Abstract
Purpose
As protection from COVID-19 following two doses of the BNT162b2 vaccine showed a time dependent waning, a third (booster) dose was administrated. This study aims to compare the antibody response following the third dose versus the second and to evaluate post-booster seroconversion.
Methods
A prospective observational study conducted in Maccabi Healthcare Services. Serial SARS-CoV-2 Spike IgG tests, 1,2,3 and 6 months following the second vaccine dose and one month following the third were obtained. Neutralizing antibody levels were measured in a subset of participants. Per individual SARS-CoV-2 Spike IgG titer ratios were calculated one month after the booster administration compared to titers one month following the second dose and prior to booster.
Results
Among 110 participants, 56 (51%) were women. Mean age was 61.7 ± 1.9 years and 66 (60%) were immunocompromised. One month after third dose, IgG titers were induced 7.83 (95 %CI 5.25–11.67) folds and 2.40 (95 %CI 1.90–3.03) folds compared to one month after the second, in the immunocompromised and immunocompetent groups, respectively. Of the 17 immunocompromised participants who were seronegative after the second dose, 4 (24%) became seropositive following the third. Comparing the titers prior to the third dose, an increase of 50.7 (95 %CI 32.5–79.1) fold in the immunocompromised group and 25.7 (95 %CI 19.1–34.7) fold in and immunocompetent group, was observed.
Conclusion
A third BNT162b2 vaccine elicited robust humoral response, superior to the response observed following the second, among immunocompetent and immunocompromised individuals.
Keywords: BNT162b2 vaccine, COVID-19, Humoral response, Third vaccine dose, Booster, Immunocompromised
Abbreviations: ICIs, Immunocompromised individuals; MHS, Maccabi Healthcare Services; PCR, Polymerase chain reaction; CLL, Chronic lymphatic leukemia; MM, Multiple myeloma; NHL, Non Hodgkin lymphoma; BMI, Body mass index; AU/mL, Arbitrary units/ milliliter; GM, Geometric mean
1. Introduction
The COVID-19 pandemic has imposed burdens of morbidity and mortality worldwide, placing unpredictable, large-scale health challenges in the hospital and community settings globally [1], [2]. Immunocompromised individuals (ICIs), those with lymphoproliferative or myeloproliferative disorder, or on immunosuppressive or immunomodulating therapy, are at a higher risk for severe COVID-19 disease, including hospital and intensive care unit admission, intubation, and mortality [3], [4].
On December 2020 Israel initiated a massive COVID-19 vaccination campaign, during which mRNA BNT162b2 (Pfizer–BioNTech) vaccines were administered [5]. A few months following receipt of the second vaccine dose, a relative decrease in the long-term protection of the BNT162b2 vaccine against the Delta variant of SARS-CoV-2 was observed [6]. Thereafter, on July 2021, the Israeli Ministry of Health approved a third (booster) SARS-CoV-2 BNT162b2 vaccine dose for individuals who received the second dose at least 5 months before. Real world data showed effectiveness of the booster administration in lowering confirmed case, disease severity and mortality due to COVID-19 [7], [8].
Previous studies evaluated the humoral response following second and third BNT162b2 vaccine doses among immunocompromised and immunocompetent individuals [9], [10], [11], [12]. However, data comparing longitudinal IgG anti-Spike SARS-CoV-2 response following the second and third doses administration are scarce, and it is not clear yet whether the pattern of humoral response is personalized and connected between the second and third dose, particularly when comparing between these two subpopulations.
In this study we longitudinally followed the antibody levels in a cohort of Immunocompromised and immunocompetent individuals, starting from 30 days after second vaccine dose administration and up to 90 days after the third (booster) vaccine dose (a total period of seven months). Using this data, we evaluated the time- dependent decrease in antibody levels in both subgroups. Furthermore, we quantified the response to the third (booster) vaccine dose in comparison with both the response to the second vaccine and the antibody levels prior to the third dose in the two subgroups.
2. Methods
2.1. Setting
A prospective observational longitudinal study, conducted in Maccabi Healthcare Services (MHS). MHS is the second largest Health Maintenance Organization in Israel, serving over 2.5 million citizens, representing a quarter of the Israeli population.
