Abstract
Attention-Deficit/Hyperactivity Disorder (ADHD) is a heterogeneous disorder. Current subtypes lack longitudinal stability or prognostic utility. We aimed to identify data-driven biotypes using multiple cognitive measures, then to validate these biotypes using EEG, ECG, and clinical response to atomoxetine as external validators. Study design was a double-blind, randomized, placebo-controlled crossover trial of atomoxetine including 116 subjects ages 6 through 17 with diagnosis of ADHD and 56 typically developing controls. Initial features for unsupervised machine learning included a cognitive battery with 20 measures affected in ADHD. External validators included baseline mechanistic validators (using electroencephalogram/EEG and electrocardiogram/ECG) and clinical response (ADHD Rating Scale and correlation with cognitive change). One biotype, labeled impulsive cognition, was characterized by increased errors of commission and shorter reaction time, had greater EEG slow wave (theta/delta) power and greater resting heart rate. The second biotype, labeled inattentive cognition, was characterized by longer/more variable reaction time and errors of omission, had lower EEG fast wave (beta) power, resting heart rate that did not differ from controls, and a strong correlation (r = −0.447, p < 0.001) between clinical response to atomoxetine and improvement in verbal memory immediate recall. ADHD comprises at least two biotypes that cut across current subtype criteria and that may reflect distinct arousal mechanisms. The findings provide evidence that further investigation of cognitive subtypes may be at least as fruitful as symptom checklist-based subtypes for development of biologically-based diagnostics and interventions for ADHD.
Keywords: ADHD, Cognition, EEG, Atomoxetine, Arousal, Machine learning, Biotype
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a highly prevalent, impairing, chronic disorder. It is heterogeneous in clinical presentation and course [1]. Theories regarding its pathophysiology are similarly heterogeneous and it is unlikely that a single, underlying deficit exists [2–5]. Current subtypes defined by the Diagnostic and Statistical Manual (DSM) criteria have limited longitudinal validity [6,7]. Many of the theories of ADHD pathophysiology involve disruption of cognitive domains thought to be subserved primarily by prefrontal cortex, including sustained attention, inhibitory control, and executive function [1,8]. Theories have also implicated the physiologic construct of arousal, which involves the coordination of both central and peripheral response via norepinephrine release including effects on cognitive performance [9,10,8,11].
ADHD is associated with impairment in several of these cognitive and arousal constructs, but no single measure is clearly helpful in diagnosis or treatment selection [12,1,13–16]. The clinical application of these measures has likely been limited by phenotypic heterogeneity. Therefore, an improved classification schema that is relevant to treatment selection—ideally differentiating affected individuals by subgroups, or biotypes, with closer relationships to underlying biologic processes than the macro-level behavioral observations that are currently used—is needed. Furthermore, research to date has, in general, examined these constructs independently of one another, leading to difficulties placing conflicting results in context.
Data-driven analytic tools such as unsupervised machine learning techniques are ideal for identifying biotypes given the current state of knowledge because they separate groups across multiple measures without an a priori theoretical taxonomy [17–19]. To our knowledge, no study has applied such techniques to parse the phenotypic heterogeneity of ADHD using multiple cognitive and physiologic markers with clinical trial outcome data in the same subjects. Our objective was to use a data-driven approach to identify potential biotypes in ADHD via cognitive markers, then to validate these types mechanistically, via physiologic markers, and clinically, via treatment response.
Feature selection (choice of input measures) and external validation (comparison of the identified groups across different measures not included as features) are vital to these techniques [20]. We chose several widely available and cost-effective measures that are relevant to cognitive and physiologic arousal theories of ADHD pathophysiology as input and validation features. Performance measures from a cognitive battery were selected as input features [1,14]. Measures of central and peripheral arousal systems were used as external validators. These included quantified electroencephalogram (EEG) spectral power and theta/beta ratio, and electrocardiogram (ECG)-derived resting heart rate and heart rate variability [16,21]. Furthermore, EEG power is highly heritable [22], making it a good candidate biotype marker. Finally, treatment response to atomoxetine, a highly selective norepinephrine reuptake inhibitor, was used as a clinical external validator with both direct clinical importance and a specific relationship to arousal via norepinephrine modulation. Medications that improve clinical ADHD symptoms also improve cognitive performance, but these outcomes are often uncorrelated [23]. This dissociation may also be due to between-subject heterogeneity. We therefore assessed correlation between behavioral rating scale response and cognitive response.
The present study was grounded in a clinical biomarker trial of atomoxetine that included each of our primary measures of interest [24]. We hypothesized that ADHD would comprise multiple distinct biotypes with characteristic cognitive profiles. Further, we expected that these types would be differentiated mechanistically by central and peripheral arousal assessed by the EEG and ECG, and clinically by extent of symptom response following treatment with atomoxetine. Our secondary clinical hypothesis was that symptom response would differentially correlate with improvements in cognition within each identified biotype [25].
Material and methods
Study design
Data were collected in a double-blind, randomized, placebo-controlled crossover study of atomoxetine. The ADHD (Attention-Deficit/Hyperactivity Disorder) Controlled Trial Investigation Of a Non-stimulant (ACTION) protocol was previously reported [24]. The study was conducted at three academic medical centers in Australia between February 2008, and April 2010. It included a 2-week washout lead-in and three assessments: a blinded baseline assessment at the start of the first of two 6-week treatment phases separated by a 1-week washout, and a blinded assessment at the end of each phase. Cognitive testing and clinical rating scales were performed at each assessment and all sites. EEG and ECG recordings were performed only at baseline at the Sydney site. Referral identified 198 subjects, 140 were randomized and 116 completed cognitive and EEG/ECG testing in the first phase (Fig. S1). Of these 116, four ADHD subjects were removed from analysis due to reporting fewer than 4 h of sleep the night before the baseline assessments because sleep deprivation significantly affects cognition and EEG [26,27]. This left 112 ADHD subjects and 56 typically developing subjects. Typically developing controls participated in baseline measurement of EEG/ECG at Sydney.
