Abstract
Simple Summary
There is currently a global research interest in reducing off-farm input of chemical pesticides and fertilizers by using green alternative practices. Fungi belonging to the genus Trichoderma colonize plant roots and activate systemic plant defenses against the attack of pests and pathogens. Trichoderma spp. have positive impacts on the environment and guarantee food security, which, in turn, offer important economic benefits in agriculture. The purpose of the present study was to investigate the effects of the inoculation of a commercial Trichoderma strain on the arthropod community, downy mildew, and the agronomic performance of tomato plants in an experimental field. Our results showed that inoculation with Trichoderma positively influenced tomato fruit yields and could reduce the abundance of specific pests under field conditions.
Abstract
Fungi belonging to the genus Trichoderma have received much attention in recent years due to their beneficial effects on crop health and their use as pest control agents. Trichoderma activates direct plant defenses against phytophagous arthropods and reinforces indirect plant defense through the attraction of predators. Although the plant defenses against insect herbivores were demonstrated in laboratory experiments, little attention has been paid to the use of Trichoderma spp. in open field conditions. In the present study, we investigated the effects of the inoculation of the commercial Trichoderma harzianum strain T22 on the arthropod community associated with tomato plants and on the crop performance in an experimental field located in South Italy. Our results showed that inoculation with T. harzianum could alter the arthropod community and reduce the abundance of specific pests under field conditions with respect to the sampling period. The present study also confirmed the beneficial effect of T. harzianum against plant pathogens and on tomato fruit. The complex tomato–arthropod–microorganism interactions that occurred in the field are discussed to enrich our current information on the possibilities of using Trichoderma as a green alternative agent in agriculture.
Keywords: Trichoderma, field experiment, QBSar, plant pathogens, tomato aphids, Chaetocnema tibialis, Tetranychus urticae, leaf miner, integrated pest management
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most important vegetable crops cultivated in the world, second only to potato, and one of the basic foods in the Mediterranean diet [1,2]. Fresh and processed tomatoes are widely consumed in the Mediterranean area, and Italy is one of the main producers and suppliers of processed tomatoes in the world [3,4,5]. Agrochemicals, such as synthetic fertilizers, pesticides, and herbicides, are usually used in tomato production to maximize yield and product quality and to achieve low production costs [6,7]. However, their overuse can cause environmental pollution and human health problems. There is currently a global interest in reducing off-farm input of chemical pesticides and fertilizers by using green alternative practices. Among all alternatives, numerous biological products based on beneficial plant microbes, such as bacteria (Bacillus, Pseudomonas) [8,9] or fungi (Trichoderma, Beauveria, mycorrhizae) [10,11,12,13], are receiving a lot of attention in agricultural farming systems due to their valuable properties. These products are used for pest control and for their potential to increase crop health and fitness; they also do not have any negative impacts on the environment and guarantee food security.
Fungi belonging to the genus Trichoderma have received much attention in recent years due to their beneficial effects on host plants [14,15,16,17,18,19]. These fungi are distributed throughout the world and are capable of colonizing plant roots and establishing chemical communication with the host plant [20]. Moreover, Trichoderma spp. have many possible uses and were investigated for their direct effects on the host plants, such as the increase in nutrient uptake, efficiency of nitrogen use, and seed germination rate, which offer important economic benefits in agriculture. These fungi also promote plant growth and resistance against biotic and abiotic stresses [14,21,22]. It was found that the plant systemic acquired resistance (SAR) and/or the induced systemic resistance (ISR) against biotic and abiotic stress agents could be activated by some Trichoderma strains [14,21,23].
Following the Trichoderma roots colonization, the plant reacts when it is attacked by a potential root endophytic pathogen, thus activating local and systemic defense mechanisms. Therefore, the plant limits the fungus penetration inside the root, restoring its integrity and antimicrobial activity to the pre-infection levels [20,21,23]. Once this equilibrium is reached, the plant receives protection and more available nutrients, while the fungus receives organic compounds. In this way, Trichoderma activates systemic plant defenses against the attack of pests and/or pathogens.
The feeding activity of phytophagous insects also elicits the release of attractive compounds [24]. Volatile organic compound (VOC) blends released in response to a pest attack have direct and indirect defensive effects on insect performance [25]. Plant responses to herbivores, induced by the different modes in which these organisms attack the plant, were shown to be affected by Trichoderma colonization [26,27,28,29]. Several studies were focused on the role of Trichoderma spp. (and their metabolites) on multitrophic interactions or on plant growth and defence responses [16,18,30,31].
Although direct and indirect plant defenses against insect herbivores were demonstrated in different plant species in greenhouses or pot experiments, little attention has been paid to the use of Trichoderma spp. in open field conditions [16]. The interaction between the plant and Trichoderma spp. directly confers some degree of protection against nematodes [32] and insects, such as aphids [33,34], thrips [26], and caterpillars [27,33]. For example, the survival of the aphid Macrosiphum euphorbiae (Thomas) in tomato plants could be significantly reduced using the P1 strain of T. atroviride as a consequence of the upregulation of genes involved in the oxidative burst reaction [33]. Similar results were obtained when the T. harzianum strain T22 was used in the same context [34]. The tomato defense responses against the green stink bug Nezaria viridula L. were enhanced by the T. harzianum strain T22 through an early increase in transcript levels of JA marker genes [35]. In onions, the performance of Thrips tabaci L. was reduced after colonization by Trichoderma spp. [26]. Among the chewing insects, the T. atroviride P1 strain was associated with reduced survival and development of Spodoptera littoralis (Boisduval) larvae and with an enhanced expression of genes encoding for protective enzymes in tomato plants [33]. Maize inoculation with T. atroviride increased plant growth, altered the feeding pattern of Spodoptera frugiperda (JE Smith) larvae, and was correlated with an increased emission of volatile terpenes and accumulation of JA [27]. In an in vitro assay, the secondary metabolite 6-pentyl-α-pyrone produced by the bioactivity of T. asperellum caused a high mortality rate in the two-spotted spider mite Tetranychus urticae Koch [36].
Trichoderma spp. also affect above-ground plant–insect interactions, reinforcing indirect plant defense barriers against phytophages through the production and release of VOCs that are involved in the attraction of predators and parasitoids [16,23]. For example, T. longibrachiatum MK1 influences the quantity and quality of VOCs released by the tomato plant (such as methyl salicylate), improving the attractiveness and performance of the aphid parasitoid Aphidius ervi Haliday and the predator Macrolophus pygmaeus (Rambur) [37]. In tomato, colonization by T. atroviride P1 significantly increases the attraction of A. ervi [33]. In a multitrophic interaction system, Trichoderma atroviride IMI 206040 associated with maize roots was shown to increase the parasitism rate of Campoletis sonorensis (Carlson) on S. frugiperda [38]. As suggested by Battaglia et al. [37], the improved attractiveness and performance of insect predators on Trichoderma-colonized plants could be considered a result of the “increased fitness flow” modulated through the interaction between the primary and secondary metabolism of plants [39].
However, below- and above-ground plant–insect–microorganism interactions are very complex and may be very different under field conditions. Contreras-Cornejo et al. [40] showed that, in a maize field, the community of native foliage arthropods could be altered after plant inoculation with T. harzianum strain 38. The authors found that the number of arthropods per plant did not differ between the inoculated and control plants. Nevertheless, T. harzianum inoculation decreased the number of piercing-sucking insects but increased the abundance of chewing herbivores and predators. The presence of Trichoderma was also shown to influence JA-mediated VOCs production in a vineyard, attracting parasitoid wasps of the Mymaridae family [41].
Trichoderma harzianum can also modulate soil arthropod biodiversity. For example, a higher abundance of collembolans was found under optimal conditions but not under suboptimal or adverse ones [42].
In many cases, the outcomes of plant–Trichoderma interactions are species-specific and even strain-specific [20,43,44,45,46]. Trichoderma–plant interactions with pests and their natural enemies are influenced by environmental conditions when experiments are performed in the field. Recently, it was demonstrated that the tomato defense response against insect pests induced by diverse Trichoderma species is influenced by the temperature [47]. Therefore, the use of Trichoderma as a biocontrol agent in agriculture depends not only on the targeted use (pests and pathogens) but also on local climatic conditions, soil properties, the availability of water and nutrients, and crop species.
The aim of the present study was to investigate the effects of the inoculation of a commercial Trichoderma harzianum strain T22 on the arthropod community associated with tomato plants in an experimental field located in South Italy. The beneficial effects of T. harzianum on the soil arthropod biodiversity, as well as on the agronomic performance of tomato plants (improved yield) and the behavior toward downy mildew (one of the main phytopathogens of tomato in South Italy) were also investigated. The complex tomato–arthropod–microorganism interactions that occurred in the field are also discussed to enrich our current information on the possibilities of using Trichoderma as a green alternative agent in agriculture.
