Abstract
Stachybotrys chartarum is an indoor air, toxigenic fungus that has been associated with a number of human and veterinary health problems. Most notable among these has been a cluster of idiopathic pulmonary hemorrhage cases that were observed in the Cleveland, Ohio, area. In this study, 16 strains of S. chartarum isolated from case (n = 8) or control (n = 8) homes in Cleveland and 12 non-Cleveland strains from diverse geographic locations were analyzed for hemolytic activity, conidial toxicity, and randomly amplified polymorphic DNA banding patterns. In tests for hemolytic activity, strains were grown at 23°C on wet wallboard pieces for an 8-week test period. Conidia from these wallboard pieces were subcultured on sheep’s blood agar once a week over this period and examined for growth and clearing of the medium at 37 or 23°C. Five of the Cleveland strains (all from case homes) showed hemolytic activity at 37°C throughout the 8-week test compared to 3 of the non-Cleveland strains. Five of the Cleveland strains, compared to two of the non-Cleveland strains, produced highly toxic conidia (>90 μg of T2 toxin equivalents per g [wet weight] of conidia) after 10 and 30 days of growth on wet wallboard. Only 3 of the 28 strains examined both were consistently hemolytic and produced highly toxic conidia. Each of these strains was isolated from a house in Cleveland where an infant had idiopathic pulmonary hemorrhage.
Beginning in 1993, some infants, living in water-damaged homes in Cleveland, Ohio, developed cases of idiopathic pulmonary hemorrhage (IPH) (2). An investigation by the Centers for Disease Control and Prevention concluded that Stachybotrys chartarum was likely connected to this disease (10, 11, 23). Stachybotrys chartarum (Ehrenb. ex Link) Hughes (= S. atra Corda) is a toxigenic fungus that grows on wet, cellulose-based products such as paper, cardboard, and wallboard (12) and has been associated with human health problems (4, 6, 18, 19).
Since 1993, there have been more than 40 IPH cases in the Cleveland metropolitan area, with the majority of cases clustered in a small geographic area. These cases have resulted in a 30% mortality rate (7). For a 3-year period (1993 to 1995), 12% of all sudden infant death syndrome deaths in the cluster area were actually IPH deaths (7). The IPH problem is not limited to Cleveland. In an informal survey, more than 140 cases of IPH have been reported in infants across the United States during the past 5 years (8).
The first cluster of IPH cases was reported from Greece in the early 1980s (5). Other reported clusters of the illness have occurred in Chicago, Ill., between 1992 and 1994 (3); in Detroit, Mich., between 1992 and 1995 (24); in Ann Arbor, Mich., between 1988 and 1993 (27); and in Milwaukee, Wis., between 1993 and 1996 (15). No apparent etiology was found for these other clusters of IPH. But clusters of Stachybotrys-related illness and death are well documented in the veterinary literature.
In the 1930s, an epizootic of stachybotryotoxicosis (ST) in horses began in Eastern Europe and Russia and ended about 1944 (13). No similar major outbreak seems to have occurred since then, but incidents of ST still occur in domestic animals in Eastern Europe and Russia and some other parts of the world (28). Toxins produced by this fungus are considered the cause of the disease (9).
When viewed in relation to the widespread occurrence of S. chartarum around the world (21), the outbreak of disease suspected to be caused by this organism can be considered to be sporadic. The goal of this study was to determine if the strains of S. chartarum isolated from homes in Cleveland where infants developed IPH are unique compared to strains from control homes or strains from other diverse geographic locations.
MATERIALS AND METHODS
Hemolytic test of S. chartarum strains on SBA.
Conidia of each strain were inoculated onto and grown on 5- by 5-cm pieces of sterile, 9.4-mm (3/8 in.)-thick wallboard placed in 100- by 15-mm petri dishes containing 20 ml of sterile water and incubated at 23°C. More sterile water was added to the petri dishes after 4 weeks of incubation, so that there was always free water around the wallboard pieces during the 8-week incubation.
Every 7 days for 8 weeks, conidia from each strain were recovered with a sterile cotton swab from each wallboard piece. The conidia were transferred to plates of 5% sheep blood agar (SBA; Becton Dickinson, Sparks, Md.) and incubated at 37 and 23°C. After 10 days of incubation, the plates were checked for hemolytic activity. Clearing of the medium beyond the edge of the fungal growth was considered to be an indication of hemolytic activity (hemolytic positive).
Hemolytic activity after heat treatment.
After 5 weeks of growth on wallboard, conidia from each of the 28 strains were directly blotted onto four sets of duplicate sterile, borosilicate filters (Millipore, Bedford, Mass.). One set of filters was heat treated at 86°C for 20 min, and another set was held at 23°C. Finally, each filter was placed on SBA and incubated at 37°C for 10 days, and hemolysis was noted for each strain.
Toxicity of S. chartarum strains.