Sampling procedure
Subjects were assessed in a comprehensive clinical interview (by MRK, SC, DE) at a tertiary referral behavioral pediatrics center that focuses on diagnosis and treatment of ADHD. Diagnosis of ADHD subtypes were made by referring clinicians according to DSM-IV criteria [28]. Diagnosis was confirmed using the ADHD Rating Scale IV (ADHD-RS) and via clinical interview by an independent, trained research psychologist (symptoms counted as present if rated at 2 or greater) [28]. Comorbid disorders were identified by clinical interview, using the Anxiety Disorders Interview Schedule for Children (ADISC) [29], administered by the trained psychologist. Anxiety disorders included Generalized Anxiety Disorder, Social Anxiety Disorder, and Separation Anxiety Disorder. Severity of symptoms of depression and anxiety was assessed by the State-Trait Anxiety Inventory (STAI) and the Depression Anxiety Stress Scale (DASS) [30,31].
Inclusion criteria for ADHD subjects and controls included: age 6–17 years, normal body mass for age/gender, and English fluency. Exclusion criteria for both groups included IQ ≤ 80, physical brain injury, neurologic disorder, concurrent stimulant use, cardiac abnormalities, psychosis, or history of drug abuse or dependence [14,24]. ADHD subjects had to meet ADHD criteria for inclusion, while controls were excluded if they had a personal or family history of an Axis I psychiatric disorder. Subject guardians provided informed consent and study subjects assented to participation in the study. Each site’s Institutional Review Board approved the protocol.
Cognitive battery
Cognitive measures were assessed using a computerized, touchscreen test battery with audio instructions, IntegNeuro (Brain Resource Ltd., Sydney, Australia), that has been validated in thousands of subjects [32]. The specific cognitive measures are described elsewhere and have been established as sensitive for ADHD relative to healthy controls [14,24]. Briefly, this battery included the following test paradigms: Continuous Performance Test (CPT), Go/No-go (GNG), switching of attention (analogous to Trails A and B), maze, verbal memory recall (analogous to California Verbal Learning Test), verbal interference (analogous to Stroop), motor tapping, digit span, and choice reaction time [33,14]. Performance on each cognitive test was standardized to age- and gender-normed Z scores, using a large database of typically developing children [34]. The direction of effect for normed scores was standardized so that negative scores indicated worse performance.
EEG and ECG acquisition & data reduction
EEG and ECG acquisition followed established protocols [35,36]. Subjects were seated comfortably in a sound- and light-controlled testing room. Data were acquired under a resting eyes-open condition for 2 min, followed by separate task-evoked conditions. Only eyes-open EEG was analyzed for this study. EEG data were acquired from the F7, F3, FC3, Fz, FC4, F4, F8, Cz, T5, P3 CP3, Pz, CP4, P4 and T6 electrode sites (10–20 International System). Data were recorded relative to the average of A1 and A2 (mastoid) electrode sites. Two ECG channels were collected and a common reference channel was obtained at C7.
Established procedures for quality control were followed, including for dealing with artifact due to eye blinks or voltage swings over 100 uV [37]. Following Fast Fourier Transform of the EEG we quantified absolute power averaged by region for beta (14.5–30 Hz), alpha (8–13 Hz), theta (4–7.5 Hz), and delta (1.5–3.5 Hz), and computed the theta/beta ratio. These power values were natural log-transformed to better approximate a normal distribution for linear models [38]. From the ECG, heart rate was quantified by computing average RR interval. Heart rate variability was computed as the root mean square of successive differences between RR intervals (rMSSD). Both EEG and ECG values were quantified separately for resting and task conditions.
Statistical analysis
Cognitive biotype identification
Agglomerative hierarchical clustering is a robust algorithm that performs well across a variety of biomedical applications and different measures of performance [39]. Results are reproducible and the algorithm is more robust to outliers than many alternatives [40,20]. We used the agglomerative nesting algorithm described by Kaufman and Rousseeuw and implemented in the R package, cluster [41,42]. Baseline cognitive performance measures were used as cluster input features. Median replacement was performed for missing data and outliers >5 SD from the mean as a conservative approach to constructing the correlation matrix. To minimize co-linearity, among cognitive variables with >0.90 correlations, one measure was included such that overlap of tests was avoided where possible. A measure of model fit for agglomerative nesting is the agglomerative coefficient (AC) [42]. We used Euclidean distance and compared 3 different commonly used linkage methods (complete, average, and Ward’s) and chose the solution with the highest AC. AC varies from 0 to 1, with values close to 1 indicating very clear cluster structure [42]. AC is defined as the average of all values for 1 – d(i) where d(i) represents the dissimilarity of each object i to the first cluster with which it is merged, divided by dissimilarity of the merger of the last step of the algorithm. The approach reported by Malika, et al. using the R package, NbClust, was adopted for determination of the number of clusters [43].
Mechanistic validation
To investigate biotype differences across convergent validators, we used linear mixed models (LMM) with restricted maximum likelihood estimation implemented in the R package, lme4 [44]. Fixed effect terms for biotype were used to determine whether the biotypes differed significantly on the dependent variable of interest. For each dependent variable, LMMs including the final term of interest were compared with models including all other terms using a log-likelihood Chi-square test. For models with significant overall effects, the 95% confidence intervals for the beta coefficients were used to determine which individual contrasts were statistically significant. Age and subject ID were included as random effects in all models. For EEG measures, one model was constructed per power band (beta, alpha, theta and delta) and a fifth for theta/beta ratio. Fixed effects of biotype, region (frontal, temporal, central, parietal), and biotype * region interaction were added to each model. Separate LMMs were constructed for RR interval and rMSSD. Identical procedures were followed including DSM subtype rather than biotype.
Clinical validation
Difference in ADHD-RS total scores between treatment and placebo phases was the primary dependent measure of treatment response [45]. The model for ADHD-RS difference included a fixed effect for baseline ADHD-RS score. Age, site, and subject ID were included as random effects. We also calculated Pearson’s correlation coefficients between the ADHD-RS difference score and the atomoxetine – placebo score across the cognitive measures to determine whether there was a dissociation between the relationship of clinical improvement to cognitive improvement across biotypes and applied Bonferroni correction for multiple hypothesis testing [46,47].
Demographic characteristics
To assess whether demographic factors also contributed to biotype differences, two-tailed independent sample t-tests, Pearson’s Chi-square test, and Fisher’s exact test were used to compare means and proportions across clinical (and demographic) characteristics between biotypes.
