Abstract
Bacterial fruit blotch caused by Acidovorax citrulli is a serious disease of cucurbit crops. Here we report characterization of a mutant strain of A. citrulli M6 defective in lip1, a gene encoding a lipolytic enzyme. The M6-lip1- mutant was detected in a mutant library screen aimed at identifying M6 mutants with altered levels of twitching motility. In this screen M6-lip1- was the only mutant that showed significantly larger twitching motility haloes around colonies than wild-type M6. Sequence analyses indicated that lip1 encodes a member of the GDSL family of secreted lipolytic enzymes. In line with this finding, lipolytic assays showed that the supernatants of M6-lip1- had lower lipolytic activity as compared with those of wild-type M6 and a lip1-complemented strain. The mutant was also affected in swimming motility and had compromised virulence on melon seedlings and on Nicotiana benthamiana leaves relative to wild-type and complemented strains. Lip1 contains a predicted N-terminal signal sequence for type II secretion. Evidence from our study confirms Lip1 is indeed secreted in a type II secretion-dependent manner, and this is required for full virulence of A. citrulli. To the best of our knowledge this is the first study reporting contribution of lipolytic activity to virulence of a plant-pathogenic Acidovorax species.
Keywords: Acidovorax citrulli, bacterial fruit blotch, lipase, esterase, virulence, type IV pili, twitching motility, type II secretion
1. Introduction
The Gram-negative bacterium Acidovorax citrulli belongs to the Betaproteobacteria class and causes bacterial fruit blotch (BFB), a threatening disease of cucurbits worldwide, and mainly of melon and watermelon [1]. Acidovorax citrulli is a seed borne pathogen and seed transmission has been responsible for the fast spread of BFB to many parts of the world [2]. The bacterium infects all aerial parts of the plant and at all developmental stages, from the young seedling to the fruit. To date, there are no reliable sources of BFB resistance and the available disease management strategies have limited efficacy [2,3]. Based on genetic and biochemical features, A. citrulli can be divided into two main groups that also differ in terms of host preference: while group I strains were mainly isolated from melon and are moderately to highly aggressive to this plant, and to a wide range of cucurbit species, most group II strains were isolated from watermelon, are highly aggressive to this plant, and only mildly so to other cucurbits [4,5,6,7].
The successful establishment of a pathogenic bacterium in the host tissue requires the coordinated expression of virulence factors. Similarly to several Gram-negative pathogenic bacteria, A. citrulli relies on a functional type III secretion system and type III-secreted effector proteins for pathogenicity [8,9,10]. Type IV pili (T4P) also play an important role in A. citrulli virulence as well as in surface adhesion, biofilm formation and colonization [11,12]. T4P are hair-like appendages found on the poles of the cell surface. They mediate twitching motility, which is an effective flagellum-independent form of bacterial surface motility [13].
Acidovorax citrulli M6 was isolated from a BFB outbreak of melon in Israel in 2002 [6] and in the following years became a model group I strain for fundamental and applied investigation of BFB. In 2016, we reported the draft sequence of the M6 genome [14], and three years later we were able to fully assemble the genome of this strain using single molecule real time (SMRT; PacBio) sequencing [15]. We recently screened an EZ-Tn5 mutant library with ~10,000 mutants in the background of strain M6 [11] for mutants altered in twitching motility. This screen revealed fifty twitching defective mutants that were disrupted in twenty different genes. These included genes involved in T4P biogenesis, regulation and chemotaxis [16]. In agreement with previous findings supporting an important contribution of T4P to A. citrulli virulence [11], all mutants were significantly impaired in this trait [16].
In the aforementioned screen, we also identified a mutant that produced significantly larger twitching haloes around bacterial colonies as compared with wild-type M6. Sequence analysis of this mutant revealed that the transposon insertion disrupted a gene encoding a lipolytic enzyme. Lipolytic enzymes have been extensively studied due to the high interest for biotechnological applications [17,18,19]. However, only relatively few studies have dealt with their contribution to virulence of phytopathogenic bacteria. With that said, several lipases and esterases were shown to be important virulence factors in some plant-pathogenic bacteria [20,21,22,23,24,25]. The objective of this study was to assess the contribution of this lipolytic enzyme, which we named Lip1, to the virulence and other traits of A. citrulli.
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
Bacterial strains and plasmids used in this study are listed in Table 1. Acidovorax citrulli strains were routinely grown at 28 °C in nutrient broth (NB; Difco Laboratories, Detroit, MI, USA), NA (NB containing 15 g/L agar) or Lysogeny Broth (LB; Difco Laboratories). XVM2 minimal medium, which resembles to some extent the plant apoplast environment [26], was used for growth curve experiments and for biofilm formation and lipolytic assays (see below). Escherichia coli strains were cultured in LB at 37 °C. Antibiotics were added at the following concentrations: ampicillin (Ap), 100 μg/mL; kanamycin (Km), 50 μg/mL; and gentamicin (Gm), 10 or 30 μg/mL (for E. coli and A. citrulli, respectively).
Table 1.
Strains and plasmids used in this study.
| Strains/Plasmids | Characteristics * | Reference or Source |
|---|---|---|
| Acidovorax citrulli | ||
| M6 | Wild-type strain; ApR | [6] |
| M6-lip1- | M6 Tn5 mutant disrupted in gene APS58_1588 (lip1) by the insertion of EZ-Tn5 transposon; ApR, KmR | This study |
| M6-comp-lip1 | M6 lip1- mutant carrying pBBRlip1 (lip1 complemented strain); ApR, KmR, GmR | This study |
| M6-comp-ompW | M6 lip1- mutant carrying pBBRompW; ApR, KmR, GmR | This study |
| M6-comp-lip1-HA | M6 lip1- mutant carrying pBBRlip1-HA; ApR, KmR, GmR | This study |
| M6-comp-lip135-383-HA | M6 lip1- mutant carrying pBBRlip135-383-HA; ApR, KmR, GmR | This study |
| AAC00-1 | Wild-type strain; ApR | [4] |
| AAC00-1-lip1-HA | AAC00-1 carrying pBBRlip1-HA; ApR, GmR | This study |
| AAC00-1-T2SS- | AAC00-1 mutant impaired in type II secretion (double ΔgspG1/ΔgspG2 mutant); ApR | [27] |
| AAC00-1-T2SS--lip1-HA | AAC00-1-T2SS- carrying pBBRlip1-HA; ApR, GmR | This study |
| Escherichia coli | ||
| S17-1 λ pir | λ lysogenic S17-1 derivative producing π protein for replication of plasmids carrying oriR6K; recA pro hsdR RP4-2-Tc::Mu-Km::Tn7 λ– pir |
[28] |
| Plasmids | ||
| pUC-4K | pUC4 derivative (pMB1 ori), containing the KmR gene from Tn903 that was used to generate the EZ-Tn5 transposon for mutagenesis; ApR, KmR | [29] |
| pMOD-3<R6Kγori/MCS> | transposon construction vector; ApR | Epicentre, Madison, WI, USA |
| pBBR1-MCS-5 | broad host range vector; GmR | [30] |
| pBBRlip1 | pBBR1-MCS-5 containing the promotor region (250-bp) of the lip1-ompW operon followed by the lip1 open reading frame (ORF) from M6; GmR | This study |
| pBBRompW | pBBR1-MCS-5 containing the promotor region (250-bp) of the lip1-ompW operon followed by the ompW ORF from M6; GmR | This study |
| pBBRlip1-HA | pBBR1-MCS-5 containing the lip1 ORF fused to the HA epitope at its C-terminus, under the control of the lac constitutive promoter; GmR | This study |
| pBBRlip135-383-HA | pBBR1-MCS-5 containing the lip1 ORF without the N-terminal signal peptide (first 102 bp of the ORF) fused to the HA epitope at its C-terminus, under the control of the lac constitutive promoter; GmR | This study |
* KmR, ApR and GmR indicate kanamycin, ampicillin and gentamicin resistance, respectively.
2.2. Molecular Biology Techniques
Genomic DNA was extracted with the GeneElute Bacterial Genomic DNA Kit (Sigma-Aldrich, St. Louis, MO, USA). Plasmids were extracted using the BioNeer AccuPrep Plasmid Mini Extraction kit (Daejeon, Republic of Korea). For preparation of cDNA, RNA extractions from NB-grown bacteria were carried out using TRI Reagent (Sigma-Aldrich). Then, genomic DNA was eliminated using DNA-free DNase (Ambion, Austin, TX, USA). cDNA was generated using random primers with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Each sample contained 1 µg RNA in 20 µL of a reaction mix. All enzymes and kits were used according to the manufacturer’s instructions. The primers used in this study were purchased from Sigma (Rehovot, Israel) and are listed in Table S1.
2.3. Isolation of the A. citrulli M6-lip1- Mutant from a Transposon Mutant Library
The M6-lip1- mutant was screened out of a random transposon library that contains about 10,000 mutants, and was generated in the background of A. citrulli M6 using the Ez-Tn5 kit (Epicentre, Madison, WI, USA), as described [11,16]. Mutants altered in twitching motility were directly screened on NA/Km selection plates by the naked eye, and verified by colony microscopy observations using an Axio Scope light microscope (Carl Zeiss, Jena, Germany) equipped with a DXM1200F digital camera (Nikon, Tokyo, Japan). The M6-lip1- mutant was tested by Southern blot hybridization to verify insertion of the EZ-Tn5 cassette, using the ECL Direct Nucleic Acid Labeling and Detection System (Amersham Biosciences, Buckinghamshire, UK) according to the manufacturers’ instructions, and using a region of the EZ-Tn5 cassette as probe [11]. The insertion site of the EZ-Tn5 cassette was identified following sequencing of the mutant genome by Illumina MiSeq at the Center for Genomic Technologies of the Hebrew University of Jerusalem. Quality trimming and genome assembly were conducted as described [14].
