Abstract
The electrophoretic mobilities (EPMs) of a number of Escherichia coli O157:H7 and wild-type E. coli strains were measured. The effects of pH and ionic strength on the EPMs were investigated. The EPMs of E. coli O157:H7 strains differed from those of wild-type strains. As the suspension pH decreased, the EPMs of both types of strains increased.
Surface properties are important in understanding how microorganisms behave and interact with their environment. Surface properties have provided information about cell surface composition, isoelectric point, the rates of uptake of nutrients and antimicrobial drugs, agglutination, and adhesion to surfaces. Charged colloids or particles (including bacteria) suspended in water attempt to achieve electroneutrality by attracting ions of opposite charge. The charge of a particle’s surface cannot be measured directly. However, the rate of movement of a particle when placed in an electric field, or the electrophoretic mobility (EPM), can be used to calculate the potential for movement at a given distance from the particle (1).
EPMs, hydrophobicity measurements, and microscopic analyses have been used to describe the surface and adhesive properties of a variety of Escherichia coli strains under many conditions (2, 4, 6, 12, 13). Although several researchers have reported the surface charge of E. coli, the extrapolation and usefulness of this information are limited by the specificity of the conditions under which the experiments were conducted. E. coli O157:H7 is well established as an etiological agent of hemorrhagic colitis (5, 7, 10, 15, 16) and hemolytic-uremic syndrome (8, 11). There is little information regarding the EPM of this pathogen. The objective of this study was to measure and compare the EPMs of a number of E. coli O157:H7 and wild-type strains in aqueous suspensions. The impacts of pH and ionic strength on the surface charges of both E. coli types of strains were examined.
E. coli O157:H7 isolates were obtained from the culture collection of the U.S. Environmental Protection Agency (EPA) (Cincinnati, Ohio) (Table 1). Wild-type E. coli strains were isolated from a variety of sources, including domestic sewage sludge, drinking water, and dairy-cattle manure (Table 1). E. coli O157:H7 isolates exhibited characteristic phenotypic traits (14). Wild-type E. coli isolates were characterized by biochemical test kits (bioMerieux Vitek, Hazelwood, Mo.). All bacterial cultures used in the EPM experiments were grown for 18 to 20 h at 35°C in brain heart infusion broth, concentrated by centrifugation, and washed three times in phosphate buffer (9).
TABLE 1.
Bacterial strains and sources
|
E. coli isolate and description (EPA study identification no.) |
Source |
|---|---|
| O157:H7 | |
| Dairy isolate USDAa N009-6-1, WI (EPA 1) | David Miller, USDA; Ames, Iowa |
| Dairy isolate USDA N6059-7-2, MO (EPA 2) | David Miller |
| Dairy isolate USDA 6104-5-9, FL (EPA 3) | David Miller |
| Dairy isolate USDA N6021-5-1, ID (EPA 4) | David Miller |
| Dairy isolate USDA N6049-26-1, IL (EPA 5) | David Miller |
| Dairy isolate USDA N6001-8-10, WA (EPA 6) | David Miller |
| Dairy isolate USDA N6114-7-2, TX (EPA 7) | David Miller |
| 43894, produces Shiga-like toxins I and II | American Type Culture Collection, Manassas, Va. |
| 43895, produces Shiga-like toxins I and II | American Type Culture Collection |
| 43890, produces Shiga-like toxin I | American Type Culture Collection |
| 43888, produces neither Shiga-like toxin | American Type Culture Collection |
| 43889, produces Shiga-like toxin II | American Type Culture Collection |
| Wild type | |
| EPA 8 | Water main break, June 1997, Louisville, Ky. |
| EPA 9 | Dairy manure, Kenton County, Ky. |
| EPA 174 | Sewage sludge, Maryland |
| EPA 175 | Sewage sludge, Maryland |
| —b | Dairy manure, Kentucky |
| —b | Dairy manure, Kentucky |
| —b | Dairy manure, Ohio |
USDA, U.S. Department of Agriculture.
—, unnamed isolate.
EPM was measured with a Zetasizer 4 (Malvern Instruments Ltd., Malvern, England). The system measures EPM in micrometers centimeters per volt per second by laser doppler electrophoresis. Negative EPM values represent bacterial movement toward the positive electrode and a net negative surface charge. The instrument was calibrated daily with a Minusil ground quartz powder solution (3). Samples were injected directly into the 1.5-ml quartz capillary electrophoresis measurement cell with 10-ml disposable syringes. Prior to the measurement of each new sample, at least 20 ml of deionized water, followed by 10 ml of the sample to be measured, was rinsed through the capillary cell. Subsequent replicate measurements (typically four or five, depending on the sample volume) were made in 4-ml increments. Measurements were made at 25 ± 2°C.