Results
Cognitive biotype identification
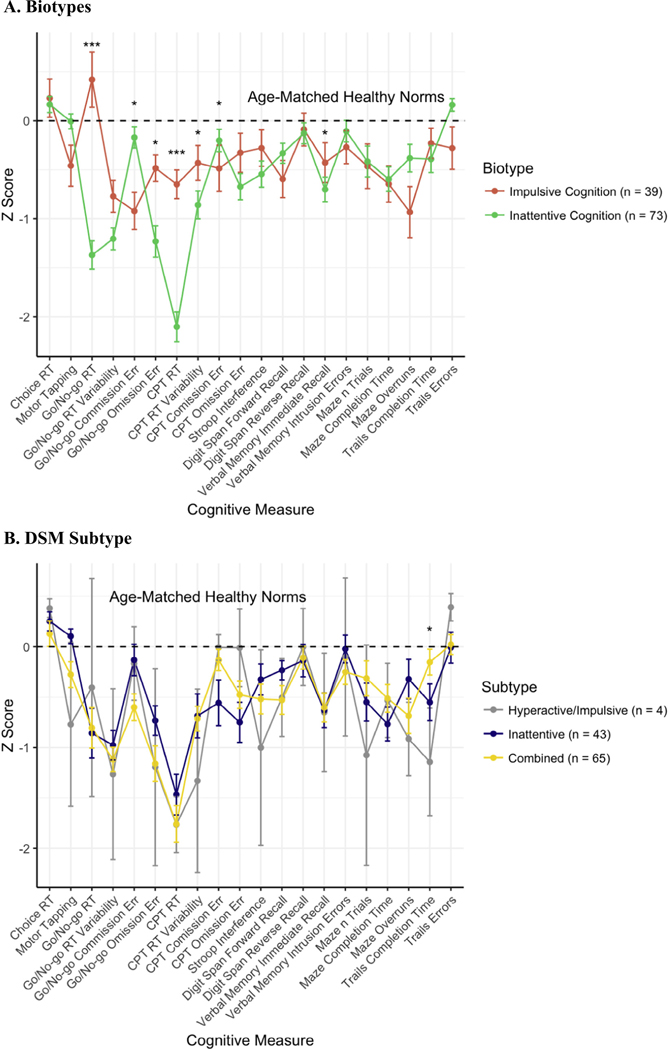
Ward’s method yielded the solution with the highest AC (0.89). The AC of the complete linkage solution was 0.71 and of the average linkage solution was 0.52. The solution was determined to have two biotypes. The average cognitive testing scores across the cognitive battery are presented in Fig. 1, with relevant scores by DSM subtype for comparison. Biotype 1, termed “impulsive cognition” was differentiated by shorter reaction times and fewer errors of omission, but more errors of commission and worse performance on tasks requiring inhibition (maze overruns, Stroop interference score). Biotype 2, termed “inattentive cognition” was characterized by longer reaction time on GNG and the CPT as well as more errors of omission on GNG.
Fig. 1.
Neuropsychological Profiles Note: Comparison of identified biotypes and DSM subtypes cognitive profiles. Errors bars represent standard errors. Dashed horizontal lines represent the averages for age-matched healthy norms from which Z-scores for all individual subjects were calculated. Asterisks indicate statistical significance of pairwise t-tests (Biotypes) and one-way ANOVA (DSM Subtype) for each cognitive measure between groups; *p < 0.05, **p < 0.01, ***p < 0.001.
Mechanistic validation: EEG
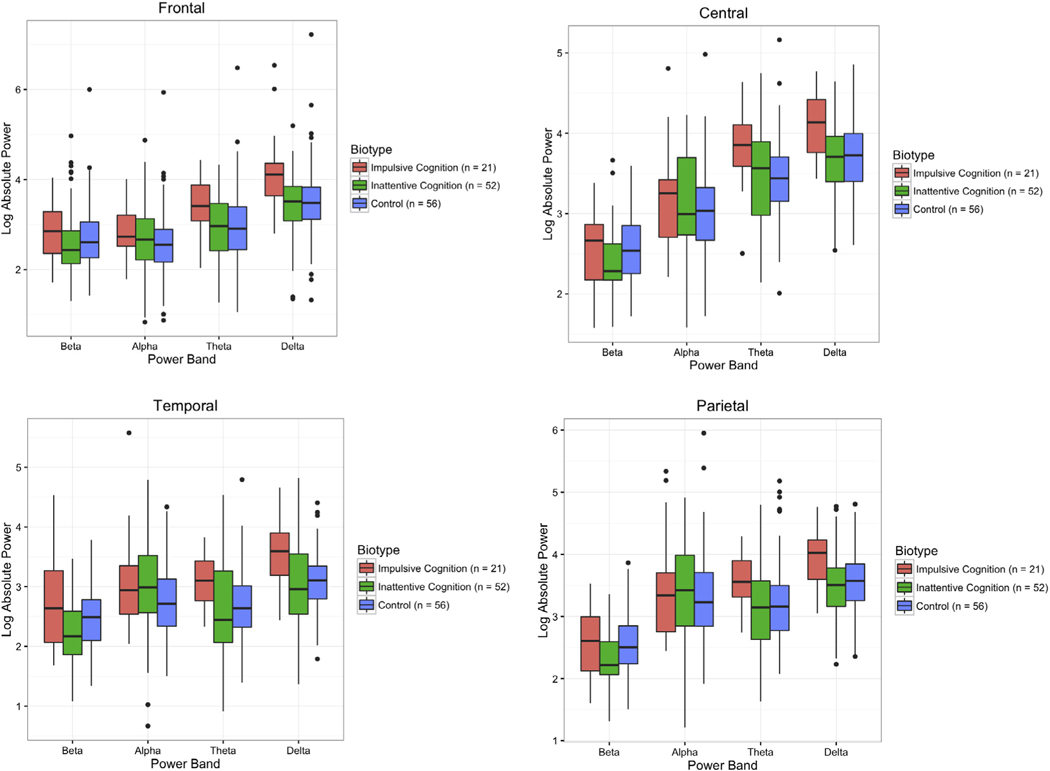
Log power by biotype is presented in Fig. 2. For eyes-open EEG, the most marked difference between biotypes was a significant effect of biotype on delta power (Χ2(2) = 10.18, p = 0.006). Impulsive cognition subjects had significantly higher delta power than both inattentive cognition subjects (b = −0.48, SE = 0.16, CI = −0.81 to −0.17) and controls (b = −0.45, SE = 0.16, CI = −0.76 to −0.14). Inattentive cognition subjects and controls did not differ from one another, and biotype * region interactions were nonsignificant. There was also a significant effect of biotype in the theta band (Χ2(2) = 6.68, p = 0.035). Again, both inattentive cognition subjects (b = −0.41, SE = 0.17, CI = −0.77 to −0.07) and controls (b = −0.42, SE = 0.18, CI = −0.76 to −0.08) had significantly lower power than impulsive cognition subjects, but inattentive cognition subjects and controls did not differ from one another. There was also no significant effect of biotype * region interactions in the theta band. There was a significant biotype * region interaction for the beta band (Χ2(6) = 15.18, p = 0.019). These contrasts for inattentive cognition * frontal (b = −0.22, SE = 0.10, CI = −0.42 to −0.02), inattentive cognition * temporal (b = −0.35, SE = 0.12, CI = −0.58 to −0.13), control * frontal (b = −0.21, SE = −0.10, CI = −0.40 to −0.15), and control * temporal (b = −0.23, SE = 0.11, CI = −0.44 to −0.01) coefficients were all significantly different from impulsive cognition subjects. There were no significant effects of biotype in the alpha band region or theta/beta ratio.
