Abstract
Phosphatidylserine (PS) translocation to the external membrane leaflet represents a key mechanism in the pathophysiology of human erythrocytes (RBC) acting as an “eat me” signal for the removal of aged/stressed cells. Loss of physiological membrane asymmetry, however, can lead to adverse effects on the cardiovascular system, activating a prothrombotic activity. The data presented indicate that structurally related olive oil phenols prevent cell alterations induced in intact human RBC exposed to HgCl2 (5–40 µM) or Ca2+ ionophore (5 µM), as measured by hallmarks including PS exposure, reactive oxygen species generation, glutathione depletion and microvesicles formation. The protective effect is observed in a concentration range of 1–30 µM, hydroxytyrosol being the most effective; its in vivo metabolite homovanillic alcohol still retains the biological activity of its dietary precursor. Significant protection is also exerted by tyrosol, in spite of its weak scavenging activity, indicating that additional mechanisms are involved in the protective effect. When RBC alterations are mediated by an increase in intracellular calcium, the protective effect is observed at higher concentrations, indicating that the selected phenols mainly act on Ca2+-independent mechanisms, identified as protection of glutathione depletion. Our findings strengthen the nutritional relevance of olive oil bioactive compounds in the claimed health-promoting effects of the Mediterranean Diet.
Keywords: calcium, endothelium, erythrocytes, glutathione, hydroxytyrosol, mercury, olive oil, oxidative stress, phosphatidylserine, tyrosol
1. Introduction
Erythrocytes (RBC) are highly deformable cells that can rapidly change their shape to fit through the blood vessels in the body. Moreover, their peculiar morphology of biconcave disc by increasing the surface/volume ratio facilitates gas exchanges, the main function of these highly specialized cells [1]. Besides the physiological role of oxygen and CO2 transport, these cells exert additional non-canonical functions, including regulation of nitric oxide (NO) bioavailability. Indeed, these cells express the eNOS isoform and show the ability to biosynthesize NO under oxygen deprivation and store it bound to specific cysteine residues of hemoglobin, contributing to cardiovascular homeostasis [2,3,4,5,6]. In mammals, mature cells come from a finally regulated process called erythropoiesis, involving loss of cell organelles. In addition, at the end of maturation, erythroblasts expel their nuclei, giving rise to reticulocytes released in the bloodstream and pyrenocytes, rapidly eliminated by macrophages, where phosphatidylserine (PS) exposure acts as an “eat me” signal [7,8]. Late enucleation of erythroblasts defines a starting point of an average aging process of 120 days until their elimination from circulation through recognition and phagocytosis mediated by tissue macrophages within the reticuloendothelial system in the spleen, liver (Kupffer cells), and bone marrow. Interestingly, this process is also associated with exposure of PS to the outer membrane leaflet. Thus, losing the physiological membrane asymmetric PS distribution turns out to be a key initiation signal in the erythrophagocytosis of aged RBC, thereby regulating the lifespan of circulating mature cells as well as the removal of damaged RBC in pathological situations [9,10,11]. In this respect, this membrane alteration is regarded as one of the mechanisms underlying the onset of anemia associated with chemotherapeutic treatments [12,13] as well as a broad range of diseases [14,15]. As a matter of fact, PS translocation to the cell surface represents a key hallmark of erythrocyte programmed cell death, also called eryptosis [16,17,18,19]. Similarly to apoptosis of nucleated cells, RBC can enter a programmed suicidal death that allows cell degradation without producing toxic by-products, preventing the release of harmful cellular components such as hemoglobin, heme and free iron that can favor plaque formation [20]. This specific membrane alteration, however, can lead to adverse effects on the cardiovascular system, by providing a binding site for prothrombinase complex as well as by inducing unusual adhesion between RBC with both endothelial cells and other cells in the bloodstream, thus resulting in clot formation and microvascular occlusion [21,22,23]. Increased microvesicles (MVs) formation has also recently been recognized as a contributing factor to thrombotic events [24,25].
Eryptosis is triggered by oxidative stress (OS) [26] as well as endogenous and exogenous substances, such as lysophosphatidic acid and heavy metal, respectively [27,28]. In this respect, our data and data in the literature show that RBC are particularly sensitive to mercury (Hg), acting as a preferential site for its accumulation [29,30]. In particular, Hg mainly binds to sulfhydryl groups of cellular thiols, mainly glutathione (GSH), thus impairing the endogenous defense system [31]. In addition, Hg rapidly reacts with accessible cysteine residues of both membrane as cytosolic proteins, resulting in severe metabolic and morphological alterations [30]. Interestingly, beside the well-known neuro and nephro toxic effects, this highly toxic wide-spread pollutant [32] can significantly affect cardiovascular health [33,34]. RBC have been proposed as contributing to endothelial dysfunction and thrombotic events [35].
Based on these observations, it would therefore be interesting to screen compounds able to reduce PS-related membrane modification. In this respect, recent data from our group showed that hydroxytyrosol (3,4-diidroxyphenylethanol, HT), an antioxidant phenol present in high concentrations in virgin olive oil, has the potential to inhibit PS exposure in human RBC, induced by different stressors, including Hg [36]. The aim of this study was to further evaluate the possible protective effects played by natural phenolic compounds in human RBC, particularly related to metabolic as well as morphological alterations associated with increased prothrombotic activity of these cells. In the search for an effective tool to counteract this damage, we performed a comparative analysis of the effects of HT and its natural olive monophenolic analogue tyrosol (Tyr) [37], as well as its in vivo metabolite homovanillic alcohol (HA) [38,39]. Intact human RBC have been subjected in vitro to treatment with mercuric chloride (HgCl2) or Ca2+-ionophore. It is well known, indeed, that alterations in calcium homeostasis are associated with Hg toxicity, and increased intracellular Ca2+ induces PS exposure [35]. The protective effect of the selected phenols and the underlying mechanism(s) were evaluated by measuring several hallmarks including PS exposure, reactive oxygen species (ROS) generation, GSH depletion and MVs formation.
2. Results
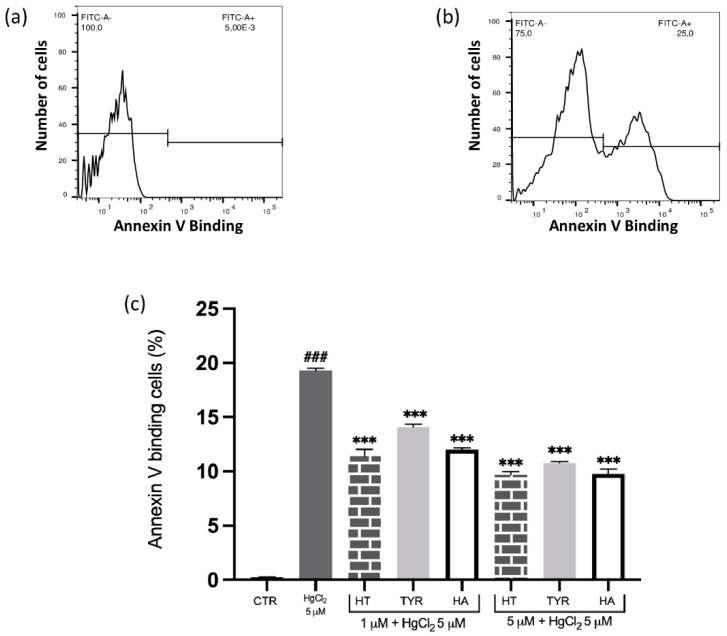
To explore the ability of olive oil bioactive components in preventing Hg-induced cytotoxicity in human RBC and the underlying mechanism(s), several phenolic compounds, endowed with different antioxidant activities, were selected. Among them, HT and its in vivo metabolite HA show effective and comparable scavenging properties [39,40,41,42], while Tyr, which lacks the o-diphenolic structure, is characterized by a weak activity [43,44]. Intact RBC were exposed in vitro to HgCl2 and several hallmarks were evaluated, including PS translocation at the cell surface; PS-exposing RBC were identified utilizing annexin-V-binding, as determined by flow cytometry. As reported in Figure 1, and according to our previous findings, cell exposure to the heavy metal induces an increase of PS exposure. Under the same experimental conditions, treatment with all selected phenols results in a decrease of annexin-V-binding RBC in a dose-dependent manner, a significant protection occurring at a concentration as low as 1 µM. Still, Tyr appears to be less effective than HT and HA (Figure 1).
