Abstract
The aim of this study was to assess the changes in microbiota composition during a gluten-free diet (GFD) in coeliac disease (CD) patients. The systematic search followed databases such as PUBMED (MEDLINE), SCOPUS, WEB OF SCIENCE and EMBASE. Out of 843 initially screened papers, a total number of 13 research papers were included. A total of 212 patients with CD on GFD, in comparison to 174 healthy individuals and 176 untreated patients with CD, were examined. Analysis of the microbial community based primarily on faecal samples and duodenal biopsies. Bifidobacterium was noticed to be less abundant in the study group than in both control groups, while the abundance of Bacteroides was more numerous in the group of CD patients on GFD. Staphylococcaceae prevailed in untreated CD patients. Despite the fact that the GFD was not able to fully restore commensal microorganism abundance, the treatment was associated with the greater abundance of selected beneficial bacteria and lower presence of pathogenic bacteria associated with worsening of CD symptoms.
Keywords: coeliac disease, gluten-free diet, microbiome
1. Introduction
Coeliac disease (CD), described as a chronic autoimmune gluten intolerance, is becoming an increasingly important health problem for modern medicine in developed countries [1,2]. The occurrence of CD is strongly related to genetic factors, among which HLA class II plays a major role—HLA-DQ2 heterodimers are expressed in over 90% of patients, the remnant express HLA-DQ8 [3]. Nowadays, diagnosis of CD is primarily based on anti-tissue transglutaminase antibodies (tTG-IgA) testing and duodenal biopsy [4]. The symptom manifestations in patients suffering from CD especially concern the gastrointestinal tract, therefore differentiated dietary strategies are tested in the clinical setting i.e., for patients’ quality of life (QoL) improvement. Microbiological approaches have been considered as able to modulate the gluten-specific immune response. Furthermore, different biotechnological approaches based on the use of chemically/enzymatically modified gluten molecules have been proven effective in different models of CD.
A gluten-free diet (GFD) seems to be an effective way of CD treatment and enables the majority of patients to achieve clinical and histological remission [5]. According to recent data [6], children’s populations with CD are able to reconstruct up to 95% of their intestinal architecture within two years by following a GFD. However, some data suggest that treatment response in the adult population (30–60 years old) is less effective [7]. Among positive aspects of GFD application can be listed: positive influence on bone mineral density, increased weight-for-age z scores in the paediatric population diagnosed with both CD and type 1 diabetes and lower risk of depression in the female population [8,9,10]. A recent study suggested that the disturbance of the intestinal microbiota might be involved in the pathogenesis of CD. Altered microbiota might have an impact on immune response to gluten, with high release of proinflammatory cytokines [11]. Observed changes in intestinal microbiota composition that occur during the application of a GFD provide promising support for better QoL and outcomes in CD patients [12].
The aim of this study was to assess the changes in microbiota composition during a GFD in CD patients.
2. Experiment
2.1. Search Strategy, Inclusion and Exclusion Criteria
From August 2021 to December 2021, the literature of the following databases: PUBMED (MEDLINE), SCOPUS, WEB OF SCIENCE and EMBASE were searched in order to identify the interventional and observational studies that investigate differences in the gut microbiome in patients suffering from CD during the GFD application.
The search strategy was limited to the human population and English language. Original articles were included. No restrictions regarding the date of the publication or age of patients were used. Articles with low quality data or incomplete data that could not be fully obtained from authors were excluded.
The search strategy included the following index terms: #1 Diet, Gluten Free OR Gluten-Free Diet OR Diets, Gluten-Free OR Gluten Free Diet OR Gluten-Free Diets; #2 Gastrointestinal Microbiomes OR Microbiome, Gastrointestinal OR Gut Microbiome OR Gut Microbiomes OR Microbiome, Gut OR Gut Microflora OR Microflora, Gut OR Gut Microbiota OR Gut Microbiotas OR Microbiota, Gut OR Gastrointestinal Flora OR Flora, Gastrointestinal OR Gut Flora OR Flora, Gut OR Gastrointestinal Microbiota OR Gastrointestinal Microbiotas OR Microbiota, Gastrointestinal OR Gastrointestinal Microbial Community OR Gastrointestinal Microbial Communities OR Microbial Community, Gastrointestinal OR Gastrointestinal Microflora OR Microflora, Gastrointestinal OR Gastric Microbiome OR Gastric Microbiomes OR Microbiome, Gastric OR Intestinal Microbiome OR Intestinal Microbiomes OR Microbiome, Intestinal OR Intestinal Microbiota OR Intestinal Microbiotas OR Microbiota, Intestinal OR Intestinal Microflora OR Microflora, Intestinal OR Intestinal Flora OR Flora, Intestinal OR Enteric Bacteria OR Bacteria, Enteric; #3 Disease, Celiac OR Gluten Enteropathy OR Enteropathies, Gluten OR Enteropathy, Gluten OR Gluten Enteropathies OR Gluten-Sensitive Enteropathy OR Enteropathies, Gluten-Sensitive OR Enteropathy, Gluten-Sensitive OR Gluten Sensitive Enteropathy OR Gluten-Sensitive Enteropathies OR Sprue, Celiac OR Sprue, Nontropical OR Nontropical Sprue OR Celiac Sprue OR Sprue.
#1 AND #2 AND #3.
2.2. Data Extraction and Analysis
Initial revision to the titles of the articles was made by four researchers. Each researcher was responsible for searching one database. In the further stage abstracts were analysed and assessed for eligibility. Subsequently, the decision on the article inclusion was made collaboratively by all groups after the full text review.
From each qualified study the following data were extracted: a title, a main author, a publication year, a study name and design, countries involved, a total number of patients, age, sex, time of GFD treatment, antibiotic treatment, duration of the disease, presence of antigliadin and anti-transglutaminase antibodies (tGA) in serum and haplotypes within the HLA-DQ serotyping system. In order to examine the microbiome structure following methods were used: 16S rDNA sequencing, 16S rRNA sequencing, microscopic analysis and identification of Bifidobacteria by determination of fructose-6-phosphate phosphoketolase, Fluorescence in situ hybridization (FISH), flow cytometry, Fuzzy c-means (FCM), quantitative real-time polymerase chain reaction (qPCR), Short Chain Fatty Acid Analysis (SCFAs), Denaturing gradient gel electrophoresis (DGGE) and bacterial culture. Additionally, pH of stool samples was obtained.
Patients were assigned to one out of three groups according to health condition and diet: individuals affected by CD and following a GFD (CD on GFD); healthy individuals without CD and other known food intolerance who did not follow any particular diet (healthy control group, HC); individuals affected by CD who did not exclude gluten from the diet (untreated, UCD).