2.2. Study population and design
As soon as BNT162b2 mRNA vaccination was authorized in Israel, we recruited the study participants from hematological and primary care clinics mainly in 'Hasharon' district. The inclusion criteria for the patients’ recruitment were: adults aged of 18 years and above; were not infected with SARS-CoV-2 (without a positive SARS-CoV-2 polymerase chain reaction (PCR) assay test; along the study period, participants with relevant clinical symptoms symptoms or exposure to a positive case were referred to PCR test and if tested positive were excluded) and signed an informed consent form. For the analysis described in this study we included only the patients who received three doses of the BNT126b2 vaccine according to the interval set by national guidelines.
The first serology test was done 30 ± 7 days following receipt the second vaccine dose. The second, third and fourth IgG antibodies samples were planned to be taken at the following time: two, three and six, months after completing the two-dose regimen of BNT126b2, respectively. Following the administration of the booster dose, participants were referred to another serology test.
Participants were divided into immunocompromised and immunocompetent subgroups according to their immunosuppression status. ICIs were defined as follows: 1. Lymphoproliferative diseases including chronic lymphatic leukemia (CLL), multiple myeloma (MM), low and high grade non Hodgkin lymphoma (NHL); treatment naïve or being currently treated, or were treated in the previous year. 2. Myeloproliferative and myelodysplastic diseases; treatment naïve or being currently treated. 3. Patients suffering from solid tumors who are receiving or have received in the previous 3 months chemotherapy or radiotherapy or immunosuppressive or immunomodulating therapy. 4. Recipients of solid organ transplant or patients with autoimmune diseases who are receiving or have received in the previous 3 months immunosuppressive agents or immune modifying treatment.
We evaluated the IgG anti-Spike SARS-CoV-2 kinetics during time frames from the second vaccine dose administration (i.e 1, 2, 3, 6 months) in the two subgroups. Later, we compared the antibody response 30 days after the second dose and third vaccine doses in the two groups. In addition, we compared the antibody response before and after the third vaccine dose in the two groups. Participants who did not elicit an antibody response after the second dose were considered “non-responders” and were analyzed separately for antibody response after the third dose.
2.3. Data collection
Data from the medical records were retrieved from the nationwide centralized database of MHS, spanning over 20 years, included demographics, body mass index (BMI) comorbidities and laboratory test results. Additional information was retrieved by two of the authors (SBBD and SS), who manually reviewed records of participants for immunosuppression status during enrolment and along the study period included medication, laboratory test results and hematologic background.
2.4. Laboratory methods
A commercial assay was used to detect IgG antibodies against SARS-CoV-2 RBD portion of the spike protein - Quant II IgG anti-Spike SARS-CoV-2 by Abbott (Illinois, U.S.A.) and reported as AU/mL (Arbitrary units). The cutoff for serology reactivity (i.e. response to vaccine) is 50 AU/mL according to the manufacturer’s instructions. Values above 40,000 AU/mL were truncated to 40,000 AU/mL.
SARS-CoV-2 Pseudoneutralization Assay:
A SARS-CoV-2 pseudovirus neutralization assay was performed as previously described [13] to detect SARS-CoV-2 neutralizing antibodies using a green fluorescent protein reporter–based pseudotyped virus with a vesicular stomatitis virus backbone coated with the SARS-CoV-2 spike (S) protein, which was generously provided by Dr Gert Zimmer (Institute of Virology and Immunology, Mittelhäusern, Switzerland). The level of detection for neutralizing antibodies was above 8. Due to the limited availability, 25% of randomly selected samples were tested by Pseudo Neutralization Assay.
2.5. Statistical analysis
Comparison of the humoral response following the second and third (booster) vaccine doses:
P-value for IgG anti-Spike SARS-CoV-2 titers following the second dose versus the third was calculated using the non-parametric Mann-Whitney U test. In order to validate that the observed difference is not due to the time interval between the vaccination and the antibody testing Student's t-test was used to compare the testing time intervals. Fold change and confidence interval were calculated by taking the exponent of the arithmetic mean and 1.96 standard deviation of the log of the second/ third dose IgG ratios (equivalent to the geometric mean of the non-log transformed ratios).
