Abstract
In recent years, important changes have occurred in the field of diabetes treatment. The focus of the treatment of diabetic patients has shifted from the control of blood glucose itself to the overall management of risk factors, while adjusting blood glucose goals according to individualization. In addition, regulators need to approve new antidiabetic drugs which have been tested for cardiovascular safety. Thus, the newest class of drugs has been shown to reduce major adverse cardiovascular events, including sodium-glucose transporter 2 (SGLT2) and some glucagon like peptide 1 receptor (GLP1) analog. As such, they have a prominent place in the hyperglycemia treatment algorithms. In recent years, the role of DPP4 inhibitors (DPP4i) has been modified. DPP4i have a favorable safety profile and anti-inflammatory profile, do not cause hypoglycemia or weight gain, and do not require dose escalation. In addition, it can also be applied to some types of chronic kidney disease patients and elderly patients with diabetes. Overall, DPP4i, as a class of safe oral hypoglycemic agents, have a role in the management of diabetic patients, and there is extensive experience in their use.
Keywords: DPP4i, T2DM, GLP1, GIP
1. Introduction
Dipeptidyl peptidase 4 (DPP4) enzyme is a type II transmembrane glycoprotein, expressed ubiquitously in many tissues, including the immune cells, kidney, liver, pancreas, fat cells, and presents as a soluble form in the circulation [1]. Dipeptidyl peptidase 4 is a serine protease, can cleave and inactivate incretin hormones, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), neuropeptides, and chemokines [2]. In addition, DPP4 has been shown to have a direct pro-inflammatory role in lymphocytes, macrophages, and smooth muscle cells [3,4]. Dipeptidyl peptidase 4 plays a major role in glucose and insulin metabolism, but its functions are not fully understood yet. On one hand, DPP4 degrades incretins such as GLP-1 and GIP, ultimately leading to reduced insulin secretion and abnormal visceral adipose tissue metabolism; on the other hand, DPP4 regulates postprandial glucose through degradation of GLP-1. Due to its ability to prevent the inactivation of GLP-1, DPP4 inhibition (DPP4i) was explored as a target for the treatment and management of type 2 diabetes mellitus (T2DM) in the 1990s [5,6,7].
T2DM is the most common type of diabetes and associated with a low-grade chronic inflammation induced by the excessive visceral adipose tissue. This inflammatory status results in dysregulation of homeostatic glucose regulation and peripheral insulin sensitivity. Dipeptidyl peptidase 4 activity is correlated with the onset and severity of obesity and diabetes [8]. The levels of plasma DPP4 activity are elevated in diseases, including T2DM [9], obesity [9], chronic diabetic kidney disease [10], cardiovascular diseases [7] and atherosclerosis [11]. Recently, the circulating levels of endogenous soluble DPP4 were found to be dissociated from the extent of systemic inflammation, glucose intolerance and white adipose tissue inflammation [12]; therefore, the search for DPP4 inhibitors is a viable approach. Sitagliptin may have potential therapeutic agent for the treatment of cardiovascular diseases via suppressing activation of p38/NF-κB signaling [7].
This review discusses the role of DPP4i in the treatment of diabetes, highlighting their benefits and risks. The article will focus primarily effect of the approved DPP4i: sitagliptin, vildagliptin, saxagliptin, alogliptin, and linagliptin.
2. DPP4 and DPP4 Inhibitions in Diabetes
2.1. Mechanisms of Effect of DPP4i
Diabetes mellitus (DM) is a worldwide health problem, which is a major cause of blindness, chronic kidney disease (CKD), stroke, lower extremity amputations, coronary heart disease and heart failure (HF) [13]. T2DM has changed from a chronic disease of the elderly in the traditional concept to a chronic disease of middle-aged and even children and adolescents [14,15]. Excess body fat along with age constitute the two most important risk factors for the premature development of T2DM [15,16]. Early onset T2DM relative to late-onset disease is associated with a more rapid deterioration of β-cell function, emphasizing the importance for early diagnosis and treatment initiation [17]. Obesity-related mechanisms that are potentially linked to the severity of the disease include adipocyte lipid spillover, ectopic fat accumulation and tissue inflammation [18]. Therapies aiming to decrease body weight are consequently a valuable strategy to delay the onset and decrease the risk of T2DM, as well as managing established disease [19].
In the past few decades, drug therapy for T2DM has developed greatly and involves several new strategies [20,21,22]. These new strategies include more patient-friendly ways to use the drug, such as improving weight loss. However, animal studies have demonstrated that a key barrier to the development of anti-obesity drugs is the large inability to predict human cardiovascular safety [23,24,25]. In tolerable doses, they rarely achieve 10% weight loss. Although the clinical success of these agents has laid the foundation for a new era of anti-obesity drugs, there is considerable debate as to how GLP1/GIP regulates metabolism and whether its receptor agonists or antagonists can be the drugs of choice for treating obesity and T2DM. At present, DPP4 inhibitors are widely used for the treatment of T2DM [26,27,28,29]. The basis for this approach lies with the finding that DPP4 has a key role in determining the clearance of the incretin hormone, GLP1 [5]. GLP1 is an intestinal peptide, which was known to have a role in glucose homeostasis via actions that include the potentiation of glucose-induced insulin secretion and the suppression of glucagon secretion [30].
Dipeptidyl peptidase 4 inhibitor (DPP4i) itself has no hypoglycemic activity. Instead, their anti-hyperglycemia effect is achieved primarily by altering levels of endogenous substrates. Once the catalytic activity of DPP4 is inhibited, the levels of these substrates change. To date, GLP1 has been considered to play a major role in the therapeutic effect of DPP4i [23]. GLP1 has been shown to be a physiological DPP4 substrate [23,25]. In vivo, endogenous levels of intact, biologically active peptides increase with DPP4 inhibition and are associated with improved glucose homeostasis [31,32]. Some studies found that GLP1 receptor antagonist inhibited GLP1 signaling pathway, and the hypoglycemic effect of DPP4i decreased [33,34], thus confirming the role of GLP1 in the mechanism of action of DPP4i. It also indicates that GLP1 is not the only regulatory factor, and even in the absence of GLP1 receptor activation, the hypoglycemic activity of DPP4i is still significant [33,34].
Another physiological substrate of DPP4 is glucose-dependent insulin polypeptide (GIP), also known as incretin, and the level of GIP increases with inhibition of DPP4 activity [35,36]. Similar to GLP-1, GIP enhances insulin secretion in pancreatic beta cells in a glucose-dependent manner but appears to act in a different way on glucagon secretion [37,38]. The response to GIP was also impaired in T2DM patients. In the past, views on the possible role of GIP in the treatment of T2DM have been largely ignored, because early studies have shown that GIP′s ability to stimulate insulin secretion is severely impaired. However, in T2DM patients, further studies to explore this problem were unable to be carried out due to the lack of appropriate GIP receptor antagonists. Recent studies have shown that GIP can improve glycemic control in patients with T2DM [39,40] and have revived studies on the development of novel antagonists [41,42]. These studies have led to a re-evaluation of the role of GIP in the anti-hyperglycemia of DPP4i. In addition, GLP1′s ability to inhibit glucagon secretion is weakened when blood glucose levels drop below normal fasting levels, while GIP enhances glucagon response to hypoglycemic levels. Thus, during insulin-induced hypoglycemia, glucagon secretion is increased due to GIP use [43]. Therefore, the increase in intact GIP levels observed after inhibition of DPP4 may help maintain the counter-regulatory response of glucagon when glucose levels are controlled at hypoglycemia [44,45]. Thus, GIP′s role in improving glucagon counter-regulation may further contribute to reducing the risk of hypoglycemia associated with DPP4i.
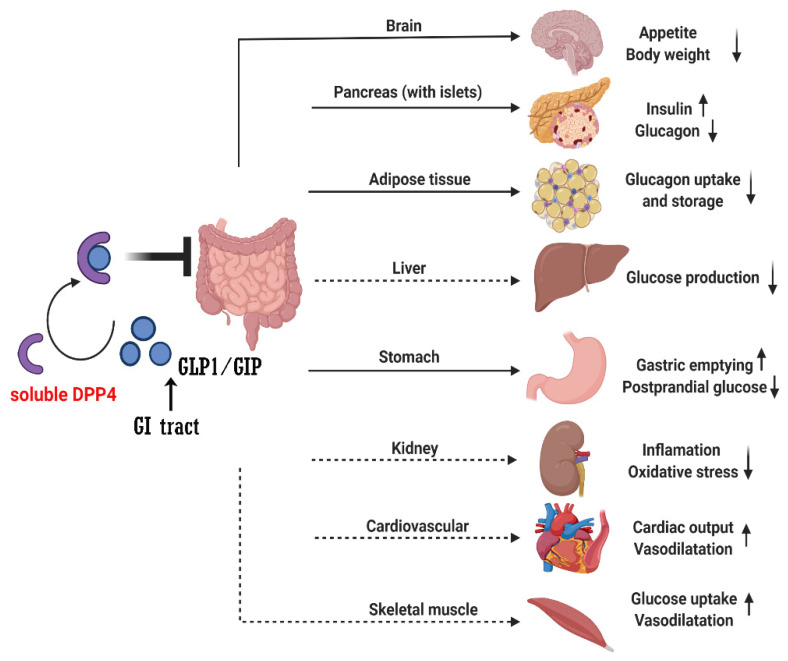
Recent studies found the direct or indirect role of soluble DPP4 in brain, gastric, liver, kidney, adipose tissue, pancreas (with islet), cardiovascular system and muscle through GLP1/GIP signaling (Figure 1). However, whether other DPP4 substrates also contribute to the therapeutic effect of DPP4i remains to be determined. In vitro, many peptide hormones and chemokines are susceptible to DPP4 cleavage when incubated with DPP4 at high concentrations [25,45]. However, there is not much evidence that they are altered in vivo by DPP4i and there have been no adverse reactions or safety issues caused by off-target effects of DPP4i on other endogenous substrates [46,47].
