Abstract
Simple Summary
Tuckerella pavoniformis (Ewing) (Acari: Tuckerellidae) was found to be solidly associated with the tropical fruit mamey, Mammea americana L. (Calophyllaceae), for the first time in northwestern Peru. The highest T. pavoniformis population density was located on the epicarps of fruits. Biometric data was collected from mite-infested fruit for future comparisons with mite-free fruit. The localized commercialization of this fruit could play an important role in the spread of this mite within Peru.
Abstract
The family Tuckerellidae, or peacock mites, is a monogeneric group comprising approximately 32 species, which are usually collected from the fruits or woody parts of their host plants. Fruits and branchlets of mamey, Mammea americana L. (Calophyllaceae) trees in north-western Peru were sampled for peacock mites throughout spring and summer for two consecutive years. This is the first record of Tuckerella pavoniformis (Ewing) (Acari: Tuckerellidae) feeding on mamey. Aggregations of mites were much higher and more common on the fruit epicarps than on branchlets. Recommendations for the development of an Integrated Pest Management strategy for this peacock mite are included.
Keywords: agroforestry system, exotic fruit, emerging pest, peacock mites, Tetranychoidea
1. Introduction
Tuckerella pavoniformis (Ewing) (Acari: Tetranychoidea: Tuckerellidae), the first peacock mite ever recorded, was originally described in 1922 as Eupalopsis pavoniformis. The description was based on material collected on Hibiscus sp. (Malvaceae) imported into San Francisco from Hawaii, USA [1]. Shortly after that, Tuckerella ornata (Tucker) was described as Tenuipalpus ornatus in the family Tetranychidae from South Africa in 1926, based on material collected from oranges [2]. Womersley [3] erected the new genus Tuckerella for T. ornata, keeping it within the Tetranychidae. In 1953, Baker and Pritchard [4] evaluated and updated the superfamily Tetranychoidea, and in doing so proposed the new family Tuckerellidae to accommodate the only two species known at that time, T. ornata and T. pavoniformis, and included host plant records for both species. According to Baker and Pritchard [4], T. pavoniformis had been recorded from Hibiscus sp. and papaya fruit (Caricaceae) in Hawaii; and from Citrus sp. (Rutaceae), cypress pine (Callitris sp., Cupressaceae), Eucalyptus sp. (Myrtaceae), and privet (Ligustrum sp., Oleaceae) in Australia. However, we now know that the Australian mite species records were based on misidentifications made by Womersley [3], and are known to actually represent T. flabellifera Miller [5]. Baker and Pritchard [4] also provided a host list for T. ornata, which included the tropical fruit called mamey or mamey apple, Mammea americana L. (Calophyllaceae) which had been imported into San Diego, USA, from Guatemala. Additionally, Ochoa et al. [6] reported T. pavoniformis damaging the fruit of Psidium cattleianum Sabine (Myrtaceae) in Central America.
Mammea americana is native to the West Indies and northern South America. The tree is grown in small-scale local commercial plantations in various Central and South American countries for beverage manufacture, and the edible pulp [7] which is exported to various countries around the world. This fruit tree is frequently used in border plantings and windbreaks for other commercial plantations (such as banana, cocoa and mango), and is domestically grown as an ornamental or backyard tree [8,9,10]. Mammea americana is known to have many medicinal, and pharmaceutical uses, which include insect-repellent properties [8,11,12]. Additionally, endophytic fungi of M. americana have been identified as a source of active secondary metabolites with non-toxic antimicrobial properties [13]. Interestingly, even the shell of the fruit is useful as an effective bioadsorbent of chrome [14]. In Singucate, Piura, Peru, mamey is grown in cocoa plantations within agroforestry systems.
This tropical tree has dark green, shiny, leathery, elliptical leaves. The creamy white, fragrant flowers [15], are borne singly or in clusters of two or three on short stalks in the axils of young branches [16]. Recent studies indicate that this species exhibits a “cryptic″ androdioecy in that there are individuals with male flowers and individuals with functionally female hermaphroditic flowers [17]. Young mamey trees begin to produce flowers and fruits between eight and 13 years of age, and fruit production is then consistent from year to year [18]. The fruit is a large globose berry [19,20] attached to the plant by a short, thick stem, and takes more than a year to mature [21]. The epicarp is grayish brown, verrucose, and rough to the touch due to small latex globules of variable thickness and consistency; and it remains hard until fully mature at which point it softens slightly. The edible endocarp, is light yellow to golden orange, non-fibrous, and may have one to four seeds [9,15]. At maturation, the endocarp separates from the rest of the pericarp and testa [19].