2. Materials and Methods
2.1. Crop Cultivation
The present study was performed in an experimental tomato field located in Pignola (40°34′06.2″ N, 15°45′35.4″ E; 780 m above sea level), Potenza, Italy, during the 2021 tomato growing season. According to the FAO world reference base for soil resources, the soil was a dystric cambisol (Bd68-2bc), with the following characteristics: particles smaller than 2 mm in size, 935 g/kg; particles larger than 2 mm, 65 g/kg; apparent density, 1.294 kg/dm3; texture composition of sand, 481 g/kg; clay, 149 g/kg; silt, 370 g/kg at depth of 0–30 cm. The contents of total carbonate and total organic matter were 16 g/kg and 32.8 g/kg, respectively. The composition of the soil was as follows: total N, 2 g/kg; P, 29 mg/kg; Ca 11.1 meq/100 g; Mg, 4.6 meq/100 g; Na, 1.8 meq/100 g; soil pH (H2O), 6.2.
The soil was left fallow the year before the experiment and then plowed to a depth of 30 cm, rotavated, and leveled before planting the crop. Tomato seedlings (Solanum lycopersicum L.) of the commercial cultivar San Marzano Kero were used in this experiment. Tomato plants placed in alveolate containers were purchased from a nursery and were transplanted into the field on 1 June 2021. Fertigation was carried out with ammonium nitrate (YaraTera© AMNITRATM, N 34.2%) applied three times in the recovery and blossoming stage (20 kg ha−1 at an interval of one week from each other), calcium nitrate (YaraTera© CACINITTM, CaO 26.5%, N 15.5%) applied twice during the fruit-bearing stage (30 kg ha−1 at an interval of one week from each other) and potassium nitrate (YaraTera© KRISTA KTM plus, N 13.7%, K2O 46.3%) applied once (20 kg ha−1) at the fruit-enlargement stage. The use of fertilizers was developed during the present experiment and was appropriate to the needs of the plants, considering the soil characteristics. The tomato plants were not treated with pesticides during the entire field trial.
2.2. Meteorological Data
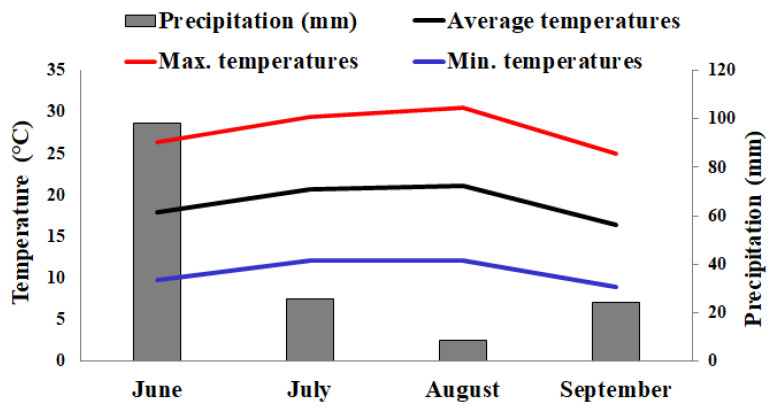
The Agrometeorological Service of the Agenzia Lucana per lo Sviluppo e l’Innovazione in Agricoltura (ALSIA) of the Basilicata Region provided the meteorological data for the area in which the experimental farm is located. The temperature and rainfall data recorded during the experiment are shown in Figure 1. During the period of interest, the average temperature remained less than or equal to 20 °C. The temperatures reached a maximum of 30 °C in August. Precipitations recorded in July, August, and September were low.
Figure 1.
Meteorological data for Pignola, Potenza, Italy, registered from June to September 2021. Data were provided by the Agrometeorological Service of the Basilicata Region, Italy. June was the month when the T. harzianum T22 inoculation and transplantation of tomato seedlings were carried out; July: vegetative growth and flowering stages; August: stages of establishment and development of fruit; September: fruit maturity stage and harvesting of tomato fruit.
2.3. Experimental Design
Two treatments were compared: non-inoculated tomato plants (control) and inoculated tomato plants with Trichoderma harzianum strain Rifai KRL-AG2 biocontrol agent (T-22) (KOPPERT B.V., Berkel en Rodenrijs, The Netherlands). Forty-day-old tomato plants were inoculated with T. harzianum following the manufacturer’s instructions (1 × 109 cfu/g of viable T. harzianum T-22 spores) one week before transplantation. The alveolate containers with 320 seedlings were then watered with 3 g of commercial T. harzianum dissolved in 3 liters of water. Treatment was repeated after three days and then the seedlings were transplanted. The control plants were not inoculated.
The experiment was carried out on a strip of soil about 58 m long and 9 m wide, divided into 6 plots of 54 m2 (6 m × 9 m) separated from each other by a strip 3.6 m wide left without plants. Thus, 3 plots treated with T. harzianum T22 and 3 control plots were obtained, alternating along the length of the field. A 10 m strip around the field was plowed and then left uncultivated throughout the entire duration of the experiment. The plants in each plot were manually transplanted on 1 June 2021 in 5 rows 1.8 m apart from each other. Each row was 6 m long with a plant spacing of 33 cm, with a total of 20 plants/row (100 plants/plot).
Six root samples of control or T.-harzianum-T22-inoculated plants (two per plot) were taken at the end of the experiment to determine the presence of the fungus. The tomato roots were dissected, mounted on a slide, and then observed with a stereomicroscope. The presence of the fungus was confirmed by the observation of hyphae in the secondary roots.
2.4. Insect Sampling
During the first month after transplantation, the tomato seedlings are very small and in a critical phase for their vegetative growth. However, seedlings are susceptible to attack by insect pests, such as aphids and beetles. For this reason, the presence/absence of phytophages was recorded via direct observation of the seedlings, without damaging them, on two dates (22 June and 1 July). Ten plants were randomly sampled within each plot, with a total of 30 plants per treatment/date. The presence/absence of insect pests on the plant was recorded; some of the observed specimens were gently removed, placed in an Eppendorf tube filled with ethanol, and transported to the laboratory for identification. On 10 June, yellow sticky traps were randomly placed between two adjacent seedlings in a row (5 traps/plot). After 20 days (1 July), the traps were collected and wrapped in a transparent PVC plastic film. Captured tomato pests were subsequently counted and identified using a stereomicroscope in the laboratory.
After the first month, as the plants were larger, leaf samples were collected for observation in the laboratory under a stereoscopic microscope. This allowed for more accurate sampling of small arthropods, thus obtaining quantitative data on their abundance. Six different samplings were carried out from 12 July to 21 September (more specifically, 12 and 22 July, 3 and 26 August, 8 and 21 September). Within each plot, ten plants were randomly sampled at 9.00 am for a total of 30 plants/date. For each plant, a composite sample was used, consisting of three leaves taken from the apical, middle, and basal parts of the tomato plant. The leaves were detached and placed in plastic cylinders (150 mL). The cylinders were then maintained in darkness at 5 °C and transported to the laboratory for the identification of the arthropods. The arthropods were transferred to 50 mL sterile Falcon tubes, which were filled with ~30 mL of 70% ethanol in water and refrigerated at 4 °C until identification. Individuals were then observed under a stereomicroscope. Arthropods from each sample were classified at the order, family, and, when possible, at the genus and species levels. Furthermore, the presence of damage caused by leaf miners on the leaves was noted and analyzed.
2.5. Soil Sampling and Microarthropod Extraction
On 21 September, within each experimental plot, three soil samples were randomly collected between two tomato plants with and without T. harzianum T22. A sample was composed of three soil clods (10 × 10 × 15 cm depth), which were taken using a hand auger in three different rows. After collection, soil samples were placed in a plastic bag, kept in darkness at 5 °C, and transported to the laboratory for arthropods extraction. Microarthropod extraction was carried out by gently placing soil clods on mesh-covered funnels (mesh 2 mm, 20 cm in diameter). A plastic jar containing 50 mL of hydroalcoholic solution (70%) was placed at the bottom of the funnel to store the extracted arthropods. Incandescent lamps (40 watts) were placed 20 cm above the soil clods. After 14 days, the extracted specimens were observed under a stereomicroscope, the biological morphs were determined, and the ecological–morphological index (EMI) was assigned. Finally, the QBSar (soil biological quality—arthropod) index was computed as the sum of the EMI values [48,49].
2.6. Evaluation of Downy Mildew on Tomato Plants
We focused only on the presence of downy mildew since it is one of the diseases that can cause major damage to tomato in the considered area. All plants were screened for the presence/absence of downy mildew at the beginning of August. For this purpose, each tomato plant was visually inspected and the presence of the abovementioned disease was recorded if at least its initial symptoms were present, such as small pale yellow spots with indefinite borders on the upper leaf surface. At an advanced stage of downy mildew development, other parts of the plant, such as stems, flowers, and fruit, are also attacked, and thus the damage could be very high. The evolution of the downy mildew disease during the entire period of tomato cultivation was also recorded.
2.7. Agronomic Performance Estimation
Fruit sampling was performed during the experiment. Within each experimental plot, ten plants were marked and followed throughout their development. Ripe tomato fruit were manually collected from the same plants on three different dates (30 August, 8 and 21 September). All red tomatoes on a plant were harvested, counted, and divided into marketable and non-marketable fruit. Marketable tomatoes were counted, weighed, and measured (maximum length and width), while unmarketable fruit were divided into two groups, rotten and insect-damaged fruit, and finally counted.
