Abstract
Introduction
Inflammatory response-induced coagulopathy is a common complication associated with severe form of covid-19 infection. Evidences suggest that neutrophil extracellular traps (NETs) play a significant role in triggering the immunothrombosis in this condition. We aimed to evaluate the diagnostic value of surface neutrophilic myeloperoxidase (MPO) as NETosis biomarker for predicting the risk of covid-19-associated coagulopathies.
Methods
Covid-19 infection was assessed by real-time-PCR and plasma d-dimer levels were measured by ELFA. Based on the covid-19 infection and d-dimer level outcomes, patients were categorized into four groups. Any alteration in the serum level of IL-6, H3Cit and neutrophilic surface MPO were analyzed by CLIA, ELISA, and flow cytometry, respectively.
Results
H3Cit variations and different d-dimer values confirmed the association between NETosis and coagulopathies. Findings showed that the expression of neutrophilic MPO reduced in cases with NETosis, which was correlated with increased levels of H3Cit. ANC/MPO ratio was signified as a valuable marker to discriminate the covid-19 and non covid-19-associated coagulopathies and could be considered as a prognostic factor due to its noteworthy correlation with serum IL-6 concentration.
Conclusion
Declined levels of surface neutrophilic MPO in NETosis correlate with covid-19-associated coagulopathies and increased IL-6 levels, as a potential biomarker of covid-19 disease severity.
Keywords: Surface myeloperoxidase, NETosis, Coagulopathies
Abbreviations: SnMPO, Surface neutrophilic myeloperoxidase; ANC, Absolute neutrophil count; NET, Neutrophil extracellular trap
1. Introduction
On December 2019, a novel coronavirus-induced infection reported in Wuhan, China, which contributed to a global pandemic in many countries few months later [1], [2]. The disease is classified as coronavirus-induced severe acute respiratory syndrome (COVID-19) with clinical symptoms similar to common cold and mild respiratory infections. However, in some patients it may appear with more systemic symptoms with progress to advanced acute respiratory distress syndrome (ARDS), higher risk of developing coagulopathy, sepsis and multi-organ dysfunction, which can ultimately result in death [3], [4], [5].
Several lines of evidence elucidate that inflammatory response-induced coagulopathy and disseminated intravascular coagulopathy (DIC) are common complications observed in severe form of covid-19 disease which might give rise to elevated rates of thrombotic complications [6], [7]. According to the recent researches, DIC has been detected in 70% of covid-19 patients with advanced stage of the disease which may eventually be fatal [1], [2].
One of the hallmark of laboratory observations in association with DIC, also identified as coagulation dysfunction marker, is enhanced level of d-dimer concentrations in covid-19 patients which directly correlates with poor prognosis of the illness severity or even higher risk of overall mortality [2], [8].
Still, the precise mechanisms of coagulopathies in covid-19 infected patients remained elusive, it is also still rudimentary whether the detected coagulation complications is directly induced by the virus or is a secondary indication promoted by increased proinflammatory immune response define as cytokines storm [9], [10], [11].
It is believed that in covid-19 patients and in the severe cases in particular, profound inflammatory cytokine responses lead to compromised immune response which along with lymphocyte cell death, endothelial cell apoptosis and hypoxia are involved in hyper activation of the coagulation system that plays a role in multi-organ failure and final death. [1], [2], [12]
Several lines of evidence indicated that increased activity of neutrophils, the first line of immune defense against intracellular pathogens, induce the generation of sustained and aberrant Neutrophil Extracellular Traps (NETs) which plays a detrimental role in covid-19 pathogenesis and covid-19 infection appears to be even defined by peripheral blood neutrophilia [5], [13].
Nevertheless, neutrophils have not been previously demonstrated to be implicated as an antiviral immune defense. Scientific reports evidenced that some types of virus have been identified within neutrophils activating them to form excessive NETs that might be performed through pattern recognition receptors (PRRs) [14].
NETs are web-like structures released by activated neutrophils and comprised intracellular components such as nuclear DNA, histones, oxidant enzyme and granule proteins like myeloperoxidase (MPO). This process is defined as NETosis, which has an important function in fighting pathogens [5], [15].
Although under normal condition NETs serve as effective tools in eliminating pathogens, several studies have documented the role of excessive NETs in promoting procoagulant state and vasculature complications in covid-19 infection [15], [16], [17]. In fact, the extended inflammation linked with higher levels of NET generation result in procoagulant activities and damage the tissues and organs in this disease [5], [15]. As described by scientific literature, NETs generally initiate the coagulation through two possible pathways of either extrinsic or contact process where it initiates coagulation through activation of tissue factor (TF) and factor XII (FXII) respectively [18]. All these findings specify a potential cross-talk of this disease with excessive NETs.
Consequently, as DIC is frequently detected among severe cases of covid-19 infected patients and as the exact mechanism underlying the associated coagulopathy (intravascular clot formation) has not yet been fully characterized, in this study we aimed to study whether NETosis play a role in covid-19-associated coagulopathies. We evaluated the markers which could be utilized as an indicator of NETosis while we were also investigating their potential importance in predicting the risk of developing thrombosis in covid-19 cases.
