Abstract
Severely ill COVID-19 patients are at high risk of nosocomial infections. The aim of the study was to describe the characteristics of candidemia during the pre-pandemic period (January 2019–February 2020) compared to the pandemic period (March 2020–September 2021). Antifungal susceptibilities were assessed using the EUCAST E.Def 7.3.2 broth dilution method. Fluconazole-resistant C. parapsilosis isolates (FRCP) were studied for sequencing of the ERG11 gene. The incidence of candidemia and C. parapsilosis bloodstream infection increased significantly in the pandemic period (p = 0.021). ICU admission, mechanical ventilation, parenteral nutrition and corticosteroids administration were more frequent in patients with candidemia who had been admitted due to COVID-19. Fifteen cases of FRCP fungemia were detected. The first case was recorded 10 months before the pandemic in a patient transferred from another hospital. The incidence of FRCP in patients admitted for COVID-19 was 1.34 and 0.16 in all other patients (p < 0.001). ICU admission, previous Candida spp. colonization, arterial catheter use, parenteral nutrition and renal function replacement therapy were more frequent in patients with candidemia due to FRCP. All FRCP isolates showed the Y132F mutation. In conclusion, the incidence of candidemia experienced an increase during the COVID-19 pandemic and FRCP fungemia was more frequent in patients admitted due to COVID-19.
Keywords: COVID-19, candidemia, Candida parapsilosis, drug resistance, microbial, fluconazole
1. Introduction
The COVID-19 pandemic has represented a substantial burden on the health care activity of hospitals [1]. Admission to medical wards and ICUs, together with the frequent invasive procedures to which these patients are subjected, could facilitate the occurrence of nosocomial infections and the emergence of antimicrobial-resistant infections [2,3].
The increase in fungal infections has been one of the major concerns in the management of patients with COVID-19 [4,5]. Although these patients do not usually present some typical risk factors for candidemia, such as neutropenia or abdominal surgery, several studies have shown a high incidence of candidemia. This fact has been related to treatment with steroids or immunosuppressants, ICU admission and mechanical ventilation [6,7]
Candida parapsilosis ranges between the second and third most frequent species causing invasive yeast infections [8]. This species is characterized by a tendency to form biofilms on medical devices and colonize the hands of healthcare personnel, which may contribute to invasive infections and nosocomial outbreaks [9,10]. Although azoles (especially fluconazole and voriconazole) are the treatment of choice for invasive infections due to C. parapsilosis, recent studies noted an increase in the number of hospital outbreaks due to azole-resistant C. parapsilosis [10,11]. The emergence of those outbreaks may be associated with previous use of fluconazole and/or antibiotics, or the clonal spread of isolates across the hospital [9].
We recently observed an increase in the number of cases of candidemia alongside the beginning of COVID-19 pandemic in our institution. Some of these bloodstream infections were produced by fluconazole-resistant Candida parapsilosis (FRCP). This study describes the clinical profile of candidemia before and during the COVID-19 pandemic and the incidence of fungemia due to fluconazole-resistant Candida parapsilosis.
2. Methods
2.1. Setting, Patients and Study Design
This retrospective, observational, single-institution study was conducted in a 620-bed tertiary university hospital located in Madrid, Spain. Between January 2019 and September 2021, blood cultures positive for Candida spp. were studied. The onset of the COVID-19 pandemic outbreak in March 2020 in Spain led to consider two distinct periods in the study. A pre-pandemic period (period 1), comprising January 2019–February 2020, and a pandemic period (period 2), from March 2020 to September 2021.
We retrospectively reviewed the electronic medical records of all adult patients (aged 18 years or older) with candidemia. An episode of candidemia was defined as the detection of at least one blood culture positive for Candida species. In addition to demographic data, we collected medical history, underlying diseases, Charlson index [12], treatments prior to the episode of candidemia, admission to the ICU, and the most important risk factors for candidemia, including previous antibiotherapy, catheters, parenteral nutrition, renal replacement techniques and previous surgery. The treatment of the candidemia episode was also recorded. Given the observational nature of the research, patients were managed according to routine clinical care.
2.2. Definitions
In the case of a patient presenting more than one episode of candidemia, only the first one was considered. Obesity was defined as a body mass index greater than 30 kg/m2. Among the category of immunosuppressive treatment, any treatment with immunosuppressive agents administered prior to admission for COVID-19 due to chronic disease or transplantation was considered.
2.3. Microbiological Studies
The patient was considered to have COVID-19 if they presented consistent symptoms (fever, cough and radiological infiltrate) together with a positive reverse transcriptase polymerase chain reaction (RT-PCR) result performed by one of the diagnostic systems available in the hospital [13,14].
2.3.1. Blood Cultures and Antifungal Susceptibility Testing
Blood cultures were obtained by standard procedures and processed with the BD BACTEC FX (Becton Dickinson, Sparks, MD, USA). All systems were applied according to the manufacturer’s instructions. When the blood culture was positive and the Gram stain demonstrated the presence of a yeast, a subculture was performed in BBL CHROMagar Candida Medium (Becton Dickinson TM). The yeasts were identified by MALDI-TOF MS (Bruker Daltonic TM).
2.3.2. Antifungal Susceptibility Testing and ERG11 Gene Sequencing
In vitro antifungal susceptibilities to amphotericin B, fluconazole, voriconazole, and posaconazole (Sigma-Aldrich, Madrid, Spain) of isolates were assessed by the EUCAST EDef 7.3.2 broth dilution method. Isolates were categorized as resistant and/or non-wild type according to EUCAST clinical breakpoints. Fluconazole-resistant C. parapsilosis isolates were further studied for ERG11 gene sequencing, as previously reported [15].
