Abstract
Alkaline phosphatase activity is a common marker of phosphate stress in many phytoplankton, but it has been difficult to attribute alkaline phosphatase activity to specific organisms or groups of phytoplankton in the field with traditional biochemical procedures. A new alkaline phosphatase substrate, ELF-97 (enzyme-labeled fluorescence), shows promise in this regard. When a phosphate group is cleaved from the ELF-97 reagent, the remaining molecule precipitates near the site of enzyme activity, thus fluorescently tagging cells with alkaline phosphatase activity. We characterized ELF-97 labeling in axenic cultures of a common dinoflagellate, Prorocentrum minimum, in order to understand ELF-97 labeling dynamics when phosphate nutrition varies. Enzyme activity, as detected by ELF-97 labeling, appears to be induced in late-log- or early-stationary-phase cultures if cells are grown in low-phosphate media and is lost when phosphate-stressed cells are refed with phosphate. ELF-97 appears to label an inducible intracellular alkaline phosphatase in P. minimum based on confocal microscopy studies. This may limit the use of this reagent to organisms that lack high levels of constitutive intracellular phosphatases. After laboratory cultures were characterized, ELF-97 was used to assay field populations of P. minimum in Narragansett Bay during two 1-week periods, and 12 to 100% of the P. minimum cells were labeled. The level of cell labeling was reduced by 3 days of incubation with added inorganic phosphate. Our results indicate that ELF-97 is an excellent new tool for monitoring phytoplankton phosphate stress in the environment when the data are supported by appropriate laboratory studies.
There is a long-standing debate as to whether phosphorus constrains primary production in marine environments. This debate can be traced back to Redfield, who concluded in his seminal 1958 paper that over geological time scales phosphorus is the critical nutrient in marine systems because of the ability of microorganisms to fix atmospheric nitrogen (27). In 1971 Ryther and Dunstan countered that P stress is common in freshwater systems, whereas N stress is found in marine systems (30). However, recent evidence indicates that P may play an important role in controlling primary production in a number of both coastal and open-ocean environments (20).
The N-versus-P debate has been perpetuated because the ability to assess the in situ physiology of phytoplankton populations has been limited and because physiological inferences based on nutrient concentration ratios and nutrient addition bioassay results are difficult to interpret (32). Data obtained by using advances in techniques for measuring in situ physiological characteristics, such as antibodies to a phosphate binding protein in Synechococcus sp. (31) and detection of phosphate-inducible bacterial porin P homologues (25, 34), have suggested that P stress occurs in microbial populations in two marine environments. Clearly, there is a need to develop these and other approaches for monitoring phytoplankton physiology at the single-cell level in order to increase our understanding of nutritional constraints on primary production.
One marker for phosphate stress in many phytoplankton is the enzyme alkaline phosphatase. This enzyme is often induced via de novo synthesis when the concentration of inorganic P drops below some threshold level so that the cells can utilize organic phosphate sources (4). Extracellular phosphatases cleave the phosphate moiety from dissolved organic phosphorus compounds, such as sugar phosphates, nucleotide phosphates, and phospholipids (26). Once released from the organic component, the free phosphate is taken up by the cells. Intracellular phosphatases may also be produced during phosphate stress and used for mobilization of internal P stores (3). Unfortunately, using alkaline phosphatase activity as an indicator of P stress in primary producers has been problematic because most substrates and products are soluble and can be hydrolyzed by a variety of organisms, including heterotrophic bacteria and zooplankton (4, 14, 33). Also, it appears that the mechanisms that regulate alkaline phosphatase activity, particularly when organisms are refed with P, can be different in different phytoplankton groups (4, 5, 7, 28, 29), so it is difficult to interpret bulk activities in relation to specific taxa that are experiencing P stress. Some of these problems have been addressed with the development of a single-cell assay for alkaline phosphatase activity in which the substrate ELF-97 (enzyme-labeled fluorescence; Molecular Probes, Eugene, Oreg.) is used. The product resulting from hydrolysis of this substrate can fluorescently label individual phytoplankton cells expressing the enzyme (8), and thus single-cell activity can be distinguished within a mixed assemblage, in contrast to bulk activity assays.
ELF-97 [2-(5′-chloro-2′-phosphoryloxyphenyl)-6-chloro-4-(3H)-quinazolinone] was originally developed for use in immunohistochemistry and in situ hybridization studies (17, 23). In the presence of alkaline phosphatase, a phosphate group is cleaved from this molecule, which results in conversion of the soluble colorless substrate to an intensely fluorescent and insoluble product, 2-(5′-chloro-2′-hydroxyphenyl)-6-chloro-4-(3H)-quinazolinone (Molecular Probes) (13, 17). This reaction can result in precipitation of the fluorescent compound at or near the site of enzyme activity and thus specifically label cells expressing the enzyme. ELF-97 appears to brightly label phytoplankton expressing alkaline phosphatase (8), and it has been used to detect in situ activity in bacterial colonies and biofilms (12).
In this research we developed and tested the use of ELF-97 as a diagnostic tool for identifying P stress in field populations of phytoplankton. In this paper we refer to phosphate stress and distinguish this condition from phosphate limitation of the growth rate or yield, which may or may not occur (22). The dinoflagellate Prorocentrum minimum was used as a model in this study because it is known to produce a cell surface alkaline phosphatase in response to P stress in culture (6). Moreover, P. minimum grows or has been found in a variety of potentially P-stressed environments, including Narragansett Bay in Rhode Island, and blooms of this species are of concern because of potential toxin production (9, 16, 19) and harm to shellfish (18). In this study we characterized the regulation and location of the enzyme labeled with the ELF-97 substrate and then used the substrate to look for evidence of P stress in P. minimum from Narragansett Bay.
MATERIALS AND METHODS
Cell culture.
Axenic P. minimum (Pavillard) Schiller CCMP 1329 was obtained from the Provasoli-Guillard Center for Culture of Marine Phytoplankton, Bigelow Laboratories. Axenic cell cultures were grown at 20°C by using a cycle consisting of 16 h of light and 8 h of near darkness; the light was provided by cool white fluorescent bulbs (797 mol of quanta · m−2 · s−1). Sterility was confirmed by microscopic examination and by testing for growth of contaminating organisms with a tryptone-fortified medium (1). P replete (+P) cells were grown in f/2 medium. Locally collected seawater was filtered (pore size, 0.2 μm) and autoclaved along with inorganic nutrients (853 μM NO3−, 36.3 μM PO4−3) and trace metals (10). Filter-sterilized thiamine (final concentration, 0.1 mg · liter−1), biotin (final concentration, 0.5 μg · liter−1), and vitamin B12 (final concentration, 0.5 μg · liter−1) were added after autoclaving. Low-phosphate (−P) medium was prepared by reducing the f/2 medium PO4−3 concentration to 1 μM. Low-nitrate medium was prepared by reducing the f/2 medium nitrate concentration to 50 μM. Most phytoplankton were grown in batch cultures that were harvested 24 h after the number of cells plateaued; the only exception was +P cells, which were harvested in the mid-log phase. Numbers of cells were determined with a hemocytometer.