2.4. Sequence Analyses and Homology Modeling
BlastN and BlastP analyses were conducted using the National Center for Biotechnology Information (NCBI) Blast server (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 10 December 2021). BlastP was also conducted against the UniProtKB/SwissProt database at the UniProt server (http://www.uniprot.org/blast/, accessed on 10 December 2021). Analysis of conserved domains was conducted using the Conserved Domains server (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 10 December 2021; [31]) with default parameters. A three dimensional (3D) homology modeling of Lip1 was generated using the I-TASSER server (https://zhanggroup.org/I-TASSER/, accessed on 15 April 2020) [32], using default parameters with no additional restraints. The model chosen for analysis was model1, which had a confidence score of −0.93. Visualization of protein structures and 3D figures were created using Discovery Studio 4.5 Visualizer (Dassault Systèmes Biovia, San Diego, CA, USA). Signal peptide prediction of Lip1 was done with Pred-Tat (http://www.compgen.org/tools/PRED-TAT/, accessed on 1 March 2022) [33] and Phobius (http://phobius.sbc.su.se/, accessed on 1 March 2022) [34].
2.5. Construction of Plasmids for Complementation and Assessment of Lip1 Secretion
Several complementation strains were created on the background of the A. citrulli M6-lip1- mutant. The first complementation strains were M6-comp-lip1 and M6-comp-ompW, carrying plasmid pBBR1-MCS-5 with the complete ORF of lip1 or ompW (plasmids pBBRlip1 and pBBRompW, respectively; Table 1). In both cases, the constructs included a 250-bp region upstream of the lip1 ORF, thus carrying the promoter region of the lip1-ompW operon (Figure 1A). These plasmids were generated using the restriction free (RF) cloning method (www.rf-cloning.org using primers that were designed based on the M6 genome annotation (GenBank accession CP029373.1) and are listed in Table S1. We first used the RF primers lipComp_F and lipComp_R to clone the promoter region and the lip1 ORF in pBBR1-MCS-5 generating pBBRlip1. Further, the resulting plasmid was used to generate pBBRompW, by replacement of the lip1 ORF by the ompW ORF using RF primers ompWcomp_F and ompWcomp_R. To assess whether Lip1 is secreted in a type II secretion-dependent manner, we generated plasmids pBBR1lip1-HA and pBBRlip135-383-HA, carrying the complete ORF of lip1 or the lip1 ORF without the predicted, N-terminal signal peptide, respectively. In both cases, the ORFs were fused to the HA epitope at their C-terminus (Table 1 and Table S1). All PCR amplifications were conducted using High-Fidelity DNA polymerase Phusion (New England Biolabs, Beverly, MA, USA). The resulting plasmids were transformed into E. coli S17-1 λpir as described [11]. The plasmids were extracted and the cloned fragments were verified by sequencing at Hy Laboratories Ltd. (Rehovot, Israel). Conjugations with A. citrulli strains were performed by bi-parental mating as described [11]. Transconjugants were selected on the basis of Gm resistance and verified by PCR.
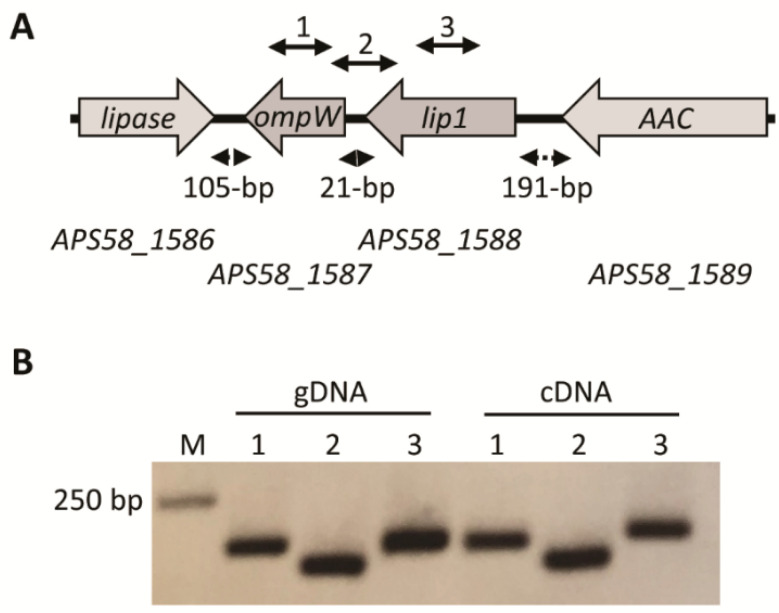
Figure 1.
The lip1-ompW operon. (A) Schematic organization of the operon and neighboring genes according to the annotation of the Acidovorax citrulli M6 genome (GenBank accession CP029373.1). Grey arrows represent the open reading frames (ORFs) and transcription direction of the following genes (numbers between parentheses indicate the position of start and stop codons in the A. citrulli M6 chromosome): APS58_1586, hypothetical protein but having high similarity to GDSL lipases (1,779,911–1,780,861); APS58_1587, outer membrane protein OmpW (1,780,966–1,781,658, complement); APS58_1588, Lip1 (1,781,679–1,782,830; complement); and APS58_1589, D-alanyl-D-alanine carboxypeptidase (AAC; 1,783,021–1,784,559; complement). Dashed double arrows indicate the distance between the ORFs. Solid double arrows above the genes indicate the location of PCR targets for cDNA amplification (see Table S1). (B) Polymerase chain reaction (PCR) using A. citrulli M6 genomic DNA (gDNA), or cDNA synthesized from RNA extraction from an overnight NB-grown culture of A. citrulli M6. PCR reactions were conducted with primers described in Table S1 and the lane numbers correspond to the solid double arrows in panel A: (1) primers ompW_F and ompW_R for amplification of an internal fragment of ompW; (2) primers 21between_F and 21between_R for amplification of a region containing part of the lip1 and ompW ORFs and their intergenic region; and (3) lip1_F and lip1_R for amplification of an internal fragment of ompW. Negative controls with no reverse transcriptase were used to verify that RNA samples do not contain genomic DNA contamination, and did not yield PCR products.
2.6. Transmission Electron Microscopy (TEM) Observations
TEM was used to visualize T4P and polar flagella in A. citrulli M6-lip1- and wild-type M6. Bacterial samples were prepared from 48-h-old, NA-grown colonies. A 5-µL sterile water drop was placed on a selected colony for 2 min, then a 300 mesh carbon grid was placed on the top of each colony for 30 s and the grid was negatively stained in a 1% uranyl drop for 10 s. The grids were examined with a FEI Tecnai-12 electron microscope (FEI, Eindhoven, The Netherlands) equipped with a F224HD 2k × 2k CCD camera (TVIPS, Gauting, Germany).
2.7. Growth Curves and Biofilm Formation Assays
Growth of A. citrulli M6 and M6-lip1- was assessed by incubation at 28 °C in rich (NB) and minimal (XVM2) media for 48 h. Bacteria were grown in 96-well polystyrene microplates (Nunc, Roskilde, Denmark; 100 µL of media in each well), in an Infinite F200 plate reader (Tecan, Männedorf, Switzerland), with linear shaking for 15 s every 30 min, and optical density was measured at OD600. Each experiment included 24 replicates per strain/medium. Biofilm assays were performed as described [35]. Briefly, overnight cultures of A. citrulli were diluted in a 1:100 ratio with fresh XVM2 media. Then, 200 μL bacterial suspensions were transferred into wells of 96-well polystyrene microplate (Nunc) and incubated at 28 °C for 48 h. The media were discarded and wells were washed twice with sterile distilled water (SDW). Two hundred microliters of 0.1% crystal violet were then added to each well and the plate was incubated at room temperature for 30 min. The crystal violet solution was discarded and the wells were washed gently with SDW (twice). Then 200 µL of 95% ethanol were added to the wells to solubilize the stained biofilms. After 2 h of incubation at room temperature the optical density of each well was measured at OD595 using the Infinite F200 plate reader.
2.8. Measurement of Twitching Haloes
Acidovorax citrulli strains were grown at 28 °C for 48 h on NA plates, and twitching haloes around the colonies were measured using EVOS microscope and software (Thermo Fisher Scientific, Waltham, MA, USA). In each experiment, twitching halo lengths of 30 colonies per strain were measured.
2.9. Virulence Assays
Seed transmission experiments were performed as described [11] with minor modifications. Acidovorax citrulli strains were grown on NA for 48 h, resuspended from plates with SDW, adjusted to an OD600 of 0.2 [about 108 colony forming units (CFU)/mL] using a Helios Gamma spectrophotometer (Thermo Electron Corp., Rochester, NY, USA), and then diluted to 106 CFU/mL. Inoculum concentrations were verified by dilution plating. Melon (Cucumis melo) cv. Ophir (Zeraim Gedera, Kibutz Revadim, Israel) seeds were placed into 50 mL-Falcon tubes containing 20 mL bacterial suspensions and incubated at room temperature for 2 h with gentle agitation. The bacterial suspensions were discarded, and the seeds were dried under a laminar hood and sown in 11-cm diameter poly pots (Tefen, Nahsholim, Israel) containing a peat-based commercial soil mixture (Shacham Givat Ada, Givat Ada, Israel). The pots were kept in a greenhouse at 26–28 °C. Disease severity was evaluated 10 days after sowing, using a 0–7 scale (0, healthy plants; 7, highest disease severity), which is based on the percentage of the foliage weight of infected plants relative to non-infected controls [36]. For infiltrations of Nicotiana benthamiana leaves, A. citrulli strains were grown on LB medium with the appropriate antibiotics at 28 °C for 24 h. Bacterial cells were collected by centrifugation (4500× g, 5 min), resuspended in a 10 mM MgCl2 solution to an OD600 of 0.6, and infiltrated with a needleless syringe into the abaxial part of the leaves of 4-week-old N. benthamiana plants. Symptoms were observed after 36 h.
