Abstract
The importance of circulating tumor cells (CTC) is well recognized. However, the biological characteristics of CTC in the bloodstream have not yet been examined in detail, due to the limited number of CTC cell lines currently available. Thirty-nine CTC cell lines were reported by 2021. For successful cell culturing, these CTC cell lines were reviewed. Previous studies on short-term cultures of CTC also analyzed approaches for establishing the long-term culture of CTC. Negative selection, hypoxic conditions, three-dimensional conditions, and careful management are preferable for the long-term culture of CTC. However, the establishment of CTC cell lines is dependent on the specific characteristics of each cell type. Therefore, a method to establish CTC cell lines has not yet been developed. Further efforts are needed to resolve this issue.
Keywords: cell line, circulating tumor cell, long-term culture, short-term culture
1. Introduction
1.1. Recent Issues in Circulating Tumor Cell Research
The importance of circulating tumor cells (CTC) has recently been recognized. Although the cultivation of CTC is very promising as a preclinical model for human cancer, difficulties are associated with establishing CTC cell lines due to the low number of CTC in blood and the majority of these cells not being viable. Only a limited number of permanent cell lines of CTC are currently available and their management is more challenging than that of standard cell lines.
CTC cell lines are important for examining the biology of CTC, but not for providing quick feedback to cancer patients. Furthermore, the translational relevance of cell lines is often questioned because prolonged cultures and multiple passages lead to phenotypes that are no longer representative of the original tumor in terms of cell epigenetics and gene expression profiles [1]. Therefore, the focus of research has recently shifted to the clinical use of short-term cultures of CTC. Therefore, the biology of CTC has not yet been investigated in detail.
1.2. Our Previous Experience
We previously established cancer cell lines from several tissue specimens, including esophageal and pancreatic cancer [2,3]. We identified monolayer epithelial growth potential and cell line establishment capability as significant prognostic factors in patients with esophageal squamous cell cancer (ESCC) [4,5].
The systematic detection of CTC was initially reported by other research groups. Racila et al. [6] used an immunomagnetic separation and flow cytometry protocol that relied on epithelial cell adhesion molecule (EpCAM)-positive expression. We subsequently developed a short-term culture of CTC using the same type of bead selection method and demonstrated that the colonies that formed contained a slightly higher number of CTC [7].
The majority of conventional cancer cell lines generally proliferate on a two-dimensional (2D) environment, in contrast to the 3D environment of most CTC cell lines, such as spheroids or organoids. We established a small cell esophageal cancer cell line (TYUC-1) that actively grew with sphere formation in a standard culture flask from a tissue specimen [8]. This experience prompted us to establish CTC cell lines.
1.3. Aim of This Review
Concerning the approaches to long-term culture, we also examined the literature for short-term cultures. Our aim was to obtain a more detailed understanding of the characteristics of CTC and several methods that support the establishment of long-term cultures from short-term cultures. Based on previous findings and our own research, we herein discussed critical issues facing the development of CTC culture methodologies and proposed a rational strategy for the long-term culture of CTC.
In this review, a long-term culture (CTC cell line) is defined as a culture period of longer than 1 year or continuous passages for more than 6 months. A short-term culture is defined as a culture period of longer than 1 week. We briefly discussed culture-related factors, such as methods for enriching CTC, the characteristics of CTC in the bloodstream, and stem cell biology. We excluded methods for ultra-short term (less than 1 week) cultures and expression assays because the focus of these methods is clinical evaluation, not long-term cultures. We mainly concentrated on the development of a reproducible protocol for establishing CTC cell lines. We also excluded details on the CTC capture system, clinical applications, genetic analyses, CTC-derived xenografts, and cultures of bone marrow disseminated tumor cells.
2. Long-Term Culture of CTC
2.1. Overview
Thirty-nine CTC cell lines were reported by 2021: 11 from colon cancer, 13 from breast cancer, 2 from prostate cancer, 2 from gastroesophageal cancer, 5 from small cell lung cancer, 2 from non-small cell lung cancer, and 4 from malignant melanoma. Establishment rates ranged between 1 and 17%, with most studies achieving a rate <10% (Table 1 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]).
Table 1.
Summary of CTC cell lines.
| Cell Line | Type of Cancer | Establishing Rate | Culture Period or Passage | Doubling Time | Xenograft | Specific Characteristics | References |
|---|---|---|---|---|---|---|---|
| CTC-1, CTC-2, CTC-3 | Breast cancer | 3/38 | Several passages? | N/A | Yes (all 3 CTC lines) | EpCAM(−) | Zang et al. (2013) [9] |
| BRx33, BRx07, BRx68, BRx50, BRx42, BRx61 | Breast cancer | 6/36. Several cell lines from the same patient at different times | >6–12 months | 3 days to 3 weeks | Yes (3 out of 5 CTC cells lines) | ER(+), PIK3CA, TP53, KRAS, FGFR2 | Yu et al. (2014) [10] |
| MSK-PCa5 | Prostate cancer | 1/17 | Passage was performed weekly at a 1:3 ratio | 1 week | Yes | CK(+), AR(+),High PSA, PTEN(−), RB(−) | Gao et al. (2014) [11] |
| CTC-MCC-41 | Colon cancer | 1/50 (CTC-positive patients) | >16 months | 20 h | Yes | EpCAM(+), Snail, ALDH1, CD133, | Cayrefourcq et al. (2015) [12] |
| BHGc7, BHGc10 | Small cell lung cancer | 2/30 | >4 months. Long-term culture was confirmed by two follow-up studies | N/A | Yes | Carbonic anhydrase (CAIX) | Hamilton et al. (2015) [13], (2016) [14] |
| N/A (one line was named CTC-TJH-01 by Que [20]) | Lung adenocarcinoma | 2/35 | >6 months | N/A | N/A | Wang et al. (2016) [15] | |
| BHGc16, BHGc26, UHGc5 | Small cell lung cancer | N/A | N/A | N/A | N/A | CD133, CD24, SOX-2 | Klameth et al. (2017) [16] |
| CTC44, CTC45 (both established from the same patient) | Colorectal cancer | 3/4. Several cell lines from the same patient at different times | >20 passages | N/A | Yes | CD133, CD26, ALDH1A1, CD44 | Grillet et al. (2017) [17] |
| BRx82, BRx142 | Breast cancer | N/A | N/A | 101.2 and 59.7 h | N/A | Able to cryopreserve | Sandlin et al. (2017) [18] |
| CTC-MCC-41.4, 41.5A. 41.5B. 41.5C, 41.5D. 41.5E, 41.5F, 41.5G (established from the same patient) | Colon cancer | N/A. Several cell lines from the same patient at different times (8 cell lines) | N/A | N/A | N/A | ALDH1, CD44, panCD66, EpCAM | Soler et al. (2018) [19] |
| CTC-TJH-01 | Non-small cell lung cancer | 1/89 | 24 months | N/A | Yes | CXCL5, CD44, ALDH1 | Que et al. (2019) [20] |
| CTC-3 | Breast cancer | 1/16 | >2 years | N/A | Yes | CD44 | Zhao et al. (2019) [21] |
| CTC-ITB-01 | Breast cancer | 1/50 | >4 years | N/A | N/A | E-Cadherin, EpCAM, K19, CD24, Twist 1 | Koch et al. (2020) [22] |
| UWG01CTC, UWG02CTC | Gastroesophageal cancer | 2/23 | >12 months | N/A | Yes | EpCAM(−), CD56, (UWG01CTC) or EpCAM(+), CK(+), CD44, E-Cadherin | Brungs et al. (2020) [23] |
| Mel-167, PEM-22, Mel-182, PEM-78 | Melanoma | 4/37. Several cell lines from the same patient at different times (Mel 182-1, Mel 182-2) | N/A | N/A | Yes | BRAF-mutant NG-2, MLANA | Hong et al. (2021) [24] |
| EMC-Pca-41 | Prostate cancer | 1/40 | >1 year, 10 passages | N/A | N/A | TMPRSS2-ERG fusion, loss of PTEN | Mout et al. (2021) [25] |
N/A: not available.
Several CTC cell lines were established from the same patients at different time points during their follow-up [17,19]. Therefore, these patients had specific CTC that were adapted for in vitro culture conditions. Cayrefourcq et al. [12] reported that two spheroids from the same cell line exhibited different characteristics, which revealed the non-uniform characteristics of CTC.
Epithelial–mesenchymal transition (EMT) cells should survive in the blood because they negligibly express EpCAM. Therefore, EpCAM is not a standard marker for the establishment of cell lines. However, some cells express EpCAM and maintain epithelial characteristics. Additionally, many cells express stem cell markers, such as CD133, CD44, CD24, and ALDH1. These findings indicate that CTC have a mixed epithelial phenotype, an intermediate epithelial/mesenchymal phenotype, and stem cell-like properties.
Stem cell concepts have been employed for long-term CTC cultures. CTC as non-adherent spheres may reflect the intrinsic properties of cancer stem cells that remain viable in the bloodstream after the loss of their attachment to the basement membrane [26]. However, CTC with these stem cell features have not yet been isolated for CTC cultures. We were also unable to establish a primary culture of sorted stem marker (p75NTR+) CTC from 23 patients [27].
2.2. Culture Protocol
2.2.1. Blood Samples
The majority of CTC cell lines have been established using 6 to 18 mL of blood. A blood volume >10 mL is a suitable source for CTC cultures. Furthermore, since the establishment of cell lines was found to be slightly easier from patients with a high CTC count (>30/mL), a CTC count >300 is preferable for the success of cultures. However, a high CTC count only does not guarantee the establishment of a permanent cell line. Furthermore, a conventional CTC count is generally assessed using EpCAM(+) cells. Since EpCAM(−) CTC have been overlooked, CTC counts are not accurate. The most recent study on CTC used a 96 mL sample obtained from leukapheresis for a cell culture [25]. Leukapheresis is used in clinical settings and is not harmful; however, more than 2 h is needed to obtain larger numbers of CTC from patients (Table 2 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]).
Table 2.