Figure 1.
The action of DPP4 degrades the GLP1 and GIP. GI: gastrointestinal; GIP: glucose-dependent insulinotropic polypeptide, GLP1: glucagon-like peptide-1, DPP4: dipeptidyl peptidase-4. Solid arrows mean the direct regulation effects by GLP1 or GIP; dashed arrows mean the indirect regulation effects by GLP1 or GIP.
2.2. DPP4 Inhibitors
When DPP4 was identified as a therapeutic target, the search began for compounds suitable for clinical use, namely the progressive development of DPP4 inhibitors such as sitagliptin [48] and saxagliptin [49]. Currently, several structures oriented to target-specific interaction with DPP-4 are already known and officially approved by the United States Food & Drug Administration (FDA), including sitagliptin [50], saxagliptin [51], alogliptin [52], and linagliptin [53], and vildagliptin 12801240 is authorized in Europe (Table 1).
Table 1.
The features of approved dipeptidyl peptidase 4 inhibitors (DPP4i).
| DPP4i | Chemistry | Metabolism | Half-Life | Elimination Method |
|---|---|---|---|---|
| Sitagliptin | β-amino acid based | Minimal | 12.5 h | Predominantly (>80%) renal |
| Vildagliptin | Cyanopyrrolidine | Hydrolysis (cytochromeindependent) to form an inactive metabolite |
~2 h | Metabolism (parent) and renal (metabolite) |
| Saxagliptin | Cyanopyrrolidine | Hydrolysis (cytochrome P450 3A4 or P450 3A5) to form an activemetabolite |
2.5 h (parent), 3 h (metabolite) |
Metabolism (parent) and renal (metabolite) |
| Alogliptin | Modified pyrimidinedione |
Minimal | 20 h | Predominantly (>70%) renal |
| Linagliptin | Xanthine based |
Minimal | ~12 h (effective), >100 h (terminal) |
Predominantly biliary (<6% renal) |
Sitagliptin. Sitagliptin was the first DPP4i to receive marketing approval. The apparent terminal elimination half-life of sitagliptin is 12.4 h and renal clearance is 350 mL/min [26,54]. In healthy adult volunteers, sitagliptin is rapidly absorbed orally after a single 100 mg dose and reaches peak plasma concentration 14 h after the dose. The pharmacokinetic characteristics of sitagliptin in T2DM are generally similar with those of healthy volunteers [26,54]. Earlier results showed that sitagliptin was mainly eliminated by renal excretion, and the renal clearance rate accounted for about 70% of the plasma clearance rate of sitagliptin in healthy volunteers [55]. Absolute bioavailability of sitagliptin is 87%, oral absorption is not affected by food, and drugs can be taken regardless of food [26,54,56].
Vidagliptin. Vildagliptin was the second DPP4i, which to be approved by Europe [57]. About 57% of the circulation of Vildagliptin in vivo is cytochromatin-independent, with a large amount of hydrolysis to produce an inactive molecule (LAY151). The remaining 18% is circulated as an active drug [58,59]. Therefore, compared with sitagliptin, it has a shorter half-life (~2 h) and is administered in a twice-daily regimen. This metabolism is the main route of elimination of maternal drugs; however, LAY151 is cleared by the kidney and administered in a manner that increases the risk of exposure in patients with impaired renal function [60].
Saxagliptin. Saxagliptin is an effective anti-diabetes drug, which expands the inhibitory effect of DPP4 enzyme [61] and metabolized via cytochrome P450 3A4/A5 [62]. The use of saxagliptin at a dose of 2.5 mg, poor membrane permeability and solubility in water may further lead to its elimination with a short half-life (4–6 h) and therefore need to be dosed more than once daily [25]. The parent molecules of saxagliptin are cleared by metabolism in the liver and its metabolites are cleared by the kidneys [61,63]. However, the effect of liver damage on drug exposure is small, meaning that the therapeutic dose does not need to be changed; however, consistent with the elimination of DPP4i in other kidneys, some dose reductions are recommended when renal function declines [64].
Linagliptin. Linagliptin is the latest to come to market, approved for glycemic management of type 2 diabetes [65,66,67]. The metabolism of Linagliptin is not obvious, and its half-life is long (effective half-life is about 12 h, terminal decay is greater than or equal to 100 h), but compared with other DPP4i, the kidney plays a very small role in its elimination, only less than 6% of the drug is cleared in the kidney, most of the drug is excreted into bile then eliminated in the feces [68,69,70]. Therefore, linagliptin is not affected by changes in renal function and dose is not adjusted according to renal function [65]. Although it is eliminated by the biliary pathway, there is no clinically significant change in liver damage to drug exposure nor dose adjustment [71]. In the latest randomized trial, in patients with T2D and high cardiovascular (CV) risk, linagliptin showed non-inferiority compared with placebo in the risk of major CV events, with a median time of 2.2 years [72]. Linagliptin is well tolerated in Asian T2DM, with low risk for adverse events [73].
Alogliptin. Like sitagliptin, it has no significant metabolism and has a half-life of about 12.4–21.4 h [52,74]. Due to a long half-life, alogliptin is generally prescribed once a day. Alogliptin is cleared primarily by the kidney through glomerular filtration and active secretion mechanisms; therefore, it is recommended to reduce the dose in patients with reduced renal function [74]. Alogliptin alone or in combination with metformin, pioglitazone, glyburide, or insulin significantly improved glycemic control in adults or elderly T2DM compared with placebo. Alogliptin will primarily be used to avoid hypoglycemic events in patients with congestive heart failure, kidney failure and liver disease, as well as in the elderly. Alogliptin protects against cyclophosphamide induced lung toxicity by reducing oxidation, inflammation and fibrosis, making it a promising pharmacological treatment for reducing lung toxicity [75].
2.3. Benefits of DPP4i
As above discussed, the efficacy of DPP4i in inhibiting the catalytic activity of DPP4 is clearly related to its efficacy as an antidiabetic agent. Marketed in the United States, DPP4i has also been evaluated for cardiovascular safety in large cardiovascular outcome trials (CVOTs) and neither type of DPP4i increases the risk of major adverse cardiovascular events [72,76]. DPP4i reduces long-term cardiovascular risk after percutaneous coronary intervention in patients with diabetes via the insulin-like growth factor-1 axis [77]. However, in CVOT′S there was not any cardiovascular benefit and saxagliptin increase heart failure hospitalization, vildagliptin is not marketed in the USA, so there is no data associated with CVOT (Table 2).
Table 2.
Cardiovascular outcome trials with DPP4 inhibitors.
| DPP4i | Trial (Year) | Median Follow-Up, Years |
Mean/Median Age, Years |
Female (Total) | BMI, kg/m2 * | HbA1c, mmol/mol (%) * | Baseline Metformin, % |
Baseline eGFR, mL/min/ [1.73 m]2 * |
Prior ASCVD, % |
Prior CHF, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Sitagliptin | TECOS (2015) | 3.0 | 65 | 4212 | 30.2 | 55 (7.2) | 81 | 75 | 100 | 18 |
| (14,523) | ||||||||||
| Saxagliptin | SAVOR-TIMI (2013) | 2.1 | 65 | 5590 | 31.2 | 64 (8.0) | 69 | 73 | 78 | 13 |
| (16,492) | ||||||||||
| Alogliptin | EXAMINE (2013) | 1.5 | 61 | 1722 | 28.7 | 64 (8.0) | NA | 71 | 100 | 28 |
| (5380) | ||||||||||
| Linagliptin | CARMEL (2019) | 2.2 | 66 | 2582 | 31.4 | 64 (8.0) | 54 | 55 | 57 | 27 |
| (6979) |
NA, not available; * These are expressed as mean values.
Previously discussed reports suggest that DPP4i may be more effective in the Asian population than in the white population [78,79]. It has been suggested that this difference may be related to pathological differences in T2DM between the two groups (emaciation and impaired beta cell function phenotype in Asian patients and obesity and insulin resistance phenotype in white patients) [80,81]. DPP4i all benefit by being highly orally available and well tolerated anti-hyperglycemic medications. They are also easy to use, requiring no dose titration and can be taken at any time of day without regard to mealtimes. Sitagliptin, alogliptin, and linagliptin interact noncovalently with residues in the catalytic bag, then decompose unchanged as parent inhibitor molecules, and then interact freely with the enzyme again plus their inherent long half-life, resulting in sustained DPP4 inhibition, which is compatible with a once-daily dosing regimen [82,83]. While saxagliptin is covalently bound by cyanopyrrolidine fragments, prolongating the inhibitor′s interaction with the enzyme until hydrolysis releases the major metabolite 5-hydroxysaxagliptin [82,83]. Accordingly, in short-term studies (1–9 days in duration), head-to-head comparisons in patients with T2DM have shown that when used at their therapeutic doses, sitagliptin (100 mg once daily) and saxagliptin (5 mg once daily) all achieve the same maximal and trough levels of DPP4 inhibition [84] and are associated with similar enhancements of intact incretin hormone concentrations [85,86]. It follows, therefore, that if the extent and duration of DPP4 inhibition is similar, the improvement in glycemic control should also be similar. Indeed, in several studies, direct comparisons have been made between DPP4 inhibitions, glucose excursions [85,86] and HbA1c levels [85,87] are reduced to similar extents (Table 3). These inhibitors achieve similar degrees of DPP4 inhibition to the shorter-acting DPP4i discussed already, in line with this finding, head-to-head comparisons have also shown them to be non-inferior with respect to HbA1c control [88].
Table 3.