ELF-97 labeling.
Cells were labeled with the ELF-97 product (referred to below as ELF-97 labeled or ELF-97 fluorescence) by using a procedure modified from the method of González-Gil et al. (8). Typically, a 9-ml sample of a culture containing about 5 × 104 cells · ml−1 was harvested by centrifugation at 3,000 × g for 10 min at room temperature. The resulting cell pellet was incubated in the dark in 1 ml of 70% ethanol for 30 min and then centrifuged at 1,500 × g for 5 min at room temperature in an IEC Micro Max microcentrifuge. The supernatant was discarded, and the remaining phytoplankton cells were incubated with 95 μl of sterile seawater and 5 μl of ELF-97 (Endogenous Phosphatase Detection Kit; Molecular Probes) for 30 min at room temperature in the dark. The cells were centrifuged again, resuspended in 100 μl of sterile seawater, centrifuged, and then resuspended in 100 μl of seawater prior to microscopic evaluation. The cells were examined for ELF-97 fluorescence by using a Zeiss Axioskop microscope equipped with a 200-W mercury arc lamp and a type G365 UV excitation filter set (catalog no. 487902; Zeiss). Chlorophyll autofluorescence was visualized with the same system by using a type H546 green excitation filter set (catalog no. 487915; Zeiss). Negative controls were treated as described above except that they were incubated in 100 μl of sterile seawater without ELF-97. Cells were scored as either negative or positive based on the absence or presence of the fluorescent green precipitate, without regard to the relative brightness of the individual cells. Unless otherwise noted, at least 100 cells were counted in triplicate and scored as either labeled or unlabeled, and the values were averaged when percentages were determined.
Alkaline phosphatase activity regulation.
To investigate induction of alkaline phosphatase activity, 1-liter cultures of P. minimum in −P medium were monitored daily after the initial inoculation. Aliquots were removed, and the inorganic phosphate concentration, cell number, and alkaline phosphatase activity were determined. P concentrations were determined with a Skalar SanPlus autoanalyzer by using standard methods as described below, and alkaline phosphatase activities were determined by using the ELF-97 substrate. The cell growth rate under the conditions used was about 0.3 day−1. To monitor the loss of activity after phosphate refeeding, 1-liter cultures were grown in −P medium until the onset of the stationary phase and then divided. Inorganic phosphate was added to one-half of each culture to a final concentration of approximately 36 μM. We assessed the cell number and ELF-97 labeling of the divided cultures over time.
Biotinylation experiments.
ELF-97 labeling was assessed by using P. minimum cells in which cell surface alkaline phosphatase activity was inhibited by biotinylation of surface-associated proteins. In this analysis −P cells were harvested from 1-liter cultures which were biotinylated. Biotin labeling of cell cultures with succinimidyl 6-(biotinamido) hexanoate was performed as described previously (6, 21). Cells were also harvested from an unlabeled culture by centrifugation at 3,000 × g at 18°C for 10 min. The resulting cells were assayed (as described above) with ELF-97 for alkaline phosphatase activity.
Confocal microscopy.
The location of ELF-97 labeling was studied by using confocal microscopy. Cells grown under −P conditions were labeled with ELF-97 as described above. Control cells were subjected to the labeling protocol without ELF-97. Microscopic slides were prepared by using 1 drop of mounting medium (Endogenous Phosphatase Detection Kit; Molecular Probes) and 10 μl of labeled cell suspension (in seawater). Coverslips were fixed to the slides with nail polish. Cells on these slides gave bright and consistent images for more than 4 weeks when they were stored in a damp container in the dark at 4°C. Pecorino et al. reported that the ELF-97 signal can be detected with the 488-nm line of the argon ion laser typically found in confocal and flow cytometry systems (24). However, in the study of Pecorino et al. the samples were not autofluorescent; in our study it was impossible to distinguish labeled cells from unlabeled cells either with the confocal microscope or with a Becton Dickinson FACSORT flow cytometer by using a 488-nm line. As a result, samples were processed with a model ARC 1024 confocal system (Bio-Rad Laboratories, Hercules, Calif.) mounted on a Nikon inverted microscope. A 450-nm long-pass emission filter was used for ELF-97 detection with the UV laser (excitation wavelength, 363 nm). A krypton-argon laser (excitation wavelength, 586 nm) equipped with a type 640 long-pass filter set was used to detect autofluorescence. A type B1 photo tube was used to collect sequential images with depth if chlorophyll and ELF-97 images were examined at the same time. ELF-97 images were obtained with a ×40, 1.7 numerical aperture oil objective with a gain of 1,400, a black level of −1, and an iris of 0.7 in a range of 0.7 to 8. Images were Kalman averaged at setting 5. The chlorophyll autofluorescence was faint compared to the ELF-97 fluorescence, most likely because of the ethanol fixation step, which visibly extracted pigment. To maximize the brightness of the autofluorescent images, the iris and gain were set to maximum values with the low signal settings and a −4 black level. The confocal images were exported to Adobe Photoshop.
Field site.
Narragansett Bay is located in the southeastern corner of Rhode Island. This relatively small bay is just south of Cape Cod and north of Long Island Sound. It is bordered by both major metropolitan areas, such as Providence, and agricultural land. There is a large tidal volume; the bay is consistently well-mixed, and there is a slight salinity gradient from about 20 ppt in the inner reaches of the bay to about 31 ppt at the mouth. For many decades this area has been experiencing blooms of several phytoplankton species (15), including P. minimum.
Water samples were collected daily from 1 June 1998 to 7 June 1998 and again from 27 June 1998 to 3 July 1998 from piers at two locations on Prudence Island in Narragansett Bay. Samples were collected in the morning at Potter’s Cove on the northern end of the island and in the afternoon at the T-Wharf, which is at the southernmost end of the island. Both sampling locations are part of the Narragansett Bay National Estuary Research Reserve, and as a result Potter’s Cove is monitored weekly for salinity, temperature, and dissolved oxygen content. Water was obtained with a Niskin bottle at a depth of 1 m.
Nutrient analysis of field samples.
