Abstract
Objectives:
To characterize neurofilament light levels in children who achieved return of spontaneous circulation following cardiac arrest compared with healthy controls and determine an association between neurofilament light levels and clinical outcomes.
Design:
Retrospective cohort study.
Setting:
Academic quaternary PICU.
Patients:
Children with banked plasma samples from an acute respiratory distress syndrome biomarker study who achieved return of spontaneous circulation after a cardiac arrest and healthy controls.
Interventions:
None.
Measurements and Main Results:
Neurofilament light levels were determined with a highly sensitive single molecule array digital immunoassay. Patients were categorized into survivors and nonsurvivors and into favorable (Pediatric Cerebral Performance Category score of 1–2 or unchanged from baseline) or unfavorable (Pediatric Cerebral Performance Category score of 3–6 or Pediatric Cerebral Performance Category score change ≥1 from baseline). Associations between neurofilament light level and outcomes were determined using Wilcoxon rank-sum test. We enrolled 32 patients with cardiac arrest and 18 healthy controls. Demographics, severity of illness, and baseline Pediatric Cerebral Performance Category scores were similar between survivors and nonsurvivors. Healthy controls had lower median neurofilament light levels than patients after cardiac arrest (5.5 [interquartile range 5.0–8.2] vs 31.0 [12.0–338.6]; p < 0.001). Neurofilament light levels were higher in nonsurvivors than survivors (78.5 [26.2–509.1] vs 12.4 [10.3–28.2]; p = 0.012) and higher in survivors than healthy controls (p = 0.009). The four patients who survived with a favorable outcome had neurofilament light levels that were not different from patients with unfavorable outcomes (21.9 [8.5––35.7] vs 37.2 [15.4–419.1]; p = 0.60) although two of the four patients who survived with favorable outcomes had progressive encephalopathies with both baseline and postcardiac arrest Pediatric Cerebral Performance Category scores of 4.
Conclusions:
Neurofilament light is a blood biomarker of hypoxic–ischemic brain injury and may help predict survival and neurologic outcome after pediatric cardiac arrest. Further study in a larger, dedicated cardiac arrest cohort with serial longitudinal measurements is warranted.
Keywords: biomarkers, neurofilament light, neurologic outcomes, survival, pediatric cardiac arrest
Pediatric cardiac arrest (CA) affects more than 20,000 children each year in the United States (1, 2). For children who achieve return of spontaneous circulation, neurologic prognostication is strengthened by integration of physical examination findings, electroencephalography, neuroimaging, and biochemical markers (3). Circulating biochemical markers are objective measures of brain injury that may assist in gauging the severity of injury and predicting outcome after pediatric CA (4). Peripheral blood biomarkers such as neuron-specific enolase, S100B, and glial fibrillary acidic protein are associated with unfavorable outcomes after CA but do not reliably predict degree of neurologic disability and are not solely expressed in neuronal structures (4–7).
Neurofilaments are scaffolding proteins that are exclusively expressed in both central and peripheral neurons and are released into the cerebrospinal fluid (CSF) and blood after neuroaxonal injury (8, 9). Neurofilament light (NfL) chain is an intermediate neurofilament protein subunit primarily expressed in myelinated subcortical white matter axons and is detectable in peripheral blood after hypoxic-ischemic brain injury from CA in adults and neonates (10–16). NfL levels obtained from adults in the Target Temperature Management After CA Trial more accurately predicted outcome than other biochemical markers including tau, neuron-specific enolase, and S100B (11, 17). It is unknown whether NfL has the same predictive value for outcomes after CA in children as in adults.
The goal of this exploratory pilot study was to characterize NfL levels in children following CA compared with healthy controls and to determine if NfL levels are associated with clinical outcomes. We hypothesized that NfL levels in children post CA would be higher than healthy controls and that NfL levels would be associated with patient outcome.
METHODS
Patients who were enrolled in a pediatric acute respiratory distress syndrome (ARDS) biomarker study at Children’s Hospital of Philadelphia (CHOP) (18, 19) were eligible for this study if they received cardiopulmonary resuscitation (CPR) for greater than or equal to 1 minute either prior to or during admission to the PICU. Plasma samples were drawn within 24 hours of ARDS onset. Patients were excluded if samples were obtained more than 48 hours after CA. The study was approved by the CHOP Institutional Review Board. Only patients who consented to their specimens being used for future research were eligible.