3. Results
3.1. Search Results
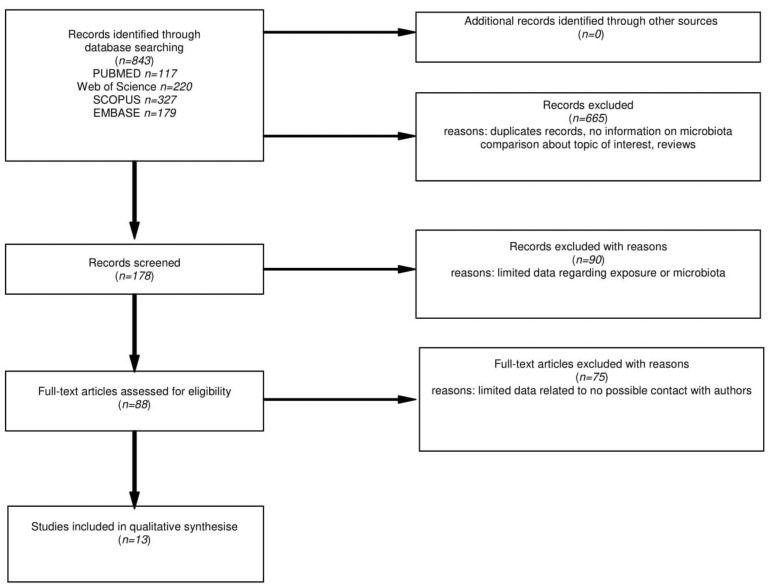
The flow chart for the literature search is presented in Figure 1. After the title database search, 843 articles were found, where further 178 abstracts were carefully examined. Finally, 88 full-texts were assessed and where needed, an attempt to contact the authors of papers with incomplete information was made. The detailed analysis of selected positions led to the acquisition of 13 papers that met all the criteria.
Figure 1.
Flow chart of the databases search on microbiota changes in CD patients on GFD.
3.2. Characteristics of the Included Studies and Study Population
All papers were original intervention studies (Table 1). Most research was conducted on the European population [13,14,15,16,17,18,19,20,21,22,23]; however, patients from North America [24] and South America [25] were also included. Finally, gut microbiota of 492 patients with diagnosed CD and 299 healthy individuals was analysed. CD diagnosis was based on the presence of CD-specific antibodies and duodenal biopsy examination. Time of intervention with a GFD in individual studies varied widely: between 6–36 months based on the consumption of certified gluten-free food only [15].
Table 1.
Characteristics of the included studies (n = 13).
| Study | Year | Country | Study Design | CD on GFD | Healthy | UCD | Duration of Treatment with Gluten-Free Diet (Months) |
|---|---|---|---|---|---|---|---|
| Nadal I. et al. [16] | 2007 | Spain | CSS | 10 | 8 | 20 | 12–24 |
| Collado M. et al. [22] | 2008 | Spain | CSS | 18 | 30 | 30 | min 24 |
| Di Cagno R. et al. [19] | 2009 | Italy | CSS | 7 | 7 | 7 | min 24 |
| Schippa S. et al. [14] | 2010 | Italy | CSS | 20 | 10 | 20 | 9 |
| De Palma G. et al. [17] | 2010 | Spain | CSS | 18 | 20 | 24 | min 24 |
| Di Cagno R. et al. [13] | 2011 | Italy | CSS | 19 | 15 | 0 | min 24 |
| Kalliomäki M. et al. [18] | 2012 | Finland | CSS | 6 | 9 | 10 | min 12 |
| Nistal E. et al. [20] | 2012 | Spain | CSS | 11 | 11 | 10 | min 24 |
| Sanchez E. et al. [23] | 2013 | Spain | CSS | 17 | 8 | 32 | min 24 |
| Pirjo W. et al. [15] | 2014 | Finland | CSS | 34 | 0 | 0 | min 36 |
| Lorenzo Pisarello M.J. et al. [25] | 2015 | Argentina | CSS | 15 | 15 | 0 | min 6 |
| Serena G. et al. [24] | 2019 | USA | CSS | 8 | 10 | 10 | min 6 |
| Panelli S. et al. [21] | 2020 | Italy | CSS | 29 | 31 | 13 | 36 (median) |
CD—coeliac disease, GFD—gluten-free diet, CSS—cross-sectional study, CD on GFD—patients with CD on GFD, Healthy—healthy individuals without CD and other known food intolerance, UCD—untreated CD patients.
The detailed clinical characteristic of studied patients was included in Table 2. The group of examined patients included both children [14,16,17,18,22,23,25] and adults [15,18,20,21]. The proportion of men and women in the eligible studies was comparable (50:50). In nine papers, antibiotic treatments were not allowed at least 1 month before collection of stool or/and duodenal biopsy samples [14,15,16,17,19,20,21,22,23,25]. Clinical symptoms of active CD such as bloating, abdominal pain, diarrhoea and weight loss were observed in untreated individuals in only two studies [22,23]. An anti-gliadin antibodies (AGA) test was positive in patients on a GFD in two [14,16] out of five studies, while in the untreated CD group, the AGA test was positive in two studies [22,23] reporting its presence. tGA test was positive in 25% of the research in which the test was carried out [14,16], whereas in the untreated CD group this ratio reached 100% of individuals [18,20,23]. Taking into account available data relating to haplotypes within the HLA-DQ serotyping system, more than 50% of patients affected by CD were DQ2+ or/and DQ8+ [21,22,23], while in all studies, less than 35% of the healthy control group was DQ2+ or/and DQ8+ [20,21]. Iron deficiency, which is one of the non-classical CD symptoms [15], was featured in only two papers [22,23], in an untreated CD group of patients.
Table 2.
Characteristics of the study population (n = 212).