2. Materials and methods
2.1. Sample collection
We recruited a total of 100 patients in this study, including 60 covid-19 positive patients and 40 covid-19 negative cases. Among them, 20 individuals had normal d-dimer levels from each group, while 40 and 20 persons showed high d-dimer levels in covid-19 positive and negative cases respectively (Table S1).
The study participants who had a history of SARS-Cov2 infection with less than 4 months prior to sampling, were excluded from groups C and D. The patients who were negative for covid-19 but indicated high d-dimer levels have previously experienced strokes or thrombosis.
Informed consent from participants, and ethics committee approval (IR.SUMS.REC.1400.482) obtained prior to beginning the study. 10 ml of venous blood was drawn from each participant using venipuncture. 3 ml of the whole blood was transferred into K3-EDTA vacutainer tube, while 4 ml was used for “no additive” clot tubes to separate the serum and finally 3 ml was drawn to citrated tube to isolate plasma. Serum and citrated plasma were obtained by centrifugation at 3000 revolutions per minute (rpm) for 5 min and 15 min, respectively.
2.2. Measurement of soluble citrullinated histone H3
Serum levels of soluble citrullinated histone H3, a known biomarker of NETosis, were measured in all the studied cases. The citrullinated histone H3 (Clone 11D3) ELISA kit (Cayman Chemical, USA) was used to quantify this biomarker according to the plotted standard curve and results were correlated with d-dimer values (the coagulopathy marker) and levels of surface neutrophilic MPO.
2.3. Plasma d-dimer level
Citrated plasma d-dimer, the soluble fibrin degradation product and critical indicator of deep vein thrombosis was automatically measured using VIDAS® D-Dimer Exclusion™ II kit (bioMérieux, France) and through enzyme-linked fluorescence assay (ELFA) technique. The values lower than 500 ng FEU/ml were considered as normal.
2.4. Complete blood counts (CBC)
Mindray 5 Differential Part BC-5800 Hematology Analyzer (Mindray, China) was applied to measure the hematological parameters and absolute count for different leukocyte populations of blood samples. Peripheral blood smears prepared from the anticoagulated blood samples were assessed microscopically for blood cells morphology.
2.5. IL-6 assay
Serum levels of IL-6 was measured using Immulite 2000 IL-6 kit on IMMULITE® 2000 XPi Immunoassay System (Siemens Healthcare GmbH, Germany) through solid-phase chemiluminescent sequential immunometric assay (CLIA) method. According to the manufacturer's guideline, the levels from non-detectable amount to 5.9 pg/ml were considered as absolute normal range.
2.6. Surface neutrophilic MPO (snMPO)
100 μl of EDTA-anticoagulated whole blood (including ~106 leukocytes) was washed by phosphate-buffered saline (PBS) to remove any possible interfering soluble myeloperoxidase in plasma. Cells were subsequently mixed with 10 μl of FITC-conjugated monoclonal mouse anti-human MPO antibody (clone MPO-7; Dako, Denmark) and incubated for 25 min at room temperature (RT) in the dark. Also, a FITC-conjugated mouse IgG1 Kappa isotype control (bioscience, USA) was used to set the negative area for FL1 fluorescent channel in every individual assay. Following this step, 100 μl of RBC lysing reagent (EXBIO, Czech Republic) was added to the cells and incubated for 3 min at RT. Then, 1 ml distilled water was added to the cells, thoroughly mixed and incubated for 3 more minutes.
The stained and RBC-lysed samples were applied for flow cytometry analysis. A total of 10,000 WBCs was acquired using FACSCalibur™ instrument (BD, USA). Neutrophil populations were gated according to their side scatter (SSC) and forward scatter (FSC) features and their related fluorescent intensity were measured in FL1 channel. Ultimately, acquired data were analyzed by FlowJo software (version 7.6 Tree Star Incorporated, Ashland, OR, USA).
2.7. Real time-PCR for covid-19
Samples were collected from upper respiratory tract, including nasopharyngeal and oropharyngeal by sterile Dacron swabs. Swabs were placed in viral transport medium (VTM) for up to 24 h and used for subsequent processing.
Viral RNAs were extracted by BehPrep viral RNA extraction kit (BehGene, Iran) according to the supplier's instruction and isolated RNAs were reverse transcribed and subjected to TaqMan real-time PCR through a multiplex One-Step reaction using covid-19 RT-PCR kit (Biorex Fars; Iran). Reaction mixture was prepared by mixing 10 μl of 2× master mix with 2 μl of primer and probe (containing three sets of primers and probes which specifically target RdRp gene, N gene and RnaseP gene as an internal control). PCR was performed by applying Applied Biosystem StepOnePlus™ Real-Time PCR machine (ABI, USA) according to the following procedure: an RNA reverse transcription step at 50 °C for 20 min and an initial denaturation step at 95 °C for 3 min, followed by 45 cycles (10 s at 94 °C for denaturation and 40 s at 55 °C for annealing, extension, and fluorescence measurement). Results were interpreted based on the kit's provided guideline and the Ct value of over 40 for each gene was considered as a negative result.
2.8. Statistical analysis
Statistical comparison of the data among the groups was performed by unpaired t-test and One-Way analysis of variance (ANOVA) methods, whereas the correlations between parameters were evaluated by Pearson correlation analysis. The graphs were plotted and analyzed using Graph Pad Prism software, version.9 (Graph Pad Software, CA, USA). The data were expressed as mean ± standard deviation (SD) and the P-value of less than 0.05 was considered as statistically significant.