Water samples were analyzed to determine their phosphate, silicate, nitrate, and nitrite contents. The samples were collected in 40-ml polypropylene, screw-cap centrifuge tubes which were cleaned with 10% HCl and then rinsed with sample water twice before they were filled. The samples were stored frozen at −20°C and then brought to room temperature prior to analysis. Nutrient analyses to determine phosphate, silicate, nitrate, and nitrite contents were performed with a Skalar SanPlus autoanalyzer by workers at the Ocean Data Facility (Scripps Institution of Oceanography); the methods recommended by the manufacturer were used. Calibration tests were performed at the beginning of each group of analyses by using mixed nutrient standards that were prepared prior to each analysis from a secondary standard in a low-nutrient seawater matrix. The samples used for the total P analysis were UV irradiated for 12 h in quartz tubes containing 30 μl of 30% H2O2 (Fisher, Fair Lawn, N.J.) to liberate inorganic P from organic sources, as described elsewhere (2). Then the samples were processed as P samples with the autoanalyzer. The organic P content was calculated by determining the difference between the total P and inorganic P values.
Counting cells in field samples.
Water samples were collected in 60-ml polypropylene bottles and fixed with 1% Lugol’s iodine solution (Sigma Chemical Co., St. Louis, Mo.). These samples were stored at room temperature in the dark until analyses were performed. Cells were counted and identified based on morphology by using a depression slide calibrated with a stage micrometer so that a total area of 265 mm2 representing 25 ml was counted. Samples were left for 48 h in a 50-ml settling chamber as described elsewhere (11), and cells were counted by using a Leitz inverted microscope at ×640 magnification.
ELF-97 labeling of field samples.
One liter of each water sample was filtered under a low vacuum through a 0.8-μm-pore-size Gelman SUPOR filter (Sigma) until the filter was just dry. The filter was then transferred to a sterile petri dish and washed gently with 1 ml of 70% ethanol. The ethanol cell suspension was stored at 4°C in the dark until it was processed as described above. Cells were considered either labeled or unlabeled based on the presence or absence of the bright fluorescent green ELF-97 precipitate. Several samples were counted as described above, and the average standard error for triplicate counts was about 3%. Subsequently, 10 μl of a 100-μl (final volume) preparation was examined to determine the percentage of labeled cells; this procedure was used rather than counting and averaging 100 cell replicates because of the difficulty involved in finding P. minimum in a mixed assemblage.
Incubation experiments.
Clear polycarbonate bottles were filled with 2 liters of seawater at the T-Wharf on 27 June 1998. Three bottles were left untreated as controls, and filter-sterilized inorganic P was added to three bottles so that the final concentration of P in each bottle was approximately 36 μM. Each bottle was sealed and incubated in situ. After 3 days of incubation, water was removed to count the cells and was processed and used for ELF-97 labeling. The percentages of P. minimum ELF-97 labeling in control and treatment bottles were compared by using an unpaired Student t test.
RESULTS
ELF-97 labeling.
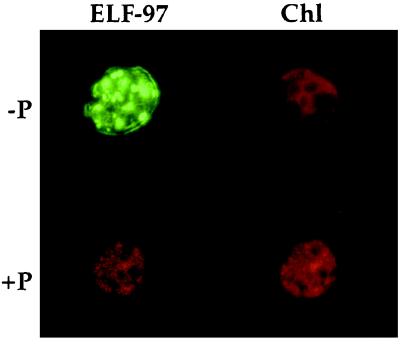
When our labeling protocol was used, ELF-97 fluorescence was associated with −P P. minimum but not with +P P. minimum (Fig. 1). Also, no fluorescence was associated with low-nitrate-grown cells (data not shown). Control samples of −P P. minimum which were subjected to the labeling protocol with seawater in place of the ELF-97 substrate looked similar to the +P samples. Microscopic visualization of ELF-97-treated cells often revealed a punctated labeling pattern, which was not always reflected in photographs. In many cases it was not clear whether the labeling should be considered intracellular or extracellular or both. Only rarely was the fluorescent ELF-97 product faintly associated with free thecal plates.
FIG. 1.
−P P. minimum cell labeled with green fluorescent precipitate after incubation with the ELF-97 alkaline phosphatase substrate, as visualized with a UV excitation filter set (ELF-97). Chlorophyll (Chl) fluorescence is included for reference. +P cells exhibited no green fluorescence after they were similarly labeled.
Alkaline phosphatase regulation.
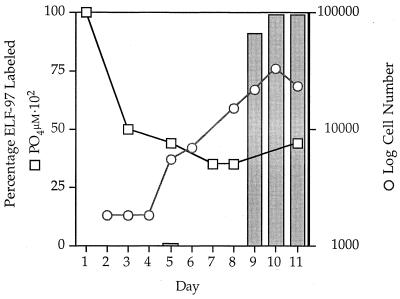
Induction of alkaline phosphatase activity was monitored by using ELF-97 labeling. The percentage of ELF-97-labeled cells increased from 0% on day 8 to more than 90% on day 9 after inoculation into −P medium (Fig. 2). It was impossible to distinguish the late log phase from the early stationary phase in this experiment because of the error involved in counting cells. The level of ELF-97 labeling appeared to be close to 100% as the cells entered the stationary phase, and P. minimum appeared to be consistently labeled at this high level well into the stationary phase. The inorganic P concentration was around 0.35 μM when the ELF-97 alkaline phosphatase activity increased on day 9.
FIG. 2.
Cell number (○), inorganic P concentration (□), and ELF-97 activity (expressed as a percentage of labeled cells) (bars) after inoculation into −P medium. ELF-97 activity was detected in either the late log or early stationary phase around day 9; at this time the P concentration was about 0.35 μM.
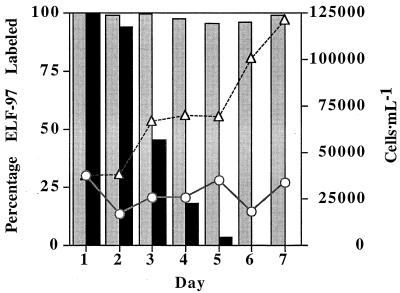
The loss of alkaline phosphatase activity after phosphate refeeding was studied. In a stressed culture, the number of cells remained relatively constant, and nearly 100% of the cells were ELF-97 labeled even after 7 days in the stationary phase. Conversely, the number of cells in the refed culture began to increase and the percentage of ELF-97-labeled cells steadily declined, reaching 0% after 6 days (Fig. 3). In these experiments, over time many of the cells which were labeled in P-refed cultures appeared to be less bright than cells in −P cultures. Since a microspectrofluorometer was not used, however, there was no straightforward way to quantify this change.
