Abstract
Previous studies assessing the antibody response (AbR) to mRNA COVID-19 vaccines in solid organ transplant (SOT) recipients are limited by short follow-up, hampering the analysis of AbR kinetics. We present the ORCHESTRA SOT recipients cohort assessed for AbR at first dose (t0), second dose (t1), and within 3 ± 1 month (t2) after the first dose. We analyzed 1062 SOT patients (kidney, 63.7%; liver, 17.4%; heart, 16.7%; and lung, 2.5%) and 5045 health care workers (HCWs). The AbR rates in the SOTs and HCWs were 52.3% and 99.4%. The antibody levels were significantly higher in the HCWs than in the SOTs (p < 0.001). The kinetics showed an increase (p < 0.001) in antibody levels up to 76 days and a non-significant decrease after 118 days in the SOT recipients versus a decrease up to 76 days (p = 0.02) and a less pronounced decrease between 76 and 118 days (p = 0.04) in the HCWs. Upon multivariable analysis, liver transplant, ≥3 years from SOT, mRNA-1273, azathioprine, and longer time from t0 were associated with a positive AbR at t2. Older age, other comorbidities, mycophenolate, steroids, and impaired graft function were associated with lower AbR probability. Our results may be useful to optimize strategies of immune monitoring after COVID-19 vaccination and indications regarding timing for booster dosages calibrated on SOT patients’ characteristics.
Keywords: COVID-19, mRNA vaccines, serology, antibody response, solid organ transplantation
1. Introduction
COVID-19 (coronavirus disease 2019) is caused by the severe acute respiration syndrome coronavirus 2 (SARS-CoV-2). Due to the rapid spread and high morbidity and mortality burden of COVID-19, major efforts were undertaken regarding the development of vaccines using preexisting or novel technologies [1,2]. Overall, the mRNA vaccines showed higher rates of protection than other vaccine types against severe disease and mortality, as well as against new variants of concern, and possessed an optimal safety profile [2,3]. This type of vaccine contains a nucleoside-modified mRNA that encodes the SARS-CoV-2 spike glycoprotein, eliciting both B- and T-cell responses and inducing a prolonged antibody production [2]. Due to their efficacy and safety profiles, mRNA vaccines have been considered the first choice for protecting immunocompromised patients, such as hematological patients and solid organ transplant recipients, by some health systems (https://www.aifa.gov.it/ (accessed on 17 April 2022). In addition, mRNA vaccines were approved and introduced before other vaccine types; thus, the majority of the early data on COVID-19 vaccination in prioritized categories were based on the use of mRNA vaccines.
Indeed, to date, several reports have underlined low rates of antibody response to mRNA COVID-19 vaccines in solid organ transplant (SOT) recipients [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The largest report is that from Boyarsky et al., including 658 recipients of different types of SOT (322 kidney, 129 liver, 97 heart, 71 lung, 22 multiorgan, and 5 pancreas) recruited across several US hospitals [5]. The antibody response after the first and second dose of an mRNA COVID-19 vaccine was assessed at the second dose and within 21 days thereafter, showing a response rate of 15% and 54%, respectively [5]. The major flaws of previous studies were a small sample size, a lack of a control group, and a limited follow-up period, hampering the analysis of the antibody response. The only study assessing the kinetics of the antibodies is a sub-study of 305 SOT recipients from the Boyarsky cohort, where the patients were sampled at three timepoints (before the second dose, 1 month, and 3 months after the second dose of mRNA vaccine). The study showed that the antibody response was largely stable after 3 months following vaccination [26]. The understanding of the kinetics of the antibody response over time in SOT recipients can play a pivotal role both for public health officials when developing recommendations for vaccination needs and booster schedules and for physicians in terms of optimizing monitoring studies and designing tailored preventive strategies. The aim of this study was to analyze, in a large multicenter cohort of SOT recipients, the kinetics of the serological response over time compared with a cohort of healthcare workers (HCWs) and drivers of the immune response to two doses of mRNA COVID-19 vaccines.
2. Materials and Methods
The study is part (Workpackage 4) of the Horizon2020 ORCHESTRA project (https://orchestra-cohort.eu/ (accessed on 17 April 2022), which aims to create a new pan-European cohort to rapidly advance the knowledge on the COVID-19 infection. The project currently includes multiple cohorts, e.g., individuals at risk of infection, COVID-19 patients, HCWs, and fragile populations, such as SOT recipients, reaching a current total of more than 1,300,000 subjects at the time of writing of this manuscript.
The prospective observational multicenter cohort study of SOT recipients includes all consecutive adult (≥18 years) SOT patients who received two doses of mRNA COVID-19 vaccine, according to national health system guidance documents, between January and May 2021, and it is running at six hospitals, five from Italy (Bologna, Verona, Padova, Vicenza, and Treviso) and one from Spain (Seville). For the purpose of this analysis, patients with clinical and/or immunological evidence of prior COVID-19 were excluded. As control group, a cohort of 5045 HCWs from the Bologna ORCHESTRA cohort (Workpackage 5) vaccinated with two doses of mRNA COVID-19 vaccine in the same study period and without history of SARS-CoV-2 infection were analyzed. Primary endpoints were the probability of positive AbR at 3 ± 1 month of first vaccine dosage in SOT recipients and the kinetics of AbR in SOT recipients compared with HCWs. A positive AbR was defined as an anti-rapid binding domain (RBD) titer ≥5 U/mL or ≥45 BAU/mL for the Elecsys and MSD assays, respectively (see following serology section for details). Secondary endpoints included the clinical and epidemiological drivers of positive AbR in SOT recipients. According to the study protocol, all SOT recipients had serological response assessed at the following timepoints: the day of first vaccine dose administration (t0); the day of second dose administration (t1, 21 or 28 days after t0 for BNT162b2 or mRNA-1273, respectively); and 3 ± 1 month after t0 (t2) (see Supplemental Figure S1).
