Abstract
From the rat intestinal microflora we isolated a gram-positive rod, termed HDCA-1, that is a member of a not previously described genomic species and that is able to transform the 3α,6β,7β-trihydroxy bile acid β-muricholic acid into hyodeoxycholic acid (3α,6α-dihydroxy acid) by dehydroxylation of the 7β-hydroxy group and epimerization of the 6β-hydroxy group into a 6α-hydroxy group. Other bile acids that were also transformed into hyodeoxycholic acid were hyocholic acid (3α,6α,7α-trihydroxy acid), α-muricholic acid (3α,6β,7α-trihydroxy acid), and ω-muricholic acid (3α,6α,7β-trihydroxy acid). The strain HDCA-1 could not be grown unless a nonconjugated 7-hydroxylated bile acid and an unidentified growth factor produced by a Ruminococcus productus strain that was also isolated from the intestinal microflora were added to the culture medium. Germfree rats selectively associated with the strain HDCA-1 plus a bile acid-deconjugating strain and the growth factor-producing R. productus strain converted β-muricholic acid almost completely into hyodeoxycholic acid.
The major biliary bile acids in the rat are tauro-conjugated cholic acid, chenodeoxycholic acid, and α- and β-muricholic acid. In germfree male and female rats fed a purified casein-starch diet, the major bile acids in the cecum are cholic acid (16 and 25%, respectively) and β-muricholic acid (73 and 46%, respectively) (6). In conventional rats, the bile acids are extensively modified by the intestinal microflora in the terminal ileum and the large intestine (15). Bile acids in feces of female and male conventional rats are deoxycholic acid (9.3 and 8.8%, respectively), 3β,6α-dihydroxy-5β-cholanoic acid (16.8 and 19.1%, respectively), and hyodeoxycholic acid (47.2 and 55.6%, respectively) (7). Deoxycholic acid is the 7α-dehydroxylation product of cholic acid, hyodeoxycholic acid is thought to derive from β-muricholic acid through 7β-dehydroxylation and 6β-hydroxy epimerization, and 3β,6α-dihydroxy-5β-cholanoic acid is the 3β-epimer of hyodeoxycholic acid. Feces from conventional rats also contain variable amounts of ω-muricholic acid (1, 20, 31), which is thought to derive from β-muricholic acid through epimerization of the 6-hydroxy group (4, 24, 25).
Bacterial 7-dehydroxylation of the biliary bile acids is crucial in the formation of secondary bile acids. Following 7-dehydroxylation, secondary bile acids may undergo various other oxido-reduction reactions. Bacteria that can 7α-dehydroxylate cholic acid and chenodeoxycholic acid are found in many intestinal genera, such as Clostridium, Eubacterium, Lactobacillus, and Bacteroides (9, 11, 21). However, 7β-dehydroxylating activity is less common. It was suggested that 7β-hydroxy groups had to be epimerized to 7α-hydroxy groups prior to dehydroxylation (8). A 7β-dehydrogenating Clostridium absonum strain was isolated by MacDonald and Roach (18). Because many 7α-dehydroxylating strains are also capable of reducing 7-keto groups to 7α-hydroxy groups, intestinal bacterial 7β-dehydroxylation could be the result of a combination of the activities of 7β-dehydrogenating and 7α-dehydroxylating strains. However, White et al. (30) and Takamine and Imamura (28) isolated from the rat intestinal tract 7β-dehydroxylating Eubacterium sp. strains that were capable of 7β-dehydroxylating the 3α,7β-dihydroxy bile acid ursodeoxycholic acid. We also isolated from the rat intestinal microflora a Clostridium sp. strain with 7α- and 7β-dehydroxylating activity in the presence of chenodeoxycholic, cholic, ursodeoxycholic, and ursocholic acids (unpublished data). No strain, however, that can dehydroxylate the 7α-hydroxy group of hyocholic acid or the 7β-hydroxy group of β-muricholic acid has yet been described.
To transform β-muricholic acid into hyodeoxycholic acid, the 6β-hydroxy group of β-muricholic acid also has to be epimerized into a 6α-hydroxy group. Several intestinal strains capable of performing this reaction have been described (4, 24, 25). A combined action of intestinal bacteria that perform the 7β-dehydroxylation reaction and other strains that epimerize the 6β-hydroxy group could therefore also lead to the formation of hyodeoxycholic acid. Einarsson (2) suggested that hyodeoxycholic acid was formed from lithocholic acid by hepatic enzymes that converted lithocholic acid into a 3α,6β-dihydroxy bile acid and bacteria that further oxidized it into a 3α-hydroxy, 6-keto bile acid and reduced it to hyodeoxycholic acid. In this paper we report the isolation of an unidentified strain that can transform hyocholic acid and α-,β-, and ω-muricholic acids directly into hyodeoxycholic acid.
MATERIALS AND METHODS
Culture media used and incubation conditions.