FIG. 3.
A P-stressed P. minimum culture was divided, and one-half was refed with inorganic P. The number of cells increased and the level of ELF-97 alkaline phosphatase activity decreased over time, reaching 0% after about 6 days in the culture to which P had been added. The level of ELF-97 labeling in the half of the culture which was not refed was consistently near 100%. The ELF-97 activity of refed (+P) cultures (solid bars), the ELF-97 activity of −P cultures (shaded bars), the +P cell number (▵), and the −P cell number (○) are shown.
Enzyme location.
As mentioned above, labeling of P. minimum (and other phytoplankton [8]) is punctate, and it is very difficult to visually identify the location of the fluorescence. Additionally, free thecal plates from lysed cells which were expected to have attached plasma membranes and thus membrane-bound alkaline phosphatase very rarely exhibited faint green fluorescence.
Biotinylation of −P P. minimum whole cells depresses cell surface alkaline phosphatase activity, as measured with the soluble alkaline phosphatase substrate p-nitrophenyl phosphate (p-NPP) (6). In contrast, the percentages of biotinylated and nonbiotinylated P. minimum cells that were labeled with ELF-97 were high and virtually identical (99.9 and 98.3%, respectively) (data not shown). Any differences in relative brightness were not distinct enough to quantify without a microfluorometer.
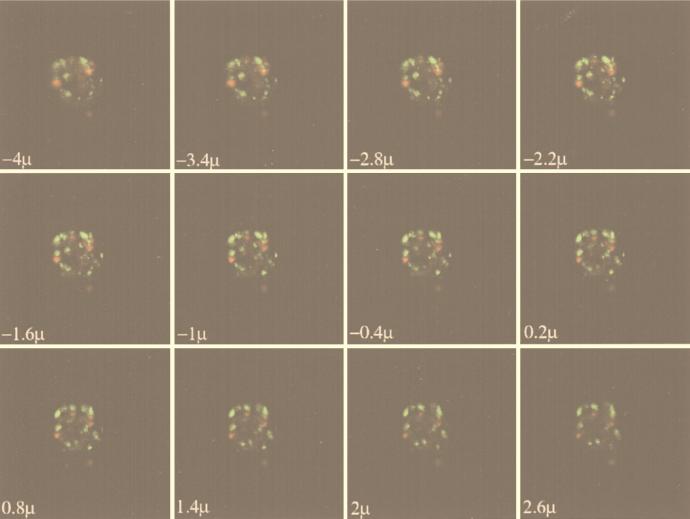
To further pursue the location of ELF-97 labeling, we used confocal microscopy. Confocal images with depth through several ELF-97-labeled cells revealed clear intracellular labeling (Fig. 4). This labeling was due to ELF-97 and was not due to autofluorescence, based on sequential overlaid images taken with depth of both autofluorescence and ELF-97 fluorescence. Moreover, the crystalline appearance and punctate labeling pattern were visually distinct from pigment autofluorescence in these cells. There did not appear to be any positive correlation between chloroplast location and ELF-97 labeling, and in general the autofluorescence of the cells was very dim compared to the fluorescence of ELF-97. Unlabeled cells exhibited no detectable green fluorescence when they were visualized with the same detection parameters; the autofluorescence was dim, and the pattern was similar to the background pattern observed with ELF-97-labeled cells.
FIG. 4.
Confocal images through a cell, showing clear intracellular labeling of P-stressed P. minimum as indicated by the appearance and disappearance of several areas of green ELF-97 labeling. Autofluorescence is represented by the color red and does not appear to be spatially associated with the green ELF-97 labeling. The images are of 0.3-μm sections taken through a cell (−5.8 to 4.4 μm) with a model 1024 confocal system (Bio-Rad). The actual section depth is indicated on each image. The cells used had stopped growing for 24 h in −P medium, and all of the labeled cells that were sectioned appeared to be similar. Unlabeled cells exhibited no ELF-97 fluorescence with the same detection parameters.
It has been reported previously that ELF-97 is very photostable compared to the common fluorochrome fluorescein (17, 23). In our study ELF-97 was also very photostable, both during weeks of storage and during an experiment, even at 100% laser power. Despite the intensity of the intracellular ELF-97 labeling, there did not appear to be any bright labeling directly associated with the cell surface. However, because of the presence of intracellular labeling and the resolution capabilities of the confocal system we cannot rule out the possibility that ELF-97 labeling of the surface-associated enzyme occurred as well.
Field study.
After the assay conditions, regulation, and location of ELF-97 labeling of P. minimum were determined, the ELF-97 substrate was used to assay a field population of the dinoflagellate in Narragansett Bay in Rhode Island. Samples were taken from two locations on Prudence Island, Potter’s Cove and the T-Wharf.
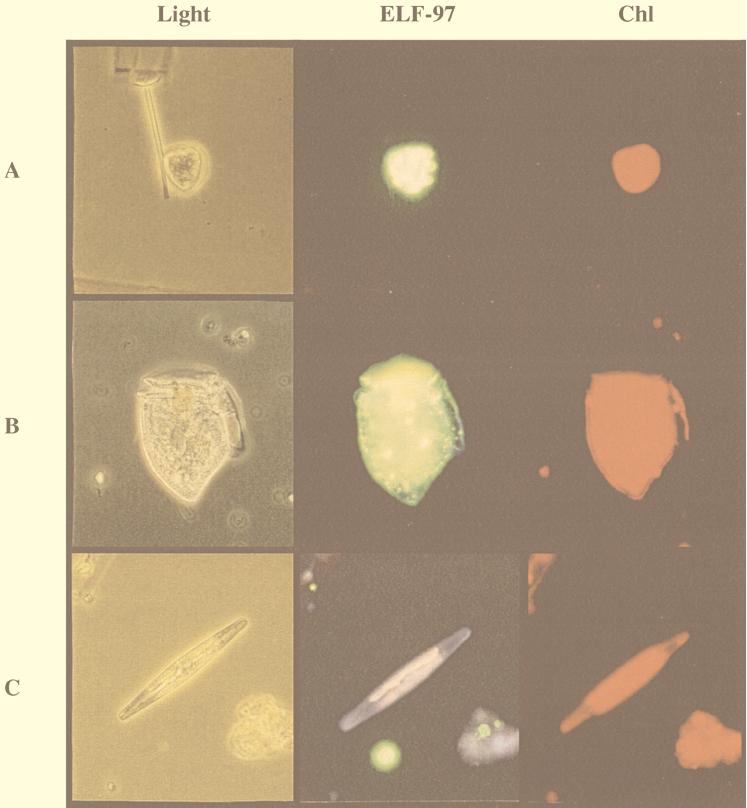
Clear ELF-97 labeling of P. minimum was evident in every sample obtained from both sampling sites in Narragansett Bay, indicating that P. minimum experienced inorganic P stress in this environment (Table 1). Several other dinoflagellates were also labeled with ELF-97, including Prorocentrum gracile, Dinophysis sp., and Ceratium sp. However, it is not known whether alkaline phosphatase activity is induced only under phosphate-stressed conditions in these species. Many other phytoplankton taxa were commonly found, particularly diatoms, but only very rarely were diatoms labeled. Figure 5 shows representative images of ELF-97-labeled P. minimum and Dinophysis sp. and an unlabeled diatom species.