The effect of DPP4 inhibitors in HbA1c.
| DPP4i | Dose (mg/Day) | HbA1c Reduction |
|---|---|---|
| Sitagliptin | 100 | 0.5–1.0 |
| Saxagliptin | 5 | 0.5–1.0 |
| Alogliptin | 25 | 0.6 (mean value) |
| Linagliptin | 5 | 0.5–0.7 |
Their mechanism of action, involving both insulinotropic and glucagon-promoting effects, means that they combine well with other anti-diabetic agents to give additional HbA1c-lowering efficacy. The potency of DPP4i in A1C reduction is moderate. In this regard, the individual DPP4i have a low propensity for drug–drug interactions, meaning that they can be used with any other medications without the need for dose adjustment. Similarly, doses of other agents used together with DPP4i do not generally require adjustment; however, reduction in concomitant sulfonylurea or insulin doses is recommended to minimize the hypoglycemic risk associated with sulfonylureas and insulin [58,89,90]. The discovery that metformin also stimulates GLP1 secretion, further explaining the special efficacy of metformin in combination with DPP4i [91]. In a retrospective study in Korea, metformin and DPP4i was found to be effective in reducing HbA1c below 7%, with a low incidence of hypoglycemia [92].In tacrolimus-induced SD rat model and nephrotoxicity test, DPP4i and sodium–glucose transporter 2 inhibitors (SGLT2i) reduced blood glucose level and HbA1C level, and increased plasma insulin level and islet size of rats, and improved renal function and reduced interstitial fibrosis and pro-fibrotic cytokines, which providing a theoretical basis for the combination of SGLG2i and DPP4i in the treatment of tacrolimus-induced DM and nephrotoxicity [93]. The dual effect of DPP4i on α- and β- cells means that they also bind well to the islet independence of SGLT2i [94]. In addition, when DPP4i is combined with insulin secretin or insulin itself, DPP4i provides a complementary effect due to its inhibition of glucagon secretion and reduction in hepatic glucose production, which also means that a beneficial effect on glucose control can be achieved even with reduced β cell function [94]. In Chinese trials with T2DM, the addition of linagliptin and insulin improved glycemic control and was well tolerated with no increased risk of hypoglycemia or weight gain [95].
As described earlier, the effect of DPP4i on the internal environment of glucose is not direct, mediated through the action of the substrates they protect, especially GLP1. Therefore, considering that the activity of DPP4 is already completely inhibited when DPP4i is used at its therapeutic dose, any increase in exposure to the drug will not have a further hypoglycemic effect (since the enzyme cannot be inhibited by more than 100%). Combined with the fact that the role of GLP1 and GIP is itself glucose dependent, the inherent risk of treating hypoglycemia with DPP4i is particularly low [96,97]. Therefore, DPP4i is particularly suitable for use in elderly, frail and/or vulnerable patients whose long-term type 2 diabetes and its complications often result in multidrug therapy. In addition, patients with liver and kidney damage may be contraindications to other antidiabetic drugs [98]. Studies have shown that DPP4i is safe and effective in elderly patients with T2DM (65 or 70 years and above), and CVOTs including elderly patients with comorbidities (≥75 years), indicating that DPP4i is safe and effective in this population and showed a similar glycemic effect as the younger participants [99,100]. In addition, DPP4i has been shown to be effective and well tolerated in patients with renal dysfunction (including end-stage renal disease patients on dialysis) and DKD [101,102]. Since sitagliptin, alogliptin, and saxagliptin are cleared through the kidney, it is recommended that DPP4i doses be reduced [64,74,103] in patients with reduced renal function, while linagliptin is cleared through the biliary tract without dose adjustment [65]. Dose adjustment for renal elimination of DPP4i was based on pharmacokinetics rather than safety concerns. Therefore, any increase in dose will not cause hypoglycemia or other mechanism-based adverse reactions because the enzyme is already maximally inhibited, and compound specific adverse reactions are unlikely; earlier dosing studies found no adverse events [55,104,105] when using 4–32 times the therapeutic dose.
2.4. Anti-Inflammation Effects of DPP4i
DPP4 plays an important role in the maturation and activation of T cells and immune responses, with independent catalytic activity [106,107]. DPP4, released from hepatocytes and adipose tissue and exogenously administered, promotes inflammatory responses in multiple tissues, often associated with the development of insulin resistance [12,107]. In vivo sitagliptin can protect the endothelial function of renal artery in spontaneously hypertensive rats by GLP-1 signaling [103]. DPP4 inhibitors play a direct role in anti-atherosclerosis by improving endothelial dysfunction, inhibiting inflammation and oxidative stress, and improving plaque instability [104,105]. Our previous studies have demonstrated that sitagliptin may protect diabetic fatty liver by reducing ROS production and NF-κB signaling pathway activation [106]. Recent studies found the anti-inflammation of DPP4 inhibitors in non-diabetes and diabetes model, which summaries in Table 4. Although DPP4i has shown many anti-inflammatory effects in diabetes complications and other disease, this effect has not been shown in clinical trials. Therefore, more human population studies are needed to verify the anti-inflammatory effects of DPP4 in liver, lung, heart, kidney, and nerve in the future.
Table 4.
The anti-inflammatory effects of DPP4 inhibitors.
| DPP4i | Experimental Model | Mechanism of the Effects | Ref. |
|---|---|---|---|
| Sitagliptin | HFD-fed diabetic mice | Inhibited fatty liver inflammation; downregulates HMGB1/TLR4/NF-κB signaling pathway |
[108,109] |
| Diet-induced NAFLD | Inhibited pro-fibrotic and pro-inflammatory changes | [110] | |
| HFD-fed rats | Ameliorated apoptosis via alleviating ROS and ER stress | [111] | |
| Hepatic ischemia-reperfusion rat | Modulates oxidative, nitrative and halogenative stress and inflammatory response | [112] | |
| High glucose-induced human renal glomerular endothelial cells | Reversed the high glucose-induced oxidative stress, inflammation, and increased permebility via regulating KLF6 | [113] | |
| Hypoxia-induced damages in endometrial stromal cells | Suppressed the expressions of the proinflammatory cytokines including TNF-α, IL-6 and MCP-1; mitigated the activation of the p-38 MAPK and NF-κB pathways | [114] | |
| Severe acute pancreatitis companied with acute lung injury | Reduced oxidative stress and excessive autophagy through the p62–Keap1–Nrf2 signaling pathway | [115] | |
| Depressive symptoms in T2DM | No effect | [116] | |
| Human rheumatoid arthritis synovial fibroblasts | Increased proinflammatory cytokine production, enhanced the risk of RA development (sitagliptin and vildagliptin) | [117] | |
| Chlorhexidine gluconate induced peritoneal dialysis rats | Reversed the EMT process, angiogenesis, oxidative stress, and inflammation | [118] | |
| Low-density lipoprotein cholesterol in diabetes (REASON) Trial | Did not affect the levels of inflammatory markers | [119] | |
| Total body irradiation induced hematopoietic cells injury | Inhibited NOX4-mediated oxidative stress and alleviated inflammation | [120] | |
| Breast cancer | Reprograms tumor microenvironment via a ROS–NRF2–HO-1–NF-kB–NLRP3 axis | [121] | |
| Obese mice | Inhibited adipose tissue inflammation, metabolic syndrome, and fatty liver via regulation of adiponectin and AMPK levels | [122] | |
| Vildaliptin | Rheumatoid arthritis | Increased proinflammatory cytokine IL-1β, IL-6, and IL-13 production | [117] |
| Septic rats with myocardial injury | Inhibited the activation of NF-κB by promoting Nrf2 to alleviate the inflammatory response | [123] | |
| Acetic acid-induced colitis in rats | Inhibited the expression of lncRNA IFNG-AS1 and miR-146a, PI3K/Akt/NFκB pathway, and activated CREB and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathways | [124] | |
| Carbon tetrachloride-induced liver fibrosis | Attenuates liver fibrosis by targeting ERK1/2, p38α, and NF-κB signaling. | [125] | |
| Bleomycin-induced pulmonary fibrosis | Attenuated inflammation and fibrosis in bleomycin-induced pulmonary tissue via inhibiting the activity of CD26/DPP4 | [126] | |
| HFD-fed rats with impaired renal function | Attenuated insulin resistance and renal lipid accumulation-induced lipotoxicity | [127] | |
| Saxagliptin | Chronic unpredictable mild stress induced depression in rats | Increased the incretin hormones, GLP-1 and GIP, and the activation PI3K/AKT signaling pathway | [128] |
| Breast cancer | Reprogramed tumor microenvironment via a ROS–NRF2–HO-1–NF-kB–NLRP3 axis | [121] | |
| H9c2 cardiomyocyte cell line | Ameliorated hypoxia-induced inflammation via upregulation of Nrf2 and HO-1 | [129] | |
| Angiotensin II kidney injury model | Improved Angiotensin II suppressed anti-inflammatory regulatory T cell and T helper 2 lymphocyte activity | [130] | |
| Young and old SD rats | Improved endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway | [131] | |
| Alogliptin | Cyclophosphamide-induced lung toxicity in rats | Ameliorated lung toxicity by mitigating the oxidative, inflammatory, and fibrotic impacts | [75] |
| Lipopolysaccharide-induced neuroinflammation in mice | Attenuated neuroinflammation through modulation of TLR4/MYD88/NF-κB and miRNA-155/SOCS-1 signaling pathways | [132] | |
| Cyclophosphamide-induced nephrotoxicity Wistar rats | Attenuated nephrotoxicity through modulating MAP3K/JNK/SMAD3 signaling cascade | [133] | |
| Fibroblast-like synoviocytes | Inhibited IL-1β-induced inflammatory response | [134] | |
| Linagliptin | Sepsis mouse | Suppressed expressions of IL-1β and intercellular adhesion molecule 1 via a NF-κB-dependent pathway | [135] |
| Acetic acid-induced colitis rats | Activated AMPK-SIRT1-PGC-1α pathway and suppressed JAK2/STAT3 signaling pathway | [136] | |
| LPS induced U937 cells | Inhibited inflammation around the TLR-4-mediated pathway. | [137] | |
| Acute kidney injury in rats | Decreased inflammatory cytokines and ROS | [138] | |
| Early T2DM | Not altered plasma nitrate levels | [139] | |
| Experimental autoimmune myocarditis mice | Suppressed oxidative stress in EAM hearts | [81] | |
| Trinitrobenzene sulfonic acid-evoked colitis in rats | Curbed inflammation through the suppression of colonic IL-6, TNF-α, and upregulation of IL-10 | [140] | |
| Anti-glomerular basement membrane antibody induced in nephritis rats | Improved resolution of glomerular injury and healing in non-diabetic renal disease | [141] | |
| OSI-906-induced hepatic steatosis | Improved hepatic steatosis via an insulin-signaling-independent pathway | [142] | |
| Diabetic injured kidney | Inhibited the CRP/CD32b/NF-kB-driven renal inflammation and fibrosis | [143] | |
| Oxidized LDL-induced THP-1 macrophage foam cell formation | Decreased the expression of CD36 and LOX-1 and increased the expression of the cholesterol transporter ABCG1 | [144] | |
| HFD and streptozotocin (STZ) induced diabetic rats: liver fibrosis with T2DM | Improved insulin sensitivity and lipid profile and reduced inflammatory mediators, and collagen depositions | [145] | |
| Atherosclerosis and T2D mice | Improved glucose tolerance and reduced hepatic inflammation but had no effect on plaque burden or atherosclerotic inflammation | [146] | |
| Hyperglycemic mice with stroke | Exerted a neuroprotective effect through activation of the Akt/mTOR pathway along with anti-apoptotic and anti-inflammatory mechanisms | [147] | |
| Mouse bone marrow macrophages | Increased M2 macrophage polarization by inhibiting DPP-4 expression and activity | [130] |
2.5. Adverse Effects
FAERS data mining helped examine adverse events associated with DPP-4 inhibitors, all of which were disproportionately associated with four types of adverse events: “gastrointestinal nonspecific inflammation and dysfunction”, “allergy”, “severe skin adverse reactions”, and “noninfectious diarrhea” [148]. As for the analysis of the level of preferred terms specified, DPP4i was associated with higher reports of gastrointestinal, pancreatic, malignancies, infections, musculoskeletal disorders, systemic diseases, allergies, and cutaneous adverse reactions [149].