The strains of S. chartarum were evaluated for their toxicity by the method of Yike et al. (30), which uses luciferase translation in a mammalian cell-free system to assess protein synthesis inhibition, an activity characteristic of trichothecene mycotoxins. Strains were grown on wallboard as described above. After 10 and 30 days of growth on wallboard, conidia from each of the strains were blotted directly onto 9-mm-diameter sterile, borosilicate filters (Millipore). The wet weight of the conidia on the filters was determined, and the filters and conidia were then freeze-dried and held at 4°C prior to performing the toxicity assays.
The filters with adhering conidia were extracted with 10 ml of 95% ethanol at room temperature overnight and then sonicated at room temperature for 30 min. The sonication was repeated with 5 ml of fresh ethanol, and the combined extracts were filtered through 0.22-μm-pore-size Millex-GV filters (Millipore) and evaporated in a Speed Vac Plus centrifuge (model SC210A; Savant Instrument, Farmingdale, N.Y.). Extracts were reconstituted in 200 μl of 95% ethanol, diluted, and passed through Ultrafree-MC 5000 NMWL microcentrifuge filters (Millipore).
In vitro translation of firefly luciferase mRNA by using Flexi rabbit reticulocyte lysate (Promega, Milwaukee, Wis.) was carried out in the presence or absence of the standard toxin, T-2 (Sigma, St. Louis, Mo.), and conidial extracts. Inhibition of protein translation caused by the extracts and purified T-2 toxin was evaluated by measuring the luminescence of newly synthesized luciferase. Dose-response curves for the standard toxin and conidial extracts were generated, and the concentrations causing 50% inhibition were determined by using the TableCurve curve-fitting program (Jandel Scientific, Chicago, Ill.). T-2 toxin equivalents in the conidial extracts were derived from comparison of the 50% inhibitory concentrations for the standard toxin.
DNA extraction and randomly amplified polymorphic DNA (RAPD) analysis of S. chartarum strains.
The 12 non-Cleveland strains used in this study are listed in Table 1. Each strain was grown on potato dextrose agar (Becton Dickinson, Sparks, Md.) for 7 days at 23°C. Conidia were collected from each strain by using a sterile cotton swab and resuspended in sterile water to give a final concentration of 107 conidia per ml (as determined by hemocytometer counts). The DNA of each strain was extracted by the bead-beating method (20) as previously modified (16). Briefly, this involves adding 0.3 g of glass beads (G-1277; Sigma, St. Louis, Mo.) to 2 ml of a conical-bottom, screw-cap tube (PGC Scientifics, Gaithersburg, Md.) and autoclaving. Then, 100 μl of lysis buffer and 300 μl of binding buffer from the Elu-Quik Kit (Schleicher & Schuell, Keene, N.H.), followed by 10 μl (i.e., 105 conidia) of each conidial suspension was added to each tube. The tubes were shaken rapidly in a Mini-Beadbeater (Biospec Products, Bartlesville, Okla.) for 1 min at the maximum rate. The released DNA was processed as described in the directions for the Elu-Quik Kit.
TABLE 1.
Non-Cleveland S. chartarum strains used in this study
| Strain no. | Culture collection and no.a | Yr isolated | Isolation location | Substrate |
|---|---|---|---|---|
| NC-1 | ATCC 16026 | Unknown | United Kingdom | Unknown |
| NC-2 | UAMH 6715 | 1991 | Saskatchewan, Canada | Indoor air |
| NC-3 | UAMH 7900 | 1995 | Alberta, Canada | Indoor air |
| NC-4 | ATCC 26303 | 1973 | Finland | Cardboard |
| NC-5 | ATCC 48610 | 1977 | Finland | Unknown |
| NC-6 | ATCC 46994 | 1982 | Egypt | Wheat straw |
| NC-7 | UAMH 3228 | 1969 | California, United States | Feathers |
| NC-8 | UAMH 6417 | 1986 | Namibia | Desert sand |
| NC-9 | UAMH 7568 | 1994 | British Columbia, Canada | Cardboard |
| NC-10 | UAMH 7598 | 1994 | Alberta, Canada | Wood molding |
| NC-11 | EPA 395b | 1996 | Prince Edward Island, Canada | Indoor air |
| NC-12 | UAMH 6425 | 1988 | Ontario, Canada | Wood, paper |
ATCC, American Type Culture Collection; UAMH, University of Alberta Microfungus Collection and Herbarium.
Gift from Richard Summerbell.
