBONEL and other colleagues, TIBOLA. These agents are also involved as etiological agents of SENLAT. The terminology can lead to confusion and it would be time to look for a consensus name. TIBOLA does not reference to the eschar, which is the main clinical sign along with the lymphadenopathy. Since not all patients are bitten on the scalp, not all patients with the involved Rickettsia spp. can be included under the acronym SENLAT.
Lastly, since DEBONEL is a prevalent rickettsiosis in the areas where Dermacentor spp. are distributed; its diagnosis should be considered in patients with the clinical manifestations herein described.
4. Materials and Methods
4.1. Case Definition
The diagnosis of DEBONEL was made in patients who met, at least, the two following criteria: 1. A focus of necrosis (eschar) or a crusted lesion at the site of the tick attachment, surrounded by erythema and painful regional lymphadenopathy; and 2. Tick-bite by a Dermacentor sp. or a large tick during the period of maximum activity for D. marginatus (in La Rioja, mainly from the end of October to the beginning of May). Diagnosis of infections by Rickettsia spp. up to species level were based on PCR detection from clinical specimens, including the removed engorged ticks [1,6,43].
4.2. Patients and Samples
From January 2001 to December 2020, we prospectively studied all patients referred to SPUH (which serves all 314,000 inhabitants of the region) with suspicion of DEBONEL. Epidemiological data (age, sex, habits, contact with animals, rural/urban place of residence, etc.) were collected. Biological samples (EDTA-blood, sera, skin biopsies, eschar swabs and ticks removed from patients) were taken whenever possible, according to the moment of diagnoses. All these clinical samples are part of the “Zoonosis collection” registered in the National Registry of Biobanks of the Carlos III Health Institute (Reference: C.0006409), located in the Center of Rickettsiosis and Arthropod-Borne Diseases (CRETAV), Infectious Diseases Department, SPUH-Center for Biomedical Research from La Rioja (CIBIR), La Rioja, Spain. Patients were re-examined after one week and, whenever possible, at 4–12 weeks from the initial visit, depending on the severity of the clinical picture and to take sera samples in convalescent phases. We also evaluated the clinical response to the treatment (doxycycline or azithromycin for some children, pregnant women, and those patients allergic to doxycycline), according to our clinical experience and recommendations [35].
Approval of the regional ethics committee was obtained (Comité Ético de Investigación Clínica-Consejería de Sanidad de La Rioja, Ref. CEICLAR PI-37). Informed consent was obtained from all participants. All procedures were in accordance with the ethical standards of the research committee and with the 1964 Helsinki declaration and its later amendments.
4.3. Microbiological Tests
4.3.1. Serological Assays
Whenever possible, acute-phase and convalescent sera (between 4–12 weeks), or at least acute sera, were tested by IFA for the presence of IgG antibodies against R. conorii [in-house (CRETAV) and/or commercial antigens (Vircell Microbiologists, Granada, Spain)] and R. slovaca [in-house (CRETAV) antigen]. Seroconversion or a fourfold rise in titer obtained from the late phase was considered evidence of recent infection by SFG Rickettsia.
4.3.2. Molecular Methods (PCR)
The acute-phase sera, the EDTA-blood samples, and the eschar swabs, as well as all D. marginatus removed from patients, were analyzed by PCR assays. DNA was extracted using the DNeasy blood & tissue kit (QIAGEN, Hilden, Germany), according to the manufacturer’s recommendations. The presence of Rickettsia spp. in human samples and in ticks was determined by PCR assays targeting ompA, ompB, and gltA genes, as detailed in Table 2. Subsequently, Bartonella spp., F. tularensis, B. burgdorferi s.l., and C. burnetii were screened by PCR assays (Table 3) when negative results for SFG Rickettsia were obtained and in selected samples. Quality controls included both positive ones, grown in Vero cells [R. conorii Malish # 7 (up to year 2009), or Rickettsia amblyommatis (during 2010–2020) from CRETAV collection, and negative controls (containing sterile water instead of template DNA) that were extracted and tested in parallel with all specimens. Other positive controls, such as B. henselae DNA extracted from a cat flea —Ctenocephalides felis— from La Rioja (Spain), double stranded synthetic gBlock of F. tularensis DNA (Integrated DNA Technologies, Coralville, IA, USA), Borrelia spielmanii DNA (kindly provided by Dr. Volker Fingerle, German National Reference Centre for Borrelia, Germany), and commercially available Amplirun® C. burnetii DNA control (Vircell Microbiologists, Granada, Spain) were included in PCR assays. Sequencing reactions were carried out and results were analyzed through GenBank database using BLAST utility (National Center for Biotechnology Information; available from: URL; http://www.ncbi.nlm.nih.gov, accessed on 29 April 2022).