Medium A consisted of 200 mg of freeze-dried feces from male, germfree rats in 5 ml of distilled water, supplemented with 0.5 ml of a 2-mg/ml solution of β-muricholic acid. Medium B contained 5 ml of PN medium supplemented with 125 mg of freeze-dried, fat-free ground beef and 0.5 ml of the bile salt solution tested (6 mg/ml). The PN medium consisted of 0.5% (wt/vol) Proteose Peptone 3 (Difco); 1% (wt/vol) Bacto Tryptone Yeast (Difco); 0.4% (wt/vol) brain heart infusion (BBL); 0.1% (wt/vol) MgSO4 · 7H2O; 1% (wt/vol) K2HPO4 · 3H2O; 0.1% (wt/vol) Na2CO3 · 10 H2O; 0.15% (wt/vol) Trizma 7.6; 0.02% (wt/vol) Tween 80; 0.0005% (wt/vol) hemin; 0.0001% (wt/vol) vitamin K (Konakion; Roche, Basel, Switzerland); 0.04% (wt/vol) cysteine HCl; and 4% (vol/vol) water extract of feces from germfree rats (supernatant of 6 g of freeze-dried feces from male germfree rats in 100 ml of water supplemented with 0.2 ml of 30% NaOH, boiled for 10 min and centrifuged at 3,000 × g for 10 min). Medium C was PN medium supplemented with 2% agar (Difco) and 0.6 mg of hyocholic acid per ml. Medium D contained 2% (wt/vol) Special Peptone (Oxoid), 1% (wt/vol) K2HPO4 · 3H2O, 0.2% (wt/vol) glucose, 0.1% (wt/vol) NaCl, 0.1% (wt/vol) MgSO4 · 7H2O, 0.002% (wt/vol) hemin, 0.0001% (wt/vol) vitamin K, and 0.04% (wt/vol) cysteine HCl and was supplemented with 0.5 ml of a 6-mg/ml solution of hyocholic acid. The dilution solution contained 1% (wt/vol) Bacto Tryptone Yeast, 0.9% (wt/vol) Na2HPO4 · 2H2O, and 0.05% (wt/vol) dithiothreitol. Autoclaved liquid and solid culture media were prereduced in the anaerobic chamber (Anaerobic System; Forma Scientific, Marietta, Ohio) for at least 48 h prior to inoculation in the anaerobic chamber. Agar plates were poured inside the anaerobic chamber. The atmosphere in the anaerobic chamber consisted of 90% N2 and 10% H2. Inoculated liquid and agar plate cultures were incubated at 37°C for 3 or 4 days.
Bile acids used and analysis of bile acids in culture media and feces.
Muricholic acids (α-, β-, and ω-muricholic acids) were obtained after hydrolysis of the respective methyl esters in 5% KOH (23) in methanol at 70°C for 1 h. 3α,7β-Dihydroxy-6-oxo-5β-cholanoic acid was isolated after enzymatic deconjugation of fecal bile acids from cholesterol-fed germfree rats (23). Hyocholic acid, chenodeoxycholic acid, and the tauro- and glycoconjugates of cholic acid and chenodeoxycholic acid were from Calbiochem-Behring (La Jolla, Calif.). Ursodeoxycholic, hyodeoxycholic, and 3α-hydroxy-6-oxo-5β-cholanoic acids were from Steraloids (Wilton, N.H.); cholic acid was from Aldrich Chemical Company (Dorset, England); ursocholic and 3α,6α-dihydroxy-7-oxo-5β-cholanoic acids were synthesized as described (16, 32). The procedures used to quantify bile acids in fecal samples from rats and mice have already been described (5, 23). Bile acid transformations in bacterial cultures were analyzed by mixing 1 ml of culture with 2 ml of distilled water, 1 ml of ethyl alcohol and 1 ml of a 0.1-mg/ml solution of 23-nor-deoxycholic acid in methyl alcohol as an external standard. This mixture was acidified to a pH of <2 with 2 N HCl and extracted twice with 6 ml of diethyl ether. Pooled extracts were evaporated to dryness, and the bile acids were derivatized to methylester trimethylsilylethers with diazomethane and TriSyl (Pierce, Rockford, Ill.). The reaction mixtures were washed with 5 ml of 0.1 N HCl and extracted twice with 4 ml of hexane. Finally, the extracts were concentrated by partial evaporation.
To analyze conjugated bile acids, samples were subjected to alkaline deconjugation with 20% (wt/vol) KOH–ethylene glycol as described (5). Gas-liquid chromatography was used to identify and quantify bile acids. Methylester trimethylsilylethers were analyzed isothermally at 260°C on a 3% OV1- or 1% QF1-packed column with N2 at a flow rate of 30 to 40 ml/min. Identification and quantification of bile acid metabolites was by capillary gas-liquid chromatography with standards as described (5, 23) on a 30 m by 0.32 mm DB-5 ms column (Alltech, Deerfield, Ill.). The carrier gas was helium and the flow rate was 1.5 ml/min. The temperature was kept initially at 80°C for 2 min, increased at 30°C/min to 275°C, and after 1 min at 275°C again raised to 280°C at 0.5°C/min. Bile acids were analyzed as acetylated derivatives or as methylester trimethylsilylether derivatives.
Study of bile acid transformations in rats.