The recovered DNA was randomly amplified by using the R28 primer (5′-ATGGATCCGC) and the PCR protocol described by Fujimori and Okuda (14), modified as follows. The R28 primer was synthesized by PE Applied Biosystems (Foster City, Calif.). The RAPD reaction was done in a total volume of 50 μl containing 0.2 μl of a 50 nM solution of the R28 primer, 1 μl of PCR nucleotide mix (1581295; Boehringer, Indianapolis, Ind.), 0.3 μl of Expand High Fidelity PCR System DNA polymerase (84367521; Boehringer), 5 μl of 10× PCR buffer with MgCl (83073623; Boehringer), 5 μl of a sterile bovine serum albumin (Fraction V; Sigma) solution containing 2 mg of water per ml, 7 μl of a 50% solution of glycerol in water, 26.5 μl of sterile deionized water, and 5 μl of the purified DNA solution. The PCR was conducted at 92°C for 1 min, followed by 30 cycles of denaturation at 92°C for 45 s, annealing at 34°C for 60 s, and extension at 72°C for 90 s; then, the final extension was done at 72°C for 10 min in a thermal cycler (PTC-200; MJ Research, Watertown, Mass.).
The PCR products were separated on a 0.7% agarose gel. The gels were run at 100 V for 6 h and stained for 40 min with SYBR Green (FMC, Rockland, Maine) at a concentration of 10 μl per 100 ml of water. The gels were imaged with the FluorImager 595 (Molecular Dynamics, Sunnyvale, Calif.), and the molecular weight of each band was determined by using the associated image analysis program, Fragment NT. Molecular size standards consisting of 2,000-, 1,200-, 800-, 400-, 200-, and 100-bp fragments (Life Technologies, Grand Island, N.Y.) were run in triplicate on each gel.
RAPD analysis of each strain’s DNA was replicated three to seven times in separate gels. Only bands that appeared in at least 80% of the analyses were considered positive for that strain. The results were assembled in a 1-0 matrix depending upon whether a band was present (1 matrix) or absent (0 matrix), as described by Fujimori and Okuda (14). Phylogenetic relationships of the strains and strain distances were inferred from these data by using the branch and bond option of the Phylogenetic Analysis Using Parsimony (PAUP) program, version 3.1 (Sinauer Associates, Sunderland, Mass.). A distance analysis was performed on each strain to determine which was the least similar. This strain was used as the outgroup in the phylogenetic evaluation. The PAUP bootstrap with a branch-and-bound search (1,000 times replication) was used to estimate the distance between strains, and the levels of support for the branches of the most parsimonious trees with at least 90% occurrence were used as the cutoff for significance.
RESULTS
Hemolysis of SBA by S. chartarum strains.
Tables 2 and 3 show the results of the weekly hemolytic assays on SBA at 37 and 23°C, respectively. When incubated at 37°C, five of the Cleveland strains (all from case homes) and three of the non-Cleveland strains were consistently capable of demonstrating hemolytic activity throughout the 8-week test (Table 2). By week 5 of the incubation, all 28 strains were demonstrating hemolytic activity. None of the strains were consistently hemolytic when incubated at 23°C (Table 3).
TABLE 2.
Weekly observations of S. chartarum strains for hemolytic activity and growth at 37°C on SBAa
| Strain | Hemolytic activity and growth at (wks on wet wallboard):
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
|||||||||
| H | G | H | G | H | G | H | G | H | G | H | G | H | G | H | G | |
| 51-05 (Ca) | − | PG | − | PG | + | + | + | + | + | + | + | + | + | + | + | + |
| 51-06 (Ca) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 51-08 (Ca) | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| 51-11 (Ca) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 51-19 (Ca) | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + |
| 58-02 (Ca) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 58-06 (Ca) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 63-07 (Ca) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 58-07 (Ct) | − | PG | − | PG | − | − | + | + | + | + | − | + | − | + | − | PG |
| 58-08 (Ct) | − | − | − | PG | − | PG | − | + | + | + | + | + | + | + | + | + |
| 58-15 (Ct) | − | + | − | + | − | + | + | + | + | + | + | + | + | + | + | + |
| 58-16 (Ct) | − | PG | − | PG | − | + | − | + | + | + | + | + | − | + | − | + |
| 58-17 (Ct) | − | + | − | PG | − | + | + | + | + | + | + | + | + | + | + | + |
| 58-18 (Ct) | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 58-19 (Ct) | − | + | − | PG | − | + | + | + | + | + | + | + | + | + | + | + |
| 63-01 (Ct) | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-1 | − | PG | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-2 | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-3 | − | PG | − | PG | − | − | − | + | + | + | + | + | + | + | + | + |
| NC-4 | − | − | − | + | − | + | + | + | + | + | + | + | + | + | + | + |
| NC-5 | − | − | − | PG | − | + | − | + | + | + | + | + | + | + | + | + |
| NC-6 | − | PG | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-7 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-8 | − | − | + | + | + | + | + | + | + | + | + | + | − | + | − | PG |
| NC-9 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-10 | − | + | − | + | − | + | − | + | + | + | + | + | − | + | − | + |
| NC-11 | − | PG | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-12 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
Abbreviations: H, hemolysis; G, growth on SBA; PG, poor growth; Ca, case house strain; Ct, control house strain.