E. coli levels of more than 106 cells/ml, based upon colony counts prepared in test water samples, were used in the electrophoresis measurements. Appropriate amounts of KH2PO4, NaCl, KCl, and BaCl2 (Fisher Scientific, Fair Lawn, N.J.) were dissolved in test water samples to achieve concentration ranges representative of drinking water. Acid or base titrations with 1 M HCl and 5 N NaOH were used to adjust the sample pH.
EPM measurements of a number of wild-type E. coli and E. coli O157:H7 strains in 9.15 mM KH2PO4-buffered deionized water (pH 6.45 to 6.55; ionic strength, 0.007 M) were made. The results showed clear differences between the two groups (Table 2). The EPMs of wild-type E. coli strains ranged from −0.82 to −1.94 μm cm V−1 s−1 and averaged −1.38 μm cm V−1 s−1. The EPMs of E. coli O157:H7 strains were considerably less negative than those of the wild-type strains. The EPMs ranged from −0.22 to −0.46 μm cm V−1 s−1 and averaged −0.36 μm cm V−1 s−1.
TABLE 2.
EPMs of E. coli isolatesa
| E. coli isolate and description (EPA study identification no.) | n | EPM (μm cm V−1 s−1)
|
|
|---|---|---|---|
| Mean | SD | ||
| O157:H7 | |||
| Dairy isolate USDA N009-6-1, WI (EPA 1) | 4 | −0.28 | 0.08 |
| Dairy isolate USDA N6059-7-2, MO (EPA 2) | 4 | −0.42 | 0.04 |
| Dairy isolate USDA 6104-5-9, FL (EPA 3) | 4 | −0.46 | 0.05 |
| Dairy isolate USDA N6021-5-1, ID (EPA 4) | 4 | −0.44 | 0.13 |
| Dairy isolate USDA N6049-26-1, IL (EPA 5) | 4 | −0.40 | 0.07 |
| Dairy isolate USDA N6001-8-10, WA (EPA 6) | 4 | −0.22 | 0.11 |
| Dairy isolate USDA N6114-7-2, TX (EPA 7) | 4 | −0.32 | 0.08 |
| Wild type | |||
| Isolate from water main break, Louisville, Ky. (EPA 8) | 4 | −1.40 | 0.05 |
| Isolate from Kenton County, Ky. (EPA 9) | 4 | −1.44 | 0.05 |
| Isolate from sewage sludge, Maryland (EPA 174) | 4 | −0.82 | 0.08 |
| Isolate from sewage sludge, Maryland (EPA 175) | 4 | −1.94 | 0.22 |
| Dairy manure, Kentucky | 2 | −1.30 | 0.00 |
Isolates were suspended in 9.15 mM KH2PO4-buffered deionized water (pH 6.45 to 6.55; ionic strength, 0.007 M). USDA, U.S. Department of Agriculture.
The impact of ionic strength (0.00061 to 0.049 M) on the EPMs of one representative E. coli O157:H7 strain (EPA 1) and one wild-type E. coli strain (EPA 8) was determined by varying the KH2PO4 buffer concentrations between 0.915 and 91.5 mM. Results (Table 3) showed that the E. coli O157:H7 strain did not appear to be significantly impacted by increased ionic strength adjusted by phosphate buffer. The EPM of the wild-type E. coli strain, however, was strongly impacted by ionic strength. As the ionic strength increased, the EPM increased in the positive direction from −1.54 to −0.1 μm cm V−1 s−1.
TABLE 3.