2.3.3. Microsatellite Typing Procedure
Species-specific microsatellite markers were used to genotype all C. parapsilosis isolates (CP1, CP4a, CP6 and B), as previously reported [16].
2.4. Data Analysis
Quantitative variables were reported as median and interquartile range (IQR), and categorical variables as counts (%). The chi-square test or Fisher exact test were used to compare the distribution of categorical variables, and Student’s t-test or Mann–Whitney U test were used for quantitative variables. Significance was set at a p value of less than 0.05.
2.5. Ethical Statement
The study was approved by the Institutional Review Board (CEIm) at Hospital Universitario Puerta de Hierro-Majadahonda, and a waiver for the informed consent was granted (PI_154_2020). The study complied with the provisions in EU and Spanish legislation on data protection and the Declaration of Helsinki 2013.
3. Results
During the study period, we observed 88 episodes of candidemia in 88 patients: 29 episodes in period 1 (January 2019–February 2020) and 56 episodes in period 2 (March 2020–September 2021). The incidence rate per 10,000 patient days during period 1 was 1.36 (0.93–1.93) and 2.55 (2.01–3.19) during period 2 (p = 0.002). The incidence in this second period was 3.18 (1.82–4.89; p = 0.006) in patients admitted for COVID-19, 13 and 2.43 (1.85–2.43) in patients without COVID-19 (p = 0.006).
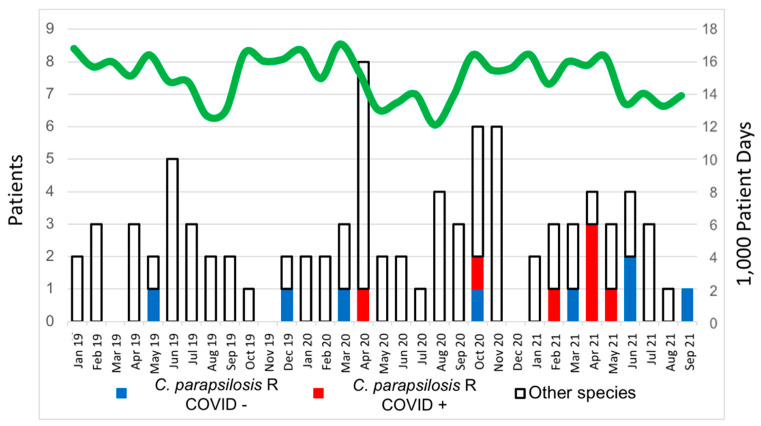
The 91 isolates of Candida species from the 88 positive blood cultures were distributed as follows: C. albicans (n = 38; 41.8%), C. parapsilosis (n = 31 isolates; 34.1%), C. glabrata (n = 15; 16.5%), C. tropicalis (n = 3; 3.3%) and C. krusei (n = 3; 3.3%) (Figure 1).
Figure 1.
Incidence of candidemia from January 2019 to September 2021. R—resistant.
During period 1, 31 isolates were identified in 29 patients and distributed as C. albicans (n = 13; 34.2%), C. parapsilosis (n = 6; 19.4%) and C. glabrata (n = 5; 16.1%). During period 2, 60 Candida isolates were identified in 59 patients and distributed as C. albicans (n = 25; 41.7%), C. parapsilosis (n = 24; 40%) and C. glabrata (n = 7; 11.7%). Incidence of C. parapsilosis bloodstream infection significantly increased in period 2 (p = 0.024). However, the comparison of the incidence of blood tract infection by C. albicans and C. glabrata between the two periods showed no significant differences (p = 0.431 and p = 0.276, respectively).
When comparing patients without COVID from both periods (before and after the onset of the pandemic), no differences were detected in the most relevant variables. Differences reaching statistical significance were not found in any of the variables studied: median age, 70 years (IQR 59–76 years) vs. 65 years (59–75 years; p = 0.653); solid tumor (34.5% vs. 25.6%; p = 0.290); chronic renal disease (10.3% vs. 7%; p = 0.462); Charlson comorbidity index [2 (IQR 1–4) vs. 3 (IQR 1–5); p = 0.251)]; hospital stay before candidemia [20 days (IQR 9–36 days) vs. 20 days (IQR 7–37 days); p = 0.595]; ICU admission (10.3% vs. 16.3%; p = 0.561); central venous catheter as source of candidemia (42.3% vs. 46.5%; p = 0.443); recurrent candidemia (4% vs. 7.5%; p = 0.500); mortality (58.6% vs. 55.8%; p = 0.504). Likewise, there were no significant differences regarding the species causing the bloodstream infections: C. albicans (44.8% vs. 46.5%; p = 0.44.6%); C. parapsilosis (24.1% vs. 34.9%; p = 0.174); C. glabrata (17.2% vs. 16.3%; p = 0.456).
3.1. Candidemia Episodes in Patients Hospitalized for COVID-19
The clinical characteristics of patients with candidemia in patients admitted for COVID-19 compared to non-COVID-19 patients are shown in Table 1.
Table 1.