Male inbred germfree, gnotobiotic, and conventional Fisher rats from our own germfree animal breeding center (Rega Institute, Leuven, Belgium) were used. The rats were originally obtained from the Laboratoire des Animaux sans Germes CNRS, Gif-sur-Yvette, France. The animals were kept in Trexler flexible-film plastic isolators (Standard Safety Equipment, Palatine, Ill.). The conventional rats were ex-germfree Fisher rats that were conventionalized by exposure in the same cage to commercially acquired Fisher rats (Animalium KULeuven, Leuven, Belgium). Due to coprophagy, the germfree rats acquired conventional intestinal flora over the course of 4 to 6 weeks. This was established through analysis of fecal bile acids and comparison of the fecal bile acid patterns to those already published (6, 7). The gnotobiotic rats were ex-germfree Fisher rats that received rectal instillation of 1 ml of 2-day-old bacterial culture fluid. Germfree rats were first associated with Clostridium perfringens ATCC 19574 and subsequently with Ruminococcus productus and strain HDCA-1, and finally, the rats in one group were also associated with Clostridium sp. strain S1 (14). After instillation of each strain, feces were checked for the presence of all introduced strains by microscopic examination and culture of fecal homogenates. The next strain was introduced only after confirmation of the presence of the preceding strain. Fecal bile acid profiles were analyzed every week for several weeks after the introduction of all the species of the defined microflora. A gradual change in the composition of the fecal bile acids was observed; there was evolution from the pattern found in the germfree animals towards a climax pattern that remained stable for the rest of the rats’ lives. This climax pattern is reported in Table 1. The gradual change in the fecal bile acid pattern took from 2 to 3 weeks to develop and spanned the interval from the time of inoculation to the time of the stable climax pattern.
TABLE 1.
Composition of fecal bile acid from germfree, gnotobiotic, and conventional ratsa
| Bile acid | Germfree rats | Gnotobiotic ratsb | Gnotobiotic ratsc | Conventional rats |
|---|---|---|---|---|
| Lithocholic acid | 2.0 | 2.0 | 3.5 | |
| Deoxycholic acid | 7.6 | 23.4 | 17.3 | |
| Chenodeoxycholic acid | 2.9 | 1.4 | 1.4 | |
| 3β,6α-Dihydroxy-5β-cholanoic acid | 5.4 | |||
| Hyodeoxycholic acid | 19.9 | 39.7 | 23.4 | |
| Cholic acid | 34.1 | 29.3 | 8.7 | 6.9 |
| α-Muricholic acid | 4.8 | 4.7 | ||
| β-Muricholic acid | 58.2 | 29.3 | 1.9 | 8.1 |
| ω-Muricholic acid | 0.5 | 12.6 | ||
| Δ22-β-Muricholic acid | 11.3 | |||
| Unidentified | 5.8 | 12.5 | 21.4 | |
| Total bile acidsd | 10.2 | 10.3 | 8.7 | 12.6 |
| % Deconjugated | 0 | 29.1 | 98.7 | 100 |
Amounts of bile acids are given as percentages of total bile acids; each value is the mean of 10 samples, and each sample consisted of a 48-h collection of feces from three different rats.
Ex-germfree rats associated with a defined mixed microflora consisting of C. perfringens ATCC 19574, the growth factor-producing R. productus strain, and strain HDCA-1.
Ex-germfree rats associated with a defined mixed microflora consisting of C. perfringens ATCC 19574, the growth factor-producing R. productus strain, and strain HDCA-1 plus Clostridium sp. strain S1 (14).
Total fecal bile acids expressed as milligrams/rat/24 h.
All rats used in our experiments received a steam-sterilized commercial diet (SRMA1210; Hope Farms, Woerden, The Netherlands) and water ad libitum. For the analysis of bile acids, feces were collected every 2 or 3 days from 30 rats in each group, homogenized with equal volumes of water, and freeze-dried.
16S rDNA sequence analysis of HDCA-1.
Genomic DNA was prepared according to the protocol of Niemann et al. (22). 16S rRNA genes were amplified by PCR by using the following primers: 5′-AGTTTGATCCTGGCTCAG-3′ and 5′-TACCTTGTTACGACTTCACCCCA-3′. PCR-amplified 16S rDNAs were purified by using a QIAquick PCR Purification kit (Qiagen GmbH, Hilden, Germany). Complete sequencing was performed by using an Applied Biosystems Inc. 377 DNA Sequencer and the protocols of the manufacturer (Perkin-Elmer, Foster City, Calif.) by using an ABI Prism Dye Terminator Cycle Sequencing Ready Reaction kit. The following five forward primers and three reverse primers were used to get an optimal overlap of sequences, enhancing the reliability of the assembled data: 5′-CTCCTACGGGAGGCAGT-3′, 5′-CAGCAGCCGCGGTAATAC-3′, 5′-AACTCAAAGGAATTGACGG-3′, 5′-AGTCCCGCAACGAGCGCAAC-3′, 5′-GCTACACACGTGCTACAATG-3′, 5′-ACTGCTGCCTCCCGTAGGAG-3′, 5′-GTATTACCGCGGCTGCTG-3′, and 5′-GTTGCGCTCGTTGCGGGACT-3′. Sequence assembly was performed by using the program AutoAssembler (Perkin-Elmer). The resulting consensus sequence was 1,443 bp long. Phylogenetic analysis was performed by using the software package GeneCompar (Applied Maths, Kortrijk, Belgium) after including the 1,443-bp-long consensus sequence in an alignment of small ribosomal subunit sequences collected from the international nucleotide sequence library of the European Molecular Biology Laboratory (EMBL) via the FASTA search program. This alignment was pairwise calculated with an open gap penalty of 100% and a unit gap penalty of 0%. A similarity matrix was created by homology calculation with a gap penalty of 0% and after discarding unknown bases. The resulting tree was constructed by using the neighbor-joining method.
RESULTS
Isolation of a minimal hyodeoxycholic acid-forming culture.