Demographics, severity of illness using the Pediatric Risk of Mortality III, CA characteristics, and outcomes were abstracted from the medical record (20). Patients were primarily categorized as PICU survivors and nonsurvivors. Neurologic outcome was secondarily categorized using the Pediatric Cerebral Performance Category (PCPC) score, a validated scale that categorizes functional impairment (1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma and vegetative state, and 6 = death) (21). Baseline PCPC scores were assigned based on level of neurologic functioning prior to PICU admission. PCPC scores were retrospectively assigned by two raters via medical record review and discrepancies resolved by consensus. Favorable outcome was defined as a PICU discharge PCPC score 1 or 2 or no change from baseline. We used existing samples from patients enrolled as healthy controls in a biomarker after pediatric traumatic brain injury study at CHOP. These patients had blood samples drawn during routine outpatient surgical procedures (e.g. tonsillectomy, hernia repair). All samples were stored in −80° centigrade freezers prior to analysis.
We used the highly sensitive single molecule array (SiMoA) digital immunoassay (Quanterix Corporation, Lexington, MA) for the quantitative determination of NfL levels (22–24). The analytical sensitivity of SiMoA is 100 to 1,000-fold higher than that obtained using the same antibodies in an enzyme-linked immunosorbent assay format. We validated each SiMoA kit before running specimens to ensure there were no technical deficits.
We described the cohort using descriptive statistics with percent for categorical variables and median with interquartile range (IQR) for continuous variables. Demographic and clinical characteristics were compared between survivors and nonsurvivors using Wilcoxon rank-sum and Fisher exact tests. Associations between NfL level and outcomes (survival vs nonsurvival and favorable vs unfavorable) were determined using Wilcoxon rank-sum test. Given the small sample sizes, multivariate adjustments to assess the independent relationship between NfL levels and outcomes were not performed. We computed area under the receiver operating characteristic (AUROC) to determine how well NfL predicted survival.
RESULTS
Thirty-four patients were eligible (Table 1). Two patients were excluded because samples were collected greater than 48 after CA, resulting in 32 evaluable patients. The 18 healthy controls were older than CA patients (median 13.3 vs 8.0 yr). Demographics were similar between survivors and nonsurvivors (Table 1). Three patients were managed with extracorporeal membrane oxygenation (two venovenous and one venoarterial) for respiratory failure after CA. Baseline PCPC scores, severity of illness, and high frequency oscillatory ventilation utilization did not differ between survivors and nonsurvivors. Five patients had baseline PCPC scores greater than or equal to 3 (Table 1) due to a chronic static encephalopathy (e.g., genetic syndrome, prematurity-related brain injury, or prior CA), and three patients had baseline PCPC scores of 4 due to a progressive encephalopathy (e.g., metabolic disease, neurodegenerative disease, or leukodystrophy).
TABLE 1.
Patient Characteristics
| Characteristics | Controls | Cardiac Arrest | p | ||
|---|---|---|---|---|---|
| All | Survivors | Nonsurvivors | |||
| Number of patients, n (%) | 18 | 32 | 13 (41) | 19 (59) | |
| Demographics | |||||