2.8. Statistical Analysis
Row data used in the analyses of the presence/absence of phytophages on tomato seedlings were the number of plants/experimental plot colonized by insects. Since these data have a discrete probability distribution, a binomial generalized linear model (GLM) with a logit link function was considered the best model for these analyses, thus avoiding transforming the data. For each of the insect species identified, the p-values for differences between treatments and sampling dates, as well as their interactions, were obtained through analyses of deviance (type III chi-squared tests). The following model was applied:
| Y = μ + Treatment + Date + Treatment × Date + ε |
where Y is the binomial trait studied (number of plants with the presence/absence of a phytophagous), Treatment (two levels: T. harzianum and control), and Date (two levels: 22 June and 1 July) are the fixed effects.
Insect abundances on yellow sticky traps were analyzed via nested analysis of variance (ANOVA) since the homoscedasticity and normality assumptions for ANOVA were checked and met for these data. The following model was applied:
| Y = μ + Treatment + Plot {Treatment} + ε |
where Y is the abundance of a phytophagous, Treatment (two levels: T. harzianum and control) is the main effect, and the Plot is nested within the Treatment (three levels/treatment).
Row data used in the analyses of the arthropod community on the tomato leaves were the number of insects per plant sampled over time. To test whether T. harzianum modulated the arthropod community on tomato leaves, a Poisson generalized linear mixed model (GLMM) with a log-link function fitted with ML (maximum likelihood) and Laplace approximation was used. The Poisson distribution best approximated the process that generated the observed data since it is a discrete distribution that measures the probability that a given number of events occur in a specified period. The p-values for differences between the treatments, sampling dates, and their interactions were obtained through analyses of deviance (type III Wald chi-square tests). The following general model was applied:
| Y = μ + Treatment + Date + Treatment × Date + Plot {Treatment {Date}} + ε |
where Y is the studied group of arthropods with a Poisson distribution, Treatment and Date (six levels: 12 and 22 July, 3 and 26 August, 8 and 21 September) are the fixed factors, and Plot is the random effect consisting of the three experimental plots nested in Treatment and Date. This model accounts for the non-independence of the data (pseudoreplication of measures) due to the different experimental plots (the random effect) that were part of the present design.
Data of the mean number of tomato fruit (marketable, rotten, and insect-damaged fruit) were also analyzed using Poisson GLMMs with Treatment and Date as fixed effects and Plot as a random effect nested in Treatment and Date. The data on fruit weight, length, and width were analyzed by linear mixed-effects models (LMMs) fitted with REML (restricted maximum likelihood). The homoscedasticity and normality assumptions for ANOVAs were checked and met on these data. p-values for differences between treatments, sampling dates, and their interactions were obtained through ANOVAs (type III Wald F tests using the Kenward–Roger approximation for the degrees of freedom [50]). The general model applied for the analysis of the arthropod community was also applied to the analysis of tomato fruit measures, but in these analyses, the factor Date consisted of three levels (30 August, 8 and 21 September).
For all the analyses described so far, the model distributions were also chosen as the best fitting based on AIC criteria [51] and the full models were presented.
Additionally, a two-way permutation multivariate analysis of variance (PERMANOVA) was also presented as a supplemental analysis to test for differences between treatments, sampling dates, or their interaction. The PERMANOVA (based on 9999 permutations) was performed on arthropods grouped according to their feeding behavior using the software PAST version 4.0 [52].
Differences between treatments for the QBSar index were analyzed using a two-sample t-test. Differences between treatments for the presence of Peronospora spp. on the tomato leaves were analyzed using a Pearson’s chi-square test for independence.
All statistical analyses (except the PERMANOVA) were performed in R version 4.1.2 “Bird Hippie” [53] with the lme4 [54] and lmerTest [55] packages.
3. Results
In this study, only the roots of the inoculated plants showed the presence of Trichoderma, while in the control plants, the presence of hyphae was not observed. Furthermore, the phenotypic fruit response to inoculation is proof of fungal establishment, as well as the differences in Peronospora spp. and in the associated community of arthropods that were collected at the same time from each treatment.
3.1. First Month after Transplantation: Seedling Growth Phase
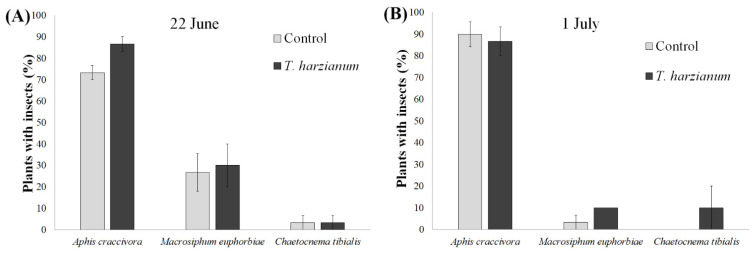
During the first month after transplanting, the presence/absence of phytophages was investigated via direct observation of the seedlings. Since the sampling method was not destructive and the plants were very small, an accurate quantitative measurement of the number of insects was not possible. At this time, we mainly found winged morphs of aphids and several specimens of a beetle identified as Chaetocnema tibialis Illiger (Coleoptera: Chrysomelidae). The aphid species were identified as Macrosiphum euphorbiae, Aphis craccivora Koch, and Aphis gossypii Glover (Hom., Aphididae). No aphid colonies were detected at this stage. Since A. gossypii was only observed on two plants during the first sampling date, it was excluded from subsequent analyses. The percentage of tomato seedlings with M. euphorbiae, A. craccivora, and C. tibialis sampled on 22 June and on 1 July (that is, during the vegetative growth stage) is reported in Figure 2.
Figure 2.
Presence of phytophages on tomato seedlings. Mean values (±standard errors) of the percentage of tomato seedlings showing the presence of phytophagous insects, as recorded on 22 June (A) and on 1 July (B).
The binomial GLMs performed on the presence/absence of A. craccivora, M. euphorbiae, and C. tibialis showed no significant differences between treatments (T. harzianum vs. control), between the two sampling periods (except for M. euphorbiae), nor in their interaction. The number of tomato seedlings with M. euphorbiae was higher on 22 June than on 1 July (χ2 = 7.16, df = 1, p < 0.001).
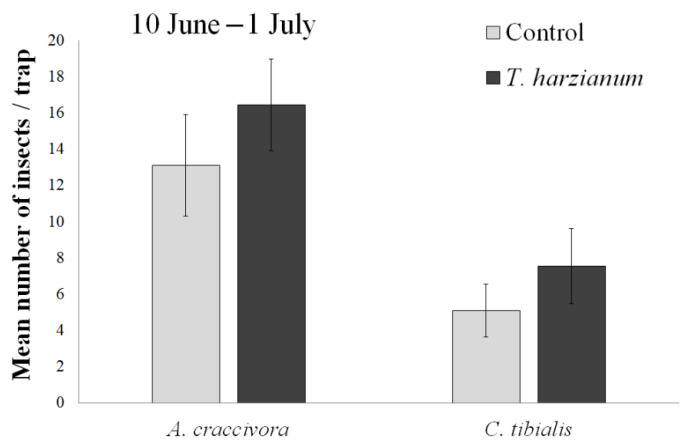
Chaetocnema tibialis, M. euphorbiae, and A. craccivora were also captured by the yellow sticky traps. From 10 June to 1 July, a total of 114 individuals of C. tibialis, 266 of A. craccivora, and 14 of M. euphorbiae were collected in yellow sticky traps. Because of the low number of M. euphorbiae specimens captured, this species was excluded from the statistical analysis. The mean numbers of C. tibialis and A. craccivora caught per trap/treatment are shown in Figure 3.
Figure 3.
Presence of phytophages on yellow sticky traps. Mean values (± standard errors) of A. craccivora and C. tibialis individuals collected per trap for each treatment from 10 June to 1 July.
For both A. craccivora and C. tibialis, no significant differences were found between treatments (F1,12 = 0.82 and F1,12 = 0.9, respectively) nor between experimental plots within treatments (F4,12 = 1.77 and F4,12 = 0.87, respectively). Even if not significant, the yellow sticky traps placed in the Trichoderma plots captured more A. craccivora and C. tibialis than the traps placed in the control plots.
3.2. Second–Fourth Month after Transplantation: Vegetative Growth, Flowering, Fruit Set, and Fruit Ripening
At this stage, since the tomato plants were large enough to tolerate the shedding of a few leaves, leaf samples were collected for observation in the laboratory (see Section 2 Materials and Methods). During this sampling period, 2473 arthropod specimens were collected, of which, 1108 and 1365 were obtained from plants with and without T. harzianum T22, respectively. The collected arthropods were grouped according to the following categories: natural enemies, piercing-sucking insects, chewing insects, and mites. We identified four families of piercing-sucking insects (Figure S1), two families of chewing insects (Figure S2), and four families of natural enemies of herbivores (predators and parasitoids, Figure S3). Among the phytophagous mites, only the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) was recorded. The PERMANOVA indicated that the abundance of these arthropod groups was affected by the sampling date (F5,348 = 55.2, p < 0.001) but not by the treatment (F1,348 = 2.01, p = 0.14). More interestingly, the interaction “Treatment × Date” was found significant (F5,348 = 2.7, p < 0.05), indicating that the arthropod community was differently affected by inoculation with T. harzianum T22 in relation to the sampling period.