TABLE 1.
Field data for two sampling sites in Narragansett Bay, Rhode Island
| Location | Date (mo/day/yr) | Cell no. (cells ml−1) | % of ELF-97-labeled cells | NO3 concn (μM) | NO2 concn (μM) | PO4 concn (μM) | Organic P concn (μM) | Ratio of PO4 to organic P | Si concn (μM) |
|---|---|---|---|---|---|---|---|---|---|
| Potter’s Cove | 6/1/98 | 0.44 | 91 | 0.17 | 0.02 | 0.46 | 0.62 | 0.74 | 3.9 |
| 6/2/98 | 0.44 | 69 | 0.1 | 0.01 | 0.4 | 0.60 | 0.67 | 3.0 | |
| 6/3/98 | 0.28 | 90 | 0.1 | 0.03 | 0.67 | 0.70 | 0.96 | 3.5 | |
| 6/4/98 | 0.16 | 70 | 0.05 | 0.01 | 0.79 | 0.48 | 1.65 | 4.65 | |
| 6/5/98 | 0.08 | 58 | 0.07 | 0.03 | 0.97 | 0.71 | 1.37 | 5.2 | |
| 6/6/98 | 0.16 | 80 | 0.33 | 0.07 | 0.87 | 0.86 | 1.01 | 3.6 | |
| 6/7/98 | 0.4 | 94 | 0.19 | 0.03 | 0.97 | 0.76 | 1.28 | 4.3 | |
| 6/27/98 | 10.64 | 63 | 0.1 | 0.03 | 1.28 | 0.39 | 3.28 | 2.8 | |
| 6/28/98 | 28.44 | 65 | 0.03 | 0.03 | 0.85 | 0.96 | 0.89 | 0.6 | |
| 6/29/98 | 9.72 | 51 | 0.16 | 0.06 | 1.08 | 0.30 | 3.6 | 2.7 | |
| 6/30/98 | NDa | ND | ND | ND | ND | ND | ND | ND | |
| 7/1/98 | 1.44 | 44 | 0.2 | 0.05 | 1.37 | 0.31 | 4.2 | 2.5 | |
| 7/2/98 | 1.8 | 74 | 7.38 | 0.74 | 1.22 | 0.39 | 3.13 | 10.3 | |
| 7/3/98 | ND | ND | ND | ND | ND | ND | ND | ND | |
| T-Wharf | 6/1/98 | 0.24 | 79 | 0.13 | 0.02 | 0.55 | 0.52 | 1.06 | 7.05 |
| 6/2/98 | 0.52 | 56 | 0.03 | 0.01 | 0.60 | 0.67 | 0.9 | 3.1 | |
| 6/3/98 | 0.6 | 88 | 0.20 | 0.02 | 0.62 | 0.49 | 1.27 | 5.0 | |
| 6/4/98 | 0.36 | 69 | 0.10 | 0.02 | 0.62 | 0.32 | 1.94 | 4.4 | |
| 6/5/98 | 0.52 | 62 | 0.07 | 0.02 | 0.61 | 0.58 | 1.05 | 4.7 | |
| 6/6/98 | 0.68 | 84 | 0.05 | 0.01 | 0.55 | 0.68 | 0.81 | 2.9 | |
| 6/7/98 | 0.4 | 100 | 0.25 | 0.04 | 0.99 | 0.40 | 2.48 | 2.7 | |
| 6/27/98 | 1.84 | 64 | 0.23 | 0.05 | 0.98 | 0.76 | 1.29 | 4.0 | |
| 6/28/98 | 14.32 | 17 | 0.02 | 0.02 | 0.78 | 0.36 | 2.1 | 1.45 | |
| 6/29/98 | 1.68 | 57 | 0.12 | 0.03 | 0.73 | 0.41 | 1.78 | 1.3 | |
| 6/30/98 | 0.48 | 78 | 0.32 | 0.04 | 0.77 | 0.40 | 1.93 | 2.6 | |
| 7/1/98 | 1.48 | 28 | 0.22 | 0.04 | 0.84 | 0.30 | 2.8 | 2.18 | |
| 7/2/98 | 19.64 | 12 | 0.05 | 0.02 | 0.74 | 0.97 | 0.76 | 1.5 | |
| 7/3/98 | 4.24 | 48 | 0.09 | 0.03 | 0.92 | 0.67 | 1.38 | 0.5 |
ND, not determined.
FIG. 5.
Micrographs of cells from field samples. (Light) Light micrographs of P. minimum (A), Dinophysis sp. (B), and an unidentified diatom species (C) from field samples obtained in Narragansett Bay, Rhode Island. (ELF-97) Cells visualized with epifluorescence to detect the ELF-97 label with a UV excitation filter set. Note the distinct punctate labeling of the fluorescent green ELF-97 precipitate associated with the two dinoflagellates and not with the diatom. (Chl) Chlorophyll autofluorescence visualized with a green excitation filter set.