The correlation between Quant II IgG anti-Spike SARS-CoV assay and neutralizing antibodies and the correlation between IgG values after the second dose and after the booster were calculated using Spearman’s rank correlation test.
Second dose waning effect estimation:
To estimate the rate of IgG decline following the second dose vaccination we used a mixed linear model. The model included the fixed effect variables: gender, age group (<65 years or ≥ 65 years) and immunosuppression status, with intercept and time trend as per subject random effects. Samples were grouped into 4 distinct time points (30, 60,90 and 180 days after the second dose) by using all samples taken in a window of ± 15 days. The dependent variable was log transform of IgG value. The mixed linear models were conducted by Statsmodels Mixedlm package.
All analyses were performed using python (version 3.8.8) and Statsmodels (version 0.12.2).
The study was approved by Maccabi Health Services’ institutional review board (0016–21-MHS), written informed consent was obtained from all participants.
3. Results
The study was conducted from February 10, 2021, to October 20, 2021. Among the 110 study participants, 66 (60%) were ICI and 44 (40%) were immunocompetent. The mean (SD) age was 61.7 ± 1.9 and 56 individuals (51%) were women (Table 1 ).
Table 1.
Participants' baseline characteristics.
| Variable |
All participants n = 110 (100%) |
Immunocompromised n = 66 (60%) |
Immunocompetent n = 44 (40%) |
||
|---|---|---|---|---|---|
| Responders to vaccine 2nd and 3rd vaccine doses n = 49 (74%) |
Responders only to 3rd vaccine dose n = 4 (6%) |
Non responders n = 13 (20%) |
|||
| Age, years, mean (SD) | 61.7 (11.9) | 63.5 (11.0) | 53.5 (15.2) | 65.5 (9.9) | 59.3 (12.2) |
| Female sex, n (%) | 56 (51%) | 19 (39%) | 3 (75%) | 5 (38%) | 29 (66%) |
| Time between recipient of 1st and 2nd vaccine doses, days (mean, SD) | 21 (1) | 21 (1) | 21 (1) | 22 (1) | 21 (1) |
| Time between recipient of 2nd and 3rd vaccine doses, days (mean, SD) | 195 (19) | 192 (17) | 195 (20) | 194 (19) | 199 (20) |
| Time from recipient of 2nd vaccine dose to antibody test, days (mean, SD) | 34 (5) | 34 (6) | 32 (4) | 34 (6) | 33.1 (4) |
| Time from recipient of 3rd vaccine dose to antibody test, days (mean, SD) | 33 (19) | 37 (21) | 34 (19) | 29 (20) | 29 (16) |
| Antibody response after 2nd vaccine | 93 (85%) | 49 (100%) | 0 | 0 | 44 (100%) |
| Antibody response after 3rd vaccine | 97 (88%) | 49 (100%) | 4 (100%) | 0 | 44 (100%) |
| Number of antibody tests after 2nd vaccine dose | 3.6 (0.7) | 3.6 (0.6) | 3.8 (0.4) | 3.6 (0.5) | 3.5 (0.9) |
| Underling diseases | |||||
| Diabetes | 11 | 4 | 0 | 2 | 5 |
| Hypertension | 27 | 14 | 1 | 3 | 9 |
| Cardiovascular disease | 13 | 9 | 0 | 0 | 4 |
| Inflammatory bowel disease | 1 | 0 | 0 | 1 | 0 |
| Chronic Kidney Disease GFR < 60 | 24 | 12 | 1 | 2 | 9 |
| BMI mean (SD) | 25.8 (4.7) | 26.6 (3.3) | 29.6 (1.7) | 24.1 (2.4) | 25.9 (4.6) |
| Immunosuppression | |||||
| Lymphoproliferative disorder (on active treatment)a | 22 (4) | 3 (2) | 12 (8) | ||
| Myeloproliferative disorder (on active treatment) | 5 (2) | 0 | 0 | ||
| Solid cancer, on active treatment | 9 | 0 | 0 | ||
| Immunosuppressive treatment non– oncologic | 13 | 1 | 1 | ||
| Laboratory results | |||||