Fig. 2.
EEG Power By Biotype Across Regions and Spectral Bands. Note: Each panel represents averaged power across the indicated leads, with the elevated global slow wave profile of the impulsive cognition biotype clearly visible. Note also the generally elevated frontal beta and relatively decreased frontal beta of the inattentive cognition biotype. Multiple regions are presented for thoroughness, though the trends are similar across regions. In contrast, there are not consistent differences by DSM subtype (not pictured). Center lines represent median values, colored areas interquartile range, whiskers the last point within 1.5 times the interquartile range of the upper/lower quartiles, and points further outliers.
The only significant effect of DSM subtype across models was a subtype * region interaction term in the alpha power band (Χ2(9) = 26.7, p = 0.002). Only the combined type * temporal lobe interaction term compared with controls was significant (b = 0.65, SE = 0.31, CI = 0.04–1.25). ADHD-I and ADHD-C did not differ significantly from one another.
Mechanistic validation: ECG
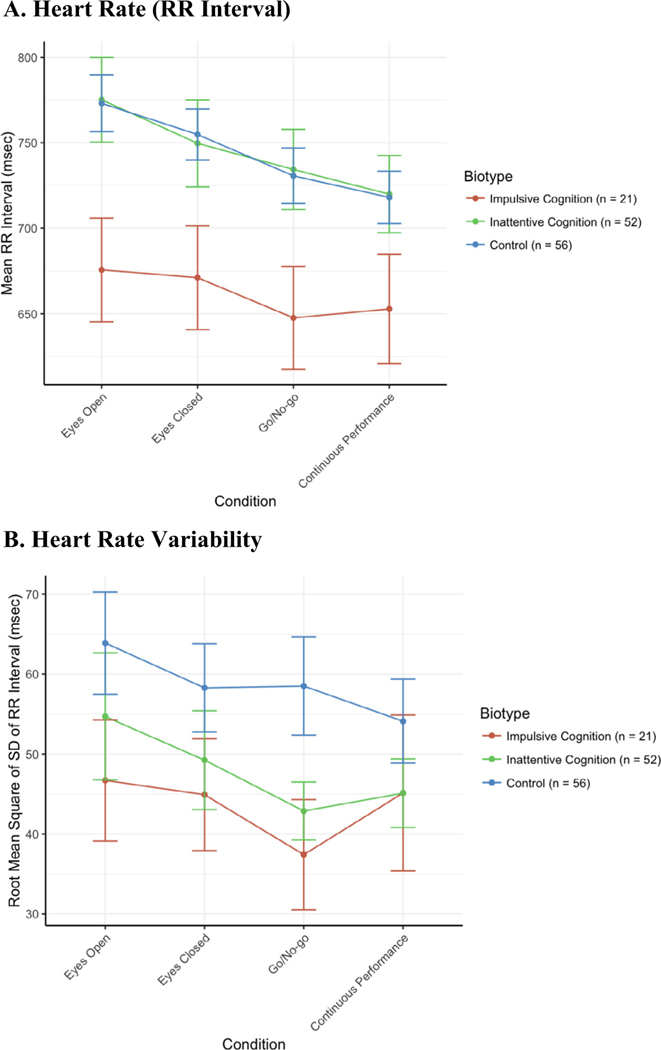
Average RR interval by biotype is presented in Fig. 3A and heart rate variability in Fig. 3B. The model for RR interval revealed a significant effect of biotype (Χ2(2) = 6.90, p = 0.032). Individual contrasts indicated that impulsive cognition subjects had a lower RR interval (greater resting heart rate) than both inattentive cognition subjects (b = 99.5msec, SE = 40.9) and controls (b = 97.5msec, SE = 39.1). There was no significant effect of biotype * task interaction. Inattentive cognition subjects did not differ from controls. For rMSSD, the biotypes did not differ significantly (Χ2(2) = 2.03, p = 0.362). There were no significant differences by DSM Subtype for RR interval or rMSSD.
Fig. 3.
ECG Note: Consistent differences are seen in resting heart rate across task conditions in the impulsive cognition biotype compared with the inattentive biotype or with controls, which are indistinguishable from one another. The separation is less clear looking at heart rate variability. Errors bars represent standard error of the mean.