Culture protocols of CTC cell lines.
| Cell Line | Blood Volume | CTC Count/mL | CTC Enrichment and Isolation | Culture Type | Environment | Culturing Conditions | Culture Medium | References |
|---|---|---|---|---|---|---|---|---|
| CTC-1, CTC-2, CTC-3 | 20 to 45 mL | Undetectable by CellSearch | FACS (CD45, ALDH1, EpCAM) | 2D. single cell to colony formation | 5% CO2. Normoxic conditions | Adherent (1–8 days), medium change (8–21 days). The EpCAM(−)/ALDH1(+)/CD45(−) population was transferred to 24- or 6-well plates | Stem cell culture medium. Insulin, hydrocortisone, B-27, EGF, FGF-2, (1–8 days). EpiCult-C medium supplemented with 10% FCS (8–21 days). DMEM/F12 supplemented with 10% FCS (from day 22) | Zang et al. (2013) [9] |
| BRx33, BRx07, BRx68, BRx50, BRx42, BRx61 | 6 to 18 mL | 3–3000/6 mL | CTC iChip | 3D Spheroid | 4% O2 | Ultra-low attachment plate. Medium changes were performed under a microscope | Serum-free, EGF, FGF, B-27 | Yu et al. (2014) [10] |
| MSK-PCa5 | 8 mL | >100 count/8 mL | RosetteSep CD45-depleted Cocktail | 3D Organoid. Start to grow as spheroids after 10 days | N/A | Matrigel | DMEM/F12, EGF, R-spondin 1, Noggin, FGF10, FGF2, DHT, Nicotinamide Acros, A83-01, SB202190, Y-27632, B27, N-Acetyl-L-cysteine, Glutamax, HEPES, Primocin | Gao et al. (2014) [11] |
| CTC-MCC-41 | 10 mL | 302 count/7.5 mL | RosetteSep CD45-depleted Cocktail | 3D Spheroid | Initial environment: 2% O2. Maintenance: 5% CO2, Normoxic conditions | 24-well non-adherent plate. T25 flask (maintenance) | Initial medium: Stem cell culture medium, DMEM/HamF12 2% FCS, insulin, N2 component, EGF, L-Glutamine, FGF2. Second medium: RPMI1640 EGF, FGF2, insulin-transferrin-selenium supplement, L-Glutamine. Maintenance: N/A | Cayrefourcq et al. (2015) [12] |
| BHGc7, BHGc10 | N/A | N/A | Ficoll-Hypaque density gradient | 3D and 2D | Normoxic conditions | 12-well adherent plate. Normoxic conditions | Initial medium: Serum-free, RPMI-1640, insulin, IGF-1, transferrin, selenite, Maintenance: RPMI-1640, 10% FCS | Hamilton et al. (2015) [13] (2016) [14] |
| N/A (one line was named as CTC-TJH-01 in Que Z paper [20]) | 2 mL | 130 count/2 mL | Microfluidics-based immunomagnetic isolation. EpCAM coated and EGFR coated immunomagnetic microbeads | N/A | 3% O2, 5% CO2 (1–14 days). 5% CO2 Normoxic conditions | 96-well non-adherent plate. | Initial medium: RPMI1640, EGF, FGF, B27. Maintenance: RPMI-1640, 10% FCS | Wang et al. (2016) [15] |
| BHGc16, BHGc26, UHGc5 | N/A | N/A | N/A | 3D and 2D | Normoxic conditions | N/A | Initial medium: N/A. Maintenance: RPMI-1640, 10% FCS | Klameth et al. (2017) [16] |
| CTC44, CTC45 (both established from same patient) | 8–10 mL | N/A | RosetteSep CD45-depleted Cocktail | 3D | N/A | Ultra-low attachment 24-well plate | DMEM/F12, 2% FCS, L-Glutamine, N2 supplement, EGF, FGF2 | Grillet et al. (2017) [17] |
| BRx82, BRx142 | N/A | N/A | N/A | 3D | N/A | 6-well ultra-low adhesion plate | Initial medium: N/A, Maintenance: RPMI1640 EGF, FGF2, B-27 | Sandlin et al. (2017) [18] |
| CTC-MCC-41.4, 41.5A. 41.5B. 41.5C, 41.5D. 41.5E, 41.5F, 41.5G, (established from same patient) | 10 mL | 286/7.5 mL–3278/7.5 mL | RosetteSep CD45-depleted Cocktail | 3D | N/A | 24-well non-adherent plate (1st week). New 24-well non-adherent plate (2nd week). T25 flask (maintenance) | RPMI 1640, EGF, FGF-2, insulin-transferrin-selenium supplement, L-Glutamine | Soler et al. (2018) [19] |
| CTC-TJH-01 | 5 mL | 130 count | A mixture of EpCAM and EGFR coated immunomagnetic microbeads in microfluidic Herringbone-Chip | 2D | N/A | Non-adherent plate | Initial medium: RPMI1640, EGF, FGF, B27. Maintenance: RPMI-1640, 10% FCS | Que et al. (2019) [20] |
| CTC-3 | 6 mL | N/A | RosetteSep CD45-depleted Cocktail | 2D | Normoxic conditions, 5% CO2 | 6-well Matrigel-coated plate (2 weeks), normal 6-well plate (medium change every 2–3 days). Final culture in a T25 flask | DMEM/RPMI1640, 10% FCS, EGF, FGF, Nu-Serum, L-Glutamine | Zhao et al. (2019) [21] |
| CTC-ITB-01 | 7.5 mL | 1547/mL | RosetteSep CD45-depleted Cocktail | 3D and 2D | Normoxic conditions, 5% CO2 (1–14 days) | 96-well normal plate, then transferred to a 12-well culture dish | RPMI1640 10% FCS, L-Glutamine, insulin-transferrin selenium-A, FGF2, EGF, hydrocortisone, cholera toxin | Koch et al. (2020) [22] |
| UWG01CTC, UWG02CTC | 15 mL | 3/mL (UWG01CTC), 109/mL (UWG02CTC) | RosetteSep CD36-depleted Cocktail | 2D or 3D | Normoxic or hypoxic conditions | 24-well ultra-low attachment plate | DMEM/F12, EGF, FGF, N2 supplement (normoxic conditions) or DMEM/F12, 10% FCS (hypoxic conditions) | Brungs et al. (2020) [23] |
| Mel-167, PEM-22, Mel-182, PEM-78 | 10 mL | N/A | CTC iChip | 3D | Hypoxia, 5% CO2, 4% O2 | 24-well ultra-low attachment plate (4–8 weeks). Use of a 3D fibrin Matrigel culture. Finally, a switch to an anchorage-independent culture | RPMI 1640, EGF, FGF2, B-27, Heparin, Y-27632 | Hong et al. (2021) [24] |
| EMC-PCa-41 | 5 L | 5312/96 mL | Leukapheresis, RosetteSep CD45-depleted Cocktail | 3D Organoid | Normoxic conditions | 24-well plate Matrigel droplets. Organoids were collected and resuspended in a new plate | Initial medium: Prostate growth medium (PGM) or adjusted prostate cancer organoid medium (APCOM). Maintenance: AdMEM/F12 | Mout et al. (2021) [25] |
EGF: Epidermal growth factor, FGF: Fibroblast growth factor.
2.2.2. CTC Enrichment
An enrichment step for CTC is indispensable before cells are cultured. Viable CTC isolation may be divided into three types: antibody-based, physical property-based, and function-based approaches. The most successful enrichment method for long-term cultures is the negative selection method (the density gradient method or RosetteSep®). Although antibody-based approaches were employed in three studies, capture with antibodies against surface antigens is harmful, precluding its use for CTC cultures [9,15,20]. CTC analysis differs from CTC culture [28,29]. Our failure to establish a stem cell marker-selected CTC culture appeared to be partially attributed to the disadvantages associated with the positive selection method [27].
The use of a microfluidic device and nanotechnology-based method may be less harmful to CTC. One group used microfluidics-based immunomagnetic isolation [15,20] and another group used the CTC iChip [10,24]. Thus, microfluidic devices are also useful for long-term culture.
The RosetteSep® antibody cocktail, which crosslinks unwanted cells in human whole blood to multiple red blood cells to form immune rosettes, has been used to enrich viable CTC and has contributed to the successful establishment of long-term cultures of CTC. Although negative selection will affect CTC purity, this is not critical for CTC cultures because leukocytes do not have a negative impact on cell cultures and are eliminated during the culture [30,31]. Nevertheless, since non-cancer cells may inhibit CTC growth, mimicking the in vivo tumor microenvironment as closely as possible may be important for the successful establishment of CTC cultures [21] (Table 2 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]).
2.2.3. Culture Conditions
Three-dimensional conditions with a low attachment plate or gel formation are generally employed for cultures. Cells have been cultured in spheroid or organoid 3D models. Yu et al. [10] previously suggested that non-adherent culture conditions were critical because CTC senesced after a few cell divisions in adherent monolayer cultures. They used ultra-low attachment culture dishes, while the other five conditions involving Matrigel and feeder cells failed [10]. However, attachment or Matrigel-coated plates were utilized for one-third of the cultures of CTC cell lines. Therefore, non-adherent conditions are not the gold standard (Table 2 [9,11,13,21,22,25]).
Hypoxic conditions have been recommended for stem cell cultures. Two-thirds of studies initially cultured cells under hypoxic conditions for several days. Hypoxic conditions generally use low attachment plates in the first step. However, one-third of studies used normoxic conditions [9,13,16,21,22,23,25]. Therefore, hypoxic conditions are also not always inevitable. Furthermore, several different hypoxic conditions (2, 4, and 8%) have been used during the establishment step for CTC cell lines, and the most suitable conditions have not yet been identified. Hypoxic conditions are used to maintain CTC and deplete normal cells. Cells are then transferred to the second stage using normoxic conditions [12,15] (Table 2).
2.2.4. Culture Medium
Specific growth factor-enriched medium with or without fetal calf serum (FCS) has been used as the start medium. The culture medium is then changed to ordinal standard medium. The culture medium is important during the initial 2 weeks. The proliferation of most cells peaks after approximately 21 days. CTC with proliferative potential may then grow without special growth supplement, except for FCS (Table 2).
Epidermal growth factor (EGF) and basic fibroblast growth factor (FGF) are often listed in recipes for stem cell cultures. Recipes for serum-free CTC culture media mostly include proprietary B27 and N2 supplements, together with insulin, transferrin, and selenium. Y27632, a cell-permeable inhibitor of Rho-associated coiled-coil containing protein kinase, is also used. However, the optimal conditions for ex vivo CTC cultures have yet to be established.