3. Conclusions
Dipeptidyl peptidase 4 plays a key role in the regulation of glucose metabolism. The DPP4 inhibitors are effective and safe hypoglycemic therapy for type 2 diabetes that are effective orally and associated with a low risk of hypoglycemia, weight gain, or other adverse events on a solid basis. There is also a large body of clinical and experimental data suggesting that improved islet function is the key mechanism behind the inhibitory hypoglycemic effect of DPP4i, both of which are associated with increased insulin secretion and glucagon secretion inhibition.
Additionally, DPP4i′s ability to reduce the risk of hypoglycemia and to work in complementarity with other antidiabetic drugs makes them widely used second-line drugs and means they are particularly useful for other drugs or contraindications that may not be preferred. Co-action of DPP4i with other drugs such as metformin, SGLT2i, and pioglitazone can provide additional glycemic efficacy without increasing the burden of pills [150,151,152]. However, unlike GLP1 receptor agonists and SGLT2i, DPP4i was insignificant in terms of cardiovascular benefit and reduced risk of major adverse cardiovascular events. In summary, DPP4i is safe and effective in the majority of T2DM patients, and we hope that DPP4i can help patients achieve glycemic goals in an overall favorable therapeutic setting. In addition, with the deepening of some DPP4i related research, more excellent efficacy may be developed.
Author Contributions
Conceptualization, R.Y. and D.Z.; writing—original draft preparation, R.Y.; writing—review and editing, L.Y.; visualization, X.W.; supervision, Y.X.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This review collected from open access web-source PubMed.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by The National Natural Science Foundation of China grant number 82170454.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kos K., Baker A.R., Jernas M., Harte A.L., Clapham J.C., O’Hare J.P., Carlsson L., Kumar S., McTernan P.G. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes. Metab. 2009;11:285–292. doi: 10.1111/j.1463-1326.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 2.Mentlein R. Dipeptidyl-peptidase IV (CD26)—Role in the inactivation of regulatory peptides. Regul. Pept. 1999;85:9–24. doi: 10.1016/S0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 3.Wronkowitz N., Gorgens S.W., Romacho T., Villalobos L.A., Sanchez-Ferrer C.F., Peiro C., Sell H., Eckel J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim. Biophys. Acta. 2014;1842:1613–1621. doi: 10.1016/j.bbadis.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Ghorpade D.S., Ozcan L., Zheng Z., Nicoloro S.M., Shen Y., Chen E., Bluher M., Czech M.P., Tabas I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555:673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holst J.J., Deacon C.F. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47:1663–1670. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 6.Ahren B. Dipeptidyl peptidase-4 inhibitors: Clinical data and clinical implications. Diabetes Care. 2007;30:1344–1350. doi: 10.2337/dc07-0233. [DOI] [PubMed] [Google Scholar]
- 7.Fan L., Zhou W., Zhang L., Jiang D., Zhao Q., Liu L. Sitagliptin protects against hypoxia/reoxygenation (H/R)-induced cardiac microvascular endothelial cell injury. Am. J. Transl. Res. 2019;11:2099–2107. [PMC free article] [PubMed] [Google Scholar]
- 8.Kirino Y., Sato Y., Kamimoto T., Kawazoe K., Minakuchi K., Nakahori Y. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: A streptozotocin-induced model using wild-type and DPP4-deficient rats. J. Endocrinol. 2009;200:53–61. doi: 10.1677/JOE-08-0424. [DOI] [PubMed] [Google Scholar]
- 9.Kirino Y., Sato Y., Kamimoto T., Kawazoe K., Minakuchi K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2011;300:E372–E379. doi: 10.1152/ajpendo.00319.2010. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Xiang J., Huang G., Kang L., Yang G., Wu H., Jiang K., Liang Z., Yang S. Inhibition of Podocytes DPP4 Activity Is a Potential Mechanism of Lobeliae Chinensis Herba in Treating Diabetic Kidney Disease. Front. Pharmacol. 2021;12:779652. doi: 10.3389/fphar.2021.779652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng T.P., Liu Y.H., Yang L.X., Qin S.H., Liu H.B. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of subclinical atherosclerosis in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Atherosclerosis. 2015;242:580–588. doi: 10.1016/j.atherosclerosis.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Varin E.M., Mulvihill E.E., Beaudry J.L., Pujadas G., Fuchs S., Tanti J.F., Fazio S., Kaur K., Cao X., Baggio L.L., et al. Circulating Levels of Soluble Dipeptidyl Peptidase-4 Are Dissociated from Inflammation and Induced by Enzymatic DPP4 Inhibition. Cell Metab. 2019;29:320–334.e5. doi: 10.1016/j.cmet.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2011;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 15.Candler T.P., Mahmoud O., Lynn R.M., Majbar A.A., Barrett T.G., Shield J.P.H. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018;35:737–744. doi: 10.1111/dme.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lascar N., Brown J., Pattison H., Barnett A.H., Bailey C.J., Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- 17.Magliano D.J., Sacre J.W., Harding J.L., Gregg E.W., Zimmet P.Z., Shaw J.E. Young-onset type 2 diabetes mellitus—Implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020;16:321–331. doi: 10.1038/s41574-020-0334-z. [DOI] [PubMed] [Google Scholar]
- 18.Goossens G.H., Blaak E.E. Adipose tissue dysfunction and impaired metabolic health in human obesity: A matter of oxygen? Front. Endocrinol. 2015;6:55. doi: 10.3389/fendo.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller T.D., Bluher M., Tschop M.H., DiMarchi R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022;21:201–223. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser A.S., Attwood M.M., Rask-Andersen M., Schioth H.B., Gloriam D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rask-Andersen M., Almen M.S., Schioth H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011;10:579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- 22.Kaur P., Mittal A., Nayak S.K., Vyas M., Mishra V., Khatik G.L. Current Strategies and Drug Targets in the Management of Type 2 Diabetes Mellitus. Curr. Drug Targets. 2018;19:1738–1766. doi: 10.2174/1389450119666180727142902. [DOI] [PubMed] [Google Scholar]
- 23.Deacon C.F., Hughes T.E., Holst J.J. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764–769. doi: 10.2337/diabetes.47.5.764. [DOI] [PubMed] [Google Scholar]
- 24.Xiang X., Lang M., Li Y., Zhao X., Sun H., Jiang W., Ni L., Song Y. Purification, identification and molecular mechanism of dipeptidyl peptidase IV inhibitory peptides from discarded shrimp (Penaeus vannamei) head. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021;1186:122990. doi: 10.1016/j.jchromb.2021.122990. [DOI] [PubMed] [Google Scholar]
- 25.Mulvihill E.E., Drucker D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohrborn D., Wronkowitz N., Eckel J. DPP4 in Diabetes. Front. Immunol. 2015;6:386. doi: 10.3389/fimmu.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahren B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019;10:376. doi: 10.3389/fendo.2019.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De S., Banerjee S., Kumar S.K.A., Paira P. Critical Role of Dipeptidyl Peptidase IV: A Therapeutic Target for Diabetes and Cancer. Mini-Rev. Med. Chem. 2019;19:88–97. doi: 10.2174/1389557518666180423112154. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S., Hosooka T., Matsuda T., Asahara S., Koyanagi-Kimura M., Kanno A., Bartolome A., Etoh H., Fuchita M., Teruyama K., et al. DPP4 inhibitor vildagliptin preserves beta-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J. Mol. Endocrinol. 2012;49:125–135. doi: 10.1530/JME-12-0039. [DOI] [PubMed] [Google Scholar]
- 30.Jonik S., Marchel M., Grabowski M., Opolski G., Mazurek T. Gastrointestinal Incretins-Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease-State of the Art. Biology. 2022;11:288. doi: 10.3390/biology11020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekiari E., Rizava C., Athanasiadou E., Papatheodorou K., Liakos A., Karagiannis T., Mainou M., Rika M., Boura P., Tsapas A. Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes. Endocrine. 2016;52:458–480. doi: 10.1007/s12020-015-0841-1. [DOI] [PubMed] [Google Scholar]