TABLE 3.
Weekly observations of S. chartarum strains for hemolytic activity and growth at 23°C on SBAa
| Strain | Hemolytic activity and growth at (wks on wet wallboard):
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
|||||||||
| H | G | H | G | H | G | H | G | H | G | H | G | H | G | H | G | |
| 51-05 (Ca) | − | + | − | + | − | + | − | + | + | + | + | + | − | + | − | + |
| 51-06 (Ca) | − | + | − | + | − | + | − | + | + | + | − | + | − | + | − | + |
| 51-08 (Ca) | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 51-11 (Ca) | − | + | − | + | − | + | − | + | − | + | + | + | − | + | − | + |
| 51-19 (Ca) | − | + | − | + | + | + | + | + | + | + | + | + | − | + | − | + |
| 58-02 (Ca) | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 58-06 (Ca) | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 63-07 (Ca) | − | + | − | + | + | + | − | + | + | + | + | + | + | + | + | + |
| 58-07 (Ct) | − | + | − | + | − | + | − | + | + | + | − | + | − | + | − | + |
| 58-08 (Ct) | − | + | − | + | + | + | − | + | + | + | − | + | + | + | + | + |
| 58-15 (Ct) | − | + | − | + | − | + | − | + | + | + | − | + | − | + | − | + |
| 58-16 (Ct) | − | + | − | + | − | + | − | + | + | + | − | + | − | + | − | + |
| 58-17 (Ct) | − | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + |
| 58-18 (Ct) | − | + | − | + | + | + | − | + | + | + | + | + | + | + | + | + |
| 58-19 (Ct) | − | + | − | + | − | + | + | + | + | + | − | + | − | + | − | + |
| 63-01 (Ct) | − | + | − | + | − | + | − | + | + | + | + | + | + | + | + | + |
| NC-1 | − | + | − | + | − | + | + | + | + | + | − | + | − | + | − | + |
| NC-2 | − | + | − | + | − | + | − | + | + | + | + | + | + | + | + | + |
| NC-3 | − | + | − | + | + | + | − | + | − | + | + | + | − | + | − | + |
| NC-4 | − | + | − | + | − | + | − | + | + | + | − | + | − | + | − | + |
| NC-5 | − | + | − | + | − | + | − | + | + | + | + | + | + | + | + | + |
| NC-6 | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-7 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NC-8 | − | + | − | + | + | + | + | + | + | + | + | + | − | + | − | + |
| NC-9 | − | + | − | + | − | + | + | + | + | + | + | + | + | + | + | + |
| NC-10 | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + |
| NC-11 | − | + | − | + | − | + | − | + | − | + | − | + | + | + | + | + |
| NC-12 | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
Abbreviations are as defined in Table 2, footnote a.
Hemolytic activity after heat treatment.
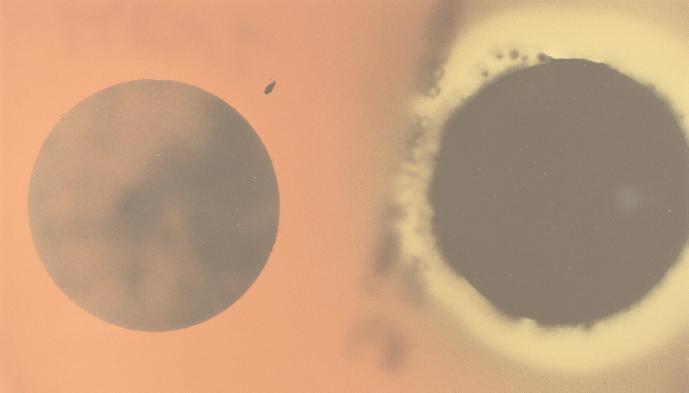
None of the strains on the filters heat treated at 86°C for 20 min produced a hemolytic response on SBA at 37°C, but the conidia of all strains on SBA that were not heat treated grew and produced a hemolytic response (Fig. 1).
FIG. 1.
Appearance of strain 58-06 conidia on SBA after conidia on filters were heat treated (left) or not heat treated (right). After 5 weeks of growth on wallboard, conidia from strain 58-06 were directly blotted onto borosilicate filters. The filter on the left was heat treated at 86°C for 20 min, and the one on the right was held at 23°C. Filters were placed on SBA and incubated at 37°C for 10 days.
Toxicity of S. chartarum strains.
The conidia of all 28 strains of S. chartarum tested in this study showed some level of toxicity, as measured by protein synthesis inhibition, when grown on wet wallboard (Table 4). However, only seven strains (five from Cleveland and two non-Cleveland strains) were consistently highly toxic, i.e., toxicities were greater than 90 μg of T-2 toxin equivalents per g (wet weight) of conidia. One Cleveland strain (58-08) and three non-Cleveland strains (NC-1, NC-2, and NC-4) were intermediate in toxicity. The other 17 strains had toxicities consistently less than 20 μg of T-2 toxin equivalents per g (wet weight) of conidia.
