Abstract
Chile is in the extreme southwestern part of America, and it has an extreme length, of approximately 4300 km that increases to 8000 km considering the Chilean Antarctic Territory. Despite the large extent of its coastal territory and the diversity of geographic environments and climates associated with Chilean coasts, the research on marine resources in Chile has been rather scarce. From marine organisms found in Chilean coastal waters, algae have been the most studied, since they contain a wide range of interesting secondary metabolites that have some structural traits that make them unique and uncharacteristic. Thus, a wide structural variety of natural products including terpenoids (monoterpenes, sesquiterpenes, diterpenes, and meroterpenoids), furanones, and C15-acetogenins have been isolated and identified. This review describes the existing literature on bioprospecting and exploration of secondary metabolites from Chilean coasts.
Keywords: marine natural products, secondary metabolites, Chilean algae, biological activities, biosynthesis, structure elucidation
1. Introduction
Marine natural products have served as a rich source of new bioactive agents [1,2,3]. The diversity of marine habitats and unique sea environmental conditions have enabled marine organisms to develop mostly untapped sources of potential drugs with superior chemical novelty [4]. During the last decades, much effort has been dedicated to isolating and identifying new compounds from marine organisms, with the interesting outcome that many of these derivatives exhibited biological activities [5,6,7,8,9,10]. Algae are one of the simplest organisms containing chlorophyll, and, therefore, are found in almost every place where there is light to perform photosynthesis, namely in seas, lakes, rivers, animals and plants (as symbiotic species) [11]. They can be found as colonies of single-celled or multicellular organisms, and in some cases collaborating as simple tissues. Consequently, their size varies from 3–10 µm (unicellular algae) to 70 m long, i.e., giant kelp species growing up to 50 cm per day. Algae are classified into two major groups: microalgae, found both in benthic environments (littoral) and in the ocean (phytoplankton), and macroalgae (marine algae) red, brown and green algae, established in the littoral zone [12]. Phytoplankton comprise organisms such as diatoms (Bacillariophyta), dinoflagellates (Dinophyta), green and yellow-brown flagellates (Chlorophyta; Prasinophyta; Prymnesiophyta, Cryptophyta, and Rhaphidiophyta) and blue-green algae (Cyanophyta). These photosynthetic organisms play an important role in the productivity of oceans and are at the base of the food chain [11].
Marine macroalgae have been used in a number of important areas including the food industry [13], agriculture [14] and as raw materials on third-generation bioplastics [15]. However, despite their extended use for decades in traditional medicinal therapies, and the huge number of bioactive compounds that have been extracted and identified, algae are still underrepresented in the pharmaceutical industry. For example, compounds isolated from seaweeds showed interesting biological activities such as: antiprotozoal [16], antimicrobial [17,18,19], antifouling [20,21], anticancer [22], antileishmanial [23] and anti-inflammatory properties [24]. Between these compounds are: terpenoids, sterols, phenols, peptides, polysaccharides, acrylic acid, vitamins, proteins, heterocyclic compounds, chlorophyllides, halogenated ketones and alkanes as well as cyclic polysulfides [25]. From the large variety of metabolites isolated from algae, the most abundant compounds are terpenes, including monoterpenes, sesquiterpenes and diterpenes. These compounds, which are formed by different numbers of isoprene units (2-methylbuta-1,3-diene), have been found in volatile oils of terrestrial plants as well as in seaweed [26,27]. All of them possess great potential for further development in pharmacological applications [28].
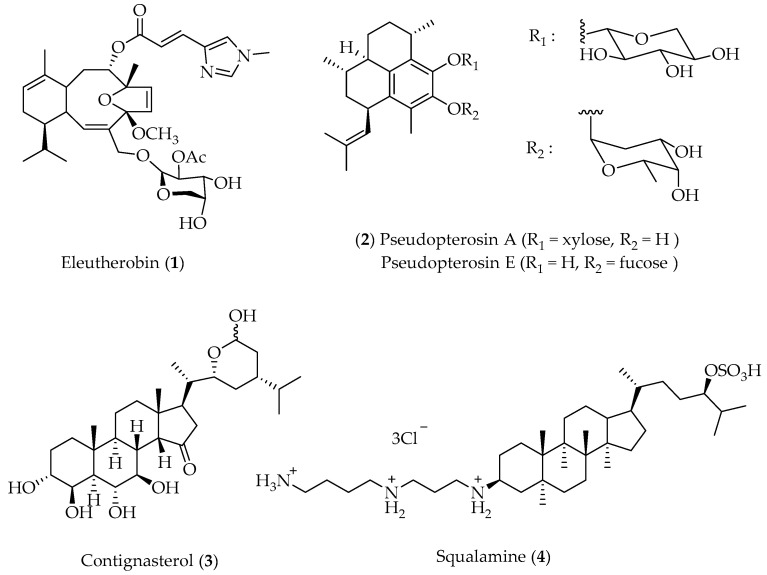
The biological importance of marine terpenes, as illustrated by their ecological role, may also be exploited in terms of their pharmacology. From this point of view, several biologically active terpenoids have been reported with biomedical potential, and some of them are already in preclinical or clinical development [29]. Eleutherobin (1) (Figure 1) originates from a soft coral of the genus Eleutherobia collected from Australian waters [30] and has been re-isolated along with congeners from another octocoral from the Caribbean [31], which can be maintained by aquaculture [32]. In preclinical experiments, eleutherobin has been employed as stabilizer of microtubuline, and it competes for the paclitaxel binding site on the microtubule polymer [33]. The anti-inflammatory pseudopterosins A–E (2) (Figure 1) are diterpene glycosides characterized by the presence of an amphilectane type skeleton. They were obtained from the gorgonian coral Pseuodopterogorgia elisabethae by Fenical’s group in the late 1980s [4,34]. Marine organisms, mainly sponges, contain unusual sterols such as contignasterol (3) (Figure 1), isolated from Petrosia contignata [35,36,37,38,39]. Contignasterol and its derivatives exhibit anti-inflammatory effects. Squalamine (4) (Figure 1) is a water-soluble cationic amino sterol occurring in the liver and stomach tissues of Squalus acanthias. The structure was published in 1993 and initially, it was reported as a potent antimicrobial agent with antibacterial, antifungal and anti-protozoic properties [40,41]. Subsequent studies demonstrated that this compound inhibits angiogenesis and tumor growth in various models [42], making it a good candidate for drug development as an innovative anticancer agent. Thus, a large quantity of terpenic compounds with important biological activity, isolated from different marine organisms, have been described in the literature [43].
Figure 1.
Terpenoid drug leads.
It is worth noting that the reason why seaweeds produce such a vast spectrum of secondary metabolites is because they live in nonfriendly environments, and, therefore, they are forced to synthesize protective compounds and to develop protective mechanisms [44]. Abiotic stresses to which algae are exposed include rapid fluctuations in light intensity, temperature, osmotic stress and desiccation, which induce the formation of free radicals and oxidizing agents leading to photodynamic damage [45].
The benthic habitat maps of continental Chile show special topographic characteristics that have joined with coastal currents and winds to enhance the spread of algae, and consequently, around 440 species have been identified [46]. Therefore, the study of Chilean algae is a challenging task, and they are probably a unique source of new compounds with interesting biological activities [47]. Thus, in this work we present a review of the literature on terpenes, C15-acetogenins, and furanones as secondary metabolites isolated from Chilean marine algae. Scifinder databases as well as the repositories of the Pontificia Universidad Católica de Chile and Universidad Técnica Federico Santa María were used to search reports published from 1967 to the present. Regarding the search methods, these involved filtering by authors, e.g., Blunt, J.W.; San-Martín, A.; Darias, J.; Silva, M.; Norte, M., as well as by keywords, e.g., secondary metabolites, brown algae, and red algae, to name a few. The search criteria focused on algae obtained from Chilean coasts and the South Shetland Islands and reports of novel marine natural products. The latter were spectroscopically characterized and present interesting biological and pharmaceutical properties. Reports involving vegetable extracts or primary metabolites were omitted. If some contributions (works or results) were omitted, it was due to an unintended error that we deeply regret.
2. Secondary Metabolites Isolated from Chilean Algae
2.1. Terpenoids
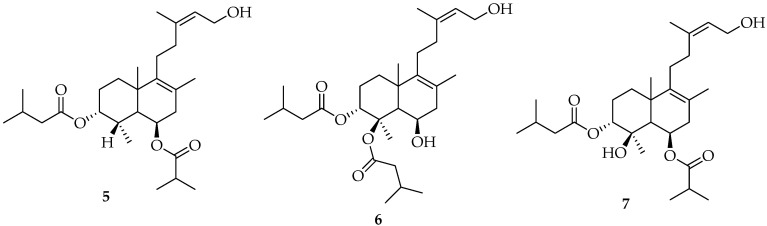
Chilean waters and coasts have turned out to be a fertile source of a variety of marine organisms from which new terpenes have been obtained. For instance, the terpenes shown in Figure 2 were isolated and identified from Trimusculus peruvianus, a marine mollusk collected near Antofagasta coast, Chile. Compound 5, identified as a new terpene, and previously reported compounds, 6 and 7 were assayed for their toxicity against Artemia saline [1,48,49,50].
Figure 2.
Terpenoids isolated from Trimusculus peruvianus.
2.1.1. Structural Features of Marine Terpenes
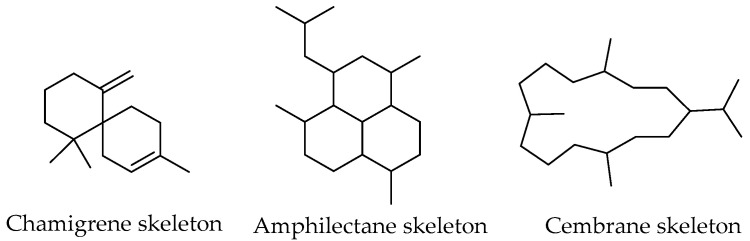
Some marine terpenes are known to have unique structural features. For instance, chamigrene [51], amphilectane [52], and cembrane [53] (Figure 3) exhibit unusual structures as well as uncommon functionalities, such as dichloroimine, isonitrile, isocyanate, isothiocyanate, and halogenated functions that are predominantly found in marine organisms. However, these functional groups are not exclusively marine [43].