Table 2.
Primer pairs used for amplification of rickettsial genes.
| Gene | Primers | Primer Sequence (5′→3′) | Fragment Size (bp) | Tm (°C) | Reference |
|---|---|---|---|---|---|
|
gltA (nested) |
RpCS.877p | GGGGGCCTGCTCACGGCGG | 381 | 48 | [46,47] |
| RpCS.1258n | ATTGCAAAAAGTACAGTGAACA | ||||
| RpCS.896p | GGCTAATGAAGCAGTGATAA | 337 | 54 | ||
| RpCS.1233n | ATTGCAAAAAGTACAGTGAACA | ||||
|
ompA (semi nested) |
Rr190.70p | ATGGCGAATATTTCTCCAAAA | 631 | 46 | [46,48] |
| Rr190.701n | GTTCCGTTAATGGCAGCATCT | ||||
| Rr190.70p | ATGGCGAATATTTCTCCAAAA | 532 | 48 | ||
| Rr190.602n | AGTGCAGCATTCGCTCCCCCT | ||||
|
ompB (nested) |
rompB OF | GTAACCGGAAGTAATCGTTTCGTAA | 511 | 54 | [47] |
| rompB OR | GCTTTATAACCAGCTAAACCACC | ||||
| rompB SFG IF | GTTTAATACGTGCTGCTAACCAA | 420 | 56 | ||
| rompB SFG/TG IR | GGTTTGGCCCATATACCATAAG |
Table 3.
PCR primer pairs used for screening of Bartonella spp., Francisella tularensis, Borrelia burgdorferi sensu lato, and Coxiella burnetii.
| Bacteria | Target Gene | Primer Sequence (5′→3′) | Fragment Size (bp) |
Tm (°C) |
Reference |
|---|---|---|---|---|---|
| Bartonella spp. | rpoB | CGCATTGGCTTACTTCGTATG GTAGACTGATTAGAACGCTG | 825 | 53 | [49] |
| Francisella tularensis | 17 KDa lipoprotein |
ATGGCGAGTGATACTGCTTG GCATCATCAGAGCCACCTAA |
250 | 56 | [50] |
| Borrelia burgdorferi sensu lato |
Flagellin (nested) |
AARGAATTGGCAGTTCAATC GCATTTTCWATTTTAGCAAGTGATG |
497 | 52 | [51] |
| ACATATTCAGATGCAGACAGAGGTTCTA GAAGGTGCTGTAGCAGGTGCTGGCTGT |
389 | 55 | [51,52] | ||
| Coxiella burnetii | IS1111 | TATGTATCCACCGTAGCCAGTC CCCAACAACACCTCCTTATTC |
685 | 48 | [53] |
W: A/T; R: A/G.
Acknowledgments
To Pablo Villoslada, from Infectious Diseases, Microbiota and Metabolism Unit (CIBIR, La Rioja, Spain) for carrying out the biostatistical analysis.