| Study | Age (Years) Mean ±SD |
Sex (% Male) | Antibiotic Treatment | AGA | tGA | HLA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD on GFD | Healthy | UCD | CD on GFD | Healthy | UCD | CD on GFD | Healthy | UCD | CD on GFD | Healthy | UCD | CD on GFD | Healthy | UCD | ||
| Di Cagno R. et al. [13] | 9.7 (6–12) 1 | 10.4 (6–12) 1 | N/A | 42 | 47 | N/A | no last 3 mo | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lorenzo Pisarello M.J. et al. [25] | 7.5 | 6.5 | N/A | N/A | N/A | N/A | no last 1 mo | negative | N/A | N/A | negative | N/A | N/A | N/A | N/A | N/A |
| Schippa S. et al. [14] | N/A | 11.7 (7.8–20.8) | 8.3 (1.2–16.1) | 40 | 30 | 40 | no last 3 mo | positive | N/A | N/A | positive | N/A | N/A | N/A | N/A | N/A |
| Pirjo W. et al. [15] | N/A | N/A | N/A | N/A | N/A | N/A | No | N/A | N/A | N/A | 0.3 | 0.8 | N/A | 65% DQ2/DQ8 | N/A | N/A |
| Nadal I. et al. [16] | 5.1 | 4.1 | N/A | N/A | N/A | N/A | no last 1 mo | positive | N/A | N/A | positive | N/A | N/A | N/A | N/A | N/A |
| De Palma G. et al. [17] | 5.5 | 5.3 | 5.5 | N/A | N/A | N/A | no last 1 mo | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kalliomäki M. et al. [18] | 46 | 8.5 | 9.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | negative | negative | positive | N/A | N/A | N/A |
| Di Cagno R. et al. [19] | N/A | N/A | N/A | N/A | N/A | N/A | no last 1 mo | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Nistal E. et al. [20] | 40.4 | 30.9 | 38.5 | N/A | N/A | N/A | no last 1 mo | N/A | N/A | N/A | negative | negative | positive | N/A | 0% DQ2/DQ8 | N/A |
| Panelli S. et al. [21] | 37+/−6 | 44+/−17 | 35+/−6 | 31 | 23 | 15 | no last 1 mo | N/A | N/A | N/A | N/A | N/A | N/A | 58% DQ2+, 3% DQ8+, 7% DQ2/DQ8+ | 32% DQ2+, 10% DQ2+, 3% DQ2/DQ8+, 26% DQ2/DQ8- | 62% DQ2+, 0% DQ8+, 23% DQ2/DQ8+ |
| Serena G. et al. [24] | N/A | N/A | N/A | N/A | N/A | N/A | no last 1 mo | N/A | N/A | N/A | negative | N/A | positive | N/A | N/A | N/A |
| Collado M. et al. [22] | 5.43 | 3.75 | 4.7 | 44.4 | 43.3 | 40 | no last 1 mo | negative | negative | positive | negative | negative | positive | DQ2+-100% | N/A | DQ2+-100% |
| Sanchez E. et al. [23] | 5.9 | 6.9 | 5.1 | 47.1 | 50 | 43.7 | no last 1 mo | negative | negative | positive | negative | negative | positive | DQ2/DQ8 100% |
N/A | DQ2/DQ8 100% |
N/A—not applicable, AGA—anti-gliadin antibodies, tGA—anti-tissue transglutaminase antibodies, HLA—haplotypes within the HLA-DQ serotyping system (genetic test), CD—coeliac disease, GFD—gluten-free diet, CD on GFD—patients with CD on GFD, Healthy—healthy individuals without CD and other known food intolerance, UCD—untreated CD patients. 1 Median age (range).
3.3. Assessment of Microbiota Changes Related to GFD Treatment
Analysis of the microbial community was based on faecal samples and duodenal biopsies [13,14,15,16,17,18,19,20,21,22,23,24,25]. Two studies also used saliva [21] and blood [24] samples as biological material. The main route utilised by commensal bacteria to migrate to the bloodstream is through a damaged, inflamed, and therefore, permeable epithelium. It has been suggested that active CD patients, characterised by increased intestinal permeability, could acquire a unique blood microbiome reflecting the intestinal damage and that this phenomenon could influence their response to gluten. The differentiated microbiota composition with possible changes in three groups of individuals (CD on GFD, healthy controls and UCD) are presented in Table 3. Because of lack of taxonomic uniformity in studies included in the current research, a comparison of microbial communities between three groups of patients on one, we failed to obtain a common taxonomic rank. In general, Bifidobacterium genus, was the most frequently examined of all groups of bacteria [12,13,14,16,17,18,19,20,21,22,26,27] and occurred significantly less abundant in the study group than in healthy individuals according to faecal samples [13,19,20,22]. The same genus isolated from duodenal biopsy showed greater abundance in the healthy group than in the UCD [22]. Separate analysis on children and adult populations indicated that Bifidobacterium occurred less abundant in CD children in the GFD group and UCD group than in the healthy controls [13,17,22]. However, a similar tendency in the adult population was not observed. Studies on Lactobacillus changes [22,25] indicated significantly greater abundance in the GFD group than the HC [22,25]. Additionally, Lactobacillus Sakei was less abundant in CD for the GFD group than in both UCD and HC groups in the children’s population. The Staphylococcus genus isolated from duodenal biopsy was more abundant in UCD than CD for the GFD group and healthy group in only one study [22]. The same pattern of changes in faecal samples has been observed. Significant changes in abundance of Bacteroides genus assessed in faecal samples of children was found to be more pronounced in CD patients on a GFD than in healthy individuals [13,19,22]. In the case of duodenal biopsy, material tendency was observed in two out of three studies [13,22]. Significant changes in E. coli abundance was observed in three studies based on children’s populations [14,16,22], where two out of three studies showed domination of E. coli species in CD on the GFD group over remaining groups of patients [14,16,22]. After analysis of changes in Firmicutes phylum, no clear conclusions can be drawn. Two studies with faecal samples as biological material present opposite tendencies—domination of Firmicutes in the UCD group [21] and domination of Firmicutes in other groups over UCD group [24]. One study attached duodenal samples and a domination of Firmicutes in the HC over the CD for the GFD group was observed [15]. Data on Bacteroidetes phylum provided by Panelli et al. [21] showed larger abundance of Bacteroidetes in the CD for the GFD group than in the UCD and HC groups in both duodenal and faecal samples. Four studies reported statistically significant changes in the Clostridium group [17,19,21,22]; however, each of them investigated changes on different taxonomic ranks. Greater abundance of Lactobacillus and Bacteroides in the study group was found to be beneficial and presumably correlated with clinical remission [28]. Bifidobacterium genus was also proved to be beneficial in CD [29,30,31]; however, it was less abundant in comparison to the UCD and healthy controls in this study group.
Table 3.
Gut microbiota diversity based on GFD application in CD patients.