Figure 3.
Common skeletons of terpenes isolated from marine organisms.
Algae are frequently found in marine habitats, where they are exposed to environmental microorganisms. However, algae survive in these hostile environments, probably due to an inherently available chemical defense mechanisms. Therefore, many novel terpenes, such as monoterpenes [54], sesquiterpenes [55,56], diterpenes [57,58], meroterpenoids [59], and steroids [60,61], have been normally isolated from different seaweeds [62]. Hence, this review will be focused on terpenes isolated from marine algae collected in Chilean coasts, classified according to the number of carbon atoms present in their structural nuclei, and on their biological activities.
2.1.2. Monoterpenes
Monoterpenes with multiple halogen substitution and uncommon carbon ring structures have been obtained mainly from red algae (Rhodophyta), and from green and brown algae, as well. They can be linear or cyclic (even heterocyclic) compounds [62].
Linear Polyhydroxylated Monoterpenes
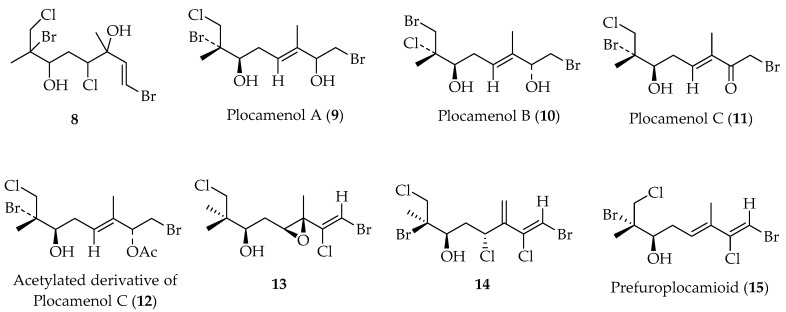
Linear polyhalogenated monoterpene (8) and three plocamenols A–C (9–11) (Figure 4), are new compounds that were isolated from P. cartilagineum [63,64].
Figure 4.
Linear polyhydroxylated monoterpenes isolated from Chilean algae.
Compound 10 contains a terminal bromohydrin, whereas 11 is the corresponding keto derivative. To verify the correct HMBC correlations, 11 was acetylated using acetic anhydride in pyridine, leading to compound 12 [64]. Compounds 9 and 10, along with costatol, are the first reported metabolites having a double bond conjugate bromohydrin [64]. Additionally, empirical rules based on 13C and 1H NMR spectroscopic analysis have been proposed to determine the regiochemistry and geometry of the 1,2-bromochloro vinyl portion. This is extremely relevant since it can be applied to any compound of natural or synthetic origin containing this functionality [65].
This structural feature was also found in two novel monoterpenes, 13 and 14, (Figure 4), isolated from P. cartilagineum. The effect of γ-substituents on chemical shifts of C-1 and H-1 of the 1,2-dihalo vinylic portion was observed and used to validate the previously reported empirical rules for determining regio- and stereochemistry of substituted vicinal vinyl dihalide [66].
Prefuroplocamioid (15) (Figure 4) was isolated from P. cartilagineum, collected along the coast of Chile. This compound has been considered as a precursor of furoplocamioids (see below, compounds 34 and 35) [65], suggesting that biosynthesis of the 1,2-bromochloro vinyl system occurs previously to oxetane ring formation of furoplocamioids.
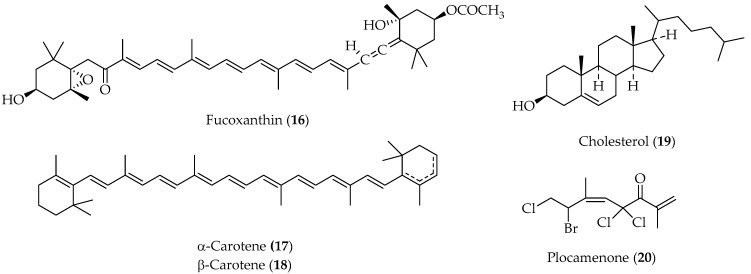
Several known compounds, including fucoxanthin (16), α and β-carotene (17 and 18), cholesterol (19) and plocamenone (20) (Figure 5) were isolated from the red algae, Ceramium rubrum, and spectroscopically characterized. The halogenated monoterpene 20 had been previously isolated and identified from the red algae Plocamium sp. [67].
Figure 5.
Secondary metabolites isolated from Chilean algae species Ceramium rubrum.
Cyclohexane Polyhalogenated Monocyclic Monoterpenes
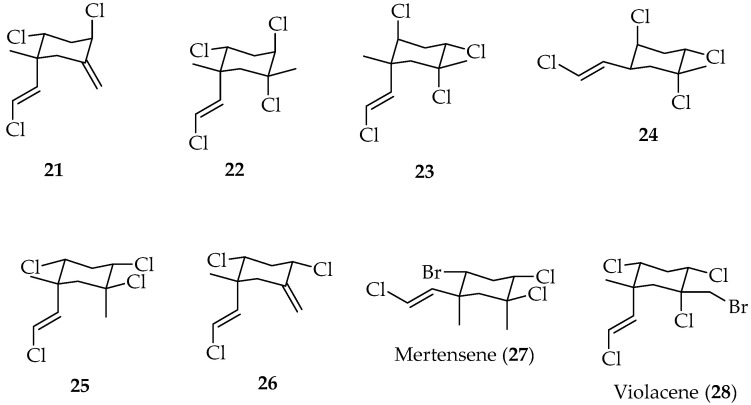
Polyhalogenated monocyclic monoterpenes, 21–28 (see Figure 6), were identified from red algae Plocamium cartilagineum collected at different points on the central Chilean coast (La Herradura (IV Region), Montemar and El Tabo (V Region), La Boca and Punta de Perros (VI Region), and Pumillahue (Chiloé, X Region)). Mertensene (27) and violacene (28), two brominated compounds, were identified spectroscopically by comparison with authentic samples [68].
Figure 6.
Polyhalogenated monocyclic monoterpenes, isolated from Chilean algae.
A study carried out with samples of P. cartilagineum, collected in two different geographical areas (Quintay (V Region) and La Boca (VI Region)) showed very interesting results. The plant materials, divided into carposporophyte, tetraspomphyte and gametophyte-bearing plants, exhibited the same qualitative chemical composition but important quantitative differences for these three reproductive phases. Depending on their capacity to incorporate bromine, the collections were classified as α chemotype, terpenes with no bromine, and β chemotype, brominated monoterpenes. Compounds 21, 22 and 26 were identified only in La Boca samples, whereas compounds 27 and 28 were characteristic of Quintay samples, and compounds 23–25 (Figure 6) were common to both collections [69]. The fungicide and insecticide/acaricide activities of several isolated derivates were determined; 28 showed the most potent insecticide activity among the compounds tested, mainly against Macrosteles facifrons [70].
Halogenated monoterpenes, 21–25 (Figure 6), were also isolated from Shottera nicaensis, which was collected intertidally at La Boca (VI Region), Chile. The total amount of compounds isolated from S. nicaensis was one order of magnitude lower than that obtained from P. cartilagineum, i.e., 0.04% and 0.5% dry weight, respectively. Even though for both algae the mixture composition turned out to be identical, small changes in the relative composition were observed. Both algae grow together, but their morphology and taxonomy are so different that it is almost impossible to mix them up. Even more unlikely is the idea that algae belonging to two different families have a common enzymatic system that allows the elaboration of identical compounds of almost the same composition. These facts seem to agree with Crew’s hypothesis about the algae Microcladia and Shottera growing in association with Plocamium, i.e., Microcladia and Shottera are able to concentrate the halogenated metabolites produced by Plocamium algae [71].
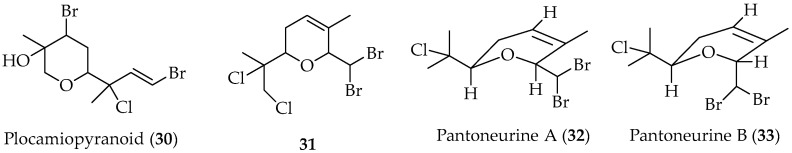
Finally, compound 29 (Figure 7) was isolated from the endemic Antarctic species Pantoneura plocamioides and P. cartilaginuem L. (Dixon), marine algae with a wide geographic distribution [72].
Figure 7.
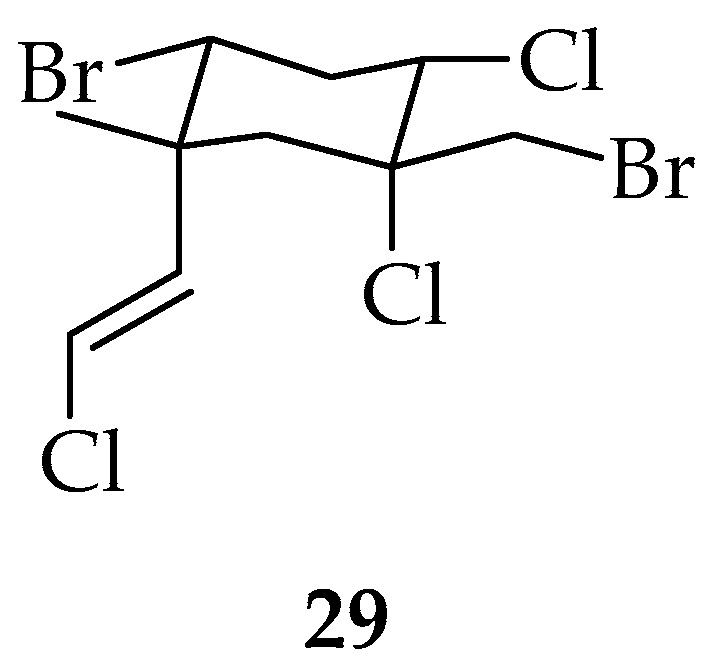
Polyhalogenated monocyclic monoterpene, obtained from endemic Antarctic species Pantoneura plocamioides and P. cartilaginuem L. (Dixon).