3.2.1. Piercing-Sucking Herbivores
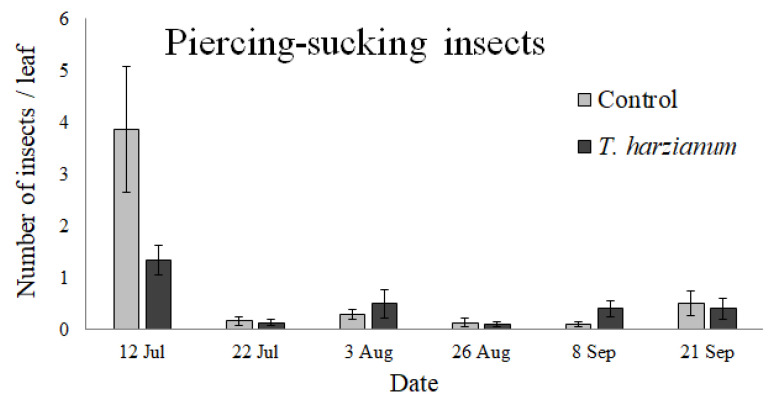
The piercing-sucking insects collected on tomato leaves belonged to the families Aphididae (two species identified: winged morph of A. craccivora and apterous morph of M. euphorbiae), Cicadellidae, Thripidae, and Pentatomidae (identified as eggs). The abundance of insects in the piercing-sucking group is shown in Figure 4. The GLMM shows that the abundance of the piercing-sucking community was affected by the sampling dates (χ2 = 48.1, df = 5, p < 0.001) but not by the inoculation of T. harzianum T22, although the probability value was close to being statistically significant (χ2 = 3.48, df = 1, p = 0.06), nor by the interaction Treatment × Date (χ2 = 6.69, df = 5, p = 0.25). It is interesting to note that during the first sampling date (12 July—flowering stage), a general increase in the abundance of piercing-sucking insects was observed on the control tomato plants. During subsequent sampling dates, the abundance of piercing-sucking arthropods was extremely low.
Figure 4.
Mean values (±standard errors) of piercing-sucking insects collected on tomato leaves during the experiment.
3.2.2. Chewing Insects
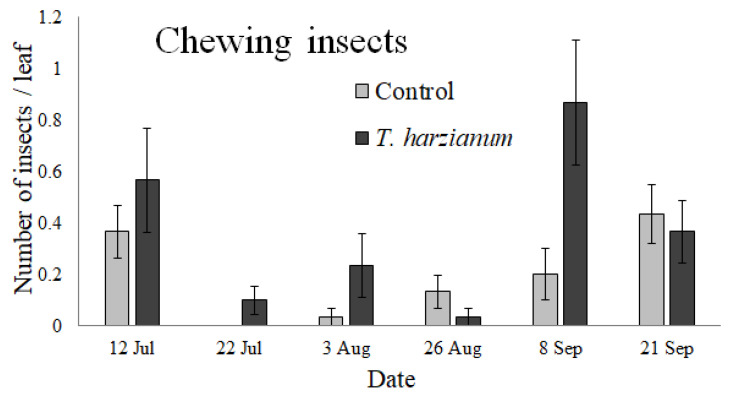
The chewing insects collected on tomato leaves belonged to the families of Noctuidae (identified as eggs and larvae) and Chrysomelidae (one species: Chaetocnema tibialis). The abundance of insects in the chewing group over time is shown in Figure 5.
Figure 5.
Mean values (±standard errors) of insect abundance in the chewing group collected on tomato leaves during the experiment.
The GLMM performed on the whole chewing insect community indicated that its abundance was not affected by the inoculation of T. harzianum T22 (χ2 = 0.75, df = 1, p = 0.38) and by the sampling dates (χ2 = 9.1, df = 5, p = 0.1). Although the probability value was close to the threshold, the interaction Treatment × Date was not significant (χ2 = 9.8, df = 5, p = 0.08). In general, the abundance of chewing arthropods increased (even if not statistically significant) in plants inoculated with T. harzianum T22 compared with control ones (this trend was particularly evident on 8 September).
3.2.3. Natural Enemies of Insects
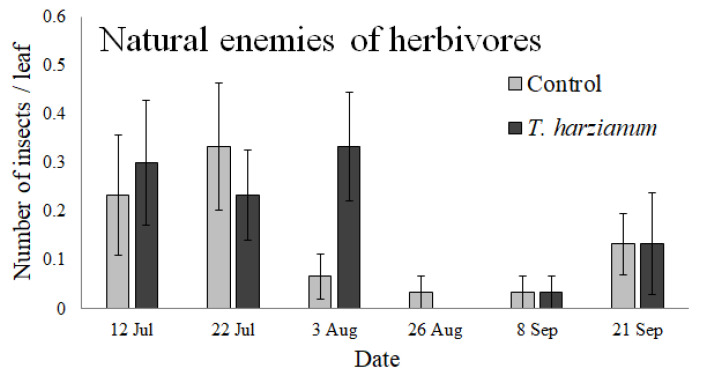
The natural enemies collected on tomato leaves belonged to the families of Syrphidae (identified as eggs or larvae), Braconidae (identified as mummies), Trichogrammatidae (adults), and Miridae (adults). We also identified two individuals belonging to the order of Araneae and one mite belonging to the family of Phytoseiidae. The abundances of natural enemies of herbivores over time are shown in Figure 6.
Figure 6.
Mean values (±standard errors) of natural enemies of herbivores (predators and parasitoids) collected on tomato leaves during the experiment.
The GLMM performed on the entire community of natural enemies indicated that its abundance was affected by the sampling dates (χ2 = 12.9, df = 5, p < 0.032), but not by T. harzianum (χ2 = 0.25, df = 1, p = 0.62) or by the interaction Treatment × Date (χ2 = 4.7, df = 5, p = 0.45). In general, the abundance of predators and parasitoids was higher in July and then decreased in the following months.
3.2.4. Spider Mites
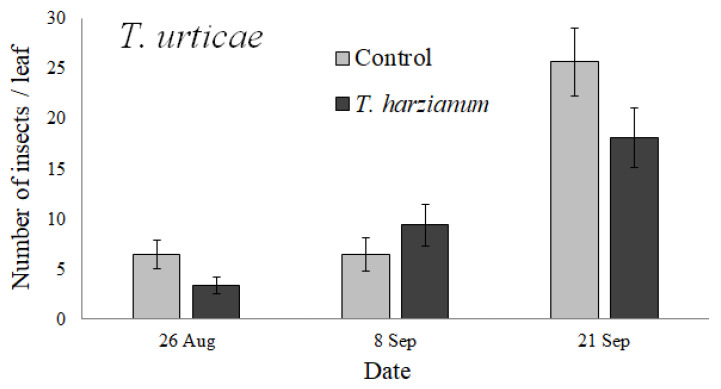
Tetranychus urticae was detected on tomato leaves only from 26 August to 21 September (Figure 7).
Figure 7.
Mean values (±standard errors) of the abundance of Tetranychus urticae collected on tomato leaves during the experiment.
The GLMM performed on T. urticae indicated that its abundance was affected by inoculation with T. harzianum T22 (χ2 = 6.9, df = 1, p < 0.01) and by Date (χ2 = 39.9, df = 2, p < 0.001); also, the interaction Treatment × Date was significant (χ2 = 9.6, df = 2, p < 0.01). For this analysis, the Date factor consisted of only three levels (fruit development and fruit ripening stages: 26 and 8 August, 21 September).
3.2.5. Leaf Miners
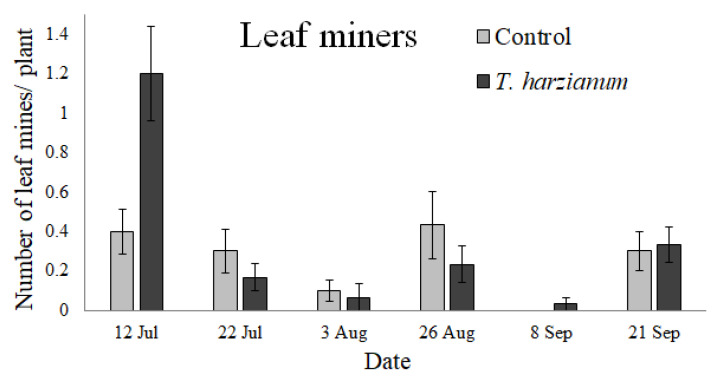
The mean number of leaf mines per plant observed in the tomato experimental field during the sampling period is reported in Figure 8. Both mines of Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae) and Liriomyza trifolii Burges (Diptera, Agromyzidae) were found.
Figure 8.