2.2.5. Growth Progression of Each CTC Cell Line
To appropriately culture CTC, it is important to understand the progression of CTC growth in each step. In this section, we focus on the growth progression of selected CTC cell lines.
-
1.
CTC-1, CTC2, and CTC3
Zhang et al. [9] previously reported the culture of captured cells in a suspension using stem cell medium for the first week, and this was followed by a medium for epithelial cells. Cells were monitored for survival and growth over 28 days, and colonies were initiated from a single cell. Thirteen, seven, and eleven colonies were present by day 21 and were named CTC-1, CTC-2, and CTC-3, respectively.
-
2.
CTC-MCC-41
In the case of CTC-MCC-41 cells, viable CTC were still observed after 4 days of culture in serum-reduced medium under hypoxic conditions. After 10 days, CTC started to proliferate and formed spheres. After a few weeks, the cell culture was switched to normoxic conditions. Single colonies from the CD45(−) population that appeared during the first week of the cell culture were transferred into a 24-well plate for further growth [12].
-
3.
BHGc7 and BHGc10
BHGc cells initially grew as typical small spheroids that eventually showed the outgrowth of adherent tumor cells and shedding of apoptotic cell fragments [14]. Proliferating CD56-positive CTC cultures displayed loosely attached or compact spheroids as well as an adherent morphology from the same source [13]. Furthermore, all small cell lung cancer (SCLC) CTC cultures showed the spontaneous formation of large multicellular aggregates that increased in diameter to 1–2 mm, designated as tumor spheres, under regular cell culture conditions [16].
-
4.
CTC-TJH-01
During an ex vivo culture of CTC-TJH-01 cells, contaminating blood cells and a small number of CTC underwent rapid cell death. The majority of surviving CTC adhered to the plate, but remained dormant throughout the initial two weeks, suggesting the low in vitro clonogenic potential of CTC. CTC started to proliferate after two weeks, and the culture was switched back to normoxic conditions. CTC slowly proliferated, formed clusters, and then exhibited sustained proliferation [15,20].
-
5.
CTC-ITB-01
CTC-ITB-01 cells grew in parallel as adherent and non-adherent cell fractions with various cell sizes. Non-adherent cells grew from adherent cells. CTC showed an interchangeable adherent and non-adherent cell population similar to cancer stem cells [22].
-
6.
UWG01CTC and UWG02CTC
In the case of UWG01CTC and UWG02CTC cells, viable, relatively pure cultures were observed within 3 weeks and rapidly expanded. UWG01CTC is adherent and requires trypsinization for passaging; however, a loose adherent spheroid phenotype was induced in a hypoxic environment and serum-free media. In contrast, UWG02CTC grew in long mucinous, loosely aggregated, and weakly adherent strands that only required gentle mechanical dissociation for passaging [23].
-
7.
CTC-3
CTC-3 cells began to proliferate and form clusters after 14 days of culture [21]. However, other detailed descriptions were not provided.
2.2.6. Timing of and Transfer Protocol for Each Cell Line
Several conditions were used for the expansion of CTC. However, suitable conditions depended on the characteristics of each cancer cell. Furthermore, the optimal timing and culture conditions for CTC expansion have not yet been established. Representative cases are described below.
-
1.
CTC-1, CTC-2, and CTC-3
Adherent plates were used during the first week with growth factor-enriched stem cell culture medium. The medium was changed to EpiCult-C medium between 8 and 21 days. Standard medium with 10% FCS was then used for maintenance [9].
-
2.
Mel-167, PEM-22, Mel-182, and PEM-78
CTC were cultured in 24-well ultra-low attachment plates for between 4 and 8 weeks. A 3D fibrin Matrigel culture was then used, and this was followed by a switch to an anchorage-independent culture [24].
-
3.
CTC-MCC-41
CTC were cultured in 24-well non-adherent plates for the 1st week. Cells were then transferred to new 24-well non-adherent plates in the 2nd week and maintained in a T25 flask [19].
-
4.
CTC-3
A Matrigel-coated plate was used in the first 2 weeks, and cells were then transferred to a normal 6-well plate [21].
3. Short-Term Culture
To establish approaches to long-term culture, short-term culture was reviewed. Information that will contribute to the establishment of long-term cultures of CTC was obtained (Table 3 and Table 4).
Table 3.
Summary of short-term cultures.
| Research Group | Cancer Type | Establishing Rate | CTC Count | CTC Detection | Culture Period | Additional Information |
|---|---|---|---|---|---|---|
| Makino et al. (1999) [7] | Gastrointestinal cancer | 4/20 | 1–123/mL by cytospin | HEA(+), CK(+) | 14 days | High CTC were more likely to form spheroids |
| Paris et al. (2009) [32] | Prostate cancer | 5/8 | 150–740/mL (CRPC), 0–100/mL (CSPC) | Pan CK(+), CD45(−), EpCAM | 1 week to 3 months | |
| Lu et al. (2010) [33] | Breast cancer | 10/10 | 18–256/mL | EpCAM, Pan CK, CD45 | 1–33 days | Collagen adhesion matrix (CAM) |
| Pizonet al. (2013) [34] | Breast cancer | 31/39 | 1700–9360/mL | EpCAM(+) | 28 days | Number of spheres was 0–29/mL |
| Bobek et al. (2014) [35] | Pancreatic cancer | 16/24 | NA | Nuclear size, NC ratio, Irregularity, CK7, DAPI, CD45(−) | >14 days | |
| Bobek et al. (2014) [36] | Esophageal cancer | 27/43 | NA | Nuclear size, NC ratio, Irregularity, CK18, DAPI, CD45(−) | <14 days | |
| Bobek et al. (2014) [37] | Pleural Mesothelioma | 4/5 | NA | Nuclear size, NC ratio, Irregularity, MPF, OPN, DAPI, CD45(−) | 10–14 days | |
| Ceganet et al. (2014) [38] | Urinary bladder cancer | 25/39 | 1–50/8 mL | Nuclear size, NC ratio, Irregularity, CK18, DAPI, CD45(−) | 14 days | |
| Kolostova et al. (2014) [39] | Urothelial tumors | NA | NA | Nuclear size, NC ratio, Irregularity, CK7, DAPI | 10 to 14 days | |
| Kolostova et al. (2014) [40] | Prostate cancer | 18 (proliferative capacity)/28 (CTC-positive)/55 (total cases) | NA | Nuclear size, NC ratio, Irregularity, CK7, DAPI | 14 days | |
| Sheng et al. (2014) [41] | Pancreatic cancer | 0/12 | 0–7/mL | CK(+), CD45(−), DAPI | no proliferation | Capture and release |
| Zhang et al. (2014) [42] | Lung cancer | 14/19 | 1 to 11/mL | CK(+), CD45(−) | NA | p53 mutation did not always match between primary tissue and CTC |
| Khoo et al. (2015) [43] | Breast cancer | 7/18 (early stage) | NA | CK(+), CD45(−) | 2–8 weeks | |
| Kolostova et al. (2015) [44] | Ovarian cancer | 77/118 | NA | Nuclear size, NC ratio, Irregularity, CK, DAPI, CD45(−) | 3–14 days | CA125-positive |
| Kolostova et al. (2015) [45] | Gynecological cancer | 3/3 | NA | Nuclear size, NC ratio, Irregularity, CK7, DAPI, CD45(−), Muc1 | 3–10 days | |
| Chen et al. (2016) [46] | Colorectal cancer | NA | 0–1000/mL | DAPI, CK20(+), CD45(−) | 10 days to 2 months | Maintenance for up to 2 months, but no growth was observed. p53 mutation, APC |
| Kolostova et al. (2016) [47] | Gastric cancer | 13/22 | NA | Nuclear size, NC ratio, Irregularity, CK18, 19, DAPI, CD45(−) | >14 days | |
| Kulasinghe et al. (2016) [48] | Head and neck cancer | 7/25 | 1–15 CTC/5 m by cytospin | EpCAM(+), CK(+) CD45(−), morphologically larger than background cells. High NC ratio | 21 days to 63 days. 3D is longer than 2D | Successfully obtained higher CTC counts. HPV-positive |
| Malala et al. (2016) [49] | Colon cancer | 7/7 | 5/mL (CD133(+)), 29/mL (CK20(+)) | CK20(+), CD45(−) | 14 days | The characterization of CTC revealed changes in their phenotypes during their cultivation in vitro |
| Zhang et al. (2016) [50] | Hepatocellular carcinoma | 31/36 (>100 μm defined as spheroids) | 1–42/2 mL | ASGPR, CK(+), CD45(−) | >7 days | Capture and release. ASGPR(+) |
| Eliasova et al. (2017) [51] | Colorectal cancer | 81/98 | NA | Nuclear size, NC ratio, Irregularity, CK, DAPI, CD45(−) | 3–5 days, some were able to grow for 6 months | |
| Lambros et al. (2018) [52] | Prostate cancer | 2/14 | 12546/7.5 mL | DAPI, CK(+), CD45(−) | >4–6 weeks | Two organoids from the same patient |
| Franken et al. (2019) [53] | Breast cancer | 2/8 (without cryopreserved samples), 3/9 (cryopreserved samples) | 1–2913 CTC/7.5 mL | CK(+), CD45(−) | >3 months | Successfully cultured from cryopreserved samples |
| Kapeleris et al. (2020) [54] | Non-small cell lung cancer | 9/70 | 0–385/7.5 mL | DAPI, CK(+), CD45(−), high NC ratio, larger than background cells | 20 to 50 days | Culturability was not affected by an increased number of CTC. EGFR mutation |
| Lee et al. (2020) [55] | Small cell lung cancer | 18/22 | 8–277/mL | CK(+), CD45(−), EpCAM, TTF-1, Synaptophysin | 2 to 6 weeks | BCC, Platelet lysate |
| Xiao et al. (2020) [56] | Breast cancer | 12/12 | NA | CK5, CK8, Mammaglobin | >30 days (6 cases), <30 days (6 cases) | Presence of CD45(+) cells exhibited higher growth potential ex vivo. Does not exclude CD45(+) cells |
| Carmona-Ule et al. (2021) [57] | Breast cancer | 36/50 | 0−1000/7.5 mL | EpCAM, Pan CK, CD45 | >23 days (up to 291 days, mean 8 weeks) | Nanoemulsions support CTC. Some cells express CD45 (+), CD36(+) |
| Hu et al. (2021) [58] | Hepatocellular carcinoma | 55 (spheroid)/60 (CTC)/106 (total) | NA | ASGPR/CPS1, DAPI, EpCAM, CD45(−) | 12–14 days | Beta-catenin (+), a spheroid was defined as a 3D cell structure >100 μm. ASGPR(+) |
| Yang et al. (2021) [59] | Gastrointestinal cancer | 13 (colony)/38 (viable cell)/81 (total) | NA | hTERT | 4 weeks | J2 feeder cell-coated plate |
HEA: Human epithelial antigen. CRPC: Castration-resistant prostate cancer. CSPC: Castration-sensitive prostate cancer. ASGPR: Asialoglycoprotein receptor 1.