Compound 28 exhibited the greatest insecticidal activity, while 27 showed a moderate activity against Aphis fabae [73].
Tetrahydropyran Monoterpenes
Four new tetrahydropyran monoterpenes, 30–33 (Figure 8), have been isolated, and their structures and relative stereochemistry were determined using spectroscopic evidence [74]. Compounds 30 and 31 were obtained from P. cartilagineum, collected in El Yeco (V Region, Chile), whereas 32 and 33 were isolated from P. plocamioides, collected off King George Island (South Shetland, Antarctic).
Figure 8.
Tetrahydropyran monoterpenes isolated from Chilean algae.
Tetrahydrofuran Monoterpenes
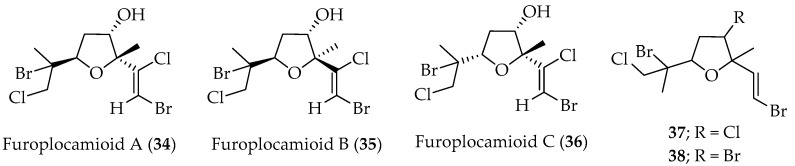
Tetrahydrofuran monoterpenes carrying non-common functional substituents, i.e., chloro or bromo vinyl groups, were identified in samples of P. cartilagineum collected off the central coast (V Region) of Chile. These compounds, 34–36 (Figure 9), are closely related to pantofuranoids obtained from the endemic Antarctic algae P. plocamioides, which indicates a close relationship between these species. The relative stereochemistry of these compounds was determined by spectroscopic experiments and molecular mechanics (MM2) calculations [75].
Figure 9.
Tetrahydrofuran monoterpenes isolated from Chilean algae.
Two new related tetrahydrofuran halogenated monoterpenes, 37 and 38 (Figure 9), were isolated from P. cartilagenium, collected at El Quisco, in the V Region of Chile. Structural elucidation of these compounds has been reported [63] and indicates the presence of unusual vicinal vinyl dihalide, like that observed in furoplocamioids (A–C) (34–36).
Antifeedant activities of halogenated monoterpenes, i.e., 21, 22, 24, 27, 28, 31, 32–34, 36, 37, and 39, were tested against Myzus persicae, Leptinotarsa decemlineata and Ropalosiphum padi. It is worth noting that none of these derivatives showed phytotoxic effects [72].
In the same line, compounds 21, 22, 24, 27, 28, 31, and 34 have been tested for their cytotoxic activity on tumor cell lines CT26, SW480, HeLa and SkMel28 with several multidrug resistance mechanisms, and on mammalian non-tumor cell line CHO (Chinese hamster ovary cells). Results showed that compound 31 presents selective activity against SW480 and HeLa cells. An analysis of cellular extracts posterior to incubation with the assayed compounds and rotenone (positive uptake control) showed intracellular accumulation of 22, 27, 31 and 34 [76].
On the other hand, the effect of photon flux density (PFD) and temperature on the relative growth rate (RGR) of P. cartilagineum and formation of three halogenated monoterpenes, 22, 27 and 28, has been assessed [77].
2.1.3. Sesquiterpenes
Sesquiterpenes isolated from seaweeds can be classified, according to their carbon skeletons, into the following groups: laurene, chamigrane, brasilane, bisabolene, cuparane, and others [62]. Additionally, sesquiterpenes isolated from red algae are characterized by an elevated number of halogenated substitutions. These compounds play important roles, such as to defend algae against predators, fouling organisms and pathogens, as well as reproduction and protection against UV radiation, and serve as allelopathic agents.
Laurencia (Rhodophyceae) is the marine macroalgae genus that represents the most important source of sesquiterpenes. The reasons for this are, in the first place, that algae belonging to this genus are extremely widespread in the world, mainly from tropical and subtropical regions; second, they present a notable ability to biosynthesize a diversity of structurally different sesquiterpenes with new skeletons, such as (seco)- or (9,10-friedo)-chamigrane, (cyclo) perforane, guimarane, and poitane. Brown algae and green algae also partly contribute to marine sesquiterpenes. However, the presence of halogenated compounds is very unusual.
Chamigrene Sesquiterpenes
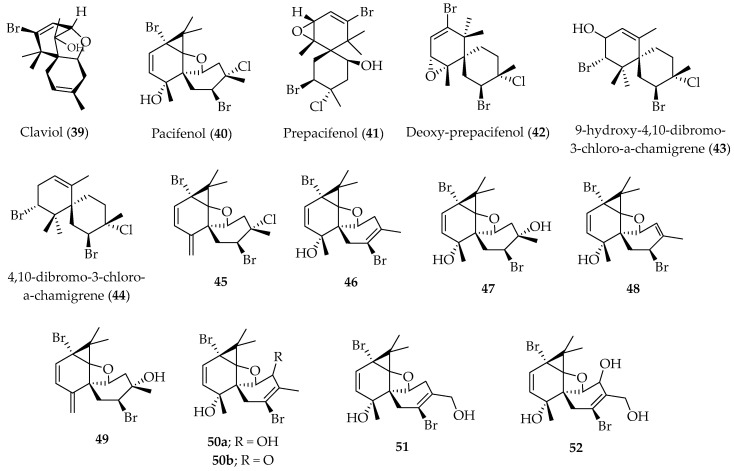
Claviol (39) and sesquiterpenes with the chamigrene skeleton, namely pacifenol (40), prepacifenol (41), deoxy-prepacifenol (42), 9-hydroxy-4,10-dibromo-3-chloro-α-chamigrene (43), and 4,10-dibromo-3-chloro-α-chamigrene (44), have been isolated from the red alga Laurencia claviformis, an endemic Easter Island species (see Figure 10) [78].
Figure 10.
Claviol (39) and sesquiterpenes with chamigrene skeleton isolated from Chilean red alga Laurencia claviformis.
All of these compounds were assayed for inhibition of cytokinesis in the sea urchin Tetrapygus niger embryos, and 43 was identified as the most active compound. However, its activity could be considered mild as compared to that shown by stypoldione, another active marine compound with ED50 = 1.1 µg/mL. Nevertheless, this test seems to be a reasonable prescreen to determine which substances merit further evaluation for antineoplastic properties [78].
Finally, microbial transformation of pacifenol (40), and two semisynthetic derivatives, 45 and 46 (Figure 10), by Aspergillus níger, Gibberella fujikuroi and Mucor plumbeus, yielded new hydroxylated derivatives, 47–52 (Figure 10) [79].
2.1.4. Diterpenes
Diterpenoids are found in higher plants, insects, fungi, and marine organisms. Several of these compounds present antimicrobial, antitumor, cytotoxic, anti-inflammatory, antifungal, molluscicide, antifeedant, and antifouling activities [43]. With more than 40 species reported, the genus Dictyota has been presented as a powerful resource for diterpenic compounds with novel chemical structures. Cyclic diterpenes are produced by many members of this genus, just like typical diterpenes with a 6-methyl-5-hepten-2-yl side chain A. Three types of main carbon skeletons have been reported: dolabellanes (including dolastanes), xenicanes, and extended sesquiterpenes [62].
Perhydroazulene Diterpenes
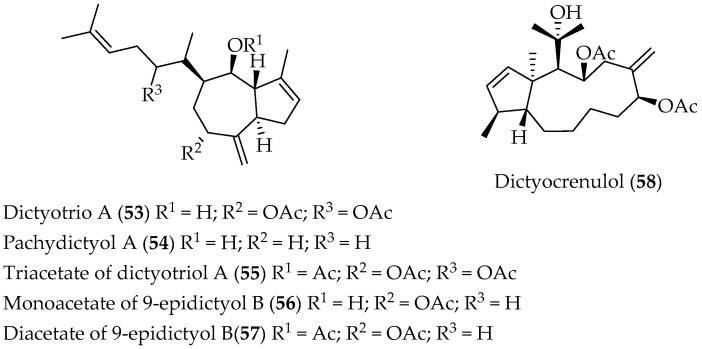
Phytochemical study of brown algae Dictyota crenulata, collected at Vaihú, Easter Island, allowed chromatographic isolation of five diterpenes, 53–57 and 58 (Figure 11), whose chemical structures were determined by spectroscopic techniques [80,81].
Figure 11.
Perhydroazulene diterpenes isolated from the Chilean brown alga Dictyota crenulata.
Compounds 53 and 55 were tested against Schizaphis graminum and Artemia salina, and insecticidal activity against Tomato moth (Tuta absolute) was assayed as well [81].
Xenicane Diterpenes
A new diterpene with xenicane skeleton, 59 (Figure 12), has been obtained from Glossophora kunthii, collected at Valparaiso, Chile [82].
Figure 12.
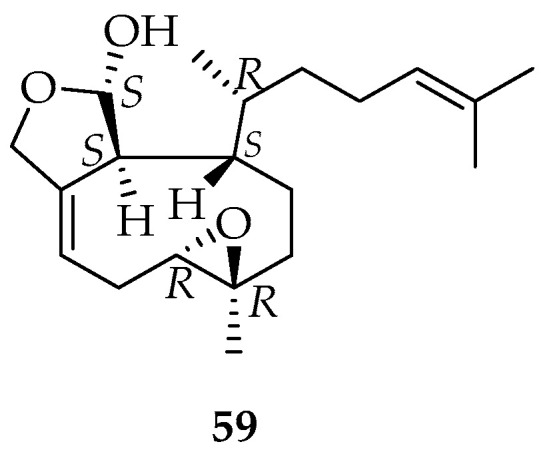
New xenicane diterpene isolated from the Chilean brown alga Glossophora kunthii.
This new metabolite was fully characterized, including absolute stereochemistry. Other diterpenes, such as pachydictyol A and dictyotriol A C-12 monoacetate, were identified in this alga and their absolute configuration was determined by CD studies (exciton chirality method). These diterpenes are frequently isolated in the Dictyota genus [83].