Figure 1.
Effect of HT and its analogue on Hg-induced PS exposure in RBC. Cells were treated with HgCl2 in the presence of increasing concentrations of HT, Tyr and HA and PS exposure was evaluated as annexin-V-binding cells. (a) Original histogram of control cells (CTR). (b) Original histogram of Hg-treated cells. (c) Histogram of phenols effect on Hg treatment. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates a significant difference from CTR. *** (p < 0.001) indicates a significant difference from Hg treatment. PS: phosphatidylserine; RBC: red blood cell, HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol.
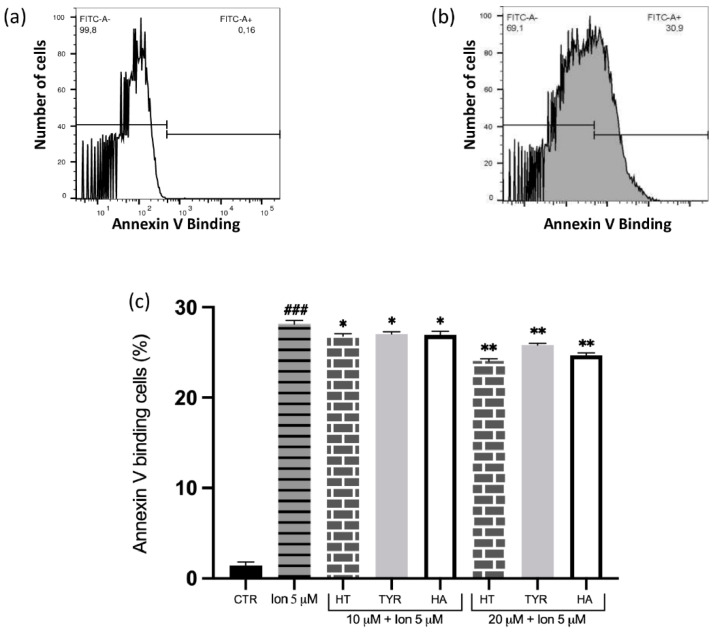
It is known that Hg toxicity is associated with alteration in calcium homeostasis and that increased intracellular Ca2+ induces PS exposure. We therefore investigated whether the tested phenolic compounds had a similar protective effect on Ca2+-induced PS exposure. As expected, cell incubation with 5 μM Ca2+ ionophore in the presence of 1 mM extracellular calcium significantly increases the percentage of annexin-V-binding RBC (Figure 2). Also in this case, in RBC treated with micromolar concentrations of the phenolic compounds, a significant reduction of PS exposing cells are observed, even though higher concentrations are required (10–20 µM).
Figure 2.
Effect of HT and its analogue on Ca2+-induced PS exposure in RBC. Cells were treated with Ca2+ ionophore in the presence of increasing concentrations of HT, Tyr and HA and PS exposure was evaluated as annexin-V-binding cells. (a) Original histogram of control cells (CTR). (b) Original histogram of Ca2+ ionophore-treated cells. (c) Histogram of phenols effect on Ca2+ ionophore treatment. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates a significant difference from CTR. ** (p < 0.01) and * (p < 0.05) indicates a significant difference from Ca2+ ionophore treatment. PS: phosphatidylserine; RBC: red blood cell, HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol.
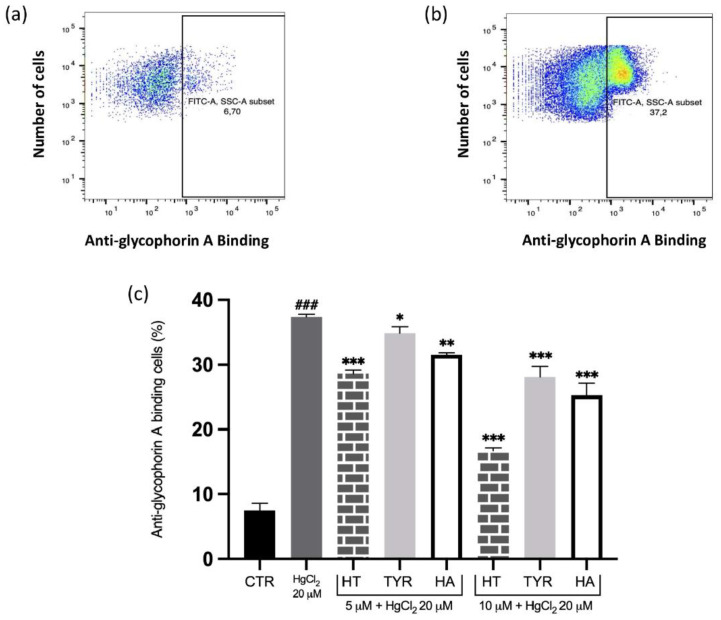
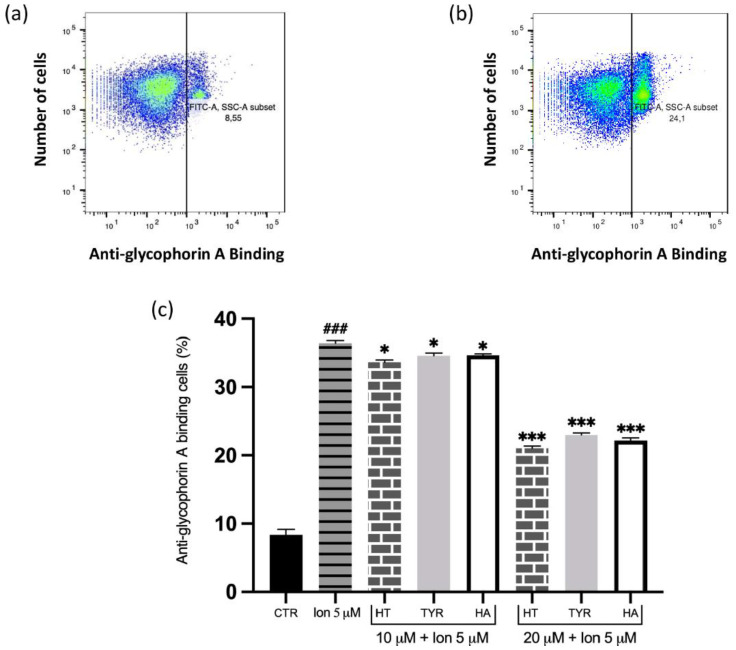
To further elucidate the PS-related membrane alterations, we verified the possible protective effect exerted by HT and its analogues in the prevention of MVs formation induced by Hg and increased intracellular calcium, analyzed by flow cytometry using anti-human anti-glycophorin A FITC antibody. The data reported in Figure 3 and Figure 4 reveal that all the selected phenols prevent MVs formation and that the protective effect parallels that observed for PS exposure.