Clinical characteristics of patients with candidemia according to hospital admission due to COVID-19 or not.
| Patients with COVID-19 (n = 16) | Patents without COVID-19 (n = 72) | p-Value | |
|---|---|---|---|
| Age (years), (median (IQR)) | 73.5 (66.5–77.5) | 66 (59–76.5) | 0.077 |
| Male gender | 12 (75) | 49 (68.1) | 0.413 |
| Obesity | 4 (25) | 2 (2.8) | 0.009 |
| Diabetes mellitus | 7 (43.8) | 20 (27.8) | 0.169 |
| Heart failure | 2 (12.5) | 9 (12.5) | 0.682 |
| Chronic lung disease | 5 (31.3) | 9 (12.5) | 0.076 |
| Dementia | 0 | 7 (9.7) | 0.252 |
| Chronic liver disease | 1 (6.3) | 9 (12.5) | 0.270 |
| Chronic renal failure | 1 (6.3) | 6 (8.3) | 0.626 |
| Solid tumor | 1 (6.3) | 21 (29.2) | 0.047 |
| Hematologic malignancy | 0 | 6 (8.3) | 0.288 |
| Charlson comorbidity index, (median (IQR)) | 1 (0–3) | 2 (1–4) | 0.110 |
| Solid organ transplantation | 0 | 6 (8.3) | 0.288 |
| Hospital stay before candidemia, (median (IQR)) | 22.5 (14–53.5) | 20 (4–35) | 0.915 |
| ICU admission | 15 (93.7) | 10 (13.9) | <0.001 |
| Previous corticosteroids treatment | 14 (87.5) | 10 (13.9) | <0.001 |
| Previous tocilizumab treatment | 14 (87.5) | 10 (13.9) | <0.001 |
| Previous immunosuppressive treatment | 0 | 6 (8.3) | 0.288 |
| Previous antifungal treatment | 5 (31.3) | 19 (26.4) | 0.454 |
| Previous antibiotic treatment | 16 (100) | 62 (86.1) | 0.119 |
| Central venous catheter | 16 (100) | 53 (73.6) | 0.013 |
| Arterial catheter | 8 (50) | 16 (22.2) | 0.018 |
| Parenteral nutrition | 12 (75) | 30 (41.7) | 0.016 |
| Renal replacement therapy | 4 (26.7) | 16 (21.9) | 0.459 |
| Candida spp. previous colonization | 5 (31.3) | 15 (20.8) | 0.276 |
| Candida Score | 2 (1–2.5) | 2 (0–3) | 0.885 |
| Abdominal surgery | 1 (6.3) | 21 (29.2) | 0.047 |
| Surgery, other site | 0 | 14 (19.4) | 0.046 |
| Candidemia source | |||
| Venous catheter | 13 (81.3) | 34 (47.2) | 0.013 |
| Intraabdominal | 1 (6.3) | 15 (20.8) | 0.156 |
| Urinary tract | 1 (6.3) | 8 (11.1) | 0.316 |
| Unknown | 3 (18.7) | 22 (30.6) | 0.184 |
| Fever | 15 (93.8) | 65 (90.3) | 0.553 |
| Endophtalmitis 1 | 3 (18.7) | 5 (7.1) | 0.094 |
| Endocarditis 2 | 1 (6.3) | 5 (6.9) | 0.701 |
| Recurrent candidemia | 4 (26.7) | 3 (6.2) | 0.019 |
| Acute kidney failure | 4 (25) | 35 (48.6) | 0.073 |
| Mortality | 7 (43.8) | 42 (58.3) | 0.216 |
| Mortality attributable to candidemia | 1 (6.25) | 6 (8.3) | 0.660 |
IQR—interquartile range. 1 Fundoscopy was performed in 61 patients. 2 Echocardiography was performed in 73 patients.
Patients with COVID-19 tended to be older (p = 0.077), more frequently had obesity and had a lower incidence of neoplastic diseases (Table 1). Certain risk factors for candidemia, such as catheter use and parenteral nutrition, were more frequent in patients admitted for COVID-19, while previous abdominal surgery was less frequent (Table 1). The use of a central venous catheter as the source of candidemia was more frequent in COVID-19 patients. A total of 15 patients with COVID-19 (93.7%) were admitted to the ICU when candidemia was detected (all required mechanical ventilation) vs. only 11 patients (13.3%) without COVID-19 (p < 0.001); additionally, 15 out of 16 patients admitted for COVID-19 (93.7%) received corticosteroids prior to the onset of candidemia (p < 0.001). There was 1 patient admitted due to COVID-19 (6.25%) and 8 additional non-COVID-19 patients (11.1%) who did not receive antifungal treatment for the episode of candidemia because it was considered a futile measure due to the extreme worsening of the patient. The hospital mortality rate was high in both groups (43.8% in COVID-19 and 58.3% in non-COVID-19 patients, respectively; p = 0.216). Distribution of Candida species causing candidemia according COVID-19 status at admission is presented in Table 2.
Table 2.
Candida species causing candidemia in patients with and without COVID-19.
| Patients with COVID-19 (n = 16) |
Patients without COVID-19 (n = 75) 1 |
p-Value | |
|---|---|---|---|
| C. albicans | 6 (37.5) | 32 (42.7) | 0.315 |
| C. parapsilosis | 9 (56.2) | 22 (29.3) 2 | 0.019 |
| FR C. parapsilosis | 7 (43.7) | 8 (10.7) | 0.003 |
| C. glabrata | 1 (6.2) | 12 (16) | 0.175 |
| C. tropicalis | 0 | 3 (4) | 0.280 |
| C. krusei | 0 | 3 (4) | 0.280 |
| C. lusitaniae | 0 | 1 (1.3) | 0.412 |
| C. africana | 0 | 1 (1.3) | 0.412 |
| C. blankii | 0 | 1 (1.3) | 0.412 |
| Mixed 3 | 0 | 3 (4) | 0.280 |
1 A total of 75 Candida species were isolated from 72 patients. FR—fluconazole resistant. 2 One case with blood cultures growing Candida orthopsilosis was included in the C. parapsilosis group. 3 Mixed: C. albicans plus C. africana, C. albicans plus C. parapsilosis and C. parapsilosis plus C. krusei (one patient each).