2.10. Swimming Motility Assays and Measurement of Swimming Speed
Swimming motility assays were performed on soft NA plates, containing 0.3% agar as described [11]. Briefly, cells from single NA-grown colonies were transferred to the center of soft NA agar plates using a toothpick. The plates were incubated at 28 °C and swimming haloes were measured after 48 h. Swimming speed of A. citrulli cells was measured after overnight growth in NB at 28 °C, using an Eclipse T1-E inverted microscope equipped with a Digital Sight cooled monochrome CCD camera (Nikon), at a magnification of ×100 with BX51 phase-contrast. Time-lapse pictures were taken and swimming speed was calculated using FIJI, an open source platform for biological image analysis [37]. In each experiment swimming speed averages were calculated from 150 cells per strain.
2.11. Lipolytic Activity Assays
Assessment of lipolytic activity by A. citrulli cultures was carried out as described [23] with few modifications. Briefly, M6, M6-lip1- and M6-comp-lip1 strains were grown for 48 h at 28 °C with shacking (200 rpm) in 5 mL XVM2 medium. Cells were pelleted by centrifugation (4500× g, 5 min, 4 °C; twice). The precipitated cells were resuspended in SDW, serially diluted and plated on NA plates for CFU counts. The supernatants were collected and assessed fluorometrically for quantitative lipolytic activity using the lipase substrate 4-methylumbelliferyl oleate (MUO; Sigma-Aldrich) [38]. One-milliliter supernatant samples were used with measurements being taken every 10 min, from time “zero” (just before addition of MUO) for 90 min. Standard curves were prepared using different concentrations of the fluorescent standard 4-methylumbelliferine (MU; Sigma-Aldrich).
2.12. Secretion Assays
Secretion assays were performed as described by Bernal et al. [39]. Briefly, bacteria were grown on LB with appropriate antibiotics at 28 °C for 24 h. Bacterial pellets were collected by centrifugation (10,000× g, 20 min; 3 times), normalized and added directly to 1× Laemmli buffer. The supernatants were collected and precipitated with trichloroacetic (TCA) acid overnight and then washed with acetone and resuspended in 1× Laemmli buffer. Proteins were separated by SDS–polyacrylamide gel electrophoresis containing 15% (w/v) acrylamide and electro-transferred to 0.45 µm-nitrocellulose membranes (Cytiva, Marlborough, MA, USA) using a Trans-Blot Turbo transfer system (Bio-Rad, Hercules, CA, USA). Immunodetection was performed using monoclonal antibodies directed against the HA epitope (Cell Signalling Technology, Danvers, MA, USA). The secondary antibody, anti-rabbit IgG HRP-linked (Cell Signalling Technology), was detected with the Immobilon Forte HRP substrate (Merck Millipore, Burlington, MA, USA) using an Odyssey XF Imaging System (Li-Cor, Lincoln, NE, SA, USA).
2.13. Statistical Analysis
Quantitative assays were analyzed by Tukey–Kramer test for mean comparison in conjunction with analysis of variance (ANOVA) using JMP software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Identification of an Acidovorax citrulli M6 Mutant with Increased Twitching Haloes
Acidovorax citrulli cells are motile on solid surfaces by means of T4P-mediated twitching motility. In this bacterium, twitching haloes around colonies growing on nutrient agar (NA) can be easily visualized by the naked eye after ~72 h of growth at 28 °C [11]. We exploited the simplicity of this phenotype to screen a library of about 10,000 EZ-Tn5 mutants in the background of the group I model strain, M6. As aforementioned, we identified fifty mutants impaired in twenty different genes that lacked twitching ability [16]. In these screens we also identified a single mutant, which was later named M6-lip1- (see below), that produced twitching haloes substantially larger than those generated by wild-type M6 colonies.
Southern blot analysis supported that the transposon was inserted only once in the M6-lip1- genome (Figure S1). To identify the site of transposon insertion, we tried a plasmid rescue approach [11,16] but in the case of this mutant we were not able to get any plasmid following digestion with several restriction enzymes. As an alternative approach, we sequenced the mutant genome by Illumina MiSeq. Following its assembly and comparison with the M6 genome (GenBank accession CP029373.1), we detected a single insertion of the EZ-Tn5 cassette, thus confirming Southern blot results. The transposon disrupted the open reading frame (ORF) of gene APS58_1588 (Figure 1A).
The APS58_1588 gene has an ORF of 1152 base pairs, encoding a 383 amino-acid protein with a calculated molecular weight of 38.8 KDa. Although this gene is annotated as encoding a hypothetical protein, BlastN against the NCBI nucleotide collection (nr/nt) matched with genes that were mostly annotated as phospholipases. The highest hits were genes from other A. citrulli strains (at 100% identity) and from strains of two other plant-pathogenic Acidovorax species, A. avenae and A. cattleyae (at 87 to 89% identity). Relatively high identity (75%) was also found for genes from two strains of Melaminivora sp., which like A. citrulli, belong to the Comamonadaceae family. Similarly, BlastP analysis of the APS58_1588 product against the NCBI Protein Reference Sequences’ database matched with proteins from various plant-pathogenic Acidovorax species at full coverage. The highest identities were for proteins from A. citrulli (100%), A. avenae (89–90%), A. cattleyae (90%), A. oryzae (89%) and A. konjaci (82%). Relatively high levels of protein identity were found for other Comamonadaceae members including Diaphrobacter polyhydroxybutyrativorans (77% identity), Melaminivora sp. (67%), Simplicispira psychrophila (63%) and Delftia acidovorans (62%). Similar to the BlastN results, the BlastP hits were mostly annotated as phospholipases, although some were annotated as lipases or hypothetical proteins.
Further observations under a light microscope revealed that differences between M6 and M6-lip1- haloes around the colonies were clearly visible after 48 h of growth (Figure 2A,B). Wild-type and mutant strains did not differ in the production of T4P and polar flagella, as observed by TEM (Figure 2C,D). Furthemore, no differences were observed between the strains in their growth ability in rich (NA) and minimal (XVM2) media, and in biofilm formation ability (Figures S2 and S3, respectively).
Figure 2.
Twitching motility and type IV pilus (T4P) formation of A. citrulli M6 and an M6 mutant disrupted in gene APS58_1588 (M6-lip1-). Images of bacterial colonies of M6 (A) and M6-lip1- (B) seen by light microscopy after 48 h of growth on NA plates at 28 °C. Typical haloes surrounding the bulk colonies (indicated by the white double arrows) are formed by bacteria migrating via twitching motility. Pictures were taken using an Axio Scope light microscope equipped with a DXM1200F digital camera. Transmission electron microscopy observations of M6 (C) and M6-lip1- (D). The strains were observed in a FEI Tecnai-12 electron microscope after growth on NA at 28 °C for 48 h. Solid and dashed arrows indicate polar flagellum and T4P, respectively. Bars at the bottom of each panel: 100 µm for (A,B); and 0.5 µm for (C,D).
3.2. Sequence Analyses of APS58_1588 Support It Encodes a Lipolytic Enzyme from the GDSL Family
Although APS58_1588 and most homologous genes are annotated as phospholipases, analysis of the APS58_1588 product at the NCBI Conserved Domains (CD) server [31] suggested this gene encodes a lipase belonging to the triacylglycerol lipase-like subfamily (cd01847) of the SGNH hydrolase superfamily (cluster cl01053). One of the representatives of this subfamily is a protein annotated as lipase 1 (Lip1) from Photorhabdus luminescens (GenBank locus LIP1_PHOLU). Moreover, BlastP analysis of APS58_1588 against the UniProtKB/SwissProt database revealed P. luminescens Lip1 as its highest hit (though at only 23% identity for a query coverage of 89%). Based on this finding, on lipolytic assays of mutant and wild-type supernatants (see below), and on the fact that, to the best of our knowledge, this is the first lipase gene to be characterized in A. citrulli, we named the APS58_1588 gene, lip1.
