Abstract
Alzheimer’s disease (AD) is one of the most complex progressive neurological disorders involving degeneration of neuronal connections in brain cells leading to cell death. AD is predominantly detected among elder people (> 65 years), mostly diagnosed with the symptoms of memory loss and cognitive dysfunctions. The multifarious pathogenesis of AD comprises the accumulation of pathogenic proteins, decreased neurotransmission, oxidative stress, and neuroinflammation. The conventional therapeutic approaches are limited to symptomatic benefits and are ineffective against disease progression. In recent years, researchers have shown immense interest in the designing and fabrication of various novel therapeutics comprised of naturally isolated hybrid molecules. Hybrid therapeutic compounds are developed from the combination of pharmacophores isolated from bioactive moieties which specifically target and block various AD-associated pathogenic pathways. The method of designing hybrid molecules has numerous advantages over conventional multitarget drug development methods. In comparison to in silico high throughput screening, hybrid molecules generate quicker results and are also less expensive than fragment-based drug development. Designing hybrid-multitargeted therapeutic compounds is thus a prospective approach in developing an effective treatment for AD. Nevertheless, several issues must be addressed, and additional researches should be conducted to develop hybrid therapeutic compounds for clinical usage while keeping other off-target adverse effects in mind. In this review, we have summarized the recent progress on synthesis of hybrid compounds, their molecular mechanism, and therapeutic potential in AD. Using synoptic tables, figures, and schemes, the review presents therapeutic promise and potential for the development of many disease-modifying hybrids into next-generation medicines for AD.
Keywords: Alzheimer’s disease, Pathogenesis, Neuronal molecular targets, Cellular pathways, Targeted hybrid therapeutics
Introduction
Alzheimer’s disease (AD) is one of the progressive neurodegenerative disorder characterized by brain cognitive impairment which influence speech, behavior and motor function and is most common form of dementia [1]. Since the recognition of the first case of AD by Alois Alzheimer (a German psychiatrist) in 1906, the significant progress has been made in understanding the cellular and molecular mechanisms on pathogenesis of AD [2, 3]. It is prevalent among elderly people, mostly belonging to age ≥65, that comprises one of the significant parts of the population. Ageing is one of the major risk factor and there are no specific feature of diagnosis except pathological diagnosis. AD prompts a poor prognosis with a low chance of early detection [4]. It is pathologically characterized by extracellular amyloid plaque and intracellular neurofibrillary tangles (NFTs) constituting hyperphosphorylated tau-protein deposited in human brain tissues [ 5, 6]. The census data (1900–2050) have shown that the population of the people belonging to this group are growing rapidly, therefore AD is becoming a matter of concern, mostly due to high cost of conventional therapeutics and unattainability of targeted therapeutics [4]. As of now, AD is considered one of the debilitating and devastating disease in the ageing population and represent a therapeutic enigma with being completely incurable [7]. The conventional therapeutics available for the management of AD mainly includes cholinesterase inhibitors (rivastigmine, galantamine, donepezil, and tacrine) that effectively shows benefits in a set of symptoms and target late aspects of AD. Among many approaches, the most effective approach includes inhibiting or preventing the aggregation of Aβ (β-amyloid) plaques in the brain cells [8]. Because of reduced absorption in neuronal cell membranes, these conventional agents mainly affect progression of the disease [9, 10]. There is still necessity of the drugs which can offer a cure for the disease. Despite significant advances in understanding the pathogenesis of AD, still there is scarcity of knowledge regarding the exact molecular mechanism which limit development of targeted and effective therapy. The recognition of targets is essential for intervention to prevent the onset and development of disease as well as direct therapy for the reversal of the modifications in the brain tissue [11, 12]. Numerous investigations have demonstrated linkage between several factors which leads to the onset of AD and hypertension appears one of the most common contributing factor leads to increased plaque formation in the brain [13–15]. Homocysteine, a sulfur-containing amino acid, contribute in the development of Aβ plaques and plays a crucial role in the pathogenesis of AD [16, 17]. Moreover, high sugar levels and obesity also exhibited potential associations with some non-conformities to the risk of developing AD [18, 19].
The single drugs or bioactives available for the treatment of AD have exhibited insufficient effectiveness owing to the polygenic etiology of AD. Therefore, the focus of the researchers and neuroscientists has shifted to “one molecule-multi targeting” molecules, more precisely recognized as hybrid therapeutic compounds, which are known to consist two or more than two bioactive moieties with complementary pharmacophoric and pharmacological properties facilitating a synergistic effect [20–22]. These engineered molecules, often defined as “multi-target-directed ligands” (MTDLs), enable simultaneous delivery of the bioactive moieties to the targeted organs with superior targeting, reduced drug-drug interactions, and lower drug resistance, leading to negligible toxicity and low-cost preclinical trials [20, 23]. In this review, we comprehensively discussed the contributing factors, molecular pathogenesis and development of targeted hybrid therapeutic compounds for the better management of AD.
Molecular Pathogenesis of AD
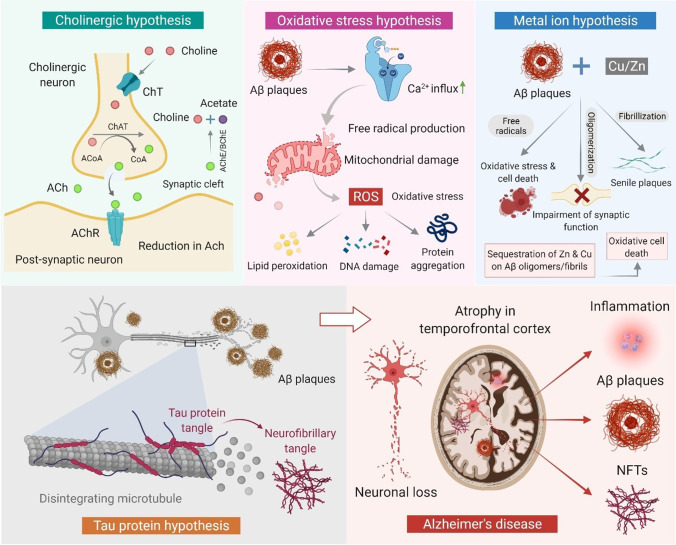
The etiopathogenesis of AD is primarily characterized by the accumulation of two protein markers: extracellular Aβ plaques and NFTs of hyperphosphorylated tau protein (Fig. 1) [5, 6]. Additionaly, other hypotheses, including the loss of synapses, oxidative stress, and nerve cell death are recognized among the pathogenic players and are frequently observed to coexist [24–26].
Fig. 1.
Schematic showing various hypotheses and pathomechanism of AD
In late 1970s, the researchers mainly focused on identifying the contribution of two putative enzymes: acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) responsible for the hydrolysis of cholinergic neurotransmitters, and demonstrated their potential roles in AD therapy [27–29]. For effective AD therapy, dual inhibition of AChE and BChE has been endorsed to provide a superior therapeutic benefit, few specific behavioral benefits, and the avoidance of AChE upregulation. This investigation, thus, gradually shifted the attention of the researchers towards developing dual inhibitors (AChE and BChE) to avoid the inefficacy of AChE [1]. Another etiological characteristic of AD is the amyloid cascade, which centers on the accumulation of the Aβ peptide (Fig. 1) in the brain parenchyma. The aberrant synthesis of amyloid precursor protein (APP) by β-secretases and γ-secretases produces Aβ40 and Aβ42 monomers respectively, which oligomerize into biochemically insoluble fibrils and aggregate within the Aβ plaques [30]. These plaques block proteosome functions, alter intracellular calcium (Ca2+) levels, and limit the mitochondrial activities, leading to increased neurotoxicity and fibrilization rate [31]. Tau protein, another hallmark of AD (Fig. 1), is a microtubule-associated protein that accomplishes the role of stabilizing microtubules. The hyperphosphorylated tau protein produces an imbalance and deteriorates the axonal transport of the APP in nerve cell bodies by forming NFTs. Further, the collapse of microtubules causes interruptions in the neuronal communications lead to the neuronal cell death [32].
The central nervous system (CNS) is secured by the blood–brain barrier (BBB) and the cerebrospinal fluid (CSF) barrier [33]. The proteins through membrane transporters at the BBB are discarded into two major categories, named as uptake and efflux transporters. Moreover, at the level of BBB many uptake transporters play significant role in transporting solutes through circulation into the endothelial cells and then into the brain cells/tissues across the basolateral membranes. Also, the BBB regulates both inward and outward movement of biomolecules to and from the brain neuronal system and contributes to the transport of Aβ to the brain. Thus, BBB plays an important function in the pathogenesis of AD by regulating the aggregation of Aβ peptides within the brain tissue. These cellular processes makes BBB as one of the most important targets that need to be studied in order to improve the delivery and targetability of the drugs to the brain [34, 35]. The CNS is easily susceptible to oxidative stress which is considered one of the major contributors to the neuronal cell death in AD. Monoamine oxygenase (MAO) targets the enzymatic metabolism of neurotransmitters by altering the level of lipids, proteins, and DNA and suppressing respiration. The abundance of Cu2+ and Fe2+ potentiate several reactive oxygen species (ROS) and reactive nitrogen species (RNS) leading to Aβ neurotoxicity. Aβ aggregation also increases the production of ROS (Fig. 1) and causes oxidative stress in mitochondria [36, 37].
