Abstract
Maintenance of pathogenicity of viable but nonculturable Salmonella typhimurium cells experimentally stressed with UV-C and seawater, was investigated relative to the viability level of the cellular population. Pathogenicity, tested in a mouse model, was lost concomitantly with culturability, whereas cell viability remained undamaged, as determined by respiratory activity and cytoplasmic membrane and genomic integrities.
In aquatic environments, most of the human pathogenic bacteria are known to enter the viable but nonculturable (VBNC) state in response to adverse environmental conditions (8, 16). However, there is some uncertainty regarding the health risk posed by such VBNC forms (17). Some authors have demonstrated pathogenic effects caused by VBNC cells (3, 4, 6), whereas others have claimed the concomitant losses of culturability and pathogenicity, independently of the level of viability (9). This uncertainty calls into question the use of traditional microbiological media to monitor the quality of water.
In this preliminary study, we investigated the maintenance of pathogenicity of Salmonella typhimurium experimentally stressed by exposure to UV-C in seawater. The pathogenicity of these culturable or nonculturable cells, following characterization of their viability level, was tested by intraperitoneal injection into mice. UV-C was chosen because it permitted us to attain variable levels of cell damage by altering the exposure time. All experiments were conducted in seawater in order to apply an additional stress factor. UV-C, which is used in some water and wastewater treatment plants, and salinity may contribute to the release of VBNC cells into the natural aquatic environment. As the purpose of this study was not to investigate the individual roles of these particular stress factors, we did not attempt to characterize separately their effects on cell damage.
Bacterial strain.
S. typhimurium C52, an isogenic variant of the wild-type strain, C5, was isolated from the parent strain after 11 subcultures at 45°C (10). Strain C52, which carries the 90-kb virulence-associated plasmid plPl350, causes a systemic infection (murine typhoid). S. typhimurium C52 was grown in Trypticase soy broth (bioMérieux) at 37°C for 18 h, centrifuged (4,000 × g for 10 min), washed three times, and resuspended in NaCl solution (salinity, 9‰).
Physiological cellular tests.
Culturable cells were enumerated on Trypticase soy agar (bioMérieux) following 10-fold dilution in NaCl solution (9‰) for high concentrations or following membrane filtration for low concentrations. Total counts and measures of cellular physiological states were determined with an Olympus Provis epifluorescence microscope coupled with an image analysis system (IDES, Toulouse, France) (1, 5). Respiring bacteria, assessed by CTC (5-cyano-2,3-ditolyl tetrazolium chloride; Polysciences Europe, Eppelheim, Germany), were enumerated according to the method described by Rodriguez et al. (15). Direct viable counts (DVC) were obtained by using the division-inhibiting antibiotic mixtures described by Kogure et al. (7). The percentage of metabolic activity of the cellular population was calculated as the degree of cell elongation postexposure divided by the degree of elongation preexposure and multiplied by 100. Elongation was calculated as the average length of cells following elongation (DVC method) minus the average length of cells prior to elongation. Both the DVC and CTC procedures were modified according to the methods described by Baleux et al. (2). For DVC and CTC tests, total cells were enumerated after staining with DAPI (4′,6-diamidino-2-phenylindole; Sigma) as described by Porter and Feig (12). The structural integrity of the bacterial cytoplasmic membrane was evaluated with the Live/Dead Baclight bacterial viability kit (LD) provided by Molecular Probes, Inc. (2). The genomic integrity of the cells, stained with DAPI as described above, was evaluated based on estimates of the levels of fluorescence emitted by the DAPI-DNA complex (DDF) (2). Genomic integrity was calculated as the ratio of the level of fluorescence of cells after exposure to stress factors versus the level prior to exposure.
Experimental protocol.
Pyrex beakers (1 liter) acted as microcosms. Each contained 300 ml of sterilized artificial seawater (salinity, 37‰ [sea salt; Sigma]). The bacterial cells were suspended in sterilized artificial seawater at a concentration of ∼107 total bacteria ml−1. The maximum thickness of cellular suspensions to be penetrated by UV-C in the microcosms was 4 cm. The experimental UV-C apparatus for exposure of the cellular suspensions consisted of four germicidal lamps (8 mW s−1 cm−2) (linear disposition) situated 30 cm above the surface of the microcosms. The UV-C dose received by the cells was measured with an IL 1400A radiometer (International Light), with a submersible detector placed at the bottom of each microcosm. Several distinct cellular populations were exposed from one to three times to UV-C in artificial seawater, at times ranging from 30 s to 24 h, to obtain cellular populations presenting a wide range of culturability and viability levels. Each cellular population was exposed for a single length of time. Following exposure to UV-C, the cellular suspensions in artificial seawater were diluted in sterile water to suppress the effect of salinity (dilution ratio, 1:4). These diluted cellular suspensions (∼2.106 total cells/ml) were used to monitor culturability and viability levels in the physiological cellular tests described above.