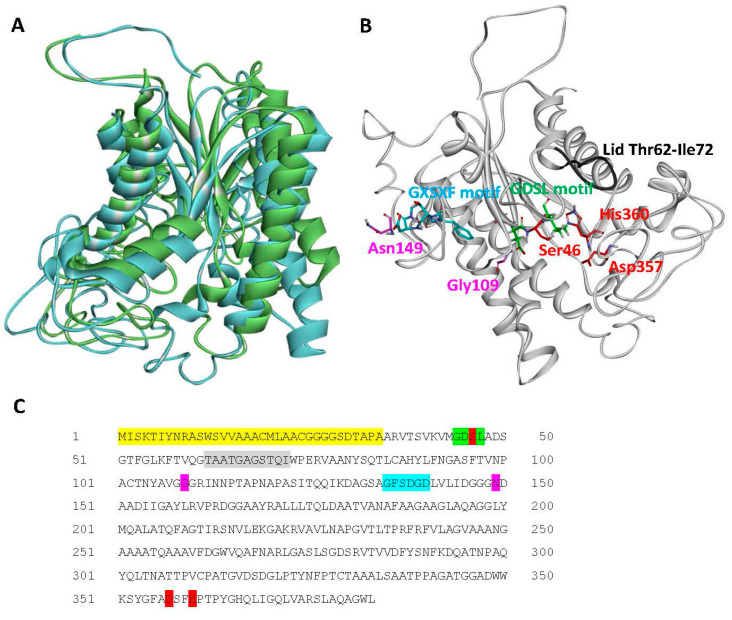
We generated a 3D model of Lip1 using the I-TASSER server [32]. High similarity was found between Lip1 and the passenger domain of the autotransporter esterase EstA from Pseudomonas aeruginosa [40,41]. EstA is a member of the GDSL family of lipolytic enzymes. It also belongs to the autotransporter (type V secretion) protein family, being composed of two domains, the N-terminal passenger domain that also harbors the enzymatic activity, and the C-terminal domain that forms a β-barrel pore in the outer membrane, through which the passenger domain is secreted to the cell surface [40,42,43,44]. A structural alignment of Lip1 and the passenger domain of EstA are shown in Figure 3A. Sequence and structural analyses revealed that Lip1 contains the conserved catalytic triad residues, Ser46-Asp357-His360 (Figure 3B,C) in positions that are relatively similar to those found in P. aeruginosa EstA. In addition, typical conserved motifs in members of the GDSL family of lipolytic enzymes- GDSL (residues 44–47) and GXSXG (residues 135–139)- are found in Lip1 (Figure 3B,C). The GXSXG motif is referred to as the nucleophile elbow [45], which allows the activity of the catalytic triad. Based on structure similarity with the passenger domain of P. aeruginosa EstA, and on information available for the lid structure [46], we hypothesize that the lid region is a loop comprising residues Thr62-Ile72 (Figure 3B). Additional analysis using the CD server pointed at Gly109 and Asn149, which together with Ser46 of the catalytic triad and the aforementioned GDSL motif, could form the oxyanion hole of the active site (Figure 3B).
Figure 3.
Structural analysis of A. citrulli M6 Lip1. (A) Structural alignment between Lip1 (blue ribbon), and the passenger domain of the autotransporter EstA from Pseudomonas aeruginosa (green ribbon; PDB code 3KVN). (B) Predicted structural model of Lip1 generated by I-TASSER, and (C) Lip1 sequence. Conserved residues and motifs are highlighted and shown in sticks as follows: red, catalytic triad; green (and red for Ser46), GDSL motif; blue, GXSXG motif; purple, oxyanion hole residues; and grey, putative lid loop (ribbon only). The secretion signal peptide as predicted by Pred-Tat and Phobius is highlighted yellow in the protein sequence.
3.3. The lip1 Gene Is Part of an Operon with ompW
In A. citrulli M6, the lip1 gene appears to be part of an operon with APS58_1587 (Figure 1A). This gene encodes an outer membrane protein, OmpW, which in other bacteria was suggested to be involved in the transport of small hydrophobic molecules [47,48]. To assess whether lip1 and ompW indeed comprise an operon, primers were designed to amplify internal regions of the two ORFs as well as a region spanning the two genes and their intergenic region (Figure 1A; Table S1). Acidovorax citrulli M6 was grown overnight in NB medium, after which RNA was extracted. cDNA synthesized from the RNA extract was used as a template for PCR reactions. The results confirmed that both genes are expressed under tested conditions, and that lip1 and ompW are indeed part of an operon (Figure 1B). Remarkably, an RNA-Seq approach that measured expression of M6 genes in the apoplast-mimicking medium XVM2, showed that both genes are expressed in this medium, and confirmed their operon organization as cDNA reads were detected that spanned the lip1 and ompW ORFs [10; RNA-Seq data available under NCBI BioProject PRJNA565338].
3.4. Increased Twitching Haloes in the lip1- Mutant Is due to lip1 Disruption
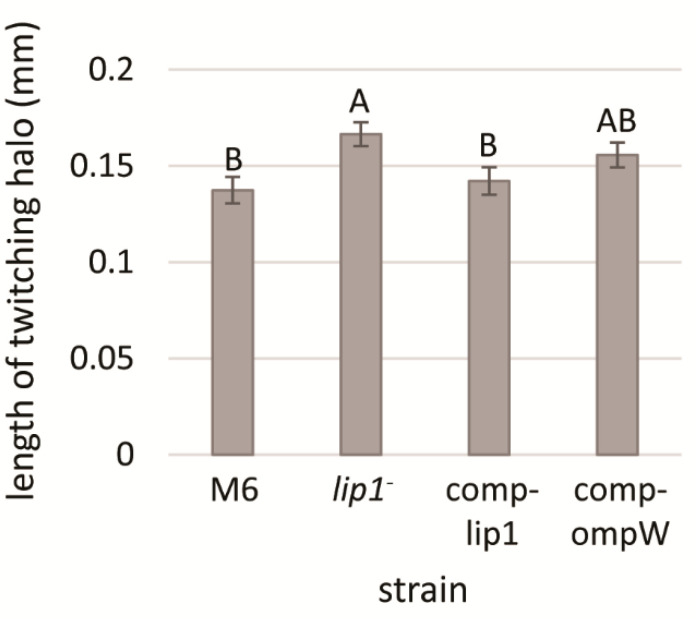
To assess whether the increased twitching haloes in the M6-lip1- mutant is a direct result of disruption of the lip1 gene or a polar effect of the mutation on ompW, we generated two complementation strains in the background of the mutant: M6-comp-lip1 and M6-comp-ompW. These strains were generated by transforming the M6-lip1- mutant with plasmids pBBRlip1 and pBBRompW carrying lip1 and ompW, respectively, under the control of the lip1-ompW operon promoter (Table 1). Complementation of the mutant with pBBRlip1 restored the wild-type phenotype in terms of twitching halo size (Figure 4). The M6-lip1- mutant carrying pBBRompW yielded intermediate values of twitching haloes between the wild-type and the mutant strain; however, they did not significantly differ from those recorded for the M6-lip1- mutant (Figure 4). Overall, these results support that the increased twitching haloes observed around M6-lip1- colonies are due to disruption of the lip1 gene.
Figure 4.
Length of twitching haloes of A. citrulli M6, the M6-lip1- mutant, and M6-lip1- carrying plasmid pBBR-MCS-5 expressing lip1 or ompW (M6-comp-lip1 and M6-comp-ompW, respectively). Bacteria were grown for 48 h on NA plates at 28 °C, and twitching haloes around the colonies were measured from 30 colonies per strain. Data represent averages and standard errors (SE) from one experiment, out of three with similar results. Different letters indicate significant differences (p < 0.05) among treatments by Tukey–Kramer and ANOVA.
3.5. The M6-lip1- Mutant Has Reduced Virulence Relative to Wild-Type M6
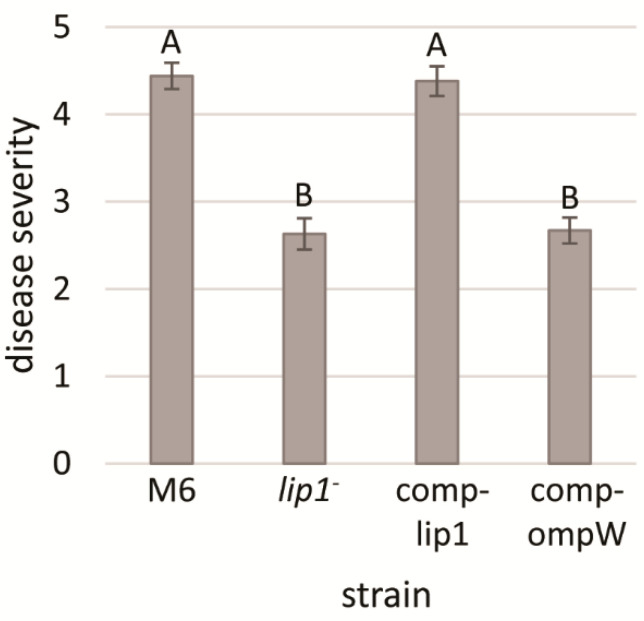
Acidovorax citrulli is a seed-borne pathogen and melon seedlings are highly susceptible to the pathogen [2]. Therefore, we carried out seed transmission assays to assess whether lip1 contributes to A. citrulli virulence. Disease severity was evaluated 10 days after inoculation (d.a.i), using a scale based on seedling weight [36]. Seedlings emerging from seeds that were inoculated with the M6-lip1- mutant exhibited significantly (p < 0.05) lower disease severity as compared with seedlings emerging from M6-inoculated seeds (Figure 5). Complementation of the mutant by expression of intact lip1 gene in plasmid pBBRlip1 (M6-comp-lip1 strain) restored wild-type levels of virulence. In contrast, wild-type levels of virulence could not be restored when the mutant carried pBBRompW, expressing the ompW gene (Figure 5). These results demonstrate that lip1 significantly contributes to A. citrulli M6 virulence.
Figure 5.
Seed transmission assays of melon inoculated with A. citrulli M6, the M6-lip1- mutant, and M6-lip1- carrying plasmid pBBR-MCS-5 expressing lip1 or ompW (M6-comp-lip1 and M6-comp-ompW, respectively). Melon cv. Ophir seeds were inoculated with bacteria at 106 CFU/mL, sown in a peat-based commercial soil mixture and maintained in the greenhouse at 26–28 °C for 10 days. Disease severity was determined using a 0–7 scale (zero, healthy; seven, highest severity) based on the percentage of the foliage weight of infected plants relative to non-infected controls. Data represent averages and SE from one experiment (15 plants per treatment), out of three with similar results. Different letters indicate significant differences (p < 0.05) among treatments by Tukey–Kramer and ANOVA.