| Absolute Neutrophil Count, micl/3*10 median (range) | 3.6 (0.6–12.4) | 3.5 (0.6–12.4) | 5.1 (4.7–6.4) | 3.9 (1.5–6.8) | 3.3 (1.8–6.5) |
| Absolute Lymphocyte Count, micl/3*10, median (range) | 2.2 (0.5–148.2) | 2.3 (0.5–132.5) | 13.1 (2.9–109.1) | 1.4 (0.7–148.2) | 2.2 (1.4–4.6) |
| Number of patients with immunoglobulin measures | 20 | 4 | 9 | ||
| IgG, mg/ Lmedian (range) |
44.3 (20.0–830.7) | 42.4 (38.4–81.1) | 45.9 (20.0–384.8) | ||
| IgM, mg/ Lmedian (range) ,<20, |
789.1 (461.5–4505.2) 4 |
665.9 (526.1–934.1) 0 |
699.1 (368.1–1144.7) 3 |
||
| IgA, mg/ Lmedian (range) , <10 |
79.8 (10.0–1058.2) 1 |
118.0 (28.0–151.8) 0 |
73.3 (11.6–200.1) 0 |
||
a Lymphoproliferative disorders: Chronic Lymphocytic Leukemia- 23, indolent lymphoma- 11, myeloma, Waldenstrom’s macroglobulinemia and other plasma cell dyscrasia- 4.
Abbreviations: BMI, Body mass index; GFR, glomerulofiltration rate; SD, standard deviation, micl microliter, mg milligrams, L liter.
Among the ICIs group, 49 (74%) developed antibody response following 34 6 days since the second BNT162b2 vaccine dose administration, as well as all 44 (100%) of the immunocompetent group. A month (33 19 days) after the third (booster) dose of the homologous BNT162b2 vaccine administration, 53 (80%) of ICI and all 44 (100%) of the immunocompetent individuals developed antibody response.
The geometric mean (GM) ratio (95 %CI) of IgG anti-Spike SARS-CoV-2 titers after the second vaccine dose administration among the ICI group who developed a humoral response was 1,056 (686 – 1,627) AU/mL versus 7,691 (5,949–9,943) AU/mL in the immunocompetent group (Table2 ). 178 ± 6 days following recipient the second vaccine dose, the antibody titer diminished in both groups, with a GM (95 %CI) of 173 (118–255) AU/mL and 718 (526–982) AU/mL in the ICIs and immunocompetent groups, respectively (Supplementary Fig. S1).
Table 2.
IgG anti-Spike SARS-CoV-2 response one month after the administration of second and one month after the third (booster) BNT162b2 mRNA vaccine doses.
| Immunocompromised |
All Immunocompetent n = 44 (100%) |
|||
|---|---|---|---|---|
| All Immunocompromised n = 49 (100%) |
Lymphoproliferative disorder /Myeloproliferative disorder n = 27 (55%) |
Solid cancer/ Immunosuppressive treatment non-oncologic n = 22 (45%) |
||
| Days between 2nd vaccine dose recipient to IgG test a | 34 6 | 34 4 | 34 7 | 33 4 |
| IgG titer following 2nd vaccine dose (GM, 95 %CI) | 1,056 (686–1,627) |
814 (473–1,403) |
1,454 (734–2,881) |
7,691 (5,949–9,943) |
| Days between 3rd vaccine dose recipient to IgG test a | 36 21 | 34 18 | 40 22 | 29 16 |
| IgG titer following 3rd vaccine dose (GM, 95 %CI) | 8,269 (5,904–11,582) |
7,855 (4,721–13,070) |
8,807 (5,744–13,506) |
18,438 (15,210–22,350) |
| P-value for IgG following 2nd dose versus 3rd | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fold ratio (GM 95 %CI)b between IgG titter following 3rd vaccine and 2nd vaccine doses | 7.83 (5.25–11.67) |
9.65 (5.55–16.77) |
6.06 (3.3–11.12) |
2.40 (1.90–3.03) |
aNo statistical difference was found between the time period after 2nd and 3rd vaccine dose recipient to IgG anti-Spike SARS-CoV-2 tests among immunocompromised (p = 0.42) and immunocompetent (p = 0.13).
bFold ratio- Rate of IgG titer one month following 3rd vaccine dose recipient (GM, 95 %CI)/ IgG titer one month following the 2nd vaccine dose recipient (GM, 95 %CI).