Author Contributions
Conceptualization, J.A.O.; methodology, S.S. and A.P.; formal analysis, S.S. and A.P.; investigation, S.S., V.I., A.P., P.S., A.M.P., C.C.-A., L.M., C.G.-G., J.A., J.R.B. and J.A.O; resources, S.S., V.I., A.P., P.S., A.M.P., C.C.-A., L.M., C.G.-G., J.A., J.R.B. and J.A.O.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, S.S., A.P. and J.A.O.; visualization, S.S., V.I., A.P., P.S., A.M.P., C.C.-A., L.M., C.G.-G., J.A., J.R.B. and J.A.O.; supervision, J.A.O.; project administration, J.A.O.; funding acquisition, J.A.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the “Comité Ético de Investigación Clínica-Consejería de Sanidad de La Rioja (protocol code CEICLAR PI-37).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Portillo A., Santibáñez S., García-Álvarez L., Palomar A.M., Oteo J.A. Rickettsioses in Europe. Microbes Infect. 2015;17:834–838. doi: 10.1016/j.micinf.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Lakos A. Tick-borne lymphadenopathy—A new rickettsial disease? Lancet. 1997;350:1006. doi: 10.1016/S0140-6736(05)64072-X. [DOI] [PubMed] [Google Scholar]
- 3.Raoult D., Lakos A., Fenollar F., Beytout J., Brouqui P., Fournier P.E. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks Clin. Infect. Dis. 2002;34:1331–1336. doi: 10.1086/340100. [DOI] [PubMed] [Google Scholar]
- 4.Oteo J.A., Ibarra V. DEBONEL (Dermacentor-borne-necrosis-erythema-lymphadenopathy). ¿Una nueva enfermedad transmitida por garrapatas? Enferm. Infecc. Microbiol. Clin. 2002;20:51–52. doi: 10.1016/S0213-005X(02)72740-7. [DOI] [PubMed] [Google Scholar]
- 5.Cazorla C., Enea M., Lucht F., Raoult D. First isolation of Rickettsia slovaca from a patient, France. Emerg. Infect. Dis. 2003;9:135. doi: 10.3201/eid0901.020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oteo J.A., Ibarra V., Blanco J.R., Martínez de Artola V., Márquez F.J., Portillo A., Raoult D., Anda P. Dermacentor-borne necrosis erythema and lymphadenopathy: Clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 2004;10:327–331. doi: 10.1111/j.1198-743X.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 7.Ibarra V., Oteo J.A., Portillo A., Santibáñez S., Blanco J.R., Metola L., Eiros J.M., Pérez-Martínez L., Sanz M. Rickettsia slovaca Infection: DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2006;1078:206–214. doi: 10.1196/annals.1374.040. [DOI] [PubMed] [Google Scholar]
- 8.Komitova R., Lakos A., Aleksandrov A., Christova I., Murdjeva M. A case of tick-transmitted lymphadenopathy in Bulgaria associated with Rickettsia slovaca. Scand. J. Infect. Dis. 2003;35:213. doi: 10.1080/0036554021000027016. [DOI] [PubMed] [Google Scholar]
- 9.Selmi M., Bertolotti L., Tomassone L., Mannelli A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008;14:817–820. doi: 10.3201/eid1405.070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porta F.S., Nieto E.A., Creus B.F., Espín T.M., Casanova F.J., Sala I.S., García S.L., Aguilar J.L., Vilaseca M.Q. Tick-borne lymphadenopathy: A new infectious disease in children. Pediatr. Infect. Dis. J. 2008;27:618–622. doi: 10.1097/INF.0b013e31816b1947. [DOI] [PubMed] [Google Scholar]
- 11.Parola P., Rovery C., Rolain J.M., Brouqui P., Davoust B., Raoult D. Rickettsia slovaca and R. raoultii in tick-borne Rickettsioses. Emerg. Infect. Dis. 2009;15:1105–1108. doi: 10.3201/eid1507.081449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieg S., Schmoldt S., Theilacker C., de With K., Wölfel S., Kern W.V., Dobler G. Tick-borne lymphadenopathy (TIBOLA) acquired in Southwestern Germany. BMC Infect. Dis. 2011;11:167. doi: 10.1186/1471-2334-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmielewski T., Rudzka D., Fiecek B., Maczka I., Tylewska-Wierzbanowska S. Case of TIBOLA/DEBONEL (tick-borne lymphadenopathy/Dermacentor spp.-borne necrosis-erythema-lymphadenopathy) in Poland. Przegl. Epidemiol. 2011;65:583–586. [PubMed] [Google Scholar]