3. Results
In the current study, based on d-dimer levels and the covid-19 RT-PCR results, cases were categorized into four different groups including: A) Covid-19 positive cases with elevated d-dimer level, B) Covid-19 positive with normal level of d-dimer, C) Patients with high level of d-dimer and negative for covid-19, and D) Covid-19 negative cases with normal d-dimer level as the control group.
3.1. Neutrophilic surface MPO expression
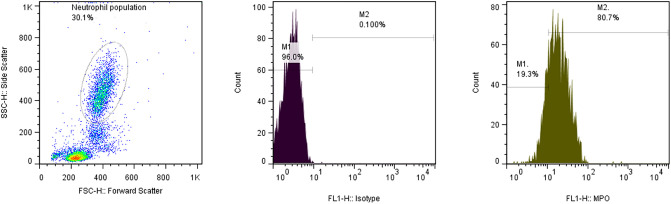
Surface MPO expression of neutrophil population in peripheral blood samples was measured versus their related isotype controls by flow cytometry (Fig. 1 ) and the results were reported as mean fluorescent intensity (MFI).
Fig. 1.
Representative gating strategy of neutrophil population (Dot-plot), IgG1 FITC isotype control (Histogram, Left), and FITC-anti-MPO treated sample (Histogram, Right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Results illustrated that the mean of MFI for MPO in covid-19 positive cases was 38.51 ± 14.85 which was significantly low when compared with covid-19 negative group with the mean MFI level of 58.96 ± 36.68. (**P value < 0.01) (Fig. S1).
Also, the surface neutrophilic MPO was compared among cases with normal and high levels of d-dimer (regardless of their covid-19 RT-PCR results). Findings revealed a considerable decrease in the surface MPO of neutrophil populations in patients with a high level of d-dimer in relation to cases with normal d-dimer values. (*P value < 0.05) (Fig. 2A).
Fig. 2.
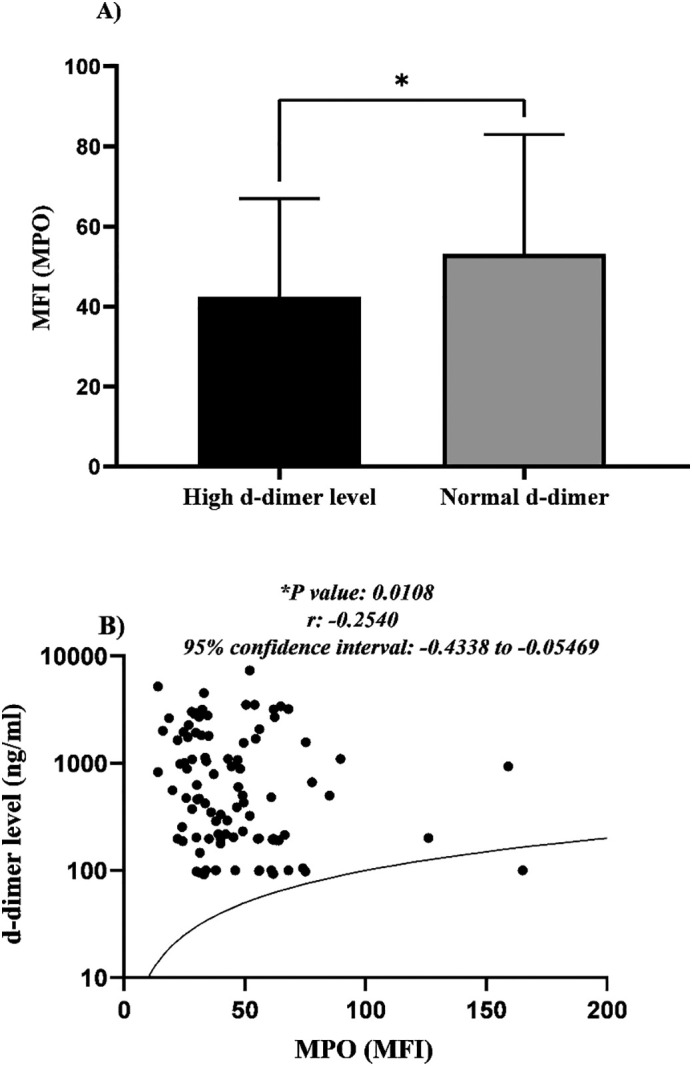
Bar chart demonstrates the significant reduction in MPO expression in the surface of neutrophils in cases with higher d-dimer level (*P value < 0.05) (A). Pearson correlation analysis of MFI obtained by flow cytometry and d-dimer levels illustrated a significant reverse correlation between these two variables (Pearson r: −0.3, *P value < 0.05) (95% confidence interval: −0.4338 to −0.05469) (B).
Evaluating the correlation between d-dimer and neutrophilic MPO expression in all studied cases evidenced a reverse and considerable correlation between these two parameters (*P value < 0.05, r: −0.2540) (Fig. 2B). In other words, the cases with high d-dimer level expressed lower level of MPO on the surface of their neutrophils (Fig. 2B).