3.2. Candidemia Episodes Due to Fluconazole-Resistant Candida parapsilosis
Regarding resistance to antifungal agents, 20 isolates were resistant to fluconazole (22%); of those, 15 isolates corresponded to FRCP, 3 isolates were C. krusei, (intrinsically resistant to fluconazole), 1 isolate C. glabrata and 1 isolate C. blankii, respectively. One isolate of C. glabrata was resistant to posaconazole. There were no cases of resistance to liposomal amphotericin B. Of the 15 FRCP isolates, 10 (66.7%) were also resistant to voriconazole. There were two FRCP isolates concurrently resistant to caspofungin.
FRCP was detected on 2 blood cultures (6.7%) during period 1 compared with 13 in period 2 (21.7%, p = 0.054). The first patient with FRCP candidemia was detected in May 2019 in a patient with a ventricular assist system transferred from a hospital in a different region (Extremadura) to be assessed for cardiac transplantation. FRCP candidemia was detected in 14 additional patients throughout the study period (Figure 1). The incidence rate per 10,000 patient days during period 1 was 0.0937, while it was 0.389 during period 2 (p = 0.032). The incidence of FRCP in patients admitted due to COVID-19 was 1.34, whereas it was 0.16 in the rest of the patients (p < 0.001).
The clinical characteristics of patients with FRCP bloodstream infection as compared to the remaining patients are shown in Table 3. A total of 9 episodes of candidemia due to FRCP (53.3%) were detected in the ICU compared with 16 episodes produced by other Candida strains (15.3%, p < 0.001). Candidemia due to FRCP was more frequent in the surgical ICU than in the medical ICU and was associated with previous Candida spp. colonization, arterial catheter use, parenteral nutrition and renal function replacement therapy (Table 3). Candidemia due to FRCP was not associated with higher hospital mortality. The characteristics of patients with fungemia caused by FRCP compared to those produced by fluconazole-susceptible C. parapsilosis are illustrated in Table 4.
Table 3.
Clinical characteristics of patients with candidemia caused by fluconazole-resistant C. parapsilosis (FRCP) compared to non-FRCP.
| Fluconazole-Resistant C. parapsilosis (n = 15) | Other Strains (n = 73) |
p-Value | |
|---|---|---|---|
| Age, (years) (median (IQR)) | 67 (50–75) | 69 (61–77) | 0.063 |
| Male gender | 13 (86.7) | 48 (65.8) | 0.094 |
| Obesity | 1 (6.7) | 5 (6.8) | 0.730 |
| Diabetes mellitus | 5 (33.3) | 22 (30.1) | 0.514 |
| Heart failure | 1 (6.7) | 10 (13.7) | 0.402 |
| Chronic lung disease | 4 (26.7) | 10 (13.7) | 0.189 |
| Dementia | 0 | 7 (9.7) | 0.252 |
| Chronic liver disease | 1 (6.7) | 5 (6.8) | 0.478 |
| Chronic renal failure | 1 (6.7) | 6 (8.2) | 0.659 |
| Solid tumor | 2 (13.3) | 22 (27.4) | 0.211 |
| Hematologic malignancy | 0 | 6 (8.2) | 0.314 |
| Charlson (median (IQR)) | 1 (0–2) | 2 (1–4) | 0.134 |
| Solid organ transplantation | 2 (13.3) | 4 (5.5) | 0.270 |
| Hospital stay (median (IQR)) | 31 (17–51) | 20 (5–36) | 0.985 |
| ICU admission | 9 (60) | 16 (21.9) | 0.005 |
| ICU stay 1 (median (IQR)) | 41.5 (6–60.5) | 14 (4.5–27) | 0.139 |
| Medical ICU admission | 2 (13.3) | 9 (12.3) | 0.438 |
| Surgical ICU admission | 7 (46.7) | 7 (9.6) | <0.001 |
| Previous corticosteroids treatment | 8 (53.3) | 16 (22.2) | 0.019 |
| Previous immunosuppressive treatment | 2 (13.3) | 4 (5.5) | 0.270 |
| Previous antibacterial treatment | 14 (93.3) | 64 (87.7) | 0.460 |
| Previous antifungal treatment | 7 (46.7) | 17 (23.3) | 0.066 |
| Previous azole treatment | 4 (26.7) | 8 (11) | 0.118 |
| Previous fluconazole treatment | 3 (20) | 6 (8.2) | 0.178 |
| Previous echinocandin treatment | 4 (26.7) | 9 (12.3) | 0.152 |
| Previous liposomal amphotericin B treatment | 1 (6.7) | 3 (4.1) | 0.533 |
| Central venous catheter | 14 (93.3) | 55 (75.3) | 0.110 |
| Arterial catheter | 7 (77.8) | 17 (29.8) | 0.009 |
| Parenteral nutrition | 12 (80) | 30 (41.1) | 0.006 |
| Renal replacement therapy | 8 (53.3) | 12 (16.4) | 0.004 |
| Candida spp. previous colonization | 7 (46.7) | 13 (17.8) | 0.023 |
| Candida Score | 2 (1–3) | 2 (0–3) | 0.499 |
| Abdominal surgery | 2 (13.3) | 20 (27.4) | 0.211 |
| Surgery, other site | 4 (26.7) | 10 (13.7) | 0.189 |
| COVID-19 | 7 (46.7) | 8 (11) | <0.001 |
| Fever | 14 (93.3) | 66 (90.4) | 0.589 |
| Endophtalmitis 2 | 1 (6.7) | 7 (10) | 0.570 |
| Endocarditis 3 | 0 | 6 (8.2) | 0.314 |
| Recurrent candidemia | 4 (26.7) | 3 (4.1) | 0.015 |
| Acute kidney failure | 8 (53.39 | 31 (42.5) | 0.312 |
| Candidemia source | |||
| Venous catheter | 10 (66.7) | 37 (50.7) | 0.200 |
| Intraabdominal | 3 (20) | 13 (17.8) | 0.545 |
| Urinary tract | 0 | 9 (12.3) | 0.170 |
| Unknown | 2 (13.3) | 14 (19.2) | 0.455 |
| Treatment 4 | |||
| Azole | 9 (60) | 32 (43.8) | 0.195 |
| Liposomal amphotericin B treatment | 7 (46.7) | 16 (21.9) | 0.055 |
| Echinocandin treatment | 11 (73.3) | 26 (35.6) | 0.028 |
| Mortality | 7 (46.7) | 42 (57.5) | 0.312 |
| Mortality attributable to candidemia | 2 (22.2) | 5 (8.9) | 0.247 |
IQR—interquartile range. 1 ICU stay until the onset of candidemia. 2 Fundoscopy was performed in 61 patients. 3 Echocardiography was performed in 73 patients. 4 Some patients received more than one type of antifungal drug.