| Study | Type of Samples | Method | Abundance | p-Value | ||
|---|---|---|---|---|---|---|
| CD on GFD | Healthy | UCD | ||||
| Di Cagno R. et al. [13] | Duodenal biopsy and faecal samples | 16S rRNA sequencing | Bifidobacteria 5.34 | Bifidobacteria 6.72 | N/A | p = 0.023 |
| Lactobacilli 8.1 | Lactobacilli 8.6 | p > 0.05 | ||||
| Enterococci 7.83 | Enterococci 8.23 | p > 0.05 | ||||
| Bacteroides 6.02 | Bacteroides 5 | p = 0.014 | ||||
| Staphylococci 7.6 | Staphylococci 7 | p > 0.05 | ||||
| Salmonella, Shigella, Klebsiella 7.26 | Salmonella, Shigella, Klebsiella 7.3 | p > 0.05 | ||||
| Enterobacteria 6.7 | Enterobacteria 6.4 | p > 0.05 | ||||
| Clostridium 1 1 | Clostridium 1 1 | p > 0.05 | ||||
| Lorenzo Pisarello M.J. et al. [25] | Faecal samples | bacterial culture | Anaerobic (1.37 ± 5.47) × 109 CFU/g | Anaerobic (2.09 ± 9.08) × 109 CFU/g | N/A | p > 0.05 |
| Aerobic (1.54 ± 5.47) × 109 | Aerobic (3.31 ± 2.57) × 109 | p > 0.05 | ||||
| Enterobacteria (1.18 ± 7.69) × 106 CFU/g | Enterobacteria (6.60 ± 5.23) × 105 CFU/g | p > 0.05 | ||||
| Lactobacillus (4.38 ± 3.14) × 105 | Lactobacillus (4.00 ± 2.45) × 106; | p < 0.05 | ||||
| Schippa S. et al. [14] | Duodenal biopsy | 16S rDNA Sequencing | Bacteroides vulgatus 85% | Bacteroides vulgatus 94.7% | Bacteroides vulgatus 20% | p < 0.001 |
| E. coli 95% | E. coli 20% | p < 0.001 | ||||
| Bifidobacterium 30% | Parabacteroides distasonis 0 6 | Bifidobacterium 20% 6 | p > 0.05 | |||
| Parabacteroides distasonis 31.6% 6 | p = 0.009 | |||||
| Pirjo W. et al. [15] | Duodenal biopsy | 16S rDNA Sequencing | Bacteroidetes 15% | Bacteroidetes 25% | N/A | p = 0.01 |
| Firmicutes 33% | Firmicutes 46% | p = 0.05 | ||||
| Proteobacteria 40% | Proteobacteria 21% | p = 0.04 | ||||
| 72 OTUs per sample | 106 OTUs per sample | |||||
| Nadal I. et al. [16] | Duodenal biopsy | FISH and flow cytometry | Bacteroides–Prevotella 12.98% 2 | Bacteroides–Prevotella 6.07 | Bacteroides–Prevotella 4.52 | p = 0.027 |
| Escherichia coli 10.98% 2 | Escherichia coli 5.04 | Escherichia coli 4.1 | p = 0.027 | |||
| Streptococcus–Lactococcus 10.88% 2 | Streptococcus–Lactococcus 7.18 | Streptococcus–Lactoco 9.44 | p > 0.05 | |||
| Bifidobacterium 9.24% 2 | Bifidobacterium 10.55 | Bifidobacterium 4.32 | p > 0.05 | |||
| De Palma G. et al. [17] | Faecal samples | FISH and FCM | Bifidobacterium 9.20% 2 | Bifidobacterium 12.54% 2 | Bifidobacterium 7.73% 2 | p = 0.009 |
| C. histolyticum 9.41% 2 | C. histolyticum 11.61% 2 | C. histolyticum 5.26% 2 | p = 0.031 | |||
| C. lituseburense 4.41% 2 | C. lituseburense 6.83% 2 | C. lituseburense 3.23% 2 | p = 0.024 | |||
| Lactobacillus-Enterococcus 1.12% 2 | Lactobacillus-Enterococcus 1.76% 2 | Lactobacillus-Enterococcus 1.94% 2 | p > 0.05 | |||
| Staphylococcus 16.49% 2 | Staphylococcus 18.04% 2 | Staphylococcus 10.36% 2 | p > 0.05 | |||
| Bacteroides-Prevotella 2.61% 2 | Bacteroides-Prevotella 2.32% 2 | Bacteroides-Prevotella 3.54% 2 | p = 0.033 | |||
| E. coli 6.39% 2 | E. coli 7.32% 2 | E. coli 5.2% 2 | p > 0.05 | |||
| F. prausnitzii 11.09% 2 | F. prausnitzii 13.88% 2 | F. prausnitzii 6.03% 2 | p = 0.045 | |||
| Sulphate-reducing bacteria 9.82% 2 | Sulphate-reducing bacteria 10.02% 2 | Sulphate-reducing bacteria 9.58% 2 | p > 0.05 | |||
| Kalliomäki M. et al. [18] | Small intestinal biopsy | qPCR | Bacteroides-Prevotella-Porphyromona group 1682 | Bacteroides-Prevotella-Porphyromonas group 684 | Bacteroides-Prevotella-Porphyromonas group 834 | p > 0.05 |
| Bifidobacterium genus 140 | Bifidobacterium genus 190 | Bifidobacterium genus 234 | p > 0.05 | |||
| Bifidobacterium adolescentis 5 5 | Bifidobacterium adolescentis 14 5 | Bifidobacterium adolescentis 10 5 | p > 0.05 | |||
| Di Cagno R. et al. [19] | Faecal samples | 16S rRNA sequencing RAPD-PCR analysis | Lactic acid bacteria 8.09 | Lactic acid bacteria 8.89 | Lactic acid bacteria 8.02 | p > 0.05 |
| Bifidobacterium 6.83 | Bifidobacterium 7.88 | Bifidobacterium 5.51 | p = 0.03 | |||
| Bacteroides 8.31 | Bacteroides 7.05 | Bacteroides 8.69 | p = 0.045 | |||
| Clostridium 8.07 | Clostridium 5.50 | Clostridium 8.04 | p = 0.045 | |||
| Staphylococcus/Micrococcus 7.42 | Staphylococcus/Micrococcus 8.05 | Staphylococcus/Micrococcus 6.00 | p > 0.05 | |||
| Enterobacteriaceae | Enterobacteriaceae 8.05 | Enterobacteriaceae 6.69 | p > 0.05 | |||
| Total anaerobes 9.63 1 | Total anaerobes 10.03 1 | Total anaerobes 9.87 1 | p > 0.05 | |||
| Nistal E. et al. [20] | Faecal samples | SCFAs, DGGE |
Lactobacillus sakei 0% | Lactobacillus sakei 45% | Lactobacillus sakei 40% | p < 0.05 |
| Bifidobacterium bifidum 18% | Bifidobacterium bifidum 9% | Bifidobacterium bifidum 60% | p < 0.05 | |||
| Bifidobacterium catenulatum 18% | Bifidobacterium catenulatum 36% | Bifidobacterium catenulatum 80% | p < 0.05 | |||
| Bifidobacterium sp.0% 6 | Bifidobacterium sp. 45% 6 | Bifidobacterium sp. 20% 6 | p < 0.05 | |||
| Panelli S. et al. [21] | Saliva samples, duodenal biopsy and faecal samples |
16S rRNA sequencing | Duodenal samples: | duodenal samples | duodenal samples: | |
| Bacteroidetes 28.08% | Bacteroidetes 20.76% | Bacteroidetes 18.20% | p < 0.05 | |||
| Actinobacteria 7.94% | Actinobacteria 11.1% | Actinobacteria 4.15% | p < 0.05 | |||
| Proteobacteria 19.21% | Proteobacteria 17.89% | Proteobacteria 35.48% | p < 0.05 | |||
| Streptococcaceae 18.34% | Streptococcaceae 25.77% | Streptococcaceae 22.77% | p < 0.05 | |||
| Gemellaceae 1.51% | Gemellaceae 2.17% | Gemellaceae 0.83% | p < 0.05 | |||
| Veillonellaceae 8.95% | Veillonellaceae 7.37% | Veillonellaceae 4.50% | p < 0.05 | |||
| Lachnospiraceae 3.26% | Lachnospiraceae 2.71% | Lachnospiraceae 2.00% | p < 0.05 | |||
| Prevotellaceae 17.8% | Prevotellaceae 12.1% | Prevotellaceae 6.80% | p < 0.05 | |||