Crenulides Diterpenes
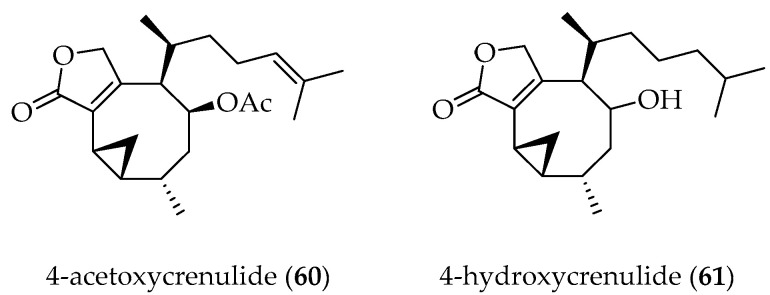
Two new crenulide diterpenes, 60 and 61 (Figure 13), have been obtained from the brown alga G. kunthii, collected at Horcones Bay, V Region, Chile. Their structures have been elucidated by spectral analysis and chemical correlation [84].
Figure 13.
Crenulide diterpenes isolated from the Chilean brown alga Glossophora kunthii.
Plastoquinone Diterpenes
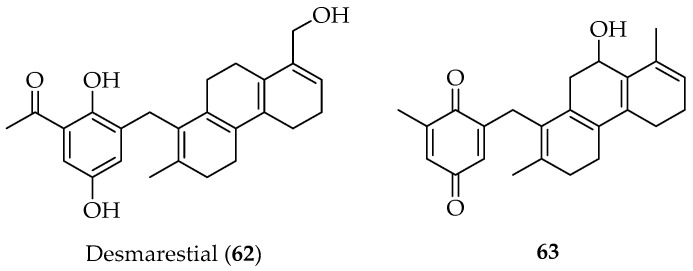
Finally, two new plastoquinone diterpenes of mixed biogenesis, 62 and 63 (Figure 14), were isolated from the brown alga Desmarestia menziesii, collected near the Antarctic Peninsula (Chilean Base Arturo Prat) [85].
Figure 14.
Plastoquinone diterpenes isolated from the Chilean brown alga Desmarestia menziesii.
2.1.5. Meroterpenoids
Meroterpenoids are prenylated aromatic compounds of mixed biogenesis combining acyclic, monocyclic, and bicyclic terpenes with aromatic or substituted aromatic groups possessing different degrees of oxidation. Many meroterpenoids from seaweeds have interesting biological activities such as antibacterial, antiviral, and antifeeding properties [62]. Plants in the genera Humulus and Cannabis produce these metabolites [86].
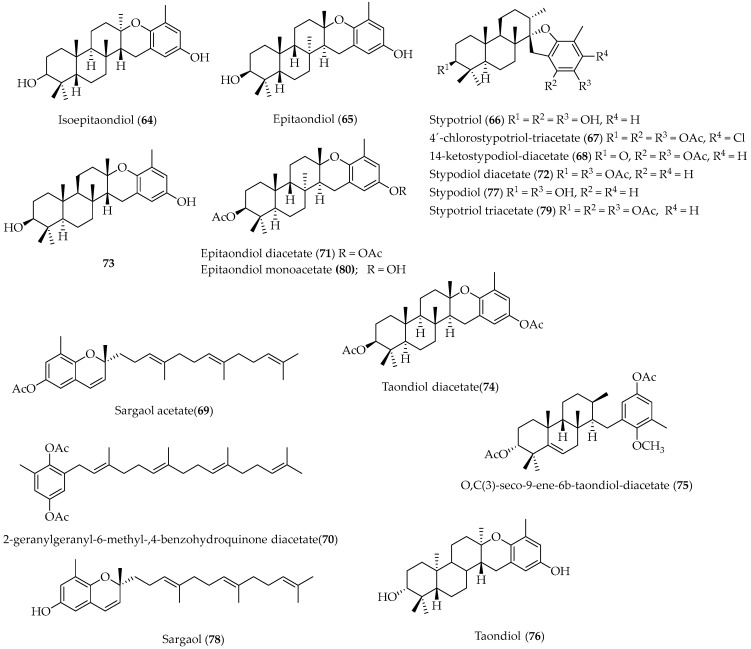
Seventeen meroterpenoids, 64–80 (Figure 15), were isolated from alga Stypopodium flabelliforme, collected at Easter Island [87,88,89,90,91,92]. The epitaondiol (65) structure was fully revised, and a meroditerpenoid containing an unusual two fused six-membered rings forced into the twist-boat conformation was demonstrated [87].
Figure 15.
Meroterpenoids isolated from Chilean brown alga Stypopodium flabelliforme.
Structural elucidation of 67 and 68 (Figure 15) was carried out through spectroscopic analysis and theoretical studies. Remarkably, 67 was the first metabolite isolated from the Stypopodium genus that presents one halogen in its structure. At this point, it is worth noting that based on published NMR data of isoepitaondiol (64) (Figure 15), it was observed that the structure of compound 64 was wrongly assigned, and that the right structure of this compound corresponds to 65. The relative configuration of this compound was confirmed by single-crystal X-ray diffraction, while the absolute configuration was evidenced by vibrational circular dichroism in combination with DFT B3LYP/DGDZVP calculations [91].
Taondiol (76) (Figure 15) has been isolated previously from this Stypopodium species. The absolute configurations of (−)-taondiol diacetate (74) and (+)-epitaondiol diacetate (71) isolated previously were determined using vibrational circular dichroism (VCD). For verification, their relative stereochemistry was determined by X-ray diffraction. Additionally, the crystal stereo structure of meroditerpenoid (79) (Figure 15) was reported [92].
Insecticidal activities of compounds 65, 66 and 71 (Figure 15) were tested, mainly against Spodoptera frugiperda, and 71 showed the highest anti-insect activity. On the other hand, compound 71 showed no activity towards the National Cancer Institute’s test (U.S.A.) for agents active against HIV (killing of T lymphocytes by HIV) [88]. The inhibitory effects of 66 (Figure 15) were studied. Additionally, the molecular action of this compound on microtubule assembly was also analyzed [89,90].
The meroditerpenoids stypodiol (77), isoepitaondiol (64), and epitaondiol (65) exhibited gastroprotective activity in mice [93].
Compound 65 and sargaol (78) were tested on HCl/ethanol-induced gastric lesions in mice and compared with lansoprazole. Both 65 and 78 showed gastroprotective activity with ED50 values between 35 mg/kg and 40 mg/kg. The results suggest that 65 and 78 protect the gastric mucosa in the HCl/EtOH model in mice [94].
Pacifenol (43) (Figure 10), stypotriol triacetate (79) and epitaondiol (65) (Figure 15) were assayed for their anti-inflammatory effects. Compound 65 showed an important topical anti-inflammatory activity, whereas the other compounds showed a non-significant effect. Compound 65 inhibited human recombinant synovial phospholipase A2 activity in a concentration-dependent manner, whereas 40 effectively inhibited the degranulation response, but none of these compounds affected superoxide generation by human neutrophils [95].
Six meroditerpenoids, 65, 68, 71, 77, 79 and 80 (Figure 15), were tested for their cell proliferation inhibitory activity in five cell lines: Caco-2, SH-SY5Y, RBL-2H3, RAW.267 and V79. Overall, these compounds showed good activity against all cell lines, with SH-SY5Y and RAW.267 being the most susceptible. Their antimicrobial activity was also evaluated against Enterococcus faecalis, Staphylococcus aureus, Proteus mirabilis, Salmonella typhimurium, Bacillus cereus, and Micrococcus luteus. Antimicrobial capacity was observed for 77, 79 and 80, with the first being the most active [96].
2.2. C15-Acetogenins
Acetogenins are compounds biosynthesized from ethyl acetate or acetyl coenzyme A. Several halogenated C15-acetogenins, possessing acetylenes, allenes, and oxygen heterocycles, have been isolated from seaweeds [62]. Both linear and cyclic C15-acetogenins have been reported.
2.2.1. Linear Polyhalogenated C15-Acetogenins
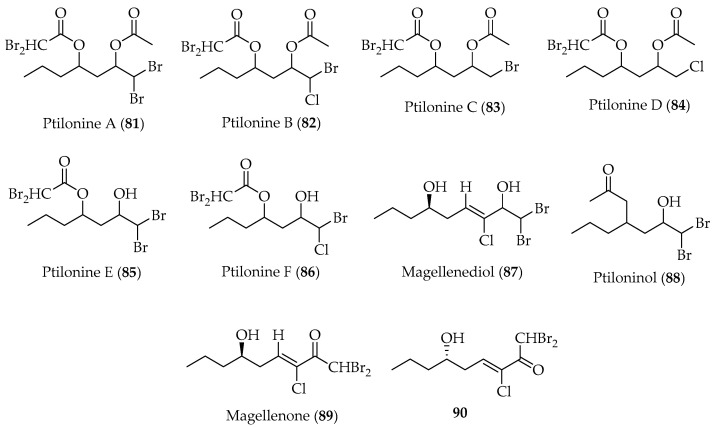
Studies carried out on the red alga Ptilonia magellanica, collected around Fuerte Bulnes (Punta Arenas, XII region, Chile) at 3 m depth, led to isolation and identification of fifteen metabolites belonging to a single biosynthetic class [97,98]. Ptilonines A–F (81–86), magellenediol (87), magellenone (88) and ptiloninol (89) (Figure 16) are novel linear acetogenins that are described for the first time. Compounds 81–84 and 89 (Figure 16) were tested for their antimicrobial activity [97]. Results show that only compound 89 exhibited antibacterial activity against K. pneumoniae (MIC ≈ 100 mg/mL). It is worth noting that ptilonines present an unusual halogenation substitution pattern, which may confer evolutionary advantages to P. magellanica, for which a biogenetic origin is proposed [97].
Figure 16.
Linear polyhalogenated C15-acetogenins isolated from the Chilean marine alga Ptilonia magellanica.