Mamey fruits are marketed nationally in Peru and the main productive regions are Tumbes, Piura, Lambayeque, La Libertad and Cajamarca. In 2020, Peru exported 27,548 kg of mamey pulp to Europe, mostly to Spain, Netherlands, France, Germany, and Italy [22]. This article reports M. americana as a new host tree association for T. pavoniformis in Peru, with observations on the ecology of the mite and mamey trees, and offers contributions to the development of Integrated Pest Management strategies.
2. Materials and Methods
2.1. Studied Area
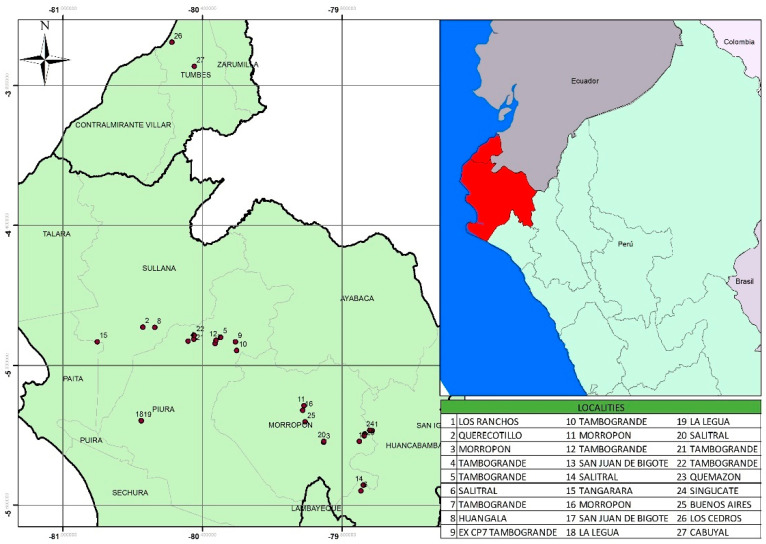
In this study, T. pavoniformis mites were collected in agricultural plots in 27 localities of two Departments, Tumbes and Piura (Figure 1), located in north–western Peru. The fruits were harvested in the years 2020 and 2021 during the period of fruit maturity that coincides with spring and summer. The climate in Tumbes and Piura is considered to be tropical and subtropical desert, or a BWh type according to the Köppen–Geiger climate classification system.
Figure 1.
Current peacock mite Tuckerella pavoniformis distribution in north–western Peru.
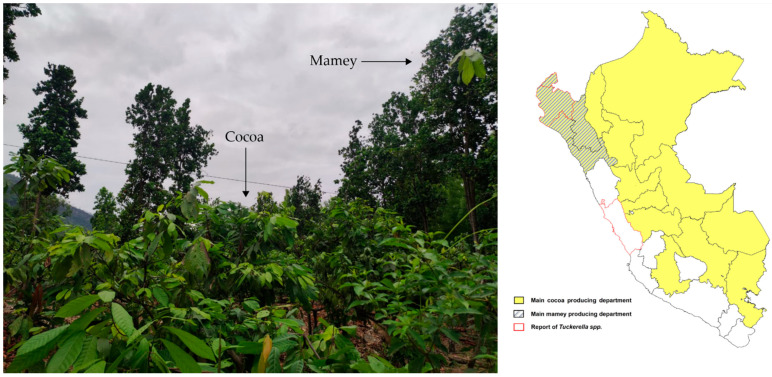
The sampled mamey trees (Figure 2) were between 15 and 20 m tall, 20 to 25 years old, and individual trees were numbered from one to ten within each agricultural plot surveyed. The one exception was the town of Singucate where mamey is used in cocoa cultivation as part of an agroforestry system (Figure 3). A single tree was surveyed within each plot, when multiple trees were present one was randomly selected. Each mamey fruit takes more than a year to mature, and the timing of maturation in the surveyed localities occurred between October and February. Local management practices for mamey trees in the surveyed region included selective pruning of dry branches, irrigation, and manual weed control every month. The trees were pesticide free because tree height made it impractical to spray.
Figure 2.
(A) Adult tree of Mammea americana L. (Calophyllaceae). (B) Detail of the fruits and leaves of mamey, with insertion of detail of the flower. (C) Cross sections of halved mamey apple fruit, M. americana.
Figure 3.
Current Tuckerella spp. distribution in Perú on mamey and cocoa crops.