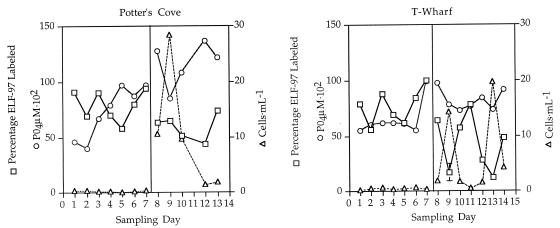
ELF-97 labeling of P. minimum, the inorganic P concentration, and the number of cells varied over time and between the two sites. Both at the sheltered Potter’s Cove site and at the more exposed T-Wharf site there appeared to be two different regimes delineated by the two distinct 1-week sampling trips (1 June 1998 to 7 June 1998 and 27 June 1998 to 3 July 1998). For the first sampling trip in general we obtained higher percentages of ELF-97 labeling in the P. minimum population, low cell numbers, and low inorganic P concentrations. The second regime, as reflected by data from the second sampling trip, was characterized by lower percentages of ELF-97 labeling, higher cell numbers, and higher inorganic P concentrations (Fig. 6). Within the two different general regimes there were often large fluctuations in the ELF-97-labeled alkaline phosphatase activity and cell numbers (Fig. 6 and Table 1). The organic P concentrations did not appear to be correlated with either an increase or a decrease in the level of ELF-97 labeling and were consistently low in all of the samples. However, the ratio of inorganic P to organic P did change; it was generally low for the first sampling trip and higher for the second trip (Table 1). The nitrate and nitrite concentrations were both consistently low with the exception of the 2 July 1998 sample collected at the Potter’s Cove site; in all cases the ratio of dissolved inorganic nitrogen (DIN) to dissolved inorganic phosphate (DIP) was less than 15 (Table 1), which suggests that the system was nitrate stressed.
FIG. 6.
Cell number (▵), ELF-97 labeling (□), and inorganic P concentration (○) at the two different sites on Prudence Island, showing that there were two distinct regimes delineated by days 1 to 7 (1 June 1998 to 7 June 1998) and days 8 to 14 (27 June 1998 to 3 July 1998). The first regime was characterized by high levels of ELF-97 labeling, low cell numbers, and low inorganic P concentrations. The second regime was characterized by lower levels of ELF-97 labeling, higher cell numbers, and higher inorganic P concentrations. The standard errors were low for the ELF-97-labeled samples for which they were calculated, and thus the standard error is shown for only one sample.
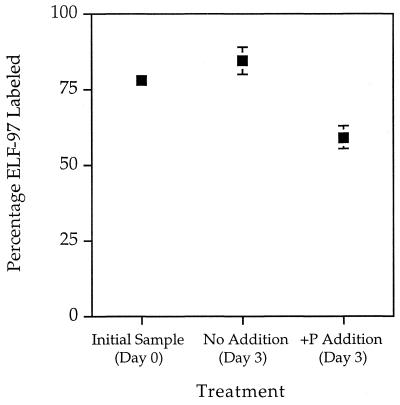
Triplicate incubation experiments were performed to assess the change in ELF-97 labeling after P refeeding. There was a significant difference in the levels of ELF-97 labeling between the control and treatment bottles (84.67 and 59.33%, respectively) (P < 0.05) (Fig. 7). This difference was about what we expected based on the rate of ELF-97 signal decline observed in laboratory experiments performed with cultures. The average number of cells after the +P treatments (2.36 cells · ml−1) was about 1.5 times higher than the average number of cells in the control bottles (1.49 cells · ml−1).
FIG. 7.
Seawater samples were taken on 30 June 1998 and assayed for P. minimum ELF-97 labeling (Initial sample, Day 0). Water was then sealed in triplicate clear polycarbonate bottles and incubated in situ with either nothing added (No Addition, Day 3) or inorganic P added (+P Addition, Day 3) to a final concentration of approximately 36 μM. After 3 days, cell numbers and ELF-97 labeling were assessed. There was a significant difference in the levels of ELF-97 labeling between the control and +P treatments (84.6 and 59.3%, respectively; P < 0.05). This indicates that the activity of ELF-97-labeled alkaline phosphatase, our marker for P stress, was repressed when cells from field populations were refed with phosphate.
DISCUSSION
Alkaline phosphatase activity detected by ELF-97 labeling is clearly regulated by phosphate. −P cells are brightly fluorescently labeled, and +P cells are not. ELF-97-labeled alkaline phosphatase activity appears to be rapidly induced in the late log or early stationary phase in −P cells, and stressed cells eventually lose activity if they are refed with P. The loss of alkaline phosphatase activity after refeeding appears to be relatively slow; there is about a 50% decrease after 3 days. This rate is similar to the rate observed for a cell surface alkaline phosphatase assayed with p-NPP in the same species (5). Phytoplankton alkaline phosphatases appear to have different regulatory mechanisms, and at this time it is difficult to say whether the ELF-97-labeled enzyme is actively degraded or simply diluted out as new, unstressed cells continue to grow.
The data presented here indicate that ELF-97 is able to label intracellular alkaline phosphatase activity. This was shown by the dramatic intracellular ELF-97 labeling in the confocal images and is consistent with biochemical evidence which shows that cell surface biotinylation inhibits most p-NPP-detected activity but not ELF-97 activity.
P. minimum is known to have an inducible cell surface alkaline phosphatase (6), yet the majority of ELF-97 labeling observed in our experiments appeared to be intracellular. It appears that either the surface-associated enzyme is not labeled with ELF-97 or the ELF-97 precipitate associated with surface activity is washed away from the cells during the labeling protocol. The latter is possible as the method used for ELF-97 labeling requires several centrifugations and ELF-97 was originally developed for thin-section immunohistochemistry.
Many algal alkaline phosphatases are surface associated (4), and the results of previous studies have implied that ELF-97 labeling is surface associated in Isochrysis sp. and Amphidinium carterae (8), although photographs of Isochrysis sp. and A. carterae samples appear to be similar to photographs of P. minimum in which the labeling is predominately if not exclusively intracellular. In Alexandrium fundyense, an important harmful algal bloom species, very low levels of ELF-97 activity were detected regardless of the P content of the media, and González-Gil et al. suggested that this organism had a constitutively expressed, intracellular alkaline phosphatase (8). The location of ELF-97 labeling is important because whereas numerous surface-associated alkaline phosphatases are inducible, intracellular alkaline phosphatases can be constitutively expressed (4, 26). This suggests that it is important to test induction and repression with cultured representatives before ELF-97 is applied to field populations of other phytoplankton species as a diagnostic tool for P stress.
The primary goal of the Narragansett Bay field work was to test ELF-97 with natural populations of P. minimum. P. minimum populations from both sampling locations appeared to experience P stress, as detected by ELF-97 labeling. The presence of unlabeled cells in ELF-97-labeled samples served as an internal control, and samples processed without ELF-97 as negative controls did not exhibit any ELF-97 fluorescence. To our knowledge, this is the first field application of this technique for detecting in situ P stress in phytoplankton. Few diatoms were labeled, but numerous other phytoplankton taxa were labeled with ELF-97, including P. gracile, Ceratium sp., and Dinophysis sp. The labeling of these and other species is encouraging for broad application of the ELF-97 substrate. However, Dinophysis sp. is difficult to culture, and P regulation of the ELF-97 alkaline phosphatase activity in the other dinoflagellates has not been assessed. Therefore, P stress cannot truly be attributed to these organisms at this time.