3.6. The M6-lip1- Mutant Swims Slower than the Wild-Type Strain
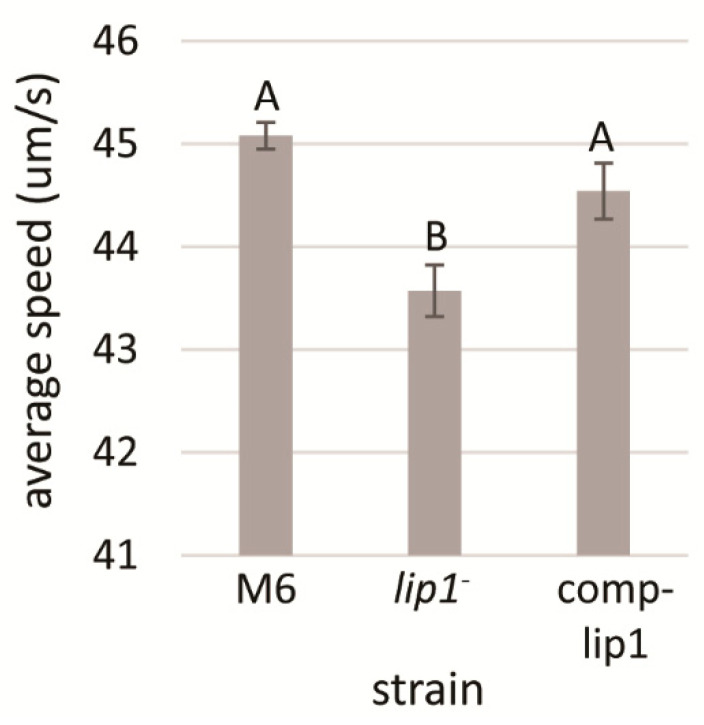
Polar flagellum is an important virulence factor of A. citrulli [49]. Therefore, we assessed whether the lip1- mutant has altered flagellum-mediated swimming motility relative to wild-type M6. No differences in this trait could be observed between the strains in swimming motility assays performed on soft agar plates (NA containing 0.3% agar) after 48 h of incubation at 28 °C. Since this assay may not be sufficiently sensitive, we carried out microscope monitoring of swimming speed of both strains after overnight growth of bacteria in NB. Time laps microscopic tracking and calculations of swimming speed revealed that, under tested conditions, M6-lip1- cells had significantly (p < 0.05) lower swimming speed than wild-type cells. In contrast, the lip1 complemented strain (M6-comp-lip1) did not significantly differ from the wild-type strain in this trait (Figure 6).
Figure 6.
Analysis of swimming speed of A. citrulli M6, the M6-lip1- mutant and the lip1 complemented mutant (M6-comp-lip1). Swimming speed measurements were calculated from NB-overnight cultures, as average of 150 bacterial cells for each strain, using a Nikon Eclipse T1-E inverted microscope equipped with a Digital Sight cooled monochrome CCD camera. Time-lapse pictures were taken and swimming speed was calculated using FIJI. Data represent averages and SE from one experiment out of three with similar results. Different letters indicate significant differences (p < 0.05) among treatments by Tukey–Kramer and ANOVA.
3.7. Lip1 Possesses Lipolytic Activity
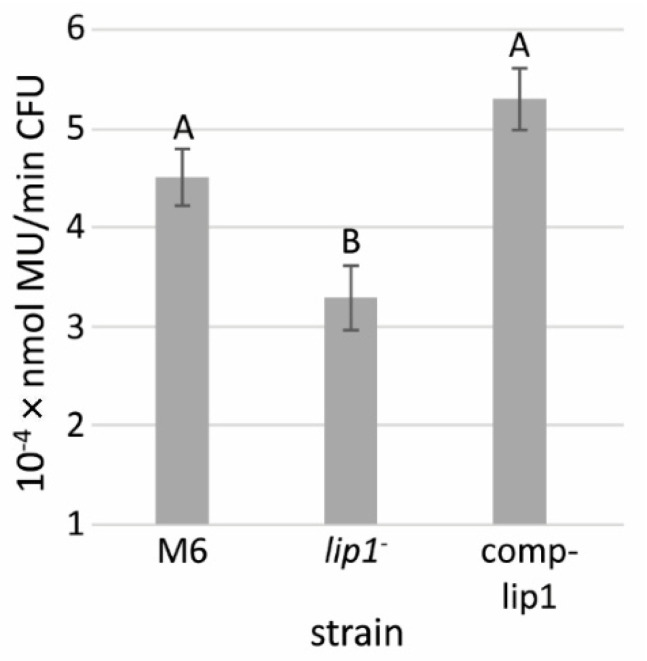
Sequence analyses strongly indicated that Lip1 belongs to the GDSL family of lipases/esterases. We compared the lipolytic activities of the supernatants of XVM2-grown M6-lip1-, wild-type and lip1 complemented strains, using the lipase substrate, 4-methylumbelliferyl oleate (MUO). These experiments showed a significantly (p < 0.05) lower lipolytic activity in the supernatants of M6-lip1- cultures relative to those of wild-type and complemented strains (Figure 7). Lipolytic activity was still detected in the mutant supernatant, probably due to the activity of other lipolytic enzymes.
Figure 7.
Lipolytic activity of supernatants from A. citrulli M6, the M6-lip1- mutant, and M6-lip1- carrying plasmid pBBR-MCS-5 expressing lip1 (M6-comp-lip1). The strains were grown in XVM2 medium for 48 h at 28 °C with shacking (200 rpm). The supernatants were collected and used for measurement of lipolytic activity using 4-methylumbelliferyl oleate (MUO) as a substrate. Data represent averages and SE from three experiments with similar results (five replicates per strain in each experiment). Different letters indicate significant differences (p < 0.05) among treatments by Tukey–Kramer and ANOVA. MU, 4-methylumbelliferine; CFU, colony forming units.
3.8. Lip1 Is Secreted in a Type II Secretion-Dependent Manner
In Proteobacteria, lipolytic enzymes are commonly secreted by type II secretion (T2S) systems, which are generally referred to as the main branch of the general secretory pathway (Gsp; Sec-pathway) [50,51]. We analyzed the Lip1 sequence using the Pred-Tat server, which predicts N-terminal Sec-pathway or twin-arginine translocation (Tat) signal peptides with Hidden Markov models [33]. This analysis revealed the presence of a Sec-pathway signal peptide composed by the first 34 amino acids in the Lip1 N-terminus, with a reliability score of 0.997. According to this prediction, the cleavage site is between two alanine residues, Ala34 and Ala35 (Figure 3C). An identical prediction supporting the 34-amino acid-signal peptide in the N-terminus of Lip1 was obtained using the Phobius prediction tool [34].
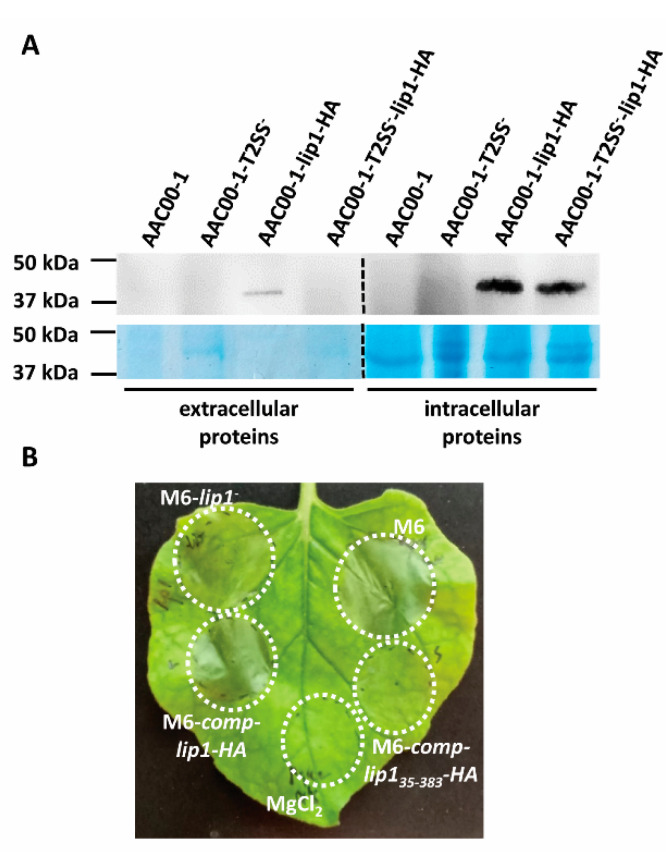
We introduced plasmid pBBRlip1-HA, carrying the lipA ORF fused to an HA tag, to A. citrulli AAC00-1, and to a mutant of this strain impaired in T2S [2,27]. Western blot analysis showed that while the LipA-HA recombinant protein was produced and detected in the intracellular fraction of both strains, the protein could be detected in supernatants of wild-type AAC00-1 cultures but not in those of the T2S mutant (Figure 8A and Figure S4). The LipA-HA signal was weaker in supernatants than in the intracellular fractions due to the lower concentration of proteins in the former.
Figure 8.
Type II secretion (T2S) of Lip1. (A) Western blot analysis of extracellular and intracellular fractions of A. citrulli strains (AAC00-1, wild-type; AAC00-1-T2SS-, T2S mutant) expressing or not recombinant Lip1-HA (see details in Materials and Methods). Representative images of one out of two experiments with similar results are shown. Dashed lines between extracellular and intracellular proteins indicate that the images were composed for comprehensive visualization of treatments. The original images are shown in Figure S4. (B) Infiltration of four-week-old N. benthamiana leaves with strains M6, M6-lip1- and M6-lip1- expressing full length Lip1-HA or the Lip1 ORF lacking the N-terminal secretion signal (strains M6-comp-lip1-HA and M6-comp-lip135-383-HA, respectively). The picture was taken 36 h after infiltration and is representative of three independent experiments with similar results (with each experiment involving at least five leaves).