The level of MPO on neutrophil's surface was compared among all the groups and results confirmed that it was excessively high in the control group (covid-19 negative/normal d-dimer) in relation to other groups. Accordingly, the average MFI for this biomarker in the control group was 59.1 ± 15.14, which was also significantly high when compared with groups A, B, and C, where they showed an average of 39.24 ± 15.19 (****P value: <0.0001), 38.75 ± 15.0 (***P value: <0.001) and 40.15 ± 9.76 (**P value: <0.01) respectively. However, among non-control groups, we did not find any noticeable alteration in this variable (P value > 0.05) (Fig. 3 ).
Fig. 3.
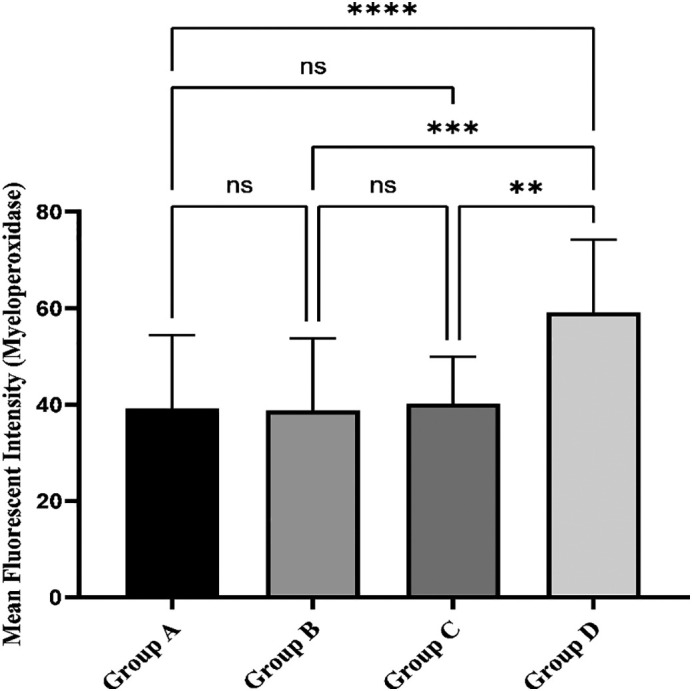
Comparison of neutrophilic surface MPO expression in different groups by flow cytometry. Results evidenced a significant decrease in neutrophilic MPO expression in all studied groups when compared with healthy controls. Meanwhile, this parameter did not reveal any significant change among different patient groups.
3.2. Evaluation of NETosis through assessing circulating citrullinated histone H3 (H3Cit)
Serum levels of H3Cit, the NETosis biomarker, were measured in all studied cases. Results demonstrated that compare to the control group which showed an average of 18.8 ± 8.4 ng/ml in this biomarker, there was a significant increase in groups A (57.6 ± 27.5 ng/ml), B (38.4 ± 13.31 ng/ml), and C (39.2 ± 12.8 ng/ml). Intriguingly, the highest level was found in covid-19 positive patients who also designated significantly elevated d-dimer levels. NETosis level, however, was not noticeably different between group B and C (P value > 0.05) (Fig. 4 ).
Fig. 4.
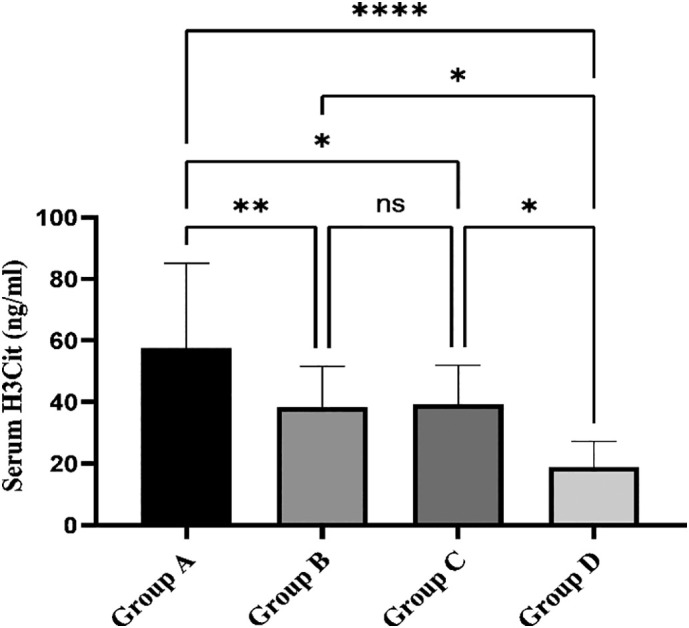
Bar chart compares the serum H3Cit level among the different groups. The level of H3Cit in group A was expressively higher than those of the other three groups. The difference was not significant between groups B and C, while their related H3Cit levels were meaningfully greater than the control group.