2.2. Peacock Mite Sampling and Identification
Selected mamey trees in 27 separate plots were sampled for peacock mites (Figure 1). Each sample consisted of two fully developed fruit, and two 15 cm long branchlets taken from the lower third of one tree. Fruit and branchlets were put in separate paper bags, transported to the laboratory inside a portable cooler, and examined under a stereomicroscope (ZEISS Stemi 508 with a digital camera AixocamERc 5s). The numbers of mites on a randomly chosen 1 cm2 area of fruit epicarp, and on the entire branchlets, were recorded. The mites were stored in 2 mL microtubes with 70% alcohol. After one month, were mounted in Hoyer’s medium [23], and stored in an oven at 50 °C for 5 days. Mites were identified using taxonomic keys provided by Meyer and Ueckermann [24], and their most important taxonomic characters, using an Omax 40X–2500X phase contrast trinocular microscope with an OMAX S35180U3–18Mp camera. Voucher specimens of the peacock mites collected in this study were deposited in the Acarology Collection of the Entomology Laboratory (SL01LA68) at the Universidad Nacional de Piura, Peru.
2.3. The Biometric Characteristics of the Fruits Infested by Peacock Mites
Fifty of the mamey fruit collected in Section 2.2 were selected to undertake biometric analyses. The biometric characteristics measured are as follows:
− weight of fruit (Wfruit), seeds (Wseed) and epicarp (Wepicarp) (digital scale, precision 0.10 g),
− length of fruit measured using a slide caliper (precision 0.01 mm),
− circumference of fruit determined with a meter ribbon (precision 1 mm).
− weight of pulp (Wpulp) established using the equation,
| Wpulp = [Wfruit − (Wepicarp + Wseed)]. |
− percentage of pulp (P%) using the equation:
3. Results and Discussion
This is the first record of Tuckerella pavoniformis feeding on Mammea americana trees (Figure 1, Table 1). This peacock mite is widely distributed throughout the Americas (Table 2), and has been collected from a taxonomically broad range of hosts. It is interesting to note that not only is T. pavoniformis restricted to the new world, and is not known from outside the Americas, a large proportion of its hosts plants are endemic to the Americas also (Table 2). Following from this, T. pavoniformis is the most widely established tuckerellid species in cocoa producing areas in Piura, Peru, where it occurs in populations large enough to cause significant economic damage to cocoa fruit that results in reduced harvestable yield [25]. The populations of T. pavoniformis on mamey reported here are comparably large in size, and further dedicated research on potential yield reduction is warranted.
Table 1.
Locality records for Tuckerella pavoniformis in North-western Peru.
| Localities/Departament | Collection Date | Coordinates | Average Mites/Branchlets | Average Mites/cm2 Fruit Surface | |
|---|---|---|---|---|---|
| South | West | ||||
| Los Ranchos/Piura | 25–Nov.–2020 | 5°16′50.28″ | 79°40′16.44″ | 3.0 | 17.0 |
| Querecotillo/Piura | 03–Dec.–2020 | 4°50′14.31″ | 80°39′22.82″ | 3.5 | 18.5 |
| Morropón/Piura | 03–Dec.–2020 | 5°19′49.82″ | 79°52′44.55″ | 2.0 | 13.5 |
| Tambogrande/Piura | 03–Dec.–2020 | 4°53′49.50″ | 80°27′41.40″ | 3.5 | 15.5 |
| Tambogrande/Piura | 03–Dec.–2020 | 4°52′51.70″ | 80°19′19.40″ | 2.5 | 16.0 |