Fresh feces from 2- to 4-month-old ex-germfree conventionalized Fisher rats were transferred to the anaerobic chamber, and approximately 0.5 g was homogenized in 10 ml of the dilution fluid. Anaerobic subcultures of 0.5 ml of this fecal suspension in medium A converted β-muricholic acid and hyocholic acid into hyodeoxycholic acid, whereas aerobic subcultures did not. Heating of the fecal suspension in the dilution fluid for 20 min at 80°C followed by anaerobic subculturing did not impede transformation of β-muricholic acid and hyocholic acid into hyodeoxycholic acid. However, the hyodeoxycholic acid-producing microflora could not be maintained on any of the commonly used culture media, such as brain heart infusion broth, Columbia broth, or thioglycolate broth. Two essential conditions for maintaining the hyodeoxycholic acid-producing microflora were a pH of > 6.5 and the presence in the culture medium of 7-hydroxy bile acids. Although bile acids were found to be essential to sustain the hyodeoxycholic acid-producing microflora, bile acid concentrations of 0.1 mg/ml or more led to the disappearance of the hyodeoxycholic acid-forming activity. Addition of 125 mg of freeze-dried ground beef per 5 ml of the strongly buffered PN culture medium (medium B) permitted addition of up to 1 mg of bile acids per ml and led to stable hyodeoxycholic acid formation.
Subculturing of pure cultures of bacterial strains picked after plating out of the mixed hyodeoxycholic acid-forming cultures on medium C never led to the isolation of pure cultures of hyodeoxycholic acid-forming bacteria. The minimal mixed culture, in which stable hyodeoxycholic acid-forming activity was found, consisted of two strains. The first of these two strains was a gram-positive coccus identified as R. productus, and the other strain was an unidentified gram-variable rod named HDCA-1. Identification of R. productus was based on the VPI Anaerobe Laboratory Manual (12) and Bergey’s Manual of Systematic Bacteriology (26). These bacteria presented as gram-positive coccobacilli, lying in pairs or short chains. They fermented arabinose, cellobiose, fructose, glucose, lactose, maltose, mannose, melibiose, raffinose, rhamnose, ribose, sorbitol, sucrose, trehalose, and xylose. Gas-liquid chromatography of fermentation products in peptone-yeast extract-glucose broth showed that these bacteria produced acetate, succinate, and lactic acid (13). Gas-liquid chromatography of whole-cell fatty acids and comparison to the database via the Microbial Identification System (MIDI, Inc., Newark, Del.) confirmed the identification. The R. productus strain was grown as a pure culture but did not transform hyocholic acid or β-muricholic acid.
Characteristics and phylogeny of strain HDCA-1.
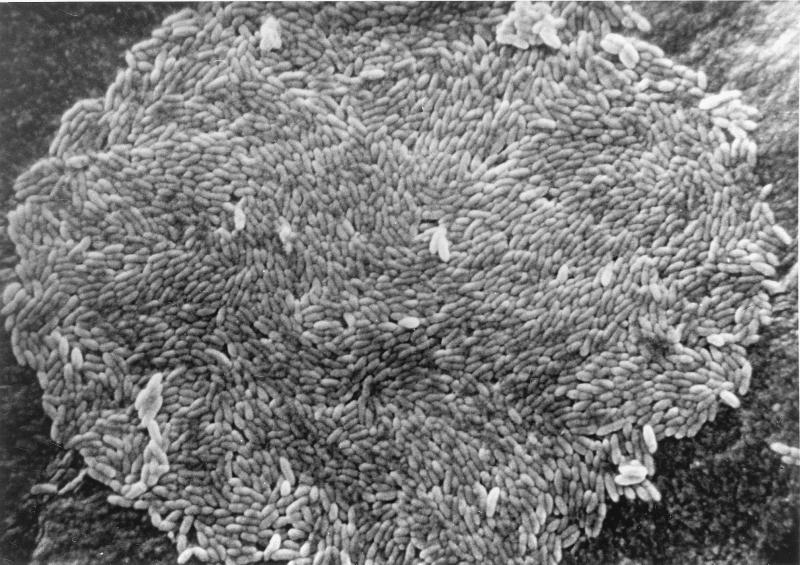
Cells of strain HDCA-1 presented as small, gram-variable, nonmotile, tapered rods lying in pairs or short chains. HDCA-1 in fecal suspensions obtained from gnotobiotic rats selectively associated with HDCA-1, R. productus, and C. perfringens ATCC 19574 survived heat shock for 20 min at 80°C. Spore formation could not be induced in vitro in any of the culture media that we tested. Colonies on medium C were less than 0.1 mm in diameter, circular, entire, and glistening, and consisted of a monolayer of bacteria (Fig. 1).
FIG. 1.
Scanning electron micrograph of a colony of strain HDCA-1, showing a monolayer of bacteria.