| Age, median (IQR) | 13.3 (8.7−15.0) | 8.0 (3.8−13.3) | 8.0 (5.2−13.0) | 8.0 (3.6−13.4) | 0.969 |
| Male gender, n (%) | 6 (33) | 17 (53) | 8 (62) | 9 (47) | 0.491 |
| Race, n (%) | |||||
| African American | 6 (33) | 8 (25) | 4 (31) | 4 (21) | 0.053 |
| White | 9 (50) | 14 (44) | 8 (61) | 6 (32) | |
| Other | 3 (17) | 10 (31) | 1 (8) | 9 (47) | |
| Hispanic ethnicity, n (%) | 2 (11) | 4 (13) | 4 (21) | 0.128 | |
| Baseline PCPC, median (IQR) | 1.0 (1.0− 2.3) | 2.0 (1.0− 2.0) | 1.0 (1.0− 2.0) | 0.308 | |
| 1, n (%) | 20 (63) | 6 (46) | 14 (74) | ||
| 2, n (%) | 4 (12) | 4 (31) | |||
| 3, n (%) | 2 (6) | 1 (8) | 1 (5) | ||
| 4, n (%) | 6 (19) | 2 (15) | 4 (21) | ||
| Severity of illness | |||||
| Pediatric Risk of Mortality 3, median (IQR) | 27.5 (22.5− 33.0) | 24.0 (21.0− 30.0) | 31.0 (24.0− 35.0) | 0.087 | |
| Worst oxygenation index in first 24 hr, median (IQR) | 27.9 (17.9−45.9) | 27.3 (12.4−39.3) | 26.7 (20.7−54.6) | 0.527 | |
| High-frequency oscillatory ventilation, n (%) | 13 (41) | 6 (46) | 7 (37) | 0.670 | |
| Extracorporeal membrane oxygenation, n (%) | 3 (9) | 3 (23) | 0.058 | ||
| Cardiac arrest characteristics | |||||
| Out-of-hospital, n (%) | 22 (69) | 6 (46) | 16 (84) | 0.049 | |
| Cardiopulmonary resuscitation duration, median (IQR) | 11.0 (6.5−25.0) | 8.0 (4.8−11.8) | 17.0 (10.0−30.0) | 0.014 | |
| Rhythm, n (%) | |||||
| Asystole/pulseless electrical activity | 13 (41) | 3 (23) | 10 (53) | 0.032 | |
| Bradycardia | 9 (28) | 7 (54) | 2 (10) | ||
| Unknown | 10 (31) | 3 (23) | 7 (37) | ||
| Defibrillation, n (%) | 3 (9) | 3 (16) | 0.579 | ||
| Etiology, n (%) | |||||
| Respiratory failure | 9 (28) | 5 (38) | 4 (21) | 0.373 | |
| Drowning | 9 (28) | 3 (23) | 6 (32) | ||
| Shock/arrhythmia | 4 (13) | 2 (15) | 2 (11) | ||
| Trauma | 7 (22) | 2 (15) | 5 (26) | ||
| Other/unknown | 3 (9) | 1 (8) | 2 (11) | ||
| Initial pH, median (IQR) | 7.1 (7.0−7.2) | 7.1 (6.9−7.2) | 7.1 (7.0−7.2) | 0.628 | |
| Initial lactate, median (IQR) | 5.6 (4.9−7.8) | 5.0 (2.9−7.0) | 5.6 (5.5−9.5) | 0.486 | |
| Postarrest Glasgow Coma Scale, median (IQR) | 3 (3−3) | 3 (3−3) | 3 (3−3) | 0.570 | |
| Outcomes | |||||
| PICU length of stay, median (IQR) | 7.5 [3.0−15.8] | 24.0 [13.0−67.0] | 4.0 [2.0−6.0] | < 0.001 | |
| Death, n (%) | |||||
| Brain death | 9 (47) | ||||
| Withdrawal of life-sustaining therapies | 9 (47) | ||||
| Rearrest | 1 (5) | ||||
| PICU discharge PCPC, median (IQR) | 4.0 (3.0−4.0) | 6.0 (6.0−6.0) | |||
| 2, n (%) | 2 (15) | ||||
| 3, n (%) | 3 (23) | ||||
| 4, n (%) | 8 (62) | ||||
| 6, n (%) | 19 (100) | ||||
| Change in PCPC, median (IQR) | 2.0 (1.0−2.0) | 5.0 (4.0−5.0) | |||
| 0, n (%) | 3 (23) | ||||
| 1, n (%) | 3 (23) | ||||
| 2, n (%) | 4 (31) | 4 (21) | |||
| 3, n (%) | 3 (23) | 1 (5) | |||
| 4 | |||||
| 5 | 14 (74) | ||||
| Discharge, n (%) | |||||
| Rehabilitation | 7 (54) | ||||
| Facility | 3 (23) | ||||
| Home | 2 (15) | ||||
| Still admitted | 1 (8) | ||||
IQR = interquartile range, PCPC = Pediatric Cerebral Performance Category.
A greater percentage of nonsurvivors had an out-of-hospital CA (84% vs 46%; p = 0.049). Nonsurvivors received CPR for a longer duration although initial pH and lactate were similar between survivors and nonsurvivors. Survivors had longer PICU lengths of stay than nonsurvivors (24.0 [13.0–67.0] vs 4.0 d [2.0–6.0 d]; p ≤ 0.001). Modes of death (i.e., brain death, withdrawal for poor neurologic prognosis, rearrest) for nonsurvivors and discharge disposition (i.e., rehabilitation, chronic care facility, home) for survivors are summarized in Table 1.