EPMs of E. coli suspended in KH2PO4-buffered deionized water
| E. coli isolate (EPA study identification no.) | Buffer concn (mM) | pH | Ionic strength (M) | n | EPM (μm cm V−1 s−1)
|
|
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| O157:H7 (EPA 1) | 0.915 | 6.27 | 0.00061 | 4 | −0.30 | 0.07 |
| 9.15 | 5.86 | 0.0052 | 5 | −0.46 | 0.15 | |
| 91.5 | 5.53 | 0.049 | 5 | −0.14 | 0.11 | |
| Wild type (EPA 8) | 0.915 | 6.29 | 0.00061 | 5 | −1.54 | 0.09 |
| 9.15 | 5.86 | 0.0052 | 5 | −1.02 | 0.04 | |
| 91.5 | 5.70 | 0.050 | 5 | −0.10 | 0.00 | |
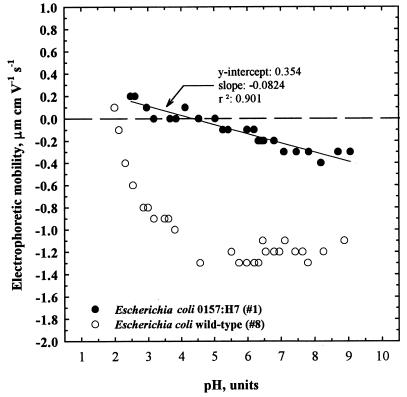
The impact of pH on the EPMs of an E. coli O157:H7 strain (EPA 1) and a wild-type E. coli strain (EPA 8) was determined by titrating the samples suspended in 9.15 mM KH2PO4 buffer with HCl or NaOH. One-liter suspensions were divided for separate acid and base titrations to avoid discrepancies between desorption and adsorption of cations (3). The results (Fig. 1) showed that the impact of pH was more significant with the wild-type E. coli strain. The curve describing the pH was nonlinear and appeared to consist of two regions. The EPMs decreased more sharply (grew more negative) between pH 2 and 5 in a near-linear fashion and reached an isoelectric point (zero point of charge [ZPC]) at pH 2.1. The EPM appeared to be independent of pH at pH values above 5 and had a value of approximately −1.2 μm cm V−1 s−1. The EPMs of the E. coli O157:H7 strain increased linearly (grew more positive) with decreasing pH values over the entire pH range; however, the rise was more gradual. The isoelectric point was observed to occur at a pH of approximately 4.3.
FIG. 1.
Impact of pH on the EPM of E. coli suspended in 9.15 mM KH2PO4 buffer. The pH was adjusted with 1.0 M HCl and 5 N NaOH.
The EPMs of different Shiga-like-toxin-producing E. coli O157:H7 strains were measured in 9.15 mM phosphate buffer at pH 5.76 to 5.85. The EPMs of bacteria capable of producing Shiga-like toxins I, II, and I and II and of bacteria producing neither toxin were compared. The results (Table 4) did not suggest a relationship between the electrostatic properties of the cell wall and the ability of the bacteria to produce Shiga-like toxins.
TABLE 4.
EPMs of Shiga-like-toxin-producing E. coli O157:H7 strainsa
| E. coli O157:H7 isolate | Shiga-like toxin | pH | n | EPM (μm cm V−1 s−1)
|
|
|---|---|---|---|---|---|
| Mean | SD | ||||
| 43894 | I and II | 5.85 | 5 | −0.74 | 0.22 |
| 43895 | I and II | 5.76 | 5 | −0.36 | 0.05 |
| 43890 | I | 5.78 | 5 | −0.64 | 0.05 |
| 43888 | Neither | 5.78 | 5 | −0.82 | 0.18 |
| 43889 | II | 5.78 | 5 | −0.82 | 0.04 |
Strains were suspended in 9.15 mM KH2PO4-buffered deionized water (pH 5.76 to 5.85; ionic strength, 0.005 M).
Electrostatic studies of E. coli in aqueous suspensions showed marked differences between the EPMs of various wild-type E. coli strains and E. coli O157:H7 strains. The EPMs of wild-type strains were more positive than those of O157:H7 strains by as much as 1.7 μm cm V−1 s−1 in the suspensions tested, signifying a greater net negative surface charge. Electrostatic attraction of cations to the cell surface would also have been expected to be greater. E. coli O157:H7 strains had a near-neutral charge in the suspensions tested; thus, electrostatic repulsive forces between E. coli O157:H7 strains and negatively charged ions, colloids, particles, and surfaces would have been weaker. The tendency for attractive interactions, such as hydrophobic and van der Waals forces, to occur would have been greater, making it more likely that the pathogens would have aggregated and adsorbed to hydrophobic materials.
Increasing ionic strength had a strong impact on the electrostatic properties of wild-type E. coli strains but little effect on those of the E. coli O157:H7 strains. This observation can be explained by the electrostatic attraction of cations (Na+) added in the phosphate buffer to the anionic bacterial surface. The attraction was stronger for the wild-type strains, which exhibited a greater net negative surface charge, and was barely observable with the O157:H7 strains, whose surface charges were relatively close to neutral.