Table 4.
Clinical characteristics of patients with bloodstream infection due to Candida parapsilosis according to resistance to fluconazole.
| Fluconazole-Resistant C. parapsilosis (n = 15) | Fluconazole-Susceptible C. parapsilosis (n = 16) | p-Value | |
|---|---|---|---|
| Age (years) (median (IQR)) | 67 (50–75) | 70.5 (57–75) | 0.309 |
| Male gender | 13 (86.7) | 14 (87.5) | 0.675 |
| Obesity | 1 (6.7) | 0 | 0.484 |
| Diabetes mellitus | 5 (33.3) | 5 (31.3) | 0.602 |
| Heart failure | 1 (6.7) | 3 (18.8) | 0.325 |
| Chronic lung disease | 4 (26.7) | 2 (12.5) | 0.295 |
| Chronic liver disease | 1 (6.7) | 0 | 0.484 |
| Chronic renal failure | 1 (6.7) | 2 (12.5) | 0.525 |
| Solid tumor | 2 (13.3) | 5 (31.3) | 0.224 |
| Hematologic malignancy | 0 | 2 (12.5) | 0.258 |
| Neutropenia | 1 (6.7) | 3 (18.8) | 0.325 |
| Charlson (median (IQR)) | 1 (0–2) | 3 (2–3) | 0.256 |
| Solid organ transplantation | 2 (13.3) | 0 | 0.226 |
| Hospital (stay median (IQR)) | 31 (17–51) | 21 (3–30) | 0.489 |
| ICU admission | 9 (60) | 3 (18.8) | 0.023 |
| Medical ICU admission | 2 (13.3) | 1 (6.3) | 0.475 |
| Surgical ICU admission | 7 (46.7) | 2 (12.5) | 0.043 |
| Previous corticosteroids treatment | 8 (53.3) | 3 (18.8) | 0.050 |
| Previous immunosuppressive treatment | 2 (13.3) | 1 (6.3) | 0.475 |
| Previous antibacterial treatment | 14 (93.3) | 15 (93.8) | 0.742 |
| Previous antifungal treatment | 7 (46.7) | 6 (37.5) | 0.439 |
| Previous azole treatment | 4 (26.7) | 2 (12.5) | 0.295 |
| Previous fluconazole treatment | 3 (20) | 1 (6.3) | 0.275 |
| Previous echinocandin treatment | 4 (26.7) | 4 (25) | 0.618 |
| Previous liposomal amphotericin B treatment | 1 (6.7) | 0 | 0.484 |
| Central venous catheter | 14 (93.3) | 15 (93.8) | 0.742 |
| Arterial catheter | 7 (77.8) | 3 (23.1) | 0.017 |
| Parenteral nutrition | 12 (80) | 10 (62.5) | 0.250 |
| Renal replacement therapy | 8 (53.3) | 2 (12.5) | 0.019 |
| Candida spp. previous colonization | 7 (46.7) | 2 (12.5) | 0.044 |
| Candida Score | 2 (1–3) | 2 (0–2) | 0.413 |
| Abdominal surgery | 2 (13.3) | 4 (25) | 0.359 |
| Surgery, other site | 4 (26.7) | 3 (18.8) | 0.461 |
| COVID-19 | 7 (46.7) | 2 (12.5) | 0.044 |
| Candidemia source | |||
| Venous catheter | 10 (66.7) | 10 (62.5) | 0.553 |
| Intraabdominal | 3 (20) | 3 (18.8) | 0.642 |
| Urinary tract | 0 | 2 (12.5) | 0.258 |
| Unknown | 2 (13.3) | 1 (6.3) | 0.475 |
| Fever | 14 (93.3) | 15 (93.8) | 0.742 |
| Endophtalmitis 1 | 1 (6.7) | 1 (6.3) | 0.742 |
| Endocarditis 2 | 0 | 1 (6.3) | 0.516 |
| Recurrent candidemia | 4 (26.7) | 0 | 0.043 |
| Acute kidney failure | 8 (53.3) | 5 (31.3) | 0.189 |
| Mortality | 7 (46.7) | 7 (43.8) | 0.578 |
| Mortality attributable to candidemia | 2 (13.3) | 0 | 0.226 |
IQR—interquartile range. 1 Fundoscopy was performed in 21 patients. 2 Echocardiography was performed in 27 patients.
The 15 fluconazole-resistant C. parapsilosis isolates were subjected to sequencing of the ERG11 gene and the A395T mutation, conferring the amino acid substitution Y132F was found in all of them. Such mutation was never found in fluconazole-susceptible isolates studied. All isolates harboring the A395T resulted to be a single genotype that demonstrated that all of them belonged to the same clone.
4. Discussion
Our investigation has shown an increase in the incidence of candidemia during the pandemic period, mainly due to an increase in C. parapsilosis cases. The most remarkable finding was the increase FRCP bloodstream infection episodes in patients admitted due to COVID-19, which, to our knowledge, has not been reported to date.