- 14.Gaston J., Durox H., Sparsa A., Bonnetblanc J.M., Doffoel-Hantz V. Dermohypodermitis on the face revealing TIBOLA. Arch. Pediatr. 2011;18:565–567. doi: 10.1016/j.arcped.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Oteo J.A., Portillo A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012;3:271–278. doi: 10.1016/j.ttbdis.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 16.de Sousa R., Pereira B.I., Nazareth C., Cabral S., Ventura C., Crespo P., Marques N., da Cunha S. Rickettsia slovaca infection in humans, Portugal. Emerg. Infect. Dis. 2013;19:1627–1629. doi: 10.3201/eid1910.130376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rigal E., Dorcier D., Lesens O., Texier C., D’Incan M. TIBOLA: An emerging clinically polymorphous rickettsiosis. Ann. Dermatol. Venereol. 2014;141:186–191. doi: 10.1016/j.annder.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Pietzsch M.E., Hansford K.M., Cull B., Jahfari S., Sprong H., Medlock J.M. Detection of Dermacentor marginatus and a possible Rickettsia slovaca case in the United Kingdom-the risk of the visiting traveller. Travel Med. Infect. Dis. 2015;13:200–201. doi: 10.1016/j.tmaid.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Silva J.T., López-Medrano F., Fernández-Ruiz M., Foz E.R., Portillo A., Oteo J.A., Aguado J.M. Tickborne Lymphadenopathy Complicated by Acute Myopericarditis, Spain. Emerg. Infect. Dis. 2015;21:2240–2242. doi: 10.3201/eid2112.150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlozzari G., Romiti F., Zini M., Magliano A., De Liberato C., Corrias F., Capponi G., Galli L., Scarpulla M., Montagnani C. Scalp eschar and neck lymphadenopathy by Rickettsia slovaca after Dermacentor marginatus tick bite case report: Multidisciplinary approach to a tick-borne disease. BMC Infect. Dis. 2021;21:103. doi: 10.1186/s12879-021-05807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raoult D., Berbis P., Roux V., Xu W., Maurin M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet. 1997;350:112–113. doi: 10.1016/S0140-6736(05)61814-4. [DOI] [PubMed] [Google Scholar]
- 22.Oteo J.A., Ibarra V., Blanco J.R. Zubía Monográfico. Volume 12. Instituto de Estudios Riojanos; Logroño, Spain: 2000. Eritema, Necrosis y Linfedenopatía. Una nueva enfermedad (DEBONEL) transmitida por Dermacentor marginatus Sulzer, 1776; pp. 49–58. [Google Scholar]
- 23.Ibarra V., Portillo A., Santibanez S., Blanco J.R., Pérez-Martínez L., Márquez F.J., Oteo J.A. DEBONEL/TIBOLA: Is Rickettsia slovaca the only etiological agent? Ann. N. Y. Acad. Sci. 2005;1063:346–348. doi: 10.1196/annals.1355.056. [DOI] [PubMed] [Google Scholar]
- 24.Portillo A., Ibarra V., Santibáñez S., Pérez-Martínez L., Blanco J.R., Oteo J.A. Genetic characterisation of ompA, ompB and gltA genes from Candidatus Rickettsia rioja. Clin. Microbiol. Infect. 2009;15:307–308. doi: 10.1111/j.1469-0691.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 25.Mediannikov O., Matsumoto K., Samoylenko I., Drancourt M., Roux V., Rydkina E., Davoust B., Tarasevich I., Brouqui P., Fournier P.E. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008;58:1635–1639. doi: 10.1099/ijs.0.64952-0. [DOI] [PubMed] [Google Scholar]