The pH of the water had a large impact on the EPMs of the bacterial surfaces. The effect was more pronounced in the wild-type strains, owing to the greater net negative charge at near-neutral pH values. The pHs of the ZPC of wild-type and O157:H7 strains occurred at significantly different values and differed by more than 1 pH unit. The ZPCs of the cell surfaces reflect the ZPCs of the surface groups and therefore suggest differences in outer cell wall composition.
In summary, wild-type E. coli strains and E. coli O157:H7 strains have considerably different surface charges, which suggests differences in surface characteristics. The discrepancy could translate into differences in how the microorganisms interact with their environment. The suspension pH impacted the EPMs of both types of strains; as the pH decreased, the EPMs increased (became more positive). Increasing the ionic strength impacted the EPMs of wild-type E. coli strains to a greater extent than those of the E. coli O157:H7 strains.
Acknowledgments
We acknowledge Christina Feld and Jeffery Gerkin of the EPA for their assistance in making electrophoretic mobility measurements. We also thank Donald Reasoner, Armah de la Cruz, Robert Clark, and Robert Thurnau (also of the EPA) for reviewing the manuscript and providing useful suggestions and comments.
REFERENCES
- 1.American Water Works Association. Water quality and treatment. 4th ed. New York, N.Y: McGraw-Hill Inc.; 1990. [Google Scholar]
- 2.Bayer M E, Sloyer J L. The electrophoretic mobility of gram-negative and gram-positive bacteria: an electrokinetic analysis. J Gen Microbiol. 1990;136:867–874. doi: 10.1099/00221287-136-5-867. [DOI] [PubMed] [Google Scholar]
- 3.Bier M. Electrophoresis: theory, methods and application. New York, N.Y: Academic Press; 1959. [Google Scholar]
- 4.Buggs C W, Green R G. Electrophoretic phenomena of bacteria. II. Electrophoretic velocities of virulent and non-virulent C. diphtheriae. J Bacteriol. 1935;30:447–451. doi: 10.1128/jb.30.5.447-451.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dev V J, Main M, Gould I. Waterborne outbreak of Escherichia coli O157. Lancet. 1991;337:1412. doi: 10.1016/0140-6736(91)93092-n. [DOI] [PubMed] [Google Scholar]
- 6.Dyar M T, Ordal E J. Electrokinetic studies on bacterial surfaces. I. The effects of surface-active agents on the electrophoretic mobilities of bacteria. J Bacteriol. 1946;51:149–167. [PMC free article] [PubMed] [Google Scholar]
- 7.Geldreich E E, Fox K R, Goodrich J A, Rice E W, Clark R M, Swerdlow D L. Searching for a water supply connection in the Cabool, Missouri disease outbreak of Escherichia coli O157:H7. Water Res. 1992;26:1127–1137. [Google Scholar]
- 8.Gransden W R, Damm M A S, Anderson J D, Carter J E, Lior H. Further evidence associating hemolytic uremic syndrome with infection by verotoxin-producing Escherichia coli O157:H7. J Infect Dis. 1986;154:522–524. doi: 10.1093/infdis/154.3.522. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg A E, editor. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C: American Public Health Association; 1995. [Google Scholar]
- 10.Isaacson M, Canter P H, Effler P, Arntzen L, Bomans P, Heenan R. Hemorrhagic colitis epidemic in Africa. Lancet. 1993;341:961. doi: 10.1016/0140-6736(93)91253-i. [DOI] [PubMed] [Google Scholar]
- 11.Karmali M, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 12.Moyer L S. Changes in the electrokinetic potential of bacteria at various phases of the culture cycle. J Bacteriol. 1936;32:433–464. doi: 10.1128/jb.32.4.433-464.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyer L S. A suggested standard method for the investigation of electrophoresis. J Bacteriol. 1936;31:531–546. doi: 10.1128/jb.31.5.531-546.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice E W, Johnson C H, Reasoner D J. Detection of Escherichia coli O157:H7 in water from coliform enrichment cultures. Lett Appl Microbiol. 1996;23:179–182. [PubMed] [Google Scholar]
- 15.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow D L, Woodruff B A, Brady R C, Griffen P M, Tippen S, Donnell H D, Geldreich E E, Payne B J, Meyer A, Wells K D, Greene K D, Bright M, Bean N H, Blake P A. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann Intern Med. 1992;117:812–817. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]