Abstract
A gene encoding a putative 150-amino-acid methylglyoxal synthase was identified in Clostridium acetobutylicum ATCC 824. The enzyme was overexpressed in Escherichia coli and purified. Methylglyoxal synthase has a native molecular mass of 60 kDa and an optimum pH of 7.5. The Km and Vmax values for the substrate dihydroxyacetone phosphate were 0.53 mM and 1.56 mmol min−1 μg−1, respectively. When E. coli glycerol dehydrogenase was coexpressed with methylglyoxal synthase in E. coli BL21(DE3), 3.9 mM 1,2-propanediol was produced.
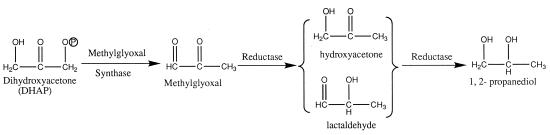
Methylglyoxal synthase, which catalyzes the conversion of dihydroxyacetone phosphate (DHAP) to methylglyoxal and inorganic phosphate, has been found in many organisms, including enteric bacteria (6, 8, 11), some gram-positive bacteria (7), a number of archaebacteria (18), several yeast species (1), and goat liver (19). This enzyme provides bacteria with an alternative to triosphosphate isomerase for metabolizing DHAP. Phosphate acts as an allosteric inhibitor of the enzyme, which suggests that the methylglyoxal bypass may have significant activity under phosphate starvation conditions; methylglyoxal is also known to be cytotoxic, and it has been suggested that methylglyoxal is a growth regulator. In bacteria, this compound may function as an antibiotic; in mammals, it has been implicated in diabetic complications (3). We are interested in the use of metabolic engineering for developing processes for microbial conversion of sugars to diols, such as 1,2-propanediol, in Clostridium acetobutylicum ATCC 824, which is a gram-positive, spore-forming, saccharolytic bacterium that is capable of fermenting a wide variety of sugars. Figure 1 shows the metabolic pathway to 1,2-propanediol from DHAP, an intermediate in sugar metabolism. A number of bacteria, such as Clostridium pasteurianum (17), Klebsiella spp. (4, 21), and Lactobacillus species (24), ferment glycerol and produce many fermentation products, including 1,2-propanediol and 1,3-propanediol. In this study, we overexpressed, purified, and characterized methylglyoxal synthase from C. acetobutylicum ATCC 824 and produced 1,2-propanediol in recombinant Escherichia coli by coexpression of E. coli glycerol dehydrogenase.
FIG. 1.
Metabolic pathways to 1,2-propanediol from DHAP.
Amino acid sequence analysis.
Newly available sequence data from the C. acetobutylicum genome sequence database (9a) made possible identification of a gene coding for a putative methylglyoxal synthase. The amino acids of the predicted gene products deduced from the DNA sequence were similar to the amino acids of E. coli methylglyoxal synthase, which was recently cloned and expressed (22, 27), and other putative methylglyoxal synthases (Fig. 2). A comparison of the sequences of methylglyoxal synthase and related open reading frames from other bacterial species revealed that four aspartic acids (Asp-17, Asp-68, Asp-88, and Asp-98), which were suggested to be involved in protecting the enzyme from the substrate DHAP or reactive intermediates in the catalytic pathway (22), are highly conserved.
FIG. 2.
Alignment of the amino acid sequences of C. acetobutylicum ATCC 824, E. coli, Bacillus subtilis, Haemophilus influenzae, Synechocystis, and Bacillus abortus methylglyoxal synthases. Positions at which more than 50% of the sequences have identical amino acids are shaded.
Cloning and expression of the methylglyoxal synthase gene.