Figure 3.
Effect of HT and its analogue on Hg-induced MVs formation from RBC. Cells were treated with HgCl2 in the presence of increasing concentrations of HT, Tyr and HA and MVs formation was evaluated using FITC anti-human glycophorin A antibody. (a) Original histogram of control cells (CTR). (b) Original histogram of Hg-treated cells. (c) Histogram of phenols effect on Hg treatment. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates a significant difference from CTR. *** (p < 0.001), ** (p < 0.01) and * (p < 0.05) indicates a significant difference from Hg treatment. MVs: microvesicles; RBC: red blood cell, HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol; FITC: fluorescein isothiocyanate.
Figure 4.
Effect of HT and its analogue on Ca2+-induced MVs formation from RBC. Cells were treated with Ca2+ ionophore in the presence of increasing concentrations of HT, Tyr and HA and MVs formation was evaluated using FITC anti-human glycophorin A antibody. (a) Original histogram of control cells (CTR). (b) Original histogram of Ca2+ ionophore-treated cells. (c) Histogram of phenols effect on Ca2+ ionophore treatment. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates a significant difference from CTR. *** (p < 0.001) and * (p < 0.05) indicates a significant difference from Ca2+ ionophore treatment. MVs: microvesicles; RBC: red blood cell, HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol; FITC: fluorescein isothiocyanate; DFC: dichlofluorescein.
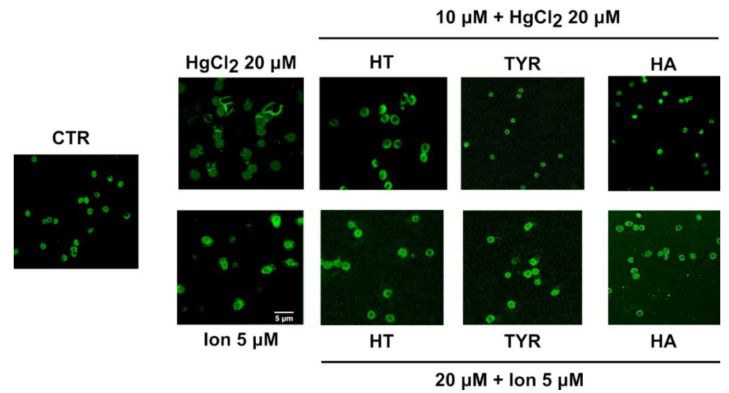
These data have been further supported by confocal fluorescence microscopy (Figure 5).
Figure 5.
Effect of HT and its analogue on Hg or Ca2+-induced MVs release from RBC. Cells were treated with HgCl2 and Ca2+-ionophore in the presence of HT, Tyr and HA and MVs release were stained with FITC anti-human glycophorin A antibody.
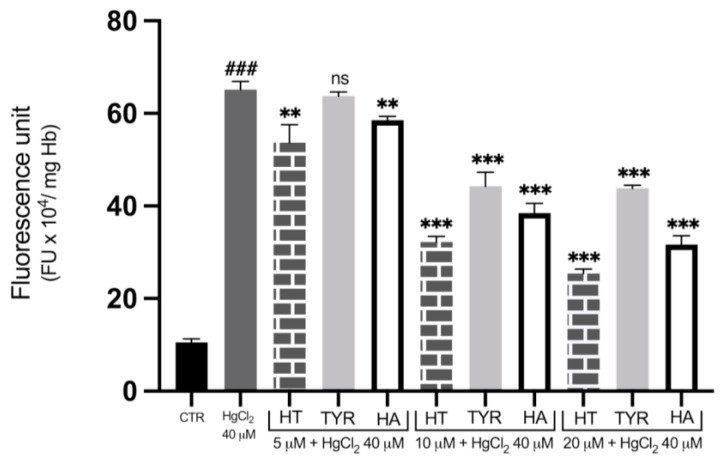
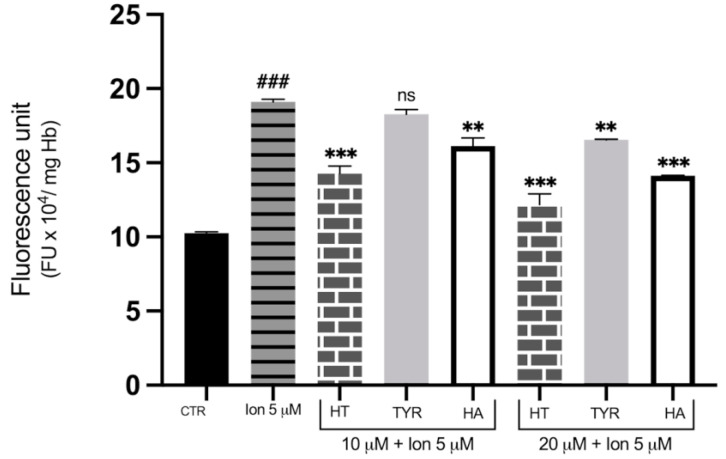
To investigate the role of OS in Hg-induced cytotoxicity and the possible protective effects of HT, Tyr and HA, the fluorescent dichlofluorescein (DCF) assay was utilized to measure ROS formation. Figure 6 shows that incubation with 40 μM HgCl2 causes an increase in DCF fluorescent signal, which is indicative of ROS formation after 4 h of treatment, confirming our previous data that Hg treatment exposes cells to an oxidative microenvironment. To test the efficiency of HT and its analogues in reducing ROS generation, cells were subjected to metal exposure in the presence of increasing concentrations of the three phenols. As shown in Figure 6, HT and HA decrease the fluorescent signal of the metal-exposed RBC, with a significant reduction observed at all concentrations tested, starting at a concentration as low as 5 μM. Tyr also appears protective against Hg-induced ROS formation, although to a lesser extent, with a significant value at 10 μM. Cell treatment with HT and its analogues also results in prevention of ROS generation induced by incubation in the presence of 5 µM of Ca2 + ionophore, the protective effect being less efficient compared with Hg-induced ROS production (Figure 7).
Figure 6.
Effect of HT and its analogues on Hg-induced ROS production in RBC. Cells were treated with HgCl2 in the presence of increasing concentrations of HT, Tyr and HA and ROS production was evaluated by means of the fluorescent probe DCF. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates significant difference from control (CTR). *** (p < 0.001), ** (p < 0.01), indicate a significant difference from Hg treatment. ns (p > 0.05) indicates no significant difference from Hg treatment. ROS: Reactive oxygen species; RBC: red blood cell; HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol; DFC: dichlofluorescein.
Figure 7.
Effect of HT and its analogues on Ca2+-induced ROS production in RBC. Cells were treated with Ca2+ ionophore in the presence of increasing concentrations of HT, Tyr and HA and ROS production was evaluated by means of the fluorescent probe DCF. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates a significant difference from control (CTR). *** (p < 0.001), ** (p < 0.01), indicate a significant difference from Ca2+ ionophore treatment. ns (p > 0.05) indicates no significant difference from Ca2+ ionophore treatment. ROS: Reactive oxygen species; RBC: red blood cell; HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol; DFC: dichlofluorescein.