GM- geometric mean, CI- confidence interval.
Using mixed model regression (see methods) the estimated rate of monthly antibody titer decline found to be 0.57 (95 %CI 0.54–0.60) P-value < 0.0001 among all participants. Immunosuppression status was found to decrease antibody titers by 0.30 (95 %CI 0.19–0.48, supplementary Table S1).
3.1. Comparison of antibody response a month after the second and a month after third vaccine dose administration
Seropositivity one month following the second vaccine dose, was found in 49 of ICI 49 patients and 44 immunocompetent individuals. These participants were also evaluated for serologic response one month after the third (booster) vaccine dose. As expected, the IgG anti-Spike SARS-CoV-2 titer levels were lower in the ICI group compared to immunocompetent group) after the second and the third vaccine dose (P-value < 0.0001, P-value = 0.0007 respectively). The GM (95 %CI) of IgG anti-Spike SARS-CoV-2 titer following 36 ± 21 days from the third dose administration was 8,269 (5,904–11,582) in the ICI group and 18,38 (15,210–22,350) in the immunocompetent group. Comparison between antibody titers after the second and the third doses is shown in Figs. 1 and S2. The third dose elicited more than seven-fold titer elevation in the ICI groups, reaching 7.83 (5.25–11.67) in the ICIs and 2.40 (1.90–3.03) fold in the immunocompetent participants. For both groups the difference is significant (Table 2).
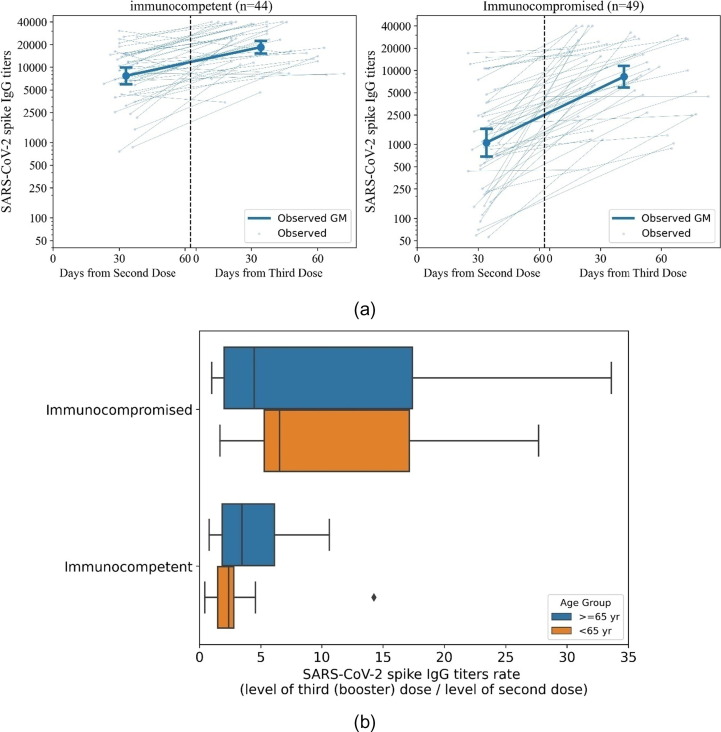
Fig. 1.
Comparison of SARS-CoV-2 spike IgG titers following the second and three (booster) vaccine doses. Fig. 1a: SARS-CoV-2 spike IgG response after second and third (booster) vaccine doses. GM with 95% CI was calculated for IgG titers after the second and third (booster) vaccine doses for the immunocompetent (n = 44) and immunocompromised (n = 49) participants. Individual per participant observed values are shown in thin lines, and GM are shown in bold. X-axis represents time from second dose (left of dashed line) and time from third vaccine (right of dashed line). Fig. 1b: SARS-CoV-2 spike IgG ratio between the third (booster) and second doses. The ratio of the SARS-CoV-2 spike IgG titers after the third (booster) dose to the IgG titers after the second dose, calculated per patient. Rates are shown for each subgroups- the immunocompromised (top) and immunocompetent (bottom), stratified by age groups (≥65 years - blue, <65 years - orange).