The “neuroinflammatory hypothesis” which serves as the first layer of protection in the event of an injury or infection, frequently responds disproportionately and injures brain cells further leads to rapid neurodegeneration [38]. Ca2+ signaling plays an important role in many intracellular and extracellular activities. Ca2+ triggers synaptic vesicle exocytosis, essential for synaptic transmission, thus releases the neurotransmitters [39]. Ca2+ also manipulates the cytoskeleton and associated proteins aiding neuroplasticity and neuronal development. However, prolonged elevation of cytosolic Ca2+ levels triggers the activation of several kinase-dependent signaling cascades that are significantly responsible for the generation of ROS and loss of synaptic plasticity [40]. In this event, the aggregation of MAO-B observed high around senile plaques, leads to the pathogenesis of AD. The catalytic properties of MAO produce H2O2 inside reactive microglia in brain tissue and trigger the onset of oxidative stress and consequent degeneration of the brain cell [41].
Significant Role of Hybrid Therapeutic Compounds in AD
The complex etiology of AD has reinvigorated the development and establishment of novel therapeutic strategies comprised of multitargeted therapeutics targeting specific diseased sites in the brain. Because of the ambiguity, compounds targeting single molecular targets have exhibited limited impact over the complicated pathogenic networks. Till date, studies have shown that various pathogenic pathways are responsible for the initiation and progression of AD, and they are also associated with various cellular response and sequence of events that deteriorate the diseased state while exponentially enhances the chances neuronal death. In many instances, the “one-target one-molecule” strategy is found to be ineffective in influencing the pathogenic events, resulting in limited clinical benifits. Therefore, one single moiety comprised of specific targets focusing important molecular sites could be the most effective method for establishing a potential therapeutic strategy in AD therapeutics. As compared to co-treatment or “single pill” medication combos, these targeted strategies have exhibited substantial benefits as demonstrated by reduced adverse effects of drugs/bioactives and improved pharmacokinetic as well as pharmacodynamic profile with ADME optimization. However, the advancements of “multi-target-directed ligands” continue to be a challenge that need to be addressed in a variety of ways [42]. The most common method is to develop combinatorial therapeutic approaches by combining numerous therapeutic active moieties to form a single hybrid complex. Combining two or more therapeutic active compounds into a single hybrid complex is considered to be one of the typical strategy.
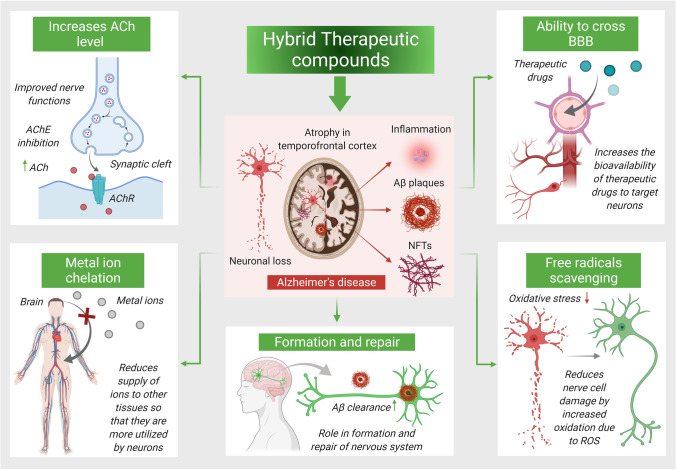
There are numerous options for designing hybrid therapeutic compounds, mostly possible due to their common structural properties at molecular level, with better targeting to the specific molecular sites. Hybrid derivatives can combine a variety of chemical components from different resources (organic moieties, peptides, prodrugs, and others). These hybrid compounds have shown high affinity against a specific disease by exerting complementary actions such as dual mode of action, carrier or barrier crossing attributes, cellular subclass targeting, or other complementary actions (Fig. 2) [43].
Fig. 2.
Hybrid multitargeted therapeutic compounds against multifactorial character of AD
Various Hybrid Therapeutic Compounds Targeting AD
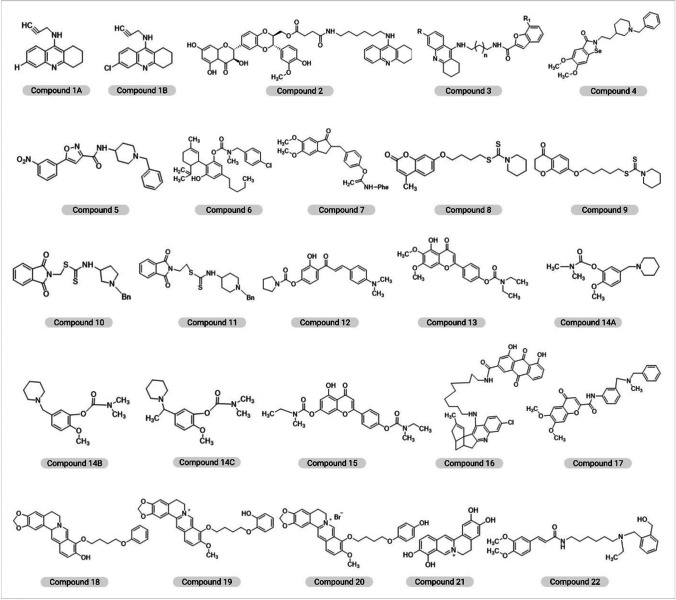
Lack of disease-altering medications to treat AD has generated a flurry of research and the numerous studies are under-going for the development of multi-target therapies in order to tackle the complications occurring during AD progression [44]. Tacrine (TAC), donepezil (DP), and rivastigmine (RSM) are some of the most widely explored drugs that can be conjugated or hybridized to target additional AD targets. The chemical structure of hybrid compounds along with their serial number is depicted in Fig. 3.
Fig. 3.
The chemical structure of hybrid compounds along with their serial number
Tacrine-Conjugated Hybrids
Acetylcholine (ACh) is a neurotransmitter that encodes memory, and a reduced Ach activity is indicative of the severity of AD [45–47]. TAC is a FDA-approved acetylcholinesterase inhibitor (AChEI) for the management of AD [48]. Even though TAC possesses various adverse effects, it is directed to patients suffering from AD for its enhanced ability to block AChE [49]. Moreover, to overcome the hepatotoxicity and neurotoxicity issues associated with TAC, its hybrid compounds are synthesized.
The first TAC-based hybrids were utilized via THA-propargylamine (compound 1), a compound containing a propargylamine group for neuroprotective effects. The THA moiety and the propargylamine in combinations were used as a novel heterodimers for enhancing the AChEI activity and limiting the hepatotoxicity, making it as a potential candidate for treating AD [50]. Chen et al. designed and prepared another hybrid (compound 2) combining THA and silibinin via silibinin (silymarin complex) having anti-inflammatory, anti-cancer, and neuroprotective and hepatoprotective properties. Compound 2 displayed potent cholinesterase inhibitory activity but appears less potent AChEI than THA, though it was able to downregulate AchE activity with neuroprotective effects and reduced hepatotoxicity, both in vitro and in vivo [51]. Tang et al. developed an avant-garde hybrid of oxoisoaporphine and THA, possessing Aβ aggregation and anticholinergic activity for self or induced AChEI activity. Oxoisoaporphine alkaloids isolated from the rhizome of Menispermum dauricum showed high AChEI inhibitory activity and better selectivity of AChE over BChE. An amino alkyl ether comprised of a secondary amine and amide bond was connected using spacer, THA fragment with the oxoisoaporphine moiety [52]. Eriksen et al. synthesized novel multifunctional drug candidates by combining THA with flurbiprofen, a non-steroidal anti-inflammatory drug (NSAID) reduced the production of Aβ40-42 via flurbiprofen that targets the complex, γ-secretase, responsible for the formation of Aβ from APP [53]. Fancellu et al. synthesized another novel hybrid by coupling TAC to benzofuran (BF) derivatives. The BF derivatives imparts the conjugate molecules have ability to inhibit AChE and Aβ peptide aggregation. Moreover, it improved the ability to chelate metals ( Cu2+ and Fe2+) and associated extra antioxidant activity, for the hybrids with hydroxyl substitution. The novel TAC-BF hybrids (compound 3) enhanced AChEI activity and significantly prevented self- and Cu-mediated Aβ aggregation which is dependent on linker size and substituent groups of each moiety along with neuroprotective effects [54].
Ferulic acid (FA), an antioxidant of natural origin found in esterified forms with saccharides, sterols, and lignin, is taken up via the gastrointestinal tract, metabolized by the liver. Moreover, it makes THA-FA derivative moderately antioxidant and a potent reversible, non-competitive AChEIs [55]. Rodriguez-Franco et al. synthesized a novel THA melatonin derivative as a potential multi-targeting drug ligand that could be utilized in the treatment of AD. Melatonin is a pineal neurohormone that not only possesses potent antioxidant activities and aids in scavenging ROS but also provide protection against Aβ-triggered apoptosis in microglial cells [56]. Furthermore, antioxidant capacities were also tested in an oxygen radical absorbance capacity assay using fluorescein. The compound developed by the group act as a strong inhibitor of human AChE and showed high oxygen radical absorbance capacity. Thus, various TAC-based hybrids exhibited therapeutic activities with multiple relevant properties and could be considered potential multitargeting anti-AD agents.