| Salitral/Piura | 03–Dec.–2020 | 5°32′21.50″ | 79°43′10.70″ | 0.0 | 4.0 |
| Tambogrande/Piura | 04–Dec.–2020 | 4°54′28.00″ | 80°20′45.49″ | 2.0 | 19.0 |
| Huangala/Piura | 04–Dec.–2020 | 4°50′19.36″ | 80°36′18.78″ | 0.0 | 2.5 |
| Ex CP7 Tambogrande/Piura | 04–Dec.–2020 | 4°54′1.20″ | 80°15′29.24″ | 0.0 | 7.0 |
| Tambogrande/Piura | 04–Dec.–2020 | 4°56′14.48″ | 80°15′11.50″ | 0.0 | 5.0 |
| Morropón/Piura | 04–Dec.–2020 | 5°10′23.28″ | 79°57′50.40″ | 2.0 | 6.0 |
| Tambogrande/Piura | 06–Dec.–2020 | 4°53′35.31″ | 80°20′32.85″ | 0.0 | 13.0 |
| San Juan De Bigote/Piura | 06–Dec.–2020 | 5°17′43.08″ | 79°42′11.58″ | 1.5 | 1.0 |
| Salitral/Piura | 06–Dec.–2020 | 5°30′51.40″ | 79°42′33.30″ | 10.5 | 6.0 |
| Tangarara/Piura | 06–Dec.–2020 | 4°54′0.31″ | 80°51′8.84″ | 2.5 | 5.5 |
| Morropón/Piura | 06–Dec.–2020 | 5°11′34.80″ | 79°58′11.22″ | 9.0 | 8.0 |
| San Juan De Bigote/Piura | 06–Dec.–2020 | 5°19′37.68″ | 79°43′33.84″ | 4.0 | 5.0 |
| La Legua/Piura | 06–Dec.–2020 | 5°14′15.9″ | 80°39′48.8″ | 2.0 | 9.0 |
| La Legua/Piura | 06–Dec.–2020 | 5°14′18.2″ | 80°39′47.2″ | 4.0 | 10.5 |
| Salitral/Piura | 07–Dec.–2020 | 5°19′35.10″ | 79°52′44.02″ | 5.0 | 15.0 |
| Tambogrande/Piura | 07–Dec.–2021 | 4°53′20.72″ | 80°26′14.85″ | 0.0 | 9.5 |
| Tambogrande/Piura | 07–Dec.–2022 | 4°52′20.94″ | 80°26′11.73″ | 14.0 | 23.5 |
| Quemazon/Piura | 09–Dec.–2020 | 5°18′8.83″ | 79°42′18.55″ | 7.0 | 17.5 |
| Singucate/Piura | 09–Dec.–2020 | 5°16′44.44″ | 79°40′53.59″ | 4.0 | 16.0 |
| Buenos Aires/Piura | 21–Jan.–2021 | 5°14′35.63″ | 79°57′33.25″ | 8.5 | 10.0 |
| Los Cedros/Tumbes | 11–Oct.–2021 | 3°36′51.22″ | 80°31′53.10″ | 1.5 | 5.5 |
| Cabuyal/Tumbes | 03–Nov.–2021 | 3°43′6.12″ | 80°26′7.74″ | 0.0 | 8.5 |
Table 2.
Tuckerella pavoniformis in the Americas—distribution and host plants (hosts in bold are endemic to the Americas).
| Location | Host (Family) | References |
|---|---|---|
| Brazil |
Malpighia emarginata D.C. (Malpighiaceae) Litchi chinensis Sonn (Sapindaceae) |
[26] [27] |
| Caribbean |
Annona muricata L. (Annonaceae)
Lantana camara L. (Verbenaceae) Turnera ulmifolia L. (Passifloraceae) |
[28] [28] [28] |
| Costa Rica |
Psidium cattleianum Sabine (Myrtaceae)
Malpighia glabra L. (Malpighiaceae) Persea americana Mill. (Lauraceae) |
[6] [29] [30] |
| Cuba |
Achras sapote L. (Sapotaceae) Casuarina equisetifolia L. (Casuarinaceae) Persea americana |
[31] [31] [32] |
| Peru |
Theobroma cacaoL. (Malvaceae) Cydonia oblonga Mill. (Rosaceae) Mammea americana L. (Calophyllaceae) |
[25] [33] present study |
| Republica Dominicana | Persea americana | [Ochoa, pers.obs.] |
| USA |
Hibiscus spp. (Malvaceae) Carica papaya (Caricaceae) Persea americana Quercus spp. (Fagaceae) |
[1,4] [4] [Ochoa, pers.obs.] [34] |
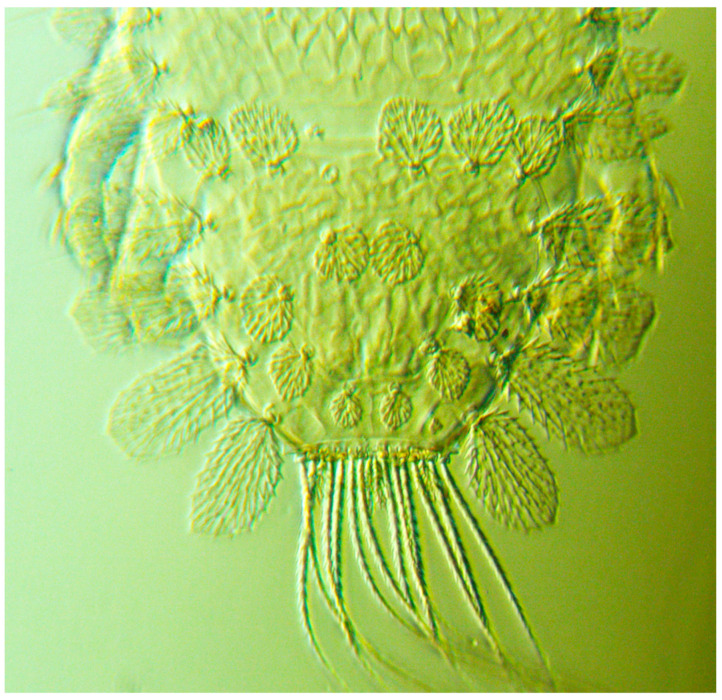
Peacock mites are easily recognized by their outstanding broadly flattened dorsal setae that are usually white in colour and ovate to obovate in shape (i.e., leaf-like in appearance), in conjunction with their striking “tail” setae. Tuckerella pavoniformis is separated from other peacock mite species by having six pairs of elongate flagellate caudal setae equal in length (h2, h3, h4, h5, h7, h8) and two pairs of short broad ovate setae (h1 and h6); dorsal cuticle in the pygidial region with mostly regular reticulations; and setae f2 larger than, and inserted anterior to, setae f1 (Figure 4).