Two sampling trips which were approximately 1 month apart revealed two different regimes with regard to the P status of the P. minimum population in Narragansett Bay. During the first trip the high level of ELF-97 labeling and the low number of cells indicated that P stress may have constrained P. minimum abundance. This hypothesis was supported by the relatively low concentrations of inorganic and organic P. It appears that the P concentrations were low enough to induce alkaline phosphatase activity but that there was not enough organic P available to release the constraint on cell numbers or to downregulate the enzyme activity.
The data obtained from the second sampling trip revealed a different regime, in which the number of cells was higher and in general the level of ELF-97 labeling was lower. These data support a scenario in which the cells were released from P stress, which repressed ELF-97 alkaline phosphatase activity and increased the number of cells. This idea is supported by the increased inorganic P concentrations characteristic of the second sampling trip. What is unclear is whether the release from P stress was due to inorganic P or organic P. It is important to note that alkaline phosphatase activity can also drive the P supply by liberating inorganic P from organic sources. Long-term monitoring of a more southern site near the Narragansett Bay mouth indicated that the number of P. minimum cells increases each year from about mid-June to early July (15), which is consistent with our abundance data. These dynamics may reflect a common yearly pattern, in which increased P. minimum abundance is driven in part by P nutrition.
Incubation experiments confirmed, however, that ELF-97-labeled alkaline phosphatase present in field populations can be downregulated by inputs of inorganic P based on the significant decreases in the percentages of the P. minimum population that were ELF-97 labeled in the treated bottles. Cells in the control bottles appeared to have slightly increased percentages of ELF-97 labeling compared to the water samples obtained before incubation, which may have been due to bottle effects. However, the inorganic P concentration was relatively low (near 0.77 μM) prior to incubation, and the increases in the level of ELF-97 labeling may have been due to decreases in the P concentration in the bottles over the course of the incubation. P addition also resulted in an increase in the average total cell number, which also indicated that the cells were released from P stress. In the bottle experiments the average cell number was about 1.5 times higher in +P bottles.
It is interesting that nutrient data alone did not predict P stress without ELF-97 labeling as biochemical evidence of in situ physiology because the N concentrations were fairly low and the DIN/DIP ratios were less than the Redfield ratio (27), which suggests N stress. This again demonstrated that it can be difficult to infer nutrient stress in phytoplankton from nutrient concentrations in the water due to the variable nutritional history of the organisms and to the fact that nutrient cycling rates may not be reflected by one-time assessments of nutrient concentrations.
The field data described above represent a brief snapshot of P. minimum physiological ecology at the two study sites. The data indicate that P nutrition may have an important role in regulating the abundance of this organism, but this role cannot be substantiated without more extensive time series studies. Clearly, more detailed spatial and temporal sampling is needed in Narragansett Bay to definitively address how P influences growth and bloom dynamics in this species. Regardless of the P-related P. minimum dynamics, ELF-97-based alkaline phosphatase activity assays are clearly feasible in field populations of phytoplankton, which is an important step forward in our ability to study phytoplankton physiology in situ.
In summary, we used the alkaline phosphatase substrate ELF-97 as a tool for monitoring P stress in natural assemblages of P. minimum. It seems likely that ELF-97-based detection of alkaline phosphatase activity may be applicable to numerous phytoplankton taxa; however, our direct evidence of intracellular labeling and the potential for differential regulation of the enzyme in different species mandate that laboratory studies of cultured, representative organisms precede the use of ELF-97 assays with field populations. The use of ELF-97 is not likely to immediately reveal which nutrients constrain primary production in the marine environment. However, tools such as ELF-97 should allow in situ measurement of P physiology and should increase our ability to understand how phytoplankton nutrition influences the physiological ecology of important phytoplankton species.
ACKNOWLEDGMENTS
This research was supported in part by NSF Biological Oceanography grant OCE96-33111 to B.P. Funds were also provided to S.T.D. by the University of California Toxicology Research and Teaching Program, the Scripps Institution of Oceanography Graduate Department, the PEO Chapter International, and the ARCS Foundation.
We especially thank Allan Beck of the Narragansett Bay National Estuarine Research Reserve for assistance, equipment, laboratory space, and housing during the field component of this study. Field assistance was also provided by Jennifer Hall and Michelle Moore. We also acknowledge Jeffrey Price of the UCSD Confocal Microscopy and Imaging Center for training of S.T.D. in the use of the confocal microscope.
REFERENCES
- 1.Anderson R A, Jacobson D M, Sexton J P. Provasoli-Guillard Center for Culture of Marine Phytoplankton: catalogue of strains. West Boothbay Harbor, Maine: Provasoli-Guillard Center for Culture of Marine Phytoplankton; 1991. [Google Scholar]
- 2.Armstrong F A J, Williams P M, Strickland J D H. Photo-oxidation of organic matter in sea water by ultra-violet radiation, analytical and other applications. Nature. 1966;211:481–483. [Google Scholar]
- 3.Cembella A D, Antia N J, Harrison P J. The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective. Part 2. Crit Rev Microbiol. 1984;11:13–81. doi: 10.3109/10408418409105902. [DOI] [PubMed] [Google Scholar]
- 4.Cembella A D, Antia N J, Harrison P J. The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective. Part 1. Crit Rev Microbiol. 1984;10:317–391. doi: 10.3109/10408418209113567. [DOI] [PubMed] [Google Scholar]
- 5.Dyhrman, S. T., and B. Palenik. Unpublished data.