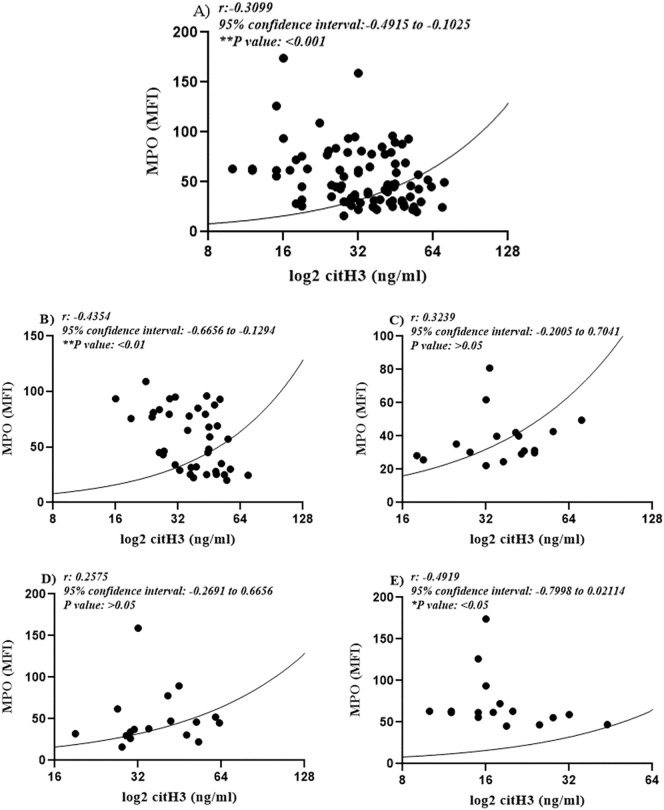
Assessments also showed a reverse and interestingly, significant association between circulating H3cit and surface neutrophilic MPO which was evaluated by flow cytometry (**P value < 0.01) (Fig. 5A).
Fig. 5.
The Pearson correlation analysis of neutrophilic surface MPO and serum H3Cit in all studied cases revealed a noteworthy reverse association between these two variables (**P value < 0.01) with a 95% confidence interval of −0.4915 to −0.1025 (A). Evaluating the correlation between these two variables within different groups showed significant association in group A (B) and D (E), while this association was not evident in group B (C) and C (D).
These findings could signify the role of NETosis in either covid-19 or non-covid-19 associated coagulopathies. Furthermore, the decreased levels of surface neutrophilic MPO might be considered as a valuable biomarker in appraisal of NETosis process.
Further analysis of correlations between pairs of variables within each group revealed significant negative correlations in groups A (**P value < 0.01) (Fig. 5B) and D (*P value < 0.05) (Fig. 5E). However, no substantial correlation was observed in groups B (Fig. 5C) and C (P value > 0.05) (Fig. 5D).
3.3. NETosis and ANC
We also evaluated absolute neutrophil count, one of the key elements associated with NETosis, its correlation with surface neutrophilic MPO and the d-dimer levels.
Based on our results, the highest ANC was observed in group A (6.77 ± 3.03), which was significantly higher than that of other groups. Nevertheless, ANC in groups B (4.46 ± 1.45) and C (3.92 ± 1.2) did not illustrate any substantial changes in comparison with the control group (group D) which exhibited an average ANC of 3.8 ± 1.67 (Fig. S2 A).
We detected a distinct increase in ANC index in cases with high d-dimer levels when compared to those with normal d-dimer values (the groups included both covid-19 positive and negative cases) (**P value < 0.01) (Fig. S2 B).
Evaluating this parameter in covid-19 positive and negative groups further indicated a noteworthy upsurge in patients with covid-19 positive outcomes (***P value < 0.001) (Fig. S2 C). Accordingly, these findings signify the importance of neutrophils function and NETosis phenomenon, in stimulating coagulopathies in both covid-19 and non-covid-19 cases.
3.4. NETosis and N/L ratio
Neutrophil to lymphocyte ratio (N/L ratio) has been known as a valuable prognostic marker in covid-19 patients. In the current study, we measured this ratio while also appraised its related correlation with MPO levels in different experimental groups.
We observed a noticeably elevated N/L ratio in covid-19 positive cases, who had also signified a high level of d-dimer in their plasma (group A) compare to other groups.
3.5. ANC/MPO ratio index
Based on findings obtained in current study and since there was no marked differences in surface neutrophilic MPO among covid-19 positive patients either with normal (group B) or high-level d-dimer (group A), this parameter could not be a prognostic marker to evaluate the covid-19 associated coagulopathies.
However, considering the direct and of course significant association between d-dimer levels and ANC (d-dimer ∝ ANC) as well as the reverse correlation between d-dimer and NsMPO (d-dimer ∝ 1/NsMPO), we established a valuable formula by calculating the ratio of ANC to MPO to estimate the risk of covid-19-induced coagulopathy in patients with positive SARS-CoV-2 diagnostic results. This notion is supported by our finding that there was a significant alteration in ANC/MPO ratio among the covid-19 positive patients with high (group A) and normal d-dimer levels (group B) (0.2 ± 0.09 vs 0.13 ± 0.06, *P value < 0.05) as well as other studied groups.
Meanwhile, this value did not show noteworthy variation among covid-19 positive group with normal d-dimer level and other covid-19 negative groups (P value > 0.05).
Interestingly, this parameter could differentiate between the covid-19-associated coagulopathies and other leading causes of coagulopathies. This is due to the fact that the mean value of ANC/MPO ratio was significantly higher (approximately two-fold) in cases with high level of d-dimer who were also positive for covid-19 in comparison with those who were negative for covid-19 (0.20 ± 0.09 vs 0.10 ± 0.07, **P value < 0.01) (Fig. 6A).