TABLE 4.
Protein translation toxicity of S. chartarum strains grown at 23°C on wet wallboard
| Strain | Toxicity (T-2 equivalents/g [wet wt] of conidia) after (days of incubation):
|
|
|---|---|---|
| 10 | 30 | |
| Cleveland | ||
| 51-05 | 3.7 | 7.3 |
| 51-06 | 104.5 | 109.2 |
| 51-08 | 8.3 | 2.0 |
| 51-11 | 192.0 | 95.1 |
| 51-19 | 6.6 | 0.6 |
| 58-02 | 146.8 | 111.5 |
| 58-06 | 7.8 | 1.5 |
| 58-07 | 14.0 | 1.0 |
| 58-08 | 56.6 | 323.6 |
| 58-15 | 8.3 | 1.5 |
| 58-16 | 6.3 | 1.1 |
| 58-17 | 909.1 | 444.0 |
| 58-18 | 555.5 | 173.0 |
| 58-19 | 3.8 | 4.5 |
| 63-01 | 5.7 | 7.4 |
| 63-07 | 3.7 | 1.3 |
| Non-Cleveland | ||
| NC-1 | 63.7 | 134.0 |
| NC-2 | 13.9 | 228.0 |
| NC-3 | 5.3 | 1.8 |
| NC-4 | 17.5 | 559.0 |
| NC-5 | 556.4 | 469.0 |
| NC-6 | 9.8 | 2.0 |
| NC-7 | 3.0 | 0.9 |
| NC-8 | 15.1 | 1.2 |
| NC-9 | 1.9 | 2.0 |
| NC-10 | 422.7 | 122.0 |
| NC-11 | 10.1 | 1.5 |
| NC-12 | 6.5 | 2.7 |
RAPD analysis of S. chartarum strains.
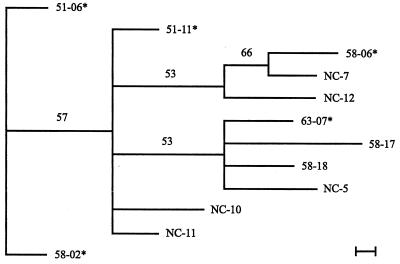
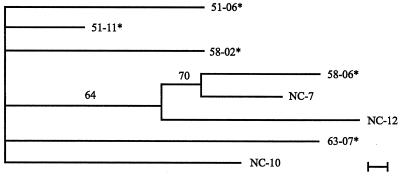
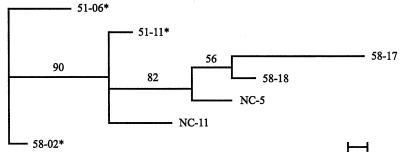
The RAPD analysis of S. chartarum strains produced 26 bands. The matrices resulting from this analysis are shown in Tables 5 and 6. Two bands (15 and 21) were found in all strains of S. chartarum analyzed in this study. When the highly toxic and/or consistently hemolytic strains are analyzed as a group for phylogenetic relationships, strains 51-06 and 58-02 are most distant from the other nine strains in this group (Fig. 2). If the consistently hemolytic strains are analyzed as a group (Fig. 3), none of the strains show significant relationships. If the highly toxic strains are analyzed as a group, then 51-06 and 58-02 are significantly different from the other highly toxic strains (Fig. 4).
TABLE 5.
Matrix showing the presence (“1”) or absence (“0”) of the 26 bands produced by RAPD analysis of the strains of S. chartaruma
| Strain | Matrix of band:
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (2026– 1970) | 2 (1923– 1889) | 3 (1818– 1750) | 4 (1708– 1657) | 5 (1601– 1592) | 6 (1553– 1517) | 7 (1509– 1451) | 8 (1438– 1416) | 9 (1399– 1367) | 10 (1357– 1347) | 11 (1300– 1251) | 12 (1211– 1153) | 13 (1094– 1004) | 14 (995– 956) | 15 (931– 884) | 16 (872– 822) | 17 (754– 702) | 18 (700– 664) | 19 (656– 610) | 20 (599– 566) | 21 (534– 481) | 22 (469– 421) | 23 (402– 381) | 24 (367– 332) | 25 (310– 282) | 26 (223– 206) | |
| Cleveland case strains | ||||||||||||||||||||||||||
| 51-05 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 51-06 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 51-08 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| 51-11 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| 51-19 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| 58-02 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 58-06 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| 63-07 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| Cleveland control strains | ||||||||||||||||||||||||||
| 58-07 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| 58-08 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| 58-15 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 58-16 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| 58-17 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| 58-18 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| 58-19 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| 63-01 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| Non-Cleveland strains | ||||||||||||||||||||||||||
| NC-1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| NC-2 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| NC-3 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| NC-4 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| NC-5 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| NC-6 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| NC-7 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| NC-8 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| NC-9 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| NC-10 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| NC-11 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| NC-12 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
Under each band number, the band width is shown in base pairs; e.g., band 1 goes from 2,026 to 1,970 bp, band 2 goes from 1,923 to 1,889 bp, etc.