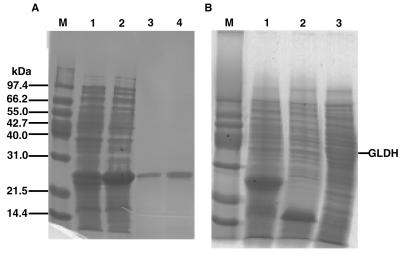
The methylglyoxal synthase gene was cloned by PCR amplification (15) by using a set of appropriate primers. C. acetobutylicum chromosomal DNA was the template used, the oligonucleotide 5′-GAATTCATATGGCACTTATAATGAATAGT was the forward primer, and the oligonucleotide 5′-CCGCTCGAGTTAAAAATTCTGTTTTCTAAT was the reverse primer. An NdeI site 5′ to the methylglyoxal synthase gene and an XhoI site 3′ to the methylglyoxal synthase gene were introduced. The resulting 450-bp DNA fragment was ligated to the corresponding cloning sites of plasmids pET30a and pET15b after digestion with NdeI and XhoI to form pMGS1 and pMGS2, respectively. Sequence integrity was confirmed by DNA sequencing, and no change due to PCR amplification was observed. In plasmid pMGS1, methylglyoxal synthase gene transcription is under control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T7 promoter, and the gene contains the affinity His-tag coding region of the plasmid (the molecular weight of the His-tag fusion methylglyoxal synthase deduced from the methylglyoxal synthase sequence plus the His-tag DNA sequence is 21,969). In pMGS2, methylglyoxal synthase gene transcription is under control of an IPTG-inducible T7 promoter, and the gene has no His-tag (the molecular weight deduced from the methylglyoxal synthase DNA sequence is 15,165). Plasmids pMGS1 and pMGS2 were transformed into E. coli BL21(DE3) (26) and were selected on Luria broth (23) supplemented with kanamycin (50 μg/ml). Addition of 0.4 mM IMPG to liquid cultures resulted in intense protein bands in crude cell extracts that migrated at molecular masses of 25 kDa for BL21(DE3)/pMGS1 cultures and 15 kDa for BL21(DE3)/pMGS2 cultures (Fig. 3) during sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% acrylamide gels (12) which were stained with Coomassie blue R-250. Thus, both construct pMGS1 and construct pMGS2 resulted in overexpression of the methylglyoxal synthase gene in E. coli.
FIG. 3.
SDS-PAGE analysis of fractions from each purification step and total protein. Samples were electrophoresed on a 12% acrylamide gel. The resulting gel was subjected to protein staining. (A) Lane M, molecular weight standards, lane 1, total protein from induced E. coli BL21(DE3)/pMGS1 (12 μg after 4 h of induction with 0.4 mM IPTG); lane 2, soluble protein from E. coli BL21(DE3)/pMGS1 (14 μg); lanes 3 and 4, purified methylglyoxal synthase (1 and 2 μg, respectively). (B) Lane M, molecular weight standards; lane 1, total protein from E. coli BL21(DE3)/pMGS1; lane 2, total protein from E. coli BL21(DE3)/pMGS2; lane 3, total protein from E. coli BL21(DE3)/pGLDH.
Purification of methylglyoxal synthase.
Recombinant methylglyoxal synthase was purified by using the procedure described in the Talon metal affinity resin manual (Clontech, Palo Alto, Calif.). The purification data were based on analysis of a 50-ml culture. Protein concentrations were determined by the method of Bradford (2) by using a Bio-Rad protein assay kit and bovine serum albumin as the standard. E. coli BL21(DE3)/pMGS1 cultures were harvested 4 h after induction with IPTG by centrifugation for 10 min at 4,000 × g. The pellet from a 50-ml culture was resuspended in 4 ml of lysis buffer containing 100 mM Tris-HCl (pH, 8.0), 10% glycerol, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 100 mM NaCl and incubated for 30 min at 4°C in the presence of 0.75 mg of lysozyme per ml. The cells were lysed by sonication (four times, 15 s each) at full power by maintaining the temperature below 8°C with an ice bath. Unlysed cells and cell debris were removed by centrifugation at 12,000 × g for 15 min. The supernatant was mixed with Talon metal affinity resin, gently agitated at 4°C for 30 min, and centrifuged at 700 × g for 5 min. The supernatant was discarded, and the resin was washed four times with 15 ml of lysis buffer. The washed resin was then resuspended in lysis buffer and transferred to a gravity column. The column was washed with 10 ml of washing buffer (0.1 M Tris-HCl, 10 mM imidazole, 10% glycerol, 1 mM PMSF), and the methylglyoxal synthase was eluted with 3 ml of elution buffer (0.1 M Tris-HCl, 10% glycerol, 0.1 M imidazole, 1 mM PMSF). The fractions collected were judged to be homogeneous based on SDS-PAGE results (Fig. 3). Methylglyoxal synthase activity was assayed by using the procedure described previously (8). The recombinant methylglyoxal synthase was readily purified to homogeneity (>90%) (Table 1). The overall yield of the purification procedure was 85%, which corresponded to 1.4 mg of purified methylglyoxal synthase obtained from a 50-ml culture. Purified methylglyoxal synthase was reasonably stable when it was stored in elution buffer at −20°C for at least 1 month. This property was different from the storage properties of E. coli methylglyoxal synthase, which lost activity quickly when the Pi concentration decreased, requiring 1 mM Pi in the buffer to stabilize E. coli methylglyoxal synthase during purification (11).