Table 4.
Protocol for the short-term culture.
| Authors | Blood | CTC Enrichment and Isolation | Culture Type | Environment | Culture Condition | Culture Medium |
|---|---|---|---|---|---|---|
| Makino et al. (1999) [7] | 30 mL | Magnetic cell sorting system (MACS) anti-HEA125 (EpCAM) | 2D semi-spheroid | 37 °C, 5% CO2 | 60 mm culture dish | RPMI1640/F12, 10% FCS |
| Paris et al. (2009) [32] | 3 mL | Ficoll-Paque | 2D | N/A | 16-well chamber slide coated with Collagen adhesion matrix (CAM) | DMEM/RPMI1640, 10% FCS, Nu-serum, L-glutamine |
| Lu et al. (2010) [33] | 0.5 mL | Ficoll density gradient centrifugation, Collagen adhesion matrix (CAM) capture | 2D | 37 °C, 5% CO2 | CAM-coated 96-well microtiter plate | RPMI1640, 10% FCS, Nu-serum, L-glutamine |
| Pizon et al. (2013) [34] | 1 mL | Erythrocyte lysis | 3D | 37 °C, 5% CO2 | 25 cm2 culture flask | RPMI1640, low FCS, L-glutamine, EGF, Insulin, Hydrocortisone |
| Bobek et al. (2014) [35,36,37] | 8 mL | MetaCell: filtration using a porous polycarbonate membrane (pores with a diameter of 8 μm) | 2D | 37 °C, 5% CO2 | On the membrane and bottom of the 6-well culture plate | RPMI1640, 10% FCS |
| Cegan et al. (2014) [38] | 8 mL | MetaCell: filtration using a porous polycarbonate membrane (pores with a diameter of 8 μm) | 2D | 37 °C, 5% CO2 | On the membrane and bottom of the 6-well culture plate | RPMI1640, 10% FCS |
| Kolostova et al. (2014) [39,40,44,45,47] | 8 mL | MetaCell: filtration using a porous polycarbonate membrane (pores with a diameter of 8 μm) | 2D | 37 °C, 5% CO2 | On the membrane and bottom of the 6-well culture plate | RPMI1640, 10% FCS |
| Sheng et al. (2014) [41] | 5–10 mL | GEM chip | 2D | 37 °C, 5% CO2 | 60 mm culture dish | DMEM, 10% FCS |
| Zhang et al. (2014) [42] | 5 mL | Microfluidic CTC capture chip | 3D | 37 °C, 7.5% CO2 | On chip co-culture (fibroblast) 7 days. Well plate for 7 days | RPMI complete medium, 10% FCS |
| Khoo et al. (2015) [43] | 10 mL | RBC lysis | 3D spheroid | 37 °C, 5% CO2, 1% O2. Normoxic conditions (maintenance) | Tapered microwell (14 days). 3D (Geltrex) ultra-low adhesive dish | DMEM, 10% FCS. Then, DMEM/F12, reduced serum |
| Chen et al. (2016) [46] | 2 mL | Lipid bilayer-coated microfluidic system (CMx platform) EpCAM, gentle sweep of air foam | 2D and 3D | N/A | Normal attachment culture dish or ultra-low attachment plate | DMEM medium, EGF, FGF, Insulin, B-27 |
| Kulasinghe et al. (2016) [48] | 10 mL | RosetteSep CD45-depleted Cocktail | 2D and 3D | 2% O2, 5% CO2 | 96-well standard microplates, Spheroid microplates, Happy Cell® hydrogel for 3D expansion | DMEM/F12, EGF, R-Spondin, Noggin, FGF10, FGF2, Nicotinamide, A83-01, SB202190, Y-27632, B27, N-Acetyl-L-cysteine, Glutamax, Hepes, Primocin |
| Malala et al. (2016) [49] | 5 mL | Ficoll-Paque | 3D sphere | N/A | Culture dishes | DMEM/F12, Heparin, EGF, FGF, BSA |
| Zhang et al. (2016) [50] | 2 mL | Microfluidic chip, Capture and release | 3D Gel | 37 °C | Matrigel 24-well plate | DMEM: Matrigel 1:1, 10% FCS |
| Eliasova et al. (2017) [51] | 8 mL | MetaCell: filtration using a porous polycarbonate membrane (pores with a diameter of 8 μm) | 2D | 37 °C, 5% CO2 | On the membrane and bottom of the 6-well culture plate | RPMI1640, 10% FCS |
| Lambros et al. (2018) [52] | 40 to 100 mL | Apheresis, EasySep EpCAM-positive selection | 3D organoid | N/A | Ultra-low attachment surface-coated microplates | Growth factor-reduced Matrigel |
| Franken et al. (2019) [53] | 3.41 L | Diagnostic Leukapheresis (DLA), microfluidic Parsortix system | 3D | 5% CO2, 4% O2 | Low attachment plate | Tumor sphere medium, RPMI1640, B27, EGF, FGF, |
| Kapeleris et al. (2020) [54] | 10 mL | RosetteSep CD45-depleted Cocktail, RBC lysis | N/A | 1–2% O2 | 96-well standard microplates | DMEM/F12, EGF, R-Spondin, Noggin, FGF10, FGF2, Nicotinamide, A83-01, SB202190, Y-27632, B27, N-Acetyl-L-cysteine, Glutamax, Hepes, Primocin, 10% FCS |
| Lee et al. (2020) [55] | 7.5 mL | RosetteSep | 2D and 3D | N/A | 96-well binary colloid crystal (BCC) substrate | DMEM/F12, EGF, bFGF, B27, Platelet lysate |
| Xiao et al. (2020) [56] | 7.5 mL | Ficoll-Paque | 2D | 37 °C | Culture dishes | DMED/F12 B27, EGF, FGF, Heparin, Y-27632, Adenine, L-Glutamine |
| Carmona-Ule et al. (2021) [57] | 7.5 mL | RosetteSep CD56-depleted Cocktail | 3D | 37 °C, 5% CO2, 1–2% O2 (1 week). Then, normoxic conditions | 96-well ultra-low attachment plate. Then, 24-well ultralow attachment. 25T flask (maintenance) | MammoCult media, Progesterone, Beta-estradiol, Heparin, Hydrocortisone, UltraGRO, B-27, bFGF, EGF, Nanoemulsions |
| Hu et al. (2021) [58] | 5 mL | Ficoll-Paque, CD45 depletion by magnetic separation | 3D Gel | 37 °C, 5% CO2 | 24-well plate | DMEM, Matrigel, 10% FCS |
| Yang et al. (2021) [59] | 5 mL | RBC lysis | 2D | 37 °C, 5% CO2 | J2 feeder cell-coated plate | DMEM, 5% FCS, L-glutamine, F12 nutrient mix, Hydrocortisone, EGF, Insulin, Cholera toxin Y-27632 |
3.1. Overview of Short-Term Cultures
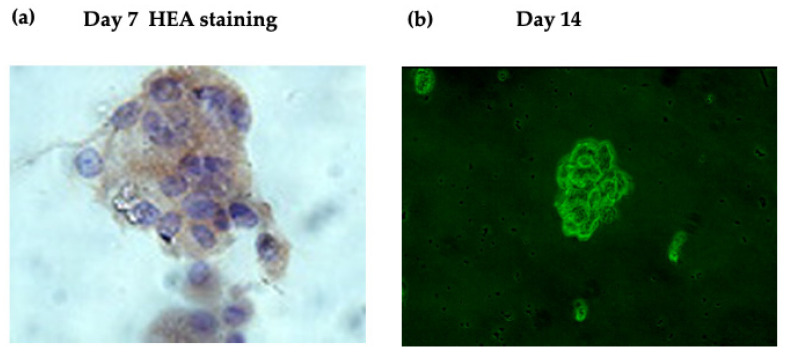
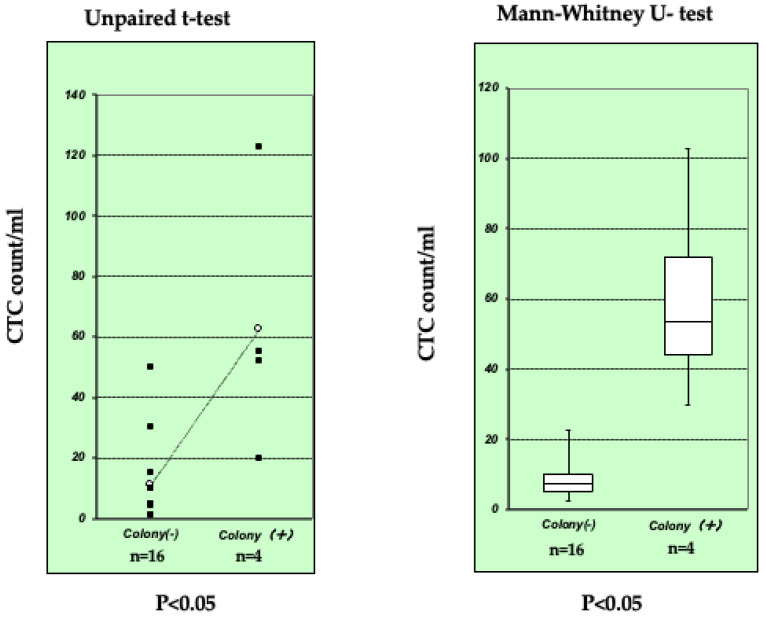
We successfully performed a short-term culture of CTC using a magnetic cell sorting system (MACS) with anti-Human Epithelial Antigen (HEA) 125 in 1999 [7]. We detected CTC (1 to 123 cells/mL) in 20/32 (63%) ESCC patients. Among 20 CTC-positive samples, 4 (20, 50, 52, and 123 CTC/mL) formed colonies after more than 14 days (Figure 1). However, we were unable to establish long-term cultures of these colonies. Although the number of samples was too small for a definite evaluation, a high CTC count was associated with colony formation (Figure 2). Approximately 30 studies on short-term cultures of CTC were subsequently published (Table 3 and Table 4 [7,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]). Ten of these studies were by the same research group and used the same method [35,36,37,38,39,40,44,45,47,51].