Data were collected at t0 and included: age, sex, comorbidities other than the cause of transplant according to Charlson index criteria, type and date of transplant, current immunosuppressive regimen, receipt of induction regimen in the last 6 months, and graft function defined as good, impaired, or failure according to the judgement of attending physicians. Occurrence of SARS-CoV-2 infection and clinical course was collected at each timepoint. Data collected for the HCWs at the same timepoint included age, sex, and AbR. Study variables were registered using a standardized electronic case report form (eCRF) managed by a centralized REDCap capture tool [27]. Data sources were clinical charts and hospital electronic records. Detection of AbR was performed at Bologna University, Italy (for the Bologna cohort of SOT recipients and HCWs), and at Antwerp University, Belgium (for all the other cohorts) with Elecsys® Anti-SARS-CoV-2 ECLIA assay (Roche Diagnostics AG, Rotkreuz, Switzerland) and V-PLEX SARS-CoV-2 Panel 6 Kit (IgG) from Meso Scale Discovery (MSD, Rockville, MD, USA), respectively. The Elecsys® Anti-SARS-CoV-2 ECLIA assay (Roche Diagnostics AG, Rotkreuz, Switzerland) was performed on the Cobas e 801 analyzer (Roche Diagnostics). The cut-off value for positive reactivity anti-N was equal to 1.0 COI (cut-off index), and anti-S (RBD) was 0.8 U/mL, according to the manufacturer’s instructions. To establish more accurate criteria for interpretation of serological results, the assay was validated in two well-defined groups of serum samples obtained from 50 HCWs before and one month after receiving the second dose of vaccine. Based on the results from the 50 true-positive and the 50 true-negative SARS-CoV-2 samples, antibody responses were stratified according to Anti-N (negative: 1.0 COI; inconclusive: ≥1 to <5 COI; positive: ≥5 COI) and Anti-S (negative: 0.8 U/mL; inconclusive: ≥0.8 to <5 U/mL; positive: ≥5 U/mL). The V-PLEX SARS-CoV-2 Panel was used according to the manufacturer’s instructions. IgG titers to the following antigens were measured: SARS-CoV-2 N, SARS-CoV-2 S1 RBD, SARS-CoV-2 Spike, SARS-CoV-2 Spike (D614G), SARS-CoV-2 Spike (B.1.1.7), SARS-CoV-2 Spike (B.1.351), SARS-CoV-2 Spike (P.1). Quantitative IgG results were measured in antibody units (AU)/mL, converted to WHO binding antibody units (BAU)/mL using a conversion factor provided by MSD. The detection range is described in Supplemental Table S1. The overall antibody responses were stratified into non-reactive, inconclusive, positive-low, positive-mild, and positive-high according to WHO criteria (see Supplemental Table S2).
3. Results
By November 2021, 1452 SOT recipients were included in the ORCHESTRA SOT cohort. From this number, 412 were excluded from this analysis for the following reasons: the results of the AbR at 3 ± 1 month were not available at the time of analysis due to logistical issues (n = 304), SARS-CoV-2 infection before vaccination (n = 76) or between dosages (n = 8), and incomplete vaccination schedule (n = 23). The eight breakthrough infections occurred in seven kidney and one liver transplant recipients within a mean of 16.62 days (range 8–33) from the first dose administration of BNT162b2 and mRNA-1273 vaccine in seven and one SOT recipient, respectively.
Therefore, a total of 1062 patients were included in the analysis. The majority of the enrolled patients were males (704, 66.3%), with a mean age (±SD) of 58.28 (±13.10) years. Concomitant comorbidities (other than the cause of SOT) were present in 715 (67.3%) patients. As for the type of SOT, most patients had kidney (n = 677, 63.7%), followed by liver (n = 182, 17.4%), heart (n = 111, 16.7%), and lung (n = 26, 2.5%) transplantation. In the majority of the patients (n = 836, 78.7%), more than 3 years had elapsed from SOT to administration of vaccination. Accordingly, only one patient had received an induction regimen in the last 6 months prior to vaccination. The most common drugs used for maintenance immunosuppressive regimen were tacrolimus (n = 763, 72%), steroids (n = 709, 66.7%), and mycophenolate mofetil (n = 626, 59%). Overall, 222 (20.9%) patients were reported as having an impaired function of the graft or graft failure. BNT162b2 andmRNA-1273 vaccines were administered to 928 (87.4%) and 134 (12.6%) patients, respectively. The cohort details are described in Table 1. The number of SOT recipients enrolled at each center and the distribution of the types of graft per center are shown in Supplemental Table S3.
Table 1.