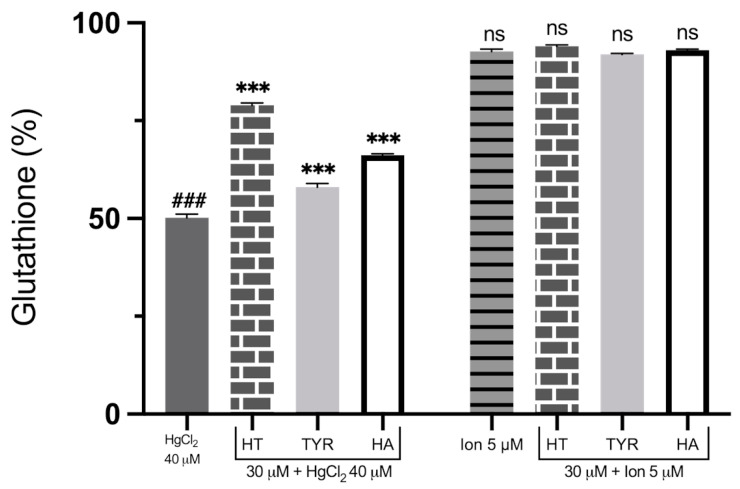
Due to the fundamental role played by GSH depletion in Hg-induced toxicity, linked to the impairment of the antioxidant defense system, we compared the possible protection of HT and its analogues on this specific metabolic alteration induced by HgCl2 and Ca2+ ionophore. As shown in Figure 8, RBC exposure to the heavy metal for 4 h drastically reduces GSH levels. All phenols are able to partially restore GSH cellular content, Tyr being the least efficient again. Interestingly, intracellular GSH was not significantly reduced when measured upon cell incubation with the Ca2+ ionophore.
Figure 8.
Effect of HT and its analogues on HgCl2 or Ca2+-induced GSH decrease in RBC. Cells were treated with HgCl2 and Ca2+ ionophore in the presence of HT, Tyr and HA. Data are the means ± SD (n = 9). Statistical analysis was performed with one-way ANOVA followed by Tukey’s test. ### (p < 0.001) indicates a significant difference from control (CTR). *** (p < 0.001) indicates a significant difference from Hg treatment. ns (p > 0.05) indicates no significant difference from Ca2+ ionophore treatment. GSH: glutathione; HT: hydroxytyrosol; Tyr: tyrosol; HA: homovanillic alcohol.
3. Discussion
RBC are the most abundant cells in the bloodstream, characterized by a simplified metabolism and amply utilized as a unique cellular model in biomedical research, due to its peculiar maturation process [7]. In particular, late enucleation is considered an advantage in the investigation of particular aspects of these cells, such as aging [45]. In vitro ageing during the blood bank storage has been also amply investigated [46,47].
Furthermore, RBC are particularly suitable for studies that investigate OS-induced events, due to the high tension of oxygen and the highly toxic free radicals deriving from them [48,49]. Accordingly, these cells are equipped with a powerful antioxidant defence system and significantly contribute to protect from oxidative insult, both other blood cells and the endothelium [22]. However, if RBC reach highly inflamed tissues, such as the endothelium with atherosclerotic lesions, their behaviour shifts from the physiological activity of scavenger to the harmful role of ROS generator, inducing an oxidative microenvironment, thus worsening endothelial dysfunction [50]. Accordingly, RBC have been identified for use as potential markers of OS-related pathologies, including cardiovascular diseases (CVD). Interestingly in this respect, RBC have been proposed as a potential link between diabetes and Alzheimer’s diseases [51]. Finally, we recently reviewed data indicating the rationale for using of RBC as a model for heavy metal-induced endothelial dysfunction, including Hg [35]. In this respect, a link between this highly toxic environmental pollutant and CVD has been proposed, and several authors indicate that Hg human exposure may act as a potential risk factor for these pathologies [52,53].
As far as the mechanisms underlying Hg toxic effects on the cardiovascular system, RBC metabolic as well as morphological changes appear to play a role, increasing the pro-coagulant activity of these cells, particularly related to alteration of cellular membrane asymmetry [21]. The maintenance of phospholipid asymmetry in the plasma membrane, resulting from the coordinated action of flippase and scamblase enzymatic activities [54,55], is essential for RBC physiopathology. In fact, besides its physiological role in erythropoiesis and erythrophagocytosis, PS exposure to the outer membrane leaflet allows the removal of stressed RBC [56]. This membrane modification, however, can induce adverse effects on the cardiovascular system, activating a prothrombotic activity [23]. It is therefore essential to explore tools to protect cells from the possible toxic effects of human exposure to heavy metals. In this regard, we considered worth to verify the potential protective role of nutrition, by testing bioactive compounds normally present in our diet.
The data presented provide experimental evidence for the efficacy of structurally related olive oil phenolic compounds in preventing metabolic and morphological damage to human RBC exposed in vitro to HgCl2 treatment and Ca2+ ionophore. Our study, in agreement with previous reports [36,57], confirms that Hg and Ca2+ induce PS exposure and MVs generation; here, we report that cell treatment with µM concentrations of olive oil phenols reduces the alteration of membrane asymmetry due to PS exposure. In particular, HT and HA show similar protection, while Tyr appears to be less effective. Interestingly, a similar effect was obtained with regard to Ca2+ ionophore-induced PS exposure and MVs formation, but the protective effect was observed at higher concentrations of the three phenols, indicating that HT and its analogues also act on non-calcium-dependent mechanisms in Hg toxicity. Accordingly, we report that the removal of calcium from the medium only partially affects the increase in PS exposure induced in RBC by Hg treatment, but HT effect is still observable. In this respect, it is well known that eryptosis is in large part, but not fully, triggered by entry of calcium [58,59].
An interesting finding is that all tested compounds are able to maintain cellular thiol homeostasis, counteracting GSH depletion in Hg-exposed RBC. In fact, although a particularly high GSH concentration may partially protect RBC from Hg toxic effects, chronic exposure might affect RBC viability and induce prothrombotic activity, also affecting CVD. In our opinion, the efficacy of the olive oil phenols in preventing Hg-induced modifications in intracellular thiols by restoring the GSH levels may represent a key mechanism underlying the protective effects. It is well known indeed that the simple intracellular thiol modifications can induce apoptosis in nucleated cells, unrelated to ROS generation [60]. Moreover, Mohan et al. reported that the ability of HT to promote the expression of Nrf2, which in turn elevates GSH levels, is crucial in ameliorating the neurotoxic effect of MeHg in IMR-32 neuroblastoma cells [61]. On the contrary, in RBC treated with calcium ionophore, only a minimal reduction in GSH level is observed.
Altogether, data from our study demonstrate that Hg treatment and an increase in intracellular calcium can induce changes in shape, MVs generation, and PS exposure on the RBC membrane, important mediators of RBC procoagulant activation [62]. This suggests that these cells represent key cellular targets of Hg toxicity, thus contributing to the cardiovascular dysfunction associated with human exposure to this heavy metal. In our opinion, the reported findings are particularly promising and clinically important and point to RBC as a target for therapeutic strategies. This aspect deserves further clinical and experimental investigation.
Finally, our findings strengthen the nutritional relevance of olive oil bioactive compounds to the claimed health-promoting effects of this component of the Mediterranean Diet. In this respect, antioxidant polyphenols are believed to play a major role in the positive correlation between adherence to the Mediterranean Dietary Habit and a low incidence of CVD [63,64]. In recent years, nutritional research as well as in vitro studies have mainly focused on the effects of HT in the progression of atherosclerosis [65]. Beside its inflammatory properties, this phenol reduces the expression of adhesion molecules [66,67], a key mechanism implicated in plaque formation. Furthermore, HT inhibits in vitro LDL oxidation and counteracts the OS-induced endothelial dysfunction [68]. The reported data expand upon the HT known beneficial effects of olive oil, particularly related to human exposure to heavy metals, indicating that prevention of metal toxicity should be regarded as an additional mechanism responsible for the health-promoting potential of olive oil intake on the cardiovascular system. In this regard, an important finding is that the in vivo HT metabolite still retains the biological activities of its dietary precursor. Our attention was also directed towards Tyr, which exerts significant protection in spite of its weak scavenging activity [37]. This phenol, which lacks the o-diphenolic structure, is unable to counteract the OS-induced cytotoxicity [69], indicating that additional mechanisms are involved in the protective effect observed in our experimental model. However, although Tyr is not as efficient as other antioxidants found in olive oil, our data suggest that it may contribute to the overall positive effect of olive oil on human health.