TABLE 6.
Frequency of occurrence of bands in the strains of S. chartarum that were both highly toxic and consistently hemolytic (A) compared to the other 25 strains in the study (B)a
| Type | Matrix of band:
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (2026– 1970) | 2 (1923– 1889) | 3 (1818– 1750) | 4 (1708– 1657) | 5 (1601– 1592) | 6 (1553– 1517) | 7 (1509– 1451) | 8 (1438– 1416) | 9 (1399– 1367) | 10 (1357– 1347) | 11 (1300– 1251) | 12 (1211– 1153) | 13 (1094– 1004) | 14 (995– 956) | 15 (931– 884) | 16 (872– 822) | 17 (754– 702) | 18 (700– 664) | 19 (656– 610) | 20 (599– 566) | 21 (534– 481) | 22 (469– 421) | 23 (402– 381) | 24 (367– 332) | 25 (310– 282) | 26 (223– 206) | |
| A | 100 | 100 | 67 | 0 | 0 | 0 | 67 | 33 | 33 | 100 | 100 | 100 | 67 | 0 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 67 | 67 | 0 | 0 |
| B | 64 | 80 | 88 | 56 | 32 | 44 | 60 | 24 | 72 | 16 | 92 | 84 | 76 | 44 | 100 | 92 | 88 | 32 | 64 | 40 | 100 | 80 | 56 | 64 | 56 | 68 |
The band numbers in boldface indicate band frequencies that differ by at least 50% in the two groups.
FIG. 2.
Phylogram of the 11 strains of S. chartarum which were highly toxic and/or consistently hemolytic. The phylogram presented is the most parsimonious tree inferred from the binary data (Table 5) by using the branch-and-bond option in PAUP 3.1. The scale bar represents the distance resulting from one character change. Distance analysis of the data set was performed in PAUP to determine which strain was the least similar. This strain was used as the outgroup in the phylogenetic evaluation. Values appearing above the branches are percentages of 1,000 bootstrap analysis replicates in which the branches were found. Only percentages greater than 50% are shown. A strain number followed by an asterisk indicates a strain that came from an IPH case house in Cleveland.
FIG. 3.
Phylogram of the seven strains of S. chartarum which were consistently hemolytic. The phylogram presented is the most parsimonious tree inferred from the binary data (Table 5) by using the branch-and-bond option in PAUP 3.1. The scale bar represents the distance resulting from one character change. Distance analysis of the data set was performed in PAUP to determine which strain was the least similar. This strain was used as the outgroup in the phylogenetic evaluation. Values appearing above the branches are percentages of 1,000 bootstrap analysis replicates in which the branches were found. Only percentages greater than 50% are shown. A strain number followed by an asterisk indicates a strain that came from an IPH case house in Cleveland.
FIG. 4.
Phylogram of the seven highly toxic strains of S. chartarum. The phylogram presented is the most parsimonious tree inferred from the binary data (Table 5) by using the branch-and-bond option in PAUP 3.1. The scale bar represents the distance resulting from one character change. Distance analysis of the data set was performed in PAUP to determine which strain was the least similar. This strain was used as the outgroup in the phylogenetic evaluation. Values appearing above the branches are percentages of 1,000 bootstrap analysis replicates in which the branches were found. Only percentages greater than 50% are shown. A strain number followed by an asterisk indicates a strain that came from an IPH case house in Cleveland. Strains 51-06 and 58-02 were significantly different from the other highly toxic strains, as indicated by the 90 on the line connecting to the rest of the strains.
DISCUSSION
S. chartarum is the etiologic agent of ST, a potentially fatal disease of horses and other large domestic animals caused by consuming moldy feed (13). Even though S. chartarum has a worldwide distribution, the incidence of ST is limited to certain geographic areas, especially Eastern Europe and Russia (28). Toxins produced by S. chartarum are generally considered responsible for the ST deaths (9), but additional factors might also have been involved. Sarkisov and Orshanskaiya (26) described infections of the horse’s liver, kidney, lymphatic system, and spleen, from which they were able to isolate Stachybotrys. This suggests that, under some circumstances, this fungus was not only toxigenic but also infectious in horses.
Currently available information on the levels of exposure to airborne S. chartarum conidia for children with diagnosed cases of IPH (11) suggests that toxic effects alone may not be sufficient to explain these illnesses. This possibility was further suggested by demonstrations that the amounts of toxins produced by isolates from the IPH case homes were no greater than those produced by isolates from control homes (17). These observations suggest that some additional factor(s) may contribute to IPH.