Nicotiana benthamiana was recently demonstrated as a suitable surrogate host for investigation of A. citrulli pathogenicity [52]. We introduced pBBRlip1-HA in the M6-lip1- mutant and used the resulting strain, M6-comp-lip1-HA, for leaf infiltration of N. benthamiana leaves, in comparison with wild-type M6 and M6-lip1- strains. While the mutant strain was severely compromised in its ability to induce symptoms in N. benthamiana leaves, strain M6-comp-lip1-HA performed as similar as the wild-type strain (Figure 8B). Remarkably, plasmid pBBRlip135-383-HA carrying the lip1 ORF without the predicted signal peptide (Table 1), was not able to complement the M6-lip1- mutant for virulence in these assays (Figure 8B). Overall, our results confirm that Lip1 is secreted in a T2S-dependent manner, and this is required for wild-type levels of virulence of A. citrulli.
4. Discussion
Twitching motility is a flagellum-independent translocation mechanism that promotes bacterial movement on solid surfaces. This type of motility is mediated by type IV pili (T4P), hair-like polar appendages that are found in a wide range of bacteria belonging to the Proteobacteria, Cyanobacteria and Firmicutes [53,54]. Besides mediating twitching motility, T4P are involved in surface adhesion, colonization, biofilm formation, genetic material uptake and virulence [13,54,55]. Among plant-pathogenic bacteria, a significant contribution of T4P to virulence has been demonstrated mainly for vascular, xylem-colonizing bacteria [54]. In these pathogens, T4P may contribute to colonization and spread in the xylem vessels by promoting biofilm formation on the vessel surfaces as well as twitching motility in this niche [54].
Due to the importance of T4P and twitching motility for virulence of A. citrulli [11], we screened a mutant library of the model A. citrulli strain M6 for altered twitching phenotypes. Fifty mutants impaired in twenty different genes were detected in this screen that showed no twitching ability and compromised virulence [16]. Interestingly, one single mutant was found to display larger twitching motility haloes in comparison with the wild-type strain (Figure 2 and Figure 4). Sequence analyses of the disrupted gene in this mutant revealed it encodes a protein belonging to the GDSL family of lipases/esterases. Lipolytic assays of bacterial supernatants with the lipase substrate MUO confirmed that the mutated gene encodes a lipolytic enzyme (Figure 7), which we named Lip1. Remarkably, the A. citrulli M6-lip1- mutant was found to be significantly compromised in virulence relative to wild-type M6 (Figure 5 and Figure 8B). In A. citrulli, mutants damaged in twitching ability and/or T4P synthesis are also compromised in biofilm formation ability [11,12,16]. Here we show that under tested conditions, the M6-lip1- mutant does not significantly differ from wild-type M6 in this trait (Figure S2).
The relationship between lipolytic enzymes and motility has been well investigated in the opportunistic pathogen Pseudomonas aeruginosa. Barker et al. [56] showed that a phospholipase, PlcB, is required for directed twitching motility in response to gradients of certain phospholipids. Wilhelm et al. [41] showed that a P. aeruginosa mutant defective in the esterase gene estA, was affected in twitching and swimming motilities as well as in biofilm formation ability. The involvement of EstA and the lipases LipA and LipC in twitching of P. aeuriginosa in response to the phospholipid phosphatidylethanolamine (PE) was further demonstrated by Miller et al. [57]. A lipC mutant of this bacterium was also affected in swimming motility [58]. Here we show that the A. citrulli M6-lip1- mutant has lower swimming speed than the wild-type strain (Figure 6).
Lipases are extracellular enzymes and must therefore be translocated through the bacterial membrane to find their way out of the cell [17]. In Gram-negative bacteria, most lipases/esterases are secreted via type I or type II secretion (T1S and T2S, respectively) systems, but an additional secretory pathway is the type V/autotransporter system [59]. The latter is the case of the aforementioned EstA esterase of P. aeruginosa, which is required for rhamnolipid production [41,60]. The crystal structure of EstA was solved by van den Berg [40]. Interestingly, structure prediction of A. citrulli Lip1 revealed that, among proteins with solved structures, the N-terminal passenger domain of EstA has the highest structural similarity to Lip1. Sequence analyses also revealed that Lip1 carries typical motifs of members of the GDSL family of lipases/esterases.
Two different prediction tools revealed that Lip1 has a predicted 34-amino acid signal peptide in its N-terminus (cleavage site between Ala34 and Ala35), which probably mediates its translocation through the cytoplasmic membrane via the general secretory pathway (Gsp; Sec-pathway). Since Lip1 does not possess an autotransporter β-barrel domain like EstA, we hypothesized that Lip1 is transferred to the periplasm via the Sec-pathway, and from the periplasm out of the cell, via T2S. This hypothesis was further validated using an A. citrulli mutant defective in T2S (Figure 8A). Remarkably, a neighbor gene of lip1, APS58_1589 (Figure 1A), encodes D-alanyl-D-alanine carboxypeptidase (DD-CPase). DD-CPases are enzymes that belong to the penicillin-binding protein (PBP) family, some of which possess Ala-Ala endopeptidase activity [61]. Further investigation is needed to assess whether the APS_1589 gene encodes a bona fide DD-CPase and if its product mediates cleavage of the Lip1 signal peptide.
Although Lip1 significantly contributes to the lipolytic activity of A. citrulli, the M6-lip1- mutant still retained this activity (Figure 7). This is probably due to the presence of additional lipolytic enzymes. Indeed, the annotated genome of A. citrulli M6 contains additional genes annotated as lipases, phospholipases and esterases. We cannot discard the possibility that some of these genes are misannotated, and that few other genes annotated as hypothetical proteins could in fact be encoding enzymes with lipolytic activity. For instance, similar to lip1, the closely located gene APS58_1586 (Figure 1A) was also automatically annotated as encoding a hypothetical protein, however Blast analysis revealed it encodes a protein with high similarity to GDSL lipases.
We showed that in A. citrulli M6, lip1 is located in an operon together with the ompW gene. This seems also to be the case for the sequenced group II strain of A. citrulli, AAC00-1 (GenBank accession NC_008752.1), where lip1 and ompW correspond to genes Aave_4567 and Aave_4566, respectively. OmpW is a member of a major family of outer membrane proteins that are widespread in Gram-negative bacteria. These proteins have been proposed to form channels for uptake of small hydrophobic molecules [47,48]. Evidence from studies with several bacterial species support that OmpW proteins are involved in adaptive responses to a variety of stresses, including salinity, temperature and exposure to antibiotics [62,63,64]. In Escherichia coli, OmpW forms part of the colicin S4 receptor [65]. Here we showed that the twitching and virulence phenotypes that were altered in the lip1- mutant could be restored to wild-type levels by in trans expression of lip1, but not of ompW. These results demonstrate that the observed phenotypes were a direct consequence of lip1 disruption and not due to a polar effect on ompW expression, as sometimes observed in transposon-mediated mutagenesis, including with EZ-Tn5 [66]. With that said, it is yet to be elucidated whether there is a functional relationship between Lip1 and OmpW.
Relatively few studies have studied the contribution of lipolytic enzymes to virulence of plant-pathogenic bacteria. In this regard, some lipases/esterases were shown to be important virulence factors in Burkholderia glumae [22], Xanthomonas oryzae pv. oryzae [20,21], Xanthomonas perforans [23], Xylella fastidiosa [25] and Pseudomonas syringae pv。actinidiae [24]. Lipolytic enzymes also play a role in virulence of several fungal plant pathogens such as Blumeria graminis [67] and Fusarium spp. [68]. Overall, there is little understanding about mechanistic aspects associated with the contribution of lipolytic enzymes to pathogens’ virulence and fitness. Several mechanisms have been proposed, including the degradation of host cell membranes, nutrient acquisition by digestion of lipids, adhesion through release of free fatty acids, interference with the host immune system, inactivation of bactericidal lipids and increased competition with other microorganisms [23,67,68,69,70,71]. Here, we showed that the A. citrulli M6-lip1- mutant has lower swimming motility speed than the wild-type strain. Since polar flagellum mutants of A. citrulli have been shown to be compromised in virulence [49], it could be that the alteration in swimming ability by the disruption of lip1 could also be associated, at least partially, with the reduced virulence ability of this mutant.
5. Conclusions
In this study we reported the characterization of an A. citrulli mutant defective in lip1, a gene encoding a GDSL-lipolytic enzyme that is expressed in both rich and minimal media. To the best of our knowledge, this is the first report on the contribution of a lipolytic enzyme to virulence of a plant-pathogenic Acidovorax species. Further investigation is needed to elucidate the mechanisms by which Lip1 contributes to A. citrulli virulence, and to assess the importance of other lipolytic enzymes in the pathogenicity of this bacterium.
Acknowledgments
We thank Ron Walcott from University of Georgia, Athens, for kindly providing us the A. citrulli AAC00-1-T2SS- mutant. We thank Rajesh Sathyamoorthy from the Department of Plant Pathology and Microbiology of the Hebrew University of Jerusalem for his helpful assistance with swimming speed calculations. We also thank Nadav Elad form the Electron Microscopy Unit of the Weizmann Institute of Science for helpful assistance with TEM imaging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10051016/s1, Figure S1: Southern blot analysis for verification of the insertion of the EZ-Tn5 cassette in the chromosome of Acidovorax citrulli M6-lip1-; Figure S2: Growth curves of A. citrulli M6 and lip1- mutant in rich and in minimal media; Figure S3: Quantification of biofilm formation of A. citrulli strains M6 and lip1- mutant; Figure S4: Type II secretion of Lip1; Table S1: Sets of PCR primers used in this study.