2.2.2. Cyclic Polyhalogenated C15-Acetogenins
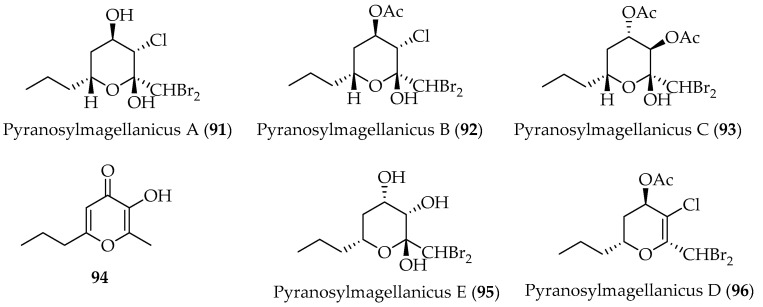
Previously reported γ-pyrone (94) [99] and five new cyclic polyhalogenated acetogenins, namely pyranosylmagellanicus A–C (91–93) and pyranosylmagellanicus D–E (95 and 96) (Figure 17), were obtained from the red alga P. magellanica [97,98]. These new metabolites are polyhalogenated pyranosyl-like hemiacetals that represent a novel structural type of acetogenin, being the first derivatives within the genus that incorporate chlorine in their structure [98]. These cyclic acetogenins present a common biosynthetic precursor, the linear acetogenin 90 (Figure 16).
Figure 17.
Cyclic polyhalogenated C15-acetogenins isolated from Chilean marine alga Ptilonia magellanica.
The absolute configuration of the known pyranosylmagellanicus A (91), was determined by treatment of 91 with (R)- and (S)-α-methoxy-α-phenylacetic acids (MPA). Compounds 91–93 were tested for their antimicrobial activity, but no activity was found [97].
2.2.3. Bromoallene C15-Acetogenins
Acetogenins that end in an enyne group are produced by algae from the genus Laurencia. Thus, (3Z)-13-epipinnatifidenyne (97) (Figure 18), a new C15-acetogenin, was obtained from the red alga L. claviformis collected at Easter Island. The structure of 97 was determined using 1D and 2D spectral analysis [100].
Figure 18.
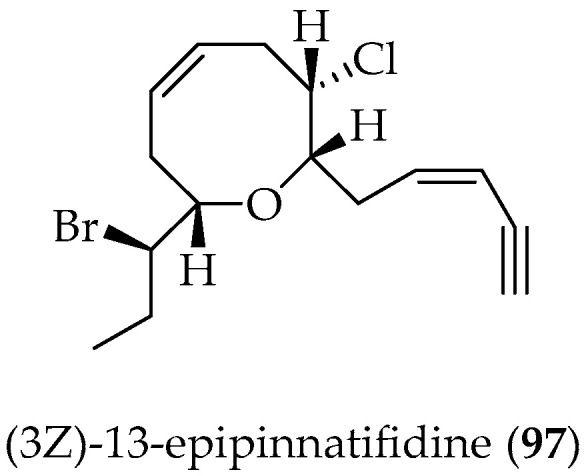
Bromoallene C15-acetogenins isolated from Chilean marine alga Laurencia claviformis.
2.3. Furanones
2-Furanone is a heterocyclic organic compound. It is also known as γ-crotonolactone (GCL), as it is formally the lactone derived from γ-hydroxyisocrotonic acid. 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®, HDMF, also 4-hydroxy-2,5-dimethyl-2,3-dihydrofuran-3-one) was identified for the first time in 1960, as a product of the Maillard reaction or nonenzymatic browning [101]. Previously, the synthesis and quorum sensing modulating effects of halogenated furanones isolated from the algae and their synthetic analogues have been reported [102].
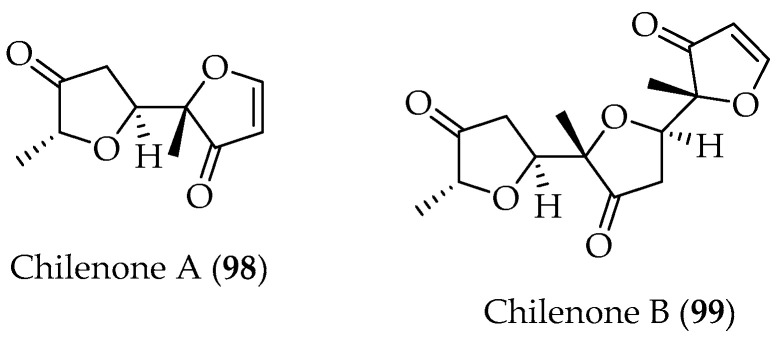
Chilenone A (98) (Figure 19) was obtained from Laurencia chilensis, collected at Horcones Bay, Chile, and its structure was determined by spectroscopic and X-ray crystallographic techniques. The structure of 98 is unusual, and there are no previously reported antecedents in this respect. The potential precursor, 2-methyl-3(2β)-furanone has been known since 1929, but it has not been reported previously from any natural sources [103]. Chilenone B (99) (Figure 19) was obtained from a posterior collection of the same algae and its structure was established by X-ray diffraction and spectroscopic experiments. Compound 99 was identified as a trimer of 2-methyl-3(2H)-furanone [104].
Figure 19.
Furanones isolated from Chilean Marine Algae Laurencia chilensis.
Finally, a new tetracyclic polyketal 100 (Figure 20) was identified from the marine red alga L. chilensis, collected in Cocholgüe, Concepción Bay, VIII region, Chile. This compound was isolated from chloroform extract, recrystallized from ethyl acetate and finally characterized by X-ray diffraction [105].
Figure 20.
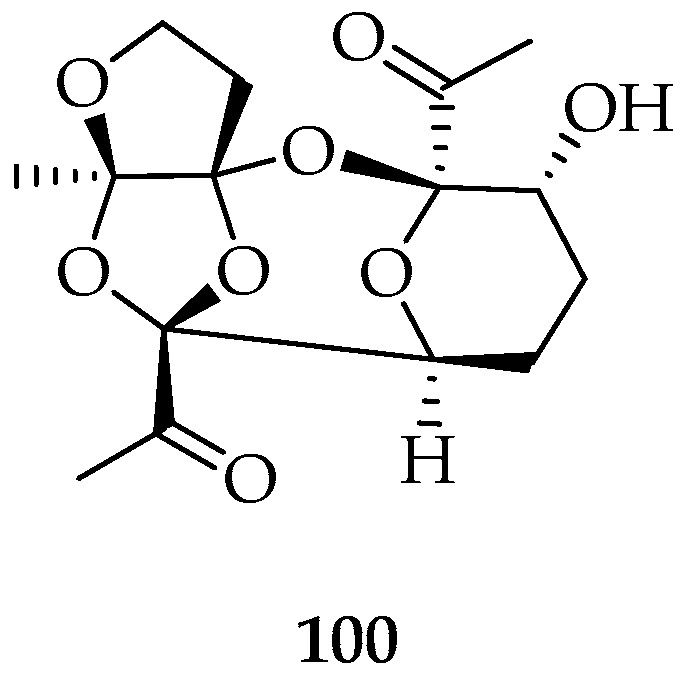
Tetracyclic polyketal isolated from Chilean marine alga Laurencia chilensis.
3. Conclusions
A first review of investigations carried out with marine algae from the Chilean coasts was published in 1989. Herein, we have updated the information by compiling all works, published up to mid-2019, on natural products isolated from algae collected along the coasts of Chile. The main species of algae that have been studied are Plocamium cartilagineum, Shottera nicaensis, Pantoneura plocamioides, Ceramium rubrum, Laurencia claviformis, Laurencia chilensis, Dictyota crenulata, Glossophora kunthii, Desmarestia menziesii, Stypopodium flabelliforme, and Ptilonia magellanica. From the total number of marine natural products identified (92), 31 are monoterpenes (8–38), 14 sesquiterpenes (39–52), 11 diterpenes (53–63), 17 meroterpenoids (64–80), 16 acetogenins (81–89 and 91–97), and three furanones (98–100). Among the biological activities studied in these compounds are the following: insecticidal activity of 27 and 28 against Aphis fabae, 53 and 55 against tomato moth (Tuta absolute), 65 against Spodoptera frugiperda; cytotoxicity of 31 against colon and cervical adenocarcinoma cells; inhibition of cytokinesis by 43 against Tetrapygus niger; toxicity against Schizaphis graminum; gastroprotective activity of 65 and 78 in mice; topical anti-inflammatory activity of 65 related to inhibition of leukocyte accumulation and human recombinant synovial phospholipase A2 activity; and inhibition of degranulation response by 40. Compounds 65, 68, 71, 77, 79 and 80 were tested for their cell proliferation inhibitory activity in five cell lines: Caco-2, SH-SY5Y, RBL-2H3, RAW.267 and V79. Antimicrobial activity by 77, 79 and 80, and antibacterial activity by 89 against K. pneumoniae were determined.
The situation observed in the genus Plocamium is striking. The characteristic metabolites of this genus have been isolated from algae of the genera Microcladia, Shotera, Pantoneura and Ceramium. All of them belong to different families. So far, no explanation has been given for this phenomenon. On the other hand, it has been proposed that in P. violaceum, there would be two chemotypes depending on whether the monoterpenes are cyclic or lineal. An analogous situation can be described for P. cartilagineum; however, the description of oxygenated monoterpenes suggests that a possible chemotaxonomy of this species could become even more confusing.
Thus, from a chemical point of view, Chilean algae are characterized by the unique structures of some of their metabolites. However, considering the great variety of Chilean alga species, the number of works that have been published is relatively scarce. The extensive Chilean coasts are bathed by the cold Humboldt current, resulting in very cold waters in the extreme south and much warmer waters in the extreme north. Easter Island is a special case because it is in the middle of the ocean, far from the influence of this current and from any other kind of external influence. For these reasons, the marine biodiversity of the Chilean coast can be considered as an important source of new bioactive marine natural products that could be the basis for the development of new drugs but that have been poorly studied and exploited to date.
Acknowledgments
The authors express thanks to the people who helped with this work and the Programa de Incentivo a la Iniciación Científica (PIIC).