- 32.Scott L.J. Sitagliptin: A Review in Type 2 Diabetes. Drugs. 2017;77:209–224. doi: 10.1007/s40265-016-0686-9. [DOI] [PubMed] [Google Scholar]
- 33.Aulinger B.A., Bedorf A., Kutscherauer G., de Heer J., Holst J.J., Goke B., Schirra J. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes. 2014;63:1079–1092. doi: 10.2337/db13-1455. [DOI] [PubMed] [Google Scholar]
- 34.Nauck M.A., Kind J., Kothe L.D., Holst J.J., Deacon C.F., Broschag M., He Y.L., Kjems L., Foley J. Quantification of the Contribution of GLP-1 to Mediating Insulinotropic Effects of DPP-4 Inhibition With Vildagliptin in Healthy Subjects and Patients With Type 2 Diabetes Using Exendin [9-39] as a GLP-1 Receptor Antagonist. Diabetes. 2016;65:2440–2447. doi: 10.2337/db16-0107. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A., Paliwal G., Upadhyay N., Tiwari A. Retraction Note: Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J. Diabetes Metab. Disord. 2015;15:34. doi: 10.1186/s40200-016-0256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A., Paliwal G., Upadhyay N., Tiwari A. Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J. Diabetes Metab. Disord. 2015;14:15. doi: 10.1186/s40200-015-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Vilsboll T., Krarup T., Madsbad S., Holst J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 38.Mentis N., Vardarli I., Kothe L.D., Holst J.J., Deacon C.F., Theodorakis M., Meier J.J., Nauck M.A. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011;60:1270–1276. doi: 10.2337/db10-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hojberg P.V., Vilsboll T., Rabol R., Knop F.K., Bache M., Krarup T., Holst J.J., Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 40.Aaboe K., Akram S., Deacon C.F., Holst J.J., Madsbad S., Krarup T. Restoration of the insulinotropic effect of glucose-dependent insulinotropic polypeptide contributes to the antidiabetic effect of dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2015;17:74–81. doi: 10.1111/dom.12395. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T., Tanimoto H., Okamoto M., Takeuchi M., Tsubamoto Y., Noda H. GIP Receptor Antagonist, SKL-14959 Indicated Alteration of the Lipids Metabolism to Catabolism by the Inhibition of Plasma LPL Activity, Resulting in the Suppression of Weight Gain on Diets-Induced Obesity Mice. Diabetes Metab. Syndr. Obes. 2021;14:1095–1105. doi: 10.2147/DMSO.S297353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasbjerg L.S., Bari E.J., Stensen S., Hoe B., Lanng A.R., Mathiesen D.S., Christensen M.B., Hartmann B., Holst J.J., Rosenkilde M.M., et al. Dose-dependent efficacy of the glucose-dependent insulinotropic polypeptide (GIP) receptor antagonist GIP(3–30)NH2 on GIP actions in humans. Diabetes Obes. Metab. 2021;23:68–74. doi: 10.1111/dom.14186. [DOI] [PubMed] [Google Scholar]
- 43.Christensen M.B. Glucose-dependent insulinotropic polypeptide: Effects on insulin and glucagon secretion in humans. Dan. Med. J. 2016;63:B5230. [PubMed] [Google Scholar]
- 44.Ahren B., Schweizer A., Dejager S., Dunning B.E., Nilsson P.M., Persson M., Foley J.E. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009;94:1236–1243. doi: 10.1210/jc.2008-2152. [DOI] [PubMed] [Google Scholar]
- 45.Farngren J., Persson M., Schweizer A., Foley J.E., Ahren B. Glucagon dynamics during hypoglycaemia and food-re-challenge following treatment with vildagliptin in insulin-treated patients with type 2 diabetes. Diabetes Obes. Metab. 2014;16:812–818. doi: 10.1111/dom.12284. [DOI] [PubMed] [Google Scholar]
- 46.Mannucci E., Nreu B., Montereggi C., Ragghianti B., Gallo M., Giaccari A., Monami M., SID-AMD Joint Panel for Italian Guidelines on Treatment of Type 2 Diabetes Cardiovascular events and all-cause mortality in patients with type 2 diabetes treated with dipeptidyl peptidase-4 inhibitors: An extensive meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021;31:2745–2755. doi: 10.1016/j.numecd.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Molina-Vega M., Munoz-Garach A., Fernandez-Garcia J.C., Tinahones F.J. The safety of DPP-4 inhibitor and SGLT2 inhibitor combination therapies. Expert Opin. Drug Saf. 2018;17:815–824. doi: 10.1080/14740338.2018.1497158. [DOI] [PubMed] [Google Scholar]
- 48.Biftu T., Feng D., Qian X., Liang G.B., Kieczykowski G., Eiermann G., He H., Leiting B., Lyons K., Petrov A., et al. (3R)-4-[(3R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoyl]-3-(2,2,2-trifluoroethyl)-1,4-diazepan-2-one, a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. Lett. 2007;17:49–52. doi: 10.1016/j.bmcl.2006.09.099. [DOI] [PubMed] [Google Scholar]
- 49.Augeri D.J., Robl J.A., Betebenner D.A., Magnin D.R., Khanna A., Robertson J.G., Wang A., Simpkins L.M., Taunk P., Huang Q., et al. Discovery and preclinical profile of Saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005;48:5025–5037. doi: 10.1021/jm050261p. [DOI] [PubMed] [Google Scholar]
- 50.Choy M., Lam S. Sitagliptin: A novel drug for the treatment of type 2 diabetes. Cardiol. Rev. 2007;15:264–271. doi: 10.1097/CRD.0b013e318123f771. [DOI] [PubMed] [Google Scholar]
- 51.Thareja S., Aggarwal S., Malla P., Haksar D., Bhardwaj T.R., Kumar M. Saxagliptin: A new drug for the treatment of type 2 diabetes. Mini-Rev. Med. Chem. 2010;10:759–765. doi: 10.2174/138955710791572424. [DOI] [PubMed] [Google Scholar]
- 52.White J.R. Alogliptin for the treatment of type 2 diabetes. Drugs Today. 2011;47:99–107. doi: 10.1358/dot.2011.47.2.1583163. [DOI] [PubMed] [Google Scholar]
- 53.Aletti R., Cheng-Lai A. Linagliptin: The newest dipeptidyl peptidase-4 inhibitor for type 2 diabetes mellitus. Cardiol. Rev. 2012;20:45–51. doi: 10.1097/CRD.0b013e31823a3afc. [DOI] [PubMed] [Google Scholar]
- 54.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergman A., Ebel D., Liu F., Stone J., Wang A., Zeng W., Chen L., Dilzer S., Lasseter K., Herman G., et al. Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm. Drug Dispos. 2007;28:315–322. doi: 10.1002/bdd.560. [DOI] [PubMed] [Google Scholar]
- 56.Thornberry N.A., Weber A.E. Discovery of JANUVIA (Sitagliptin), a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Curr. Top. Med. Chem. 2007;7:557–568. doi: 10.2174/156802607780091028. [DOI] [PubMed] [Google Scholar]
- 57.Villhauer E.B., Brinkman J.A., Naderi G.B., Burkey B.F., Dunning B.E., Prasad K., Mangold B.L., Russell M.E., Hughes T.E. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 2003;46:2774–2789. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- 58.Deacon C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020;16:642–653. doi: 10.1038/s41574-020-0399-8. [DOI] [PubMed] [Google Scholar]
- 59.He Y.L. Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin. Pharmacokinet. 2012;51:147–162. doi: 10.2165/11598080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Agency E.M. Vildagliptin: Summary of Product Characteristics. [(accessed on 1 August 2020)]. Available online: https://www.ema.europa.eu/en/documents/product-information/galvus-eparproduct-information_en.pdf.
- 61.Boulton D.W. Clinical Pharmacokinetics and Pharmacodynamics of Saxagliptin, a Dipeptidyl Peptidase-4 Inhibitor. Clin. Pharmacokinet. 2017;56:11–24. doi: 10.1007/s40262-016-0421-4. [DOI] [PubMed] [Google Scholar]
- 62.Scheen A.J. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes. Metab. 2010;12:648–658. doi: 10.1111/j.1463-1326.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 63.Boulton D.W., Li L., Frevert E.U., Tang A., Castaneda L., Vachharajani N.N., Kornhauser D.M., Patel C.G. Influence of renal or hepatic impairment on the pharmacokinetics of saxagliptin. Clin. Pharmacokinet. 2011;50:253–265. doi: 10.2165/11584350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Saxagliptin: Summary of Product Characteristics. [(accessed on 1 August 2020)]. Available online: https://www.ema.europa.eu/en/documents/product-information/onglyza-eparproduct-information_en.pdf.
- 65.Linagliptin: Summary of Product Characteristics. [(accessed on 1 August 2020)]. Available online: https://www.ema.europa.eu/en/documents/product-information/trajenta-eparproduct-information_en.pdf.