Clinical Validation: Treatment response
Clinical characteristics are listed in Table 1. The Impulsive Cognition biotype had a mean decrease of 6.1 (SD = 14.2) on the ADHD-RS, while the Inattentive Cognition biotype had a mean decrease of 3.0 (SD = 9.4). The DSM Combined Subtype had a mean decrease of 4.1 (SD = 9.7), while the DSM Inattentive Subtype had a mean decrease of 4.2 (SD = 12.4). These differences did not reach statistical significance. Biotype did not have a statistically significant effect on treatment outcome in the full mixed-effects model (Χ2(1) = 1.27, p = 0.259). Biotypes differed in the pattern of correlations between change in ADHD-RS and change in cognitive measures. Specifically, only inattentive cognition biotype had a significant correlation between change in overall ADHD-RS score and verbal memory immediate recall (r = −0.447, p < 0.001). Negative correlation here indicates that improvements correlated. No other clinical and cognitive change correlations survived Bonferroni correction.
Table 1.
Clinical Characteristics.
| Variable | Impulsive Cognition (n = 39) | Inattentive Cognition (n = 73) | Test Statistic | p |
|---|---|---|---|---|
| ADHD-RS Atomoxetine Improvement – Placebo Improvement (SD) | 6.1 (14.2) | 3.0 (9.4) | t = −1.3 | 0.186 |
| Comorbidity, No. of persons (%) | ||||
| Any Anxiety Disorder | 13 (33%) | 22 (30%) | Χ2 = 0.1 | 0.728 |
| Dysthymic Disorder | 8 (21%) | 11 (15%) | Χ2 = 0.5 | 0.599 |
| Major Depressive Disorder | 2 (5%) | 1 (1%) | Fisher’s Exact | 0.277 |
| Oppositional Defiant Disorder | 18 (46%) | 27 (37%) | Χ2 = 0.9 | 0.346 |
| Conduct Disorder | 6 (15%) | 8 (11%) | Χ2 = 0.5 | 0.500 |
| Obsessive Compulsive Disorder | 5 (13%) | 4 (5%) | Fisher’s Exact | 0.272 |
| Post-Traumatic Stress Disorder | 1 (3%) | 5 (7%) | Fisher’s Exact | 0.349 |
| STAI State, baseline score (SD) | 32 (7.5) | 31 (6.2) | t = 0.887 | 0.377 |
| STAI Trait, baseline score (SD) | 38 (9.3) | 34 (7.6) | t = 2.054 | 0.044 |
| DASS Anxiety, baseline score (SD) | 1.4 (1.7) | 1.6 (1.6) | t = −0.577 | 0.565 |
| DASS Depression, baseline score (SD) | 5.05 (4.3) | 4.6 (4.3) | t = 0.542 | 0.589 |
| DASS Stress, baseline score (SD) | 7.1 (3.9) | 7.1 (5.3) | t = −0.003 | 0.998 |
| ADHD-RS, baseline score (SD) | 37.1 (10.3) | 36.3 (10.0) | t = 0.367 | 0.714 |
| ADHD-RS Inattentive, baseline score (SD) | 20.6 (5.2) | 20.9 (4.0) | t = −0.326 | 0.745 |
| ADHD-RS Hyperactive/Impulsive, baseline score (SD) | 16.5 (6.6) | 15.4 (7.4) | t = 0.720 | 0.473 |
Note: ADISC = Anxiety Disorders Interview Schedule-Child version; STAI = State/Trait Anxiety Inventory; DASS = Depression Anxiety Stress Scales; ADHD-RS = ADHD-Rating Scale for DSM-IV. For clarity, ADHD-RS Improvement scores are listed as positive scores; this indicates a decrease in the score during the active treatment phase compared to the placebo phase.
Sample characteristics
Impulsive cognition subjects had four point higher average STAI Trait scores (p = 0.044) (Table 1). The biotypes did not differ in proportion of inattentive, combined, or hyperactive/impulsive subtypes defined by DSM. There were no other baseline differences in clinical co-morbidities between biotypes.
Impulsive cognition subjects were, on average, 1.3 years younger than inattentive cognition subjects (p = 0.010) and differed proportionally in education (Table 2). There was not a significant difference in the representation of different ethnic groups across biotypes.
Table 2.
Demographic Characteristics
| Variable | Impulsive Cognition (n = 39) | Inattentive Cognition (n = 73) | Test Statistic | p |
|---|---|---|---|---|
| Age, yrs, mean (SD) | 10.4 (2.6) | 11.7 (2.4) | t = −2.6 | 0.010 |
| Sex, No. of persons (%) | X2 = 1.3 | 0.243 | ||
| Male | 29 (74%) | 61 (84%) | ||
| Female | 10 (26%) | 12 (16%) | ||
| Education, yrs, mean (SD) | 4.9 (2.5) | 6.4 (2.4) | t = −3.1 | 0.003 |
| Ethnicity, No. of persons (%) | Fisher’s Exact | 0.299 | ||
| White | 25 (64%) | 40 (56%) | ||
| Black | 1 (3%) | 0 (0%) | ||
| Indigenous | 0 (0%) | 2 (3%) | ||
| Asian | 0 (0%) | 0 (0%) | ||
| Pacific Islander | 2 (5%) | 1 (1%) | ||
| Middle Eastern | 0 (0%) | 4 (5%) | ||
| Mixed ethnicity | 6 (15%) | 9 (12%) | ||
| Declined/left blank | 5 (13%) | 17 (23%) | ||
| Body Mass Index, kg/m2, mean (SD) | 20.6 (5) | 21.4 (4) | t = −0.7 | 0.512 |
Discussion
Our approach identified two putative biotypes amongst youth with ADHD – one with impulsive cognition and another characterized by inattentive cognition. Strikingly, the identified biotypes did not differ in proportion of inattentive, combined, or hyperactive/impulsive subtypes defined by DSM, or in average inattentive or hyperactive/impulsive ADHD symptom scores. One implication is that links between the behavioral rating scale and cognitive measures of inattention and impulsivity cannot be taken for granted. Impulsive cognition subjects had slightly higher anxiety severity than inattentive cognition subjects but they did not differ on any other clinical measures. They differed significantly across several convergent markers that validate their use as biotypes likely to have more homogeneous underlying biological differences than do current DSM subtypes. Specifically, the impulsive cognition biotype had elevated slow wave (delta and theta) resting EEG profiles and elevated resting HR, while the inattentive cognition biotype had lower frontal and temporal beta resting EEG and resting HR that did not differ from controls. The identified biotypes also differed in patterns of cognitive-clinical improvement correlation.