Figure 1.
Representative images of the short-term CTC cell culture. (a) Cytospin image. Positive staining for Human Epithelial Antigen (HEA) was observed in cell clusters on day 7. (b) Phase contrast image of cell clusters on day 14 using a green filter [7].
Figure 2.
Colony formation by 4 out of 20 samples. The number of samples was too small for a definite evaluation; however, a high CTC count/mL (anti-HEA) was associated with colony formation (an unpaired t-test and the Mann–Whitney U test < 0.05) [7].
3.2. Enrichment of CTC
Similar to long-term cultures, most enrichment methods for cultures involve the negative selection method (the density gradient method or RosetteSep®). Few studies employed RBC cell lysis only. Under optimal conditions, the introduction of an RBC lysis step does not markedly affect the viability of the nucleated cell fraction [43]. Ten studies used MetaCell® (filtration using a porous polycarbonate membrane) selection [35,36,37,38,39,40,44,45,47,51]. Four studies described the use of a microfluidic device [42,46,50,53]. Furthermore, diagnostic leukapheresis (DLA) and microfluidic enrichment obtained a large number of viable CTC from metastasized cancer patients. The average CTC yield per apheresis (mean volume, 59.5 mL) was 12,546 CTC [52].
Each method has its own advantages and limitations, and researchers have based the development of capture strategies on the specific aims of further CTC characterization studies [60] (Table 4).
-
1.
Porous polycarbonate membrane
The MetaCell® filtration device is a capillary action-driven, size-based separation method that isolates and enriches viable CTC from peripheral blood samples using a porous polycarbonate membrane (pores with a diameter of 8 μm) [35,36,37,38,39,40,44,45,47,51]. The culture with the device consists of a membrane (membrane fraction) and the bottom of a 6-well culture plate (invading fraction). CTC grow on the bottom of the culture plate after escaping the separating membrane through the pores. MetaCell® detects cancer cells not only on the separating membrane, but also on the plastic bottom of the 6-well plate [39]. Membranes with captured cells may be transferred to culture plates [51]. The success rate for short-term cultures was high.
-
2.
On-chip culture
Zhang et al. [42] described an on-chip culture of a microfluidic co-culture model. After 7 days of the on-chip culture, cells were released from the device by an incubation with collagenase. Cells were then flushed outside the device with media at a flow rate of 10 mL/h for 3 mL. Approximately 90% of cells were released from the device. The recovered population was reseeded on well plates and cultured for an additional 7–14 days. The on-chip culture successfully expanded CTC isolated from 14 out of 19 early-stage lung cancer patients.
3.3. Characteristics of Short-Term Cultures of CTC
Kapeleris et al. [54] suggested the identification of CTC as follows: (I) morphologically larger than background cells with intact nuclei; (II) a high nuclear–cytoplasmatic ratio (NC ratio); (III) positive for pan-cytokeratin; (IV) positive for DAPI; (V) negative for CD45; and (VI) cells larger than 14 μm. Kolostova et al. [40] also suggested defining CTC as cells exhibiting the following characteristics: (1) a nuclear size equal to or larger than 10 μm; (2) irregular nuclear contours; (3) the presence of a visible cytoplasm; (4) prominent nucleoli; (5) a high NC ratio; (6) changes in the NC ratio with the in vitro culture time; and (7) deformability/plasticity (growth through the membrane to the bottom and the formation of new colonies).
Regarding CD45, a significant population of “double positive” cells with hematopoietic and epithelial markers (CK+/CD45+) were detected in many patient samples [41]. Furthermore, a few reports suggested the existence of tumor–macrophage fusion cells (TMFs) in the patients’ blood [61,62]. Tumor-associated macrophages (TAMs) recruited to the stroma from circulating monocytes are required for tumor cell intravasation, migration, extravasation, and angiogenesis. TAMs have been hypothesized to fuse their membranes with those of tumor cells, forming tumor–macrophage hybrid cells [56].
Thus, most of the double positive cells may be the heterogeneous CTCs [41]. From the viewpoint of cell culture, CD45+ cells co-inhabited in the majority of the samples [57], and cultures grown in the presence of CD45+ cells exhibited higher growth potential ex vivo [56]. Furthermore, both macrophages and neutrophils associate with CTCs [56]. Thus, CD45+ cells may not always be excluded from the culture.
Khoo et al. [43] maintained CTC cultures for 2–8 weeks and noted that CTC with longer culture times exhibited more mesenchymal characteristics, with an increase in Vimentin and Fascin staining and the almost complete loss of epithelial characteristics. Malala et al. [49] attempted to characterize CTC and reported changes in their phenotype during in vitro cultivation.
The viability and morphology of expanded CTC were examined by LIVE/DEAD staining [55].
3.4. Culture Conditions and Medium
Few studies have employed low attachment plates and hypoxic conditions. Therefore, short-term cultures are generally aimed at clinical use, not long-term cultures. Therefore, they are routinely conducted under adherent and normoxic conditions. Furthermore, most studies used simple standard culture medium with FCS.
Tapered microwells are preferred over conventional cylindrical microwells for cluster formation. Cultures are sheltered within microwells with minimal disturbance during medium changes, which reduces shear stress and cell loss to allow for subsequent expansion [43]. This method takes advantage of a patient’s own white blood cells as co-culture components with CTC, and maintains their proximity within specialized microwells for maximum interaction. White blood cells from the same patient are feeder cells that promote CTC cluster formation [63].
Carmona-Ule et al. [57] suggested a culture medium supplemented with nanoemulsions composed of oleic acid and lipids for CTC growth (Table 4).
3.5. Success Rate and Culture Period
The success rate of cultures was previously reported to be more than 50%. The total success rate of MetaCell® was 65%. Despite the leukapheresis approach, CTC from only a few patients have been successfully cultured. A success rate of 100% (12/12 samples) was achieved by Ficoll-Paque selection [56].
The typical cell culture period is <14 days and few cells may be cultured for more than 3 months. Typical spheres were shown to increase in size over time from 7–21 days [34]. Khoo et al. [43] reported that the proportion of CK+/CD45− cells significantly decreased after day 14 in most samples, and thus selected day 14 as the endpoint for culture phenotyping. This time point also correlated with the highest number of Ki67-positive clusters.
Carmona-Ule et al. [57] investigated the median progression time (100 days) and days of CTC in culture, and proposed 23.5 days as the best threshold for discriminating between groups (AUC = 0.65). Therefore, a cut-off of 23 days is considered to be optimal for CTC culture. Using this criterion, approximately 50% of studies achieved successful short-term cultures (Table 3 and Table 4).
Although proliferation was not observed due to the challenging ex vivo growth environment, the maintenance of CTC viability was guaranteed for at least two months [46]. Eliasova et al. [51] reported that some isolated CTC grew in vitro for up to 6 months as a standard cell culture; however, the exact number of cases was not described.
3.6. Growth Patterns of Short-Term Cultures of CTC
3.6.1. Cell Distribution
Khoo et al. [43] showed that cytokeratin-positive (CK+) cells localized at the center of tapered microwells and were surrounded by CD45+ cells. The majority of large cells within and outside the microwells expressed CD68, which was suggestive of macrophages.
3.6.2. Presence of Undetectable CTC
Khoo et al. [43,63] demonstrated that some blood samples that did not initially contain detectable CK+ CTCs in culture were positive on day 14. This may have been due to proliferation of very few CTC with heightened survival characteristics. Therefore, initial CTC counts before culture do not always reflect the potential for cluster formation. Samples with an undetectable level of CTC before culture may still form clusters.
3.6.3. Morphology
The morphological spheroid construct typically consists of three different presentable types: large-sized, cohesive round-shaped spheroids; small-sized cohesive irregular or round spheroids; and discohesive “grape-like” spheroids [55]. Some samples presented cells growing both in suspension and adherence [57].
In the MetaCell® system, CTC grew through the membrane on the bottom of the well after 14 days in an in vitro culture exhibiting two different phenotypes, epithelial-like and stem cell-like. Stem cell-like cells formed visible cell clumps. Cells growing on the bottom showed a very plastic morphology [40]. CTC increased in size and became elongated during in vitro growth, which altered the NC ratio [44].
3.6.4. Unexpected Effects of Blood Cells
Non-adherent blood cells provide a cushion layer that keeps CTC in suspension [61]. CTC have been shown to attach to the top of adherent leukocyte cells and expand [56].
3.7. Timing of the Culture Process
After 1 week of a cell culture under hypoxic conditions, cells are generally switched to standard cell culture conditions. When 85% confluence is reached, growing cells are transferred to an ultra-low attachment 24-well plate [57]. However, the optimal timing for the transfer of cultured cells to the next step currently remains unclear.
3.8. Difference between CTC Cell Lines and Short-Term Cultures
Regarding the establishment of CTC cell lines, the majority of cultured CTC lack the ability to proliferate. Therefore, 2 months is a sufficient time period to assess whether CTC exhibit the ability to continuously proliferate. Future studies that compare CTC lines with CTC that stop proliferating after a short-term culture will facilitate the development of optimal molecular conditions for the permanent survival and growth of CTC [22].
4. Our Experience
Cell growth depends on the proliferative potential of each cell. Among 21 conventional esophageal cancer cell lines, the time after the initiation of the primary culture to the second culture varied from 2.5 weeks to 48 weeks, depending on cell growth patterns, such as the adhesion-dependent type, spheroid type, and mixed type. Adaptability to a new environment is also important [2].
We previously reported the maintenance of several cancer cells in protein-free medium without other supplements for 2 years [3]. These cell lines exhibited the ability to grow without exogenous growth factors; however, another study showed that lipids, insulin, and transferrin were essential factors for the proliferation of some carcinoma cell lines [28,57]
Based on our experience and previous findings, we preliminarily performed a culture of CTC collected from patients with prostate cancer and renal cell carcinoma. We recently developed a CTC capture system [64]. We first attempted an on-chip culture using gel formation or a culture of released CTC under a high flow. We successfully established a short-term culture of CTC (20 out of 27 samples), but not a long-term culture.