Mean values (±standard errors) of insects that cause leaf mine damage per plant associated with the tomato leaves over time.
The GLMM performed on leaf miners indicated that their abundance was affected by T. harzianum T22 (χ2 = 8.5, df = 1, p < 0.01). The sampling dates and the interaction Treatment × Date were not significant (χ2 = 5.3, df = 5, p = 0.38 and χ2 = 10.5, df = 5, p = 0.06, respectively). The leaf miners were more numerous on the inoculated tomato plants, especially during the first sampling date, then their number decreased.
3.3. QBSar
The soil samples contained several microarthropods belonging to the orders Collembola, Isopoda, Protura, and Diplura, and the class Arachnida. The sporadic presence of holometabolous insect larvae was also recorded. The differences in QBSar indexes calculated for the soil from control and Trichoderma T22 treated plots were not statistically significant (82 ± 20 and 62 ± 5, respectively; t4 = 1.9, p = 0.13).
3.4. Crop Sampling
3.4.1. Number of Fruit per Plant
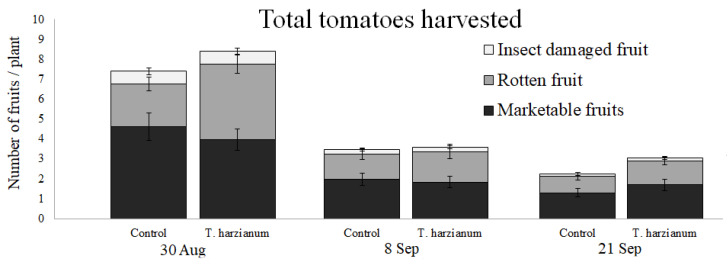
Figure 9 shows the mean number of fruit harvested per plant for the two experimental treatments during the three sampling dates. Immediately after harvesting, the tomatoes were divided into three categories: marketable, rotten, and insect-damaged fruit.
Figure 9.
Mean number of tomato fruit (±standard errors) harvested per plant in the two experimental treatments during the three sampling dates.
The GLMM indicated that the numbers of marketable, rotten, and insect-damaged tomato fruit were affected by the sampling dates (χ2 = 29.3, χ2 = 11.8, and χ2 = 17.6, respectively; df = 2, p < 0.01 in all cases). The interaction Treatment × Date was never found to be significant. Differences between treatments were found only for rotten tomato fruit (χ2 = 13.7, df = 1, p < 0.001). In general, the number of rotten fruit was a little higher in the T. harzianum T22-inoculated plants, especially during the first sampling date.
3.4.2. Weight, Length, and Width of Marketable Tomato Fruit
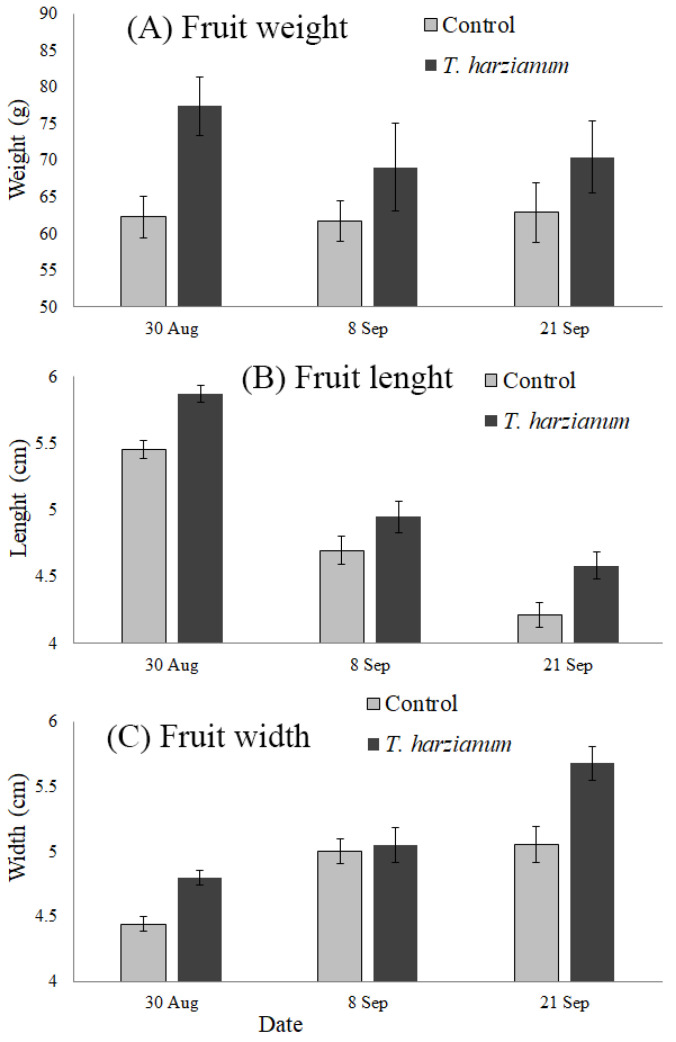
Figure 10 shows the mean values of the weight, length, and width of tomato fruit harvested from plants inoculated with T. harzianum T22 and the controls on the three sampling dates. For the fruit weight, statistically significant differences were only found between treatments (F2,12 = 8.57, p < 0.05), indicating that plants inoculated with T. harzianum T22 produced larger fruit over time.
Figure 10.
Mean values (±standard errors) of fruit weight, length, and width from tomato plants inoculated with T. harzianum T22 and control ones at the three sampling dates.
The length and width of the tomato fruit were statistically different between dates (F2,12 = 45.9 and F2,12 = 19.76, respectively; p < 0.001 in both cases) and between treatments (F1,12 = 9.86 and F1,13 = 12.26, respectively; p < 0.01 in both cases). No significant interaction Treatment × Date was found for length (F2,12 = 0.39, p = 0.69) and width (F2,12 = 2.29, p = 0.14) of tomato fruit. As for fruit weight, plants inoculated with T. harzianum T22 produced longer and wider fruit. It is interesting to note that the fruit shape of the San Marzano Kero cultivar changes with time, becoming rounder and more flattened toward the end of the production season.
3.4.3. Presence/Absence of Downy Mildew
At the beginning of August, all tomato plants observed in the field were in good health, despite the presence of downy mildew. Microscope observations in the laboratory performed on symptomatic leaves confirmed the presence of Peronospora spp. Most of the tomato leaves observed in both the control and treated plants showed only initial symptoms of downy mildew and, consequently, were less damaged. Approximately 46% of the control plants (139 out of 302) showed the initial presence of downy mildew, while only approximately 25% (48 out of 193) of the inoculated plants showed similar symptoms; this difference was statistically significant (χ2 = 22.4, df = 1, p < 0.001).
4. Discussion
Knowledge of the adverse effects of agrochemicals on human health and the environment has led to the search for environmentally friendly methods to control plant diseases and pests. Beneficial soil microbes promoting plant defenses, such as non-pathogenic bacteria [56], mycorrhizal fungi [57,58,59], and plant-growth-promoting fungi [18,60], are a possible alternative to pesticides. Fungi belonging to the genus Trichoderma are known as plant growth promoters and control agents against plant pathogens [18]. Trichoderma spp. potentiate and stimulate plant defense responses against plant pathogens [61]. Furthermore, Trichoderma fungi can directly antagonize plant pathogens through competition, antibiosis, and mycoparasitism [18,62]. After a plant is attacked by pathogenic microorganisms or herbivorous arthropods, defense mechanisms involving signal transduction pathways responding to the phytohormones salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are activated [20,21,23,63]. Plant responses induced by herbivores depend on the mode in which these organisms attack the plant, with differences between piercing/sucking and chewing organisms [28,29]. For example, the JA signaling pathway is activated in response to chewing insects, such as caterpillars, whereas piercing-sucking insects, like aphids, induce SA-related defenses [64]. SAR signaling is mainly mediated by SA-derived compounds, while ISR is regulated by the antagonistic JA/ET signaling, but dependence on SA signaling was also reported [65,66,67]. However, although the pathways regulated by JA and ET are mutually antagonistic, their synergistic interactions play a fundamental role in the ISR activation, but how plants coordinate these complex interactions is still unclear [68,69,70,71]. Although the effects of Trichoderma spp. against plant pathogens are widely documented in the laboratory and the field, fewer and mostly recent studies have addressed their effects on insect pests. Laboratory studies showed that Trichoderma spp. negatively influence both piercing-sucking [26,33,34,35,72] and chewing insects [27,33]. In addition, Trichoderma spp. activate the plant’s indirect defenses by attracting the natural enemies of pest insects [37,38,73]. Field studies confirming these results are almost absent. This is an important knowledge gap because the results obtained in the laboratory [74,75] may not be evident in more complex field conditions [76]. The implications from a practical perspective are significant.
Regarding Trichoderma spp., the only available field study investigated the effect of T. harzianum root inoculation on the community of pests and beneficial arthropods associated with maize foliage [40]. This study showed that, under field conditions, the abundance of piercing-sucking herbivores decreased, while that of natural enemies increased, confirming laboratory observations. In contrast, the abundance of chewing herbivores increased, and this result seemed to be inconsistent with what was previously observed in the laboratory.