General characteristics of study population.
| Total n = 1062 (%) |
Positive Antibody Response at 3 ± 1 Month N = 556 (%) |
Negative Antibody Response at 3 ± 1 Month N = 506 (%) |
p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (mean ± SD) (years) | 58.28 ± 13.10 | 56.47 ± 13.61 | 60.26 ± 12.23 | <0.001 |
| Age group | <0.001 | |||
| <39 y | 93 (8.76%) | 63 (67.74%) | 30 (32.26%) | |
| 40–49 y | 156 (14.69%) | 93 (59.62%) | 63 (40.38%) | |
| 50–59 y | 268 (25.24%) | 146 (54.48%) | 122 (45.52%) | |
| 60–69 y | 309 (29.10%) | 148 (47.90%) | 161 (52.10%) | |
| ≥70 y | 236 (22.22%) | 106 (44.92%) | 130 (55.08%) | |
| Sex | 0.967 | |||
| Male | 704 (66.29%) | 368 (52.27%) | 336 (47.73%) | |
| Female | 353 (33.40%) | 185 (52.41%) | 168 (47.59%) | |
| Comorbidities | 0.005 | |||
| No | 347 (32.67%) | 203 (58.50%) | 144 (41.5%) | |
| Yes | 715 (67.33%) | 353 (49.37%) | 362 (50.63%) | |
| Type of graft | <0.001 | |||
| Kidney | 677 (63.75%) | 312 (46.09%) | 365 (53.91%) | |
| Heart | 177 (16.67%) | 86 (48.59%) | 91 (51.41%) | |
| Liver | 182 (17.14%) | 144 (79.12%) | 38 (20.88%) | |
| Lung | 26 (2.45%) | 14 (53.85%) | 12 (46.15%) | |
| Type of vaccine | <0.001 | |||
| BNT162b2 | 928 (87.38%) | 463 (49.89%) | 465 (50.11%) | |
| mRNA-1273 | 134 (12.62%) | 93 (69.40%) | 41 (30.60%) | |
| Time from transplant to vaccination | <0.001 | |||
| Less than 1 year | 58 (5.46%) | 21 (36.21%) | 37 (63.79%) | |
| 1 to 3 years | 166 (15.63%) | 68 (40.96%) | 98 (59.04%) | |
| More than 3 years | 836 (78.72%) | 465 (55.62%) | 371 (44.38%) | |
| Induction regimen in the last 6 months | 0.340 | |||
| No | 1061 (99.90%) | 555 (52.31%) | 506 (47.69%) | |
| Any | 1 (0.09%) | 1 (100%) | 0 (0.00%) | |
| Immunosuppressive drugs at the time of vaccination | ||||
| Calcineurin inhibitors | 1007 (94.82%) | 520 (51.64%) | 487 (48.36%) | 0.046 |
| Tacrolimus | 763 (71.85%) | 384 (50.33%) | 379 (49.67%) | 0.035 |
| Cyclosporine | 246 (23.16%) | 136 (55.28%) | 110 (44.72%) | 0.294 |
| Anti-metabolites | 663 (62.43%) | 284 (42.84%) | 379 (57.16%) | <0.001 |
| Mycophenolate mofetil | 626 (58.95%) | 252 (40.26%) | 374 (59.74%) | <0.001 |
| Azathioprine | 37 (3.48%) | 32 (86.49%) | 5 (13.51%) | <0.001 |
| mTOR | 144 (13.56%) | 90 (62.50%) | 54 (37.50%) | 0.009 |
| Everolimus | 128 (12.05%) | 78 (60.94%) | 50 (39.06%) | 0.038 |
| Sirolimus | 16 (1.51%) | 12 (75.00%) | 4 (25.00%) | 0.068 |
| Steroids | 709 (66.76%) | 312 (44.01%) | 397 (55.99%) | <0.001 |
| Impaired graft function | <0.001 | |||
| Good | 830 (78.15%) | 475 (57.23%) | 355 (42.77%) | |
| Impaired or Failure | 222 (20.90%) | 75 (33.78%) | 147 (66.22%) | |
| Time between first dose and assessment of antibody response | 0.038 | |||
| 40–70 d | 170 (16.01%) | 74 (43.53%) | 96 (56.47%) | |
| 70–100 d | 500 (47.08%) | 262 (52.40%) | 238 (47.60%) | |
| 100–130 d | 298 (28.06%) | 170 (57.05%) | 128 (42.95%) | |
| 130–160 d | 75 (7.06%) | 37 (49.33%) | 38 (50.67%) | |
| >160 d | 19 (1.79%) | 13 (68.42%) | 6 (31.58%) |
mTOR: mammalian target of rapamycin; SD: standard deviation.
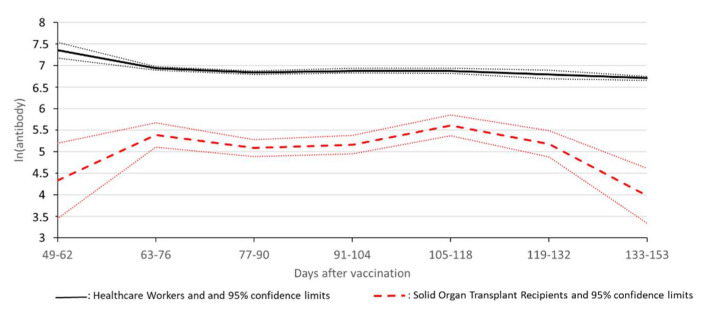
All the SOT recipients tested at t0 had a negative AbR (n = 622). The rate of positive AbR was 9.8% (62/631) at t1 and 52.3% (556/1062) at t2. The mean time from the first vaccination to t2 was 92 ± 35 days, with the majority of the patients being assessed between 70 and 100 (n = 500, 47.08%) and 100 and 130 (n = 298, 28.06%) days after the first dose (see Table 1). The analysis of the weekly trend of odds for positive AbR showed a steady increase in the probability of having a positive response from day 50 to day 110 after the first dose of vaccination. The ORCHESTRA HCWs cohort included 5045 subjects (68.9% women, mean age 43.1 years). The positions of the healthcare workers enrolled are summarized in Supplemental Table S4. A serological response was detected in 99.5% of the subjects at t2. Breakthrough infections were found in 32 (0.63%) HCWs between 40 and 150 days after the 1st dose of vaccine and in 73 (1.45%) HCWs after 14 days since full vaccination. In the multivariable logistic regression analysis, the adjusted OR of the serological response for HCWs versus SOT recipients was 120 (95% CI 73.1–199). The adjusted mean of ln (AbR) was 6.9 (±0.01) in the HCWs and 5.2 (±0.05) in SOT recipients (p < 0.001). Figure 1 shows the mean ln (AbR) in the two populations for the periods between 49 and 153 days (the overlap period of the two series of results) after vaccination in individuals with positive AbR. The ratio of ln (AbR) between HCWs and SOT recipients ranged between 1.2 and 1.7, i.e., between 3.3 and 5.5 on an arithmetic scale; the SOT recipients showed a significant increase up to 76 days (p < 0.001), then a non-significant decrease in ln (AbR) after 118 days (p = 0.1); conversely, the HCWs experienced a decrease in ln (AbR) up to 76 days (p = 0.02) and a less pronounced decrease between 76 and 118 days (p = 0.04).