4.1. Candidemia Episodes in Patients Hospitalized for COVID-19
We detected a higher incidence of candidemia during the pandemic period, and in particular, it was higher in patients admitted for COVID-19, as previously described [6], suggesting that specific risk factors may be involved in this complication.
Classic risk factors for candidemia, such as cancer or previous surgery (abdominal or of other site), were less common among patients with COVID-19, for instance, all patients who had an underlying hematologic disease were in the group of patients without COIVD-19. Similar to our findings, other authors report fewer cases of patients with neoplasms in patients with COVID-19 [7]. Not surprisingly, obesity, diabetes and chronic lung disease tended to be present more frequently in patients with candidemia and COVID-19, as they are known COVID-19 risk factors that increase the probability hospital admission [5,17]. The limited number of cases in our series may have precluded detection of the possible association of candidemia in patients with COVID-19 with chronic lung disease or diabetes.
A distinctive feature of patients with COVID-19 who developed candidemia is the association with admission to the ICU [5,6,7,17]. As expected, patients admitted to a conventional ward had a much lower incidence due to the absence of risk factors for candidemia. Patients with severe COVID-19 who required admission to the ICU presented a high incidence of antibiotic use, corticosteroid treatment, central venous catheterization, parenteral nutrition and mechanical ventilation. Reducing the duration of admission to the ICU and the risk factors present in each patient as much as possible is an elusive goal, but one that could decrease this high incidence.
The common use of corticosteroids (which is a known risk factor for candidemia) in COVID-19 patients has been involved in their immune impairment and could explain in part the increase in candidemia observed in similar investigations [17,18]. This association was to be expected considering the previously described correlation with episodes of candidemia in oncology patients as well as in patients with other underlying diseases [19].
Regarding the species causing the bloodstream infection, other studies have observed a prominent role of non-albicans Candida species in patients admitted for COVID-19 [20,21]. The present study underlines the relevant role of C. parapsilosis in these patients that has not been previously reported [5,6,17,22]. C. parapsilosis commonly colonizes the skin, so that any manipulation or alteration of the skin integrity—such as those that occur frequently during ICU admission, e.g., catheter use—would facilitate candidemia and explain the high percentage of cases in this setting. Many of these candidemia episodes were originated in catheters.
4.2. Candidemia Episodes Due to Fluconazole-Resistant Candida parapsilosis
The most relevant finding of our study was the increase in cases of candidemia caused by FRCP during the pandemic. In this study, we found the Y132F mutation in ERG11 gene only in fluconazole-resistant isolates and not in fluconazole-susceptible isolates. This study constitutes the second series of cases of FRCP due to the Y132F mutation reported in Spain [23]. Although all 15 isolates were resistant to fluconazole, susceptibility to other azoles varied. Most of them were also resistant to voriconazole. Previous use of fluconazole has been considered a risk factor for the development of resistance in several Candida species; however, no statistically significant difference was detected in our patients (Table 3 and Table 4) [24,25]. We hypothesize that the patient’s proximity to the environmental reservoir may have played a more important role than previous antifungal exposure. In relation to the other resistant species, it is worth highlighting the case of bloodstream infection by C. blankii, which was detected before the pandemic. This species could become an emerging epidemiological problem due to its reduced sensitivity to azoles and echinocandins [26].
When comparing patients with candidemia due to FRCP both to those with fluconazole-susceptible C. parapsilosis, and with non-parapsilosis candidemia, the former presented more surgical ICU admissions, arterial catheters, a renal function replacement technique and previous Candida colonization (any species). Compared with non-parapsilosis candidemia, parenteral nutrition was also associated with FRCP candidemia. Since multivariate analysis was not performed, it is not possible to establish which variables are the more commonly associated with this infection. However, other studies suggest that vascular access and surgical ICU admission are the most relevant parameters [27]. It should be taken into account that epidemiological surveillance cultures are not usually performed in conventional hospital wards, but, on the contrary, they are performed in ICUs. This may account for the high proportion of previous Candida colonization detected in patients with FRCP fungemia. Other studies with a high number of azole-resistant Candida infections have observed a clear association with prolonged hospital admission [28]. In the present study, there was a non-significant trend to a longer hospital stay in patients with FRCP.
The COVID-19 pandemic may have provided an optimal scenario for the spread of a resistant pathogen that already existed in the hospital, as a result of transferring a first patient with candidemia with FRCP from another hospital. Undoubtedly, the high number of patients admitted to the ICU has supposed a somewhat unusual clinical scenario and it cannot be ruled out that the usual aseptic measures to control the horizontal transmission of pathogens were reduced. It is noteworthy that the Candida species involved in this outbreak is characterized by its ability to adhere to catheters and to colonize the hands of healthcare personnel [9,10,24]. We also are unaware of whether there were any particularly contaminated areas that could have acted as a reservoir and were not adequately managed. The fact that these outbreaks tend to affect one of the ICUs more severely than other ICUs in the same institution suggests the existence of an environmental reservoir that is often very difficult to determine [24,29,30].
There are some similarities between candidemia outbreaks due to C. auris described to date in patients with COVID-19 and those by FRPC observed in our series, such as the identification of patients with infection or colonization by the same Candida species during the preceding months [31,32]. Other relevant characteristics of C. auris cases are the predominant involvement of certain areas of the ICUs, their relationship with prolonged hospital stays, previous administration of antifungal drugs and the high frequency of previous colonization by Candida spp. [31,32,33]. Additionally, its association with central venous catheters and the suspicion that the relaxation of infection control measures may have favored horizontal transmission is noteworthy [31]. These facts make it advisable to pursue early diagnosis and treatment of these infections in patients with COVID-19 admitted to the ICU and to intensify measures to control hospital-acquired infections.