Fig. 6.
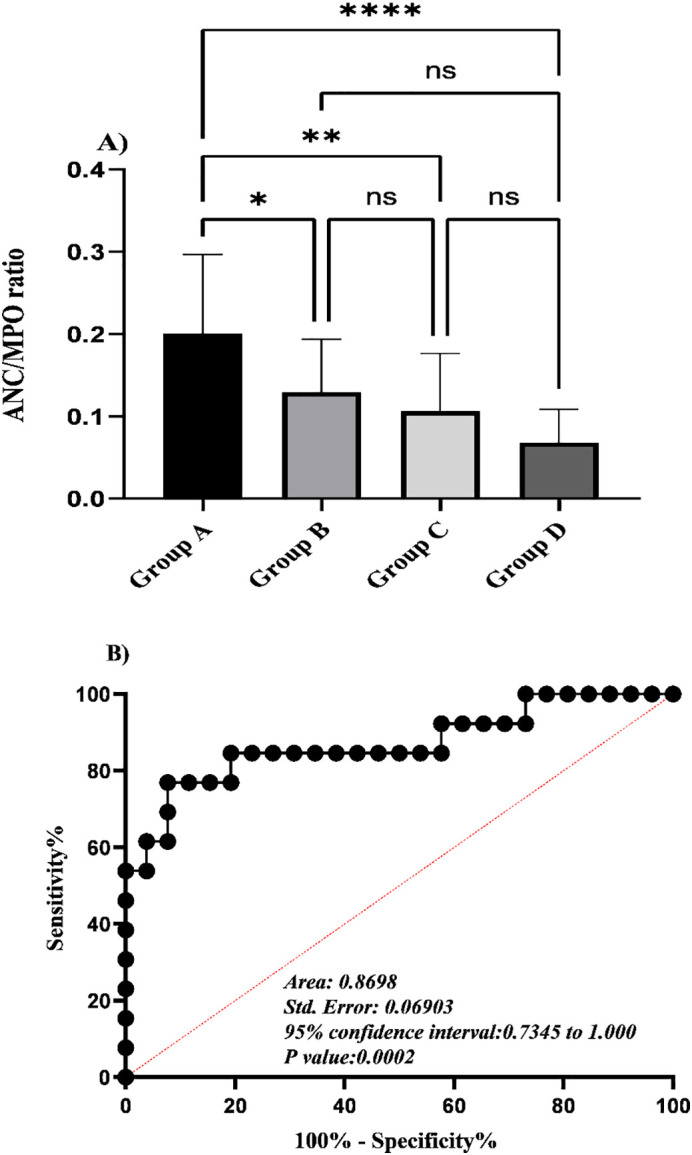
The bar chart shows One-way analysis of variance for ANC/MPO index among cases who were already grouped based on their coagulopathy status and covid-19 results. Results represent a significant increase in this index in group A. The differences are not significant when compared among other groups (A). ROC curve analysis of this index in group A and B (covid-19 positive case) suggest a cutoff value of <0.13 for predicting the coagulopathy in covid-19 positive cases with a sensitivity of 76.9% and specificity of 92.3% (***P value < 0.001) (B).
Using receiver operating characteristic (ROC) curve analysis, the ANC/MPO ratio of less than 0.13 was determined as the best cut-off value for discrimination of covid-19 associated coagulopathies. (Area Under Curve (AUC): 0.87; specificity: 92.3%; sensitivity: 76.9%) (***P value < 0.001) (Fig. 6B).
3.6. Correlation between ANC/MPO ratio and serum IL-6 levels
Based on the substantiated importance of IL-6 assessment in predicting the disease severity and particular cytokine-targeted therapy of covid-19 patients by actemra (tocilizumab), the serum levels of IL-6 measured in covid-19 positive patients, were correlated with their related ANC/MPO ratio index. Intriguingly, results obtained from Pearson correlation analysis illustrated a direct and significant relationship between these two parameters (***P value < 0.001, r: 0.5813, 95% confidence interval: 0.2917 to 0.7732) (Fig. 7 ). Independently, neither ANC index nor the MPO levels did display the notifiable correlation when compared with the serum IL-6 levels, a prognostic indicator of covid-19 disease severity.
Fig. 7.
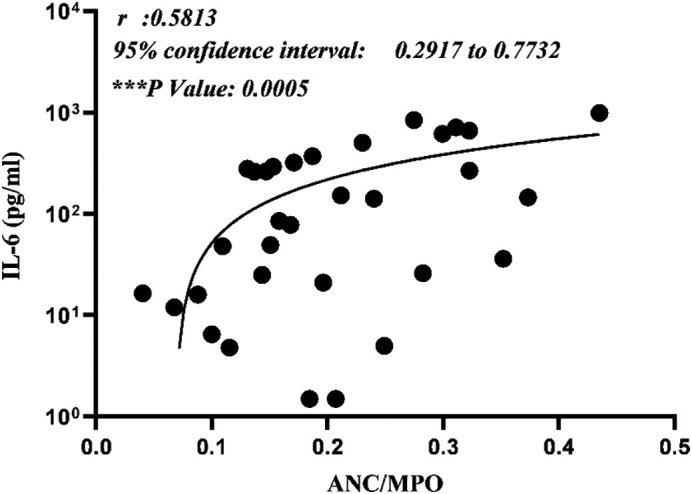
Representative of Pearson correlation analysis for ANC/MPO ratio and serum IL-6 level. Assessments confirmed a direct and considerable association between these two parameters (***P value < 0.001).