Figure 1.
Mean ln (RBD) and 95% confidence limits in HCWs (continuous line) and SOT recipients (broken line) between 49 and 153 days after vaccination, adjusted for sex and age. The mean ln (AbR) in the two populations for the periods between 49 and 153 days after vaccination in individuals with positive AbR. The ratio of ln (AbR) between HCWs and SOT recipients ranged between 1.2 and 1.7, i.e., between 3.3 and 5.5 on arithmetic scale; SOT recipients showed a significant increase up to 76 days (p < 0.001), then a non-significant decrease in ln (AbR) after 118 days (p = 0.1); conversely, HCWs experienced a strong decrease in ln (AbR) up to 76 days (p = 0.02), and a less pronounced decrease between 76 and 118 days (p = 0.04).
To predict the drivers of AbR in SOT recipients, univariable and multivariable analyses were performed. Univariable analysis found significant differences between the patients with positive and negative AbR according to age, presence of other comorbidities, type of graft, time from SOT, immunosuppressive drugs (tacrolimus, mycophenolate mofetil, azathioprine, everolimus, and steroids), impaired graft function or graft failure, and the type of mRNA COVID-19 vaccine (see Table 1). Upon multivariable analysis, liver transplant (vs. other types of SOT; OR 2.71, 95%CI 1.55 4.72, p < 0.001), ≥3 years from SOT to vaccination (OR 4.92, 95%CI 2.56–9.45, p < 0.001), mRNA-1273 vaccine (3.57, 95%CI 2.25 5.67, p < 0.001), use of azathioprine (OR 3.43, 95%CI 1.20–9.82, p = 0.02), and longer time from vaccination to serological assessment (OR 1.30, 95%CI 1.10–1.53, p < 0.001) were associated with a positive AbR. Meanwhile, older age (OR 0.68, 95%CI 0.60–0.77, p <0.001), presence of other comorbidities (OR 0.60, 95%CI 0.43 0.83,p = 0.002), use of mycophenolate mofetil (OR 0.29, 95%CI 0.20 0.43, p < 0.001), steroids (OR 0.44, 95%CI 0.30–0.65, p < 0.001), and impaired graft function or graft failure at vaccination (OR 0.38, 95%CI 0.26–0.55, p < 0.001) were associated with a lower probability of positive antibody response at t2 (see Table 2).
Table 2.
Multivariable analysis of predictors of antibody response at 3 ± 1 months after first dose administration of mRNA COVID-19 vaccine in SOT recipients.
| Variable | OR (95% CI) | p-Value (α = 0.05) |
|---|---|---|
| Sex | - | - |
| Male | 1 (ref) | - |
| Female | 0.91 (0.67 1.24) | 0.568 |
| Age | - | - |
| Categorical increase (<39 y; 40–49 y; 50–59 y; 60–69 y; ≥70 y) | 0.67 (0.60 0.76) | <0.001 |
| Type of graft | - | - |
| Kidney | 1 (ref) | |
| Heart | 0.64 (0.39 1.07) | 0.090 |
| Liver | 2.71 (1.55 4.72) | <0.001 |
| Lung | 1.16 (0.46 2.95) | 0.750 |
| Time from transplant to vaccination | - | - |
| Less than 1 year | 1 (ref) | |
| 1 to 3 years | 1.79 (0.87 3.67) | 0.111 |
| More than 3 years | 4.92 (2.56 9.45) | 0.000 |
| Time from vaccination onset to serological assessment | ||
| Categorical increase (40–70 d; 70–100 d; 100–130 d; 130–160 d; >160 d) | 1.30 (1.10 1.53) | <0.001 |
| Comorbidities | ||
| No | 1 (ref) | |
| Yes | 0.60 (0.43 0.83) | 0.002 |
| Type of vaccine | ||
| BNT162b2 | 1 (ref) | |
| mRNA-1273 | 3.57 (2.25 5.67) | <0.001 |
| Immunosuppressive drugs at the time of vaccination | ||
| Cyclosporine | 0.71 (0.30 1.67) | 0.429 |
| Tacrolimus | 0.52 (0.23 1.16) | 0.111 |
| Azathioprine | 3.43 (1.20 9.82) | 0.022 |
| Mycophenolates | 0.29 (0.20 0.43) | <0.001 |
| Sirolimus | 0.70 (0.18 2.66) | 0.598 |
| Everolimus | 0.72 (0.43 1.20) | 0.212 |
| Steroids | 0.44 (0.30 0.65) | 0.000 |
| Impaired graft function | ||
| Good | 1 (ref) | |
| Impaired, Failure, and others | 0.38 (0.26 0.55) | <0.001 |
OR: odd ratio.