Figure 4.
Dorsal view of opisthosoma of adult female Tuckerella pavoniformis.
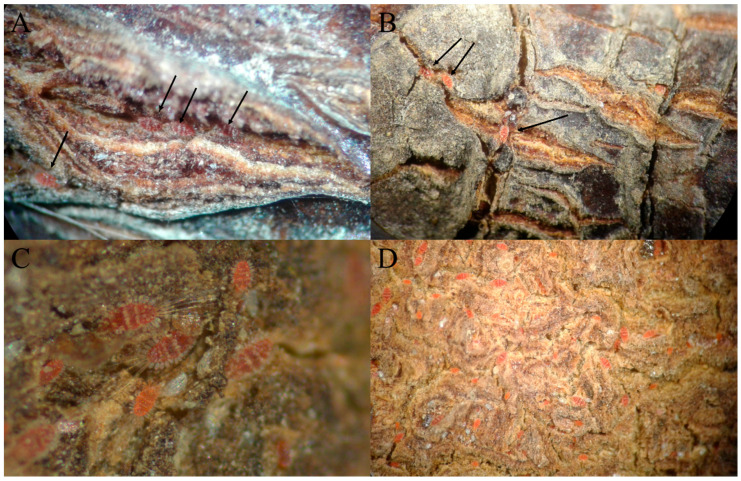
Tuckerella pavoniformis was observed and collected on the branchlets and fruit epicarps of mamey (Figure 5). As aggregations of mites were more common, and the population densities were much higher (mean 10.6 mites/cm2), on the fruit than on the branchlets (mean 3.4 mites/15 cm of branchlets) (Table 1), we assume that the fruits represent the preferred feeding site for this species. Motile stages and eggs of T. pavoniformis are readily observed wedged within the crevices found on the epicarp of the fruit and on the surface of the branchlets. The mites appear to use these crevices to access feeding and oviposition sites, and for protection from predation and climate. Due to its high respiration rate of 28–40 mg CO2 kg−1 h−1 at 27 °C, mamey is a climacteric fruit and is highly perishable with a limited shelf-life [35,36,37]. As a consequence of this, the fruit will drop from the tree once completely ripened. During this survey, large numbers of mites were observed on fallen ripe fruit that were haphazardly examined in situ with a hand lens. Additionally, incidental observations indicated that peacock mites were also present on the fruit being sold at a local market in Piura, Peru.
Figure 5.
Aggregations of adult Tuckerella pavoniformis on Mammea americana with one or more eggs and/or immatures (indicated by black arrows) on branchlets sections (A,B) and fruit epicarp (C,D).
The analysis of the biometric characteristics recorded for ripe fruits of M. americana infested by T. pavoniformis revealed an average weight of 397.23 ± 20.79 g, with individual fruit ranging in weight from 355.40–439.00 g each. In the majority of cases, the fruits of the sampled trees developed only one seed, with average seed number of 1.52 ± 0.11 (Figure 2). The average percentage of pulp per fruit observed was 56.51 ± 1.74% (Table 3). The critical next step is to evaluate the effect that the observed significant populations of peacock mites have on these biometrics, through a comparison with biometrics from healthy fruits, as has been demonstrated previously by Escobar-Garcia et al. [25] for cocoa fruits.
Table 3.
Biometric characteristics of fruits with T. pavoniformis populations (n = 50 fruit).