Several lines of evidence converge with the findings reported here in support of the validity of the identified biotypes. Latent class analysis of similar cognitive measures in a sample with ADHD identified a slow and variable group, a quick and accurate group, and a group with more errors on GNG termed a poor cognitive control group [48]. The biotypes identified here differ by a similar pattern. Another recent study clustered ADHD subjects by temperament, finding a group termed “surgent” with novelty-seeking temperament and greater sympathetic tone measured by cardiac pre-ejection period [49]. The surgent group was the only group to separate from controls on this measure and the difference was present across tasks, which is the pattern observed here in RR interval for impulsive cognition subjects. Slow wave EEG power has been linked to altered risk-taking, further suggesting a possible congruence between the surgent group and the impulsive cognition biotype [50]. The study by Karalunas, et al. did not include EEG or cognitive variables, however, and we did not measure temperament.
Integrating these studies, we conclude that underlying biotypes appear cohesive across convergent cognitive and biological measures but not consistently linked to clinical ratings of behavior. Another recent study split a sample according to number of disruptive symptoms [51]. This resulted in a group characterized by longer and more variable reaction times with lower relative delta and higher relative alpha power than the group characterized by GNG errors of commission—similar to the split observed here. In contrast to our findings, however, those groups differed across multiple behavioral ratings of psychopathology, including comorbid anxiety disorders, mood disorders, oppositional defiant disorder, and conduct disorder.
Our findings have implications for developing diagnostic tests for ADHD based on a biologic taxonomy. It has long been proposed, among the competing theories of the pathophysiology of ADHD, that children with ADHD are physiologically hypo-aroused [9]. In this context, slow wave (delta/theta) EEG power has generally been thought to indicate decreased arousal, and fast wave (alpha/beta) to indicate increased arousal during task-directed activity. Theta/beta ratio has been investigated as a diagnostic test for ADHD, but findings have been inconsistent [21]. Our results suggest that these inconsistencies may have resulted at least in part from sampling heterogeneity not captured by clinical rating scales. Rather than a uniformly elevated theta/beta ratio for ADHD, our approach to the natural structure of neural activity defines one biotype with elevated slow wave (delta/theta) and elevated fast wave (beta) EEG along with elevated heart rate and cognitive indictors of impulsivity rather than hypo-arousal. The group with longer reaction times did not differ from controls in slow wave power. In this regard, our results indicate that multiple models of dysfunction of the arousal system—rather than general hypo-arousal—are needed to understand the mechanisms of ADHD pathophysiology. They also provide a framework within which to reconcile conceptualizations of the frontoparietal cognitive control network as a driver of ADHD pathology with broader evidence suggesting an integral role for the arousal system.
This framework is aligned with neurobiological models of cognitive function that focus on the role of catecholamines in prefrontal and parietal cortex. These regions, particularly parietal cortex, receive dense innervation from locus coeruleus (LC) [52] and have been implicated in ADHD and the frontoparietal cognitive control network [53]. High-affinity alpha2 norepinephrine receptor activation improves working memory, inhibitory control, and flexible associative learning at moderate norepinephrine levels. Activation of lower-affinity alpha1 and beta receptors inhibits working memory and reduces inhibitory control, but improves sustained attention at higher norepinephrine levels [54–56].
A parsimonious explanation for the observed pattern that is concordant with the theorized role of frontoparietal cortex and the differential effects of norepinephrine at the receptor level would suggest a source or common pathway of dysfunction in the locus coeruleus-norepinephrine (LC-NE) system based on the adaptive gain theory [52]. LC has both tonic and phasic firing patterns. The relative balance between tonic and phasic firing affects cognitive performance on forced choice and signal discrimination tasks similar to CPT and GNG. Increased tonic firing—which can be situationally adaptive or can result from lesions to LC—increases baseline levels of norepinephrine in prefrontal cortex (thereby activating alpha1 and beta receptors at high levels of activity) and is associated with increased errors of commission [57]. Decreased phasic firing, which according to this model can result from decreased coupling of LC neurons influenced by many different afferent signals, is associated with slower and more variable responses, though additional task parameters such as difficulty seem also to affect this relationship [58,52]. Intriguingly, delta power has been linked to LC firing rates [59]; salience detection and inhibitory control [60]; and resting heart rate [61]. The LC-NE system is also involved in regulation of heart rate [62]. Increased tonic firing leading to increased cortical and peripheral norepinephrine levels and/or increased sensitivity of alpha1 or beta receptors could explain the observed deficits in the impulsive cognition biotype. Disruptions to the functioning of the phasic firing system, which is dynamically influenced by many regions implicated in previous functional imaging studies of ADHD, including the dopaminergic reward expectation system and prefrontal regions such as the anterior cingulate, could be implicated in the inattentive cognition biotype [63]. Future studies with greater spatial resolution could be informative here.
We did not detect the statistically significant difference in ADHD-RS response that we had hypothesized. An observed effect of phase and somewhat lower treatment effect in our study compared to previous studies of atomoxetine may have decreased our ability to detect a statistically significant effect [64,65]. Nevertheless, the dissociation in patterns of correlation between ADHD symptom response and cognitive response further validates the biotypes and suggests that clinical response to atomoxetine may be mediated by different cognitive functions within each biotype. There were no statistically significant correlations between cognitive markers and ADHD-RS improvement in either DSM subtype. A strong correlation between improvement in behavioral ratings and verbal memory immediate recall was identified only in the inattentive cognition biotype. The verbal memory immediate recall measure is an adaptation of the California Verbal Learning Test (CVLT) and depends on the cognitive constructs of working memory, encoding, and retrieval [66]. The biotypes did not differ significantly on this measure at baseline. Future work is needed to continue to explore the links between cognitive and symptomatic response to treatments for ADHD.