Virulence factors, such as hemolysins, are often associated with pathogenic microorganisms (22). In some cases, the ability of pathogenic microorganisms to acquire iron from a mammalian host by producing a hemolysin has been shown to be of critical importance in establishing infections (1, 25, 29). The four infants who had lived in the homes where the eight case strains were isolated had hemoglobinuria, a finding consistent with a hemolysin playing a role in the pathophysiology of IPH.
All 16 strains of S. chartarum studied from case and control houses produced a hemolytic agent at 37°C (Table 2). However, the strains isolated from case houses showed hemolytic activity 89% of the time (57 of 64 observations) at 37°C compared to strains isolated from control houses, which were hemolytic only 58% of the time (37 of 64 observations). Chi-square analysis of these results indicates that case strains and control strains were significantly different (P < 0.001) in the frequency of hemolytic-activity expression. When SBA plates were incubated at 23°C, the case strains were hemolytic only 53% of the time (33 of 64 observations) compared to only 30% of the time (19 of 64 observations) for control strains (Table 3). Chi-square analysis of these results indicates that case strains and control strains were significantly different (P < 0.001) in their frequency in expressing hemolysis at 23°C. Comparing all strains incubated at 37°C to all strains incubated at 23°C, the frequency of hemolytic expression was also statistically different (chi-square result of P < 0.001), indicating that 37°C is the preferred temperature for S. chartarum hemolysis expression.
The identity of the hemolysin has not been determined. Forgacs (13) indicated that S. chartarum conidia were killed by heat treatment but that toxins in the spores were unaffected by this treatment. Because heat-killed conidia in this study are not hemolytic, it seems unlikely that the hemolysin is any of the known S. chartarum toxins.
This study has demonstrated that there are highly toxic and consistently hemolytic strains of S. chartarum. The frequency of occurrence of highly toxic strains from Cleveland was about the same as for the non-Cleveland sources; i.e., 31% of the Cleveland strains were highly toxic compared to 17% of the non-Cleveland strains (Table 4). The frequency of consistently hemolytic strains at 37°C was only slightly higher among the Cleveland strains (31%) than among the non-Cleveland strains (25%) (Table 2). Despite these indications that S. chartarum strains from the Cleveland area are not much more likely to be either highly toxic or highly hemolytic than strains from other locations, as a whole, results from this study suggest that the combination of these characteristics may be unique to certain Cleveland case strains.
Only three strains of the 28 tested (51-06, 51-11, and 58-02) were both highly toxic and consistently hemolytic. All three of these strains came from homes in Cleveland where infants became sick. This observation raises the interesting possibility that a combination of hemolysins and toxins may be required to induce the IPH disorder. Two of these three strains (51-06 and 58-02) were statistically different from the other highly toxic strains based on RAPD analysis (Fig. 4).
The RAPD analysis of strains 51-06, 51-11, and 58-02 compared to that of the other 25 strains in this study (Table 6) indicates a potentially diagnostic pattern for combined highly toxic and consistently hemolytic strains. There are five bands (bands 4, 10, 18, 25, and 26) that occur in this group of three strains whose frequency of occurrence differs by more than 50% from the other set of strains. The binary pattern, bands 4(0), 10(1), 18(1), 25(0), and 26(0), is unique to this group. Knowing this pattern may help screen other strains for these combined virulence factors.
REFERENCES
- 1.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Acute pulmonary hemorrhaging/hemosiderosis among infants: Cleveland, January 1993–November 1994. Morbid Mortal Weekly Rep. 1994;43:881–883. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Acute pulmonary hemorrhage among infants: Chicago, April 1992–November 1994. Morbid Mortal Weekly Rep. 1994;44:67–74. [PubMed] [Google Scholar]
- 4.Cooley J D, Wong W C, Jumper C A, Straus D C. Correlation between the prevalence of certain fungi and sick building syndrome. Occup Environ Med. 1998;55:579–584. doi: 10.1136/oem.55.9.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossimos C D, Chryssanthopoulos C, Panagiotidou C. Epidemiologic observations in idiopathic pulmonary hemosiderosis. J Pediatr. 1983;102:698–702. doi: 10.1016/s0022-3476(83)80236-4. [DOI] [PubMed] [Google Scholar]
- 6.Croft W A, Jarvis B B, Yatawara C S. Airborne outbreak of trichothecene toxicosis. Atmos Environ. 1986;20:549–552. [Google Scholar]