The beneficial properties of olive oil bioactive components provide biochemical bases for nutritional strategies in the prevention of pathologies related to Hg exposure, by using both functional foods and nutraceutical preparations. Accordingly, the Mediterranean Dietary Habit decreases prothrombotic MVs release in asymptomatic individuals at high cardiovascular risk [70]. At least in theory, olive oil phenols may be similarly effective in the prevention of endothelial dysfunction-related erythroid and non-erythroid human pathologies [71]. In this respect, RBC-altered adhesiveness appears to play a role in complications such as thrombosis in polycythemia vera and splenic sequestration in hereditary spherocytosis. In addition, RBC adhesion to the vascular endothelium might be involved in the occurrence of vaso-occlusive crisis in sickle cell disease. Unexpectedly, recent data suggest that PS-mediated abnormal RBC adhesion might be involved in the pathophysiology of non-erythroid disorders, such as central retinal vein occlusion and Gaucher disease, which share common clinical manifestations, including thrombotic events [72,73].
4. Materials and Methods
4.1. Chemicals and Solutions
4-Bromo-calcium Ionophore A23187; DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate), DTNB (5,5-dithiobis (2-nitrobenzoic acid), or Ellman’s reagent), PBS (phosphate-buffered saline), HgCl2, HT, Tyr and HA were from Sigma Chemical Co. (St. Louis, MO, USA), Annexin V-fluorescein isothiocyanate (V-FITC) Apoptosis Detection Kit (556547, BD Pharmigen, Franklin Lakes, NJ, USA) and Anti Glicophorin A antibody (FITC) were purchased from antibodies-online.com (ABIN6253946, antibodies-online GmbH, Aachen, Germany. https://www.antibodies-online.com/antibody/6253946/anti-Glycophorin+A+GYPA+antibody+FITC/ accessed on 12 April 2022). HT, Tyr and HA were prepared in dimethyl sulfoxide (DMSO) and diluted from 10 mM or 100 mM stock Solution. The resulting solutions were preventively tested upon RBC at their final concentrations to exclude any damage.
4.2. Preparation of RBC and Treatment with HgCl2 and Ca2+-Ionophore
Whole blood was obtained with informed consent from healthy volunteers at Campania University “Luigi Vanvitelli” (Naples, Italy). It was collected in heparinized tubes and centrifuged at 2000× g for 10 min at 4 °C. After removal of the buffy coat, the RBC fraction was washed twice with isotonic saline solution (0.9% NaCl) and resuspended in Krebs solution containing (mM) NaCl 125, KCl 4, MgSO4 1, Hepes 32, CaCl2 1, glucose 2.8; pH 7.4 to obtain a 10% (v/v) hematocrit (or 0.5 and 3% according to experienced needs). RBC were incubated at 37 °C for 4 or 24 h with HgCl2 (5–40 μM) and increasing concentrations of HT, HA and Tyr (1–30 μM). In order to estimate the different impact of Hg and Ca2+, RBC were exposed for 1 h to a combination of Ca2+-ionophore (5 μM) and different concentrations of the selected phenolic compounds.
4.3. Detection of Annexin-V-Binding Cells
After incubation under the respective experimental condition (24 h for Hg treatment), RBC (1 × 10−6 for each condition) were resuspended in 600 μL of 1× binding buffer and incubated in the dark for 15 min at room temperature, with 5 μL of annexin V Apoptosis detection kit. The assessment of fluorescence was performed with BD Accuri C6 and data were analyzed by FlowJo V10 software (https://www.flowjo.com/solutions/flowjo accessed on 12 April 2022); 20,000 events were recorded for each sample.
4.4. Quantification Assay of MVs by Flow Cytometry
MVs obtained after HgCl2 (24 h treatment) and Ca2+-ionophore-stimulation were counted by flow cytometry [74]. Briefly, 95 μL of samples were mixed with 5 μL of anti-human anti glycophorin A FITC-antibody and incubated for 20 min at room temperature (RT) on a roller. Before analysis, 80 μL of labelled MVs were dissolved in 400 μL of 0.9% NaCl and transferred to 1.5 mL tubes. MVs were quantified by flow cytometry, as previously described.
4.5. Confocal Microscope Analysis
RBC were treated with HgCl2 and Ca2+ ionophore in the presence or the absence of HT, TYR and HA, as described above. After incubation (24 h for Hg treatment), RBC were washed twice with phosphate-buffered saline pH 7.4 (PBS) and counted in a Burker chamber. The confocal laser scanning microscope analyses were performed according to Nguyen [74], with few modifications. In brief, the cells were then fixed with 2% formaldehyde for 1 h at 4 °C, then washed several times and incubated with anti-human anti glycophorin A FITC antibody for 30 min at 4 °C in the dark. Afterwards, the samples were placed on glass slides and air-dried for 1 h. The slides were dipped quickly, and gently washed stepwise with ethanol from 50% to 75%, 90%, and then 100% for dehydration. Finally, cells were fixed in 2% formaldehyde and washed three times with PBS. For confocal laser scanning microscope imaging, several randomly selected frames from each sample were captured for morphological observation and statistical strength. Excitation and emission filters were set at 488 nm and 550–600 nm, respectively.
4.6. ROS Determination
ROS generation was determined using the DCF assay, according to Tagliafierro et al. [75]. Using this method, 250 µL of intact RBC (hematocrit 10%) were incubated with DCFH-DA at a final concentration of 10 µM for 15 min at 37 °C. After centrifuging at room temperature at 1200× g for 5 min, the supernatant was removed, and the hematocrit was re-adjusted to 10% with Krebs solution. RBC were then treated concurrently with HgCl2, Ca2+-ionophore and selected phenolic compounds in the dark (4 h for Hg treatment). After the incubation, 20 µL of RBC were diluted in 2 mL of water, and the fluorescence intensity of the oxidized derivative DCF was recorded (_exc502; _em520). The results were expressed as fluorescence intensity/mg of hemoglobin. Fluorescence measurements were performed on a Perkin Elmer Life Sciences LS 55 spectrofluorimeter.
4.7. Assay for Reduced GSH
By reaction with DTNB, the intracellular GSH content was assessed, according to Tortora et al. [76]. After incubation (4 h for Hg treatment), samples (0.25 mL) were centrifuged for 5 min at 800× g. After removal of supernatants, RBC were lysed using 0.6 mL of cold water and proteins were then precipitated by the addition of 0.6 mL of a cold metaphosphoric acid solution (1.67 g metaphosphoric acid, 0.2 g EDTA, and 30 g NaCl in 100 mL of water). The samples were placed at 4 °C for 5 min, and then the precipitated proteins were removed by centrifugation at 18,000× g for 10 min. Finally, 0.45 mL of supernatant were mixed with an equal volume of 0.3 M Na2HPO4. To determine the reduced GSH, 0.1 mL of a DNTB solution (20 mg DTNB plus 1% of sodium citrate in 100 mL of water) was added to the solution. After 10 min of incubation at room temperature, the absorbance of the samples was measured at a wavelength of 412 nm.