Our study was the first investigation of the effects of T. harzianum T22 on the above-ground arthropod community in a tomato field. In addition, we investigated whether soil inoculation with T. harzianum T22 may have an effect on soil micro-arthropod biodiversity, the presence and degree of attack of Peronospora spp., and some fruit traits. This study was based on a 1-year tomato cycle in the field and, as with all experiments carried out in the field, it had intrinsic limitations due to the effects of many environmental factors that could not be controlled. Environmental variables, such as temperature, can affect the performance of Trichoderma spp. Temperature influences the spore germination and the hyphal growth, and, consequently, the plant colonization of the biocontrol agent. Recently, Di Lelio et al. [47] investigated the impact of temperature on the defense response induced by insects in tomato plants inoculated with T. harzianum T22 and T. atroviride P1. Tomato plants treated with T22 exhibited enhanced resistance, mediated by SA, toward Spodoptera littoralis and Macrosiphum euphorbiae at 25 °C, while T. atroviride P1 was shown to be more effective at 20 °C [47]. Another important environmental variable is the wind. For example, depending on the turbulence and wind speed, plant VOCs rapidly become diluted within and above the plant canopy in agroecosystems [77], limiting plant protection against insects. Other factors, such as the genotype of the plant [78], may also significantly influence the trophic interaction. However, our results provide valid information on the complex plant–arthropod–microorganism interactions that occur during a season and can be fundamental to the correct development of sustainable organic agricultural systems.
During the first month after transplanting, the tomato plants were colonized by C. tibialis adults and winged aphids of different species (no aphid colonies were observed at this time). The colonization of tomato plants by these species was not significantly different from the control when compared to the treatment with Trichoderma T22. This seemed to indicate that inoculation with T. harzianum did not alter plant attractiveness to aphids and flea beetles in the field. The trap catches confirmed this information. Traps placed inside the T.-harzianum-T22-inoculated plots captured more aphids and beetles than those placed in the control plots, but the differences were not statistically significant.
In the following months, the foliage arthropod communities were quantified. We found that the total numbers of arthropod pests and natural enemies sampled on treated and control plants were almost the same. Unlike laboratory studies, these neutral effects are widespread in ecological above-below-ground field experiments [79], where there are many variables (and all their interactions) that can influence the final results. However, we observed that the effects of T. harzianum T22 on insect abundance interacted with the sampling period. This result could be related to the different attractiveness levels of the plants with T. harzianum as seen by a particular arthropod group [37,40,74] and the period of its appearance. To better understand the plant-mediated influence of T. harzianum T22 on these dynamics, the sampled arthropods were grouped into three groups: piercing-sucking or chewing herbivores and natural enemies of pests.
Our results indicated that the arthropod composition was affected differently by inoculation with T. harzianum T22 in relation to the sampling period, although the average abundance was not significantly different from the controls. Differences between different phytophagous groups, found by Contreras-Cornejo et al. [40] on maize, were confirmed in this study as trends and were highly influenced by the sampling date. This result could be related to the different attractiveness of the plants with T. harzianum as seen by a particular arthropod group [37,74] and the period of its appearance. A general increase in piercing-sucking insects was observed in control tomato plants during the flowering stage, while the abundance of chewing arthropods increased in plants inoculated with T. harzianum T22, particularly in September (i.e., during the fruit maturity stage). In our experimental field, there was a slightly greater number of Noctuidae eggs in treated plants, probably due to the insect deposition preference. Trichoderma spp. is known to promote plant N uptake [14]. As a consequence, it is possible that chewing arthropods prefer hosts with high nutritional value for mating, oviposition, and a food source for offspring [40].
Only the abundances of the spider mite T. urticae and the leaf miners were significantly different on plants inoculated with Trichoderma T22 compared to control plants. The increased abundance of leaf mines on Trichoderma-inoculated plants is consistent with the increase in chewing insects observed by Cornejo et al. [40]. Equally, the reduction in T. urticae abundance on Trichoderma-inoculated plants was consistent with the reduction in piercing-sucking herbivores reported by Cornejo et al. [40], although spider mites, compared to other piercing-sucking herbivores, such as aphids and whiteflies, produce substantial cellular damage.
To our knowledge, this is one of the first studies investigating the effects of Trichoderma plant inoculation on T. urticae. Until now, it has only been reported that the secondary metabolites of Trichoderma caused a high mortality rate in T. urticae [36] and future studies should be performed to assess the effects of Trichoderma on the performance of T. urticae.
No significant effects on natural enemies of insects and the QBSar index were apparent from our study. In general, the number of these arthropods was not affected by T. harzianum, probably as a consequence of their relatively low number of uncontrolled environmental variables operating in the field. However, even if not significant, the number of natural enemies of insects increased on 3 August. In this period, the numbers of Syrphidae eggs and Syrphidae larvae were very high, probably due to the presence of aphids.
During the first sampling date, the number of rotten fruit was slightly higher in plants inoculated with T. harzianum T22, but the number of marketable fruit was almost the same between treatments. In addition, the weight of marketable fruit in the inoculated plants was increased by about 20%. Taking these results together, the production of fresh marketable tomatoes in the field could increase with T. harzianum T22 inoculation. Our study also confirmed the beneficial effect of T. harzianum T22 against plant pathogens. Downy mildew was recorded in both the control and treated tomato plants. However, even if the control tomato plants were more attacked by Peronospora spp. (46%) than plants treated with the T. harzianum T22 strain (25%), in both cases, the disease did not develop further. It was shown that Trichoderma positively influences tomato fruit yields as a consequence of enhanced plant growth [80]. The fungus increases the availability of nutrients to the host plant, lowers the ethylene level within the plant, and enhances the production of stimulatory compounds, such as plant growth regulators [80]. Our results confirmed that Trichoderma T22 inoculation in tomato has the potential to improve fruit yields.
5. Conclusions
This is one of the few studies that explored the potential beneficial effects related to the use of the Trichoderma harzianum strain T22 in tomato under field conditions. The interaction of the complex of the T. harzianum/tomato plant with arthropods appeared to be complex. Our results confirmed that inoculation with T. harzianum T22 could alter the arthropod community and reduce the abundance of specific pests under field conditions. The discrepancies between the results obtained in the laboratory and the field should be better investigated to understand their causes. For example, it would be necessary to know whether interactions with a herbivorous insect change with its developmental stage and how the foraging behavior of the adult is affected. Finally, our study suggested the possibility of using Trichoderma as a green alternative agent in agriculture.
Acknowledgments
The authors want to thank the “Azienda Agricola Rosa Marisa”, Pignola (PZ), Italy (40°34′ N, 15°45′ E) for their support in the realization of the experimental field and the Agenzia Lucana per lo Sviluppo e l’Innovazione in Agricoltura (ALSIA) of the Basilicata Region, Italy, for providing the metereological data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13050418/s1, Figure S1: Families of piercing-sucking insects. Mean values (±standard errors) of insect abundance in the families of the piercing-sucking group on tomato leaves over time. At the family level, no significant differences in insect abundance were observed between treatments; Figure S2: Families of chewing insects. Mean values (±standard errors) of insect abundance in the families of the chewing group on tomato leaves over time. At the family level, no significant differences in insect abundance were observed between treatments; Figure S3: Families of natural enemies of insects. Mean values (±standard errors) of insect abundance in the families of natural enemies in tomato leaves over time. At the family level, no significant differences in insect abundance between treatments were observed.
Author Contributions
Conceptualization, V.T. and D.B.; methodology, V.C., P.F. (Pierluigi Forlano), S.M.M., P.F. (Paolo Fanti), M.N., D.B. and V.T.; software, V.T.; validation, V.C., V.T., P.F. (Pierluigi Forlano), P.F. (Paolo Fanti) and D.B.; formal analysis, V.T.; data curation, V.C., P.F. (Pierluigi Forlano), D.B., P.F. (Paolo Fanti) and V.T.; writing—original draft preparation, V.C. and V.T.; revising the work critically for important intellectual content and editing, V.C., P.F. (Pierluigi Forlano), S.M.M., P.F. (Paolo Fanti), M.N., D.B. and V.T.; supervision, D.B. and V.T.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was founded by “Basilicata Region” project entitled “Study of emergencies related to alien organisms in Basilicata”; the Ph.D. project entitled “Sustainable control of insect vectors of phytopathogenic viruses in the context of climate change: the role of root symbionts”; and the PON “Research and Innovation” 2014–2020, Action IV.6, project entitled “New strategies for the eco-sustainable control of harmful insects”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siracusa L., Patanè C., Rizzo V., Cosentino S.L., Ruberto G. Targeted secondary metabolic and physico-chemical traits analysis to assess genetic variability within a germplasm collection of “long storage” tomatoes. Food Chem. 2018;244:275–283. doi: 10.1016/j.foodchem.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 2.FAO Crop Information. [(accessed on 1 March 2022)]. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/tomato/en/
- 3.Elia A., Conversa G. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur. J. Agron. 2012;40:64–74. doi: 10.1016/j.eja.2012.02.001. [DOI] [Google Scholar]
- 4.Bettini O. USDA—Foreign Agricultural Service. [(accessed on 3 March 2022)]; Available online: https://www.fas.usda.gov/data/italy-italian-processed-tomato-overview-2018.