| Micrococcaceae 4.98% | Micrococcaceae 7.51% | Micrococcaceae 2.27% | p < 0.05 | |||
| Neisseriaceae 7.91% | Neisseriaceae 3.95% | Neisseriaceae 16.14% | p < 0.05 | |||
| Stool samples: | Stool samples: | Stool samples: | ||||
| Bacteroidetes 59.99% | Bacteroidetes 51.97% | Bacteroidetes 44.27% | p < 0.05 | |||
| Firmicutes 34.21% | Firmicutes 36.39% | Firmicutes 47.83% | p < 0.05 | |||
| Actinobacteria 0.82% | Actinobacteria 1.96% | Actinobacteria 2.93% | p < 0.05 | |||
| Proteobacteria 3.96% | Proteobacteria 6.9% | Proteobacteria 3.12% | ||||
| Coriobacteriaceae 0.12% | Coriobacteriaceae 0.14% | Coriobacteriaceae 1.39% | p < 0.05 | |||
| Clostridiaceae 0.57% | Clostridiaceae 0.18% | Clostridiaceae 0.63% | p < 0.05 | |||
| Veillonellaceae 6.35% | Veillonellaceae 6.35% | Veillonellaceae 2.4% | p < 0.05 | |||
| Erysipelitrichaceae 0.44% | Erysipelitrichaceae 0.30% | Erysipelitrichaceae 1.14% | p < 0.05 | |||
| Ruminococcaceae 13.94% | Ruminococcaceae 23.52% | Ruminococcaceae 23.52% | p < 0.05 | |||
| Coriobacteriaceae 0.12% | Coriobacteriaceae 0.14% | Coriobacteriaceae 1.39% | p > 0.05 | |||
| Enterobacteriaceae 2.13% | Enterobacteriaceae 0.46% | Enterobacteriaceae 1.84% | p > 0.05 | |||
| Pasteurellaceae 0.41% | Pasteurellaceae 2.32% | Pasteurellaceae 0.56% | p > 0.05 | |||
| Serena G. et al. [24] | Blood samples and faecal samples | 16S rRNA sequencing | blood samples: | blood samples: | blood samples: | |
| Proteobacteria 41.26% | Proteobacteria 42.34% | Proteobacteria 49.16% | p > 0.05 | |||
| Actinobacteria 8.16% | Actinobacteria 8.42% | Actinobacteria 9.44% | p > 0.05 | |||
| Bacteroidetes 6.39% | Bacteroidetes 5.87% | Bacteroidetes 7.87% | p > 0.05 | |||
| Firmicutes 36.36% | Firmicutes 32.07% | Firmicutes 26.44% | p > 0.05 | |||
| Other 7.83% | Other 11.3% | Other 7.09% | p > 0.05 | |||
| faecal samples: | faecal samples: | faecal samples: | ||||
| Firmicutes 71.41% | Firmicutes 77.37% | Firmicutes 57.8% | p < 0.05 | |||
| Bacteroidetes 11.87% | Bacteroidetes 13.01% | Bacteroidetes 31.86% | p < 0.05 | |||
| Other 16.72% | Other 9.62% | Other 10.34% | p > 0.05 | |||
| Collado M. et al. [22] 3 | Duodenal biopsy and faecal samples | qPCR | Study—Treated CD faecal samples: | Healthy faecal samples: | Untreated CD faecal samples: | |
| Bifidobacterium—8.77 (8.58–9.60) | Bifidobacterium—9.80 (9.23–10.33) | Bifidobacterium—8.67 (8.68–9.90) | p < 0.05 | |||
| Bacteroides—8.55 (8.30–8.90) | Bacterioides—8.13 (7.41–8.53) | Bacterioides—8.71 (8.05–9.00) | p < 0.05 | |||
| Staphylococcus—6.58 (6.28–6.88) | Staphylococcus—6.78 (6.26–7.18) | Staphylococcus—7.07 (6.06–7.35) | p < 0.05 | |||
| C. coccoides—9.00 (8.41–9.56) | C. coccoides—9.00 (8.23–9.79) | C. coccoides—9.03 (8.50–9.52) | p > 0.05 | |||
| C. leptum—9.17 (8.86–9.74) | C. leptum—8.42 (7.89–8.74) | C. leptum—8.88 (8.10–9.50) | p < 0.05 | |||
| Lactobacillus—6.68 (6.26–7.30) | Lactobacillus—6.39 (6.08–6.85) | Lactobacillus—6.34 (6.06–6.95) | p < 0.05 | |||
| E. coli—7.05 (6.20–7.64) | E. coli—6.40 (6.21–6.56) | E. coli—7.11 (6.50–8.01) | p < 0.05 | |||
| A. muciniphilia—7.01 (5.80–7.44) | A. muciniphilia—5.75 (4.96–7.40) | A. muciniphilia—7.00 (5.65–8.00) | p > 0.05 | |||
| duodenal biopsy: | duodenal biopsy: | duodenal biopsy: | ||||
| Bifidobacterium—6.15 (4.97–6.28) | Bifidobacterium—6.27 (6.03–6.80) | Bifidobacterium—5.95 (5.55–6.21) | p < 0.05 | |||
| Bacterioides—4.98 (3.98–5.00) | Bacteroides—3.28 (2.25–4.10) | Bacteroides—4.97 (4.03–5.20) | p < 0.05 | |||
| Staphylococcus—2.67 (2.12–3.00) | Staphylococcus—2.35 (1.25–2.77) | Staphylococcus—3.97 (3.44–4.06) | p < 0.05 | |||
| C. coccoides—3.70 (3.30–4.12) | C. coccoides—4.06 (3.70- 4.70) | C. coccoides—4.00 (3.65–4.25) | p > 0.05 | |||
| C. leptum—3.98 (3.23–4.15) | C. leptum—3.65 (3.05–4.52) | C. leptum—4.56 (4.42–4.70) | p < 0.05 | |||
| Lactobacillus—2.70 (2.58–3.46) | Lactobacillus—3.12 (2.74–4.14) | Lactobacillus—4.92 (4.16–5.25) | p < 0.05 | |||
| E. coli—3.18 | E. coli—3.04 | E. coli—4.23 (3.99–4.47) | p < 0.05 | |||
| A. muciniphila—N/A | A. muciniphila—2.78 (2.50–3.38) | A. muciniphila—2.95 (2.74—4.00) | p > 0.05 | |||
| Sanchez E. et al. [23] 1 | Duodenal biopsy | 16S rRNA sequencing | Enterobacteriaceae—4 | Enterobacteriaceae—0 | Enterobacteriaceae—22 | N/A |
| Actinobacteria—2 | Actinobacteria—4 | Actinobacteria—15 | ||||
| Staphylococcaceae—8 | Staphylococcaceae—2 | Staphylococcaceae—32 | ||||
| Streptococcaceae—58 | Streptococcaceae—58 | Streptococcaceae—59 | ||||
| Clostridiaceae—2 | Clostridiaceae—3 | Clostridiaceae—4 | ||||
| Lactobacillaceae—0 | Lactobacillaceae—2 | Lactobacillaceae—0 | ||||
| Enterococcaceae—0 | Enterococcaceae—0 | Enterococcaceae—2 | ||||
| Veillonellaceae—2 | Veillonellaceae—0 | Veillonellaceae—3 | ||||
| Carnobacteriaceae—4 4 | Carnobacteriaceae—1 4 | Carnobacteriaceae—2 4 | ||||
1 Cultivable cells (log cfu/g) of the main microbial groups in faecal samples. 2 Median (%). 3 Data are shown as medians and IQR of log of cell numbers per gram of samples. 4 Abundance is expressed as the absolute numbers of isolated clones belonging to one specific taxonomic group. 5 Medians of 16S rRNA gene copies per milligram of tissue. 6 Percentage of patients with bacteria detected. N/A—not applicable, CD—coeliac disease, GFD—gluten-free diet, CD on GFD—patients with CD on GFD, Healthy—healthy individuals without CD and other known food intolerance, UCD—untreated CD patients, FISH—Fluorescence in situ hybridization, SCFAs—Short Chain Fatty Acid Analysis, DGGE—Denaturing gradient gel electrophoresis, FCM—Fuzzy c-means.
4. Discussion
The impact of a GFD on the gut microbiome composition in CD patients has been proven by the obtained data. The approximation of the microbiome of CD patients to the composition of the microbiome of healthy people after excluding gluten from the diet has been found. However, the unequivocal trend of change and the resulting effects are difficult to define due to limited data.