4.8. Statistical Analyses
Data evaluations were expressed as means ± S.D. of 3 independent experiments performed in triplicate with RBC from different donors. The significance of differences was determined by one-way ANOVA followed by a post Tukey’s multiple comparisons test. GraphPad Prism 9.1 was utilized for statistical analysis.
Abbreviations
| CVD | Cardiovascular Diseases |
| DCF | Dichlofluorescein |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DMSO | Dimethyl sulfoxide |
| DNTB | 5,5-dithiobis 2-nitrobenzoic acid |
| GSH | Glutathione |
| HA | Homovanillic alcohol |
| Hg | Mercury |
| HT | Hydroxytyrosol |
| MVs | Microvesicles |
| NO | Nitric oxide |
| OS | Oxidative stress |
| PBS | Phosphate-buffered saline |
| PS | Phosphatidylserine |
| RBC | Erythrocytes |
| ROS | Reactive Oxygen Species |
| Tyr | Tyrosol |
Author Contributions
Conceptualization, C.M.; methodology, R.N.; software, P.P. and G.L.; validation, C.M. and M.P.; formal analysis, G.L. and L.M.; investigation, R.N., P.P. and L.M.; writing—original draft preparation, R.N. and P.P.; writing—review and editing, C.M.; visualization, R.N. and M.P.; supervision, C.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamidi M., Tajerzadeh H. Carrier Erythrocytes: An Overview. Drug. Deliv. 2003;10:9–20. doi: 10.1080/713840329. [DOI] [PubMed] [Google Scholar]
- 2.Kleinbongard P., Dejam A., Lauer T., Rassaf T., Schindler A., Picker O., Scheeren T., Gödecke A., Schrader J., Schulz R., et al. Plasma Nitrite Reflects Constitutive Nitric Oxide Synthase Activity in Mammals. Free Radic. Biol. Med. 2003;35:790–796. doi: 10.1016/S0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 3.Red Blood Cell and Endothelial ENOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure—PubMed. [(accessed on 6 April 2022)]; doi: 10.1161/CIRCULATIONAHA.120.049606. Available online: https://pubmed.ncbi.nlm.nih.gov/34229449/ [DOI] [PMC free article] [PubMed]
- 4.Helms C.C., Gladwin M.T., Kim-Shapiro D.B. Erythrocytes and Vascular Function: Oxygen and Nitric Oxide. Front. Physiol. 2018;9:125. doi: 10.3389/fphys.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y., Wang X., Noviana M., Hou M. Nitric Oxide in Red Blood Cell Adaptation to Hypoxia. Acta Biochim. Biophys. Sin. 2018;50:621–634. doi: 10.1093/abbs/gmy055. [DOI] [PubMed] [Google Scholar]
- 6.Gladwin M.T., Lancaster J.R., Freeman B.A., Schechter A.N. Nitric Oxide’s Reactions with Hemoglobin: A View through the SNO-Storm. Nat. Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 7.Moras M., Lefevre S.D., Ostuni M.A. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front. Physiol. 2017;8:1076. doi: 10.3389/fphys.2017.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freikman I., Fibach E. Distribution and Shedding of the Membrane Phosphatidylserine during Maturation and Aging of Erythroid Cells. Biochim. Biophys. Acta. 2011;1808:2773–2780. doi: 10.1016/j.bbamem.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Arashiki N., Takakuwa Y. Maintenance and Regulation of Asymmetric Phospholipid Distribution in Human Erythrocyte Membranes: Implications for Erythrocyte Functions. Curr.. Opin. Hematol. 2017;24:167–172. doi: 10.1097/MOH.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 10.Arias C.F., Arias C.F. How Do Red Blood Cells Know When to Die? R Soc. Open Sci. 2017;4:160850. doi: 10.1098/rsos.160850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klei T.R.L., Meinderts S.M., van den Berg T.K., van Bruggen R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017;8:73. doi: 10.3389/fimmu.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissinger R., Schumacher C., Qadri S.M., Honisch S., Malik A., Götz F., Kopp H.-G., Lang F. Enhanced Eryptosis Contributes to Anemia in Lung Cancer Patients. Oncotarget. 2016;7:14002–14014. doi: 10.18632/oncotarget.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang E., Bissinger R., Qadri S.M., Lang F. Suicidal Death of Erythrocytes in Cancer and Its Chemotherapy: A Potential Target in the Treatment of Tumor-Associated Anemia. Int. J. Cancer. 2017;141:1522–1528. doi: 10.1002/ijc.30800. [DOI] [PubMed] [Google Scholar]
- 14.Kempe-Teufel D.S., Bissinger R., Qadri S.M., Wagner R., Peter A., Lang F. Cellular Markers of Eryptosis Are Altered in Type 2 Diabetes. Clin. Chem. Lab. Med. 2018;56:e177–e180. doi: 10.1515/cclm-2017-1058. [DOI] [PubMed] [Google Scholar]
- 15.Abed M., Artunc F., Alzoubi K., Honisch S., Baumann D., Föller M., Lang F. Suicidal Erythrocyte Death in End-Stage Renal Disease. J. Mol. Med. 2014;92:871–879. doi: 10.1007/s00109-014-1151-4. [DOI] [PubMed] [Google Scholar]
- 16.Lang E., Qadri S.M., Lang F. Killing Me Softly—Suicidal Erythrocyte Death. Int. J. Biochem. Cell. Biol. 2012;44:1236–1243. doi: 10.1016/j.biocel.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Eryptosis: Programmed Death of Nucleus-Free, Iron-Filled Blood Cells—PubMed. [(accessed on 6 April 2022)]; doi: 10.3390/cells11030503. Available online: https://pubmed.ncbi.nlm.nih.gov/35159312/ [DOI] [PMC free article] [PubMed]
- 18.Lang E., Lang F. Triggers, Inhibitors, Mechanisms, and Significance of Eryptosis: The Suicidal Erythrocyte Death. Biomed. Res. Int. 2015;2015:513518. doi: 10.1155/2015/513518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qadri S.M., Bissinger R., Solh Z., Oldenborg P.-A. Eryptosis in Health and Disease: A Paradigm Shift towards Understanding the (Patho)Physiological Implications of Programmed Cell Death of Erythrocytes. Blood Rev. 2017;31:349–361. doi: 10.1016/j.blre.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Turpin C., Catan A., Meilhac O., Bourdon E., Canonne-Hergaux F., Rondeau P. Erythrocytes: Central Actors in Multiple Scenes of Atherosclerosis. Int. J. Mol. Sci. 2021;22:5843. doi: 10.3390/ijms22115843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim K.-M., Kim S., Noh J.-Y., Kim K., Jang W.-H., Bae O.-N., Chung S.-M., Chung J.-H. Low-Level Mercury Can Enhance Procoagulant Activity of Erythrocytes: A New Contributing Factor for Mercury-Related Thrombotic Disease. Environ. Health Perspect. 2010;118:928–935. doi: 10.1289/ehp.0901473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisel J.W., Litvinov R.I. Red Blood Cells: The Forgotten Player in Hemostasis and Thrombosis. J. Thromb. Haemost. 2019;17:271–282. doi: 10.1111/jth.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borst O., Abed M., Alesutan I., Towhid S.T., Qadri S.M., Föller M., Gawaz M., Lang F. Dynamic Adhesion of Eryptotic Erythrocytes to Endothelial Cells via CXCL16/SR-PSOX. Am. J. Physiol. Cell. Physiol. 2012;302:C644–C651. doi: 10.1152/ajpcell.00340.2011. [DOI] [PubMed] [Google Scholar]