Donepezil-Based Hybrids
The AChEI boosts brain cholinergic neurotransmission by increasing endogenous ACh levels. DP (2-((1-benzylpiperidin-4-yl)methyl)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one) is one of the most effective and well-known FDA-approved AChEI [57]. DP constitutes of dimethoxy indanone and a methylene linker that connects it to N-benzylpiperidine. It demonstrates AChEI activity, anti-Aβ aggregation, and antioxidant as well as metal chelating activities [58]. Luo et al. investigated a new class of chemicals by combining DP, the cholinesterase inhibitor with ebselen, an antioxidant to generate multi-target-directed ligands (MTDLs) against AD. An AChEI, compound 4 (IC50 = 0.042 μM for Electrophorus electricus AChE and IC50 = 0.097 μM for hAChE), has been discovered to be a strong BChE inhibitor (IC50 = 1.586 μM) having H2O2 and peroxynitrite scavenging activity, glutathione peroxidase-like activity (νo = 123.5 μM min−1), and found to be a substrate of class TrxR with no issues. Compound 4 potentially permeated the CNS, showed in an in vitro BBB model [44]. Arce et al. used a linker that is biocompatible, named L-glutamic acid, a novel multifunctional DP-based derivative for the treatment of AD. The distance between the αNH and the γCO2H makes L-glutamic acid a good choice to permit simultaneous interaction between two primary sites, catalytic active site (CAS) and peripheral anionic site (PAS). Cholinesterase inhibitors significantly displace propidium iodide from AChE for inhibition of amyloid aggregation and penetrate brain and improves cell viability, in vitro. They also inhibit human AChE and BChE, protect neurons from mitochondrial free radical damage, and are likely to enter the CNS through passive diffusion [59]. In another study Monjas et al. elucidated a new DP-based L- and D-glutamic acid derivative exhibiting potential neuroprotective effects that combat oxidative stress caused by a combination of rotenone and oligomycin A or oxygen and glucose deficiency [60]. In a recent investigation, Saeedi et al. synthesized N(1benzylpiperidin4yl)5 aryl isoxazole 3 carboxamide derivatives and tested it against AChE and BChE. Based on the in vitro results, compound 5 appears most potent inhibitor of AChE (IC50 = 16.07 μM) and BChE (IC50 = 15.16 μM) and exhibited selective anti-AChE activity (IC50 = 23.63 M). Thus, aryl isoxazole benzylpiperidine-based compounds could be considered potential agents for the treatment of AD [61]. A new class of multifunctional hybrids has been developed and tested by Piemontese et al. that could be potential agents for AD. These are based on the structure of the DP drug that imitates the structure of DNP by conjugating a benzylpiperidine/benzylpiperazine fraction with bioactive heterocyclics (benzimidazole or benzofuran). These endowed the hybrids with additional features like inhibition of Aβ peptide aggregation, antioxidant activity, and metal chelation. Altogether, the compounds displayed the well ability to inhibit AChE (IC50 = 4.0–30.0 μM) [62]. Camps et al. created some DP-TAC hybrids that link with AChE’s active, peripheral, and mid gorge binding sites all at the same time. AChE, BChE, and Aβ-aggregation influenced by AChE were showed inhibited by these hybrids. The hybrids of DP-TAC were made by joining 6 chloro-TAC with the indanone fraction of DP and they appear more potent than their parent compounds in inhibiting hAChE [63].
Carbamate-Conjugated Hybrids
Carbamates (CM) are an important compound due to their superior chemical stability owing to the carbamic group, and ability to increase the permeability of biological membranes. It serves as a crucial structural motif in several licensed medicines and pro-drugs. Recently, studies reported significant application in their usage for developing agents with potential to target their CM moiety. During the process of enzymatic inhibition carbamic group binds to the CAS triad in the active site of AChE to release the phenolic counterpart which exhibits therapeutic potential against AD attributed to the antioxidant mediated neuroprotective activities against AD [64].
Jiang and colleagues investigated the binding ability of a CM fragment to the active site of BChE through structural splicing approach which is based on docking. Based on the aforementioned approach, seventeen new compounds were generated via structural re-assembly but compound 6 was the only compound that exhibited a highly selective BChE inhibitory activity. It possesses the ability to penetrate BBB and offers harmless, neurological protection, antioxidant, and pseudo-irreversible BChE inhibition. In-vivo results revealed that compound 6 significantly reduced scopolamine-induced cognitive impairment and recuperated the Aβ1-42 (icv)-impaired cerebral function. Thus, compound 6 exhibited better efficacy than DP with a decent anti-amyloidogenic effect, and so it could be potentially used as a potential therapeutic agent in AD [65]. In another investigation, Gargari et al. designed a new set of indanone-CM hybrids based on the pharmacophore hybridization technique. These compounds were investigated in order to inhibit AChE and BChE, and it was observed that the compound 7 displayed the highest AChEI activities through reversible partial non-competitive inhibition and also act as a potent Aβ1-40 aggregation inhibitor [66]. Jiang et al. designed coumarin-dithiocarbamate hybrid as versatile compound for AD. Compound 8 is a type of variegated inhibitor that concomitantly interacts with the CAS and PAS in order to hinder AChE and exhibited potent inhibition of Aβ aggregation. Moreover, it possesses the ability to chelate specific metals, better BBB permeability, and reduced neurotoxicity, both in vitro and in vivo and thus may be promising compound in the quest for drugs against AD [67]. Jiang et al. developed a group of hybrids acting as unique multifunctional AChE inhibitors by attaching chromanone to dithiocarbamate moieties using flexible linkers to inhibit self- and AChE-induced aggregation of Aβ. Moreover, the potential to infiltrate the BBB and reduce neurotoxicity in neural cells highlighted the importance of compound 9 as a potential drug against AD [68]. In another investigation, inhibitory potentials of a novel set of phthalimide-dithiocarbamate hybrids were examined by Asadi et al. against AChE and BChE in vitro. The anti-cholinesterase activity of compounds 10 and 11 could also possess properties similar to drugs and cross the BBB, thereby demonstrated the capability to be used in AD [69].
Rivastigmine-Based Hybrids
Rivastigmine (RSM) has received approval from the FDA for utilization as a transdermal patch for the management of AD at a mild, moderate, and severe stage. It has both AChE and BChE inhibitory activity. Moreover, with severity of the disease stages, a surge in the BChE levels in the temporal cortex and hippocampus is observed in people with AD, however AChE activity decreases. Therefore, an inhibitor which exhibit effect on both will increase ACh levels drastically. Considering mechanism of action, it behaves similarly to ACh as it attaches to both the anionic and esteratic sites of AChE. Instead of dissociating immediately post hydrolysis, which happens in ACh, RSM gets hydrolyzed leaving the esteratic site of AChE carbamylated for a period of time, thereby inhibiting the enzyme. It is also known to inhibit the G1 enzymatic form of AChE, which is typically prevalent in the brains of AD patients [70].
RSM-caffeic acid and RSM-FA hybrids were developed, synthesized, and assessed in vitro as potential multifunctional medicines for AD by Chen et al. The newly produced hybrids showed antioxidant and neuroprotection activities, and also upregulated ChE inhibition but simultaneously decreased the aggregation of Aβ [71]. In another study, Xiao et al. developed and examined therapeutic potential of 4′-amino chalcone-RSM hybrids as multifunctional medicines in AD therapy. Compound 12 inhibited AChE and substantial anti-oxidative activity with reduced hepatotoxicity and permeated through the BBB in vitro. Furthermore, it also exhibited self-induced Aβ1-42 anti-aggregation properties as well as inhibited Aβ1-42 aggregation, induced due to Cu2+, by selectively acting as a MAO-B inhibitor and metal chelator [72]. Sang et al. reported that scutellarin-RSM hybrids exhibited cholinergic, antioxidant, bio-metal chelating, and neuroprotective properties against AD. Certain types of scutellarein carbamate derivatives were made using MDLS and the compound 13 exhibited dual inhibition of AChE and BChE, bio metal-chelating properties, antioxidant properties, and also provided neuroprotection against cell injury induced by H2O2 using in vitro murine model [73]. Li et al. created a novel series of 2-methoxy-phenyl dimethyl-carbamate derivatives to evaluate site-activated MTDLs on the basis of curcumin and RSM. Compound 14A, compound 14B, and compound 14C exhibited dual AChE and BChE inhibition with IC50 value less than a micromole against self-aggregation of Aβ. Among the compounds developed, compound 14B showed the maximum competency for AChE inhibition which was about 20-times more when compared to that of RSM and the hydrolysate of 14B exhibited the potential for Cu2+ and Fe3+ chelation in vitro [74]. A multimodal drug, Ladostigil (TV3326) [(N-propargyl-(3R) aminoindan-5yl)-ethyl methyl carbamate], was combined with rasagiline, an anti-Parkinsonian drug, and specific MAO-B inhibitor and showed that hybrid compounds potentially inhibited the ChE activity of RSM [75]. Sang et al. developed novel apigenin-RSM hybrid via MTDLs approach owing to antioxidant potency and a reversible huAChE and huBChE inhibitory potential using in vitro model. Compound 15 also acts as a selective metal chelator, exhibited self-mediated and Cu2+-mediated Aβ1-42 anti-aggregation property, causing suppression of huAChE-mediated induced Aβ1-40 aggregation. Moreover, compound 15 displayed neuroprotective and hepatoprotective activity, favorable for BBB infiltration in vitro and drug-like properties, thus could be used as a potential agent to target numerous AD-associated factors [76].