Healthy controls had lower NfL levels than patients after CA (5.49 [IQR, 4.96–8.18] vs 30.96 [11.97–338.64]; p < 0.001). NfL levels were significantly higher in nonsurvivors than survivors (p = 0.012) and higher in survivors than healthy controls (p = 0.009) (Fig. 1). AUROC for NfL and survival was 0.78 (95% CI, 0.62–0.94). The four patients who survived with a favorable outcome had NfL levels that were not significantly different from patients with unfavorable outcomes and healthy controls (Fig. 1). Two of these patients who survived with a favorable outcome had progressive encephalopathies with both baseline and post-CA PCPC scores of 4. One additional patient with a baseline PCPC score of 3 due to a chronic static encephalopathy survived with an unfavorable outcome.
Figure 1.
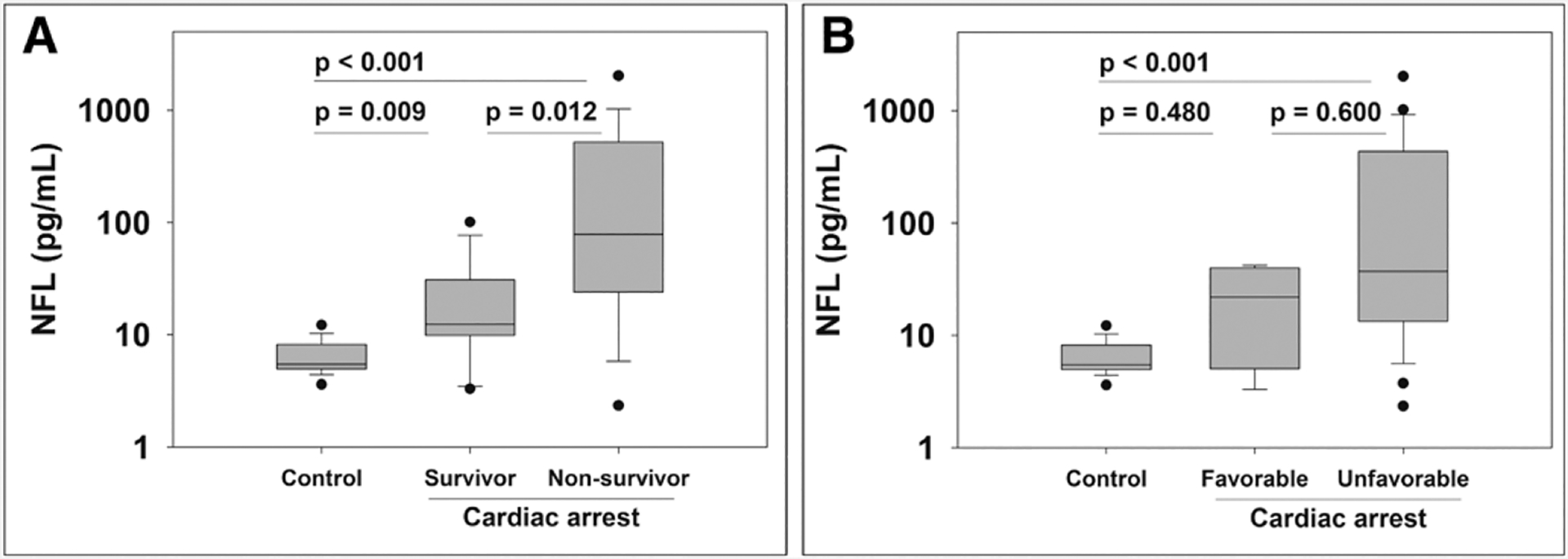
Serum neurofilament light (NfL) concentration from patients after pediatric cardiac arrest and healthy controls. Cardiac arrest patients are categorized into survivors and nonsurvivors (A) and favorable and unfavorable outcomes (B). Data are plotted on the log-scale for ease of visualization.
DISCUSSION
In a small cohort of pediatric patients with CA and ARDS, plasma NfL levels were higher for nonsurvivors than survivors. These data extend findings from adults and neonates who demonstrated that higher NfL levels after CA are associated with unfavorable neurologic outcomes (11, 15, 16).