- 24.Sudnitsyna J., Skverchinskaya E., Dobrylko I., Nikitina E., Gambaryan S., Mindukshev I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants. 2020;9:929. doi: 10.3390/antiox9100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thangaraju K., Neerukonda S.N., Katneni U., Buehler P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020;22:153. doi: 10.3390/ijms22010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bissinger R., Bhuyan A.A.M., Qadri S.M., Lang F. Oxidative Stress, Eryptosis and Anemia: A Pivotal Mechanistic Nexus in Systemic Diseases. FEBS J. 2019;286:826–854. doi: 10.1111/febs.14606. [DOI] [PubMed] [Google Scholar]
- 27.Tortora F., Notariale R., Lang F., Manna C. Hydroxytyrosol Decreases Phosphatidylserine Exposure and Inhibits Suicidal Death Induced by Lysophosphatidic Acid in Human Erythrocytes. Cell. Physiol. Biochem. 2019;53:921–932. doi: 10.33594/000000185. [DOI] [PubMed] [Google Scholar]
- 28.Lupescu A., Jilani K., Zelenak C., Zbidah M., Qadri S.M., Lang F. Hexavalent Chromium-Induced Erythrocyte Membrane Phospholipid Asymmetry. Biometals. 2012;25:309–318. doi: 10.1007/s10534-011-9507-5. [DOI] [PubMed] [Google Scholar]
- 29.Vianna A.D.S., de Matos E.P., de Jesus I.M., Asmus C.I.R.F., de Magalhães Câmara V.D. Human Exposure to Mercury and Its Hematological Effects: A Systematic Review. Cad Saude Publica. 2019;35:e00091618. doi: 10.1590/0102-311x00091618. [DOI] [PubMed] [Google Scholar]
- 30.Piscopo M., Notariale R., Tortora F., Lettieri G., Palumbo G., Manna C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules. 2020;25:3278. doi: 10.3390/molecules25143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández L.E., Sobrino-Plata J., Montero-Palmero M.B., Carrasco-Gil S., Flores-Cáceres M.L., Ortega-Villasante C., Escobar C. Contribution of Glutathione to the Control of Cellular Redox Homeostasis under Toxic Metal and Metalloid Stress. J. Exp. Bot. 2015;66:2901–2911. doi: 10.1093/jxb/erv063. [DOI] [PubMed] [Google Scholar]
- 32.Lettieri G., Notariale R., Carusone N., Giarra A., Trifuoggi M., Manna C., Piscopo M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus Galloprovincialis to Mercury-A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021;22:5893. doi: 10.3390/ijms22115893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X.F., Lowe M., Chan H.M. Mercury Exposure, Cardiovascular Disease, and Mortality: A Systematic Review and Dose-Response Meta-Analysis. Environ. Res. 2021;193:110538. doi: 10.1016/j.envres.2020.110538. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopalan S., Landrigan P.J. Pollution and the Heart. N. Engl. J. Med. 2021;385:1881–1892. doi: 10.1056/NEJMra2030281. [DOI] [PubMed] [Google Scholar]
- 35.Notariale R., Infantino R., Palazzo E., Manna C. Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components. Int. J. Mol. Sci. 2021;22:6604. doi: 10.3390/ijms22126604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Officioso A., Alzoubi K., Lang F., Manna C. Hydroxytyrosol Inhibits Phosphatidylserine Exposure and Suicidal Death Induced by Mercury in Human Erythrocytes: Possible Involvement of the Glutathione Pathway. Food Chem. Toxicol. 2016;89:47–53. doi: 10.1016/j.fct.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Karković Marković A., Torić J., Barbarić M., Jakobušić Brala C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules. 2019;24:2001. doi: 10.3390/molecules24102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manna C., Galletti P., Maisto G., Cucciolla V., D’Angelo S., Zappia V. Transport Mechanism and Metabolism of Olive Oil Hydroxytyrosol in Caco-2 Cells. FEBS Lett. 2000;470:341–344. doi: 10.1016/S0014-5793(00)01350-8. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelo S., Manna C., Migliardi V., Mazzoni O., Morrica P., Capasso G., Pontoni G., Galletti P., Zappia V. Pharmacokinetics and Metabolism of Hydroxytyrosol, a Natural Antioxidant from Olive Oil. Drug Metab. Dispos. 2001;29:1492–1498. [PubMed] [Google Scholar]
- 40.Tuck K.L., Hayball P.J., Stupans I. Structural Characterization of the Metabolites of Hydroxytyrosol, the Principal Phenolic Component in Olive Oil, in Rats. J. Agric. Food Chem. 2002;50:2404–2409. doi: 10.1021/jf011264n. [DOI] [PubMed] [Google Scholar]
- 41.Caruso D., Visioli F., Patelli R., Galli C., Galli G. Urinary Excretion of Olive Oil Phenols and Their Metabolites in Humans. Metabolism. 2001;50:1426–1428. doi: 10.1053/meta.2001.28073. [DOI] [PubMed] [Google Scholar]
- 42.Umeno A., Takashima M., Murotomi K., Nakajima Y., Koike T., Matsuo T., Yoshida Y. Radical-Scavenging Activity and Antioxidative Effects of Olive Leaf Components Oleuropein and Hydroxytyrosol in Comparison with Homovanillic Alcohol. J. Oleo Sci. 2015;64:793–800. doi: 10.5650/jos.ess15042. [DOI] [PubMed] [Google Scholar]
- 43.Bors W., Michel C. Chemistry of the Antioxidant Effect of Polyphenols. Ann. N. Y. Acad. Sci. 2002;957:57–69. doi: 10.1111/j.1749-6632.2002.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 44.Di Benedetto R., Varì R., Scazzocchio B., Filesi C., Santangelo C., Giovannini C., Matarrese P., D’Archivio M., Masella R. Tyrosol, the Major Extra Virgin Olive Oil Compound, Restored Intracellular Antioxidant Defences in Spite of Its Weak Antioxidative Effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007;17:535–545. doi: 10.1016/j.numecd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Kaestner L., Minetti G. The Potential of Erythrocytes as Cellular Aging Models. Cell. Death. Differ. 2017;24:1475–1477. doi: 10.1038/cdd.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida T., Prudent M., D’alessandro A. Red Blood Cell Storage Lesion: Causes and Potential Clinical Consequences. Blood Transfus. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abonnenc M., Tissot J.-D., Prudent M. General Overview of Blood Products in Vitro Quality: Processing and Storage Lesions. Transfus. Clin. Biol. 2018;25:269–275. doi: 10.1016/j.tracli.2018.08.162. [DOI] [PubMed] [Google Scholar]
- 48.Remigante A., Morabito R., Marino A. Band 3 Protein Function and Oxidative Stress in Erythrocytes. J. Cell. Physiol. 2021;236:6225–6234. doi: 10.1002/jcp.30322. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad S., Mahmood R. Mercury Chloride Toxicity in Human Erythrocytes: Enhanced Generation of ROS and RNS, Hemoglobin Oxidation, Impaired Antioxidant Power, and Inhibition of Plasma Membrane Redox System. Environ. Sci. Pollut. Res. Int. 2019;26:5645–5657. doi: 10.1007/s11356-018-04062-5. [DOI] [PubMed] [Google Scholar]