Author Contributions
Conception, D.A., L.T., A.S.-M., H.C., A.F.O.; Resources, D.A., H.C., A.S.-M. Writing—original draft, D.A.; Writing—review and editing, L.T., H.C., A.S.-M., L.E., A.F.O. All authors participated in similar measure in the preparation of this manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.San-Martín A., Rovirosa J., Bacho M., Gaete K., Ampuero J. A New Labdane Diterpene from the Limpet Trimusculus Peruvianus. Bol. Latinoam. Caribe Plantas. 2012;11:520. [Google Scholar]
- 2.Mayer A.M.S., Rodríguez A.D., Berlinck R.G.S., Fusetani N. Marine Pharmacology in 2007–2008: Marine Compounds with Antibacterial, Anticoagulant, Antifungal, Anti-Inflammatory, Antimalarial, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous System, and Other Miscellaneous Mechanisms of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011;153:191. doi: 10.1016/j.cbpc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer A., Rodríguez A., Taglialatela-Scafati O., Fusetani N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs. 2017;15:273. doi: 10.3390/md15090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerwick W.H., Moore B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012;19:85. doi: 10.1016/j.chembiol.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2017;34:235. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]
- 6.Blunt J.W., Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2018;35:8. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- 7.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2019;36:122. doi: 10.1039/C8NP00092A. [DOI] [PubMed] [Google Scholar]
- 8.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2020;37:175. doi: 10.1039/C9NP00069K. [DOI] [PubMed] [Google Scholar]
- 9.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2021;38:362. doi: 10.1039/D0NP00089B. [DOI] [PubMed] [Google Scholar]
- 10.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2022 doi: 10.1039/D1NP00076D. Advance Article. [DOI] [PubMed] [Google Scholar]
- 11.El Gamal A.A. Biological Importance of Marine Algae. Saudi Pharm. J. 2010;18:1–25. doi: 10.1016/j.jsps.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garson M.J. Biosynthetic Studies on Marine Natural Products. Nat. Prod. Rep. 1989;6:143. doi: 10.1039/np9890600143. [DOI] [Google Scholar]
- 13.Ścieszka S., Klewicka E. Algae in Food: A General Review. Crit. Rev. Food Sci. Nutr. 2019;59:3538. doi: 10.1080/10408398.2018.1496319. [DOI] [PubMed] [Google Scholar]
- 14.Lever J., Brkljača R., Kraft G., Urban S. Natural Products of Marine Macroalgae from South Eastern Australia, with Emphasis on the Port Phillip Bay and Heads Regions of Victoria. Mar. Drugs. 2020;18:142. doi: 10.3390/md18030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mata T.M., Martins A.A., Caetano N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010;14:217. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- 16.Torres F.A.E., Passalacqua T.G., Velásquez A.M.A., de Souza R.A., Colepicolo P., Graminha M.A.S. New Drugs with Antiprotozoal Activity from Marine Algae: A Review. Rev. Bras. Farmacogn. 2014;24:265. doi: 10.1016/j.bjp.2014.07.001. [DOI] [Google Scholar]
- 17.Cheung R.C.F., Wong J.H., Pan W.L., Chan Y.S., Yin C.M., Dan X.L., Wang H.X., Fang E.F., Lam S.K., Ngai P.H.K., et al. Antifungal and Antiviral Products of Marine Organisms. Appl. Microbiol. Biotechnol. 2014;98:3475. doi: 10.1007/s00253-014-5575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyawali R., Ibrahim S.A. Natural Products as Antimicrobial Agents. Food Control. 2014;46:412. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- 19.Pérez M., Falqué E., Domínguez H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs. 2016;14:52. doi: 10.3390/md14030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahms H., Dobretsov S. Antifouling Compounds from Marine Macroalgae. Mar. Drugs. 2017;15:265. doi: 10.3390/md15090265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva A., Silva S.A., Carpena M., Garcia-Oliveira P., Gullón P., Barroso M.F., Prieto M.A., Simal-Gandara J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics. 2020;9:642. doi: 10.3390/antibiotics9100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alves C., Silva J., Pinteus S., Gaspar H., Alpoim M.C., Botana L.M., Pedrosa R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018;9:777. doi: 10.3389/fphar.2018.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchokouaha Yamthe L., Appiah-Opong R., Tsouh Fokou P., Tsabang N., Fekam Boyom F., Nyarko A., Wilson M. Marine Algae as Source of Novel Antileishmanial Drugs: A Review. Mar. Drugs. 2017;15:323. doi: 10.3390/md15110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernando I.P.S., Nah J.-W., Jeon Y.-J. Potential Anti-Inflammatory Natural Products from Marine Algae. Environ. Toxicol. Pharmacol. 2016;48:22–30. doi: 10.1016/j.etap.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Bajpai V.K. Antimicrobial Bioactive Compounds from Marine Algae: A Mini Review. Indian J. Mar. Sci. 2016;45:10. [Google Scholar]
- 26.Cikoš A.-M., Jurin M., Čož-Rakovac R., Jokić S., Jerković I. Update on Monoterpenes from Red Macroalgae: Isolation, Analysis, and Bioactivity. Mar. Drugs. 2019;17:537. doi: 10.3390/md17090537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoubaky G.A.E., Salem E.A. Terpenes and Sterols Composition of Marine Brown Algae Padina pavonica (Dictyotales) and Hormophysa triquetra (Fucales) Int. J. Pharmacogn. Phytochem. Res. 2015;6:7. [Google Scholar]
- 28.Chojnacka K., Kim S.-K. Introduction of Marine Algae Extracts. In: Kim S.-K., Chojnacka K., editors. Marine Algae Extracts. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2015. pp. 1–14. [Google Scholar]
- 29.Newman D.J., Cragg G.M. Marine Natural Products and Related Compounds in Clinical and Advanced Preclinical Trials. J. Nat. Prod. 2004;67:1216. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- 30.Lindel T., Jensen P.R., Fenical W., Long B.H., Casazza A.M., Carboni J., Fairchild C.R. Eleutherobin, a New Cytotoxin That Mimics Paclitaxel (Taxol) by Stabilizing Microtubules. J. Am. Chem. Soc. 1997;119:8744. doi: 10.1021/ja9717828. [DOI] [Google Scholar]
- 31.Cinel B., Roberge M., Behrisch H., van Ofwegen L., Castro C.B., Andersen R.J. Antimitotic Diterpenes from Erythropodium caribaeorum Test Pharmacophore Models for Microtubule Stabilization. Org. Lett. 2000;2:257. doi: 10.1021/ol9912027. [DOI] [PubMed] [Google Scholar]
- 32.Taglialatela-Scafati O., Deo-Jangra U., Campbell M., Roberge M., Andersen R.J. Diterpenoids from Cultured Erythropodium caribaeorum. Org. Lett. 2002;4:4085. doi: 10.1021/ol026831m. [DOI] [PubMed] [Google Scholar]
- 33.Long B.H., Carboni J.M., Wasserman A.J., Cornell L.A., Casazza A.M., Jensen P.R., Lindel T., Fenica W., Fairchild C.R. Eleutherobin, a Novel Cytotoxic Agent That Induces Tubulin Polymerization, Is Similar to Paclitaxel (Taxol®) Cancer Res. 1998;58:11116. [PubMed] [Google Scholar]
- 34.Look S.A., Fenical W., Matsumoto G.K., Clardy J. The Pseudopterosins: A New Class of Antiinflammatory and Analgesic Diterpene Pentosides from the Marine Sea Whip Pseudopterogorgia elisabethae (Octocorallia) J. Org. Chem. 1986;51:5140. doi: 10.1021/jo00376a016. [DOI] [Google Scholar]
- 35.Djerassi C. Recent Studies in the Marine Sterol Field. Pure Appl. Chem. 1981;53:873. doi: 10.1351/pac198153040873. [DOI] [Google Scholar]
- 36.D’Auria M.V., Minale L., Riccio R. Polyoxygenated Steroids of Marine Origin. Chem. Rev. 1993;93:1839. doi: 10.1021/cr00021a010. [DOI] [Google Scholar]
- 37.Fujita M., Nakao Y., Matsunaga S., Seiki M., Itoh Y. Isolation and Structure Elucidation of Two Phosphorylated Sterol Sulfates, MT1-MMP Inhibitors from a Marine Sponge Cribrochalina sp.: Revision of the Structures of Haplosamates A and B. Tetrahedron. 2001;57:1885. doi: 10.1016/S0040-4020(01)00259-9. [DOI] [Google Scholar]
- 38.Rudi A., Yosief T., Loya S., Hizi A., Schleyer M., Kashman Y. Clathsterol, a Novel Anti-HIV-1 RT Sulfated Sterol from the Sponge Clathria Species. J. Nat. Prod. 2001;64:1451. doi: 10.1021/np010121s. [DOI] [PubMed] [Google Scholar]
- 39.Volkman J. Sterols in Microorganisms. Appl. Microbiol. Biotechnol. 2003;60:495. doi: 10.1007/s00253-002-1172-8. [DOI] [PubMed] [Google Scholar]