Neurofilaments are a group of intermediate-sized structural scaffolding proteins that make up the cytoskeleton of axons. These Class IV filaments are obligate heteropolymers, composed of three subunits: a light (NfL, 70 kDa), a medium (NfM, 150 kDa), and a heavy (NfH 200 KDa) chain and are exclusively found in neurons. They are important for axonal growth and transport of proteins (8, 9). The NfL subunit is predominantly expressed in myelinated axons in the CNS. Pathologic processes that cause axonal injury or degeneration release NfL into the CSF and peripheral blood. NfL levels are elevated in many forms of acute brain injury in children and adults including hypoxia-ischemia, stroke, neuroinflammatory disorders like demyelinating syndromes, opsoclonus-myoclonus syndrome, and autoimmune encephalitis (25–30). NfL is also elevated in many forms of neurodegenerative disease such as Alzheimer’s, Parkinson’s, and Amyotrophic Lateral Sclerosis (31–33), and in peripheral nervous system disorder like Charcot–Marie–Tooth disease (9, 34). Thus, when there is concomitant insult or inflammation to peripheral nerves, elevation of NfL may not reflect the extent and severity of the accompanying brain injury.
The rise in NfL levels after the hypoxic-ischemic injury of a CA may be due to several mechanisms including direct axonal or oligodendrocyte pathology, axonal degeneration after neuronal injury, or a combination (35–37). The time course of these injury mechanisms may explain NfL’s relatively later peak than other biochemical markers of brain injury. Thus, the optimal timing of NfL measurements to predict neurologic outcome after acute brain injury is unclear. In adults with unfavorable neurologic outcomes after CA NfL levels doubled between 24 and 48 hours after CA, however, were stable between 48 and 72 hours after CA (11). NfL predicted unfavorable outcome best at 5 and 7 days post CA (10). NfL kinetics post CA may be more similar to myelin basic protein which has a delayed and sustained increase in patients with unfavorable outcomes (5). Similarly, in adult patients after traumatic brain injury, NfL levels within 24 hours of injury were associated with survival and neurologic outcome. However, NfL levels continued to rise until at least 12 days after injury (38). Notably, NfL levels were stable through day 4 and then rose again at day 6 (38). The half-life of NfL in humans has not been established but has been approximated at 3 weeks in an animal model (34). NfL levels have not been assayed further than 7 days post CA (10). In our convenience sample, nearly all (97%) samples were collected within 24 hours of CA. It is possible that NfL levels at later time points after pediatric CA may better discriminate favorable from unfavorable outcomes and predict neurocognitive deficits in survivors.
NfL levels in healthy controls in our cohort (5.49 [4.96–8.18]) were similar to controls in other studies also using SiMoA technology (30, 38, 39). Concentrations of NfL in blood and CSF in healthy children have not been systematically investigated, and it is unknown whether basal NfL levels or NfL release after neuroaxonal injury is age-dependent. Additionally, comparing across studies is challenging due to differences in NfL assay techniques (8). In healthy adults, CSF NfL levels increase with age (40). NfL levels in children were found to be higher than adults at the time of a demyelinating event (28). Healthy neonates may have higher levels than older children as the median NfL levels of healthy neonates was 23.3 pg/mL in one study compared with 5.49 in our pediatric population (15).
Interestingly, NfL is elevated in children with mitochondrial disease and other progressive encephalopathies (29, 41). In our cohort, two of the four patients who survived with favorable outcomes had progressive encephalopathies with both baseline and post-CA PCPC scores of 4. These two patients had NfL levels of 34 and 42 pg/mL, compared with the other two patients whose levels were 10 and 3 pg/mL and healthy controls whose levels were a median of 5.5 pg/mL. It is likely that the underlying neurologic disease of these patients contributed to their elevated NfL levels and that postarrest NfL levels may only discriminate favorable and unfavorable outcomes after CA among patients without underlying neurologic disease. However, we are clearly underpowered to make any conclusions regarding NfL levels and neurologic prognosis from this pilot study. This also reinforces the necessity of studying NfL levels in children, rather than extrapolating from adult data, as the types of comorbidities and epidemiology of CA differ substantially.