In contrast to what has been observed in other FRCP outbreaks, candidemia due to this microorganism was not associated with increased mortality [10,24,29]. Our interpretation is that the Y132F substitution confers azole resistance but not increased virulence. It cannot be ruled out that the lower comorbidity in our patients coinfected with COVID-19 compared to other patients with candidemia may have influenced their prognosis.
Our findings should be a warning about the risk of transmission of resistant microorganisms among COVID-19 patients and the inform the appropriate preventive measures that need to be undertaken.
4.3. Limitations
Our study has several limitations. The most important limitation is the small number of patients with candidemia due to FRCP, which prevented the performance of a multivariate analysis to identify the variables most closely related to this infection. Since this is a single-center study, our conclusions may not be applicable to other institutions. The retrospective collection of patient information from the medical records could have prevented us from correctly recording some variables, such as obesity.
Author Contributions
Conceptualization, A.R.-M., Á.A., A.F.-C., J.G. and E.M.; methodology, M.M.-A., I.S.-R., J.D.-G. and J.G.; software, I.P.-P.; validation, A.R.-M. and A.F.-C.; formal analysis, Á.A., I.P.-P., A.R.-M. and A.G.-V.; resources, A.R.-M. and I.P.-P.; data curation, I.S.-R., M.M.-A., J.C.-P., E.G.-A., A.G.-V. and V.M.-T.; writing—original draft preparation, A.R.-M., A.G.-V. and E.G.-A.; writing—review and editing, A.F.-C., J.G., J.D.-G., I.S.-R., J.C.-P., R.I. and V.M.-T.; visualization, A.R.-M., I.P.-P. and E.M.; supervision, E.M. and I.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants PI18/01155 and PI19/00074 from Fondo de Investigación Sanitaria (FIS. Instituto de Salud Carlos III; Plan Nacional de I+D+I 2017–2020). The study was co-funded by the European Regional Development Fund (FEDER) ‘A way of making Europe.’ JG is a steady researcher contracted by Fundación para Investigación Sanitaria del Hospital Gregorio Marañón. JD (FI19/00021) holds a predoctoral grant by FIS.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Hospital Universitario Puerta de Hierro Majadahonda (protocol code 2/2022, 31 January 2022).
Informed Consent Statement
Informed consent was not obtained because the study was retrospective in nature and no specific diagnostic or therapeutic intervention was performed on the patients. The local clinical research committee approved this waiver.
Data Availability Statement
The data supporting the reported results are available and can be obtained upon request by email to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest related to the content of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller I.F., Becker A.D., Grenfell B.T., Metcalf C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat. Med. 2020;26:1212–1217. doi: 10.1038/s41591-020-0952-y. [DOI] [PubMed] [Google Scholar]
- 2.Grasselli G., Scaravilli V., Mangioni D., Scudeller L., Alagna L., Bartoletti M., Bellani G., Biagioni E., Bonfanti P., Bottino N., et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest. 2021;160:454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Simmonds A., Annavajhala M.K., McConville T.H., Dietz D.E., Shoucri S.M., Laracy J.C., Rozenberg F.D., Nelson B., Greendyke W.G., Furuya E.Y., et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J. Antimicrob. Chemother. 2020;76:380–384. doi: 10.1093/jac/dkaa466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohamed A., Rogers T.R., Talento A.F. COVID-19 Associated Invasive Pulmonary Aspergillosis: Diagnostic and Therapeutic Challenges. J. Fungi. 2020;6:115. doi: 10.3390/jof6030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seagle E.E., Jackson B.R., Lockhart S.R., Georgacopoulos O., Nunnally N.S., Roland J., Barter D.M., Johnston H.L., Czaja C.A., Kayalioglu H., et al. The Landscape of Candidemia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2021:ciab562. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M., Barreiros G., Guimarães L.F., Deriquehem V.A., Castiñeiras A.C., Nouér S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2020;64:152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastrangelo A., Germinario B.N., Ferrante M., Frangi C., Voti R.L., Muccini C., Ripa M., Canetti D., Castiglioni B., Oltolini C., et al. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID-19 Controls. Clin. Infect. Dis. 2020;73:e2838–e2839. doi: 10.1093/cid/ciaa1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimopoulos G., Velegraki A., Falagas M.E. A 10-Year Survey of Antifungal Susceptibility of Candidemia Isolates from Intensive Care Unit Patients in Greece. Antimicrob. Agents Chemother. 2009;53:1242–1244. doi: 10.1128/AAC.01368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tóth R., Nosek J., Mora-Montes H.M., Gabaldon T., Bliss J.M., Nosanchuk J.D., Turner S.A., Butler G., Vágvölgyi C., Gácser A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019;32:e00111-18. doi: 10.1128/CMR.00111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomaz D.Y., De Almeida J.N.J., Lima G.M.E., Nunes M.D.O., Camargo C.H., Grenfell R.D.C., Benard G., Del Negro G.M.B. An Azole-Resistant Candida parapsilosis Outbreak: Clonal Persistence in the Intensive Care Unit of a Brazilian Teaching Hospital. Front. Microbiol. 2018;9:2997. doi: 10.3389/fmicb.2018.02997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martini C., Torelli R., De Groot T., De Carolis E., Morandotti G.A., De Angelis G., Posteraro B., Meis J.F., Sanguinetti M. Prevalence and Clonal Distribution of Azole-Resistant Candida parapsilosis Isolates Causing Bloodstream Infections in a Large Italian Hospital. Front. Cell. Infect. Microbiol. 