- 66.Johansen O.E., Neubacher D., von Eynatten M., Patel S., Woerle H.J. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: A pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc. Diabetol. 2012;11:3. doi: 10.1186/1475-2840-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groop P.H., Cooper M.E., Perkovic V., Emser A., Woerle H.J., von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deacon C.F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes. Metab. 2011;13:7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 69.Graefe-Mody U., Retlich S., Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin. Pharmacokinet. 2012;51:411–427. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Snyder B., Polasek T.M., Doogue M.P. Reply to: Graefe-Mody, U.; Friedrich, C.; Port, A.; Ring, S.; Retlich, T.; Heise, A.; Halabi, H.-J. Woerle Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin. Diabetes Obes. Metab. 2011;13:939–946. doi: 10.1111/j.1463-1326.2011.01458.x. Diabetes Obes. Metab.2012, 14, 670; Author Reply: Diabetes Obes. Metab. 2012, 14, 671–672. [DOI] [PubMed] [Google Scholar]
- 71.Graefe-Mody U., Rose P., Retlich S., Ring A., Waldhauser L., Cinca R., Woerle H.J. Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br. J. Clin. Pharmacol. 2012;74:75–85. doi: 10.1111/j.1365-2125.2012.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenstock J., Perkovic V., Johansen O.E., Cooper M.E., Kahn S.E., Marx N., Alexander J.H., Pencina M., Toto R.D., Wanner C., et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katsiki N., Ofori-Asenso R., Ferrannini E., Mazidi M. Fixed-dose combination of empagliflozin and linagliptin for the treatment of patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes. Metab. 2020;22:1001–1005. doi: 10.1111/dom.13989. [DOI] [PubMed] [Google Scholar]
- 74.Alogliptin: Summary of Product Characteristics. [(accessed on 1 August 2020)]. Available online: https://www.ema.europa.eu/en/documents/product-information/vipidia-eparproduct-information_en.pdf.
- 75.Alsemeh A.E., Abdullah D.M. Protective effect of alogliptin against cyclophosphamide-induced lung toxicity in rats: Impact on PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades. Cell Tissue Res. 2022;388:417–438. doi: 10.1007/s00441-022-03593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biessels G.J., Verhagen C., Janssen J., van den Berg E., Zinman B., Rosenstock J., George J.T., Passera A., Schnaidt S., Johansen O.E., et al. Effect of Linagliptin on Cognitive Performance in Patients With Type 2 Diabetes and Cardiorenal Comorbidities: The CARMELINA Randomized Trial. Diabetes Care. 2019;42:1930–1938. doi: 10.2337/dc19-0783. [DOI] [PubMed] [Google Scholar]
- 77.Chikata Y., Iwata H., Miyosawa K., Koike T., Yasuda H., Funamizu T., Doi S., Endo H., Wada H., Naito R., et al. Dipeptidyl peptidase-4 inhibitors reduced long-term cardiovascular risk in diabetic patients after percutaneous coronary intervention via insulin-like growth factor-1 axis. Sci. Rep. 2022;12:5129. doi: 10.1038/s41598-022-09059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carr R.D., Katzeff H.L., Alexander C.M., Berger J.P., Xu S.S., Thornberry N. Reply to: Ahren, B.; Schweizer, A.; Dejager, S.; Villhauer, E.B.; Dunning, B.E.; Foley, J.E. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes. Metab. 2011, 13, 775–783 and Ahren, B.; Schweizer, A.; Dejager, S.; Villhauer, E.B.; Dunning, B.E.; Foley, J.E. Clinical evidence and mechanistic basis for vildagliptin’s action when added to metformin. Diabetes Obes. Metab. 2011;13:193–203. doi: 10.1111/j.1463-1326.2011.01542.x. Diabetes Obes. Metab.2012, 14, 383–384. [DOI] [PubMed] [Google Scholar]
- 79.Nabeno M., Akahoshi F., Kishida H., Miyaguchi I., Tanaka Y., Ishii S., Kadowaki T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem. Biophys. Res. Commun. 2013;434:191–196. doi: 10.1016/j.bbrc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 80.Tatosian D.A., Guo Y., Schaeffer A.K., Gaibu N., Popa S., Stoch A., Langdon R.B., Kauh E.A. Dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes treated with saxagliptin, sitagliptin, or vildagliptin. Diabetes Ther. 2013;4:431–442. doi: 10.1007/s13300-013-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baranov O., Kahle M., Deacon C.F., Holst J.J., Nauck M.A. Feedback suppression of meal-induced glucagon-like peptide-1 (GLP-1) secretion mediated through elevations in intact GLP-1 caused by dipeptidyl peptidase-4 inhibition: A randomized, prospective comparison of sitagliptin and vildagliptin treatment. Diabetes Obes. Metab. 2016;18:1100–1109. doi: 10.1111/dom.12706. [DOI] [PubMed] [Google Scholar]
- 82.Alsalim W., Goransson O., Tura A., Pacini G., Mari A., Ahren B. Persistent whole day meal effects of three dipeptidyl peptidase-4 inhibitors on glycaemia and hormonal responses in metformin-treated type 2 diabetes. Diabetes Obes. Metab. 2020;22:590–598. doi: 10.1111/dom.13934. [DOI] [PubMed] [Google Scholar]
- 83.Scheen A.J., Charpentier G., Ostgren C.J., Hellqvist A., Gause-Nilsson I. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2010;26:540–549. doi: 10.1002/dmrr.1114. [DOI] [PubMed] [Google Scholar]
- 84.Addy C., Tatosian D., Glasgow X.S., Gendrano I.N., 3rd, Kauh E., Martucci A., Johnson-Levonas A.O., Selverian D., Matthews C.Z., Gutierrez M., et al. Pharmacokinetic and Pharmacodynamic Effects of Multiple-dose Administration of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor, in Obese Participants With and Without Type 2 Diabetes Mellitus. Clin. Ther. 2016;38:516–530. doi: 10.1016/j.clinthera.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y.G., Hahn S., Oh T.J., Kwak S.H., Park K.S., Cho Y.M. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: A systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 86.Cai X., Han X., Luo Y., Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on beta-cell function in Asian and Caucasian type 2 diabetes mellitus patients: A meta-analysis. J. Diabetes. 2015;7:347–359. doi: 10.1111/1753-0407.12196. [DOI] [PubMed] [Google Scholar]
- 87.Gao W., Wang Q., Yu S. Efficacy, safety and impact on beta-cell function of dipeptidyl peptidase-4 inhibitors plus metformin combination therapy in patients with type 2 diabetes and the difference between Asians and Caucasians: A meta-analysis. J. Endocrinol. Investig. 2016;39:1061–1074. doi: 10.1007/s40618-016-0465-1. [DOI] [PubMed] [Google Scholar]
- 88.Kozlovski P., Fonseca M., Mohan V., Lukashevich V., Odawara M., Paldanius P.M., Kothny W. Effect of race and ethnicity on vildagliptin efficacy: A pooled analysis of phase II and III studies. Diabetes Obes. Metab. 2017;19:429–435. doi: 10.1111/dom.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seino Y., Fujita T., Hiroi S., Hirayama M., Kaku K. Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr. Med. Res. Opin. 2011;27:1781–1792. doi: 10.1185/03007995.2011.599371. [DOI] [PubMed] [Google Scholar]
- 90.Seino Y., Miyata Y., Hiroi S., Hirayama M., Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes. Metab. 2012;14:927–936. doi: 10.1111/j.1463-1326.2012.01620.x. [DOI] [PubMed] [Google Scholar]
- 91.Bahne E., Sun E.W.L., Young R.L., Hansen M., Sonne D.P., Hansen J.S., Rohde U., Liou A.P., Jackson M.L., de Fontgalland D., et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI Insight. 2018;3:e93936. doi: 10.1172/jci.insight.93936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee K.A., Jin H.Y., Kim Y.J., Kim S.S., Cho E.H., Park T.S. Real-world comparison of mono and dual combination therapies of metformin, sulfonylurea, and dipeptidyl peptidase-4 inhibitors using a common data model: A retrospective observational study. Medicine. 2022;101:e28823. doi: 10.1097/MD.0000000000028823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ko E.J., Shin Y.J., Cui S., Lim S.W., Chung B.H., Yang C.W. Effect of dual inhibition of DPP4 and SGLT2 on tacrolimus-induced diabetes mellitus and nephrotoxicity in rat model. Am. J. Transpl. 2022 doi: 10.1111/ajt.17035. [DOI] [PubMed] [Google Scholar]
- 94.Morita A., Mukai E., Hiratsuka A., Takatani T., Iwanaga T., Lee E.Y., Miki T. Distinct effects of dipeptidyl peptidase-4 inhibitor and glucagon-like peptide-1 receptor agonist on islet morphology and function. Endocrine. 2016;51:429–439. doi: 10.1007/s12020-015-0733-4. [DOI] [PubMed] [Google Scholar]
- 95.Yang W., Xu X., Lei T., Ma J., Li L., Shen J., Ye B., Zhu S., Meinicke T. Efficacy and safety of linagliptin as add-on therapy to insulin in Chinese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2021;23:642–647. doi: 10.1111/dom.14231. [DOI] [PubMed] [Google Scholar]
- 96.Esposito K., Cozzolino D., Bellastella G., Maiorino M.I., Chiodini P., Ceriello A., Giugliano D. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: Meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2011;13:594–603. doi: 10.1111/j.1463-1326.2011.01380.x. [DOI] [PubMed] [Google Scholar]
- 97.Scheen A.J. The safety of gliptins: Updated data in 2018. Expert Opin. Drug Saf. 2018;17:387–405. doi: 10.1080/14740338.2018.1444027. [DOI] [PubMed] [Google Scholar]
- 98.Wang T., McNeill A.M., Chen Y., O’Neill E.A., Engel S.S. Characteristics of Elderly Patients Initiating Sitagliptin or Non-DPP-4-Inhibitor Oral Antihyperglycemic Agents: Analysis of a Cross-Sectional US Claims Database. Diabetes Ther. 2018;9:309–315. doi: 10.1007/s13300-017-0360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leiter L.A., Teoh H., Braunwald E., Mosenzon O., Cahn A., Kumar K.M., Smahelova A., Hirshberg B., Stahre C., Frederich R., et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145–1153. doi: 10.2337/dc14-2868. [DOI] [PubMed] [Google Scholar]
- 100.Bethel M.A., Engel S.S., Green J.B., Huang Z., Josse R.G., Kaufman K.D., Standl E., Suryawanshi S., Van de Werf F., McGuire D.K., et al. Assessing the Safety of Sitagliptin in Older Participants in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) Diabetes Care. 2017;40:494–501. doi: 10.2337/dc16-1135. [DOI] [PubMed] [Google Scholar]
- 101.Walker S.R., Komenda P., Khojah S., Al-Tuwaijri W., MacDonald K., Hiebert B., Tangri N., Nadurak S.W.D., Ferguson T.W., Rigatto C., et al. Dipeptidyl Peptidase-4 Inhibitors in Chronic Kidney Disease: A Systematic Review of Randomized Clinical Trials. Nephron. 2017;136:85–94. doi: 10.1159/000454683. [DOI] [PubMed] [Google Scholar]
- 102.Trakarnvanich T., Satirapoj B., Suraamornkul S., Chirananthavat T., Sanpatchayapong A., Claimon T. Effect of Dipeptidyl Peptidase-4 (DPP-4) Inhibition on Biomarkers of Kidney Injury and Vascular Calcification in Diabetic Kidney Disease: A Randomized Controlled Trial. J. Diabetes Res. 2021;2021:7382620. doi: 10.1155/2021/7382620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sitagliptin: Summary of Product Characteristics. [(accessed on 1 August 2020)]. Available online: https://www.ema.europa.eu/en/documents/product-information/januvia-eparproduct-information_en.pdf.