Pathogenicity experiments.
The exposed cellular suspensions described above were tested for pathogenicity and virulence with a mouse model (C57BL/6j/Rj). Pathogenicity was defined by the mortality rate of infected mice. Virulence was represented by the time, in days, between infection and death (survival time). Mice were infected by intraperitoneal injection with approximately 103 total bacterial cells in 100 μl. All inoculated mice were observed for a period of 28 days. The spleens and livers of dead mice were removed for the detection of culturable Salmonella. In this study, preliminary experiments showed that the minimum number of culturable (i.e., fully pathogenic and virulent) cells necessary to cause a mortality rate of 100% in mice (with a mean survival time of 4.2 days) was as few as nine.
The positive control consisted of mice injected with a similar concentration of unstressed S. typhimurium cells (∼103 total bacterial cells/100 μl injected). The levels of culturability and viability of these unstressed cellular populations were close to 100%, and their injection into 45 mice resulted in a 100% mortality rate with a mean survival time of 4.3 days (range, 3 to 7 days).
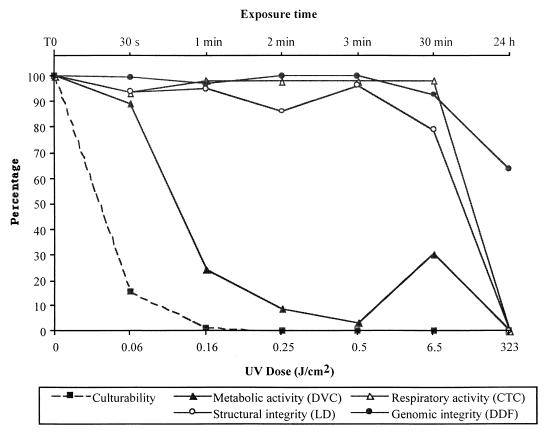
A total of 14 trials were conducted for the six exposure periods (from 30 s to 24 h). Levels of culturability and viability are presented in Table 1. Replicate samples showed a high level of agreement for each parameter and each time period, suggesting that all the physiological tests used in this study are reproducible and reliable. The greatest variability was observed for measurements of metabolic activity. This is probably due to the difficulty in discriminating between elongated and nonelongated cells. The mean values of culturability and viability for each exposure period are plotted in Fig. 1. This figure illustrates that the exposure of Salmonella cells to UV-C and seawater led to a progressive alteration of the cellular physiology. This alteration was characterized by a rapid loss of culturability and metabolic activity, followed by reduction in respiratory activity and structural integrity, and finally by a slight degree of damage to genomic integrity.
TABLE 1.
Percentages of culturability, metabolic and respiratory activities, and structural and genomic integrities of distinct cellular S. typhimurium populations exposed (in repeated and independent experiments) to UV-C in seawater
| Microcosm | Exposure time | UV-C dose (J/cm2) | Culturability (%) | Metabolic activity (%) | Respiratory activity (%) | Structural integrity (%) | Genomic integrity (%) |
|---|---|---|---|---|---|---|---|
| M1 | 30 s | 0.06 | 13.4 | 100 | 96.2 | 95.5 | 100 |
| M2 | 30 s | 0.05 | 16.8 | 100 | 94.4 | 93.4 | 100 |
| M3 | 30 s | 0.06 | 15.3 | 68.5 | 90.3 | 93.1 | 98.6 |
| M4 | 1 min | 0.25 | 2 | 21.3 | 98.9 | 96.3 | 100 |
| M5 | 1 min | 0.12 | 0.9 | 32.6 | 98.1 | 93.1 | 100 |
| M6 | 1 min | 0.11 | 1 | 19.1 | 97.8 | 96.2 | 91.6 |
| M7 | 2 min | 0.25 | 2.4 × 10−4 | 8.9 | 97.8 | 86.1 | 100 |
| M8 | 3 min | 0.7 | 3.5 × 10−3 | 96.6 | 95 | 100 | |
| M9 | 3 min | 0.4 | 7 × 10−4 | 1.1 | 97.3 | 96.7 | 100 |
| M10 | 3 min | 0.4 | 2.4 × 10−4 | 5.6 | 100 | 96.8 | 100 |
| M11 | 30 min | 6.7 | 0 | 32.6 | 98.5 | 77.7 | 85.5 |
| M12 | 30 min | 6.4 | 1.1 × 10−3 | 28.1 | 98.1 | 80.1 | 100 |
| M13 | 24 h | 319 | 0 | 0 | 0 | 0 | 55.6 |
| M14 | 24 h | 327 | 0 | 0 | 0 | 0 | 71.4 |
FIG. 1.