Physostigmine-Conjugated Hybrids
Physostigmine (PSM) or Eserine, originally isolated from Calabar beans has been found useful in AD due to its inhibitory actions on AChE. Potent novel analogs of PSM showing AChE inhibitory property (IC50 = 0.14 nM) were developed wherein alkoxy groups were utilized to attach N-methyl-N-(3-carbamoyloxyphenyl) methylamino derivatives with the tertiary amino nitrogen of parent PSM [77]. In addition to AChE inhibitory actions, they also showed the potential to improve memory [78]. Tolserine and phenserine are some of the effective ChE inhibitor derivatives.
A chemical derived from PSM, phenserine ((-)-eseroline phenyl carbamate) appears an effective, non-competitive, long-acting, and selective inhibitor of AChE [79]. Klein et al. reported that phenserine inhibits AChE and reduces the production of APP through interaction with a regulatory element in the 5′-UTR region APP gene to reduce the efficiency with which APP mRNA translates into a protein, Aβ. This process is mediated by an interaction with Fe3+ or an Fe3+-responsive element and it impact the formation of Aβ in vivo and in vitro [80].
The only difference between tolserine and phenserine is the 2-methyl substitution on the phenyl carbamoyl molecule. Mehta et al. showed that tolserine was 200 times more selective for hAChE than BChE [81]. Tolserine showed the IC50 value of 0.01 µM against AChE in human erythrocytes [82]. Eu et al. found the IC50 value using Ellman Technique was 0.0103 µM against the hAChE in RBCs [83]. It has also found that the potency of tolserine to human AChE is much higher than phenserine or PSM [84].
Galantamine-Based Hybrids
Galantamine (GAL), a tertiary alkaloid of natural origin acts as a reversible, competitive inhibitor of the AChE enzyme. It is approved by FDA in year 2001 for use in the management of mild to moderate cognitive impairment in AD patients. GAL potentially inhibits the AChE in the synapse, hence enhances the function and signaling of the cholinergic neurons. GAL found to influence the progression of the disease and facilitates retaining the functions of the cholinergic neurons [85]. The efficacy of GAL is often compromised by its adverse effects and limited success in preventing the worsening of a patient’s condition [86].
Curcumin (CU), a natural polyphenolic compound chemically known as diferuloyl methane has garnered attention for its therapeutic potential in neurodegenerative diseases including AD. It possesses numerous benefits such as delayed degradation of neurons, decreased Aβ plaques, metal-chelation, antioxidant, and reduced microglia formation and anti-inflammation, which makes it as a potential candidate in improving cognitive features and curtailing pathogenesis in AD [87]. Stavrakov et al. developed a hybrid compound composed of GAL core combined with CU fragments to obtain the synergistic effect. Moreover, CU can attach to the oligomers of Aβ and prevent plaque formation. A GAL-CU hybrid was synthesized as a new non-toxic AChEI with increased antioxidant activity that served as a lead compound and explored for therapeuric potential in AD [88, 89]. GAL attaches properly over the AChE binding gorge but the size does not seem apt enough for occupying it entirely. The peptide of Aβ attaches with the PAS at the entryway of the binding site of AChE and induces amyloid plaque production; however, PAS inhibition thwarts Aβ aggregation induced by AChE. Stavrakov et al. investigated the potential of a set of GAL-camphane hybrids in AD which act as AChEIs. Camphane is a large component that adheres to the wide opening of the gorge that is made with varying lengths of linkers attached to it, therefore the compound could attach to the gorge. The camphene fragments belonging to the best binders attach in the same position, closely located to the PAS and the site where Ω-loop of Aβ attaches to AChE. These hybrids penetrated through the BBB via passive diffusion and appears devoid of neurotoxicity, even at the inhibitory concentrations [90].
Rhein/Huprine-Conjugated Hybrids
According to Viayna et al. huprine and rhein were linked via a distinct length alkyl or aryl alkyl chain (5 to 11 carbon atoms). Both hAChE and hBChE were inhibited by the synthesized huprine-rhein hybrids, with the value of IC50 in the nanomolar and sub-micromolar to low micromolar ranges, respectively. The novel rhein-huprine hybrid compounds were physiologically assessed against AChE, BChE, dual Aβ42, BACE-1, and anti-tau accumulating properties in E. coli. The hybrids comprised of a rhein and a huprine Y structure joined through penta- to un decamethylene or 1,4-phenylene-bis(methylene) linkers. Compound 16 exhibited disease-modifying anti-Alzheimer’s therapeutic candidate with hAChE (IC50 = 2.39 nM), hBChE (IC50 = 513 nM), BACE-1 (IC50 = 80 nM), and 43% accumulation of Aβ42 at 10 μM. In vivo results showed that, it also inhibited Aβ-induced synaptic loss of protein and lowering of central Aβ in APP-PS1 mice [91]. Pérez-Areales et al. developed similar type of multitarget hybrid molecules comprised of rhein with a unit of the effective AChEI, huprine Y. In vitro studies revealed that the hybrid compound exhibited an intriguing multi-targeting property, including cholinergic activity via inhibition of hAChE (IC50 = 3.60 nM); hBChE (IC50 = 620 nM), and anti-aggregating activity for Aβ42 and tau (48% and 30% inhibition at 10 μM, respectively), in a cell-based assay utilizing E. coli. This compound also inhibited human BACE-1 (IC50 = 120 nM), the enzyme represent rate-determining stage of Aβ production from APP, leading to reduced level of Aβ in a transgenic murine strain of AD. The 3′-basicity huprine component is crucial for the inhibition of AChE and BACE-1. The huprine moiety of this hybrid reacts with the catalytic site of both AChE and BACE-1 when protonated at physiological pH. It allows cation–π interactions with Trp86’s indole ring and Tyr337’s benzene ring. It also forms a salt bridge with the catalytic dyad’s Asp32 and Asp228 residues in BACE-1 [92]. Furthermore, the role of different hybrid therapeutic compounds for the management of AD is discussed in Table 1.
Table 1.