The increase in the IgG anti-Spike SARS-CoV-2 titers was significant for the two age categories in the ICI responders' groups: 9.81 (5.64–17.07) for participants under 65 years of age and 6.70 (3.76–11.93) for participants aged over 65 years Among the immunocompetent group, the increase was more prominent in participant 65 years and over, with a 3.34 (2.16–5.19) increase in the IgG titers, versus 2.02 (1.54–2.65) fold in participants aged under 65 years (Supplementary Table S2). Personalized correlation assessment between antibody response following the second and third vaccines using the Spearman's rank found a correlation of 0.51 (95% CI 0.27–0.69) P-value < 0.0001 for ICI, and was 0.54 (95% CI 0.31–0.71), P-value < 0.0001 for the immunocompetent subpopulation.
3.2. Comparison of antibody response prior to, and after the third vaccine dose administration
Prior to the 3rd vaccine dose, approximately six months after the second vaccine dose administration, 37 immunocompromised and 31 immunocompetent participants preformed a serology test (Table 3 ). Comparing between the IgG anti-Spike SARS-CoV-2 titer prior to and after the third (booster) dose, the third dose elicited more than 50-fold titer elevation in the ICI group, reaching 50.7 (32.5–79.1) fold for ICIs and 25.7 (19.1–34.7) fold for the immunocompetent group (Figs. 2 and S3).
Table 3.
IgG anti-Spike SARS-CoV-2 response prior to and after the BNT162b2 mRNA vaccine 3rd (booster) dose.
| Immunocompromised |
All Immunocompetent n = 31 (100%) |
|||
|---|---|---|---|---|
| All Immunocompromised n = 37 (100%) |
Lymphoproliferative disorder / Myeloproliferative disorder n = 23 (62%) |
Solid cancer /Immunosuppressive treatment non-oncologic n = 14 (38%) |
||
| Days between 2nd vaccine dose recipient to IgG test | 178.1 5.4 | 178.2 6.6 | 177.8 2.4 | 177.4 6.1 |
| Days prior to 3rd vaccine dose | 20.4 11.0 | 20.7 11.0 | 19.9 11.0 | 31.3 15.9 |
| IgG titer prior to 3rd vaccine dose (GM, 95 %CI) | 173 (118–255) |
168 (99–285) |
182 (105–316) |
718 (526–982) |
| Days between 3rd vaccine dose recipient to IgG test | 37.1 19.9 | 34.2 18.9 | 41.9 20.5 | 34.7 14.6 |
| IgG titer following 3rd vaccine dose (GM, 95 %CI) | 8,793 (5,947–13,000) |
9,329 (5,482–15,875) |
7,979 (4,498–14,153) |
18,480 (15,094–22,625) |
| P-value for IgG titer prior to and after the 3rd vaccine dose | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fold ratio (GM, 95 %CI) between IgG titter prior to and after 3rd vaccine dose a | 50.7 (32.5–79.1) |
55.4 (30.5–100.6) |
43.7 (20.7–92.3) |
25.7 (19.1–34.7) |
aFold ratio- Rate of IgG titer one month following 3rd vaccine dose recipient (GM, 95 %CI)/ IgG titer prior to 3rd vaccine dose recipient (approximately six month following the 2nd vaccine dose) (GM, 95 %CI).
GM- geometric mean, CI- confidence interval.
Fig. 2.
Comparison of SARS-CoV-2 spike IgG titers prior to (5–7 month after the second dose) and after the third (booster) vaccine dose. Fig. 2a: SARS-CoV-2 spike IgG titers prior to and after the third (booster) vaccine dose. GM with 95% CI was calculated for IgG titers in the last test prior to (5–7 month after the second dose) and following the third dose. In addition, the observed titers for every patient are available at the figure. Data is shown for (n = 68) patients with samples available (5–7 months after the second dose). Fig. 2b: SARS-CoV-2 spike IgG ratio following and prior to (5–7 month after the second dose) the third vaccine dose. The ratio was calculated per patient. Rates are shown for each subgroups- immunocompromised (top) and immunocompetent (bottom) stratified by age groups (≥65 years - blue, <65 years - orange).