- 6.Dyhrman S T, Palenik B P. The identification and purification of a cell-surface alkaline phosphatase from the dinoflagellate Prorocentrum minimum (Dinophyceae) J Phycol. 1997;33:602–612. [Google Scholar]
- 7.Dyhrman S T, Palenik B P. EOS Transactions, American Geophysical Union 1998 Ocean Sciences Meeting. Washington, D.C: American Geophysical Union; 1997. Using cell-surface proteins to identify phosphate-limitation in Emiliania huxleyi, abstr. OS31M-9; p. OS103. [Google Scholar]
- 8.González-Gil S, Keafer B, Jovine R V M, Anderson D M. Detection and quantification of alkaline phosphatase in single cells of phosphorus-limited marine phytoplankton. Mar Ecol Prog Ser. 1998;164:21–35. [Google Scholar]
- 9.Grzebyk D, Deardon A, Berland B, Puochus Y F. Evidence of a new toxin in the red-tide dinoflagellate Prorocentrum minimum. J Plankton Res. 1997;19:1111–1124. [Google Scholar]
- 10.Guillard R R L. Culture of phytoplankton for feeding marine invertebrates. In: Smith W C, Chanley M H, editors. Culture of marine invertebrate animals. New York, N.Y: Plenum; 1975. pp. 29–60. [Google Scholar]
- 11.Hasle G R. The inverted microscope method. In: Sournia A, editor. Phytoplankton manual. Paris, France: UNESCO; 1978. pp. 89–96. [Google Scholar]
- 12.Huang C-T, Xu K D, McFeters G A, Stewart P S. Spatial patterns of alkaline phosphatase expression within bacterial colonies and biofilms in response to phosphate starvation. Appl Environ Microbiol. 1998;64:1526–1531. doi: 10.1128/aem.64.4.1526-1531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z, You W, Haugland R P, Paragas V B, Olson N A, Haugland R P. A novel fluorogenic substrate for detecting alkaline phosphatase activity in situ. J Histochem Cytochem. 1993;41:313–317. doi: 10.1177/41.2.8419466. [DOI] [PubMed] [Google Scholar]
- 14.Jamet D, Boge G. Characterisation of marine zooplankton alkaline phosphatase activity in relation to water quality. Hydrobiologia. 1998;374:311–316. [Google Scholar]
- 15.Karentz D, Smayda T J. Temperature and seasonal occurrence patterns of 30 dominant phytoplankton species in Narragansett Bay over a 22-year period (1959–1980) Mar Ecol Prog Ser. 1994;18:277–293. [Google Scholar]
- 16.Kat M. The occurrence of Prorocentrum species and coincidental gastrointestinal illness of mussel consumers. In: Taylor D, Seliger H, editors. Toxic dinoflagellate blooms. Amsterdam, The Netherlands: Elsevier North Holland; 1979. pp. 215–220. [Google Scholar]
- 17.Larsion R R, BreMiller R, Wells K S, Clements I, Haugland R P. Use of a new fluorogenic phosphatase substrate in immunohistochemistry applications. J Histochem Cytochem. 1995;43:77–83. doi: 10.1177/43.1.7822768. [DOI] [PubMed] [Google Scholar]
- 18.Luckenbach M. Effects of two bloom-forming dinoflagellates on the growth and survival of the eastern oyster. J Shellfish Res. 1993;12:411–415. [Google Scholar]
- 19.Okaichi T, Imatomi Y. Toxicity of Prorocentrum minimum var. mariae-lebouriae assumed to be a causative agent of short-necked clam poisoning. In: Taylor D, Seliger H, editors. Toxic dinoflagellate blooms. Amsterdam, The Netherlands: Elsevier North Holland; 1979. pp. 385–394. [Google Scholar]
- 20.Palenik B, Dyhrman S T. Recent progress in understanding the regulation of marine primary production by phosphorus. In: Lynch J P, Diekman J, editors. Phosphorus in plant biology: regulating roles in molecular, cellular, organismic and ecosystem processes. Rockville, Md: American Society of Plant Physiologists; 1998. pp. 26–38. [Google Scholar]
- 21.Palenik B, Koke J. Characterization of a nitrogen-regulated protein identified by cell surface biotinylation of a marine phytoplankton. Appl Environ Microbiol. 1995;61:3311–3315. doi: 10.1128/aem.61.9.3311-3315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palenik B, Wood A M. Molecular markers of phytoplankton physiological status and their application at the level of individual cells. In: Cooksey K E, editor. Molecular approaches to the study of the oceans. London, United Kingdom: Chapman and Hall; 1997. pp. 187–205. [Google Scholar]
- 23.Paragas V B, Zhang Y, Haughland P, Singer V L. The ELF-97 alkaline phosphatase substrate provides a bright, photostable, fluorescent signal amplification method for FISH. J Histochem Cytochem. 1997;45:345–357. doi: 10.1177/002215549704500302. [DOI] [PubMed] [Google Scholar]
- 24.Pecorino L T, Brockes J P, Entwistle A. Semi-automated position analysis using laser scanning microscopy of cells transfected in a regenerating newt limb. J Histochem Cytochem. 1996;44:559–569. doi: 10.1177/44.6.8666741. [DOI] [PubMed] [Google Scholar]
- 25.Poole K, Hancock R. Phosphate-starvation-induced outer membrane proteins of members of the families Enterobacteriaceae and Pseudomonodaceae: demonstration of immunological cross-reactivity with antiserum specific for porin protein P of Pseudomonas aeruginosa. J Bacteriol. 1986;165:987–993. doi: 10.1128/jb.165.3.987-993.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price N, Morel F. Role of extracellular enzymatic reactions in natural waters. In: Stumm W, editor. Aquatic chemical kinetics: reaction rates of processes in natural waters. New York, N.Y: Wiley-Interscience; 1990. pp. 235–258. [Google Scholar]
- 27.Redfield A C. The biological control of chemical factors in the environment. Am Sci. 1958;46:205–222. [PubMed] [Google Scholar]
- 28.Rivkin R, Swift E. Diel and vertical patterns of alkaline phosphatase activity in the oceanic dinoflagellate Pyrocystis noctiluca. Limnol Oceanog. 1979;24:107–116. [Google Scholar]
- 29.Rivkin R, Swift E. Characterization of alkaline phosphatase and organic phosphorus utilization in the oceanic dinoflagellate, Pyrocystis noctiluca. Mar Biol. 1980;61:1–8. [Google Scholar]
- 30.Ryther J H, Dunstan W M. Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science. 1971;171:1008–1013. doi: 10.1126/science.171.3975.1008. [DOI] [PubMed] [Google Scholar]
- 31.Scanlan D J, Silman N J, Donald K M, Wilson W H, Carr N G, Joint I, Mann N. An immunological approach to detect phosphate stress in populations and single cells of photosynthetic picoplankton. Appl Environ Microbiol. 1997;63:2411–2420. doi: 10.1128/aem.63.6.2411-2420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Söderström J. The significance of observed nutrient concentrations in the discussion about nitrogen and phosphorus as limiting nutrients for the primary carbon flux in coastal water ecosystems. Sarsia. 1996;81:81–96. [Google Scholar]
- 33.Taft J, Loftus M, Taylor W. Phosphate uptake from phosphomonoesterases by phytoplankton in the Chesapeake Bay. Limnol Oceanog. 1977;22:1012–1021. [Google Scholar]
- 34.Tanoue E, Nishiyama S, Kamo M, Tsugita A. Bacterial membranes: possible source of a major dissolved protein in seawater. Geochim Cosmochim Acta. 1995;59:2643–2648. [Google Scholar]