| Biometric Characteristics | Means ± SE | 95% Confidence Interval |
|---|---|---|
| Average fruit weight (g) | 397.23 ± 20.79 | 355.40–439.00 |
| Average pulp weight (g) | 228.28 ± 15.21 | 197.70–258.85 |
| Average seed weight (g) | 87.34 ± 5.36 | 76.57–98.10 |
| Average fruit height (cm) | 9.02 ± 0.13 | 8.74–9.28 |
| Average fruit circumference (cm) | 28.96 ± 0.52 | 27.90–30.00 |
| Average seed number | 1.52 ± 0.11 | 1.30–1.73 |
| Percentage of pulp (%) | 56.51 ± 1.74 | 53.02–60.00 |
| Percentage of seed (%) | 22.29 ± 0.95 | 20.37–24.20 |
| Percentage of epicarp (%) | 21.2 ± 1.03 | 19.12–23.26 |
| Ratio pulp/seed | 2.88 ± 0.17 | 2.52–3.22 |
There are few studies regarding the mite diversity on mamey. There are no records of the phytophagous spider mite family Tetranychidae [38] and the predator family Phytoseiidae [39], being collected on M. americana. However, De Leon [40] has reported the flat mite “Brevipalpus phoenicis” (Geijskes) (Tenuipalpidae) and three species of Tuckerellidae, T. ornata and T. knorri reported previously by Cao [41], and T. pavoniformis reported here from mamey.
Tuckerella pavoniformis is an important mite on fruit trees (avocado, cocoa, guava) and ornamental trees, and its presence on M. americana in Peru is of potential concern. We consider it to be of phytosanitary importance due to its association with cocoa cultivation in agroforestry systems, as occurs in Singucate, Piura (Figure 1 and Figure 3). In this case, control strategies within Integrated Pest Management programs should be considered, including equipment such as Unmanned Aerial Vehicles (UAV) to assist with efficient management and application of miticides on such tall trees (15 to 20 m). UAVs are highly capable, and their uses are rapidly expanding as they become increasingly adopted across many sectors of agriculture, including for pesticide application [42]. Finally, it is possible that the slow development rate of the fruit may play a significant role in the spread of T. pavoniformis. Since the fruit takes 12 months to fully mature, the mites have a long time to build up potentially large populations, after which the fruit is then moved to many parts of Peru for commercialization and consumption. Further research on mite distribution is needed, including sampling mite populations throughout the period of fruit development. Given that these mites often go undetected through concealment on the fruit, we encourage a greater awareness of the domestic movement of this fruit, and the implementation of a post-harvest washing process in an effort to prevent the spread of this mite throughout the rest of the country, before its commercialization is developed further. In addition to preventing or slowing its movement into the main cocoa producing areas where this species is known to cause significant damage to the fruit.
Acknowledgments
We want to thank all the team of collectors of Table 1, Morey M., Garces J., Ramos R., Silupu M., Avila J., Zapata M., Castillo B., Lazaro R., Rufino A., Neira A., Morales J., Lopez D., Colan A., Saavedra A., Alban W., Maza L., Zapata D., Calero A., Varona J., Guerrero D., Cordova E., Castillo L., Neira D., Suarez E., for their time and dedication in this research study. To the farmers of the 27 agricultural plots in Peru for their collaboration and support allowing the research personnel team to enter their agricultural areas. To Andrew Ulsamer and Debra Creel, SEL-USDA, for their help with references and suggestions in this manuscript, and to Andy Garcia for the help with Figures. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA; USDA is an equal opportunity provider and employer.
Author Contributions
Conceptualization, H.A.E.-G., J.J.B. and R.O.; methodology, H.A.E.-G., J.J.B. and R.O.; formal analysis, H.A.E.-G.; investigation, H.A.E.-G., J.J.B. and R.O.; resources, H.A.E.-G. and R.O.; data curation, H.A.E.-G., J.J.B. and R.O.; writing—original draft preparation, H.A.E.-G.; writing—review and editing, H.A.E.-G., J.J.B. and R.O.; visualization, H.A.E.-G., J.J.B. and R.O.; supervision, J.J.B. and R.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ewing H.E. Three new species of peculiar and injurious spider mites. Proc. Entomol. Soc. Wash. 1922;24:104–108. [Google Scholar]
- 2.Tucker R.W.E. Some south African mites. Mainly Tetranychidae and Eriophyidae. Union S. Afr. Dept. Agric. Div. Ent. Mem. 1926;5:1–15. [Google Scholar]
- 3.Womersley H. Studies in Australian Acarina Tetranychidae and Trichadenidae. Trans. R. Soc. S. Aust. 1940;64:233–265. [Google Scholar]
- 4.Baker E.W., Pritchard A.E. The family categories of Tetranychoid mites, with a review of the new families Linotetranidae and Tuckerellidae. Ann. Entomol. Soc. Am. 1953;46:243–258. doi: 10.1093/aesa/46.2.243. [DOI] [Google Scholar]
- 5.Miller L.W. The Papers and Proceedings of the Royal Society of Tasmania. Vol. 98. Royal Society of Tasmania; Hobart, Australia: 1964. A new species of Tuckerella (Acarina: Tetranychoidea, Tuckerellidae) from Tasmania; pp. 79–84. [Google Scholar]
- 6.Ochoa R., Aguilar H., Vargas C. Phytophagous Mites of Central America: An Illustrated Guide. CATIE; Cartago, Costa Rica: 1994. pp. 1–234. [Google Scholar]
- 7.Lim T.K. Edible Medicinal and Non-Medicinal Plants. Springer; London, UK: 2012. Mammea Americana; pp. 134–142. [Google Scholar]
- 8.Morton J.F. In: Fruits of Warm Climates. Dowling C.F., editor. ECHO Inc.; Miami, FL, USA: 1987. pp. 304–307. [Google Scholar]
- 9.Duarte O., Paull R. Exotic Fruits and Nuts of the New World. CABI; Wallingford, UK: 2015. 332p [Google Scholar]
- 10.CABI Invasive Species Compendium. 2019. [(accessed on 8 November 2021)]. Available online: https://www.cabi.org/isc/datasheet/32390.