3.7. Morphological findings during NETosis
To find out the possible morphological changes in peripheral blood cells during NETosis process, peripheral blood smears were prepared from studied cases and the cells were morphologically evaluated.
The most prominent morphological finding found in cases with active NETosis was the presence of smudged cells representing neutrophilic characteristics (Fig. S3). These cells were frequently found in the peripheral blood smear of covid-19 patients with elevated d-dimer, although they were not observed in the blood films of other studied cases.
3.8. NETosis and neutrophilic side scatter (SSC)
In addition to other functions, NETosis process likewise induces morphological changes in neutrophils which are computable throughout routine flow cytometry analysis. Side scatter parameter provides information regarding complexity and particularly granularity of cells. Neutrophil degranulation which occurs during NETosis could affect the neutrophil's SSC. Furthermore, immature neutrophils with less segmentation and granulation could also contribute to this phenomenon, as commonly found during covid-19 infection. Accordingly, the mean value of SSC parameter was compared among the neutrophil populations of different groups. Results revealed significant decrease in mean side scatter of neutrophils in group A (***P value < 0.001), B (*P value < 0.05), and C (***P value < 0.001) (groups with higher levels of H3Cit) when compared with the normal control group. However, this parameter did not show any noticeable alteration when compared among group A, B, and C (P value > 0.05) (Fig. S4).
4. Discussion
Coagulopathies are known as significant complications associated with both covid-19 infection and vaccination. Several factors have been introduced which might trigger the coagulation cascade in these cases. NETosis is one of the plausible factors involved in coagulopathies which has recently received more consideration particularly in patients infected with covid-19 [19]. Discovering the diverse molecular aspects of this phenomenon, its potential cause and effects, as well as its role in stimulating coagulopathies could be helpful in providing suitable approaches for diagnosis, monitoring, and even controlling the NETosis process and its related unfavorable coagulopathies.
NETosis is a process in which neutrophils expel their cytosolic granules and nuclear proteins on a chromatin based web-like scaffold [20].
Generally, the neutrophils that undergo NETosis process, would degenerate and their fragments will be removed from peripheral blood by enzymatic and phagocytic activities. Nevertheless, these cells could be occasionally found in the peripheral blood smear of some cases who have undergone active NETosis process.
Morphologically and based on the NETosis phase, these cells fall into two different categories. The first group includes partially disintegrating cells with segmented nucleus and pinkish cytoplasmic granules representing prominent filamentous protrusion (Fig. S3) and the second category comprises completely ruptured neutrophils with bare nuclei resembling filamentous network and with no cytoplasmic structure. These cells are barely distinguishable from smudge cell originated from other leukocytes. Vigorous NETosis may undoubtedly rise the chance of detecting smudged neutrophils (type 1) during routine peripheral blood film assessment. Accordingly, the presence of smudged neutrophils (type 1) in the peripheral blood smear could be a remarkable prognostic indicator for NETosis which is also simply accessible.
Therefore, this interesting finding from the current study not only highlights a new morphological evidence for active NETosis, but also it could be served as a valuable sign in predicting the coagulopathies in covid-19 positive cases.
Myeloperoxidase is an antimicrobial enzyme abundantly found in neutrophils' azurophilic granules. This enzyme could be released in plasma following neutrophil degranulation, which normally occurs during inflammatory and NETosis processes. The soluble myeloperoxidase could subsequently be passively absorbed via neutrophils' plasma membrane [21], [22], [23]. Several studies revealed that the level of MPO absorption by the neutrophils' surface could be affected by different factors, such as salt concentration, pH, neuraminidase, and polyanionic molecules. Consequently, the alteration in the surface neutrophilic MPO in different clinical conditions could be evident [21], [24].
In a study conducted by Bangalore N et al., it was illustrated that the higher affinity of MPO to neutrophils' surface is a charge-dependent phenomenon. Interestingly, they also indicated that the presence of basic proteins, particularly histones, could dramatically suppress MPO to be membrane bound [24].
In the current study, we evaluated the MPO levels on the surface of neutrophils in cases with activated NETosis including either covid-19 positive or negative cases and the results from the coronavirus infection were correlated with the plasma concentration of H3Cit, a specific NETosis biomarker.
Although, we expected that elevated levels of the soluble MPO in NETosis [25] could trigger the recruitment of MPO by neutrophils and lead to subsequent rise in its surface level, intriguingly, our findings suggested that cases with active NETosis (both covid-19 positive and negative cases) illustrate a noticeable decline in this parameter.
This alteration might be at least in part be explained by two distinct hypotheses. Firstly, due to different degranulation manners occur during NETosis, the surface expression of basic proteins and in particular histones might change the neutrophil surface charge and thereby inhibit cell membrane MPO binding [26]. Additionally, as released MPO is usually in complex with histones and other nuclear components [20], [27], it could highly diminish the chance of MPO passive absorption by neutrophils either through steric hindrance or charge-dependent mechanisms.