Author Contributions
Conceptualization, T.R., I.J.-G. and S.B.; data curation, T.R., I.J.-G. and S.B.; funding acquisition, S.B.; investigation, T.R., I.J.-G., D.T.-A., T.Y. and S.B.; project administration, S.B.; supervision, S.B.; writing-original draft preparation, T.R., I.J.-G. and S.B.; writing-review and editing, T.R., I.J.-G., D.T.-A., T.Y. and S.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grant number 851/14 from the Israel Science Foundation.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schaad N.W., Postnikova E., Sechler A., Claflin L.E., Vidaver A.K., Jones J.B., Agarkova I., Ignatov A., Dickstein E., Ramundo B.A. Reclassification of subspecies of Acidovorax avenae as A. avenae (Manns 1905) emend., A. cattleyae (Pavarino, 1911) comb. nov., A. citrulli Schaad et al., 1978) comb. nov., and proposal of A. oryzae sp. nov. Syst. Appl. Microbiol. 2008;31:434–446. doi: 10.1016/j.syapm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Burdman S., Walcott R. Acidovorax citrulli: Generating basic and applied knowledge to tackle a global threat to the cucurbit industry. Mol. Plant Pathol. 2012;13:805–815. doi: 10.1111/j.1364-3703.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins D.L., Thompson C.M. Seed transmission of Acidovorax avenae subsp. citrulli in cucurbits. HortScience. 2002;37:924–926. doi: 10.21273/HORTSCI.37.6.924. [DOI] [Google Scholar]
- 4.Walcott R.R., Langston D.B., Jr., Sanders F.H., Jr., Gitaitis R.D. Investigating intraspecific variation of Acidovorax avenae subsp. citrulli using DNA fingerprinting and whole cell fatty acid analysis. Phytopathology. 2000;90:191–196. doi: 10.1094/PHYTO.2000.90.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Walcott R.R., Fessehaie A., Castro A.C. Differences in pathogenicity between two genetically distinct groups of Acidovorax avenae subsp. citrulli on cucurbit hosts. J. Phytopathol. 2004;152:277–285. doi: 10.1111/j.1439-0434.2004.00841.x. [DOI] [Google Scholar]
- 6.Burdman S., Kots N., Kritzman G., Kopelowitz J. Molecular, physiological, and host-range characterization of Acidovorax avenae subsp. citrulli isolates from watermelon and melon in Israel. Plant Dis. 2005;89:1339–1347. doi: 10.1094/PD-89-1339. [DOI] [PubMed] [Google Scholar]
- 7.Yan L.C., Hu B.S., Chen G., Zhao M., Walcott R.R. Further evidence of cucurbit host specificity among Acidovorax citrulli groups based on a detached melon fruit pathogenicity assay. Phytopathology. 2017;107:1305–1311. doi: 10.1094/PHYTO-11-16-0416-R. [DOI] [PubMed] [Google Scholar]
- 8.Johnson K.L., Minsavage G.V., Le T., Jones J.B., Walcott R.R. Efficacy of a nonpathogenic Acidovorax citrulli strain as a biocontrol seed treatment for bacterial fruit blotch of cucurbits. Plant Dis. 2011;95:697–704. doi: 10.1094/PDIS-09-10-0660. [DOI] [PubMed] [Google Scholar]
- 9.Bahar O., Burdman S. Bacterial fruit blotch: A threat to the cucurbit industry. Isr. J. Plant Sci. 2010;58:19–31. doi: 10.1560/IJPS.58.1.19. [DOI] [Google Scholar]
- 10.Jiménez-Guerrero I., Pérez-Montaño F., Da Silva G.M., Wagner N., Shkedy D., Zhao M., Pizarro L., Bar M., Walcott R., Sessa G., et al. Show me your secret(ed) weapons: A multifaceted approach reveals a wide arsenal of type III-secreted effectors in the cucurbit pathogenic bacterium Acidovorax citrulli and novel effectors in the Acidovorax genus. Mol. Plant Pathol. 2020;21:17–37. doi: 10.1111/mpp.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahar O., Goffer T., Burdman S. Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli. Mol. Plant-Microbe Interact. 2009;22:909–920. doi: 10.1094/MPMI-22-8-0909. [DOI] [PubMed] [Google Scholar]
- 12.Bahar O., De La Fuente L., Burdman S. Assessing adhesion, biofilm formation and motility of Acidovorax citrulli using microfluidic flow chambers. FEMS Microbiol. Lett. 2010;312:33–39. doi: 10.1111/j.1574-6968.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 13.Jarrell K.F., McBride M.J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 14.Eckshtain-Levi N., Shkedy D., Gershovitz M., Mateus Da Silva G., Tamir-Ariel D., Walcott R., Pupko T., Burdman S. Insights from the genome sequence of Acidovorax citrulli M6, a group I strain of the causal agent of bacterial fruit blotch of cucurbits. Front. Microbiol. 2016;7:430. doi: 10.3389/fmicb.2016.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang R.Z., Garcia D.S., Pérez Montaño F., da Silva G.M., Zhao M., Jiménez Guerrero I., Rosenberg T., Chen G., Plaschkes I., Morin S., et al. Complete assembly of the genome of an Acidovorax citrulli strain reveals a naturally occurring plasmid in this species. Front. Microbiol. 2019;10:1400. doi: 10.3389/fmicb.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg T., Salam B.B., Burdman S. Association between loss of type IV pilus synthesis ability and phenotypic variation in the cucurbit pathogenic bacterium Acidovorax citrulli. Mol. Plant-Microbe Interact. 2018;31:548–559. doi: 10.1094/MPMI-12-17-0324-R. [DOI] [PubMed] [Google Scholar]
- 17.Jaeger K.E., Eggert T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002;13:390–397. doi: 10.1016/S0958-1669(02)00341-5. [DOI] [PubMed] [Google Scholar]
- 18.Littlechild J.A. Improving the ‘tool box’ for robust industrial enzymes. J. Ind. Microbiol. Biotechnol. 2017;44:711–720. doi: 10.1007/s10295-017-1920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho A.L.S., Orlandelli R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021;61:1689–1703. doi: 10.1080/10408398.2020.1764489. [DOI] [PubMed] [Google Scholar]
- 20.Aparna G., Chatterjee A., Sonti R.V., Sankaranarayanan R. A cell wall-degrading esterase of Xanthomonas oryzae requires a unique substrate recognition module for pathogenesis on rice. Plant Cell. 2009;21:1860–1873. doi: 10.1105/tpc.109.066886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jha G., Rajeshwari R., Sonti R.V. Functional interplay between two Xanthomonas oryzae pv. oryzae secretion systems in modulating virulence on rice. Mol. Plant-Microbe Interact. 2007;20:31–40. doi: 10.1094/MPMI-20-0031. [DOI] [PubMed] [Google Scholar]
- 22.Devescovi G., Bigirimana J., Degrassi G., Cabrio L., LiPuma J.J., Kim J., Hwang I., Venturi V. Involvement of a quorum-sensing-regulated lipase secreted by a clinical isolate of Burkholderia glumae in severe disease symptoms in rice. Appl. Environ. Microbiol. 2007;73:4950–4958. doi: 10.1128/AEM.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamir-Ariel D., Rosenberg T., Navon N., Burdman S. A secreted lipolytic enzyme from Xanthomonas campestris pv. vesicatoria is expressed in planta and contributes to its virulence. Mol. Plant Pathol. 2012;13:556–567. doi: 10.1111/j.1364-3703.2011.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel H.K., Ferrante P., Xianfa M., Javvadi S.G., Subramoni S., Scortichini M., Venturi V. Identification of loci of Pseudomonas syringae pv. actinidiae involved in lipolytic activity and their role in colonization of kiwifruit leaves. Phytopathology. 2017;107:645–653. doi: 10.1094/PHYTO-10-16-0360-R. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento R., Gouran H., Chakraborty S., Gillespie H.W., Almeida-Souza H.O., Tu A., Rao B.J., Feldstein P.A., Bruening G., Goulart L.R., et al. The type II secreted lipase/esterase LesA is a key virulence factor required for Xylella fastidiosa pathogenesis in grapevines. Sci. Rep. 2016;6:18598. doi: 10.1038/srep18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wengelnik K., Marie C., Russel M., Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J. Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson K.L. Ph. D. Thesis. University of Georgia; Athens, GA, USA: 2010. Elucidation of the Host-Pathogen Interactions That Influence Seed-to-Seedling Transmission of Acidovorax citrulli. [Google Scholar]
- 28.Simon R., Priefer U., Puhler A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in Gram negative bacteria. Biotechnology. 1983;1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 29.Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 30.Kovach M.E., Elzer P.H., Hill D.S., Robertson G.T., Farris M.A., Roop II R.M., Peterson K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 31.Marchler-Bauer A., Bo Y., Han L.Y., He J.E., Lanczycki C.J., Lu S.N., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45:D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J.Y., Yan R.X., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagos P.G., Nikolaou E.P., Liakopoulos T.D., Tsirigos K.D. Combined prediction of Tat and Sec signal peptides with hidden Markov models. Bioinformatics. 2010;26:2811–2817. doi: 10.1093/bioinformatics/btq530. [DOI] [PubMed] [Google Scholar]
- 34.Kall L., Krogh A., Sonnhammer E.L.L. Advantages of combined transmembrane topology and signal peptide prediction—The Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrestha R.K., Rosenberg T., Makarovsky D., Eckshtain-Levi N., Zelinger E., Kopelowitz J., Sikorski J., Burdman S. Phenotypic variation in the plant pathogenic bacterium Acidovorax citrulli. PLoS ONE. 2013;8:e73189. doi: 10.1371/journal.pone.0073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahar O., Kritzman G., Burdman S. Bacterial fruit blotch of melon: Screens for disease tolerance and role of seed transmission in pathogenicity. Eur. J. Plant Pathol. 2009;123:71–83. doi: 10.1007/s10658-008-9345-7. [DOI] [Google Scholar]