Growth of strain HDCA-1 in liquid culture medium was obtained only if the R. productus strain was cocultured or if 10 drops of culture supernatant of a 48-h-old R. productus culture were added to the culture medium. Addition of 5.5% (vol/vol) of the R. productus culture supernatant led to an HDCA-1 cell density of around 106 per ml after 3 days of incubation on medium B. Reduction of the volume of supernatant added to below 2.7% (vol/vol) led to loss of the HDCA-1 culture if subcultures were made every 3 days. On solid media, growth of strain HDCA-1 could be obtained only after streak inoculation of R. productus, presumably because of rapid inactivation of the culture supernatant by trace amounts of oxygen. The growth factor was prepared by boiling of the entire culture or filtration-centrifugation of a 48-h-old R. productus culture. It was resistant to boiling for 5 min under anaerobic conditions but was rapidly inactivated by contact with oxygen. After inactivation by oxygen it could be reactivated by addition of 6 mM dithiothreitol in the anaerobic isolator. At −20°C it could be kept for at least 6 months. It was, however, inactivated at a pH of <6.5. The growth factor was also produced by the R. productus type strain (ATCC 27340). It was not present in horse blood or feces from germfree rats. Vitamins, pantethine, glutathione, lipoic acid, or coenzyme A could not replace the activity of the growth factor. Other growth requirements of strain HDCA-1 were strict anaerobiosis (Eh below −250 mV) and a pH between 7.0 and 7.75 at the time of inoculation. Addition of dithiothreitol at concentrations up to 12 mM reduced the Eh to below −300 mV. Although this was not toxic to the HDCA-1, it did not improve growth, nor did it lead to increased bile acid transformation. Growth was impaired at pHs below 7.0, and no growth was observed at pHs below 6.5. In addition, supplementation of the culture medium with a carbohydrate (glucose, fructose, ribose, maltose, lactose, melibiose, and to a lesser extent sucrose, trehalose, and starch) and the presence of a nonconjugated 7-hydroxy bile acid were also necessary for growth. The freeze-dried ground beef (125 mg/5 ml) that was added to allow addition of bile acids in concentrations greater than 0.1 mg/ml could be replaced by 0.002% (wt/vol) hemin (medium D). However, even under optimal conditions, the number of CFU of strain HDCA-1 never exceeded 106/ml. Replacing the standard 90% N2–10% H2 atmosphere in the anaerobic chamber with an 80% N2–10% H2–10% CO2 atmosphere did not stimulate growth.
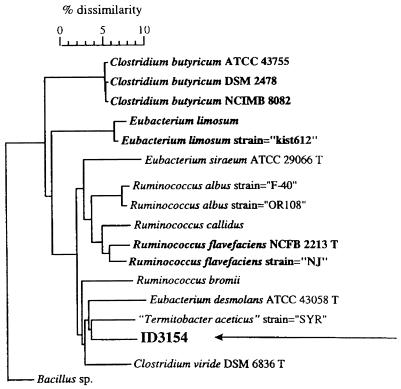
Identification of HDCA-1 through conventional methods was not possible because of our inability to grow the strain on the necessary culture media. Gas-liquid chromatography of whole-cell fatty acids and comparison to the database via the Microbial Identification System did not lead to detection of similarities with known patterns sufficient to allow identification. Sequence analysis of the 16S rRNA genes (EMBL accession no. AJ238611) and alignment with the other sequences from the EMBL sequence library by using the software package GeneCompar (Applied Maths) made it possible to determine the phylogenetic position of HDCA-1 within the spectrum of low G+C content gram-positive bacteria. HDCA-1 showed the greatest sequence similarities (91 to 92%) with unidentified rumen isolates (27). Other close relatives were Termitobacter aceticus (89.6%), Eubacterium desmolans ATCC 43058 (89.2%), Clostridium viride DSM 6836 (89.1%), Ruminococcus flavefaciens NCFB 2213 (87.6%), and Ruminococcus albus OR108 (87.5%) (Fig. 2). The low sequence similarities (less than 97%) clearly indicate that HDCA-1 is a member of a new, not previously described genomic species (29).
FIG. 2.
Phylogenetic tree of HDCA-1 (ID3154) based on the alignment of the most similar 16S rDNA sequences, using the GeneCompar software package (Applied Maths). Type species are shown in bold, designations of type strains are followed by a T, and invalid species names are given in quotes.
Bile acid transformations by strain HDCA-1.
Between 70 and 80% of 0.6-mg/ml solutions of the 7α- and 7β-trihydroxy bile acids α-muricholic acid, β-muricholic acid, ω-muricholic acid, ursocholic acid, and cholic acid were 7-dehydroxylated by strain HDCA-1. Identical concentrations of the 7α-dihydroxy bile acid chenodeoxycholic and the 7β-dihydroxy bile acid ursodeoxycholic acid were less than 50% 7-dehydroxylated.
In addition to the dehydroxylation of the 7-hydroxy group, strain HDCA-1 also epimerized the 6β-hydroxy groups of β-muricholic acid and α-muricholic acid into 6α-hydroxy groups. As a result of this combined 7-dehydroxylating and 6β-epimerizing activity, strain HDCA-1 transformed α-muricholic, β-muricholic, ω-muricholic, and hyocholic acid into hyodeoxycholic acid. We did not find dehydroxylation of 3-, 6-, or 12-hydroxy groups by strain HDCA-1.