Role of different hybrid compounds against AD
| Hybrid compound | AChE inhibitor | β-amyloid antiaggregation | Antioxidant | Other activities | IC50 value(for AChE) | Clinical study | References |
|---|---|---|---|---|---|---|---|
| N-(prop-2-yn-1-yl)-1,2,3,4-tetrahydroacridin-9-amine (Cpd1A) | ✔□ | BChE inhibitory activities | 51.3 nM | In vitro | [50] | ||
| 6-Chloro-N-(prop-2-yn-1-yl)-1,2,3,4-tetrahydroacridin-9- amine (Cpd1B) | ✔□ | BChE inhibitory activities | 11.2 nM | In vitro | [50] | ||
| Mixture of silibinin hemisuccinate and 6- aminohexamethylene tacrine (N1 -(1,2,3,4-tetrahydroacridin-9- yl)hexane-1,6-diamine)Cpd2 | ✔□ | ✔□ | BChE inhibitory activities | 53.9 nM | In vivo, In vitro | [51] | |
| Oxoisoaporphine-tacrine | ✔□ | ✔□ | NA | nM range (41–57 nM) | In vitro | [52] | |
| Tacrine–benzofuran hybrid Cpd 3 | ✔□ | ✔□ | ✔□ | Metal chelation activity | 38.6 nM | In vitro | [54] |
| Tacrine-melatonin hybrid | ✔□ | ✔□ | Able to cross BBB | 0.008 nM(40 000-fold more potent than tacrine) | In vitro | [56] | |
| Tacrine-ferulic acid hybrid | ✔□ | ✔□ | ✔□ | Inhibition of the PAS of AChE BChE inhibitory activities | 4.4 nM | In vitro | [55] |
|
Donepezil and Ebselen hybrid Cpd 4 |
✔□ | ✔□ | ✔□ | Butyrylcholinesterase inhibitor (IC50 = 1.586 μM), peroxynitrite scavenging activity and glutathione peroxidase-like activity (ν0 = 123.5 μM min–1) | 0.097 μM | In vitro | [44] |
| DNP based L-glutamic acid hybrid | ✔□ | ✔□ | ✔□ | BChE inhibitory activities, BBB permeation ability | 0.10–0.53 μM | In vitro | [59] |
| N-Cbz-L-Glu(OEt)-[NH-2-(1-benzylpiperidin-4-yl)ethyl] (L-3) | ✔□ | Protected rat hippocampal slices against oxygen–glucose deprivation, becoming promising anti-Alzheimer's and anti-stroke lead compounds | 4.99 µM | In vitro | [60] | ||
| N-Cbz-L-Glu(OEt)-[NH-2-(1-benzylpiperidin-4-yl)ethyl] (L-1) | ✔□ | Blocks the voltage-dependent calcium channels | 0.53 µM | In vitro | [60] | ||
| Donepezil-N(1benzylpiperidin4yl)5 aryl isoxazole 3 carboxamide derivative | ✔□ | ✔□ | BChE inhibitory activities | 16.07 μM | In vitro | [61] | |
| Donepezil-tacrine hybrid | ✔□ | ✔□ | BChE inhibition | Subnanomolar or low nanomolar range | In vitro and in silico | [63] | |
| Donepezil-Benzylpiperidine hybrid | ✔□ | ✔□ | ✔□ | Tau hyperphosphorylation inhibition, metal chelation activity | 4.0–30.0 μM | In silico | [62] |
| Carbamate derivative Cpd 6 | ✔□ | ✔□ | ✔□ | Penetrates BBB, offers benign safety, neuroprotection, and pseudo-irreversible BChE inhibition | 5.3 nM (for BChE) | In vivo and in silico | [65] |
| Indanone–carbamate hybrid Cpd 7 | ✔□ | ✔□ | NA | 4.64 μM | In vitro and in silico | [66] | |
| Coumarin-dithiocarbamate hybrid Cpd 8 | ✔□ | ✔□ | Metal-chelating ability, good BBB permeability and low toxicity on SH-SY5Y neuroblastoma cell | 0.027 μM | In vitro and in vivo | [67] | |
| Chromanone-dithiocarbamate hybrid Cpd 9 | ✔□ | ✔□ | Ability to penetrate the BBB and low neurotoxicity in SH-SY5Y cells | 0.10 μM | In vitro, in vivo and in silico | [68] | |
| Phthalimide-dithiocarbamate hybrid Cpd 10 | ✔□ | Anti BChE activity, possesses drug-like properties and able to cross the BBB | 4.6 μM | In vitro and in silico | [69] | ||
| 4′-aminochalcone-revastigmine hybrid | ✔□ | ✔□ | ✔□ | Selective monoamine oxidase B inhibitor and a selective biometal chelator | 4.91 μM | In vitro | [72] |
| Scutellarein carbamate derivative Cpd 13 | ✔□ | ✔□ | Bio-metal chelating and neuroprotective properties | 0.57 μM | In vitro | [73] | |
| 4′-aminochalcone-revastigmine hybrid Cpd 12 | ✔□ | ✔□ | ✔□ | Selective monoamine oxidase B inhibitor (IC50 = 0.29 μM) and a selective biometal chelator | 4.91 μM | In vitro | [72] |
| 2-methoxy-phenyl dimethyl-carbamate derivative Cpd 14B | ✔□ | ✔□ | Potent ABTS.+ scavenging and moderate copper ion chelating activity | 0.097 μM | In vitro | [74] | |
| Apigenin-rivastigmine hybrid Cpd 15 | ✔□ | ✔□ | ✔□ | Remarkable dyskinesia recovery rate and response efficiency | 6.8 μM | In vitro and in vivo | [76] |
| Phenserine | ✔□ | ✔□ | NA | 22 nM | In vitro and in vivo | [80] | |
| Tolserine | ✔□ | NA | 0.01 µM | In vivo | [82] | ||
| Galantamine (GAL) and curcumin (CU) hybrid | ✔□ | ✔□ | NA | 7.91 to 52.53 µM | In vitro | [88, 89][87] | |
| Galantamine-camphane hybrid | ✔□ | ✔□ | NA | 0.0029–0.0099 µM | In silico | [90] | |
| Cpd 16 | ✔□ | ✔□ | Tau hyperphosphorylation inhibition | 2.39 nM | In vivo | [91] | |
| Naphthyridine- and thienopyridine-based rhein-huprine hybrids | ✔□ | ✔□ | ✔□ | Tau hyperphosphorylation inhibition | 3.60 nM | In vitro | [92] |
Novel Hybrid Therapeutic Compounds Targeting AD
Along with the aforementioned hybrid therapeutic complexes, also plenty of new hybrid compounds have been synthesized exhibiting potential therapeutic efficacy in the management of AD development (Table 2). The activities of these novel hybrid therapeutic compounds have been discussed in succeeding paragraphs.
Table 2.
Novel hybrid therapeutic compounds against AD
| Hybrid compound | AChE inhibitor | β-amyloid antiaggregation | Antioxidant | Other activities | IC50 value | Clinical study | References | |
|---|---|---|---|---|---|---|---|---|
| Amentoflavone | ✔□ | NA | 0.26 µM | In vitro | [93] | |||
| N-(3-((Benzyl(methyl)amino)methyl)phenyl)-6,7-dimethoxy-4-oxo-4H-chromene-2-carboxamide Cpd 17 | ✔□ | NA | 4.5 µM | In vitro and in silico | [94] | |||
| Cpd18, berberine linked to phenol by 4-carbon spacers | ✔□ | NA | 0.097 µM | In vitro and in silico | [99] | |||
| Berberine-pyrocatechol hybrid (compound 19) | ✔□ | NA | 0.123 µM | In vitro | [100] | |||
| Berberine-hydroquinone hybrid (compound 20) | ✔□ | ✔□ | ✔□ | NA | 0.460 μM | In vitro | [100] | |
| Ber-D Cpd 21 | ✔□ | Cu chelation, reduces cellulo multifaceted toxicity in AD | NA | In vitro and in silico | [101] | |||
| Bis(9)-( −)-Meptazinol (B9M) | ✔□ | ✔□ | NA | 3.9 nM | In vitro | [102] | ||
| Ferulic acid-memoquin hybrids, Cpd 22 | ✔□ | ✔□ | ✔□ | Can cross the BBB | 3.2 μM | In vitro | [103] | |
The biflavonoids have been tested by Choi et al. and the result showed that amentoflavone (IC50 = 0.26 µM) significantly inhibited Aβ1-42 fibrillization most efficiently. According to the structure–activity relationship analysis, the –OH groups of biflavonoid molecules are crucial in their chemical interaction with the active course of Aβ1-42 fibrillization. Amentoflavone interrupted the granular shape of formed Aβ1-42 threads, causing them to form nebulous Aβ1-42 aggregates. Many such consequences revealed that amentoflavone seems to have the most potent action across equally Aβ1-42 fibrillization suppression and fully developed Aβ1-42 fibril disaggregation, indicating that it could be used to treat the disease [93]. In another study, Estrada-Valencia et al. performed a funnel-type screening of many CNS-permeable flavonoid-DBMA hybrids and found that compound 17 showed the MTD-profile in human AChE, hLOX-5, hBACE-1, and σ1R (IC50 (hAChE) = 4.5 µM; IC50 (hLOX-5) = 30 µM; IC50 (hBACE-1) = 6.7 µM; and IC50 (σ1R) = 0.5 µM). Compound 17, which could aid neurological rejuvenation and actually hinder neurodegeneration in AD was developed through molecular mechanics simulations to get an effective agent with protein relationship [94].
Berberine (BB), an isoquinoline alkaloid is one of the important ingradient of many conventional Chinese medicines which are used in the treatment of illnesses including inflammatory and diarrheal diseases, high blood pressure, and carcinomas [95–97]. BB has been shown to interfere with AD pathogenic processes through lowering the levels of Aβ by impeding activity of secretase enzymes in the APP pathway, alleviating astrocytosis, mitigating oxidative stress, and hindering neurological exaggeration [98]. Huang et al. developed and tested the inhibitory activity of three courses of its derivatives against AChE. Majority of them have been found powerful AChE suppressors, with IC50 values in the micromolar range. The far more powerful suppressor, compound 18, BB has been connected to phenol by 4-carbon inserts, hindered AChE with an IC50 of 0.097 µM. The derivatives prompted a mixed form of inhibitory activity and engaged among both CAS and PAS, following the kinetic model [99]. Jiang et al. successfully synthesized a sequence of hybrid compounds through reacting BB to Benzene-1,2-diol, N-Acetyl-5-Methoxytryptamine, and FA, agent showed potential for AD, was modified as BB-pyrocatechol hybrid (compound 19) appear more efficient in inhibiting AChE than the parent BB (IC50: 0.123 vs 0.374 μM). Not only that, it was also figured out that the berberine-hydroquinone hybrid (compound 20) showed greater antioxidant activities and was also able to inhibit AChE (IC50 of 0.460 μM), with an additional feature of inhibiting Aβ aggregation. The location of phenolic hydroxyl over the benzene ring in compound 20, a hydroquinone-BB derivative, tends to affect the chemical’s potential to block Aβ aggregation, similar to its critical role in inhibiting AChE and BChE [100]. Recently, Rajasekhar et al. reported a multifunctional natural substance derived from BB (Ber-D) that reduces cellulo multifaceted toxicity in AD. The structural characteristics of polyphenol Ber-D (compound 21) have also presented to its effective Cu2+ chelation along with redox cycle arresting properties, preventing the formation of ROS. In silico simulations show that Ber-D suppresses both metal-dependent and metal-independent Aβ aggregation and also prevented mitochondrial malfunction and neuronal toxicity, leading to early apoptosis. Ber-D can be a promising therapeutic option in mitigating complex Aβ toxicity in AD owing to these significant multifunctional properties [101].