- 104.He Y.L., Wang Y., Bullock J.M., Deacon C.F., Holst J.J., Dunning B.E., Ligueros-Saylan M., Foley J.E. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J. Clin. Pharmacol. 2007;47:633–641. doi: 10.1177/0091270006299137. [DOI] [PubMed] [Google Scholar]
- 105.Christopher R., Covington P., Davenport M., Fleck P., Mekki Q.A., Wann E.R., Karim A. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin. Ther. 2008;30:513–527. doi: 10.1016/j.clinthera.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Klemann C., Wagner L., Stephan M., von Horsten S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhong J., Rao X., Deiuliis J., Braunstein Z., Narula V., Hazey J., Mikami D., Needleman B., Satoskar A.R., Rajagopalan S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62:149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou S.T., Cui W., Kong L., Yang X. Efficacy of Sitagliptin on Nonalcoholic Fatty Liver Disease in High-fat-diet-fed Diabetic Mice. Curr. Med. Sci. 2022 doi: 10.1007/s11596-022-2573-9. [DOI] [PubMed] [Google Scholar]
- 109.Allam M.M., Ibrahim R.M., El Gazzar W.B., Said M.A. Dipeptedyl peptidase-4 (DPP-4) inhibitor downregulates HMGB1/TLR4/NF-kappaB signaling pathway in a diabetic rat model of non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2021 doi: 10.1080/13813455.2021.1975758. [DOI] [PubMed] [Google Scholar]
- 110.Ren J., Wang X., Yee C., Gorrell M.D., McLennan S.V., Twigg S.M. Sitagliptin Is More Effective Than Gliclazide in Preventing Pro-Fibrotic and Pro-Inflammatory Changes in a Rodent Model of Diet-Induced Non-Alcoholic Fatty Liver Disease. Molecules. 2022;27:727. doi: 10.3390/molecules27030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cao Q., Xu D., Chen Y., Long Y., Dai F., Gui L., Lu Y. Sitagliptin Reduces Endothelial Dysfunction and Apoptosis Induced by High-Fat Diet and Palmitate in Thoracic Aortas and Endothelial Cells via ROS-ER Stress-CHOP Pathway. Front. Pharmacol. 2021;12:670389. doi: 10.3389/fphar.2021.670389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trocha M., Fleszar M.G., Fortuna P., Lewandowski L., Gostomska-Pampuch K., Sozanski T., Merwid-Lad A., Krzystek-Korpacka M. Sitagliptin Modulates Oxidative, Nitrative and Halogenative Stress and Inflammatory Response in Rat Model of Hepatic Ischemia-Reperfusion. Antioxidants. 2021;10:1168. doi: 10.3390/antiox10081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu L., Shao F. Sitagliptin protects renal glomerular endothelial cells against high glucose-induced dysfunction and injury. Bioengineered. 2022;13:655–666. doi: 10.1080/21655979.2021.2012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y., Lv X., Jiang M., Jin Z. Sitagliptin ameliorates hypoxia-induced damages in endometrial stromal cells: An implication in endometriosis. Bioengineered. 2022;13:800–809. doi: 10.1080/21655979.2021.2012950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kong L., Deng J., Zhou X., Cai B., Zhang B., Chen X., Chen Z., Wang W. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell. Death Dis. 2021;12:928. doi: 10.1038/s41419-021-04227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moulton C.D., Rokakis A.S., Pickup J.C., Young A.H., Stahl D., Ismail K. SITAgliptin for Depressive Symptoms in Type 2 Diabetes: A Feasibility Randomized Controlled Trial. Psychosom. Med. 2021;83:913–923. doi: 10.1097/PSY.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 117.Han C.K., Lee W.F., Hsu C.J., Huang Y.L., Lin C.Y., Tsai C.H., Huang C.C., Fong Y.C., Wu M.H., Liu J.F., et al. DPP4 reduces proinflammatory cytokine production in human rheumatoid arthritis synovial fibroblasts. J. Cell. Physiol. 2021;236:8060–8069. doi: 10.1002/jcp.30494. [DOI] [PubMed] [Google Scholar]
- 118.Li Y.C., Sung P.H., Yang Y.H., Chiang J.Y., Yip H.K., Yang C.C. Dipeptidyl peptidase 4 promotes peritoneal fibrosis and its inhibitions prevent failure of peritoneal dialysis. Commun. Biol. 2021;4:144. doi: 10.1038/s42003-021-01652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teragawa H., Morimoto T., Fujii Y., Ueda T., Sakuma M., Shimabukuro M., Arasaki O., Node K., Nomiyama T., Ueda S. Effect of Anagliptin versus Sitagliptin on Inflammatory Markers: Sub-Analysis from the REASON Trial. Diabetes Metab. Syndr. Obes. 2020;13:4993–5001. doi: 10.2147/DMSO.S282968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang M., Dong Y., Wu J., Li H., Zhang J., Lu L., Zhang Y., Zhou Z., Fan S., Li D., et al. Sitagliptin Mitigates Total Body Irradiation-Induced Hematopoietic Injury in Mice. Oxid. Med. Cell. Longev. 2020;2020:8308616. doi: 10.1155/2020/8308616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li R., Zeng X., Yang M., Feng J., Xu X., Bao L., Ye T., Wang X., Xue B., Huang Y. Antidiabetic DPP-4 Inhibitors Reprogram Tumor Microenvironment That Facilitates Murine Breast Cancer Metastasis Through Interaction With Cancer Cells via a ROS-NF-small ka, CyrillicB-NLRP3 Axis. Front. Oncol. 2021;11:728047. doi: 10.3389/fonc.2021.728047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prakash S., Rai U., Kosuru R., Tiwari V., Singh S. Amelioration of diet-induced metabolic syndrome and fatty liver with sitagliptin via regulation of adipose tissue inflammation and hepatic Adiponectin/AMPK levels in mice. Biochimie. 2020;168:198–209. doi: 10.1016/j.biochi.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 123.Yang M., Chen X., Chen X., Liu H., Zhang Z. Protective effect of vildagliptin on myocardial injury in septic rats by regulating TLR-4/NF-kappaB pathway. Minerva Med. 2021;112:522–524. doi: 10.23736/S0026-4806.19.06242-6. [DOI] [PubMed] [Google Scholar]
- 124.Fouad M.R., Salama R.M., Zaki H.F., El-Sahar A.E. Vildagliptin attenuates acetic acid-induced colitis in rats via targeting PI3K/Akt/NFkappaB, Nrf2 and CREB signaling pathways and the expression of lncRNA IFNG-AS1 and miR-146a. Int. Immunopharmacol. 2021;92:107354. doi: 10.1016/j.intimp.2020.107354. [DOI] [PubMed] [Google Scholar]
- 125.Khalil R., Shata A., Abd El-Kader E.M., Sharaf H., Abdo W.S., Amin N.A., Saber S. Vildagliptin, a DPP-4 inhibitor, attenuates carbon tetrachloride-induced liver fibrosis by targeting ERK1/2, p38alpha, and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 2020;407:115246. doi: 10.1016/j.taap.2020.115246. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y., Qi Y. Vildagliptin, a CD26/DPP4 inhibitor, ameliorates bleomycin-induced pulmonary fibrosis via regulating the extracellular matrix. Int. Immunopharmacol. 2020;87:106774. doi: 10.1016/j.intimp.2020.106774. [DOI] [PubMed] [Google Scholar]
- 127.Pengrattanachot N., Cherngwelling R., Jaikumkao K., Pongchaidecha A., Thongnak L., Swe M.T., Chatsudthipong V., Lungkaphin A. Atorvastatin attenuates obese-induced kidney injury and impaired renal organic anion transporter 3 function through inhibition of oxidative stress and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165741. doi: 10.1016/j.bbadis.2020.165741. [DOI] [PubMed] [Google Scholar]
- 128.Nazeem M., Wahdan S.A., El-Naga R.N., Gad A.M. Saxagliptin ameliorated the depressive-like behavior induced by chronic unpredictable mild stress in rats: Impact on incretins and AKT/PI3K pathway. Eur. J. Pharmacol. 2021;912:174602. doi: 10.1016/j.ejphar.2021.174602. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L., Qi X., Zhang G., Zhang Y., Tian J. Saxagliptin protects against hypoxia-induced damage in H9c2 cells. Chem. Biol. Interact. 2020;315:108864. doi: 10.1016/j.cbi.2019.108864. [DOI] [PubMed] [Google Scholar]