- 26.Rydkina E., Roux V., Rudakov N., Gafarova M., Tarasevich I., Raoult D. New Rickettsiae in ticks collected in territories of the former soviet union. Emerg. Infect. Dis. 1999;5:811–814. doi: 10.3201/eid0506.990612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shpynov S., Parola P., Rudakov N., Samoilenko I., Tankibaev M., Tarasevich I., Raoult D. Detection and identification of spotted fever group rickettsiae in Dermacentor ticks from Russia and central Kazakhstan. Eur. J. Clin. Microbiol. Infect. Dis. 2001;20:903–905. doi: 10.1007/s10096-001-0638-4. [DOI] [PubMed] [Google Scholar]
- 28.Angelakis E., Pulcini C., Waton J., Imbert P., Socolovschi C., Edouard S., Dellamonica P., Raoult D. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after Tick Bite. Clin. Infect. Dis. 2010;50:549–551. doi: 10.1086/650172. [DOI] [PubMed] [Google Scholar]
- 29.Edouard S., Gonin K., Turc Y., Angelakis E., Socolovschi C., Raoult D. Eschar and neck lymphadenopathy caused by Francisella tularensis after a tick bite: A case report. J. Med. Case Rep. 2011;5:108. doi: 10.1186/1752-1947-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cascio A., Torina A., Valenzise M., Blanda V., Camarda N., Bombaci S., Iaria C., De Luca F., Wasniewska M. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013;19:836–837. doi: 10.3201/eid1905.121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foissac M., Socolovschi C., Raoult D. Update on SENLAT syndrome: Scalp eschar and neck lymph adenopathy after a tick bite. Ann. Dermatol. Venereol. 2013;140:598–609. doi: 10.1016/j.annder.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Dubourg G., Socolovschi C., Del Giudice P., Fournier P.E., Raoult D. Scalp eschar and neck lymphadenopathy after tick bite: An emerging syndrome with multiple causes. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1449–1456. doi: 10.1007/s10096-014-2090-2. [DOI] [PubMed] [Google Scholar]
- 33.Zaharia M., Popescu C.P., Florescu S.A., Ceausu E., Raoult D., Parola P., Socolovschi C. Rickettsia massiliae infection and SENLAT syndrome in Romania. Ticks Tick Borne Dis. 2016;7:759–762. doi: 10.1016/j.ttbdis.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Seo J.W., Kim C.M., Yun N.R., Kim D.M., Kim S.S., Choi S., Chu H. Scalp eschar and neck lymphadenopathy after tick bite (SENLAT) caused by Bartonella henselae in Korea: A case report. BMC Infect. Dis. 2020;20:216. doi: 10.1186/s12879-020-4940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibarra V., Blanco J.R., Portillo A., Santibáñez S., Metola L., A Oteo J. Effect of antibiotic treatment in patients with DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2005;1063:257–258. doi: 10.1196/annals.1355.040. [DOI] [PubMed] [Google Scholar]
- 36.Faccini-Martínez Á.A., García-Álvarez L., Hidalgo M., Oteo J.A. Syndromic classification of rickettsioses: An approach for clinical practice. Int. J. Infect. Dis. 2014;28:126–139. doi: 10.1016/j.ijid.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Oteo J.A., Martínez de Artola V., Casas J.M. Tick-borne diseases in Spain; Proceedings of the 6th International Congress for Infectious Diseases; Prague, Czech Republic. 26–30 April 1994. [Google Scholar]
- 38.Oteo Revuelta J.A., Blanco Ramos J.R., Martínez de Artola V., Grandival García R., Ibarra Cucalón V., Dopereiro Gómez R. Eritema migratorio (borreliosis de Lyme). Características clinicoepidemiológicas de 50 pacientes [Migratory erythema (Lyme borreliosis). Clinicoepidemiologic features of 50 patients] Rev. Clin. Esp. 2000;200:60–63. doi: 10.1016/S0014-2565(00)70564-9. [DOI] [PubMed] [Google Scholar]