- 11.Zamore R., Ebroin A. Vertus et Secrets Desplantes Médicinales des Antilles; Kolodziej, E., Ed.; Fort-de-France, Martinique, France. 1984. [(accessed on 17 March 2022)]. p. 13. Available online: https://kolibris.univ-antilles.fr/discovery/fulldisplay?vid=33UAG_INST:33UAG_INST&tab=TOUT&docid=alma991000410419705746&lang=fr&context=L&adaptor=Local%20Search%20Engine&query=sub,exact,%20speciation%20,AND&mode=advanced&offset=30.
- 12.Poupon J., Chauvin G. Les arbres de la Martinique. Off. Natl. For. Guadeloupe. 1983:210–211. [Google Scholar]
- 13.Mosquera W.G., Criado L.Y., Guerra B.E. Actividad antimicrobiana de hongos endófitos de las plantas medicinales Mammea americana (Calophyllaceae) y Moringa oleifera (Moringaceae) Biomédica. 2020;40:55–71. doi: 10.7705/biomedica.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta I., Sandoval P., Bautista D., Hernández N., Cárdenas J.F., Martínez V.M. Bioadsorción de cromo (VI) por la cáscara de mamey (Mammea americana L.) Av. Cienc. Ing. 2012;3:1–9. [Google Scholar]
- 15.Little E.L., Wadsworth F.H. Agriculture Handbook, No. 249. U.S. Department of Agriculture; Washington, DC, USA: 1964. Common trees of Puerto Rico and the Virgin Islands.548p [Google Scholar]
- 16.Mahecha V.G.E., Echeverri R.R. Arboles del Valle del Cauca. Litografia Arco; Bogotá, Colombia: 1983. 208p [Google Scholar]
- 17.Dunthorn M. Cryptic dioecy in Mammea (Clusiaceae), Plant Syst. Evol. 2004;249:191–196. [Google Scholar]
- 18.Francis J.K. Mammea americana L. Mamey, Mammee-Apple. Department of Agriculture, Forest Service, Southern Forest Experiment Station; New Orleans, LA, USA: 1989. 4p Note SO-ITF-SM-22. [Google Scholar]
- 19.Mourão K.S.M., Beltrati C.M. Morfología y anatomía de frutos y semillas en desarrollo de Mammea americana L. (Clusiaceae) Rev. Braz. J. Biol. 2000;60:70–111. doi: 10.1590/s0034-71082000000400023. [DOI] [PubMed] [Google Scholar]
- 20.Gervais L., Lavigne C. Mamey (Mammea americana L.) in Martinique Island: An inheritance to be developed. Fruits. 2007;62:237–246. doi: 10.1051/fruits:2007019. [DOI] [Google Scholar]
- 21.Liogier A.H. Arboles Dominicanos. Academia de Ciencias de la República Dominicana; Santo Domingo, Dominican Republic: 1978. 220p [Google Scholar]
- 22.Agrodataperu Exportaciones Agropecuarias del Perú. 2020. [(accessed on 8 November 2021)]. Available online: https://www.agrodataperu.com/2021/01/frutas-pulpas-aguacate-palta-arandanos-peru-exportacion-2020-diciembre.html.