Loss of culturability and physiological deterioration (as determined by DVC, CTC, LD, and DDF methods) of S. typhimurium according to the time of exposure to UV-C in seawater.
Results of the mouse infectivity experiments are shown in Table 2. After 30 s of exposure to UV-C in seawater, the culturability averaged 15.2%, while each of the measures of cellular viability remained above 90%. The mortality rate of mice injected with these cells was equal to that of mice injected with unstressed cells (100%). After 1 min of exposure, culturability decreased to 1.3% and metabolic activity decreased to 24.3%, while the other parameters remained unchanged. Even though the numbers of culturable cells injected, 41.7, 18.7, and 19.4 for M4, M5, and M6, respectively, were above the minimum number for 100% mortality (only 9 culturable unstressed cells required), the mortality rate decreased to 87% and the mean survival time increased to 9.5 days. Moreover, four mice survived the entire 28-day observation period. This suggests that cells which remain culturable following experimental stress may in fact exhibit reduced pathogenicity and virulence.
TABLE 2.
Effects of intraperitoneal injections (100 μl) into groups of mice of distinct cellular S. typhimurium populations exposed (in repeated and independent experiments) to UV-C in seawater
| Microcosm | Exposure time | Total no. of cells injected/100 μl | No. of culturable cells injected/100 μl | No. of mice (dead/injected) | Mortality rate (%) | Survival time (minimum, mean, maximum)a | No. of positive cultures (mixed spleen and liver) |
|---|---|---|---|---|---|---|---|
| M1 | 30 s | 1,870 ± 180 | 250 | 10/10 | 100 | 3, 4, 5 | 10/10 |
| M2 | 30 s | 1,990 ± 210 | 335 | 10/10 | 100 | 4, 4.2, 5 | 10/10 |
| M3 | 30 s | 2,090 ± 12 | 320 | 10/10 | 100 | 4, 7, 14 | 10/10 |
| M4 | 1 min | 2,090 ± 220 | 41.7 | 9/10 | 90 | 4, 7.5, 12 | 9/10 |
| M5 | 1 min | 2,100 ± 180 | 18.7 | 9/10 | 90 | 3, 9.5, 25 | 9/10 |
| M6 | 1 min | 1,910 ± 28 | 19.4 | 8/10 | 80 | 4, 11.5, 22 | 8/10 |
| M7 | 2 min | 1,950 ± 177 | 0.0047 | 0/20 | 0 | 28 | 0/20 |
| M8 | 3 min | 840 ± 231 | 0.0293 | 0/10 | 0 | 28 | 0/10 |
| M9 | 3 min | 1,180 ± 110 | 0.0083 | 0/10 | 0 | 28 | 0/10 |
| M10 | 3 min | 1,690 ± 190 | 0.004 | 0/10 | 0 | 28 | 0/10 |
| M11 | 30 min | 1,200 ± 430 | 0 | 0/10 | 0 | 28 | 0/10 |
| M12 | 30 min | 518 ± 42 | 0.0057 | 0/10 | 0 | 28 | 0/10 |
| M13 | 24 h | 1,250 ± 450 | 0 | 0/10 | 0 | 28 | 0/10 |
| M14 | 24 h | 3,610 ± 700 | 0 | 0/10 | 0 | 28 | 0/10 |
Survival time is shown in days.
After an exposure time of 2 min, virtually all cells were nonculturable (0.0047 culturable cells out of 1,950 total cells injected). All 20 mice survived the 28-day period. Similarly, all mice injected with cells exposed for a longer time period (3 min, 30 min, and 24 h) also survived (100% survival).
Correlation analysis (Pearson coefficient) revealed that the mortality rate was closely correlated with the number of culturable cells injected (r = 0.73, P = 0.0031). Metabolic activity (measured by DVC), which may be considered a microtest of culturability with prevention of cellular division, was also correlated with mortality (r = 0.66, P = 0.0098). None of the other cellular parameters used to monitor cellular viability (CTC, LD result, and DDF) were correlated with mortality in this study. Nevertheless, the maintenance of some physiological functions may suggest that these cells could be resuscitated (i.e., have the potential for reversibility to a culturable state) under appropriate conditions.