This study was limited by its retrospective nature and using stored blood from a previous biomarker study of ARDS. Thus, all patients in this cohort had CA and ARDS. Although it is unknown if ARDS directly or indirectly influences NfL levels, patients are often ventilated with high mean airway pressures. This could decrease cerebral venous drainage resulting in cerebral ischemia or edema with secondary neuronal injury that could increase NfL levels. Alterations in Paco2 from ventilation strategies could also contribute to cerebral ischemia or hyperemia. Additionally, it is unknown if patients may have sustained peripheral nervous system injury as a result of their CA. Furthermore, the timing of blood sampling was not standardized from CA. However, even in this limited cohort, higher NfL concentrations were associated with worse outcomes.
CONCLUSIONS
Higher NfL levels are associated with nonsurvival after pediatric CA, thus establishing NfL as a promising blood biomarker of hypoxic-ischemic brain injury after pediatric CA. Further prospective study at serial post-CA time points in a larger dedicated pediatric CA population is warranted to assess the utility of NfL as a prognostic biomarker after pediatric CA.
Acknowledgments
Supported, in part, by the Department of Anesthesiology and Critical Care Medicine at the Children’s Hospital of Philadelphia.
Footnotes
This work was performed at the Children’s Hospital of Philadelphia.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. ; Resuscitation Outcomes Consortium Investigators: Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The resuscitation outcomes consortium epistry-cardiac arrest. Circulation 2009; 119:1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators: Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019; 12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 3.Topjian AA, de Caen A, Wainwright MS, et al. : Pediatric post-cardiac arrest care: A scientific statement from the American Heart Association. Circulation 2019; 140:e194–e233 [DOI] [PubMed] [Google Scholar]
- 4.Prout AJ, Wolf MS, Fink EL: Translating biomarkers from research to clinical use in pediatric neurocritical care: Focus on traumatic brain injury and cardiac arrest. Curr Opin Pediatr 2017; 29:272–279 [DOI] [PubMed] [Google Scholar]
- 5.Fink EL, Berger RP, Clark RS, et al. : Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Crit Care Med 2014; 42:664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topjian AA, Lin R, Morris MC, et al. : Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med 2009; 10:479–490 [DOI] [PubMed] [Google Scholar]
- 7.Fink EL, Berger RP, Clark RS, et al. : Exploratory study of serum ubiquitin carboxyl-terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation 2016; 101:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil M, Teunissen CE, Otto M, et al. : Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14:577–589 [DOI] [PubMed] [Google Scholar]
- 9.Petzold A: Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 2005; 233:183–198 [DOI] [PubMed] [Google Scholar]
- 10.Rana OR, Schröder JW, Baukloh JK, et al. : Neurofilament light chain as an early and sensitive predictor of long-term neurological outcome in patients after cardiac arrest. Int J Cardiol 2013; 168:1322–1327 [DOI] [PubMed] [Google Scholar]
- 11.Moseby-Knappe M, Mattsson N, Nielsen N, et al. : Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol 2019; 76:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosén C, Rosén H, Andreasson U, et al. : Cerebrospinal fluid biomarkers in cardiac arrest survivors. Resuscitation 2014; 85:227–232 [DOI] [PubMed] [Google Scholar]
- 13.Rundgren M, Friberg H, Cronberg T, et al. : Serial soluble neurofilament heavy chain in plasma as a marker of brain injury after cardiac arrest. Crit Care 2012; 16:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas-Escobar M, Yang C, Bennett J, et al. : A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatr Res 2010; 68:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toorell H, Zetterberg H, Blennow K, et al. : Increase of neuronal injury markers Tau and neurofilament light proteins in umbilical blood after intrapartum asphyxia. J Matern Fetal Neonatal Med 2018; 31:2468–2472 [DOI] [PubMed] [Google Scholar]
- 16.Shah DK, Ponnusamy V, Evanson J, et al. : Raised plasma neurofilament light protein levels are associated with abnormal MRI outcomes in newborns undergoing therapeutic hypothermia. Front Neurol 2018; 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen N, Wetterslev J, Cronberg T, et al. ; TTM Trial Investigators: Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013; 369:2197–2206 [DOI] [PubMed] [Google Scholar]