Although it has not been thoroughly examined whether the transformation in human microbiome is either the cause or effect of CD, it certainly has an impact on inappropriate functioning of bowels and is related to the severity of clinical symptoms [29]. Microorganisms play a major role in the fermentation of indigestible food components into absorbable metabolites, the synthesis of essential vitamins, the removal of toxic compounds, the out competition of pathogens, the strengthening of the intestinal barrier and the stimulation and regulation of the immune system [32]. Gut microbiome structure more similar to healthy gut microbiome were observed in patients treated with a GFD in comparison to patients consuming gluten [14,17,19,23,24]. Distinctive mechanisms for immunomodulation by commensal microorganisms have been confirmed in the latest research. Short chain fatty acids (SCFA) that are produced by microbiota’s components affect Treg cells [33]. The abnormal butyrate production by microbiome is recognised as a cause of higher expression of non-functional form of FOXP3, which is associated with an enlarged risk of autoimmunity [34]. Disorders in T cell functions are successively underlined in the pathogenesis of CD [35,36]. T cell related production of antibodies against gluten peptides is an immune factor causing symptoms in CD. Mainly recognised antibodies that also have a main role in diagnosis are AGA and tGA. This result highlights that a reduction in gluten intake not only can alleviate clinical symptoms but can also impact the pathomechanism of disease.
The association between the microbiome composition in adult coeliac patients and the severity of clinical symptoms was demonstrated in study by Wacklin et al. [28]. Firmicutes and Bacteroides occurred to be significantly more abundant in the microbiome of asymptomatic patients, while Proteobacteria, Acinetobacter and Neisseria were more common among patients with gastrointestinal (GI) symptoms. Polled data on GFD indicated higher richness of Bacteroides [13,14,19,21,22] and Firmicutes [24] in treated patients’ samples compared to untreated individuals with CD. Furthermore, Neisseria [21] and Proteobacteria [24], which seems to be correlated with more severe symptoms, were less abundant among patients on GFD. The therapy’s potential to alleviate clinical symptoms of CD is presented by these facts.
Bifidobacterium, Lactobacillus and Bacteroides play out a significant role in being a part of intestinal microbiota. The Bifidobacterium genus, which belongs to Acinetobacter phylum, takes part in acetate synthesis and prevents E. coli colonisation as a commensal [29]. Bifidobacterium seems to be very beneficial in autoimmune GI diseases such as CD. Bifidobacterium strains have abilities to neutralise the toxicity of gliadin and alleviate mechanical damages in gut walls triggered by gluten [30]. Sjogren et al. [31] assessed that high abundance of Bifidobacterium species in the faecal samples corresponds significantly with the IgA level in the saliva of examined infants [31], which has a direct impact on increased protection against allergies and autoimmunity [37]. Nonetheless, the Bifidobacterium genus was less abundant in the study group of CD patients compared to the control groups regardless of the type of sample [13,19,20,21,22]. This fact may reveal that even long-term and strict adherence to the GFD may not be sufficient to completely restore the microbiome composition to show the similarity with a related control population. This result is partially consistent with the outcomes obtained by Sanz Y. et al. [38]. The Bacteroides genus turned out to be more abundant in the treated CD group than control groups [13,19,21,22], which seems to be beneficial as it is associated with host protection against pathogenic microbes and the delivery of nutrients for commensal microflora [39]. Lactobacillus bacteria are involved in many various functions in the human intestine, such as antibiotic production, organic acid production, bile deconjugation and carcinogen suppression [40]. Additionally, it was reported that Lactobacillus crispatus confers an anti-inflammatory phenotype to human dendritic cells, which is especially profitable in inflammatory diseases such as CD [41]. A statistically significant higher abundance of Lactobacillus in the study group than in the UCD group was described in two papers [22,25]. Furthermore, the proportion of Lactobacillus species plays an important role and its disturbance may go undetected with the preserved abundance [38]. Finally, time of intervention on a GFD might play an important role, which was already suggested by Garcia-Mazcorro JF et al. [42]. There were no significant differences between GI microbiome composition before and after GFD treatment over 4 weeks in a dietary intervention. Nevertheless, studies included in the current research can be characterised as long term if an intervention lasted at least 6 months. Unfortunately, detailed information on patients’ diets or on methods of controlling dietary compliance was provided by the authors. Only Panelli et al. [21] included information about using a five-level score to evaluate patients’ adherence to GFD. However, this scale is a subjective tool, while it is comprised of a dietary questionnaire. This is a serious obstacle to the conduct of reliable research, because strict adherence to GFD is required to obtain potential beneficial effects. Furthermore, difficulties with controlling a GFD in many CD patients are highlighted by researchers [43], which indicates the need for strict and objective control.