Statistical Analysis
For descriptive analysis, categorical variables were presented as absolute numbers, and their relative frequencies and continuous variables were presented as mean ± standard deviation (SD) if normally distributed, or as median and interquartile range (IQR) if non-normally distributed. Quantitative anti-RBD levels for positive cases were log-transformed to account for the skewness of the distribution and then normalized by dividing them by the center-specific standard error to take into account the different methods used across centers. The comparison between SOT recipients and HCWs was performed using multivariable logistic regression with AbR as dependent variable and cohort (HCWs vs. SOT recipients) as primary endpoint, after adjustment for sex, age, and time since vaccination. Sex- and age-adjusted means of anti-RBD levels were calculated among subjects with positive immune response in the two groups, based on ANOVA. Time trends in log-transformed anti-RBD levels were assessed with linear regression after application of linear splines with two knots at weeks 10 and 16. For the secondary endpoint, multivariable logistic regression models were fitted to estimate odds ratios (ORs) and 95% confidence intervals (CI) of AbR as a dichotomous variable. The main exposure variable was time between administration of first vaccine dose and the AbR assessment; models also included sex, age (categorical), comorbidities, type of graft, type of vaccine, time between transplant and vaccination (categorical), induction regimen in the last 6 months, immunosuppressive drugs at the time of vaccination (calcineurin inhibitors, anti-metabolites, mTOR inhibitors, steroids), graft function (good, impaired, failure), and time between first dose and assessment of AbR (categorical) as potential confounders. Analyses were completed using the STATA package, Version 16.1 (STATA/SE 16.0 for Windows. StataCorp Llc., College Station, TX, USA) using the commands logistic, glm, anova, and mksplines.
The study, according to the Italian legislation for SARS-CoV-2 studies, was approved by the Agenzia Italiana del Farmaco (AIFA) and the Ethics Committee of Istituto Nazionale per le Malattie Infettive (INMI) Lazzaro Spallanzani (document n. 359 of Study’s Registry 2020/2021). Informed consent was obtained from all the enrolled patients. The study was conducted in accordance with the Declaration of Helsinki.
4. Discussion
The ORCHESTRA SOT cohort is the largest cohort of SOT recipients assessed for a serological response to mRNA SARS-CoV-2 vaccines reported to date and the first providing data regarding the kinetics of the antibody response compared to that of HCWs. The assessment of the immune response at month 3 ± 1 after two doses of mRNA COVID-19 vaccine found that 52.3% among 1062 naïve SOT recipients had a positive antibodies response versus 99.5% detected in HCWs. A steady increase in the probability of having a positive response from day 50 to day 110 after first dose administration was observed. Furthermore, as expected among the serological responders, the mean levels of antibodies were significantly higher in the HCWs than in the SOT recipients, and the kinetics was different. Our results suggest that early assessment of AbR in SOT recipients could miss the subsequent increase in AbR, and, on the other hand, that SOT recipients maintain good antibody levels during a limited period of time (up to day 118) after a standard vaccination schedule (two doses of mRNA COVID-19 vaccine).
The rate of seroconversion was heterogeneous by type of SOT, as already observed [28], and varied from 46% in kidney- to 79% in liver transplant recipients. It is noteworthy that factors such as age, comorbidities, type of graft, time from SOT, graft function, and type of immunosuppressive regimen were associated with the AbR within 3 months. Therefore, prevention strategies, other than vaccination, could be considered in this population, such as reduction in immunosuppression [29] or the use of long-acting monoclonal antibodies against the spike protein of SARS-CoV-2 [30].
A higher intensity of immunosuppressive regimen, in particular the use of anti-metabolites drugs, has been associated with a lower antibody response [28]. For this reason, some authors have proposed the temporary suspension of mycophenolate during vaccine administration, although this practice has been discouraged by international transplant societies due to safety concerns and a lack of data regarding its efficacy (https://ishlt.org/ishlt/media/documents/ISHLT-AST_SARS-CoV-2-Vaccination_5-11-21.pdf (accessed on 17 April 2022)). A clinical trial is currently ongoing assessing the seroconversion rate after a third dose among patients receiving an mRNA vaccination with or without temporary suspension of mycophenolate [31]. We confirmed the negative role of mycophenolate, along with steroids, on the probability to achieve a seroconversion rate at 3 ± 1 month from vaccination, while patients on azathioprine were more likely to show a positive AbR. We deem that this result could be relevant for future strategies to improve the efficacy of vaccination in SOT recipients, considering azathioprine as a temporary alternative to mycophenolate, in order to improve the immunological response and minimize the rejection risk at the same time.
Data on the kinetics of the antibody response to SARS-CoV2 infection or vaccine, as well as to other infectious agents, such as influenza, are limited [32]. Most studies focused on the duration of the antibody response, with the waning of antibodies being the main concern of physicians and public health officials during the pandemic of SARS-CoV-2. However, the knowledge of the time needed to mount a protective AbR in SOT recipients could be very important to provide correct advice to patients and plan adequate monitoring activities. Our study shows a steady increase in the probability of having a positive antibody response between day 50 and 110 after the first vaccination, suggesting that this could be the best time interval to assess the antibody response to vaccination in SOT recipients. Similarly, Bovarsky et al. observed an increase from 13.5% to 67% at month 3 after the second dose [26], confirming that a delayed sampling could be associated with a higher probability of finding a positive antibody response to a COVID-19 vaccine in SOT recipients. The use of immunosuppressive medications that inhibit T-cell and B-cell responses to prevent transplant rejection could have a role in this delayed response [33]. Very interestingly, our data also showed a different kinetics of the antibody response in SOT recipients compared to HCWs, which merits further investigation. Our data confirmed a lower level of antibodies among SOT recipients than HCW responders, which was maintained during a limited period of time, supporting the current strategy of booster dosage in this setting. In this regard, the preliminary data were controversial, showing a moderate rate of seroconversion [34,35]. According to our and prior data, low-level responders could be those who most benefit from booster dosages [33].