We also used VIVANT-CELL®-pot (porous polycarbonate membrane selection) with Lymphoprep or negative selection using RosetteSep® CD45, CD36, or CD56. A short-term culture of CTC was successful for 16 out of 27 samples; however, expansion was very challenging to overcome. One reason is that we did not obtain a high number of viable CTC from patients because most had already received several courses of chemotherapy. The most important issue is that we have not yet established a specific protocol for individual carcinoma cells.
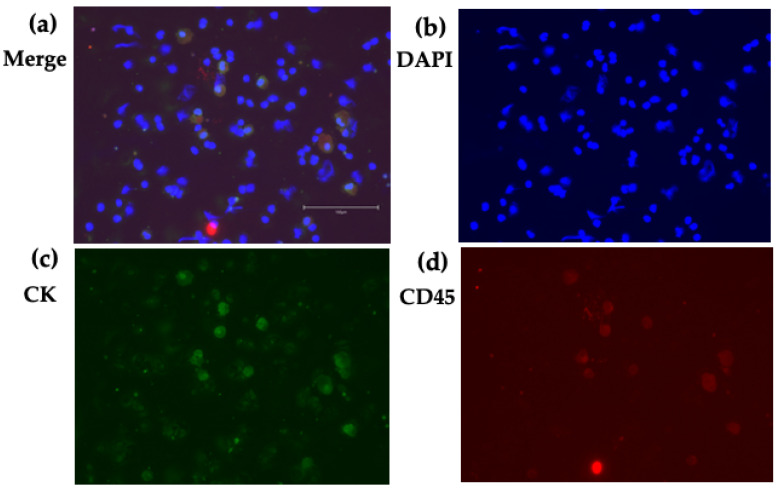
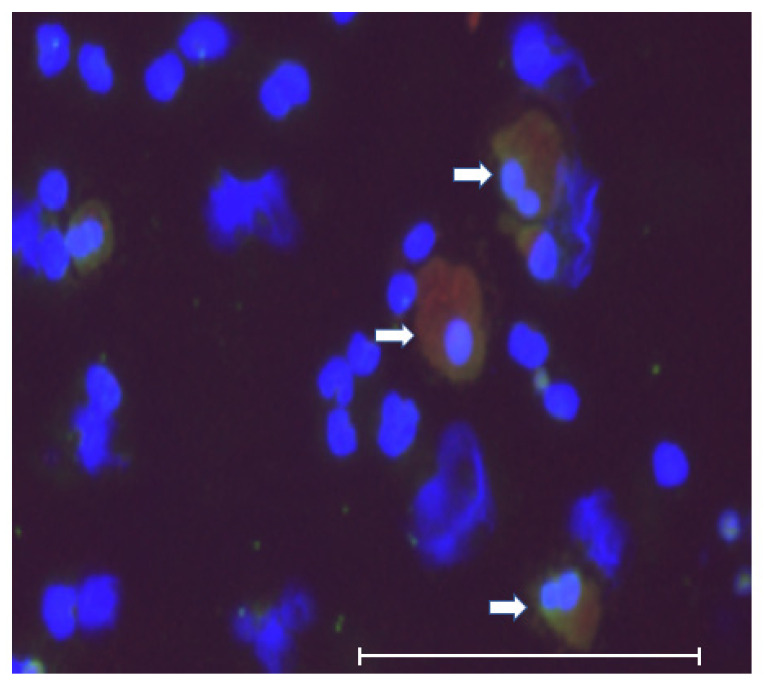
Regarding double positive (CK+/CD45+) cells, we also detected these cells in the short-term culture of CTC. Some of these cells were large, polymorphic in shape and polynuclear (Figure 3 and Figure 4). These morphological features suggested that these were tumor–macrophage fusion cells (TMFs), macrophage–tumor cell fusion cells (MTFs), or cancer-associated macrophage-like (CAMLs) cells [62].
Figure 3.
CK/CD45 double positive cells. Cells obtained from the short-term (35 days) CTC culture. Cytospin image: (a) Merge, (b) DAPI, (c) CK, and (d) CD45. The scale bar shows 150 μm. Some cells express both CK and CD45.
Figure 4.
Morphology of double positive cells. These cells were large, polymorphic, and polynuclear, which suggested that they were tumor–macrophage fusion cells (TMFs), macrophage–tumor cell fusion cells (MTFs), or cancer-associated macrophage-like (CAMLs) cells. The scale bar shows 150 μm. White arrows indicate CK/CD45 double positive cells.
In several trials, we developed three cell lines with the spontaneous immortalization of lymphocytes from different patients under conditions involving several growth supplements, such as Y-27632 and Z-VAD-fmk. We previously reported the incidental establishment of B-cell cell lines (ITSM), derived from EB virus spontaneous activation during an ordinary cell culture from pseudomyxoma peritonei [65]. The long-term use of growth supplements exerted unexpected effects on the culture. Wang et al. [28] also suggested the application of exogenous factors, but was unable to increase the success rate of CTC cultures and may result in undesired complications. Therefore, careful management is needed during cell cultures. These findings suggest the use of growth supplements for a set time period only.
5. Critical Points
CTC cell lines have unique intermediate characteristics between primary tumors and distant metastasis. CTC are not simple travelers in the bloodstream. Although most CTC are apoptotic and sensitive to stress, a few viable CTC survived.
The CTC count of samples is the most important factor for the successful establishment of CTC cell lines. A CTC count of >100 cells/mL blood is favorable. Leukapheresis may be the most powerful method for enriching CTC; however, its success rate is low.
There is currently no evidence to support the contribution of CD45+ cells to the success of long-term culture. However, a reconsideration of the parameters for CTC isolation is warranted [56].
It is important to note that because optimal CTC culture conditions have yet to be defined, current research relies on stem cell culture methods that ensure maximal CTC expansion. Each protocol is designed based on a researcher’s own expertise and empirical results, and not by a rationale based on the biological features of CTC, which are presently not defined [28].
Moreover, activated lymphocyte proliferation is transient, ending with programmed activation-induced cell death. Macrophages are the only cell type among peripheral blood mononuclear cells that are capable of surviving for weeks in culture. However, they are also a terminally differentiated cell type that is incapable of ex vivo proliferation [28].
Other key technical limitations include the maintenance of the CTC phenotype and the composition of a stable population in culture. Previous studies suggested that normal human mesenchymal stem cells are prone to genomic changes and subsequent malignant transformation in long-term cultures [66].
Therefore, the further optimization of CTC culture conditions and a more detailed understanding of the differences between non-adherent CTC cell lines and adherent cell lines as well as CTC cultures and patient samples is required [67].
6. Protocol Currently Recommended for CTC Cell Lines
As described above, the recommended protocol for CTC cell lines is as follows (Table 5). With careful optimization, these culture conditions may be applied to successful long-term cultures.
Table 5.
Protocol currently recommended for CTC cell lines.
| 1 First step |
| (1) Blood sample volume >10 mL |
| (2) Negative selection for CTC enrichment (RosetteSep®, etc.) or Ficoll-Paque density gradient centrifugation |
| (3) Hypoxic conditions within 10 days (7 days is recommended) |
| (4) Low attachment plate or gel formation |
| (5) Growth factors (such as EGF, FGF, and B-27) and anti-apoptotic supplements (including Y-27632) with FCS |
| 2 Option |
| (1) Preservation of CD45(+) cells. |
| (2) Normoxic conditions and attachment plates dependent on the cell type |
| (3) Microfluidic device or nanotechnology-based method |
| (4) Serum-free medium |
| (5) Co-culture with normal leukocytes or fibroblasts |
| (6) Culture medium changes under the microscopic ministering of cell clusters |
| (7) Exclusion criteria |
| (i) the use of chemotherapy and/or antibody-based therapy in the past 4 weeks |
| (ii) the use of radiotherapy in the past 2 weeks |
| 3 Second step (after 7 to 14 days of the initial culture) |
| (1) Transfer to normoxic conditions |
| (2) Standard culture medium |
| 4 Third step (after several weeks) |
| (1) No standard method |
| (2) Select specific conditions depending on each cell |
| (3) Careful management |
| 5 Critical steps |
| (1) Medium changes at 200 μL/min or less every 72 h [64] |
| (2) In the case of a highly proliferative culture, an increased frequency of medium changes |
| (3) To prevent the excessive stimulation of normal blood cells, the use of anti-apoptotic supplements for long periods is not recommended |
7. Conclusions
A careful review of previous studies suggested that CTC in long- and short-term cultures have different characteristics. Not only the culture method, but also the characteristics of CTC themselves affect the success of establishing CTC cell lines.
In the circulation, CTC from any type of cancer encounter the same environment, which differs from their solid tissue location. Only cells with specific characteristics, such as stem cell-like cells, may survive in the bloodstream. CTC without these characteristics are protected by surrounding cells, such as leukocytes and platelets. However, it currently remains unclear whether only CTC with stem cell features are suitable long-term culture.
Low attachment plates and hypoxic conditions may be the ideal conditions for the initial culture steps. A successful culture medium contains several growth factors and anti-apoptotic supplements. However, as described above, cancer cells have already acquired growth and survival autonomy. Therefore, CTC cultured in the presence of growth factors and small molecule inhibitors may not be ideal for modeling cancer and stromal interactions. The most important factors are the timing of CTC transfer to the next step and the careful management of medium changes.
Short-term culture is the initial approach to long-term culture; however, prolonged protocols sometimes deviate from the main expansive route for establishing CTC cell lines. The success of short-term cultures is not always affected by a high number of CTC; however, long-term cultures generally require high numbers of CTC. Therefore, the culture method may only prolong cell survival; it does not affect permanent proliferative activity. The most important issue is obtaining CTC that are suitable for long-term culture; however, a method to identify these CTC has not yet been established.
There is currently no gold standard method. The selection of an optimal method for each sample may be achieved through careful management and continued efforts. Extensive trials are needed to establish long-term cultures of CTC. Without these efforts, CTC that are suitable for expansion will not be identified.
Acknowledgments
We thank Kazuaki Watanabe, Takako Murai, and Yukari Kamimura for their technical assistance. We also thank Shin Yonehara for his special advice on growth supplements.