5. Limitations
This study has distinct limitations. Firstly, all papers included were cross-sectional. The impact on gut microbiome could evolve depending on time that elapsed since the implementation of the GFD, which requires a special caution during interpretation. Additionally, deficient data about clinical symptoms as well as serological and genetic profile did not provide a complete view of the patients. Another limitation was that there was no possibility to fully control the patients’ diets. Even though coeliac patients are well educated about GFD, there is never full assurance whether they consume (intentionally or not) even the minimum doses of gluten. Moreover, a lack of information about required time without any antibiotic interventions before being examined [13,15,18,24] or the short duration of this period [16,17,19,20,21,22,23,25] may raise doubts, as it is known that antibiotics have a significant and long-term impact on gut microbiome composition and functions [44]. Restrictions regarding the consumption of probiotics by the examined patients were introduced only in three studies [13,19,21]. Probiotics affect the composition and functioning of the microbiome in many ways [45], thus its intake should be controlled more strictly. In general, the diet quality was not evaluated in the selected studies, although it may directly influence the gut microbiota composition. Finally, different sequencing methods used in the single studies may have yielded different results and this may have impacted the overall analysis.
6. Conclusions
In conclusion, patients suffering from CD who follow a GFD correlates with the presence of a gut microbiome composition similar to healthy individuals. However, full restoration of commensal microorganism abundance in patients treated with GFD was not observed.
Author Contributions
All of the authors performed the literature search and the data extraction. P.H. and I.K. searched SCOPUS, S.K. and K.D. searched Web of Science, A.G. and M.S.-M. searched PUBMED. I.K., A.G., K.D., S.K. and P.H. created the tables and populated them with data from the articles. The manuscript was written by I.K. and K.D. Through each research stage, M.S.-M. supervised the process and promoted the work with substantive reinforcement. P.B. critically reviewed the manuscript. M.S.-M. and M.M. were responsible for the study design, primary draft correction and manuscript revision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The single study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Poznan University of Medical Science (20 March 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Detailed secondary data will be available after direct contact with stelmach@ump.edu.pl.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lohi S., Mustalahti K., Kaukinen K., Laurilla K., Collin P., Rissanen H., Lohi O., Bravi E., Gasparin M., Reunanen A., et al. Increasing prevalence of coeliac disease over time. Aliment. Pharmacol. Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 2.Caio G., Volta U., Sapone A., Leffler D.A., Giorgio R., Catassi C., Fasano A. Celiac disease: A comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trynka G., Wijmenga C., Heel D.A. A genetic perspective on coeliac disease. Trends Mol. Med. 2010;16:537–550. doi: 10.1016/j.molmed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Tapia A., Hill I.D., Kelly C.P., Calderwood A.H., Murray J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013;108:656–676. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardella M.T., Velio P., Cesana B.M., Prampolini L., Casella G., Bella C., Lanzini A., Gambarotti M., Bassotti G., Villanacci V. Coeliac disease: A histological follow-up study. Histopathology. 2007;50:465–471. doi: 10.1111/j.1365-2559.2007.02621.x. [DOI] [PubMed] [Google Scholar]
- 6.Wahab P.J., Meijer J.W.R., Mulder C.J.J. Histologic Follow-up of People with Celiac Disease on a Gluten-Free Diet. Am. J. Clin. Pathol. 2002;118:459–463. doi: 10.1309/EVXT-851X-WHLC-RLX9. [DOI] [PubMed] [Google Scholar]
- 7.Tursi A., Brandimarte G., Giorgetti G., Elisei W., Inchingolo C., Monardo E., Aiello F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: A 2-year prospective study. Endoscopy. 2006;38:702–707. doi: 10.1055/s-2006-925178. [DOI] [PubMed] [Google Scholar]
- 8.Kavak U.S., Yüce A., Koçak N., Demir H., Saltik İ.N., Gürakan F., Özen H. Bone Mineral Density in Children with Untreated and Treated Celiac Disease. J. Pediatric Gastroenterol. Nutr. 2003;37:434–436. doi: 10.1097/00005176-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Saadah O.I., Zacharin M., O’Callaghan A., Oliver M.R., Catto-Smith A. Effect of gluten-free diet and adherence on growth and diabetic control in diabetics with coeliac disease. Arch. Dis. Child. 2004;89:871–876. doi: 10.1136/adc.2002.012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zylberberg H.M., Demmer R.T., Murray J.A., Green P.H., Lebwohl B. Depression and insomnia among individuals with celiac disease or on a gluten-free diet in the USA. Eur. J. Gastroenterol. Hepatol. 2017;29:1091–1096. doi: 10.1097/MEG.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Palma G., Cinova J., Stepankova R., Tuckova L., Sanz Y. Pivotal Advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J. Leukoc. Biol. 2010;87:765–778. doi: 10.1189/jlb.0709471. [DOI] [PubMed] [Google Scholar]
- 12.Akobeng A.K., Singh P., Kumar M., Khodor S. Role of the gut microbiota in the pathogenesis of coeliac disease and potential therapeutic implications. Eur J Nutr. 2020;59:3369–3390. doi: 10.1007/s00394-020-02324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Cagno R., De Angelis M., De Pasquale I., Ndagijimana M., Vernocchi P., Ricciuti P., Gagliardi F., Laghi L., Crecchio C., Guerzoni M.E., et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011;11:219. doi: 10.1186/1471-2180-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schippa S., Iebba V., Barbato M., Di Nardo G., Totino V., Checchi M.P., Longhi C., Maiella G., Cucchiara S., Conte M.P. A distinctive ‘microbial signature’ in celiac pediatric patients. BMC Microbiol. 2010;10:175. doi: 10.1186/1471-2180-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirjo W., Pilvi L., Katri L., Pekka C. Altered Duodenal Microbiota Composition in Celiac Disease Patients Suffering From Persistent Symptoms on a Long-Term Gluten-Free Diet. Am. J. Gastroenterol. 2014;109:1933–1941. doi: 10.1038/ajg.2014.355. [DOI] [PubMed] [Google Scholar]