Shi et al. examined the effect of Bis(9)-( −)-Meptazinol (B9M), a unique putative multifunctional binding AChEI, on memory and cognitive abilities in the APP/PS1 mouse model of AD. In the Morris water maze test, nest-building test, and new object recognition experiment, B9M therapy markedly enhanced the cognitive ability of APP/PS1 transgenic mice. In early investigations, B9M was banded over CAS and PAS of AChE and showed an efficient AChE inhibitory activity (IC50 = 3.9 nM). B9M also inhibited the AChE-triggered Aβ accumulation, suggesting that it could be useful to treat AD [102].
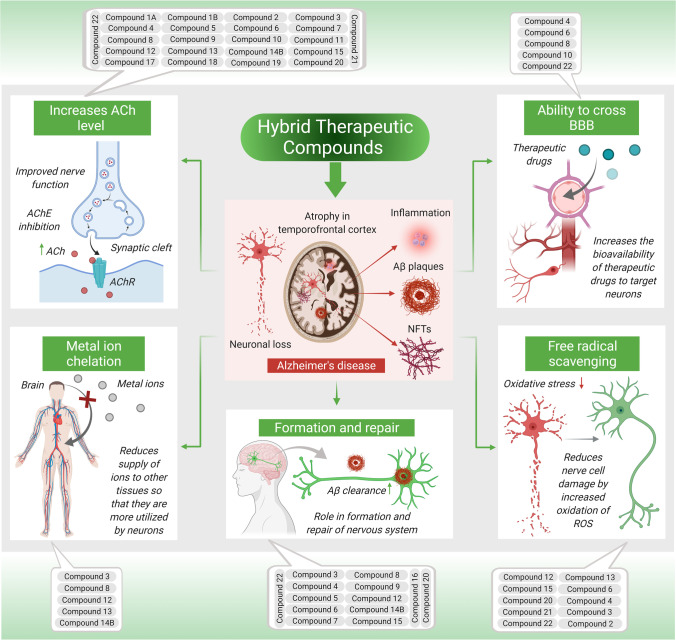
Pan et al. synthesized, and evaluated a new class of FA-memoquin hybrids as multifaceted medicines for AD. The in vitro experiments revealed that the majority of the compounds exerted a significant inhibitory effect on AChE (IC50 of 3.2–34.7 μM). Especially, compound 22 showed significant and found superior in inhibiting AChE (IC50 = 3.2 μM) and the accumulation of Aβ1-42 (30.8%). It was also an exceptional antioxidant and neuroprotectant. Additionally, compound 22 could cross the BBB in vitro while demonstrating that the benzylamines group exerted important outcome on the antagonistic effect of AChE, usually, the -CH3 group at the 2′-position of benzylamine exhibited inhibition, and the substituted groups possessing an N-ethyl group indicated improved AChE inhibition than that with an N-methyl group. Furthermore, the inhibitory activity was enhanced with the extension of the methylene linker [103]. Furthermore, as discussed in aforementioned sections, the specific role of each hybrid therapeutic compound against AD is illustrated in Fig. 4.
Fig. 4.
Illustration shows specific role of each hybrid therapeutic compound against AD
Conclusion and Future Perspectives
In summary, the pursuit of an effective and safe medications for AD continue to be a challenge for medicinal chemists and neuroscientists. Since the pathogenesis of AD includes multiple characteristics including dysregulation of diverse molecular targets and biochemical pathways, therefore achieving therapeutic benefits from a single agent and single target remains a challenge with potential therapeutic agents. The use of highly selective drugs targeting AD has made the therapeutic strategies ineffective, leaving the patients with no other options but to use multiple drugs combinations for regulating a diverse series of symptoms associated with the progression of AD. Therefore, a great deal of recent research aimed to the discovery of novel bioactive hybrid compounds targeted towards the unique, diverse, and prospective pathogenic hallmarks of AD. An effective therapy for AD may rely on if the novel developed agent exert the potential for interaction and regulate different molecular targets and simultaneously block or activate numerous biochemically linked pathogenic pathways. Moreover, the reported studies have provided adequate evidence for the favourable outcome of poly-pharmacology in controlling AD pathogenesis. This is more compelling particularly regarding the utility of a single drug showing different modes of action, with reduced toxic effects, metabolic overloads, and drug-drug interactions. In this field, numerous compounds with multi-target profiles have been discovered, and many of them have shown intriguing and promising pharmacological characteristics, making them prospective therapeutic candidates. The hybrid therapeutic complexes are mainly developed with the specific combination of two bioactive pharmacophores to produce homo- and heterodimers with an improved affinity, therapeutic efficacy, biological profile, and additional complementary effects. Thus, many disease-modifying hybrids could be potentially developed into next-generation medicines for AD. The method of designing hybrid molecules has numerous advantages over conventional multitarget drug development methods. In comparison to in silico high throughput screening, hybrid molecules generate quicker results and are also less expensive than fragment-based drug development. Designing hybrid multitargeted therapeutic compounds is thus a prospective approach in developing an effective treatment for AD. Nevertheless, several issues must be addressed, and additional researches should be conducted to develop hybrid therapeutic compounds for clinical usage while keeping other off-target adverse effects in mind.
Acknowledgements
Dr. Niraj Kumar Jha is thankful to Sharda University for the infrastructure and facility. The author would like to thank Deanship of Scientific Research at Majmaah University for supporting this work under project number No. R-2022-68. Kavindra Kumar Kesari and Janne Ruokolainen thanking Aalto University for providing open access support. The authors would like to acknowledge the support from their respective institutes throughout the review writing process.
Abbreviations
- Aβ
β-amyloid
- Ach
Acetylcholine
- AChE
Acetylcholinesterase
- AChEIs
Acetylcholinesterase inhibitors
- AD
Alzheimer’s disease
- ADME
Absorption, distribution, metabolism, and excretion
- APP
Amyloid precursor protein
- B9M
Bis(9)-( −)-meptazinol
- BACE-1
Beta-secretase 1
- BBB
Blood-brain barrier
- BChE
Butyrylcholinesterase
- BF
Benzofuran
- CAM
Camphane
- CAS
Catalytic active site
- ChEs
Cholinesterases
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CU
Curcumin
- DBMA
N,N-dibenzyl(N-methyl)amine
- DNA
Deoxyribonucleic acid
- FA
Ferulic acid
- FDA
Food & Drug Administration
- GAL
Galantamine
- hAChE/huAChE
Human acetylcholinesterase
- IC50
Half-maximal inhibitory concentration
- Icv
Intracerebroventricular
- MAO
Monoamine oxygenase
- MAOB
Monoamine oxidase B
- MTDLs
Multi-target-directed ligands
- MTT
3(4,5Dimethylthiazol2yl)2,5 diphenyltetrazolium bromide
- NFTs
Neurofibrillary tangles
- NSAID
Non-steroidal anti-inflammatory drug
- PAS
Peripheral anionic site
- PLLA
Poly (l-lactic acid)
- PLGA
Poly (lactide-co-glycolide)
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- TAC
Tacrine
- THA
Tetrahydroacridine
- TrxR
Thioredoxin reductases
Author Contribution
AB, AJ, KKK, NKJ, and GMA conceptualized, wrote, and edited the manuscript. AJ, AB, AS, AC, RB, AP, SD, AD, and AP performed the literature survey and drafted and edited the manuscript. NKJ ideated the scheme and performed artwork. AS and AJ drafted the tables. SSD, SO, SKJ, SKS, PKG, JR, and DI significantly helped in revision and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Open Access funding provided by Aalto University.
Data availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors give their consent for publication.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ankit Jana, Arkadyuti Bhattacharjee, Sabya Sachi Das and Niraj Kumar Jha contributed equally to this work.
Contributor Information
Niraj Kumar Jha, Email: nirajkumarjha2011@gmail.com.
Kavindra Kumar Kesari, Email: kavindra.kesari@aalto.fi.
Ghulam Md Ashraf, Email: ashraf.gm@gmail.com.