A limitation of our study is the lack of cellular immune response analysis. Indeed, some authors have shown, upon analyzing cellular immunity as well, that the rate of overall immunological response seems to be higher than that reported considering only the serological response, mainly among patients receiving hybrid (vector/mRNA) COVID-19 vaccination [8,14,36]. Furthermore, the association between the rates and patterns of the immunological response and clinical effectiveness of COVID-19 vaccination in SOT recipients is still an open issue that should be investigated in future studies. We did not collect the dosage of immunosuppressive drugs at vaccination, which could impact the AbR to vaccines in SOT recipients. Finally, due to the high volume of patients and in order to avoid multiple visits to SOT recipients with already scheduled visits near the predefined timepoint, we left a wider temporal range to local sites for performing the 3M visits; thus, 30% of the visits fell out of the predefined interval. However, we deem that the wide temporal range could have allowed us to capture differences in the kinetic of antibody response between SOT recipients and HCWs. The strengths of our study, in addition to the large study population, include the extensive amount of clinical data available for analysis, enabling a detailed investigation of the characteristics of the SOT recipients associated with an immune response to mRNA vaccines, the fact that a 3-month follow-up was available for all the subjects, and the use of a comparison population of HCWs.
5. Conclusions
In conclusion, we showed that a positive antibodies response at month 3 ± 1 after two doses of mRNA COVID-19 vaccine was found in 52.3% of the SOT recipients versus 99.5% in HCWs. However, the timing of the sampling was significantly associated with the probability of finding a positive antibody response to the COVID-19 vaccine in the SOT recipients, suggesting a slow increase in the antibody levels. Indeed, when compared with the HCWs, the kinetics of the antibody response in the SOT recipients was different and was maintained at good levels during a limited period of time. These results could be helpful to optimize strategies of immune monitoring after COVID-19 vaccination and to provide indications regarding the timing for booster dosages in the setting of SOT recipients.
Acknowledgments
We wish to thank ORCHESTRA study group WP-4: Federica Arbizzani: Francesca Fanì, Maria Eugenia Giacomini, Oana Vatamanu, Beatrice Tazza, Clara Solera Horna, Caterina Campoli, Michele Bartoletti, Linda Bussini, Zeno Pasquini, Giacomo Fornaro, Fabio Trapani, Luciano Attard, Milo Gatti, Piergiorgio Cojutti, Antonio Gramegna, Elena Rosselli Del Turco, Sara Tedeschi, Kristian Scolz, Gaetano La Manna, Valeria Grandinetti, Marcello Demetri, Simona Barbuto, Chiara Abenavoli, Giovanni Vitale, Laura Turco, Matteo Ravaioli, Matteo Cescon, Valentina Bertuzzo, Angela Lombardi, Alessandra Trombi, Marco Masetti, Paola Prestinenzi, Mario Sabatino, Laura Giovannini, Aloisio Alessio, Antonio Russo, Maria Francesca Scuppa, Laura Borgese, Giampiero Dolci, Gianmaria Paganelli, Liliana Gabrielli, Matteo Pavoni. IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy. Simona Granata, Alberto Verlato, and Rossella Elia. Renal Unit, Department of Medicine, University Hospital of Verona, Verona, Italy. Alex Borin. Liver Transplant Unit, Department of Surgery and Dentistry, University and Hospital Trust of Verona, Verona Italy. Livio San Biagio, Alessandra Francica, Ilaria Tropea. Division of Cardiac Surgery, University of Verona, Verona, Italy. Elisa Razzaboni, Maria Elena De Rui, Anna Gorska, Maria Mongardi, Massimo Mirandola, Mariana Nunes Pinho Guedes, Gaia Maccarone, Division of Infectious Diseases, Department of Diagnostics and Public Health, University of Verona, Verona, Italy. Francesca Russo, Michele Mongillo, Direzione Prevenzione, Sicurezza Alimentare, Veterinaria, Regione Veneto, Italy. Natalia Maldonado, Paula Olivares, David Gutiérrez-Campos, Ana BelénMartín-Gutiérrez, Virginia Palomo, AlmudenaSerna, Eduardo Reyna Villasmil, Marta Fernández-Regaña. Infectious Diseases and Microbiology Unit, Hospital Universitario Virgen Macarena, and Department of Medicine, University of Sevilla / Biomedicines Institute of Sevilla, Sevilla, Spain. Ana Belén Hidalgo, Ioana Hrom, Myriam Adorna, Rubén Murillo, Mª José Ríos, and Mª Isabel García-Sánchez. Biobank Nodo Hospital Virgen Macarena (Biobanco del Sistema Sanitario Público de Andalucía) integrated in the Spanish National biobanks Network (PT20/00069). Paolo Angeli, Alessandra Brocca, Marco Cola, Luca Beggiato, Daniel Salinas. Unit of Internal Medicine and Hepatology (UIMH), Department of Medicine—DIMED, University of Padua, Padua, Italy. Andrea Carraro, JosèIgeno San Miguel, Emanuele Vianello, Susanna Negrisolo. Pediatric Nephrology, Dialysis and Transplant Unit, Department of Women’s and Children’s Health, Padua University Hospital, Padua, Italy. Enrico Gringeri, Patrizia Boccagni, Francesco Enrico D’amico, Riccardo Boetto, Lara Borsetto. Department of Surgery, Oncology and Gastroenterology, Hepatobiliary Surgery and Liver Transplantation Unit, Padua University Hospital, Padua, Italy. Erica Nuzzolese, Marianna Di Bello, Caterina Di Bella. Kidney and Pancreas Transplantation Unit, Department of Surgical, Oncological and Gastroenterological Sciences, University of Padua, Padua, Italy. Paola Gaio. Unit of Pediatric Gastroenterology, Digestive Endoscopy, Hepatology and Care of the Child with Liver Transplantation, Department of Women’s