- 50.Minetti M., Agati L., Malorni W. The Microenvironment Can Shift Erythrocytes from a Friendly to a Harmful Behavior: Pathogenetic Implications for Vascular Diseases. Cardiovasc. Res. 2007;75:21–28. doi: 10.1016/j.cardiores.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Carelli-Alinovi C., Misiti F. Erythrocytes as Potential Link between Diabetes and Alzheimer’s Disease. Front. Aging Neurosci. 2017;9:276. doi: 10.3389/fnagi.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi T., Shimohata T. Vascular Dysfunction Induced by Mercury Exposure. Int. J. Mol. Sci. 2019;20:2435. doi: 10.3390/ijms20102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genchi G., Sinicropi M.S., Carocci A., Lauria G., Catalano A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health. 2017;14:74. doi: 10.3390/ijerph14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wesseling M.C., Wagner-Britz L., Nguyen D.B., Asanidze S., Mutua J., Mohamed N., Hanf B., Ghashghaeinia M., Kaestner L., Bernhardt I. Novel Insights in the Regulation of Phosphatidylserine Exposure in Human Red Blood Cells. Cell. Physiol. Biochem. 2016:1941–1954. doi: 10.1159/000447891. [DOI] [PubMed] [Google Scholar]
- 55.Nagata S., Sakuragi T., Segawa K. Flippase and Scramblase for Phosphatidylserine Exposure. Curr. Opin. Immunol. 2020;62:31–38. doi: 10.1016/j.coi.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Segawa K., Nagata S. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends Cell. Biol. 2015;25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Zierle J., Bissinger R., Lang F. Inhibition by Teriflunomide of Erythrocyte Cell Membrane Scrambling Following Energy Depletion, Oxidative Stress and Ionomycin. Cell. Physiol. Biochem. 2016;39:1877–1890. doi: 10.1159/000447886. [DOI] [PubMed] [Google Scholar]
- 58.Officioso A., Manna C., Alzoubi K., Lang F. Triggering of Erythrocyte Death by Triparanol. Toxins. 2015;7:3359–3371. doi: 10.3390/toxins7083359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Föller M., Lang F. Ion Transport in Eryptosis, the Suicidal Death of Erythrocytes. Front. Cell. Dev. Biol. 2020;8:597. doi: 10.3389/fcell.2020.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gatti R., Belletti S., Uggeri J., Vettori M.V., Mutti A., Scandroglio R., Orlandini G. Methylmercury Cytotoxicity in PC12 Cells Is Mediated by Primary Glutathione Depletion Independent of Excess Reactive Oxygen Species Generation. Toxicology. 2004;204:175–185. doi: 10.1016/j.tox.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 61.Mohan V., Das S., Rao S.B.S. Hydroxytyrosol, a Dietary Phenolic Compound Forestalls the Toxic Effects of Methylmercury-Induced Toxicity in IMR-32 Human Neuroblastoma Cells. Environ. Toxicol. 2016;31:1264–1275. doi: 10.1002/tox.22134. [DOI] [PubMed] [Google Scholar]
- 62.Qadri S.M., Donkor D.A., Bhakta V., Eltringham-Smith L.J., Dwivedi D.J., Moore J.C., Pepler L., Ivetic N., Nazi I., Fox-Robichaud A.E., et al. Phosphatidylserine Externalization and Procoagulant Activation of Erythrocytes Induced by Pseudomonas Aeruginosa Virulence Factor Pyocyanin. J. Cell. Mol. Med. 2016;20:710–720. doi: 10.1111/jcmm.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montano L., Maugeri A., Volpe M.G., Micali S., Mirone V., Mantovani A., Navarra M., Piscopo M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022;23:1568. doi: 10.3390/ijms23031568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benbrahim C., Barka M.S., Basile A., Maresca V., Flamini G., Sorbo S., Carraturo F., Notariale R., Piscopo M., Khadir A., et al. Chemical Composition and Biological Activities of Oregano and Lavender Essential Oils. Appl. Sci. 2021;11:5688. doi: 10.3390/app11125688. [DOI] [Google Scholar]
- 65.Hydroxytyrosol and Its Main Plasma Circulating Metabolites Attenuate the Initial Steps of Atherosclerosis through Inhibition of the MAPK Pathway-ScienceDirect. [(accessed on 7 April 2022)]. Available online: https://www.sciencedirect.com/science/article/pii/S1756464617306795.
- 66.Manna C., Napoli D., Cacciapuoti G., Porcelli M., Zappia V. Olive Oil Phenolic Compounds Inhibit Homocysteine-Induced Endothelial Cell Adhesion Regardless of Their Different Antioxidant Activity. J. Agric. Food Chem. 2009;57:3478–3482. doi: 10.1021/jf8037659. [DOI] [PubMed] [Google Scholar]
- 67.de Souza P.A.L., Marcadenti A., Portal V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients. 2017;9:1087. doi: 10.3390/nu9101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mateos R., Martínez-López S., Baeza Arévalo G., Amigo-Benavent M., Sarriá B., Bravo-Clemente L. Hydroxytyrosol in Functional Hydroxytyrosol-Enriched Biscuits Is Highly Bioavailable and Decreases Oxidised Low Density Lipoprotein Levels in Humans. Food Chem. 2016;205:248–256. doi: 10.1016/j.foodchem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Manna C., Galletti P., Cucciolla V., Moltedo O., Leone A., Zappia V. The Protective Effect of the Olive Oil Polyphenol (3,4-Dihydroxyphenyl)-Ethanol Counteracts Reactive Oxygen Metabolite-Induced Cytotoxicity in Caco-2 Cells. J. Nutr. 1997;127:286–292. doi: 10.1093/jn/127.2.286. [DOI] [PubMed] [Google Scholar]
- 70.Chiva-Blanch G., Sala-Vila A., Crespo J., Ros E., Estruch R., Badimon L. The Mediterranean Diet Decreases Prothrombotic Microvesicle Release in Asymptomatic Individuals at High Cardiovascular Risk. Clin. Nutr. 2020;39:3377–3384. doi: 10.1016/j.clnu.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 71.Zwaal R.F.A., Comfurius P., Bevers E.M. Surface Exposure of Phosphatidylserine in Pathological Cells. Cell. Mol. Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PubMed] [Google Scholar]
- 72.Colin Y., Le Van Kim C., El Nemer W. Red Cell Adhesion in Human Diseases. Curr. Opin. Hematol. 2014;21:186–192. doi: 10.1097/MOH.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 73.Shet A.S., Lizarralde-Iragorri M.A., Naik R.P. The Molecular Basis for the Prothrombotic State in Sickle Cell Disease. Haematologica. 2020;105:2368–2379. doi: 10.3324/haematol.2019.239350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen D.B., Ly T.B.T., Wesseling M.C., Hittinger M., Torge A., Devitt A., Perrie Y., Bernhardt I. Characterization of Microvesicles Released from Human Red Blood Cells. Cell. Physiol. Biochem. 2016;38:1085–1099. doi: 10.1159/000443059. [DOI] [PubMed] [Google Scholar]
- 75.Tagliafierro L., Officioso A., Sorbo S., Basile A., Manna C. The Protective Role of Olive Oil Hydroxytyrosol against Oxidative Alterations Induced by Mercury in Human Erythrocytes. Food Chem. Toxicol. 2015;82:59–63. doi: 10.1016/j.fct.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 76.Tortora F., Notariale R., Maresca V., Good K.V., Sorbo S., Basile A., Piscopo M., Manna C. Phenol-Rich Feijoa Sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants. 2019;8:220. doi: 10.3390/antiox8070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.