2020;10:232. doi: 10.3389/fcimb.2020.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Singanayagam A., Patel M., Charlett A., Bernal J.L., Saliba V., Ellis J., Ladhani S., Zambon M., Gopal R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance. 2020;25:2001483. doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gniazdowski V., Morris C.P., Wohl S., Mehoke T., Ramakrishnan S., Thielen P., Powell H., Smith B., Armstrong D.T., Herrera M., et al. Repeated Coronavirus Disease 2019 Molecular Testing: Correlation of Severe Acute Respiratory Syndrome Coronavirus 2 Culture With Molecular Assays and Cycle Thresholds. Clin. Infect. Dis. 2020;73:e860–e869. doi: 10.1093/cid/ciaa1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkow E.L., Manigaba K., Parker J., Barker K.S., Kelly S.L., Rogers P.D. Multidrug Transporters and Alterations in Sterol Biosynthesis Contribute to Azole Antifungal Resistance in Candida parapsilosis. Antimicrob. Agents Chemother. 2015;59:5942–5950. doi: 10.1128/AAC.01358-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinea J., Arendrup M.C., Cantón R., Cantón E., García-Rodríguez J., Gómez A., De La Pedrosa E.G.G., Hare R.K., Orden B., Sanguinetti M., et al. Genotyping Reveals High Clonal Diversity and Widespread Genotypes of Candida Causing Candidemia at Distant Geographical Areas. Front. Cell. Infect. Microbiol. 2020;10:166. doi: 10.3389/fcimb.2020.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayaaslan B., Eser F., Kaya Kalem A., Bilgic Z., Asilturk D., Hasanoglu I., Ayhan M., Tezer Tekce Y., Erdem D., Turan S., et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64:1083–1091. doi: 10.1111/myc.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riche C.V.W., Cassol R., Pasqualotto A.C. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J. Fungi. 2020;6:286. doi: 10.3390/jof6040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 20.Omrani A.S., Koleri J., Ben Abid F., Daghfel J., Odaippurath T., Peediyakkal M.Z., Baiou A., Sarsak E., Elayana M., Kaleeckal A., et al. Clinical characteristics and risk factors for COVID-19-associated Candidemia. Med. Mycol. 2021;59:1262–1266. doi: 10.1093/mmy/myab056. [DOI] [PubMed] [Google Scholar]
- 21.Macauley P., Epelbaum O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021;64:634–640. doi: 10.1111/myc.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., Pandey M., Whitaker H., May A., Morgan M., et al. A National Strategy to Diagnose Coronavirus Disease 2019–Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2020;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alcoceba E., Gómez A., Lara-Esbrí P., Ferre-Beltran O.A., Ayestarán I., Muñoz P., Escribano P., Guinea J. Fluconazole-resistant Candida parapsilosis clonally related genotypes: First report proving the presence of endemic isolates harbouring the Y132F erg11 gene mutation in Spain. Clin. Microbiol. Infect. 2022 doi: 10.1016/j.cmi.2022.02.025. in press . [DOI] [PubMed] [Google Scholar]
- 24.Arastehfar A. First Report of Candidemia Clonal Outbreak Caused by Emerging Fluconazole-Resistant Candida parapsilosis Isolates Harboring Y132F and/or Y132F+K143R in Turkey. Antimicrob. Agents Chemother. 2020;64:e01001-20. doi: 10.1128/AAC.01001-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett J.E., Izumikawa K., Marr K.A. Mechanism of Increased Fluconazole Resistance in Candida glabrata during Prophylaxis. Antimicrob. Agents Chemother. 2004;48:1773–1777. doi: 10.1128/AAC.48.5.1773-1777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhary A., Stielow J.B., Upadhyaya G., Singh P.K., Singh A., Meis J.F. Candida blankii: An emerging yeast in an outbreak of fungaemia in neonates in Delhi, India. Clin. Microbiol. Infect. 2020;26:648.e5–648.e8. doi: 10.1016/j.cmi.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Fekkar A., Blaize M., Bouglé A., Normand A.-C., Raoelina A., Kornblum D., Kamus L., Piarroux R., Imbert S. Hospital Outbreak of Fluconazole-Resistant Candida parapsilosis: Arguments for Clonal Transmission and Long-Term Persistence. Antimicrob. Agents Chemother. 2021;65:e02036-20. doi: 10.1128/AAC.02036-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao X., Qiu H., Li R., Guo F., Liu W., Kang M., Kang Y. Risk factors for fluconazole-resistant invasive candidiasis in intensive care unit patients: An analysis from the China Survey of Candidiasis study. J. Crit. Care. 2015;30:862.e1–862.e5. doi: 10.1016/j.jcrc.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Karmarkar E.N., O’Donnell K., Prestel C., Forsberg K., Gade L., Jain S., Schan D., Chow N., McDermott D., Rossow J., et al. Rapid Assessment and Containment of Candida auris Transmission in Postacute Care Settings-Orange County, California, 2019. Ann. Intern. Med. 2021;174:1554–1562. doi: 10.7326/M21-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston B.L., Schlech W.F., 3rd, Marrie T.J. An outbreak of Candida parapsilosis prosthetic valve endocarditis following cardiac surgery. J. Hosp. Infect. 1994;28:103–112. doi: 10.1016/0195-6701(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilato V.D., Codda G., Ball L., Giacobbe D.R., Willison E., Mikulska M., Magnasco L., Crea F., Vena A., Pelosi P., et al. Molecular epidemiological investigation of a nosocomial cluster of C. auris: Evidence of recent emergence in italy and ease of transmission during the COVID-19 pandemic. J. Fungi. 2021;7:140. doi: 10.3390/jof7020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moin S., Farooqi J., Rattani S., Nasir N., Zaka S., Jabeen K. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med. Mycol. 2021;59:1238–1242. doi: 10.1093/mmy/myab057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the reported results are available and can be obtained upon request by email to the corresponding author.