and Children’s Health, University Hospital of Padua, Padua, Italy. Luigi Garufi, Chiara Tessari, SaimaImran. Cardiac Surgery Unit, Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padua, Padua, Italy. Debora Bizzarro, Francesco Paolo Russo. Unit of Gastroenterology and Multivisceral Transplant, Department of Surgery, Oncology and Gastroenterology, University Hospital of Padua, Padua, Italy. Lorena Brunello, Marta Tenan, Monica Rizzolo. Nephrology Unit, Treviso Hospital, Treviso, Italy. Carlotta Caprara, Grazia Maria Virzì, Matteo Marcello. Department of Nephrology, Dialysis and Transplantation, San Bortolo Hospital, Vicenza, Italy. Angelina Konnova, Akshita Gupta, Matilda Berkell. Molecular Pathology Group, Faculty of Medicine, Laboratory of Cell Biology & Histology University of Antwerp, Antwerp, Belgium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10051021/s1: Supplemental Table S1: Lower limits of quantification (LLQ) and upper limits of quantification (ULQ) for IgG measurements; Supplemental Table S2: Stratification of antibody response according to WHO criteria; Supplemental Table S3: Number of SOT recipients enrolled at each center and the distribution of the types of graft per center; Supplemental Table S4: Positions of Healthcare Workers enrolled; Supplemental Figure S1: Study flow chart.
Author Contributions
Conceptualization, J.R.-B. and E.T.; Data curation, M.G., E.R. and P.B. (Paolo Boffetta); Formal analysis, M.G., S.K.-S., M.A. and P.B. (Paolo Boffetta); Funding acquisition, M.G. and E.T.; Investigation, M.G., E.R., R.P., M.R., N.C., C.G., Z.R.P.-B., G.C. (Giulia Caponcello), M.C.M., M.T., M.B., G.C. (Giorgia Comai), L.P., E.S., G.F., U.C., G.G., M.C., S.P., E.B., P.B. (Patrizia Burra), M.L., L.F., G.Z., F.O., A.C., F.G. and M.N.; Methodology, M.G. and P.B. (Paolo Boffetta); Project administration, M.G.; Supervision, P.B. (Paolo Boffetta), J.R.-B., T.L. and P.V.; Writing—original draft, M.G.; Writing—review & editing, E.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study, according to the Italian legislation for SARS-CoV-2 studies, was approved by the Agenzia Italiana del Farmaco (AIFA) and the Ethics Committee of Istituto Nazionale per le Malattie Infettive (INMI) Lazzaro Spallanzani (document n. 359 of Study’s Registry 2020/2021). The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
The ORCHESTRA project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 101016167.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Del Rio C., Omer S.B., Malani P.N. Winter of Omicron-The Evolving COVID-19 Pandemic. JAMA. 2022;327:319–320. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]
- 2.Patel R., Kaki M., Potluri V.S., Kahar P., Khanna D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022;18:2002083. doi: 10.1080/21645515.2021.2002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenforde M.W., Self W.H., Adams K., Gaglani M., Ginde A.A., McNeal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D., et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., Martzloff J., Perrin P., Moulin B., Fafi-Kremer S., et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky B.J., Chiang T.P.-Y., Ou M.T., Werbel W.A., Massie A.B., Segev D.L., Garonzik-Wang J.M. Antibody Response to the Janssen COVID-19 Vaccine in Solid Organ Transplant Recipients. Transplantation. 2021;105:e82–e83. doi: 10.1097/TP.0000000000003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavarot N., Ouedrani A., Marion O., Leruez-Ville M., Vilain E., Baaziz M., Del Bello A., Burger C., Sberro-Soussan R., Martinez F., et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated With Belatacept. Transplantation. 2021;105:e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 7.Cholankeril G., Al-Hillan A., Tarlow B., Abrams D., Jacobs J.S., Flores N.P., Rana A., Kanwal F., Goss J.A. Clinical Factors Associated With Lack of Serological Response to SARS-CoV-2 Messenger RNA Vaccine in Liver Transplantation Recipients. Liver Transplant. 2022;28:123–126. doi: 10.1002/lt.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucchiari D., Egri N., Bodro M., Herrera S., Del Risco-Zevallos J., Casals-Urquiza J., Cofan F., Moreno A., Rovira J., Banon-Maneus E., et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danthu C., Hantz S., Dahlem A., Duval M., Ba B., Guibbert M., El Ouafi Z., Ponsard S., Berrahal I., Achard J.M., et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. JASN. 2021;32:2153–2158. doi: 10.1681/ASN.2021040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dębska-Ślizień A., Ślizień Z., Muchlado M., Kubanek A., Piotrowska M., Dąbrowska M., Tarasewicz A., Chamienia A., Biedunkiewicz B., Renke M., et al. Predictors of Humoral Response to mRNA COVID19 Vaccines in Kidney Transplant Recipients: A Longitudinal Study-The COViNEPH Project. Vaccines. 2021;9:1165. doi: 10.3390/vaccines9101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grupper A., Rabinowich L., Schwartz D., Schwartz I.F., Ben-Yehoyada M., Shashar M., Katchman E., Halperin T., Turner D., Goykhman Y., et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett A.M., Greenberg R.S., Boyarsky B.J., Shah P.D., Ou M.T., Teles A.T., Krach M.R., López J.I., Werbel W.A., Avery R.K., et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J. Heart Lung Transplant. 2021;40:1579–1588. doi: 10.1016/j.healun.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlin J., Svorcova M., Dvorackova E., Lastovicka J., Lischke R., Kalina T., Hubacek P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transplant. 2021;40:754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera S., Colmenero J., Pascal M., Escobedo M., Castel M.A., Sole-González E., Palou E., Egri N., Ruiz P., Mosquera M., et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transplant. 2021;21:3971–3979. doi: 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itzhaki Ben Zadok O., Shaul A.A., Ben-Avraham B., Yaari V., Ben Zvi H., Shostak Y., Pertzov B., Eliakim-Raz N., Abed G., Abuhazira M., et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—A prospective cohort study. Eur. J. Heart Fail. 2021;23:1555–1559. doi: 10.1002/ejhf.2199. [DOI] [PubMed] [Google Scholar]