3.3. Antibody response after the third vaccine dose administration among “non-responders”
Seventeen (26%) of immunocompromised participants were seronegative after the second vaccine dose (IgG level below 50 AU/mL). Of them, four (24%) elicited antibody response after the third vaccine dose. IgG anti-Spike SARS-CoV-2 titers of the participants who elicited seroconversion were 64, 100, 1761 and 2018 AU/mL. The remaining ICIs who were seronegative after the second vaccine dose (76%) did not develop any antibody response after the third vaccine dose. We validated the immunocompromised status of these patients at the time of the third dose administration by their medical records, including active immunosuppressive treatment and baseline disease. We could not identify a medical reason that could explain the elicited seroconversion in some of the ICIs comparing to others.
3.4. Correlation between IgG anti-Spike SARS-CoV-2 titers and neutralizing antibody levels
Correlation between IgG anti-Spike SARS-CoV-2 titers and neutralizing antibody levels along time was 0.77 (95% CI 0.67–0.85, n = 68) with P-value < 0.0001, using Spearman’s rank (Fig. S4). Correlation was consistent with age groups: For age < 65 years correlation was 0.84 (95% CI 0.72–0.91, n = 40),P-value < 0.0001 and for age ≥ 65 years correlation was 0.71 (95% CI 0.49–0.84, n = 28) with P-value < 0.0001.
4. Discussion
After a time-dependent decline in the quantitative SARS-CoV-2 spike IgG titers following months from the second BNT162b2 vaccine dose administration, a third vaccine dose elicited a robust humoral immune response, with more than 50-fold and 25-fold increase in SARS-CoV-2 spike IgG titer, among immunocompromised and immunocompetent, respectively. Comparing SARS-CoV-2 spike IgG titers one month after the second and third BNT162b2 vaccine, the third vaccine elicited spike IgG titers more than 7- fold of the range that was achieved after the second vaccine does in the ICI group and more than 2- fold in the immunocompetent group.
Previous studies have demonstrated a peak immunity induced by the BNT162b2 vaccine, followed by a sharp decline in the antibody titers 6 months after vaccination [14]. A study by Levin et al. demonstrated a monthly decrease rate of 0.54 in the naturalizing antibody among health care worker [15]. This waning in the humoral response is in line with our findings. Real world evidence showed waning of immunity against the delta variant of SARS-CoV-2 in all age groups [16]. Although specific correlation between protection from SARS-CoV-2 and antibody titers is not yet defined, waning vaccine-elicited immunity may play a crucial component, along with the high levels of viral transmission for the increased rate of confirmed SARS-CoV-2 infections and cases of severe illness.
Of note, most of our heterogenic immunocompromised subgroup, including different immunosuppressive treatments and underlying diseases elicited a substantial humoral response after the third (booster) vaccine dose. While they had a lower baseline antibody titer after the second dose compared to the immunocompetent, the relative increase in their antibody titer was 3.5 times higher compared to the immunocompetent participants. A small study by Rottenberg et al [11] focused on oncologic patients undergoing chemotherapy found a good antibody response following the third dose as well as among Chronic Lymphocytic Leukemia (CLL) and kidney recipients [17]. About 40% of the ICI failed to elicit antibody response after two vaccine doses, most of them patients with lymphoproliferative disease without active treatment. In previous studies 60% of the patients with CLL did not elicit immune response [18], [10]. Similar results were obtained regarding patients with lymphoma and myeloma [19], [20]. The discrepancy between previous findings and this study may be explained by the fact that most of the patients with CLL who participated in our study were treatment naïve. Thus, the failing of antibodies production can be attributed solely to the CLL and not to immunosuppressive treatment In this study, 25% of non-responders managed to produce antibodies after the third (booster) dose, similar to the findings from a study of patients with CLL who failed standard two-dose Vaccination [21]. Indeed, the facts that post second dose seropositive ICIs in our study had an augmented humoral response after the booster dose recipient and that a quarter of the non-responders managed to elicit a seroconversion, underscore the importance of the booster vaccine administration for these populations. Further studies are needed to investigate the long term protection of the booster vaccine, especially with the emergence of new variants [22]. As a third vaccine dose increased antibody levels that were waning over time in the study groups, we believe that a fourth dose may improve the antibody immune response for ICI and for immunocompetent individuals.