- 40.Moore K.S., Wehrli S., Roder H., Rogers M., Forrest J.N., McCrimmon D., Zasloff M. Squalamine: An Aminosterol Antibiotic from the Shark. Proc. Nat. Acad. Sci. USA. 1993;90:1354. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehrli S.L., Moore K.S., Roder H., Durell S., Zasloff M. Structure of the Novel Steroidal Antibiotic Squalamine Determined by Two-Dimensional NMR Spectroscopy. Steroids. 1993;58:370. doi: 10.1016/0039-128X(93)90040-T. [DOI] [PubMed] [Google Scholar]
- 42.Sills A.K., Williams J.I., Tyler B.M., Epstein D.S., Sipos E.P., Davis J.D., McLane M.P., Pitchford S., Cheshire K., Gannon F.H., et al. Squalamine Inhibits Angiogenesis and Solid Tumor Growth in Vivo and Perturbs Embryonic Vasculature. Cancer Res. 1998;58:2784. [PubMed] [Google Scholar]
- 43.Gross H., König G.M. Terpenoids from Marine Organisms: Unique Structures and Their Pharmacological Potential. Phytochem. Rev. 2006;5:115. doi: 10.1007/s11101-005-5464-3. [DOI] [Google Scholar]
- 44.Gupta S., Cox S., Rajauria G., Jaiswal A.K., Abu-Ghannam N. Growth Inhibition of Common Food Spoilage and Pathogenic Microorganisms in the Presence of Brown Seaweed Extracts. Food Bioprocess. Technol. 2012;5:1907. doi: 10.1007/s11947-010-0502-6. [DOI] [Google Scholar]
- 45.Gupta S., Abu-Ghannam N. Recent Developments in the Application of Seaweeds or Seaweed Extracts as a Means for Enhancing the Safety and Quality Attributes of Foods. Innov. Food Sci. Emerg. Tech. 2011;12:600. doi: 10.1016/j.ifset.2011.07.004. [DOI] [Google Scholar]
- 46.Ministerio del Medio Ambiente Biodiversidad de Chile, Patrimonio y Desafios. 3rd ed. Volume I Ocho Libros Editores; Santiago, Chile: 2018. [Google Scholar]
- 47.Pacheco L.V., Parada J., Pérez-Correa J.R., Mariotti-Celis M.S., Erpel F., Zambrano A., Palacios M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs. 2020;18:353. doi: 10.3390/md18070353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Díaz-Marrero A.R., Dorta E., Cueto M., Rovirosa J., San-Martín A., Loyola A., Darias J. Labdane Diterpenes with a New Oxidation Pattern from the Marine Pulmonate Trimusculus Peruvianus. Tetrahedron. 2003;59:4805. doi: 10.1016/S0040-4020(03)00731-2. [DOI] [Google Scholar]
- 49.Carballo J.L., Hernández-Inda Z.L., Pérez P., García-Grávalos M.D. A Comparison between Two Brine Shrimp Assays to Detect in Vitro Cytotoxicity in Marine Natural Products. BMC Biotechnol. 2002;2:17. doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Migliore L., Civitareale C., Brambilla G., Delupis G.D.D. Toxicity of Several Important Agricultural Antibiotics to Artemia. Water Res. 1997;31:1801. doi: 10.1016/S0043-1354(96)00412-5. [DOI] [Google Scholar]
- 51.Itô S., Endo K., Yoshida T., Yatagai M., Kodama M. Chamigrene, a Sesquiterpene Hydrocarbon of a Novel Carbon Skeleton. [(accessed on 25 April 2022)];Chem. Commun. 1967 :186b. Available online: https://pubs.rsc.org/en/content/articlelanding/1967/c1/c1967000186b/unauth. [Google Scholar]
- 52.Carbone M., Ciavatta M.L., Manzo E., Li X.-L., Mollo E., Mudianta I.W., Guo Y.-W., Gavagnin M. Amphilectene Diterpene Isonitriles and Formamido Derivatives from the Hainan Nudibranch Phyllidia Coelestis. Mar. Drugs. 2019;17:603. doi: 10.3390/md17110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigues I., Miguel M., Mnif W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules. 2019;24:781. doi: 10.3390/molecules24040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kladi M., Vagias C., Roussis V. Volatile Halogenated Metabolites from Marine Red Algae. Phytochem. Rev. 2004;3:3370. doi: 10.1007/s11101-004-4155-9. [DOI] [Google Scholar]
- 55.Guella G., Öztunç A., Mancini I., Pietra F. Stereochemical Features of Sesquiterpene Metabolites as a Distinctive Trait of Red Seaweeds in the Genus Laurencia. Tetrahedron Lett. 1997;38:8261. doi: 10.1016/S0040-4039(97)10162-9. [DOI] [Google Scholar]
- 56.Tori M., Nakasklma K., Seike M., Wrigkt A.D. Revised Structure of a Brasilane-Type Sesquiterpene Isolated From the Red Alga Laurencia implicata and Its Absolute Configuration. Tetrahedron lett. 1994;35:3105. doi: 10.1016/S0040-4039(00)76841-9. [DOI] [Google Scholar]
- 57.Gedara S.R., Abdel-Halim O.B., El-Sharkawy S.H., Salama O.M., Shier T.W., Halim A.F. Cytotoxic Hydroazulene Diterpenes from the Brown Alga Dictyota Dichotoma. Z. Naturforsch. C. 2003;58:17. doi: 10.1515/znc-2003-1-203. [DOI] [PubMed] [Google Scholar]
- 58.Goez C.E., Wright A.D., König G.M., Sticher O. Diterpenes from the Brown Alga Dilophus mediterraneus. Phytochem. Anal. 1994;5:68. doi: 10.1002/pca.2800050205. [DOI] [Google Scholar]
- 59.Areche C., San-Martín A., Rovirosa J., Soto-Delgado J., Contr Eras R. An Unusual Halogenated Meroditerpenoid from Stypopodium flabelliforme: Studies by NMR Spectroscopic and Computational Methods. Phytochemistry. 2009;70:1315. doi: 10.1016/j.phytochem.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 60.Fleury B.G., Pereira M.V.G., Da Silva J.R.P., Kaisin M., Teixeira V.L., Kelecom A. Sterols from Brazilian Marine Brown Algae. Phytochemistry. 1994;37:1447. doi: 10.1016/S0031-9422(00)90430-8. [DOI] [Google Scholar]
- 61.Kamenarska Z., Gasic M.J., Zlatovic M., Rasovic A., Sladic D., Kljajic Z., Stefanov K., Seizova K., Najdenski H., Kujumgiev A., et al. Chemical Composition of the Brown Alga Padina pavonia (L.) Gaill. from the Adriatic Sea. Bot. Mar. 2002;45:339. doi: 10.1515/BOT.2002.034. [DOI] [Google Scholar]
- 62.Peng Y. Chemical Composition of Seaweeds. In: Tiwari B.K., Troy D.J., editors. Seaweed Sustainability. 1st ed. Academic Press; London, UK: 2015. p. 79. [Google Scholar]
- 63.Díaz-Marrero A.R., Cueto M., Dorta E., Rovirosa J., San-Martín A., Darias J. New Halogenated Monoterpenes from the Red Alga. Tetrahedron. 2002;58:8539. doi: 10.1016/S0040-4020(02)01019-0. [DOI] [Google Scholar]
- 64.Díaz-Marrero A.R., Rovirosa J., Darias J., San-Martín A., Cueto M. Plocamenols A−C, Novel Linear Polyhalohydroxylated Monoterpenes from Plocamium cartilagineum. J. Nat. Prod. 2002;65:585. doi: 10.1021/np010473z. [DOI] [PubMed] [Google Scholar]
- 65.Díaz-Marrero A.R., Cueto M., Dorta E., Rovirosa J., San-Martín A., Darias J. Geometry and Halogen Regiochemistry Determination of Vicinal Vinyl Dihalides by 1H and 13C NMR. Application to the Structure Elucidation of Prefuroplocamioid, an Unusual Marine Monoterpene. Org. Lett. 2002;4:2949. doi: 10.1021/ol026353f. [DOI] [PubMed] [Google Scholar]
- 66.Díaz-Marrero A.R., Dorta E., Cueto M., Rovirosa J., San-Martín A., Darias J. Supporting the NMR-Based Empirical Rules to Determine the Stereochemistry and Halogen Regiochemistry of Vicinal Vinyl Dihalides. Naturally Occurring Monoterpenes as Chemical Models. Tetrahedron. 2004;60:5049. doi: 10.1016/j.tet.2004.04.025. [DOI] [Google Scholar]
- 67.Kesternich V., Martinez R., Gutierrexz E., Ballesteros K., Mansilla H. Antibacterial Activity of Some Compounds Isolated from Ceramium rubrum against Gram Negative Bacteria. Bol. Soc. Chil. Quím. 1997;42:105. [Google Scholar]
- 68.San-Martin A., Rovirosa J. Variations in the Halogenated Monoterpene Metabolites of Plocamium cartilagineum of the Chilean Coast. Biochem. Syst. Ecol. 1986;14:459–461. doi: 10.1016/0305-1978(86)90002-5. [DOI] [Google Scholar]
- 69.Rovirosa J., Moena J., San-Martín A. Two Chemical Types of the Red Alga Plocamium cartilagineum from Chile. Biochem. System. Ecol. 1988;16:593. doi: 10.1016/0305-1978(88)90068-3. [DOI] [Google Scholar]
- 70.San-Martin A., Negrete R., Rovirosa J. Insecticide and Acaricide Activities of Polyhalogenated Monoterpenes from Chilean Plocamium Cart. Phytochem. 1991;30:2165. doi: 10.1016/0031-9422(91)83607-M. [DOI] [Google Scholar]
- 71.Rivera P., Astudillo L., Rovirosa J., San-Martín A. Halogenated Monoterpenes of the Red Alga Shottera. Nicaensis. Biochem. System. Ecol. 1987;15:3. doi: 10.1016/0305-1978(87)90072-X. [DOI] [Google Scholar]
- 72.Argandoña V.H., Rovirosa J., San-Martín A., Riquelme A., Díaz-Marrero A.R., Cueto M., Darias J., Santana O., Guadaño A., González-Coloma A. Antifeedant Effects of Marine Halogenated Monoterpenes. J. Agric. Food Chem. 2002;50:7029. doi: 10.1021/jf025857p. [DOI] [PubMed] [Google Scholar]