Using several reliable methodologies and fluorescent dyes to precisely characterize the viability of a cellular population, we demonstrated that, with these particular stress factors, pathogenicity was lost concomitantly with the ability of the cells to multiply (culturability), whereas the cellular viability according to respiratory activity and cytoplasmic membrane and genome integrities remained undamaged. As the decline of metabolic activity was comparable with the decline of culturability, we could not test the significance, in terms of pathogenicity, of nonculturable but metabolically active (as measured by DVC) cells. But the maintenance of physiological functions such as respiratory activity or the absence of structural damage for nonculturable cells seemed to be independent of the ability of these cells to cause an infection in a mouse model.
The results obtained in this study, with these particular stress factors, demonstrated that S. typhimurium in the VBNC state was not pathogenic. The physiological characteristic most associated with pathogenicity was culturability, regardless of the level of cellular viability. The simultaneous losses of culturability and pathogenicity have also been observed in Campylobacter jejuni, despite a high level of viability in the cellular population tested (9). However, some studies have demonstrated that VBNC cells retain some pathogenic effects (11, 13, 14) and it is suspected that the cells reverse into a culturable state (4, 18). We did not attempt to resuscitate nonculturable cells in this study. Consequently, we cannot say whether the loss of pathogenicity was permanent or temporary. Further studies should further characterize cellular viability and investigate whether VBNC cells can be resuscitated and regain their pathogenicity and virulence. This study examined the simultaneous effects of two experimental factors, UV-C and artificial seawater. Further studies are needed which examine the fate of stressed bacterial cells under environmental conditions such as exposure to sunlight and natural oligotrophic seawater.
Acknowledgments
This study was supported by a PNOC (Programme National D’Océanographie Cotière) grant.
We are grateful to Thomas Handzel for his assistance with the preparation of the manuscript.
REFERENCES
- 1.Baleux B, Got P. Apport de l’observation microscopique couplée à l’analyse d’images dans l’évaluation de la qualité bactériologique des eaux: approche cellulaire globale. Techniques Sci Methodes. 1996;6:430–436. [Google Scholar]
- 2.Baleux B, Caro A, Got P, Lesne J, Binard S, Delpuech B. Survie et maintien de la virulence de Salmonella Typhimurium VNC exposée simultanément à trois facteurs stressants expérimentaux. Oceanol Acta. 1998;6:939–950. [Google Scholar]
- 3.Colwell R R, Brayton P R, Grimes D J, Roszak D B, Huq S A, Palmer L M. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology. 1985;3:817–820. [Google Scholar]
- 4.Colwell R R, Brayton P, Herrington D, Tall B, Huq A, Levine M M. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J Microbiol Biotechnol. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- 5.Got P, Baleux B, Trousselier M. Dénombrements directs des bactéries des milieux aquatiques par microscopie en épifluorescence: comparaison entre un systéme d’analyse d’image automatisé (Mudicam) et l’observation visuelle. Rev Sci Eau. 1993;6:269–284. [Google Scholar]
- 6.Grimes D J, Colwell R R. Viability and virulence of Escherichia coli suspended by membrane chamber in semi-tropical ocean water. FEMS Microbiol Lett. 1986;34:161–165. [Google Scholar]
- 7.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 8.McKay A M. Viable but non-culturable forms of potentially pathogenic bacteria in water. Lett Appl Bacteriol. 1992;14:129–135. [Google Scholar]
- 9.Medema G J, Schets F M, van de Giessen A W, Havelaar A H. Lack of colonization of 1 day old chicks by viable, non-culturable Campylobacter jejuni. J Appl Bacteriol. 1992;72:512–516. doi: 10.1111/j.1365-2672.1992.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 10.Pardon P, Popoff M Y, Coynault C, Marly J, Miras I. Virulence-associated plasmids of Salmonella serotype typhimurium in experimental murine infection. Ann Inst Pasteur/Microbiol (Paris) 1986;137B:47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- 11.Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell R R, Cormier M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–949. [Google Scholar]
- 13.Rahman I, Shahamat M, Kirchman P A, Russek-Cohen E, Colwell R R. Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1994;60:3573–3578. doi: 10.1128/aem.60.10.3573-3578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman I, Shahamat M, Chowdhury M A R, Colwell R R. Potential virulence of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1996;62:115–120. doi: 10.1128/aem.62.1.115-120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez G G, Phipps D, Ishiguro K, Ridgway H F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roszak D B, Colwell R R. Metabolic activity of bacterial cells enumerated by direct viable count. Appl Environ Microbiol. 1987;53:2889–2893. doi: 10.1128/aem.53.12.2889-2893.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinert M, Emödy L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]