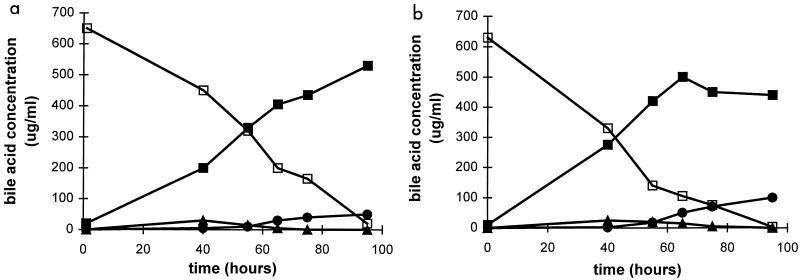
Study of the time course of β-muricholic acid and hyocholic acid transformation by strain HDCA-1 showed a rather slow formation of hyodeoxycholic acid which went on well into the stationary growth phase (Fig. 3). We also observed a weak transient formation of 3α,6α-dihydroxy-7-oxo-5β-cholanoic acid both with β-muricholic and with hyocholic acid and the slow accumulation of 3α-hydroxy-6-oxo-5β-cholanoic acid starting after 2 days of incubation.
FIG. 3.
Transformation of β-muricholic acid (a) and hyocholic acid (b) by strain HDCA-1 as a function of time. □, β-muricholic acid (panel a) and hyocholic acid (panel b); ■, hyodeoxycholic acid; ▴, 3α,6α-dihydroxy-7-oxo-5β-cholanoic acid; and ●, 3α-hydroxy-6-oxo-5β-cholanoic acid.
Association of germfree rats with strain HDCA-1.
Strain HDCA-1 alone could not be associated with germfree rats, most likely because of its absolute requirements for 7-hydroxylated nonconjugated bile acids, for the growth factor produced by R. productus, and for a low Eh. Prior association of germfree rats with the growth factor-producing R. productus strain and the bile acid-deconjugating C. perfringens strain ATCC 19574 allowed the association of these gnotobiotic rats with strain HDCA-1. Analysis of fecal bile acids from these gnotobiotic rats and comparison with fecal bile acids from germfree rats showed a 50% reduction in β-muricholic acid, a 16% reduction in cholic acid, and a 53% reduction in chenodeoxycholic acid (Table 1). Concomitantly, we found in the feces of these gnotobiotic rats 19.9% hyodeoxycholic acid, 7.6% deoxycholic acid and 2% lithocholic acid in contrast to a total absence of these bile acids for the germfree rats. α-Muricholic acid, because it is almost completely sulfated, appeared not to be transformed in the gnotobiotic rats, judging from its relative concentrations in gnotobiotic and germfree rats. Plating of serial dilutions of fresh fecal homogenates from three rats showed that R. productus and C. perfringens were present at densities of 109 per g. The numbers of cells of HDCA-1 in the feces were estimated at 103 per g as judged from the formation of hyodeoxycholic acid in serial dilutions of the same fresh fecal homogenates.
Additional association of the R. productus plus C. perfringens ATCC 19574 plus HDCA-1-associated gnotobiotic rats with the bile acid desulfating and deconjugating Clostridium sp. S1 strain (14) increased deconjugation of β-muricholic acid and cholic acid. Consequently, the transformation of β-muricholic acid and cholic acid into hyodeoxycholic and deoxycholic acid was also increased to levels that were even higher than those found in conventional rats (Table 1). In addition, 0.5% of total fecal bile acids was identified as ω-muricholic acid, and 11.3% was identified as a β-muricholic acid metabolite with a double bond in the side chain, between C-22 and C-23, as already described by Robben et al. (24) and Kayahara et al. (17).
Germfree CH3 mice (Rega Institute) were kept for up to 3 months in the same cage as the gnotobiotic rats associated with the complex microflora (R. productus, C. perfringens ATCC 19574, HDCA-1, and Clostridium sp. S1). Interestingly, we found that all the strains except for HDCA-1 became established in the ex-germfree mice. Rectal instillation of bacteria in germfree CH3 mice in the same way as used for the germfree Fisher rats also did not lead to establishment of HDCA-1 in the mice.
DISCUSSION
The transformation of α- and β-muricholic acids into hyodeoxycholic acid includes a 7α- or 7β-dehydroxylation reaction and a 6β-epimerization reaction. Hypothetical reaction mechanisms for these transformations are diaxial transelimination of the 7α-hydroxyl group and the 6β-hydrogen for α-muricholic acid and diequatorial transelimination of the 7β-hydroxyl group and the 6β-hydrogen for β-muricholic acid. The resulting 3α-hydroxy-Δ6-enol intermediate could subsequently be reduced to a 6α-hydroxyl moiety by transhydrogenation. Time course experiments on β-muricholic acid metabolism by strain HDCA-1 showed transient formation of a 3α,6α-dihydroxy-7-oxo-5β-cholanoic acid during the exponential growth phase and accumulation of 3α-hydroxy-6-oxo-5β-cholanoic acid in the stationary growth phase of the culture.
Because the 3α-hydroxy-6-oxo-5β-cholanoic acid could not be transformed by strain HDCA-1 into hyodeoxycholic acid, we suggest that this is not an intermediate in the formation of hyodeoxycholic acid but is a metabolite of hyodeoxycholic acid. ω-Muricholic acid was not found as an intermediate of the transformation of β-muricholic acid into hyodeoxycholic acid. The efficiency with which strain HDCA-1 transforms muricholic acids into hyodeoxycholic acid both in vitro and in vivo seems to exclude the combined bacterial-hepatic transformation pathway proposed by Einarsson (2). Because hyodeoxycholic acid is not very efficiently absorbed from the intestinal tract in rats, Madsen et al. (19) suggested that in the rat hyodeoxycholic acid formation might be an important mechanism for controlling the body cholesterol pools. Hence, transformation of muricholic acid by strain HDCA-1 in the rat might also be important in controlling cholesterol levels. The traces of ω-muricholic acid found in the feces of the gnotobiotic rats associated with strain HDCA-1 suggest that this bile acid could indeed be the result of a further hepatic modification of reabsorbed hyodeoxycholic acid, as suggested by Madsen et al. (19).