- 37.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts I.M. hydrolysis of 4-methylumbelliferyl butyrate—A convenient and sensitive fluorescent assay for lipase activity. Lipids. 1985;20:243–247. doi: 10.1007/BF02534195. [DOI] [Google Scholar]
- 39.Bernal P., Furniss R.C.D., Fecht S., Leung R.C.Y., Spiga L., Mavridou D.A.I., Filloux A. A novel stabilization mechanism for the type VI secretion system 33. Proc. Natl. Acad. Sci. USA. 2021;118:e2008500118. doi: 10.1073/pnas.2008500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Berg B. Crystal structure of a full-length autotransporter. J. Mol. Biol. 2010;396:627–633. doi: 10.1016/j.jmb.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm S., Gdynia A., Tielen P., Rosenau F., Jaeger K.E. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 2007;189:6695–6703. doi: 10.1128/JB.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akoh C.C., Lee G.C., Liaw Y.C., Huang T.H., Shaw J.F. GDSL family of serine esterases/lipases. Prog. Lipid Res. 2004;43:534–552. doi: 10.1016/j.plipres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Leo J.C., Grin I., Linke D. Type V secretion: Mechanism(s) of autotransport through the bacterial outer membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1088–1101. doi: 10.1098/rstb.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilhelm S., Rosenau F., Kolmar H., Jaeger K.E. Autotransporters with GDSL passenger domains: Molecular physiology and biotechnological applications. Chembiochem. 2011;12:1476–1485. doi: 10.1002/cbic.201100013. [DOI] [PubMed] [Google Scholar]
- 45.Canseco-Pérez M.A., Castillo-Avila G.M., Chi-Manzanero B., Islas-Flores I., Apolinar-Hernández M.M., Rivera-Muñoz G., Gamboa-Angulo M., Sanchez-Teyer F., Couoh-Uicab Y., Canto-Canché B. Fungal Screening on olive oil for extracellular triacylglycerol lipases: Selection of a Trichoderma harzianum strain and genome wide search for the genes. Genes. 2018;9:62. doi: 10.3390/genes9020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iqbal Khan F., Lan D., Durrani R., Huan W., Zhao Z., Wang Y. The Lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017;5:16. doi: 10.3389/fbioe.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Touw D.S., Patel D.R., van den Berg B. The crystal structure of OprG from Pseudomonas aeruginosa, a potential channel for transport of hydrophobic molecules across the outer membrane. PLoS ONE. 2010;5:e15015. doi: 10.1371/journal.pone.0015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong H.D., Patel D.R., Tamm L.K., van den Berg B. The outer membrane protein OmpW forms an eight-stranded beta-barrel with a hydrophobic channel. J. Biol. Chem. 2006;281:7568–7577. doi: 10.1074/jbc.M512365200. [DOI] [PubMed] [Google Scholar]
- 49.Bahar O., Levi N., Burdman S. The cucurbit pathogenic bacterium Acidovorax citrulli requires a polar flagellum for full virulence before and after host-tissue penetration. Mol. Plant-Microbe Interact. 2011;24:1040–1050. doi: 10.1094/MPMI-02-11-0041. [DOI] [PubMed] [Google Scholar]
- 50.Korotkov K.V., Sandkvist M., Hol W.G.J. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 2012;10:336–351. doi: 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cianciotto N.P., White R.C. Expanding role of type II secretion in bacterial pathogenesis and beyond. Infect. Immun. 2017;85:e00014–17. doi: 10.1128/IAI.00014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traore S., Eckshtain-Levy N., Miao J., Castro Sparks A., Wang Z., Wang K., Li Q., Burdman S., Walcott R., Welbaum G., et al. Nicotiana species as surrogate host for studying the pathogenicity of Acidovorax citrulli, the causal agent of bacterial fruit blotch of cucurbits. Mol. Plant Pathol. 2019;20:800–814. doi: 10.1111/mpp.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nudleman E., Kaiser D. Pulling together with type IV pili. J. Mol. Microbiol. Biotechnol. 2004;7:52–62. doi: 10.1159/000077869. [DOI] [PubMed] [Google Scholar]
- 54.Burdman S., Bahar O., Parker J.K., De La Fuente L. Involvement of type IV pili in pathogenicity of plant pathogenic bacteria. Genes. 2011;2:706–735. doi: 10.3390/genes2040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattick J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 56.Barker A.P., Vasil A.I., Filloux A., Ball G., Wilderman P.J., Vasil M.L. A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis. Mol. Microbiol. 2004;53:1089–1098. doi: 10.1111/j.1365-2958.2004.04189.x. [DOI] [PubMed] [Google Scholar]
- 57.Miller R.M., Tomaras A.P., Barker A.P., Voelker D.R., Chan E.D., Vasil A.I., Vasil M.L. Pseudomonas aeruginosa twitching motility-mediated chemotaxis towards phospholipids and fatty acids: Specificity and metabolic requirements. J. Bacteriol. 2008;190:4038–4049. doi: 10.1128/JB.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenau F., Isenhardt S., Gdynia A., Tielker D., Schmidt E., Tielen P., Schobert M., Jahn D., Wilhelm S., Jaeger K.E. Lipase LipC affects motility, biofilm formation and rhamnolipid production in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2010;309:25–34. doi: 10.1111/j.1574-6968.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 59.Rosenau F., Jaeger K.E. Bacterial lipases from Pseudomonas: Regulation of gene expression and mechanisms of secretion. Biochimie. 2000;82:1023–1032. doi: 10.1016/S0300-9084(00)01182-2. [DOI] [PubMed] [Google Scholar]
- 60.Wilhelm S., Tommassen J., Jaeger K.E. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J. Bacteriol. 1999;181:6977–6986. doi: 10.1128/JB.181.22.6977-6986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghosh A.S., Chowdhury C., Nelson D.E. Physiological functions of D-alanine carboxypeptidases in Escherichia coli. Trends Microbiol. 2008;16:309–317. doi: 10.1016/j.tim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Brambilla L., Moran-Barrio J., Viale A.M. Expression of the Escherichia coli ompW colicin S4 receptor gene is regulated by temperature and modulated by the H-NS and StpA nucleoid-associated proteins. FEMS Microbiol. Lett. 2014;352:238–244. doi: 10.1111/1574-6968.12385. [DOI] [PubMed] [Google Scholar]
- 63.Wu L.N., Lin X.M., Wang F.P., Ye D.Z., Xiao X., Wang S.Y., Peng X.X. OmpW and OmpV are required for NaCl regulation in Photobacterium damsela. J. Proteome Res. 2006;5:2250–2257. doi: 10.1021/pr060046c. [DOI] [PubMed] [Google Scholar]
- 64.Xu C.X., Lin X.M., Ren H.X., Zhang Y.L., Wang S.Y., Peng X.X. Analysis of outer membrane proteome of Escherichia coli related to resistance to ampicillin and tetracycline. Proteomics. 2006;6:462–473. doi: 10.1002/pmic.200500219. [DOI] [PubMed] [Google Scholar]
- 65.Pilsl H., Smajs D., Braun V. Characterization of colicin S4 and its receptor, OmpW, a minor protein of the Escherichia coli outer membrane. J. Bacteriol. 1999;181:3578–3581. doi: 10.1128/JB.181.11.3578-3581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millanao A.R., Mora A.Y., Saavedra C.P., Villagra N.A., Mora G.C., Hidalgo A.A. Inactivation of glutamine synthetase-coding gene glnA increases susceptibility to quinolones through increasing outer membrane protein F in Salmonella enterica serovar Typhi. Front. Microbiol. 2020;11:428. doi: 10.3389/fmicb.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng J., Wang F., Liu G.S., Greenshields D., Shen W.Y., Kaminskyj S., Hughes G.R., Peng Y.L., Selvaraj G., Zou J.T., et al. Analysis of a Blumeria graminis-secreted lipase reveals the importance of host epicuticular wax components for fungal adhesion and development. Mol. Plant-Microbe Interact. 2009;22:1601–1610. doi: 10.1094/MPMI-22-12-1601. [DOI] [PubMed] [Google Scholar]
- 68.Bravo-Ruiz G., Ruiz-Roldan C., Roncero M.I.G. Lipolytic system of the tomato pathogen Fusarium oxysporum f. sp lycopersici. Mol. Plant-Microbe Interact. 2013;26:1054–1067. doi: 10.1094/MPMI-03-13-0082-R. [DOI] [PubMed] [Google Scholar]
- 69.Rediers H., Rainey P.B., Vanderleyden J., De Mot R. Unraveling the secret lives of bacteria: Use of in vivo expression technology and differential fluorescence induction promoter traps as tools for exploring niche-specific gene expression. Microbiol. Mol. Biol. Rev. 2005;69:217–261. doi: 10.1128/MMBR.69.2.217-261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajeshwari R., Jha G., Sonti R.V. Role of an in planta-expressed xylanase of Xanthomonas oryzae pv. oryzae in promoting virulence on rice. Mol. Plant-Microbe Interact. 2005;18:830–837. doi: 10.1094/MPMI-18-0830. [DOI] [PubMed] [Google Scholar]
- 71.Jha G., Rajeshwari R., Sonti R.V. Bacterial type two secretion system secreted proteins: Double-edged swords for plant pathogens. Mol. Plant-Microbe Interact. 2005;18:891–898. doi: 10.1094/MPMI-18-0891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.