- 5.I.Stat—Coltivazioni: Ortive. [(accessed on 7 March 2022)]. Available online: http://dati.istat.it/Index.aspx?QueryId=33703.
- 6.Abdul-Baki A.A., Teasdale J.R., Korcak R., Chitwood D.J., Huettel R.N. Fresh-market tomato production in a low-input alternative system using cover-crop mulch. HortScience. 1996;31:65–69. doi: 10.21273/HORTSCI.31.1.65. [DOI] [Google Scholar]
- 7.Perring T.M., Battaglia D., Walling L.L., Toma I., Fanti P. Chapter 2—Aphids: Biology, Ecology, and Management. In: Wakil W., Brust G.E., Perring T.M., editors. Sustainable Management of Arthropod Pests of Tomato. Academic Press; San Diego, CA, USA: 2018. pp. 15–48. [Google Scholar]
- 8.Ferreira J.H., Matthee F.N., Thomas A.C. Biological control of Eutypa lata on grapevine by an antagonistic strain of Bacillus subtilis. Phytopathology. 1991;81:283–287. doi: 10.1094/Phyto-81-283. [DOI] [Google Scholar]
- 9.Walsh U.F., Morrissey J.P., O’Gara F. Pseudomonas for biocontrol of phytopathogens: From functional genomics to commercial exploitation. Curr. Opin. Biotechnol. 2001;12:289–295. doi: 10.1016/S0958-1669(00)00212-3. [DOI] [PubMed] [Google Scholar]
- 10.Lopez D.C., Sword G.A. The Endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea) Biol. Control. 2015;89:53–60. doi: 10.1016/j.biocontrol.2015.03.010. [DOI] [Google Scholar]
- 11.Rouphael Y., Franken P., Schneider C., Schwarz D., Giovannetti M., Agnolucci M., De Pascale S., Bonini P., Colla G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015;196:91–108. doi: 10.1016/j.scienta.2015.09.002. [DOI] [Google Scholar]
- 12.Sinno M., Ranesi M., Di Lelio I., Iacomino G., Becchimanzi A., Barra E., Molisso D., Pennacchio F., Digilio M.C., Vitale S., et al. Selection of endophytic Beauveria bassiana as a dual biocontrol agent of tomato pathogens and pests. Pathogens. 2021;10:1242. doi: 10.3390/pathogens10101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo M.L., Pelizza S.A., Cabello M.N., Stenglein S.A., Scorsetti A.C. Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, Hypocreales) Biocontrol Sci. Technol. 2015;25:475–480. doi: 10.1080/09583157.2014.982511. [DOI] [Google Scholar]
- 14.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 15.Woo S.L., Ruocco M., Vinale F., Nigro M., Marra R., Lombardi N., Pascale A., Lanzuise S., Manganiello G., Lorito M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014;8:71–126. doi: 10.2174/1874437001408010071. [DOI] [Google Scholar]
- 16.Poveda J. Trichoderma as Biocontrol agent against pests: New uses for a mycoparasite. Biol. Control. 2021;159:104634. doi: 10.1016/j.biocontrol.2021.104634. [DOI] [Google Scholar]
- 17.Verma M., Brar S.K., Tyagi R.D., Surampalli R.Y., Valéro J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007;37:1–20. doi: 10.1016/j.bej.2007.05.012. [DOI] [Google Scholar]
- 18.Alfiky A., Weisskopf L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi. 2021;7:61. doi: 10.3390/jof7010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira F.V., Musumeci M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021;37:90. doi: 10.1007/s11274-021-03058-7. [DOI] [PubMed] [Google Scholar]
- 20.Tucci M., Ruocco M., De Masi L., De Palma M., Lorito M. The Beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011;12:341–354. doi: 10.1111/j.1364-3703.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoresh M., Harman G.E., Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 22.Studholme D.J., Harris B., Le Cocq K., Winsbury R., Perera V., Ryder L., Ward J.L., Beale M.H., Thornton C.R., Grant M. Investigating the beneficial traits of Trichoderma hamatum GD12 for sustainable agriculture-insights from genomics. Front. Plant Sci. 2013;4:258. doi: 10.3389/fpls.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macías-Rodríguez L., Contreras-Cornejo H.A., Adame-Garnica S.G., Del-Val E., Larsen J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020;240:126552. doi: 10.1016/j.micres.2020.126552. [DOI] [PubMed] [Google Scholar]
- 24.Digilio M.C., Cascone P., Iodice L., Guerrieri E. Interactions between tomato volatile organic compounds and aphid behaviour. J. Plant Interact. 2012;7:322–325. doi: 10.1080/17429145.2012.727104. [DOI] [Google Scholar]
- 25.Volpe V., Chitarra W., Cascone P., Volpe M.G., Bartolini P., Moneti G., Pieraccini G., Di Serio C., Maserti B., Guerrieri E., et al. The association with two different arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018;9:1480. doi: 10.3389/fpls.2018.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muvea A.M., Meyhöfer R., Subramanian S., Poehling H.M., Ekesi S., Maniania N.K. Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE. 2014;9:e108242. doi: 10.1371/journal.pone.0108242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras-Cornejo H.A., Macías-Rodríguez L., Del-Val E., Larsen J. The root endophytic fungus Trichoderma atroviride induces foliar herbivory resistance in maize plants. Appl. Soil Ecol. 2018;124:45–53. doi: 10.1016/j.apsoil.2017.10.004. [DOI] [Google Scholar]
- 28.Pieterse C.M.J., Leon-Reyes A., Van der Ent S., Van Wees S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 29.Stam J.M., Kroes A., Li Y., Gols R., van Loon J.J.A., Poelman E.H., Dicke M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014;65:689–713. doi: 10.1146/annurev-arplant-050213-035937. [DOI] [PubMed] [Google Scholar]
- 30.TariqJaveed M., Farooq T., Al-Hazmi A.S., Hussain M.D., Rehman A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021;183:107626. doi: 10.1016/j.jip.2021.107626. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee M., Mukherjee P.K., Horwitz B.A., Zachow C., Berg G., Zeilinger S. Trichoderma-plant-pathogen interactions: Advances in genetics of biological control. Indian J. Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Medina A., Fernandez I., Lok G.B., Pozo M.J., Pieterse C.M.J., Van Wees S.C.M. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017;213:1363–1377. doi: 10.1111/nph.14251. [DOI] [PubMed] [Google Scholar]
- 33.Coppola M., Cascone P., Di Lelio I., Woo S.L., Lorito M., Rao R., Pennacchio F., Guerrieri E., Digilio M.C. Trichoderma atroviride P1 colonization of tomato plants enhances both direct and indirect defense barriers against insects. Front. Physiol. 2019;10:813. doi: 10.3389/fphys.2019.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coppola M., Diretto G., Digilio M.C., Woo S.L., Giuliano G., Molisso D., Pennacchio F., Lorito M., Rao R. Transcriptome and metabolome reprogramming in tomato plants by Trichoderma harzianum strain T22 primes and enhances defense responses against aphids. Front. Physiol. 2019;10:745. doi: 10.3389/fphys.2019.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alınç T., Cusumano A., Peri E., Torta L., Colazza S. Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J. Chem. Ecol. 2021;47:455–462. doi: 10.1007/s10886-021-01260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sholla S.M.E., Kottb M.R. Bioactivity of Trichoderma (6-Pentyl α-Pyrone) against Tetranychus urticae Koch (Acari: Tetranychidae) Egypt. Acad. J. Biol. Sci. 2017;10:29–34. [Google Scholar]
- 37.Battaglia D., Bossi S., Cascone P., Digilio M.C., Prieto J.D., Fanti P., Guerrieri E., Iodice L., Lingua G., Lorito M., et al. Tomato below ground-above ground interactions: Trichoderma longibrachiatum affects the performance of Macrosiphum euphorbiae and its natural antagonists. Mol. Plant. Microbe. Interact. 2013;26:1249–1256. doi: 10.1094/MPMI-02-13-0059-R. [DOI] [PubMed] [Google Scholar]
- 38.Contreras-Cornejo H.A., Macías-Rodríguez L., Alarcón A., González-Esquivel C.E., Larsen J. Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis sonorensis. Soil Biol. Biochem. 2018;122:196–202. doi: 10.1016/j.soilbio.2018.04.013. [DOI] [Google Scholar]
- 39.Neilson E.H., Goodger J.Q.D., Woodrow I.E., Møller B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013;18:250–258. doi: 10.1016/j.tplants.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Contreras-Cornejo H.A., Viveros-Bremauntz F., Del-Val E., Macías-Rodríguez L., López-Carmona D.A., Alarcón A., González-Esquivel C.E., Larsen J. Alterations of foliar arthropod communities in a maize agroecosystem induced by the root-associated fungus Trichoderma harzianum. J. Pest Sci. 2020;94:363–374. doi: 10.1007/s10340-020-01261-3. [DOI] [Google Scholar]
- 41.Parrilli M., Sommaggio D., Tassini C., Di Marco S., Osti F., Ferrari R., Metruccio E., Masetti A., Burgio G. The role of Trichoderma spp. and silica gel in plant defence mechanisms and insect response in vineyard. Bull. Entomol. Res. 2019;109:771–780. doi: 10.1017/S0007485319000075. [DOI] [PubMed] [Google Scholar]