References
- 1.Bartorelli L, et al. Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer’s disease. Curr Med Res Opin. 2005;21(11):1809–1817. doi: 10.1185/030079905X65655. [DOI] [PubMed] [Google Scholar]
- 2.Stelzmann, R.A., H. Norman Schnitzlein, and F. Reed Murtagh (1995) An english translation of alzheimer’s 1907 paper,“über eine eigenartige erkankung der hirnrinde”. Clinical Anatomy: The Official Journal of the American Association of Clinical Anatomists and the British Association of Clinical Anatomists 8(6): 429–431. [DOI] [PubMed]
- 3.Holtzman DM, Morris JC, and Goate AM (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3(77): 77sr1–77sr1 [DOI] [PMC free article] [PubMed]
- 4.Hurley AC, Wells N. Past, present, and future directions for Alzheimer research. Alzheimer Dis Assoc Disord. 1999;13:S6–10. doi: 10.1097/00002093-199904001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Scheltens P, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 6.Masters CL, et al. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 7.Nicholas LH, et al. Financial presentation of Alzheimer disease and related dementias. JAMA Intern Med. 2021;181(2):220–227. doi: 10.1001/jamainternmed.2020.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pais M, et al. Early diagnosis and treatment of Alzheimer’s disease: new definitions and challenges. Brazilian Journal of Psychiatry. 2020;42:431–441. doi: 10.1590/1516-4446-2019-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias JL (2014) Nanotechnology and drug delivery, volume one: nanoplatforms in drug delivery. Vol. 2 CRC Press
- 10.Suri K, et al. Novel approaches and strategies for biologics, vaccines and cancer therapies. Elsevier; 2015. Advances in nanotechnology-based drug delivery platforms and novel drug delivery systems; pp. 41–58. [Google Scholar]
- 11.Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clin Interv Aging. 2007;2(3):347. [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury R, Grossberg GT. Deciphering Alzheimer’s disease: predicting new therapeutic strategies via improved understanding of biology and pathogenesis. Expert Opin Ther Targets. 2020;24(9):859–868. doi: 10.1080/14728222.2020.1790530. [DOI] [PubMed] [Google Scholar]
- 13.Harilal S, et al. Advancements in nanotherapeutics for Alzheimer’s disease: current perspectives. J Pharm Pharmacol. 2019;71(9):1370–1383. doi: 10.1111/jphp.13132. [DOI] [PubMed] [Google Scholar]
- 14.Nehls M. Unified theory of Alzheimer’s disease (UTAD): implications for prevention and curative therapy. Journal of molecular psychiatry. 2016;4(1):1–52. doi: 10.1186/s40303-016-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman L, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226–234. doi: 10.1602/neurorx.1.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrak RE, Griffin WST. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8(4):369–375. doi: 10.3233/JAD-2005-8406. [DOI] [PubMed] [Google Scholar]
- 18.Anderson BM, et al. MA-[d-Leu-4]-OB3, a small molecule synthetic peptide leptin mimetic, mirrors the cognitive enhancing action of leptin in a mouse model of type 1 diabetes mellitus and Alzheimer’s disease-like cognitive impairment. Int J Pept Res Ther. 2020;26(3):1243–1249. doi: 10.1007/s10989-019-09929-w. [DOI] [Google Scholar]
- 19.Beydoun MA, et al. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. Am J Epidemiol. 2008;168(10):1179–1189. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res. 2008;41(1):69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 21.Viegas-Junior C, et al. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem. 2007;14(17):1829–1852. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- 22.Gediya LK, Njar VC. Promise and challenges in drug discovery and development of hybrid anticancer drugs. Expert Opin Drug Discov. 2009;4(11):1099–1111. doi: 10.1517/17460440903341705. [DOI] [PubMed] [Google Scholar]
- 23.Cavalli A, et al. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51(3):347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 24.Weinstock M. Selectivity of cholinesterase inhibition. CNS Drugs. 1999;12(4):307–323. doi: 10.2165/00023210-199912040-00005. [DOI] [Google Scholar]
- 25.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 26.Serrano-Pozo A, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4(2):131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 28.Greig NH, et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc Natl Acad Sci. 2005;102(47):17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giacobini E. Cholinergic function and Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18(S1):S1–S5. doi: 10.1002/gps.935. [DOI] [PubMed] [Google Scholar]
- 30.Kung HF (2012) The β-amyloid hypothesis in Alzheimer’s disease: seeing is believing. ACS Publications. [DOI] [PMC free article] [PubMed]
- 31.Inestrosa NC, et al. Acetylcholinesterase-amyloid-β-peptide interaction: effect of Congo Red and the role of the Wnt pathway. Curr Alzheimer Res. 2005;2(3):301–306. doi: 10.2174/1567205054367928. [DOI] [PubMed] [Google Scholar]
- 32.Singh M, et al. Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem. 2013;70:165–188. doi: 10.1016/j.ejmech.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 33.Pathan SA, et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Delivery Formulation. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 34.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4(2):191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 35.Fenstermacher JD, Nagaraja T, Davies KR. Blood—Brain Barrier. Springer; 2001. Overview of the structure and function of the blood-brain barrier in vivo; pp. 1–7. [Google Scholar]
- 36.Simonian N, Coyle J. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36(1):83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield DA. Amyloid β-peptide [1-42]-associated free radical-induced oxidative stress and neurodegeneration in Alzheimer’s disease brain: Mechanisms and consequences. Curr Med Chem. 2003;10(24):2651–2659. doi: 10.2174/0929867033456422. [DOI] [PubMed] [Google Scholar]
- 38.Greig NH, Giacobini E and Lahiri DK (2007) Editorial [hot topic: advances in Alzheimer therapy: development of innovative new strategies (Guest Editors: Nigel H. Greig, Ezio Giacobini and Debomoy K. Lahiri)]. Current Alzheimer Research 4(4): p. 336–339. [DOI] [PubMed]
- 39.Katz B, Miledi R. Ionic requirements of synaptic transmitter release. Nature. 1967;215(5101):651–651. doi: 10.1038/215651a0. [DOI] [PubMed] [Google Scholar]
- 40.Small DH. Dysregulation of calcium homeostasis in Alzheimer’s disease. Neurochem Res. 2009;34(10):1824–1829. doi: 10.1007/s11064-009-9960-5. [DOI] [PubMed] [Google Scholar]
- 41.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7(4):295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 42.Leon R, Garcia AG, Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med Res Rev. 2013;33(1):139–189. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- 43.Michalska P, et al. Novel multitarget hybrid compounds for the treatment of Alzheimer’s disease. Curr Top Med Chem. 2017;17(9):1027–1043. doi: 10.2174/1568026616666160927154116. [DOI] [PubMed] [Google Scholar]
- 44.Luo Z, et al. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J Med Chem. 2013;56(22):9089–9099. doi: 10.1021/jm401047q. [DOI] [PubMed] [Google Scholar]
- 45.Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14(11):494–501. doi: 10.1016/0166-2236(91)90061-X. [DOI] [PubMed] [Google Scholar]
- 46.Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. J Neurophysiol. 1992;67(5):1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- 47.Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Rev. 1997;23(1–2):28–46. doi: 10.1016/S0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 48.Tumiatti V, et al. Tacrine derivatives and Alzheimer’s disease. Curr Med Chem. 2010;17(17):1825–1838. doi: 10.2174/092986710791111206. [DOI] [PubMed] [Google Scholar]
- 49.Brodaty H (1996) Tacrine in the treatment of Alzheimer’s disease.