- 130.Nistala R., Meuth A.I., Smith C., An J., Habibi J., Hayden M.R., Johnson M., Aroor A., Whaley-Connell A., Sowers J.R., et al. DPP4 inhibition mitigates ANG II-mediated kidney immune activation and injury in male mice. Am. J. Physiol. Renal. Physiol. 2021;320:F505–F517. doi: 10.1152/ajprenal.00565.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Z., Yu J., Fu M., Dong R., Yang Y., Luo J., Hu S., Li W., Xu X., Tu L. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem. Pharmacol. 2020;177:113951. doi: 10.1016/j.bcp.2020.113951. [DOI] [PubMed] [Google Scholar]
- 132.El-Sahar A.E., Shiha N.A., El Sayed N.S., Ahmed L.A. Alogliptin Attenuates Lipopolysaccharide-Induced Neuroinflammation in Mice Through Modulation of TLR4/MYD88/NF-kappaB and miRNA-155/SOCS-1 Signaling Pathways. Int. J. Neuropsychopharmacol. 2021;24:158–169. doi: 10.1093/ijnp/pyaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salama R.M., Nasr M.M., Abdelhakeem J.I., Roshdy O.K., ElGamal M.A. Alogliptin attenuates cyclophosphamide-induced nephrotoxicity: A novel therapeutic approach through modulating MAP3K/JNK/SMAD3 signaling cascade. Drug Chem. Toxicol. 2022;45:1254–1263. doi: 10.1080/01480545.2020.1814319. [DOI] [PubMed] [Google Scholar]
- 134.Guo Q., Zhang S., Huang J., Liu K. Alogliptin inhibits IL-1beta-induced inflammatory response in fibroblast-like synoviocytes. Int. Immunopharmacol. 2020;83:106372. doi: 10.1016/j.intimp.2020.106372. [DOI] [PubMed] [Google Scholar]
- 135.Wang S.C., Wang X.Y., Liu C.T., Chou R.H., Chen Z.B., Huang P.H., Lin S.J. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Ameliorates Endothelial Inflammation and Microvascular Thrombosis in a Sepsis Mouse Model. Int. J. Mol. Sci. 2022;23:3065. doi: 10.3390/ijms23063065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El-Ghannam M.S., Saad M.A., Nassar N.N., El-Yamany M.F., El-Bahy A.A.Z. Linagliptin ameliorates acetic acid-induced colitis via modulating AMPK/SIRT1/PGC-1alpha and JAK2/STAT3 signaling pathway in rats. Toxicol. Appl. Pharmacol. 2022;438:115906. doi: 10.1016/j.taap.2022.115906. [DOI] [PubMed] [Google Scholar]
- 137.Saito H., Nakamura Y., Inagaki M., Yamadera S., Misawa H., Sato N., Oguchi T., Inagaki T., Tsuji Y., Tsuji M., et al. Linagliptin Inhibits Interleukin-6 Production Through Toll-Like Receptor 4 Complex and Lipopolysaccharide-Binding Protein Independent Pathway in vitro Model. J. Inflamm. Res. 2021;14:5681–5686. doi: 10.2147/JIR.S326382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu T.J., Hsieh Y.J., Lu C.W., Lee C.J., Hsu B.G. Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species. Int. J. Mol. Sci. 2021;22:1190. doi: 10.3390/ijms222011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reijrink M., De Boer S.A., Van Roon A.M., Slart R., Fernandez B.O., Feelisch M., Heerspink H.J.L., Van Goor H., Hillebrands J.L., Mulder D.J. Plasma Nitrate Levels Are Related to Metabolic Syndrome and Are Not Altered by Treatment with DPP-4 Inhibitor Linagliptin: A Randomised, Placebo-Controlled Trial in Patients with Early Type 2 Diabetes Mellitus. Antioxidants. 2021;10:1548. doi: 10.3390/antiox10101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arab H.H., Eid A.H., Mahmoud A.M., Senousy M.A. Linagliptin mitigates experimental inflammatory bowel disease in rats by targeting inflammatory and redox signaling. Life Sci. 2021;273:119295. doi: 10.1016/j.lfs.2021.119295. [DOI] [PubMed] [Google Scholar]
- 141.Mayer A.L., Scheitacker I., Ebert N., Klein T., Amann K., Daniel C. The dipeptidyl peptidase 4 inhibitor linagliptin ameliorates renal injury and accelerated resolution in a rat model of crescentic nephritis. Br. J. Pharmacol. 2021;178:878–895. doi: 10.1111/bph.15320. [DOI] [PubMed] [Google Scholar]
- 142.Okuyama T., Shirakawa J., Tajima K., Ino Y., Vethe H., Togashi Y., Kyohara M., Inoue R., Miyashita D., Li J., et al. Linagliptin Ameliorates Hepatic Steatosis via Non-Canonical Mechanisms in Mice Treated with a Dual Inhibitor of Insulin Receptor and IGF-1 Receptor. Int. J. Mol. Sci. 2020;21:7815. doi: 10.3390/ijms21217815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang P.M., Zhang Y.Y., Hung J.S., Chung J.Y., Huang X.R., To K.F., Lan H.Y. DPP4/CD32b/NF-kappaB Circuit: A Novel Druggable Target for Inhibiting CRP-Driven Diabetic Nephropathy. Mol. Ther. 2021;29:365–375. doi: 10.1016/j.ymthe.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H., Li Y., Zhang X., Xu Z., Zhou J., Shang W. DPP-4 Inhibitor Linagliptin Ameliorates Oxidized LDL-Induced THP-1 Macrophage Foam Cell Formation and Inflammation. Drug Des. Devel. Ther. 2020;14:3929–3940. doi: 10.2147/DDDT.S249846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aboulmagd Y.M., El-Bahy A.A.Z., Menze E.T., Azab S.S., El-Demerdash E. Role of linagliptin in preventing the pathological progression of hepatic fibrosis in high fat diet and streptozotocin-induced diabetic obese rats. Eur. J. Pharmacol. 2020;881:173224. doi: 10.1016/j.ejphar.2020.173224. [DOI] [PubMed] [Google Scholar]
- 146.Virta J., Hellberg S., Liljenback H., Stahle M., Silvola J.M.U., Huusko J., Soderstrom M., Knuuti J., Nuutila P., Yla-Herttuala S., et al. Effects of dipeptidyl peptidase 4 inhibition on inflammation in atherosclerosis: A (18)F-fluorodeoxyglucose study of a mouse model of atherosclerosis and type 2 diabetes. Atherosclerosis. 2020;305:64–72. doi: 10.1016/j.atherosclerosis.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 147.Zhang G., Kim S., Gu X., Yu S.P., Wei L. DPP-4 Inhibitor Linagliptin is Neuroprotective in Hyperglycemic Mice with Stroke via the AKT/mTOR Pathway and Anti-apoptotic Effects. Neurosci. Bull. 2020;36:407–418. doi: 10.1007/s12264-019-00446-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jedlowski P.M., Jedlowski M.F., Fazel M.T. DPP-4 Inhibitors and Increased Reporting Odds of Bullous Pemphigoid: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS) from 2006 to 2020. Am. J. Clin. Dermatol. 2021;22:891–900. doi: 10.1007/s40257-021-00625-4. [DOI] [PubMed] [Google Scholar]
- 149.Huang J., Jia Y., Sun S., Meng L. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: Data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol. Toxicol. 2020;21:68. doi: 10.1186/s40360-020-00447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bajaj H.S., Ye C., Jain E., Venn K., Stein E., Aronson R. Glycemic Improvement with a Fixed-dose combination of DPP-4 inhibitor + metformin in patients with Type 2 diabetes (GIFT study) Diabetes Obes. Metab. 2018;20:195–199. doi: 10.1111/dom.13040. [DOI] [PubMed] [Google Scholar]
- 151.Kim H.J., Jeong I.K., Hur K.Y., Kim S.K., Noh J.H., Chun S.W., Kang E.S., Rhee E.J., Choi S.H. Comparison of Efficacy of Glimepiride, Alogliptin, and Alogliptin-Pioglitazone as the Initial Periods of Therapy in Patients with Poorly Controlled Type 2 Diabetes Mellitus: An Open-Label, Multicenter, Randomized, Controlled Study. Diabetes Metab. J. 2022 doi: 10.4093/dmj.2021.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Korbut A.I., Taskaeva I.S., Bgatova N.P., Muraleva N.A., Orlov N.B., Dashkin M.V., Khotskina A.S., Zavyalov E.L., Konenkov V.I., Klein T., et al. SGLT2 Inhibitor Empagliflozin and DPP4 Inhibitor Linagliptin Reactivate Glomerular Autophagy in db/db Mice, a Model of Type 2 Diabetes. Int. J. Mol. Sci. 2020;21:2987. doi: 10.3390/ijms21082987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review collected from open access web-source PubMed.