- 73.Argandoña V., Del Pozo T., San-Martín A., Rovirosa J. Insecticidal activity of Plocamium cartilagineum monoterpens. Bol. Soc. Chil. Quím. 2000;45:371. doi: 10.4067/S0366-16442000000300006. [DOI] [Google Scholar]
- 74.Cueto M., Darias J., Rovirosa J., San-Martin A. Tetrahydropyran Monoterpenes from Plocamium cartilagineum and Pantoneura plocamioides. J. Nat. Prod. 1998;61:1466. doi: 10.1021/np9800093. [DOI] [PubMed] [Google Scholar]
- 75.Darias J., Rovirosa J., San-Martin A., Díaz A.R., Dorta E., Cueto M. Furoplocamioids A−C, Novel Polyhalogenated Furanoid Monoterpenes from Plocamium cartilagineum. J. Nat. Prod. 2001;64:1383. doi: 10.1021/np010297u. [DOI] [PubMed] [Google Scholar]
- 76.de Inés C., Argandoña V.H., Rovirosa J., San-Martín A., Díaz-Marrero A.R., Cueto M., González-Coloma A. Cytotoxic Activity of Halogenated Monoterpenes from Plocamium cartilagineum. Z.Naturforsch. C. 2004;59:339. doi: 10.1515/znc-2004-5-609. [DOI] [PubMed] [Google Scholar]
- 77.Palma R., Edding M., Rovirosa J., San-Martín A., Argandoña V.H. Effect of Photon Flux Density and Temperature on the Production of Halogenated Monoterpenes by Plocamium cartilagineum (Plocamiaceae, Rhodophyta) Z. Naturforsch. C. 2004;59:679. doi: 10.1515/znc-2004-9-1012. [DOI] [PubMed] [Google Scholar]
- 78.Rovirosa J., Soto H., Cueto M., Dárias J., Herrera J., San-Martín A. Sesquiterpenes from Laurencia claviformis. Phytochemistry. 1999;50:745. doi: 10.1016/S0031-9422(98)00617-7. [DOI] [Google Scholar]
- 79.San-Martin A., Rovirosa J., Carrasco A., Orejarena S., Soto-Delgado J., Contreras R., Chamy M.C. Microbial Transformation of Marine Halogenated Sesquiterpenes. Nat. Product. Comm. 2010;5:1859. [PubMed] [Google Scholar]
- 80.Soto H., Rovirosa J., San-Martin A., Argandoña V.H. Metabolitos secundarios de Dyctiota crenulata. Bol. Soc. Chil. Quim. 1994;13:173. [Google Scholar]
- 81.Soto H., Rovirosa J., San-Martín A. A New Diterpene from Dictyota Crenulata. Z. Naturforsch. B. 2003;58:795. doi: 10.1515/znb-2003-0812. [DOI] [Google Scholar]
- 82.Norte M., Gonzalez A.G., Arroyo P., Zarraga M., Perez C., Rodriguez M., Ruiz-Perez C., Dorta L. New xenicane diterpenes from the brown algae of Dictyotaceae. Tetrahedron. 1990;46:6125. doi: 10.1016/S0040-4020(01)87934-5. [DOI] [Google Scholar]
- 83.Arroyo P., Norte M., Vazquez J.T., Nakanishi K. Absolute Configuration of Hydroazulenoid Diterpenes Based on Circular Dichroism. J. Org. Chem. 1991;56:2671. doi: 10.1021/jo00008a018. [DOI] [Google Scholar]
- 84.Zarraga M., Amaya P., Norte M. Nuevas crenulidas de algas pardas de la familia Dictyotaceae. Bol. Soc. Chil. Quim. 1997:73. [Google Scholar]
- 85.Rivera P., Podestá F., Norte M., Cataldo F., González A.G. New Plastoquinones from the Brown Alga Desmaresti amenziesii. Can. J. Chem. 1990;68:1399. doi: 10.1139/v90-214. [DOI] [Google Scholar]
- 86.Page J.E., Nagel J. Recent Advances in Phytochemistry. Volume 40. Elsevier; Amsterdam, The Netherlands: 2006. Biosynthesis of Terpenophenolic Metabolites in Hop and Cannabis; pp. 179–210. [Google Scholar]
- 87.Sanchez-Ferrando F., San-Martin A. Epitaondiol: The First Polycyclic Meroditerpenoid Containing Two Fused Six-Membered Rings Forced into the Twist-Boat Conformation. J. Org. Chem. 1995;60:1475. doi: 10.1021/jo00110a062. [DOI] [Google Scholar]
- 88.Rovirosa J., Sepulveda M., Quezada E., San-Martin A. Isoepitaondiol, a Diterpenoid of Stypopodium Flabelliforme and the Insecticidal Activity of Stypotriol, Epitaondiol and Derivatives. Phytochemistry. 1992;31:2679. doi: 10.1016/0031-9422(92)83610-B. [DOI] [Google Scholar]
- 89.Muñoz M.A., Areche C., San-Martín A., Rovirosa J., Joseph-Nathan P. VCD Determination of the Absolute Configuration of Stypotriol. Nat. Product Commun. 2009;4:1037. doi: 10.1177/1934578X0900400804. [DOI] [PubMed] [Google Scholar]
- 90.Depix M.S., Martínez J., Santibañez F., San-Martín A., Maccioni R.B. The Compound 14-Keto-Stypodiol Diacetate from the Algae Stypopodium flabelliforme Inhibits Microtubules and Cell Proliferation in DU-145 Human Prostatic Cells. Mol. Cell. Biochem. 1998;187:191. doi: 10.1023/A:1006879308861. [DOI] [PubMed] [Google Scholar]
- 91.Areche C., San-Martín A., Rovirosa J., Muñoz M.A., Hernández-Barragán A., Bucio M.A., Joseph-Nathan P. Stereostructure Reassignment and Absolute Configuration of Isoepitaondiol, a Meroditerpenoid from Stypopodium flabelliforme. J. Nat. Prod. 2010;73:79. doi: 10.1021/np900553p. [DOI] [PubMed] [Google Scholar]
- 92.Joseph-Nathan P., Muñoz M.A., Areche C., Rovirosa J., San-Martín A., Gordillo-Román B. Absolute Configuration of the Meroditerpenoids Taondiol and Epitaondiol Diacetates by Vibrational Circular Dichroism. Heterocycles. 2012;85:1961. doi: 10.3987/COM-12-12514. [DOI] [Google Scholar]
- 93.Areche C., Benites J., Cornejo A., Ruiz L., García-Beltrán O., Simirgiotis M., Sepúlveda B. Seco-Taondiol, an Unusual Meroterpenoid from the Chilean Seaweed Stypopodium flabelliforme and Its Gastroprotective Effect in Mouse Model. Mar. Drugs. 2015;13:1726. doi: 10.3390/md13041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Areche C., San-Martín A., Rovinosa J., Sepúlveda B. Gastroprotective Activity of Epitaondiol and Sargaol. Nat. Prod. Comm. 2011;6:1073. doi: 10.1177/1934578X1100600805. [DOI] [PubMed] [Google Scholar]
- 95.Gil B., Ferrándiz M.L., Sanz M.J., Terencio M.C., Ubeda A., Rovirosa J., San-Martin A., Alcaraz M.J., Payá M. Inhibition of Inflammatory Responses by Epitaondiol and Other Marine Natural Products. Life Sci. 1995;57:PL25. doi: 10.1016/0024-3205(95)00260-D. [DOI] [PubMed] [Google Scholar]
- 96.Pereira D.M., Cheel J., Areche C., San-Martin A., Rovirosa J., Silva L.R., Valentao P., Andrade P.B. Anti-Proliferative Activity of Meroditerpenoids Isolated from the Brown Alga Stypopodium flabelliforme against Several Cancer Cell Lines. Mar. Drugs. 2011;9:852. doi: 10.3390/md9050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallardo A.B., Cueto M., Díaz-Marrero A.R., de la Rosa J.M., Fajardo V., San-Martín A., Darias J. A Set of Biogenetically Interesting Polyhalogenated Acetogenins from Ptilonia magellanica. Phytochemistry. 2018;145:111. doi: 10.1016/j.phytochem.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Lorenzo M., Cueto M., San-Martín A., Fajardo V., Darias J. Pyranosylmagellanicus a Novel Structural Class of Polyhalogenated Acetogenins from Ptilonia magellanica. Tetrahedron. 2005;61:9550. doi: 10.1016/j.tet.2005.07.083. [DOI] [Google Scholar]
- 99.Arnarp J., Bielawski J., Dahlin B.-M., Dahlman O., Enzell C.R., Pettersson T., Edwards J.V. Tobacco Smoke Chemistry. 5. Alkyl Substituted 3-Hydroxy-4-Pyrones Found in Cigarette Smoke Condensate. Acta Chem. Scand. 1990;44:963. doi: 10.3891/acta.chem.scand.44-0963. [DOI] [PubMed] [Google Scholar]
- 100.San-Martín A., Darias J., Soto H., Contreras C., Herrera J.S., Rovirosa J. A New C15 Acetogenin from the Marine Alga Laurencia claviformis. Nat. Prod. Lett. 1997;10:303. doi: 10.1080/10575639708043745. [DOI] [Google Scholar]
- 101.Schwab W. Natural 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone (Furaneol®) Molecules. 2013;18:6936. doi: 10.3390/molecules18066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hjelmgaard T., Persson T., Rasmussen T.B., Givskov M., Nielsen J. Synthesis of Furanone-Based Natural Product Analogues with Quorum Sensing Antagonist Activity. Bioorg. Med. Chem. 2003;11:3261. doi: 10.1016/S0968-0896(03)00295-5. [DOI] [PubMed] [Google Scholar]
- 103.San-Martin A., Rovirosa J., Muñoz O., Chen M.H.M., Guneratne R.D., Clardy J. The Isolation and Structure Determination of Chilenone A, a Putative Dimer of 2-Methyl-3(2H)-Furanone from the Marine Alga. Tetrahedron Lett. 1983;24:4063. doi: 10.1016/S0040-4039(00)88262-3. [DOI] [Google Scholar]
- 104.San-Martín A., Rovirosa J., Xu C., Lu H.S.M., Clardy J. Further Structural Studies on the 2-Methyl-3(2H)-Furanone Derived Metabolites of the Marine Alga Laurencia chilensis. Tetrahedron Lett. 1987;28:6013. doi: 10.1016/S0040-4039(00)96850-3. [DOI] [Google Scholar]
- 105.Bittner M., Gonzalez F., Valdebenito H., Silva M., Paul V.J., Fenical W., Chen M.H.M., Clardy J. A Novel Tetracyclic Polyketal from the Marine Red Alga. Tetrahedron Lett. 1987;28:4031. doi: 10.1016/S0040-4039(01)83853-3. [DOI] [Google Scholar]