This study has several limitations: The available cohort is small, heterogenic and not necessarily representative of the general population. In addition, COVID-19 infection before or during the study cannot be ruled out, because no pre-vaccination serology and/or PCR tests were evaluated for all participants; however, all participants' medical records were evaluated before the recruitment and during the study period and they did not have any symptoms suggestive of Covid-19 infection or a positive PCR test. Furthermore, the antibody response is only one component of the immune response to vaccines, therefore, antibody titer does not necessarily imply clinical protection.
5. Conclusion
A third (booster) BNT162b2 mRNA vaccine dose elicited robust humoral responses (compared to the second dose) among immunocompetent and immunocompromised individuals. It may also provide potential benefit for ICI who did not elicit humoral response after the second vaccine dose. This augmented immunogenic response supports the administration of an additional vaccine dose in these two populations. The durability of these responses and degree of protection from SARS CoV-2 are yet to be determined.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank the following for their contributions to our efforts: Liorit Yanay, Ateret Malachi, Dr Tehila Fisher Yosef, Dr Ruti Gorev, Dr Vered Eliyahu, Neomi broide and Amnon Amir.
Funding
None. The study was supported by the Israeli Ministry of Health, which supplied the serological testing kits.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.051.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Barber R.M., Sorensen R.J.D., Pigott D.M., Bisignano C., Carter A., Amlag J.O., et al. a statistical analysis. Lancet. 2022 doi: 10.1016/S0140-6736(22)00484-6. [DOI] [Google Scholar]
- 2.Shapiro Ben David S., Cohen D., Karplus R., Irony A., Ofer-Bialer G., Potasman I., et al. COVID-19 community care in Israel-a nationwide cohort study from a large health maintenance organization. J Public Health (Oxf) 2021;43(4):723–730. doi: 10.1093/pubmed/fdab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y.u., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen B., Waitzberg R., Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Isr J Health Policy Res. 2021;10:6. doi: 10.1186/s13584-021-00440-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizrahi B., Lotan R., Kalkstein N., Peretz A., Perez G., Ben-Tov A., et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbel R., Hammerman A., Sergienko R., Friger M., Peretz A., Netzer D., et al. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N Engl J Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., et al. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med. 2021;384(15):1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamini O., Rokach L., Itchaki G., Braester A., Shvidel L., Goldschmidt N., et al. Safety and efficacy of BNT162b mRNA Covid19 Vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2021 doi: 10.3324/haematol.2021.279196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rottenberg Y., Grinshpun A., Ben-Dov I.Z., Oiknine Djian E., Wolf D.G., Kadouri L. Assessment of response to a third dose of the SARS-CoV-2 BNT162b2 mRNA vaccine in patients with solid tumors undergoing active treatment. JAMA Oncol. 2022;8(2):300. doi: 10.1001/jamaoncol.2021.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliakim-Raz N., Leibovici-Weisman Y., Stemmer A., Ness A., Awwad M., Ghantous N., et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326(21):2203. doi: 10.1001/jama.2021.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lustig Y., Sapir E., Regev-Yochay G., Cohen C., Fluss R., Olmer L., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier A.-R., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N Engl J Med. 2021;385(21):2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marlet J., Gatault P., Maakaroun Z., Longuet H., Stefic K., Handala L., et al. Antibody responses after a third dose of COVID-19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines. 2021;9(10) doi: 10.3390/vaccines9101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry C., Luttwak E., Balaban R., Shefer G., Morales M.M., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avivi I., Balaban R., Shragai T., Sheffer G., Morales M., Aharon A., et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021;195(2):186–193. doi: 10.1111/bjh.17608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herishanu Y., Rahav G., Levi S., Braester A., Itchaki G., Bairey O., et al. Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood. 2022;139(5):678–685. doi: 10.1182/blood.2021014085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemet I., Kliker L., Lustig Y., Zuckerman N., Erster O., Cohen C., et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med. 2022;386(5):492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.