- 23.Krantz G.W., Walter D.E., editors. A Manual of Acarology. 3rd ed. Texas Tech University Press; Lubbock, TX, USA: 2009. Collecting, rearing, and preparing specimens; pp. 83–94. [Google Scholar]
- 24.Meyer M.K.P.S., Ueckermann E.A. A review of some species of the families Allochaetophoridae, Linotetranidae and Tuckerellidae (Acari: Tetranychoidea) Int. J. Acarol. 1997;23:67–92. doi: 10.1080/01647959708683103. [DOI] [Google Scholar]
- 25.Escobar-Garcia H.A., de Andrade D.J., Beard J.J., Ochoa R. Peacock mites on cocoa in Peru (Acari: Tuckerellidae: Tuckerella): Their economic importance and a key to species. Syst. Appl. Acarol. 2021;26:519–528. doi: 10.11158/saa.26.3.2. [DOI] [Google Scholar]
- 26.Mineiro J.L.C., Raga A., Lofego A.C. Ocorrência de ácaros (Arachnida: Acari) em aceroleira (Malpighia emarginata A.DC.) no Estado de São Paulo. Arq. Inst. Biol. 2004;71:282–285. [Google Scholar]
- 27.Mineiro J.L.C., Lofego A.C., Raga A., de Moraes G.J. Primeiros registros dos ácaros Amblyseiella setosa Muma (Phytoseiidae) e Tuckerella pavonformis (Ewing) (Tuckerellidae) no Brasil. Arq. Inst. Biol. 2005;72:395–396. doi: 10.1590/1808-1657v72p3952005. [DOI] [Google Scholar]
- 28.Flechtmann C.H.W., Kreiter S., Etienne J., Moraes G. Plant mites (Acari) of the French Antilles. 1. Tetranychoidea (Prostigmata) Acarologia. 1999;40:136–144. [Google Scholar]
- 29.Aguilar H., Murillo P. New hosts and records of plant feeding mites for Costa Rica: Interval 2002–2008. [(accessed on 18 February 2022)];Agron. Costarric. 2008 32:7–28. Available online: https://www.cabi.org/ISC/abstract/20093044931. [Google Scholar]
- 30.Ochoa R. The genus Tuckerella in Costa Rica (Acari: Tuckerellidae) [(accessed on 18 February 2022)];Int. J. Acarol. 1989 15:205–207. doi: 10.1080/01647958908683850. doi: 10.1080/01647958908683850. Available online: [DOI] [Google Scholar]
- 31.Cao L.J., Leal H.J.L. Ácaros Tuckerélidos (Acari, Tetranychoidea, Tuckerellidae) asociados a Casuarina equisetifolia L. en la Habana. Fitosanidad. 2011;15:69–72. [Google Scholar]
- 32.Suárez A. Catálogo de ácaros de la provincia de Guantánamo, La Habana. Fitosanidad. 2004;8:23–31. [Google Scholar]
- 33.Huanca J., Vergara C. Primer Registro del Acaro Fitófago del Genero Tuckerella Womersley (Prostigmata: Tuckerellidae) en Perú. Resúmenes, Sociedad Entolomológica de Perú; LII Convención Nacional de Entomología; Iquitos, Perú: 2010. p. 47. [Google Scholar]
- 34.Turner J.C.L. Master’s Thesis. University of Florida; Gainesville, FL, USA: 2004. Biology and Management of Allokermes kingii (Hemiptera: Kermesidae) on Oak Trees.55p [Google Scholar]
- 35.Akamine E.K., Goo T. Respiration and ethylene production in mammee apple (Mammea americana L.) J. Amer. Soc. Hortic. Sci. 1978;103:308–310. [Google Scholar]
- 36.Yahia E.M. Handbook No. 66. U.S. Department of Agriculture; Washington, DC, USA: 2004. Sapodilla and related fruits. [Google Scholar]
- 37.Yahia E.M., Guttierrez-Orozco F. Mamey Apple (Mammea americana L.) In: Yahia E.M., editor. Postharvest Biology and Technology of Tropical and Subtropical Fruits. Woodhead Publishing; Cambridge, UK: 2011. pp. 474–481. (Woodhead Publishing Series in Food Science, Technology and Nutrition). [DOI] [Google Scholar]
- 38.Migeon A., Dorkeld F. Spider Mites Web: A Comprehensive Database for the Tetranychidae. [(accessed on 8 November 2021)]. 2006–2021. Available online: http://www1.montpellier.inra.fr/CBGP/spmweb.
- 39.Demite P.R., Moraes G.J., McMurtry J.A., Denmark H.A., Castilho R.C. Phytoseiidae Database. 2021. [(accessed on 8 November 2021)]. Available online: www.lea.esalq.usp.br/phytoseiidae.
- 40.De Leon D. Some Mites of the Caribbean Area. Allen Press, Inc.; Lawrence, KC, USA: 1967. pp. 1–46. [Google Scholar]
- 41.Cao J. La familia Tuckerellidae y su presencia en Cuba. Cienc. y Técnica en la Agric. Cítricos y otros Frut. 1980;3:47–56. [Google Scholar]
- 42.Kim J., Kim S., Ju C., Son H.I. Unmanned aerial vehicles in agriculture: A review of perspective of platform, control, and applications. IEEE Access. 2019;7:105100–105115. doi: 10.1109/ACCESS.2019.2932119. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.