This study has several limitations. First and foremost, replication by independent investigators is fundamental to robustly establishing the validity of results. We cannot draw strong conclusions about longitudinal stability from our relatively short-term study. However, ADHD symptoms and EEG power have been shown to be heritable and cognitive response style has been linked to ADHD risk alleles. Unsupervised learning approaches are appropriate when the number of groups is not known, as in this case, but results can vary by algorithm and feature selection. While a strength of this study is the inclusion of multiple markers spanning multiple levels of biological organization—using patterns in the relationships between these as the focus of our analysis to maintain statistical power—no single battery can claim to be comprehensive and additional measures could be investigated in the future. Selection of different algorithms or input features could potentially yield different insights. While we had a relatively large sample powered to detect moderate between-group differences, larger samples can provide greater resolution in discovering a greater number of types if more types exist or in identifying between-group differences of lesser magnitude. Furthermore, as in any clinical trial, results should not be generalized to populations that differ significantly from study participants.
Conclusions
There is now significant evidence that cognitive measures have closer relationships to several biomarkers related to arousal and catecholamine systems than do DSM-defined behavioral symptoms. In this study, cognitive response styles were found to be associated with different patterns in EEG and ECG measures linked to the LC-NE system as well as different cognitive patterns of treatment response to a greater degree than were DSM subtypes. These patterns overlap significantly with previous cognitive, EEG, ECG, and temperament findings but less so with previous links to behavioral rating scales. The findings provide evidence that focusing on cognitive subtypes may be more fruitful than symptom checklist-based subtypes for future investigations regarding diagnostics and interventions for ADHD. Our results may explain heterogeneous past findings regarding theta/beta ratio as a diagnostic marker for ADHD and are inconsistent with general hypo-arousal as an explanation for previous observations of slow and variable response time in conjunction with increased slow wave activity in ADHD at the whole-group level.
Supplementary Material
Acknowledgements
The authors wish to thank Bradley Efron, PhD, of the Stanford University Department of Statistics for guidance in analysis of effects in the cross-over study.
Funding
This work was supported by a National Health and Medical Research Council (NHMRC) grant: ID 457424, title: “Randomised Controlled Trial Investigation of a Non-stimulant in Attention Deficit Hyperactivity.” The lead author’s time devoted to analysis and writing was supported by NIMH training grant, 2T32MH019908-23.
Abbreviations:
- LC
locus coeruleus
- NE
norepinephrine
- AC
agglomerative coefficient
Footnotes
Financial disclosures
Conflict of Interest Disclosures: JEL, KRG, MS, DSH, TWT, SC, DFH, and DE have no conflicts of interest to disclose. MRK has participated in the Adboard and received consultancy fees from Shire and Eli Lily. LMW has received consultant fees from Brain Resource and from Humana.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.pmip.2017.02.001.
References
- [1].Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol. Psychiatry 2005;57:1424–35. [DOI] [PubMed] [Google Scholar]
- [2].Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci 2006;10:117–23. [DOI] [PubMed] [Google Scholar]
- [3].Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc Psychiatr Clin N Am 2008;17:367–84. ix. [DOI] [PubMed] [Google Scholar]
- [4].Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology 2010;35:278–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 2014;171:276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol 2012;121:991–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].American Psychiatric Association. and American Psychiatric Association. Dsm-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. Ed. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- [8].Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, et al. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. J Abnorm Child Psychol 2007;35:729–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Satterfield JH, Cantwell DP, Satterfield BT. Pathophysiology of the hyperactive child syndrome. Arch Gen Psychiatry 1974;31:839–44. [DOI] [PubMed] [Google Scholar]
- [10].Rowe DL, Robinson PA, Lazzaro IL, Powles RC, Gordon E, Williams LM. Biophysical modeling of tonic cortical electrical activity in attention deficit hyperactivity disorder. Int J Neurosci 2005;115:1273–305. [DOI] [PubMed] [Google Scholar]
- [11].Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 2011;69:e89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry 1999;46:1690–7. [DOI] [PubMed] [Google Scholar]
- [13].Barry RJ, Clarke AR, Johnstone SJ, Mccarthy R, Selikowitz M. Electroencephalogram theta/beta ratio and arousal in attention-deficit/hyperactivity disorder: evidence of independent processes. Biol Psychiatry 2009;66:398–401. [DOI] [PubMed] [Google Scholar]
- [14].Williams LM, Hermens DF, Thein T, Clark CR, Cooper NJ, Clarke SD, et al. Using brain-based cognitive measures to support clinical decisions in ADHD. Pediatr Neurol 2010;42:118–26. [DOI] [PubMed] [Google Scholar]
- [15].Imeraj L, Antrop I, Roeyers H, Deschepper E, Bal S, Deboutte D. Diurnal variations in arousal: a naturalistic heart rate study in children with ADHD. Eur Child Adolesc Psychiatry 2011;20:381–92. [DOI] [PubMed] [Google Scholar]
- [16].Buchhorn R, Conzelmann A, Willaschek C, Stork D, Taurines R, Renner TJ. Heart rate variability and methylphenidate in children with ADHD. Atten Defic Hyperact Disord 2012;4:85–91. [DOI] [PubMed] [Google Scholar]
- [17].Castellanos FX, Glaser PE, Gerhardt GA. Towards a neuroscience of attention-deficit/hyperactivity disorder: fractionating the phenotype. J Neurosci Methods 2006;151:1–4. [DOI] [PubMed] [Google Scholar]
- [18].Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction. 2nd ed. New York, NY: Springer; 2009. [Google Scholar]
- [19].Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry 2016;173:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Everitt B. Cluster analysis. Ed. Chichester, West Sussex, U.K.: Wiley; 2011. [Google Scholar]
- [21].Arns M, Conners CK, Kraemer HC. A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. J Atten Disord 2013;17:374–83. [DOI] [PubMed] [Google Scholar]