The special growth factors required by HDCA-1 complicated its isolation from feces and may explain why previous efforts to isolate hyodeoxycholic acid-forming bacteria from feces did not succeed. The fact that bile acids with a 7-hydroxy group are a necessary growth factor suggests that this 7-hydroxyl functions as an electron acceptor for these bacteria. The requirement for 7-dehydroxylation of a special factor produced by another bacterial strain has been reported before (10, 28).
The sensitivity of strain HDCA-1 to changes in pH and particularly its inability to grow at pHs below 7.0 might explain the fall in hyodeoxycholic acid production in rats fed a lactose-containing diet (3). Bacterial fermentation of lactose leads to a reduced colonic pH, as reported by Eyssen et al. (3), and consequently to a reduced formation of hyodeoxycholic acid.
In conclusion, the isolation of strain HDCA-1 is an illustration of the complex interactions and metabolic interdependence that exist among the members of the intestinal microflora and again demonstrates the enormous metabolic diversity and versatility of the bacteria that constitute the intestinal microflora.
ACKNOWLEDGMENTS
We thank M. Vancanneyt and D. Janssens of the BCCM/LMG Culture Collection, Laboratory of Microbiology, University of Ghent, for performing the 16S rDNA sequence analysis of HDCA-1 and the phylogenetic analysis.
Appendix
Trivial names for bile acids are as follows: 3α-hydroxy-5β-cholanoic acid, lithocholic acid; 3α-hydroxy-5α-cholanoic acid, allolithocholic acid; 3α,6α-dihydroxy-5β-cholanoic acid, hyodeoxycholic acid; 3α,6β-dihydroxy-5β-cholanoic acid, muricholic acid; 3α,7α-dihydroxy-5β-cholanoic acid, chenodeoxycholic acid; 3α,7β-dihydroxy-5β-cholanoic acid, ursodeoxycholic acid; 3α,12α-dihydroxy-5β-cholanoic acid, deoxycholic acid; 3α,7α,12α-trihydroxy-5β-cholanoic acid, cholic acid; 3α,6α,7α-trihydroxy-5β-cholanoic acid, hyocholic acid; 3α,6β,7α-trihydroxy-5β-cholanoic acid, α-muricholic acid; 3α,6β,7β-trihydroxy-5β-cholanoic acid, β-muricholic acid; 3α,6α,7β-trihydroxy-5β-cholanoic acid, ω-muricholic acid; 3α,7β,12α-trihydroxy-5β-cholanoic acid, ursocholic acid; 3α,12α-dihydroxy-23-nor-5β-cholanoic acid, 23-nor-deoxycholic acid.
REFERENCES
- 1.Borum M L, Shehan K L, Fromm H, Jahangeer S, Floor M K, Alabaster O. Fecal bile acid excretion and composition in response to changes in dietary wheat bran, fat and calcium in the rat. Lipids. 1992;27:999–1004. doi: 10.1007/BF02535579. [DOI] [PubMed] [Google Scholar]
- 2.Einarsson K. On the formation of hyodeoxycholic acid in the rat. J Biol Chem. 1966;241:534–539. [PubMed] [Google Scholar]
- 3.Eyssen H, De Pauw G, Parmentier G. Effect of lactose on Δ5-steroid-reducing activity of intestinal bacteria in gnotobiotic rats. J Nutr. 1974;104:605–612. doi: 10.1093/jn/104.5.605. [DOI] [PubMed] [Google Scholar]
- 4.Eyssen H, De Pauw G, Stragier J, Verhulst A. Cooperative formation of ω-muricholic acid by intestinal microorganisms. Appl Environ Microbiol. 1983;45:141–147. doi: 10.1128/aem.45.1.141-147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyssen H J, Parmentier G G, Mertens J A. Sulfated bile acids in germ-free and conventional mice. Eur J Biochem. 1976;66:507–514. doi: 10.1111/j.1432-1033.1976.tb10576.x. [DOI] [PubMed] [Google Scholar]
- 6.Eyssen H, Smets L, Parmentier G, Janssen G. Sex linked differences in bile acid metabolism of germfree rats. Life Sci. 1977;21:707–712. doi: 10.1016/0024-3205(77)90079-0. [DOI] [PubMed] [Google Scholar]
- 7.Eyssen H, Van Eldere J. Metabolism of bile acids. In: Coates M E, Gustafsson B E, editors. The germ-free animal in biomedical research. London, United Kingdom: Laboratory Animals Ltd.; 1984. pp. 291–316. [Google Scholar]
- 8.Federowski T, Salen G, Colallilo A, Tint G S, Mosbach H, Hall J C. The metabolism of ursodeoxycholic acid in man. Gastroenterology. 1977;73:1131–1137. [PubMed] [Google Scholar]
- 9.Hayakawa S. Microbial transformation of bile acids. Adv Lipid Res. 1973;11:143–192. doi: 10.1016/b978-0-12-024911-4.50011-8. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Masuda N. Enhancement of the 7α-dehydroxylase activity of a gram-positive intestinal anaerobe by Bacteroides and its significance in the 7-dehydroxylation of ursodeoxycholic acid. J Lipid Res. 1982;23:1152–1158. [PubMed] [Google Scholar]