- 7.Dearborn D. Pulmonary hemorrhage in infants and children. Curr Opin Pediatr. 1997;9:219–224. doi: 10.1097/00008480-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dearborn, D. G., I. Yike, W. G. Sorenson, M. J. Miller, and R. A. Etzel. An overview of the investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. (Suppl.), in press. [DOI] [PMC free article] [PubMed]
- 9.Eppley R M, Bailey W J. 12,13-Epoxy-9-trichothecenes as the probable mycotoxins responsible for stachybotryotoxicosis. Science. 1973;181:758–760. doi: 10.1126/science.181.4101.758. [DOI] [PubMed] [Google Scholar]
- 10.Etzel R A, Montana E, Sorenson W G, Kullman G J, Miller J D. Pulmonary hemosiderosis associated with exposure to Stachybotrys atra. Epidemiology. 1996;7:S38. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- 11.Etzel R A, Montana E, Sorenson W G, Kullman G J, Allan T M, Dearborn D G. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch Pediatr Adolesc Med. 1998;152:757–762. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- 12.Flannigan B, Miller J. Health implications of fungi in indoor environments: an overview. In: Samson R, Flannigan B, Graveson S, editors. Health implications of fungi in indoor environments. Amsterdam, The Netherlands: Elsevier Science Publications; 1994. pp. 3–28. [Google Scholar]
- 13.Forgacs J. Stachybotryotoxicosis. In: Kadis S, Ciegler A, Ajl S J, editors. Microbial toxins. VIII. New York, N.Y: Academic Press, Inc.; 1972. pp. 95–128. [Google Scholar]
- 14.Fujimori F, Okuda T. Application of the random amplified polymorphic DNA using the polymerase chain reaction for efficient elimination of duplicate strains in microbial screening. I Fungi J Antibiot. 1994;47:173–182. doi: 10.7164/antibiotics.47.173. [DOI] [PubMed] [Google Scholar]
- 15.Guarih M, Rusakow L S, Gershap W M, Mischler E H. Characterization and outcome of idiopathic pulmonary hemorrhage in infants. Respir Crit Care Med. 1997;155:A711. [Google Scholar]
- 16.Haugland, R. A., J. L. Heckman, and L. J. Wymer. Evaluation of different methods for the extraction of DNA from fungal conidia by quantitative competitive PCR. J. Microbiol. Methods, in press. [DOI] [PubMed]
- 17.Jarvis B B, Sorenson W G, Hintikka E-L, Nikulin M, Zhou Y, Jiang J, Wang S, Hinkley S, Etzel R A, Dearborn D G. Study of toxin production by isolates of Stachybotrys atra and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl Environ Microbiol. 1998;64:3620–3625. doi: 10.1128/aem.64.10.3620-3625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johanning E, Morey P R, Jarvis B B. Clinical-epidemiological investigation of health effects caused by Stachybotrys atra building contamination. Indoor Air. 1993;1:225–230. [Google Scholar]
- 19.Johanning E, Biagini R, Hull D, Morey P, Jarvis B, Landsbergis P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in water-damaged office environment. Int Arch Occup Environ Health. 1996;68:297–318. doi: 10.1007/BF00381430. [DOI] [PubMed] [Google Scholar]
- 20.Lundblad V. Saccharomyces cerevisiae. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997. p. 13.13.4. [Google Scholar]
- 21.Miller J D. Fungi as contaminants in indoor air. Atmos Environ. 1992;26:2163–2172. [Google Scholar]
- 22.Mims C A. The pathogenesis of infectious disease. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 23.Montana E, Etzel R A, Dearborn D G, Sorenson W G, Hill R. Acute pulmonary hemorrhage in infancy associated with Stachybotrys atra Cleveland, Ohio, 1993–1995. Am J Epidemiol. 1995;141:S83. [Google Scholar]
- 24.Pappas M D, Sarnaik A P, Meert K L, Hasan R A, Lieh-Lai M W. Idiopathic pulmonary hemorrhage in infancy. Chest. 1996;110:553–556. doi: 10.1378/chest.110.2.553. [DOI] [PubMed] [Google Scholar]
- 25.Payne S M, Finkelstein R A. The critical role of iron in host-bacterial interactions. J Clin Investig. 1978;61:1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkisov A K, Orshanskaiya V N. Laboratory diagnosis of toxic strains of Stachybotrys alternans. Veterinariya. 1944;21:38–40. [Google Scholar]
- 27.Siden H B, Sanders G M, Moier F W. A report of four cases of acute, severe pulmonary hemorrhage in infancy and support with extracorporeal membrane oxygenation. Pediatr Pulm. 1994;18:337–341. doi: 10.1002/ppul.1950180512. [DOI] [PubMed] [Google Scholar]
- 28.Ueno Y. Toxicology. In: Ueno Y, editor. Trichothecenes: chemical biological and toxicological aspects. Developments in food science. Vol. 4. Amsterdam, The Netherlands: Elsevier Scientific Publishing; 1983. [Google Scholar]
- 29.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yike I, Allan T, Sorenson W G, Dearborn D G. Highly sensitive protein translation assay for trichothecene toxicity in airborne particulates: comparison with toxicity assay. Appl Environ Microbiol. 1999;65:88–94. doi: 10.1128/aem.65.1.88-94.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]