- [22].Olbrich S, Van Dinteren R, Arns M. Personalized Medicine: Review and Perspectives of Promising Baseline EEG Biomarkers in Major Depressive Disorder and Attention Deficit Hyperactivity Disorder. Neuropsychobiology 2015;72:229–40. [DOI] [PubMed] [Google Scholar]
- [23].Adamo N, Seth S, Coghill D. Pharmacological treatment of attention-deficit/hyperactivity disorder: assessing outcomes. Expert Rev Clin Pharmacol 2015;8:383–97. [DOI] [PubMed] [Google Scholar]
- [24].Tsang TW, Kohn MR, Hermens DF, Clarke SD, Clark CR, Efron D, et al. A randomized controlled trial investigation of a non-stimulant in attention deficit hyperactivity disorder (ACTION): rationale and design. Trials 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry 2009;65:550–5. [DOI] [PubMed] [Google Scholar]
- [26].Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 2008;1129:305–22. [DOI] [PubMed] [Google Scholar]
- [27].Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med 2011;7: S16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dupaul GJ. Parent and teacher ratings of ADHD symptoms: Psychometric properties in a community-based sample. J Clin Child Psychol 1991;20:245–53. [Google Scholar]
- [29].Silverman WK, Albano AM. Anxiety Disorders Interview Schedule (ADIS-IV) Child/Parent Clinician Manual. Ed: Graywind Publications, Oxford University Press; 2004. [Google Scholar]
- [30].Spielberger CDG, Lushene RL, Vagg R, Jacobs PR, A G. Manual for the State-Trait Anxiety Inventory (Form Y). Ed. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- [31].Lovibond SHL PF Manual for the Depression Anxiety Stress Scales Ed. Sydney, New Suth Wales, Australia: Psychology Foundation; 1995. [Google Scholar]
- [32].Williams LM, Tsang TW, Clarke S, Kohn M. An ‘integrative neuroscience’ perspective on ADHD: linking cognition, emotion, brain and genetic measures with implications for clinical support. Expert Rev Neurother 2010;10:1607–21. [DOI] [PubMed] [Google Scholar]
- [33].Delis DCK, Kaplan JH, Ober BA. CVLT-C: California Verbal Learning Test; 1994. [Google Scholar]
- [34].Mathersul D, Palmer DM, Gur RC, Gur RE, Cooper N, Gordon E, et al. Explicit identification and implicit recognition of facial emotions: II. Core domains and relationships with general cognition. J Clin Exp Neuropsychol 2009;31:278–91. [DOI] [PubMed] [Google Scholar]
- [35].Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci 2005;115:1605–30. [DOI] [PubMed] [Google Scholar]
- [36].Kozlowska K, Palmer DM, Brown KJ, Mclean L, Scher S, Gevirtz R, et al. Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom Med 2015;77:356–70. [DOI] [PubMed] [Google Scholar]
- [37].Hatch A, Madden S, Kohn MR, Clarke S, Touyz S, Gordon E, et al. EEG in adolescent anorexia nervosa: impact of refeeding and weight gain. Int J Eat Disord 2011;44:65–75. [DOI] [PubMed] [Google Scholar]
- [38].Gatt JM, Kuan SA, Dobson-Stone C, Paul RH, Joffe RT, Kemp AH, et al. Association between BDNF Val66Met polymorphism and trait depression is mediated via resting EEG alpha band activity. Biol Psychol 2008;79:275–84. [DOI] [PubMed] [Google Scholar]
- [39].Wiwie C, Baumbach J, Rottger R. Comparing the performance of biomedical clustering methods. Nat Methods 2015;12:1033–8. [DOI] [PubMed] [Google Scholar]
- [40].Mclachlan GJ, Bean RW, Ng SK. Clustering. Methods Mol Biol 2008;453:423–39. [DOI] [PubMed] [Google Scholar]
- [41].Kaufman LR PJ Finding Groups in Data: An Introduction to Cluster Analysis. Ed. New York: Wiley; 1990. [Google Scholar]
- [42].Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. cluster: Cluster Analysis basics and Extensions; 2015. [Google Scholar]
- [43].Malika C, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R Package for Determining the Relevant Number of Clusters in a Data Set. Journal of Statistical Software 2014;61:1–36. [Google Scholar]
- [44].Bates DMM; Bolker B; Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 2015;67:1–48. [Google Scholar]
- [45].Dupaul GJT J; Anastopoulos AD ADHD rating scale-IV: Checklists, norms and clinical interpretation. Ed. New York, NY: Guilford Press; 1998. [Google Scholar]
- [46].Dunn OJ. Multiple Comparisons among Means. J Am Stat Assoc 1961;56. 52-&. [Google Scholar]
- [47].Senn S. Statistics in Practice: Statistical Issues in Drug Development. Ed. Chichester, England: Wiley-Interscience; 2008. [Google Scholar]
- [48].Van Hulst BM, De Zeeuw P, Durston S. Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychol Med 2015;45:735–45. [DOI] [PubMed] [Google Scholar]
- [49].Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry 2014;71:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [50].Schutter DJ, Van Honk J. Electrophysiological ratio markers for the balance between reward and punishment. Brain Res Cogn Brain Res 2005;24:685–90. [DOI] [PubMed] [Google Scholar]
- [51].Mcgough JJ, Mccracken JT, Cho AL, Castelo E, Sturm A, Cowen J, et al. A potential electroencephalography and cognitive biosignature for the child behavior checklist-dysregulation profile. J Am Acad Child Adolesc Psychiatry 2013;52:1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005;28:403–50. [DOI] [PubMed] [Google Scholar]
- [53].Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 2012;16:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav 2011;99:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Berridge CW, Arnsten AF. Psychostimulants and motivated behavior: arousal and cognition. Neurosci Biobehav Rev 2013;37:1976–84. [DOI] [PubMed] [Google Scholar]
- [56].Arnsten AF, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress 2015;1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science 1999;283:549–54. [DOI] [PubMed] [Google Scholar]
- [58].Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol 2004;92:361–71. [DOI] [PubMed] [Google Scholar]
- [59].Safaai H, Neves R, Eschenko O, Logothetis NK, Panzeri S. Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc Natl Acad Sci USA 2015;112:12834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Harmony T. The functional significance of delta oscillations in cognitive processing. Front Integr Neurosci 2013;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev 2012;36:677–95. [DOI] [PubMed] [Google Scholar]
- [62].Wang X, Pinol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. J Neurosci 2014;34:6182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 2005;493:99–110. [DOI] [PubMed] [Google Scholar]
- [64].Clemow DB, Bushe CJ. Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients. J Psychopharmacol 2015. [DOI] [PubMed] [Google Scholar]
- [65].Childress AC. A critical appraisal of atomoxetine in the management of ADHD. Ther Clin Risk Manag 2016;12:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Delis DCK, Kaplan JH, Ober BA. CVLT, California Verbal Learning Test: Adult Version: Manual. Ed: Psychological Corporation; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.