Abbreviations
N/A: not available; FACS: fluorescent-activated cell sorting; SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer; 2D: two-dimensional culture; 3D: three-dimensional culture. HEA: Human epithelial antigen.
Author Contributions
Writing and analysis, Y.S.; methodology, T.S. (Tetsuo Sudo); patients’ treatment & sample collection, S.A. and T.S. (Takuro Sunada); investigation, K.O. & A.M.; project administration, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare financial support from Toray Industries Inc.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Kyoto university at 9 November. Approved number is R2121.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Most recent data which described in this review were preliminary data and did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khoo B.L., Grenci G., Jing T., Lim Y.B., Lee S.C., Thiery J.P., Han J., Lim C.T. Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment. Sci. Adv. 2016;2:e1600274. doi: 10.1126/sciadv.1600274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada Y., Imamura M., Wagata T., Yamaguchi N., Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::AID-CNCR2820690202>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Kato M., Shimada Y., Tanaka H., Hosotani R., Ohshio G., Ishizaki K., Imamura M. Characterization of six cell lines established from human pancreatic adenocarcinomas. Cancer. 1999;85:832–840. doi: 10.1002/(SICI)1097-0142(19990215)85:4<832::AID-CNCR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Shimada Y., Kanda Y., Shibagaki I., Kato M., Watanabe G., Tanaka H., Kano M., Imamura M. Prognostic value of monolayer culture patterning in primary cell culture of oesophageal cancer. Br. J. Surg. 2005;83:1148–1151. doi: 10.1002/bjs.1800830837. [DOI] [PubMed] [Google Scholar]
- 5.Shimada Y., Maeda M., Watanabe G., Yamasaki S., Komoto I., Kaganoi J., Kan T., Hashimoto Y., Imoto I., Inazawa J., et al. Cell Culture in Esophageal Squamous Cell Carcinoma and the Association with Molecular Markers. Clin. Cancer Res. 2003;9:243–249. [PubMed] [Google Scholar]
- 6.Racila E., Euhus D., Weiss A.J., Rao C., Mc Connell J., Terstappen L.W.M.M., Uhr J.W. Detection and characterization of carcinoma cells in the blood. Proc. Natl. Acad. Sci. USA. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino T., Shimada Y., Yamasaki S., Watanabe G., Kaganoi J., Imamura M. Capture and culture of circulating gastrointestinal tumor cell using Magnetic Cell Sorting (MACS) system; Proceedings of the Fifty Fourth Annual Meeting of The Japanese Society of Gastroenterological Surgery; Nagoya, Japan. 16 July 1999. [Google Scholar]
- 8.Okumura T., Shimada Y., Omura T., Hirano K., Nagata T., Tsukada K. MicroRNA profiles to predict postoperative prognosis in patients with small cell carcinoma of the esophagus. Anticancer Res. 2015;35:719–727. [PubMed] [Google Scholar]
- 9.Zhang L., Ridgway L.D., Wetzel M.D., Ngo J., Yin W., Kumar D., Goodman J.C., Groves M.D., Marchetti D. The Identification and Characterization of Breast Cancer CTCs Competent for Brain Metastasis. Sci. Transl. Med. 2013;5:180ra48. doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu M., Bardia A., Aceto N., Bersani F., Madden M.W., Donaldson M.C., Desai R., Zhu H., Comaills V., Zheng Z., et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao D., Vela I., Sboner A., Iaquinta P.J., Karthaus W.R., Gopalan A., Dowling C., Wanjala J.N., Undvall E.A., Arora V.K., et al. Organoid Cultures Derived from Patients with Advanced Prostate Cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrefourcq L., Mazard T., Joosse S., Solassol J., Ramos J., Assenat E., Schumacher U., Costes V., Maudelonde T., Pantel K., et al. Establishment and Characterization of a Cell Line from Human Circulating Colon Cancer Cells. Cancer Res. 2015;75:892–901. doi: 10.1158/0008-5472.CAN-14-2613. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton G., Burghuber O., Zeillinger R. Circulating tumor cells in small cell lung cancer: Ex vivo expansion. Lung. 2015;193:451–452. doi: 10.1007/s00408-015-9725-7. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton G., Hochmair M., Rath B., Klameth L., Zeillinger R. Small cell lung cancer: Circulating tumor cells of extended stage patients express a mesenchymal-epithelial transition phenotype. Cell Adhes. Migr. 2016;10:360–367. doi: 10.1080/19336918.2016.1155019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Wu W., Wang Z., Tang Y., Deng Y., Xu L., Tian J., Shi Q. Ex vivo expansion of circulating lung tumor cells based on one-step microfluidics-based immunomagnetic isolation. Anal. 2016;141:3621–3625. doi: 10.1039/C5AN02554K. [DOI] [PubMed] [Google Scholar]
- 16.Klameth L., Rath B., Hochmaier M., Moser D., Redl M., Mungenast F., Gelles K., Ulsperger E., Zeillinger R., Hamilton G. Small cell lung cancer: Model of circulating tumor cell tumorospheres in chemoresistance. Sci. Rep. 2017;7:5337. doi: 10.1038/s41598-017-05562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillet F., Bayet E., Villeronce O., Zappia L., Lagerqvist E.L., Lunke S., Charafe-Jauffret E., Pham K., Molck C., Rolland N., et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut. 2016;66:1802–1810. doi: 10.1136/gutjnl-2016-311447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandlin R.D., Wong K.H.K., Tessier S.N., Swei A., Bookstaver L.D., Ahearn B.E., Maheswaran S., Haber D.A., Stott S.L., Toner M. Ultra-fast vitrification of patient-derived circulating tumor cell lines. PLoS ONE. 2018;13:e0192734. doi: 10.1371/journal.pone.0192734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soler A., Cayrefourcq L., Mazard T., Babayan A., Lamy P.-J., Assou S., Assenat E., Pantel K., Alix-Panabières C. Autologous cell lines from circulating colon cancer cells captured from sequential liquid biopsies as model to study therapy-driven tumor changes. Sci. Rep. 2018;8:15931. doi: 10.1038/s41598-018-34365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Que Z., Luo B., Zhou Z., Dong C., Jiang Y., Wang L., Shi Q., Tian J. Establishment and characterization of a patient-derived circulating lung tumor cell line in vitro and in vivo. Cancer Cell Int. 2019;19:21. doi: 10.1186/s12935-019-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao P., Zhou W., Liu C., Zhang H., Cheng Z., Wu W., Liu K., Hu H., Zhong C., Zhang Y., et al. Establishment and Characterization of a CTC Cell Line from Peripheral Blood of Breast Cancer Patient. J. Cancer. 2019;10:6095–6104. doi: 10.7150/jca.33157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch C., Kuske A., Joosse S.A., Yigit G., Sflomos G., Thaler S., Smit D.J., Werner S., Borgmann K., Gärtner S., et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol. Med. 2020;12:e11908. doi: 10.15252/emmm.201911908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brungs D., Minaei E., Piper A.-K., Perry J., Splitt A., Carolan M., Ryan S., Wu X.J., Corde S., Tehei M., et al. Establishment of novel long-term cultures from EpCAM positive and negative circulating tumour cells from patients with metastatic gastroesophageal cancer. Sci. Rep. 2020;10:539. doi: 10.1038/s41598-019-57164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong X., Roh W., Sullivan R.J., Wong K.H.K., Wittner B.S., Guo H., Dubash T.D., Sade-Feldman M., Wesley B., Horwitz E., et al. The Lipogenic Regulator SREBP2 Induces Transferrin in Circulating Melanoma Cells and Suppresses Ferroptosis. Cancer Discov. 2021;11:678–695. doi: 10.1158/2159-8290.CD-19-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mout L., van Dessel L.F., Kraan J., de Jong A.C., Neves R.P., Erkens-Schulze S., Beaufort C.M., Sieuwerts A.M., van Riet J., Woo T.L., et al. Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur. J. Cancer. 2021;150:179–189. doi: 10.1016/j.ejca.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Luo Y.T., Cheng J., Feng X., He S.J., Wang Y.W., Huang Q. The viable circulating tumor cells with cancer stem cells feature, where is the way out? J. Exp. Clin. Cancer Res. 2018;37:38. doi: 10.1186/s13046-018-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi T., Okumura T., Hirano K., Watanabe T., Nagata T., Shimada Y., Tsukada K. Detection of circulating tumor cells by p75NTR expression in patients with esophageal cancer. World J. Surg. Oncol. 2016;14:40. doi: 10.1186/s12957-016-0793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R., Chu G.C., Mrdenovic S., Annamalai A.A., Hendifar A.E., Nissen N.N., Tomlinson J.S., Lewis M., Palanisamy N., Tseng H.-R., et al. Cultured circulating tumor cells and their derived xenografts for personalized oncology. Asian J. Urol. 2016;3:240–253. doi: 10.1016/j.ajur.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamal M., Saremi S., Klotz R., Iriondo O., Amzaleg Y., Chairez Y., Tulpule V., Lang J., Kang I., Yu M. PIC&RUN: An integrated assay for the detection and retrieval of single viable circulating tumor cells. Sci. Rep. 2019;9:17470. doi: 10.1038/s41598-019-53899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantel K., Alix-Panabières C. Functional Studies on Viable Circulating Tumor Cells. Clin. Chem. 2016;62:328–334. doi: 10.1373/clinchem.2015.242537. [DOI] [PubMed] [Google Scholar]
- 31.Guo T., Wang C.S., Wang W., Lu Y. Culture of Circulating Tumor Cells-Holy Grail and Big Challenge. Int. J. Cancer Clin. Res. 2016;3:65. doi: 10.23937/2378-3419/3/4/1065. [DOI] [Google Scholar]