- 39.Hornok S. Dermacentor marginatus (Sulzer, 1776) In: Estrada-Peña A., Mihalca A.D., Petney T.N., editors. Ticks of Europe and North Africa. A Guide to Species Identification. Springer International Publishing; Cham, Switzerland: 2017. pp. 281–286. [Google Scholar]
- 40.Silva-Pinto A., Santos Mde L., Sarmento A. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 2014;5:656–659. doi: 10.1016/j.ttbdis.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Sanogo Y.O., Davoust B., Parola P., Camicas J.L., Brouqui P., Raoult D. Prevalence of Rickettsia spp. in Dermacentor marginatus ticks removed from game pigs (Sus scrofa) in southern France. Ann. N. Y. Acad. Sci. 2003;990:191–195. doi: 10.1111/j.1749-6632.2003.tb07361.x. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Soto P., Pérez-Sánchez R., Alamo-Sanz R., Encinas-Grandes A. Spotted fever group rickettsiae in ticks feeding on humans in northwestern Spain: Is Rickettsia conorii vanishing? Ann. N. Y. Acad. Sci. 2006;1078:331–333. doi: 10.1196/annals.1374.063. [DOI] [PubMed] [Google Scholar]
- 43.Brouqui P., Bacellar F., Baranton G., Birtles R.J., Bjoërsdorff A., Blanco J.R., Caruso G., Cinco M., Fournier P.E., Francavilla E., et al. ESCMID Study Group on Coxiella, Anaplasma, Rickettsia and Bartonella; European Network for Surveillance of Tick-Borne Diseases. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004;10:1108–1132. doi: 10.1111/j.1469-0691.2004.01019.x. [DOI] [PubMed] [Google Scholar]
- 44.Santibanez S., Ibarra V., Portillo A., Blanco J.R., Martínez de Artola V., Guerrero A., Oteo J.A. Evaluation of IgG antibody response against Rickettsia conorii and Rickettsia slovaca in patients with DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2006;1078:570–572. doi: 10.1196/annals.1374.113. [DOI] [PubMed] [Google Scholar]
- 45.Santibáñez S., Portillo A., Santibáñez P., Palomar A.M., Oteo J.A. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm. Infecc. Microbiol. Clin. 2013;31:283–288. doi: 10.1016/j.eimc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Regnery R.L., Spruill C.L., Plikaytis B.D. Genotypic iden.ntification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi Y.J., Jang W.J., Kim J.H., Kim J.H., Ryu J.S., Lee S.H., Park K.H., Paik H.S., Koh Y.S., Choi M.S., et al. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg. Infect. Dis. 2005;11:237–244. doi: 10.3201/eid1102.040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roux V., Fournier P.E., Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renesto P., Gouvernet J., Drancourt M., Roux V., Raoult D. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 2001;39:430–437. doi: 10.1128/JCM.39.2.430-437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karhukorpi E.K., Karhukorpi J. Rapid laboratory diagnosis of ulceroglandular tularemia with polymerase chain reaction. Scand. J. Infect. Dis. 2001;33:383–385. doi: 10.1080/003655401750174101. [DOI] [PubMed] [Google Scholar]
- 51.Clark K., Hendricks A., Burge D. Molecular Identification and Analysis of Borrelia burgdorferi Sensu Lato in Lizards in the Southeastern United States. Appl. Environ. Microbiol. 2005;71:2616–2625. doi: 10.1128/AEM.71.5.2616-2625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson B.J.B., Happ C.M., Mayer L.W., Piesman J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am. J. Trop. Med. Hyg. 1992;47:730–741. doi: 10.4269/ajtmh.1992.47.730. [DOI] [PubMed] [Google Scholar]
- 53.Willems H., Thiele D., Frölich-Ritter R., Krauss H. Detection of Coxiella burnetii in cow’s milk using the polymerase chain reaction (PCR) J. Vet. Med. 1994;41:580–587. doi: 10.1111/j.1439-0450.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.