- 16.Nadal I., Donant E., Ribes-Koninckx C., Calabuig M., Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J. Med. Microbiol. 2007;56:1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- 17.De Palma G., Nadal I., Medina M., Donat E., Ribes-Koninckx C., Calabuig M., Sanz Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. doi: 10.1186/1471-2180-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalliomäki M., Satokari R., Lähteenoja H., Vähämiko S., Grönlund J., Routi T., Salminen S. Expression of microbiota, Toll-like receptors, and their regulators in the small intestinal mucosa in celiac disease. Pediatr. Gastroenterol. Nutr. 2012;54:727–732. doi: 10.1097/MPG.0b013e318241cfa8. [DOI] [PubMed] [Google Scholar]
- 19.Di Cagno R., Rizzello C.G., Gagliardi F., Ricciuti P., Ndagijimana M., Francavilla R., Guerzoni M.E., Crecchio C., Gobbetti M., De Angelis M. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 2009;75:3963–3971. doi: 10.1128/AEM.02793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nistal E., Caminero A., Vivas S., Ruiz de Morales J.M., Sáenz de Miera L.E., Rodríguez-Aparicio L.B., Casqueiro J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie. 2012;94:1724–1729. doi: 10.1016/j.biochi.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Panelli S., Capelli E., Lupo G.F.D., Schiepatti A., Betti E., Sauta E., Marini S., Bellazzi R., Vanoli A., Pasi A., et al. Comparative Study of Salivary, Duodenal, and Fecal Microbiota Composition Across Adult Celiac Disease. J. Clin. Med. 2020;9:1109. doi: 10.3390/jcm9041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collado M.C., Donat E., Ribes-Koninckx C., Calabuig M., Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2008;62:264–269. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez E., Donat E., Ribes-Koninckx C., Fernández-Murga M.L., Sanz Y. Duodenal-mucosal bacteria associated with celiac disease in children. Appl. Environ. Microbiol. 2013;79:5472–5479. doi: 10.1128/AEM.00869-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serena G., Davies C., Cetinbas M., Sadreyev I.R., Fasano A. Analysis of blood and fecal microbiome profile in patients with celiac disease. Hum. Microbiome J. 2019;11:100049. doi: 10.1016/j.humic.2018.12.001. [DOI] [Google Scholar]
- 25.Lorenzo Pisarello M.J., Vintiñi E.O., González S.N., Pagani F., Medina M.S. Decrease in lactobacilli in the intestinal microbiota of celiac children with a gluten-free diet, and selection of potentially probiotic strains. Can. J. Microbiol. 2015;61:32–37. doi: 10.1139/cjm-2014-0472. [DOI] [PubMed] [Google Scholar]
- 26.Golfetto L., de Senna F.D., Hermes J., Beserra B.T., França Fda S., Martinello F. Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq. Gastroenterol. 2014;51:139–143. doi: 10.1590/S0004-28032014000200013. [DOI] [PubMed] [Google Scholar]
- 27.Zafeiropoulou K., Nichols B., Mackinder M., Biskou O., Rizou E., Karanikolou A., Clark C., Buchanan E., Cardigan T., Duncan H., et al. Alterations in Intestinal Microbiota of Children With Celiac Disease at the Time of Diagnosis and on a Gluten-free Diet. Gastroenterology. 2020;159:2039–2051.e20. doi: 10.1053/j.gastro.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wacklin P., Kaukinen K., Tuovinen E., Collin P., Lindfors K., Partanen J., Mäki M., Mättö J. The Duodenal Microbiota Composition of Adult Celiac Disease Patients Is Associated with the Clinical Manifestation of the Disease. Inflamm. Bowel Dis. 2013;19:934–941. doi: 10.1097/MIB.0b013e31828029a9. [DOI] [PubMed] [Google Scholar]
- 29.Valitutti F., Cucchiara S., Fasano A. Celiac Disease and the Microbiome. Nutrients. 2019;11:2403. doi: 10.3390/nu11102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindfors K., Blomqvist T., Juuti-Uusitalo K., Stenman S., Venäläinen J., Mäki M., Kaukinen K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 2008;152:552–558. doi: 10.1111/j.1365-2249.2008.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjögren Y.M., Tomicic S., Lundberg A., Böttcher M.F., Björkstén B., Sverremark-Ekström E., Jenmalm M.C. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy. 2009;39:1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 32.Heintz-Buschart A., Wilmes P. Human Gut Microbiome: MFunction Matters. Trends Microbiol. 2018;26:563–574. doi: 10.1016/j.tim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canova C., Zabeo V., Pitter G., Romor P., Baldovin T., Zanotti R., Simonato L. Association of maternal education, early infections, and antibiotic use with celiac disease: A population-based birth cohort study in northeastern Italy. Am. J. Epidemiol. 2014;180:76–85. doi: 10.1093/aje/kwu101. [DOI] [PubMed] [Google Scholar]
- 35.Sollid L. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 36.Casellas L.R.F., Rodrigo L., Vivancos S.R.P., Riestra S., Pantiga C., Baudet J.S., Junquera F., Diví V.P., Abadia C., Papo M., et al. Factors that impact health-related quality of life in adults with celiac disease: A multicenter study. World J. Gastroenterol. 2008;14:46–52. doi: 10.3748/wjg.14.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Böttcher M.F., Häggström P., Björkstén B., Jenmalm M.C. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin. Exp. Allergy. 2002;32:1293–1298. doi: 10.1046/j.1365-2222.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 38.Sanz Y., Sanchez E., Marzotto M., Calabuig M., Torriani S., Dellaglio F. Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol. Med. Microbiol. 2007;51:562–568. doi: 10.1111/j.1574-695X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 39.Zafar H., Saier M.H. Gut Bacteroides species in health and disease. Gut Microbes. 2021;13:1848158. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandine W. Roles of Lactobacillus in the Intestinal Tract. J. Food Prot. 1979;42:259–262. doi: 10.4315/0362-028X-42.3.259. [DOI] [PubMed] [Google Scholar]
- 41.Eslami S., Hadjati J., Motevaseli E., Mirzaei R., Bonab S.F., Ansaripour B., Khoramizadeh M.R. Lactobacillus crispatus strain SJ-3C-US induces human dendritic cells (DCs) maturation and confers an anti-inflammatory phenotype to DCs. APMIS. 2016;124:697–710. doi: 10.1111/apm.12556. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Mazcorro J.F., Rivera-Gutierrez X., Cobos-Quevedo O.J., Grube-Pagola P., Meixueiro-Daza A., Hernandez-Flores K., Cabrera-Jorge F.J., Vivanco-Cid H., Dowd S.E., Remes-Troche J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients. 2018;10:1641. doi: 10.3390/nu10111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wieser H., Ruiz-Carnicer Á., Segura V., Comino I., Sousa C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients. 2021;13:2274. doi: 10.3390/nu13072274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francino M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016;6:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J.M., Dequenne I., de Timary P., Cani P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Detailed secondary data will be available after direct contact with stelmach@ump.edu.pl.