- 11.Hirano S, Nakama R, Tamaki M, Masuda N, Oda H. Isolation and characterization of thirteen intestinal microorganisms capable of 7α-dehydroxylating bile acids. Appl Environ Microbiol. 1981;41:737–745. doi: 10.1128/aem.41.3.737-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holdeman L V, Cato E P, Moore W E C. Anaerobic cocci. In: Holdeman L V, Cato E P, Moore W E C, editors. VPI anaerobe laboratory manual. 4th ed. Blacksburg, Va: Virginia Polytechnic Institute; 1977. pp. 13–21. [Google Scholar]
- 13.Holdeman L V, Cato E P, Moore W E C. Chromatographic procedures for analysis of acid and alcohol products. In: Holdeman L V, Cato E P, Moore W E C, editors. VPI anaerobe laboratory manual. 4th ed. Blacksburg, Va: Virginia Polytechnic Institute; 1977. pp. 134–140. [Google Scholar]
- 14.Huijghebaert S, Mertens J, Eyssen H. Isolation of a bile salt sulfatase-producing Clostridium strain from rat intestinal microflora. Appl Environ Microbiol. 1982;43:185–192. doi: 10.1128/aem.43.1.185-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hylemon P B, Glass T L. Biotransformation of bile acids and cholesterol by the intestinal microflora. In: Hentges D J, editor. Human intestinal microflora in health and disease. New York, N.Y: Academic Press; 1983. pp. 189–213. [Google Scholar]
- 16.Iida T, Chang F C. Potential bile acid metabolites. 7. 3,7,12-Trihydroxy-5β-cholanic acids and related compounds. J Org Chem. 1982;47:2972–2978. [Google Scholar]
- 17.Kayahara T, Tamura T, Amuro Y, Higashino K, Igimi H, Uchida K. Δ22-β-Muricholic acid in monoassociated rats and conventional rats. Lipids. 1994;29:289–296. doi: 10.1007/BF02536334. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald I A, Roach P D. Bile salt induction of 7α- and 7β-hydrosteroid dehydrogenases in Clostridium absonum. Biochim Biophys Acta. 1981;665:262–269. doi: 10.1016/0005-2760(81)90011-4. [DOI] [PubMed] [Google Scholar]
- 19.Madsen D C, Chang L, Wostmann B S. ω-Muricholate: a tertiary bile acid of the Wistar rat. Proc Indiana Acad Sci. 1975;84:416–420. [Google Scholar]
- 20.Madsen D C, Wostmann B S, Beaver M, Chang L. Effects of Aureomycin on bile acids in rats. J Lab Clin Med. 1978;91:605–611. [PubMed] [Google Scholar]
- 21.Midvedt T. Microbial bile acid transformation. Am J Clin Nutr. 1974;27:1341–1347. doi: 10.1093/ajcn/27.11.1341. [DOI] [PubMed] [Google Scholar]
- 22.Niemann S, Puehler A, Tichy H-V, Simon R, Selbitschka W. Evaluation of the resolving power of three different DNA fingerprinting methods to discriminate among isolates of a natural Rhizobium meliloti population. J Appl Microbiol. 1997;82:477–484. doi: 10.1046/j.1365-2672.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 23.Parmentier G G, Smets L M-J, Janssen G A, Eyssen H J. Effects of cholesterol feeding on the bile acids of male and female germ-free rats. Eur J Biochem. 1981;116:365–372. doi: 10.1111/j.1432-1033.1981.tb05344.x. [DOI] [PubMed] [Google Scholar]
- 24.Robben J, Parmentier G, Eyssen H. Isolation of a rat intestinal Clostridium strain producing 5α- and 5β-bile salt 3α-sulfatase activity. Appl Environ Microbiol. 1986;51:32–38. doi: 10.1128/aem.51.1.32-38.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacquet E C, Raibaud P M, Mejean C, Riottot M J, Leprince C, Leglise P C. Bacterial formation of ω-muricholic acid in rats. Appl Environ Microbiol. 1979;37:1127–1131. doi: 10.1128/aem.37.6.1127-1131.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleifer K H. Gram-positive cocci. In: Sneath P H, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1084–1097. [Google Scholar]
- 27.Tajima, K. Unpublished data.
- 28.Takamine F, Imamura T. 7β-Dehydroxylation of 3,7-dihydroxy bile acids by a Eubacterium species strain C-25 and stimulation of 7β-dehydroxylation by Bacteroides distasonis strain K-5. Microbiol Immunol. 1985;29:1247–1252. doi: 10.1111/j.1348-0421.1985.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 29.Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White B A, Fricke R J, Hylemon P B. 7β-Dehydroxylation of ursodeoxycholic acid by whole cells and cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 12708. J Lipid Res. 1982;23:145–153. [PubMed] [Google Scholar]
- 31.Wostmann B S, Beaver M, Chang L, Madsen D. Effect of autoclaving of a lactose-containing diet on cholesterol and bile acid metabolism of conventional and germ-free rats. Am J Clin Nutr. 1977;30:1999–2005. doi: 10.1093/ajcn/30.12.1999. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler P. The structure of hyocholic acid. Can J Chem. 1956;34:1528–1531. [Google Scholar]