- 42.Sotto-Alviola M.P., Lit I.L., Caasi-Lit M.T., Cuevas V.C. Springtail (Collembola) abundance in Trichoderma- enhanced and conventional cabbage (Brassica oleracea L.) plots in Sariaya, Quezon, Philippines. Philipp. Entomol. 2017;31:103–116. [Google Scholar]
- 43.Copetta A., Lingua G., Berta G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza. 2006;16:485–494. doi: 10.1007/s00572-006-0065-6. [DOI] [PubMed] [Google Scholar]
- 44.Kovach-Orr C., Fussmann G.F. Evolutionary and plastic rescue in multitrophic model communities. Philos. Trans. R. Soc. B Biol. Sci. 2013;368:20120084. doi: 10.1098/rstb.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soler R., Erb M., Kaplan I. Long distance root-shoot signalling in plant-insect community interactions. Trends Plant Sci. 2013;18:149–156. doi: 10.1016/j.tplants.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Bazghaleh N., Prashar P., Woo S.L., Vandenberg A. Effects of lentil genotype on the colonization of beneficial Trichoderma species and biocontrol of Aphanomyces root rot. Microorganisms. 2020;8:1290. doi: 10.3390/microorganisms8091290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Lelio I., Coppola M., Comite E., Molisso D., Lorito M., Woo S.L., Pennacchio F., Rao R. Temperature differentially influences the capacity of Trichoderma species to induce plant defense responses in tomato against insect pests. Front. Plant Sci. 2021;12:678830. doi: 10.3389/fpls.2021.678830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menta C., Conti F.D., Pinto S., Bodini A. Soil Biological Quality Index (QBS-Ar): 15 years of application at global scale. Ecol. Indic. 2018;85:773–780. doi: 10.1016/j.ecolind.2017.11.030. [DOI] [Google Scholar]
- 49.Parisi V., Menta C., Gardi C., Jacomini C., Mozzanica E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005;105:323–333. doi: 10.1016/j.agee.2004.02.002. [DOI] [Google Scholar]
- 50.Kenward M.G., Roger J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. doi: 10.2307/2533558. [DOI] [PubMed] [Google Scholar]
- 51.Burnham K.P., Anderson D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004;33:261–304. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 52.Hammer Ø., Harper D.A.T., Ryan P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001;4:9. [Google Scholar]
- 53.Team R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [(accessed on 10 September 2021)]. Available online: Https://Www.R-Project.Org/ [Google Scholar]
- 54.Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 55.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. LmerTest package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 56.Liu H., Brettell L.E., Qiu Z., Singh B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020;25:733–743. doi: 10.1016/j.tplants.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Jung S.C., Martinez-Medina A., Lopez-Raez J.A., Pozo M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012;38:651–664. doi: 10.1007/s10886-012-0134-6. [DOI] [PubMed] [Google Scholar]
- 58.Sanmartín N., Pastor V., Pastor-Fernández J., Flors V., Pozo M.J., Sánchez-Bel P. Role and mechanisms of callose priming in mycorrhiza-induced resistance. J. Exp. Bot. 2021;71:2769–2781. doi: 10.1093/jxb/eraa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pozo de la Hoz J., Rivero J., Azcón-Aguilar C., Urrestarazu M., Pozo M.J. Mycorrhiza-induced resistance against foliar pathogens is uncoupled of nutritional effects under different light intensities. J. Fungi. 2021;7:402. doi: 10.3390/jof7060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitti A., Sofo A., Scopa A., Nuzzaci M. Sustainable agricultural practices in disease defence of traditional crops in southern Italy: The case study of tomato cherry protected by Trichoderma harzianum T-22 against cucumber mosaic virus (CMV) BT. In: Vastola A., editor. The Sustainability of Agro-Food and Natural Res. Springer International Publishing; Cham, Switzerland: 2015. pp. 133–143. [Google Scholar]
- 61.Basińska-Barczak A., Błaszczyk L., Szentner K. Plant cell wall changes in common wheat roots as a result of their interaction with beneficial fungi of Trichoderma. Cells. 2020;9:2319. doi: 10.3390/cells9102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyśkiewicz R., Nowak A., Ozimek E., Jaroszuk-ściseł J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022;23:2319. doi: 10.3390/ijms23042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponzio C., Gols R., Pieterse C.M.J., Dicke M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 2013;27:587–598. doi: 10.1111/1365-2435.12035. [DOI] [Google Scholar]
- 64.Walling L.L. The Myriad plant responses to herbivores. J. Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- 65.Reimer-Michalski E.-M., Conrath U. Innate immune memory in plants. Semin. Immunol. 2016;28:319–327. doi: 10.1016/j.smim.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Mauch-Mani B., Baccelli I., Luna E., Flors V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017;68:485–512. doi: 10.1146/annurev-arplant-042916-041132. [DOI] [PubMed] [Google Scholar]
- 67.Bürger M., Chory J. Stressed out about hormones: How plants orchestrate immunity. Cell Host Microbe. 2019;26:163–172. doi: 10.1016/j.chom.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salas-Marina M.A., Silva-Flores M.A., Uresti-Rivera E.E., Castro-Longoria E., Herrera-Estrella A., Casas-Flores S. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 2011;131:15–26. doi: 10.1007/s10658-011-9782-6. [DOI] [Google Scholar]
- 69.Contreras-Cornejo H.A., Macías-Rodríguez L., Beltrán-Peña E., Herrera-Estrella A., López-Bucio J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin- dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungus Botrytis cinerea. Plant Signal. Behav. 2011;6:1554–1563. doi: 10.4161/psb.6.10.17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bari R., Jones J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 71.Perazzolli M., Dagostin S., Ferrari A., Elad Y., Pertot I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol. Control. 2008;47:228–234. doi: 10.1016/j.biocontrol.2008.08.008. [DOI] [Google Scholar]
- 72.Islam M.S., Subbiah V.K., Siddiquee S. Efficacy of entomopathogenic Trichoderma isolates against sugarcane woolly aphid, Ceratovacuna lanigera Zehntner (Hemiptera: Aphididae) Horticulturae. 2022;8:2. doi: 10.3390/horticulturae8010002. [DOI] [Google Scholar]
- 73.Coppola M., Cascone P., Chiusano M.L., Colantuono C., Lorito M., Pennacchio F., Rao R., Woo S.L., Guerrieri E., Digilio M.C. Trichoderma harzianum enhances tomato indirect defense against aphids. Insect Sci. 2017;24:1025–1033. doi: 10.1111/1744-7917.12475. [DOI] [PubMed] [Google Scholar]
- 74.Guerrieri E., Lingua G., Digilio M.C., Massa N., Berta G. Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol. Entomol. 2004;29:753–756. doi: 10.1111/j.0307-6946.2004.00644.x. [DOI] [Google Scholar]
- 75.Prieto J.D., Castañé C., Calvet C., Camprubi A., Battaglia D., Trotta V., Fanti P. Tomato belowground–aboveground interactions: Rhizophagus irregularis affects foraging behavior and life history traits of the predator Macrolophus pygmaeus (Hemiptera: Miridae) Arthropod-Plant Interact. 2017;11:15–22. doi: 10.1007/s11829-016-9465-5. [DOI] [Google Scholar]
- 76.Colella T., Candido V., Campanelli G., Camele I., Battaglia D. Effect of irrigation regimes and artificial mycorrhization on insect pest infestations and yield in tomato crop. Phytoparasitica. 2014;42:235–246. doi: 10.1007/s12600-013-0356-3. [DOI] [Google Scholar]
- 77.Loivamäki M., Mumm R., Dicke M., Schnitzler J.-P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc. Natl. Acad. Sci. USA. 2008;105:17430–17435. doi: 10.1073/pnas.0804488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivelli A., Trotta V., Toma I., Fanti P., Battaglia D. Relation between plant water status and Macrosiphum euphorbiae ( Hemiptera: Aphididae ) population dynamics on three cultivars of tomato. Eur. J. Entomol. 2013;110:617–625. doi: 10.14411/eje.2013.084. [DOI] [Google Scholar]
- 79.Heinen R., Biere A., Harvey J.A., Bezemer T.M. Effects of soil organisms on aboveground plant-insect interactions in the field: Patterns, mechanisms and the role of methodology. Front. Ecol. Evol. 2018;6:106. doi: 10.3389/fevo.2018.00106. [DOI] [Google Scholar]
- 80.Gravel V., Antoun H., Tweddell R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA) Soil Biol. Biochem. 2007;39:1968–1977. doi: 10.1016/j.soilbio.2007.02.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.