- 32.Paris P.L., Kobayashi Y., Zhao Q., Zeng W., Sridharan S., Fan T., Adler H.L., Yera E.R., Zarrabi M., Zucker S., et al. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 2009;277:164–173. doi: 10.1016/j.canlet.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Lu J., Fan T., Zhao Q., Zeng W., Zaslavsky E., Chen J.J., Frohman M.A., Golightly M.G., Madajewicz S., Chen W.-T. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int. J. Cancer. 2009;126:669–683. doi: 10.1002/ijc.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizon M., Zimon D., Carl S., Pachmann U., Pachmann K., Camara O. Heterogeneity of circulating epithelial tumour cells from individual patients with respect to expression profiles and clonal growth (sphere formation) in breast cancer. Ecancermedicalscience. 2013;7:343. doi: 10.3332/ecancer.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobek V., Gurlich R., Eliasova P., Kolostova K. Circulating tumor cells in pancreatic cancer patients: Enrichment and cultivation. World J. Gastroenterol. 2014;20:17163–17170. doi: 10.3748/wjg.v20.i45.17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobek V., Matkowski R., Gürlich R., Grabowski K., Szelachowska J., Lischke R., Schützner J., Harustiak T., Pazdro A., Rzechonek A., et al. Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem. Cytobiol. 2014;52:171–177. doi: 10.5603/FHC.2014.0020. [DOI] [PubMed] [Google Scholar]
- 37.Bobek V., Kacprzak G., Rzechonek A., Kolostova K. Detection and cultivation of circulating tumor cells in malignant pleural mesothelioma. Anticancer Res. 2014;34:2565–2569. [PubMed] [Google Scholar]
- 38.Cegan M., Kolostova K., Matkowski R., Broul M., Schraml J., Fiutowski M., Bobek V. In vitro culturing of viable circulating tumor cells of urinary bladder cancer. Int. J. Clin. Exp. Pathol. 2014;7:7164–7171. [PMC free article] [PubMed] [Google Scholar]
- 39.Kolostova K., Cegan M., Bobek V. Circulating tumour cells in patients with urothelial tumours: Enrichment and in vitro culture. Can. Urol. Assoc. J. 2014;8:E715–E720. doi: 10.5489/cuaj.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolostova K., Broul M., Schraml J., Cegan M., Matkowski R., Fiutowski M., Bobek V. Circulating tumor cells in localized prostate cancer: Isolation, cultivation in vitro and relationship to T-stage and Gleason score. Anticancer Res. 2014;34:3641–3646. [PubMed] [Google Scholar]
- 41.Sheng W., Ogunwobi O.O., Chen T., Zhang J., George T.J., Liu C., Fan Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip. 2013;14:89–98. doi: 10.1039/C3LC51017D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z., Shiratsuchi H., Lin J., Chen G., Reddy R.M., Azizi E., Fouladdel S., Chang A., Lin L., Jiang H., et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5:12383–12397. doi: 10.18632/oncotarget.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoo B.L., Lee S.C., Kumar P., Tan T.Z., Warkiani M.E., Ow S.G., Nandi S., Lim C.T., Thiery J.P. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget. 2015;6:15578–15593. doi: 10.18632/oncotarget.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolostova K., Matkowski R., Jędryka M., Soter K., Cegan M., Pinkas M., Jakabova A., Pavlasek J., Spicka J., Bobek V. The added value of circulating tumor cells examination in ovarian cancer staging. Am. J. Cancer Res. 2015;5:3363–3375. [PMC free article] [PubMed] [Google Scholar]
- 45.Kolostova K., Spicka J., Matkowski R., Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am. J. Transl. Res. 2015;7:1203–1213. [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J.-Y., Tsai W.-S., Shao H.-J., Wu J.-C., Lai J.-M., Lu S.-H., Hung T.-F., Yang C.-T., Wu L.-C., Chen J.-S., et al. Sensitive and Specific Biomimetic Lipid Coated Microfluidics to Isolate Viable Circulating Tumor Cells and Microemboli for Cancer Detection. PLoS ONE. 2016;11:e0149633. doi: 10.1371/journal.pone.0149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolostova K., Matkowski R., Gürlich R., Grabowski K., Soter K., Lischke R., Schützner J., Bobek V. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2015;68:1095–1102. doi: 10.1007/s10616-015-9866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulasinghe A., Perry C., Warkiani M.E., Blick T., Davies A., O’Byrne K., Thompson E.W., Nelson C.C., Vela I., Punyadeera C. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget. 2016;7:60101–60109. doi: 10.18632/oncotarget.11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malara N., Trunzo V., Foresta U., Amodio N., De Vitis S., Roveda L., Fava M., Coluccio M.L., Macrì R., Di Vito A., et al. Ex-vivo characterization of circulating colon cancer cells distinguished in stem and differentiated subset provides useful biomarker for personalized metastatic risk assessment. J. Transl. Med. 2016;14:133. doi: 10.1186/s12967-016-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Zhang X., Zhang J., Sun B., Zheng L., Li J., Liu S., Sui G., Yin Z. Microfluidic chip for isolation of viable circulating tumor cells of hepatocellular carcinoma for their culture and drug sensitivity assay. Cancer Biol. Ther. 2016;17:1177–1187. doi: 10.1080/15384047.2016.1235665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eliášová P., Pinkas M., Kolostova K., Gürlich R., Bobek V. Circulating tumor cells in different stages of colorectal cancer. Folia Histochem. Cytobiol. 2017;55:1–5. doi: 10.5603/FHC.a2017.0005. [DOI] [PubMed] [Google Scholar]
- 52.Lambros M.B., Seed G., Sumanasuriya S., Gil V., Crespo M., Fontes M., Chandler R., Mehra N., Fowler G., Ebbs B., et al. Single-Cell Analyses of Prostate Cancer Liquid Biopsies Acquired by Apheresis. Clin. Cancer Res. 2018;24:5635–5644. doi: 10.1158/1078-0432.CCR-18-0862. [DOI] [PubMed] [Google Scholar]
- 53.Franken A., Driemel C., Behrens B., Meier-Stiegen F., Endris V., Stenzinger A., Niederacher D., Fischer J.C., Stoecklein N.H., Ruckhaeberle E., et al. Label-Free Enrichment and Molecular Characterization of Viable Circulating Tumor Cells from Diagnostic Leukapheresis Products. Clin. Chem. 2019;65:549–558. doi: 10.1373/clinchem.2018.296814. [DOI] [PubMed] [Google Scholar]
- 54.Kapeleris J., Kulasinghe A., Warkiani M.E., Oleary C., Vela I., Leo P., Sternes P., O’byrne K., Punyadeera C. Ex vivo culture of circulating tumour cells derived from non-small cell lung cancer. Transl. Lung Cancer Res. 2020;9:1795–1809. doi: 10.21037/tlcr-20-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H.-L., Chiou J.-F., Wang P.-Y., Lu L.-S., Shen C.-N., Hsu H.-L., Burnouf T., Ting L.-L., Chou P.-C., Chung C.-L., et al. Ex Vivo Expansion and Drug Sensitivity Profiling of Circulating Tumor Cells from Patients with Small Cell Lung Cancer. Cancers. 2020;12:3394. doi: 10.3390/cancers12113394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao J., Mc Gill J.R., Stanton K., Kassner J.D., Choudhury S., Schlegel R., Sauna Z.E., Pohlmann P.R., Agarwal S. Efficient Propagation of Circulating Tumor Cells: A First Step for Probing Tumor Metastasis. Cancers. 2020;12:2784. doi: 10.3390/cancers12102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carmona-Ule N., González-Conde M., Abuín C., Cueva J., Palacios P., López-López R., Costa C., Dávila-Ibáñez A. Short-Term Ex Vivo Culture of CTCs from Advance Breast Cancer Patients: Clinical Implications. Cancers. 2021;13:2668. doi: 10.3390/cancers13112668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu C.-L., Zhang Y.-J., Zhang X.-F., Fei X., Zhang H., Li C.-G., Sun B. 3D Culture of Circulating Tumor Cells for Evaluating Early Recurrence and Metastasis in Patients with Hepatocellular Carcinoma. Onco Targets Ther. 2021;14:2673–2688. doi: 10.2147/OTT.S298427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang C.-S., Kim I.-H., Chae H.-D., Kim D.-D., Jeon C.-H. Detection of Circulating Gastrointestinal Cancer Cells in Conditionally Reprogrammed Cell Culture. In Vivo. 2021;35:1515–1520. doi: 10.21873/invivo.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tayoun T., Faugeroux V., Oulhen M., Aberlenc A., Pawlikowska P., Farace F. CTC-Derived Models: A Window into the Seeding Capacity of Circulating Tumor Cells (CTCs) Cells. 2019;8:1145. doi: 10.3390/cells8101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reduzzi C., Vismara M., Gerratana L., Silvestri M., De Braud F., Raspagliesi F., Verzoni E., Di Cosimo S., Locati L.D., Cristofanilli M., et al. The curious phenomenon of dual-positive circulating cells: Longtime overlooked tumor cells. Semin. Cancer Biol. 2020;60:344–350. doi: 10.1016/j.semcancer.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Manjunath Y., Porciani D., Mitchem J.B., Suvilesh K.N., Avella D.M., Kimchi E.T., Staveley-O’Carroll K.F., Burke D.H., Li G., Kaifi J.T. Tumor-Cell-Macrophage Fusion Cells as Liquid Biomarkers and Tumor Enhancers in Cancer. Int. J. Mol. Sci. 2020;21:1872. doi: 10.3390/ijms21051872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khoo B.L., Grenci G., Lim Y.B., Lee S.C., Han J., Lim C.T. Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat. Protoc. 2018;13:34–58. doi: 10.1038/nprot.2017.125. [DOI] [PubMed] [Google Scholar]
- 64.Ohnaga T., Takei Y., Nagata T., Shimada Y. Highly efficient capture of cancer cells expressing EGFR by microfluidic methods based on antigen-antibody association. Sci. Rep. 2018;8:12005. doi: 10.1038/s41598-018-30511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Japanese Collection of Research Bioresources Cell Bank. [(accessed on 4 February 2022)]. Available online: https://cellbank.nibiohn.go.jp/~cellbank/cgi-bin/search_res_det.cgi?ID=7241.
- 66.Røsland G.V., Svendsen A., Torsvik A., Sobala E., Mc Cormack E., Immervoll H., Mysliwietz J., Tonn J.-C., Goldbrunner R., Lonning P.E., et al. Long-term Cultures of Bone Marrow–Derived Human Mesenchymal Stem Cells Frequently Undergo Spontaneous Malignant Transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 67.Sharma S., Zhuang R., Long M., Pavlovic M., Kang Y., Ilyas A., Asghar W. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol. Adv. 2018;36:1063–1078. doi: 10.1016/j.biotechadv.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most recent data which described in this review were preliminary data and did not report any data.