- 18.Yehya N, Thomas NJ, Margulies SS: Circulating nucleosomes are associated with mortality in pediatric acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2016; 310:L1177–L1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yehya N, Thomas NJ, Meyer NJ, et al. : Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med 2016; 42:1137–1145 [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Patel KM, Ruttimann UE: PRISM III: An updated pediatric risk of mortality score. Crit Care Med 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 21.Fiser DH: Assessing the outcome of pediatric intensive care. J Pediatr 1992; 121:68–74 [DOI] [PubMed] [Google Scholar]
- 22.Wilson DH, Rissin DM, Kan CW, et al. : The simoa HD-1 analyzer: A novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom 2016; 21:533–547 [DOI] [PubMed] [Google Scholar]
- 23.Rissin DM, Kan CW, Campbell TG, et al. : Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010; 28:595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rissin DM, Fournier DR, Piech T, et al. : Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem 2011; 83:2279–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Marchis GM, Katan M, Barro C, et al. : Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol 2018; 25:562–568 [DOI] [PubMed] [Google Scholar]
- 26.Pranzatelli MR, Tate ED, McGee NR, et al. : CSF neurofilament light chain is elevated in OMS (decreasing with immunotherapy) and other pediatric neuroinflammatory disorders. J Neuroimmunol 2014; 266:75–81 [DOI] [PubMed] [Google Scholar]
- 27.Boesen MS, Jensen PEH, Magyari M, et al. : Increased cerebrospinal fluid chitinase 3-like 1 and neurofilament light chain in pediatric acquired demyelinating syndromes. Mult Scler Relat Disord 2018; 24:175–183 [DOI] [PubMed] [Google Scholar]
- 28.van der Vuurst de Vries RM, Wong YYM, Mescheriakova JY, et al. : High neurofilament levels are associated with clinically definite multiple sclerosis in children and adults with clinically isolated syndrome. Mult Scler 2019; 25:958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahim P, Darin N, Andreasson U, et al. : Cerebrospinal fluid brain injury biomarkers in children: A multicenter study. Pediatr Neurol 2013; 49:31–39.e2 [DOI] [PubMed] [Google Scholar]
- 30.Mariotto S, Gajofatto A, Zuliani L, et al. : Serum and CSF neurofilament light chain levels in antibody-mediated encephalitis. J Neurol 2019; 266:1643–1648 [DOI] [PubMed] [Google Scholar]
- 31.Mattsson N, Andreasson U, Zetterberg H, et al. ; Alzheimer’s Disease Neuroimaging Initiative: Association of plasma neurofilament light with neurodegeneration in patients with alzheimer disease. JAMA Neurol 2017; 74:557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson O, Janelidze S, Hall S, et al. ; Swedish BioFINDER study: Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017; 88:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feneberg E, Oeckl P, Steinacker P, et al. : Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 2018; 90:e22–e30 [DOI] [PubMed] [Google Scholar]
- 34.Barry DM, Millecamps S, Julien JP, et al. : New movements in neurofilament transport, turnover and disease. Exp Cell Res 2007; 313:2110–2120 [DOI] [PubMed] [Google Scholar]
- 35.Ginsberg MD, Hedley-Whyte ET, Richardson EP Jr: Hypoxic-ischemic leukoencephalopathy in man. Arch Neurol 1976; 33:5–14 [DOI] [PubMed] [Google Scholar]
- 36.Busl KM, Greer DM: Hypoxic-ischemic brain injury: Pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 2010; 26:5–13 [DOI] [PubMed] [Google Scholar]
- 37.Keijzer HM, Hoedemaekers CWE, Meijer FJA, et al. : Brain imaging in comatose survivors of cardiac arrest: Pathophysiological correlates and prognostic properties. Resuscitation 2018; 133:124–136 [DOI] [PubMed] [Google Scholar]
- 38.Shahim P, Gren M, Liman V, et al. : Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016; 6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anzalone AJ, Turner SM, Baleztena AC, et al. : Blood biomarkers of sports-related concussion in pediatric athletes. Clin J Sport Med 2019. Feb 26. [online ahead of print] [Google Scholar]
- 40.Vågberg M, Norgren N, Dring A, et al. : Levels and age dependency of neurofilament light and glial fibrillary acidic protein in healthy individuals and their relation to the brain parenchymal fraction. PLoS One 2015; 10:e0135886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofou K, Shahim P, Tulinius M, et al. : Cerebrospinal fluid neurofilament light is associated with survival in mitochondrial disease patients. Mitochondrion 2019; 46:228–235 [DOI] [PubMed] [Google Scholar]