- 16.Korth J., Jahn M., Dorsch O., Anastasiou O.E., Sorge-Hädicke B., Eisenberger U., Gäckler A., Dittmer U., Witzke O., Wilde B., et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13:756. doi: 10.3390/v13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinaki S., Adamopoulos S., Degiannis D., Roussos S., Pavlopoulou I.D., Hatzakis A., Boletis I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021;21:2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen R.M., Bang L.L., Tornby D.S., Kierkegaard H., Nilsson A.C., Johansen I.S., Bistrup C., Jensen T.G., Justesen U.S., Andersen T.E. The SARS-CoV-2-neutralizing capacity of kidney transplant recipients 4 weeks after receiving a second dose of the BNT162b2 vaccine. Kidney Int. 2021;100:1129–1131. doi: 10.1016/j.kint.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., Katchman E., Levi S., Houri I., Lubezky N., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashidi-Alavijeh J., Frey A., Passenberg M., Korth J., Zmudzinski J., Anastasiou O., Saner F., Jahn M., Lange C., Willuweit K. Humoral Response to SARS-CoV-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines. 2021;9:738. doi: 10.3390/vaccines9070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rincon-Arevalo H., Choi M., Stefanski A.L., Halleck F., Weber U., Szelinski F., Jahrsdörfer B., Schrezenmeier H., Ludwig C., Sattler A., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021;6:eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 22.Rozen-Zvi B., Yahav D., Agur T., Zingerman B., Ben-Zvi H., Atamna A., Tau N., Mashraki T., Nesher E., Rahamimov R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sattler A., Schrezenmeier E., Weber U.A., Potekhin A., Bachmann F., Straub-Hohenbleicher H., Budde K., Storz E., Proß V., Bergmann Y., et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021;131:e150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schramm R., Costard-Jäckle A., Rivinius R., Fischer B., Müller B., Boeken U., Haneya A., Provaznik Z., Knabbe C., Gummert J. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin. Res. Cardiol. 2021;110:1142–1149. doi: 10.1007/s00392-021-01880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shostak Y., Shafran N., Heching M., Rosengarten D., Shtraichman O., Shitenberg D., Amor S.M., Yahav D., Ben Zvi H., Pertzov B., et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir. Med. 2021;9:e52–e53. doi: 10.1016/S2213-2600(21)00184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyarsky B.J., Chiang T.P.Y., Teles A.T., Greenberg R.S., Krach M.R., Ou M.T., Massie A.B., Tobian A.A., Garonzik-Wang J.M., Segev D.L., et al. Antibody Kinetics and Durability in SARS-CoV-2 mRNA Vaccinated Solid Organ Transplant Recipients. Transplantation. 2021;105:e137–e138. doi: 10.1097/TP.0000000000003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannella M., Pierrotti L.C., Helantera I., Manuel O. SARS-CoV-2 vaccination in solid-organ transplant recipients: What the clinician needs to know. Transpl. Int. 2021;34:1776–1788. doi: 10.1111/tri.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung M., Babik J.M. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin. Infect. Dis. 2021;72:340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotton C.N. Belt and Suspenders: Vaccines and Tixagevimab/Cilgavimab for Prevention of COVID-19 in Immunocompromised Patients. Ann. Intern. Med. 2022 doi: 10.7326/M22-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahav D., Rozen-Zvi B., Mashraki T., Atamna A., Ben-Zvi H., Bar-Haim E., Rahamimov R. Immunosuppression reduction when administering a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant recipients without adequate humoral response following two vaccine doses: Protocol for a randomised controlled trial (BECAME study) BMJ Open. 2021;11:e055611. doi: 10.1136/bmjopen-2021-055611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirzel C., Ferreira V.H., L’Huillier A.G., Hoschler K., Cordero E., Limaye A.P., Englund J.A., Reid G., Humar A., Kumar D., et al. Humoral response to natural influenza infection in solid organ transplant recipients. Am. J. Transplant. 2019;19:2318–2328. doi: 10.1111/ajt.15296. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Zheng Q., Madhira V., Olex A.L., Anzalone A.J., Vinson A., Singh J.A., French E., Abraham A.G., Mathew J., et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern. Med. 2022;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparro C., Selzner N., Schiff J., McDonald M., Tomlinson G., et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werbel W.A., Boyarsky B.J., Ou M.T., Massie A.B., Tobian A.A., Garonzik-Wang J.M., Segev D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021;174:1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt T., Klemis V., Schub D., Schneitler S., Reichert M.C., Wilkens H., Sester U., Sester M., Mihm J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021;21:3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.