- 50.Mao F, et al. Tacrine–propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem. 2015;30(6):995–1001. doi: 10.3109/14756366.2014.1003212. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, et al. Tacrine-silibinin codrug shows neuro-and hepatoprotective effects in vitro and pro-cognitive and hepatoprotective effects in vivo. J Med Chem. 2012;55(11):5231–5242. doi: 10.1021/jm300246n. [DOI] [PubMed] [Google Scholar]
- 52.Tang H, et al. Hybrids of oxoisoaporphine-tacrine congeners: novel acetylcholinesterase and acetylcholinesterase-induced β-amyloid aggregation inhibitors. Eur J Med Chem. 2011;46(10):4970–4979. doi: 10.1016/j.ejmech.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Eriksen JL, et al. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J Clin Investig. 2003;112(3):440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fancellu G, et al. Novel tacrine–benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem. 2020;35(1):211–226. doi: 10.1080/14756366.2019.1689237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109(4):691–702. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Franco MI, et al. Novel tacrine− melatonin hybrids as dual-acting drugs for Alzheimer disease, with improved acetylcholinesterase inhibitory and antioxidant properties. J Med Chem. 2006;49(2):459–462. doi: 10.1021/jm050746d. [DOI] [PubMed] [Google Scholar]
- 57.Cecilia Rodrigues Simoes M, et al. Donepezil: an important prototype to the design of new drug candidates for Alzheimer’s disease. Mini Rev Med Chem. 2014;14(1):2–19. doi: 10.2174/1389557513666131119201353. [DOI] [PubMed] [Google Scholar]
- 58.Sabbagh M, et al. (2012) Novel therapeutics in Alzheimer’s Disease. Hindawi [DOI] [PMC free article] [PubMed]
- 59.Arce MP, et al. Neuroprotective and cholinergic properties of multifunctional glutamic acid derivatives for the treatment of Alzheimer’s disease. J Med Chem. 2009;52(22):7249–7257. doi: 10.1021/jm900628z. [DOI] [PubMed] [Google Scholar]
- 60.Monjas L, et al. Enzymatic and solid-phase synthesis of new donepezil-based L-and D-glutamic acid derivatives and their pharmacological evaluation in models related to Alzheimer’s disease and cerebral ischemia. Eur J Med Chem. 2017;130:60–72. doi: 10.1016/j.ejmech.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 61.Saeedi M, et al. Novel N-benzylpiperidine derivatives of 5-arylisoxazole-3-carboxamides as anti-Alzheimer’s agents. Arch Pharm. 2021;354(3):2000258. doi: 10.1002/ardp.202000258. [DOI] [PubMed] [Google Scholar]
- 62.Piemontese L, et al. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J Enzyme Inhib Med Chem. 2018;33(1):1212–1224. doi: 10.1080/14756366.2018.1491564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camps P, et al. Novel donepezil-based inhibitors of acetyl-and butyrylcholinesterase and acetylcholinesterase-induced β-amyloid aggregation. J Med Chem. 2008;51(12):3588–3598. doi: 10.1021/jm8001313. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh AK, Brindisi M. Organic carbamates in drug design and medicinal chemistry. J Med Chem. 2015;58(7):2895–2940. doi: 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang X, et al. Novel cannabidiol− carbamate hybrids as selective BuChE inhibitors: Docking-based fragment reassembly for the development of potential therapeutic agents against Alzheimer’s disease. Eur J Med Chem. 2021;223:113735. doi: 10.1016/j.ejmech.2021.113735. [DOI] [PubMed] [Google Scholar]
- 66.Shahrivar‐Gargari M, et al. (2021) Hybridization‐based design of novel anticholinesterase indanone–carbamates for Alzheimer’s disease: synthesis, biological evaluation, and docking studies. Archiv der Pharmazie e2000453 [DOI] [PubMed]
- 67.Jiang N, et al. Design, synthesis and biological evaluation of new coumarin-dithiocarbamate hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;146:287–298. doi: 10.1016/j.ejmech.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 68.Jiang N, et al. Novel chromanone-dithiocarbamate hybrids as multifunctional AChE inhibitors with β-amyloid anti-aggregation properties for the treatment of Alzheimer’s disease. Bioinorg Chem. 2019;89:103027. doi: 10.1016/j.bioorg.2019.103027. [DOI] [PubMed] [Google Scholar]
- 69.Asadi M, et al. Design, synthesis, molecular docking, and cholinesterase inhibitory potential of phthalimide-dithiocarbamate hybrids as new agents for treatment of Alzheimer’s disease. Chem Biodivers. 2019;16(11):e1900370. doi: 10.1002/cbdv.201900370. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen K, et al. Evaluation of rivastigmine in Alzheimer’s disease. Neurodegener Dis Manag. 2021;11(1):35–48. doi: 10.2217/nmt-2020-0052. [DOI] [PubMed] [Google Scholar]
- 71.Chen Z, et al. Discovery of novel rivastigmine-hydroxycinnamic acid hybrids as multi-targeted agents for Alzheimer’s disease. Eur J Med Chem. 2017;125:784–792. doi: 10.1016/j.ejmech.2016.09.052. [DOI] [PubMed] [Google Scholar]
- 72.Xiao G, et al. Design, synthesis and biological evaluation of 4′-aminochalcone-rivastigmine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg Med Chem. 2017;25(3):1030–1041. doi: 10.1016/j.bmc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Sang Z, et al. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg Med Chem. 2015;23(4):668–680. doi: 10.1016/j.bmc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Li Y, et al. Design, synthesis and evaluation of rivastigmine and curcumin hybrids as site-activated multitarget-directed ligands for Alzheimer’s disease therapy. Bioorg Med Chem. 2014;22(17):4717–4725. doi: 10.1016/j.bmc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Weinreb O, et al. Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer’s disease drugs. Neurotherapeutics. 2009;6(1):163–174. doi: 10.1016/j.nurt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sang Z, et al. Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Eur J Med Chem. 2020;187:111958. doi: 10.1016/j.ejmech.2019.111958. [DOI] [PubMed] [Google Scholar]
- 77.Recanatini M, Valenti P. Acetylcholinesterase inhibitors as a starting point towards improved Alzheimer’s disease therapeutics. Curr Pharm Des. 2004;10(25):3157–3166. doi: 10.2174/1381612043383313. [DOI] [PubMed] [Google Scholar]
- 78.Batiha GE-S, et al. Physostigmine: a plant alkaloid isolated from Physostigma venenosum: a review on pharmacokinetics, pharmacological and toxicological activities. J Drug Deliv Ther. 2020;10(1-s):187–190. doi: 10.22270/jddt.v10i1-s.3866. [DOI] [Google Scholar]
- 79.Winblad B, et al. Phenserine efficacy in Alzheimer’s disease. J Alzheimers Dis. 2010;22(4):1201–1208. doi: 10.3233/JAD-2010-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klein J. Phenserine. Expert Opin Investig Drugs. 2007;16(7):1087–1097. doi: 10.1517/13543784.16.7.1087. [DOI] [PubMed] [Google Scholar]
- 81.Mehta, M., A. Adem, and M. Sabbagh (2012) New acetylcholinesterase inhibitors for Alzheimer’s disease. Int J Alzheimer’s Dis 2012 [DOI] [PMC free article] [PubMed]
- 82.Luo W, et al. Novel anticholinesterases based on the molecular skeletons of furobenzofuran and methanobenzodioxepine. J Med Chem. 2005;48(4):986–994. doi: 10.1021/jm049309+. [DOI] [PubMed] [Google Scholar]
- 83.Yu Q-S, et al. Long-acting anticholinesterases for myasthenia gravis: synthesis and activities of quaternary phenylcarbamates of neostigmine, pyridostigmine and physostigmine. Bioorg Med Chem. 2010;18(13):4687–4693. doi: 10.1016/j.bmc.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamal MA, et al. Kinetics of human acetylcholinesterase inhibition by the novel experimental Alzheimer therapeutic agent, tolserine. Biochem Pharmacol. 2000;60(4):561–570. doi: 10.1016/S0006-2952(00)00330-0. [DOI] [PubMed] [Google Scholar]
- 85.Scott LJ, Goa KL. Galantamine. Drugs. 2000;60(5):1095–1122. doi: 10.2165/00003495-200060050-00008. [DOI] [PubMed] [Google Scholar]
- 86.Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol Med Rep. 2019;20(2):1479–1487. doi: 10.3892/mmr.2019.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stavrakov G, et al. Galantamine-curcumin hybrids as dual-site binding acetylcholinesterase inhibitors. Molecules. 2020;25(15):3341. doi: 10.3390/molecules25153341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simeonova R, et al. A novel galantamine-curcumin hybrid as a potential multi-target agent against neurodegenerative disorders. Molecules. 2021;26(7):1865. doi: 10.3390/molecules26071865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stavrakov G, et al. Docking-based design and synthesis of galantamine–camphane hybrids as inhibitors of acetylcholinesterase. Chem Biol Drug Des. 2017;90(5):709–718. doi: 10.1111/cbdd.12991. [DOI] [PubMed] [Google Scholar]
- 91.Viayna E, et al. Synthesis and multitarget biological profiling of a novel family of rhein derivatives as disease-modifying anti-Alzheimer agents. J Med Chem. 2014;57(6):2549–2567. doi: 10.1021/jm401824w. [DOI] [PubMed] [Google Scholar]
- 92.Pérez-Areales FJ, et al. Design, synthesis and multitarget biological profiling of second-generation anti-Alzheimer rhein–huprine hybrids. Future Med Chem. 2017;9(10):965–981. doi: 10.4155/fmc-2017-0049. [DOI] [PubMed] [Google Scholar]
- 93.Choi EY, et al. Polyphenolic biflavonoids inhibit amyloid-beta fibrillation and disaggregate preformed amyloid-beta fibrils. Biomolecules & therapeutics. 2020;28(2):145. doi: 10.4062/biomolther.2019.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Estrada-Valencia M, et al. New flavonoid–N, N-dibenzyl (N-methyl) amine hybrids: multi-target-directed agents for Alzheimer’s disease endowed with neurogenic properties. J Enzyme Inhib Med Chem. 2019;34(1):712–727. doi: 10.1080/14756366.2019.1581184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharmaceutica Sinica B. 2012;2(4):327–334. doi: 10.1016/j.apsb.2012.06.003. [DOI] [Google Scholar]
- 96.Ahmed T, et al. Berberine and neurodegeneration: a review of literature. Pharmacol Rep. 2015;67(5):970–979. doi: 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Zou K, et al. Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol Sin. 2017;38(2):157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Z, Wang C, Yang W. Role of berberine in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2016;12:2509. doi: 10.2147/NDT.S114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang L, et al. Synthesis, biological evaluation, and molecular modeling of berberine derivatives as potent acetylcholinesterase inhibitors. Bioorg Med Chem. 2010;18(3):1244–1251. doi: 10.1016/j.bmc.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 100.Jiang H, et al. Benzenediol-berberine hybrids: multifunctional agents for Alzheimer’s disease. Bioorg Med Chem. 2011;19(23):7228–7235. doi: 10.1016/j.bmc.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 101.Rajasekhar K, et al. Antioxidant berberine-derivative inhibits multifaceted amyloid toxicity. Iscience. 2020;23(4):101005. doi: 10.1016/j.isci.2020.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi Y, et al. Bis (9)-(−)-Meptazinol, a novel dual-binding AChE inhibitor, rescues cognitive deficits and pathological changes in APP/PS1 transgenic mice. Transl Neurodegeneration. 2018;7(1):1–11. doi: 10.1186/s40035-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pan W, et al. Design, synthesis and evaluation of novel ferulic acid-memoquin hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. 2016;26(10):2539–2543. doi: 10.1016/j.bmcl.2016.03.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.