Secondly, acquisition of MPO by neutrophils appears to be a time-dependent phenomenon and the neutrophils that have entered the bloodstream most recently, would bear a less quantity of MPO on their surface. Accordingly, considering the higher neutrophils' turnover in cases with active NETosis, it is expected that most recently released neutrophils reveal lower levels of MPO on the cell membrane.
5. Conclusions
Several conclusions could be drawn from the results of our study: 1) The NETosis process plays a prominent role in induction of coagulopathies in both covid-19 positive and negative cases. 2) Since the findings represented a noteworthy and of course reverse correlation between serum level of H3Cit and surface neutrophilic MPO, this parameter might be considered as a valuable and more accessible biomarker in evaluation of NETosis. 3) Due to the robust association between IL-6 level and ANC/MPO ratio (the proposed index of this study) in covid-19 positive cases, this index not only anticipate the prognosis of the disease but also it could discriminate between the covid-19 and non-covid-19 associated coagulopathies.
CRediT authorship contribution statement
E.J., M.A. and R.R performed study concept and design; R.R performed development of methodology and writing, review and revision of the paper; A.A.M., A.H.T., P.T., GH.T. and E.S.K. provided acquisition, analysis and interpretation of data, and statistical analysis; SH.R. provided technical and material support. All authors read and approved the final paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bcmd.2022.102676.
Appendix A. Supplementary data
Supplementary material
References
- 1.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.N T, D L, X W, Z S. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. J Thromb Haemost. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. JAMA. Vol. 323. American Medical Association; 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan,China; pp. 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. Vol. 382. Massachusetts Medical Society; 2020. pp. 1708–1720.101056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janiuk K., Jabło E. 2021. Significance of NETs Formation in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.F Z, T Y, R D, G F, Y L, Z L, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. JAMA. Vol. 180. Intern Med. American Medical Association; 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China; pp. 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucha S.R., Dugar S., McCrae K., Joseph D.E., Bartholomew J., Sacha G., et al. Coagulopathy in COVID-19: posted April 24, 2020. Cleve. Clin. J. Med. 2020;87:461–468. doi: 10.3949/ccjm.87a.ccc024. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.G., Fralick M., Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192:E583. doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S., Huang M., Li D., Tang N. J Thromb Thrombolysis. Vol. 51. Springer; US: 2021. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2; pp. 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Lancet. Vol. 395. Elsevier; 2020. Clinical features of patients infected with 2019 novel coronavirus in WuhanChina; pp. 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jm C., Jh L. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliadi V., Konstantinidou I., Aftzoglou K., Iliadis S., Konstantinidis T.G., Tsigalou C. The emerging role of neutrophils in the pathogenesis of thrombosis in COVID-19. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reshi M.L., Su Y.C., Hong J.R. RNA Viruses: ROS-mediated cell death. Int. J. Cell. Biol. 2014 doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., dos Reis M.C., de Castro G.M.M., et al. Sci Rep. Vol. 10. Nature Publishing Group; UK: 2020. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., et al. Blood. Vol. 136. American Society of Hematology; 2020. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome; pp. 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozzini C., Girelli D. Thromb Res. Vol. 191. Elsevier; 2020. The role of neutrophil extracellular traps in COVID-19: only an hypothesis or a potential new field of research? p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ME C, Y K. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc. Med. 2020;25:471–478. doi: 10.1177/1358863X20932640. Vasc Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bont C.M., Boelens W.C., Pruijn G.J.M. Cell Mol Immunol [Internet] Vol. 16. Springer; US: 2019. NETosis, complement, and coagulation: a triangular relationship; pp. 19–27. Available from: http://dx.doi.org/10.1038/s41423-018-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess C., Sadallah S., Schifferli J.A. Induction of neutrophil responsiveness to myeloperoxidase antibodies by their exposure to supernatant of degranulated autologous neutrophils. Blood. 2000;96:2822–2827. [PubMed] [Google Scholar]
- 22.Aroca R., Chamorro C., Vega A., Ventura I., Gómez E., Pérez-Cano R., et al. Immunotherapy reduces allergen-mediated CD66b expression and myeloperoxidase levels on human neutrophils from allergic patients. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Kebir D., József L., Pan W., Filep J.G. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ. Res. 2008;103:352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 24.Bangalore N., Travis J. Comparison of properties of membrane bound versus soluble forms of human leukocytic elastase and cathepsin G. Biol. Chem. Hoppe Seyler. 1994;375:659–666. doi: 10.1515/bchm3.1994.375.10.659. [DOI] [PubMed] [Google Scholar]
- 25.Guéant J.L., Fromonot J., Guéant-Rodriguez R.M., Lacolley P., Guieu R., Regnault V. Blood myeloperoxidase-DNA, a biomarker of early response to SARS-CoV-2 infection? Allergy Eur. J. Allergy Clin. Immunol. 2021;76:892–896. doi: 10.1111/all.14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan J.M. Neutrophil extracelullar traps (NETs):double-edged swords of innate immunity. 1(189) 2013. pp. 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin Oyarzún C.P., Carestia A., Lev P.R., Glembotsky A.C., Castro Ríos M.A., Moiraghi B., et al. Sci Rep. Vol. 6. Nature Publishing Group; 2016. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms; pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material