TABLE 1.
Purification of recombinant methylglyoxal synthase from E. coli BL21(DE3)/pMGS1
| Step | Total protein (mg) | Total activity (μmol/min) | Sp act (μmol/min/μg) | % Recovery |
|---|---|---|---|---|
| Supernatant | 28 | 2,570 | 92 | 100 |
| Affinity chromatography | 1.4 | 2,184 | 1,560 | 85 |
Physical properties of methylglyoxal synthase activity.
The mobilities of the recombinant methylglyoxal synthases from E. coli BL21(DE3)/pMGS1 and BL21(DE3)/pMGS2 during SDS-PAGE corresponded to Mr of 25,000 and 15,000, respectively (Fig. 3). The Mr of partially purified methylglyoxal synthase (the fraction collected from a DEAE-52 column) from BL21(DE3)/pMGS2 was estimated to be 60,000 by gel filtration high-performance liquid chromatography (HPLC) on a Bio-Sil SEC 125 column (Bio-Rad) (data not shown). We suggest that the native enzyme is a tetramer, like the E. coli methylglyoxal synthase (22, 27), which is a tetramer of a total molecular size of about 69 kDa. The reason why the His-tag (the molecular weight of the His-tag and amino acids encoded as part of the multicloning site sequences was only 5,000) affected the migration of methylglyoxal synthase obtained from pMGS1 on SDS-PAGE gels so strongly is not known.
To compare the activity of our methylglyoxal synthase with the activity of E. coli methylglyoxal synthase, we examined the activity of the enzyme under various conditions. A standard methylglyoxal synthase activity test was used; each reaction mixture contained 0.14 μg of purified protein per ml, and the temperature used was 30°C. The pH of the reaction buffer (Tris-HCl buffer) was adjusted with either HCl or NaOH. When DHAP (0.375 mM) was the substrate, the pH profile was essentially symmetrical, and optimal activity occurred at pH 7.5. These conditions were used to examine the effects of possible physiological inhibitors. Phosphate compounds have been found to inhibit bacterial methylglyoxal synthase (6, 7). Phosphate, pyrophosphate, phosphoenolpyruvate, and ADP were found to be modestly inhibitory for C. acetobutylicum methylglyoxal synthase (Table 2). This result was similar to the results obtained with goat methylglyoxal synthase (19). ATP resulted in 50% inhibition of the methylglyoxal synthase of C. acetobutylicum but had little effect on other methylglyoxal synthases (7, 19).
TABLE 2.
Effects of some phosphorylated compounds and inorganic phosphates on methylglyoxal synthase activity
| Addition | Concn (mM) | Activity (%) |
|---|---|---|
| None | 100 | |
| Orthophosphate | 40 | 34 |
| Pyrophosphate | 20 | 40 |
| Phosphoenolpyruvate | 1.5 | 24 |
| ATP | 1.5 | 51 |
| Fructose 1,6-biphosphate | 1.5 | 33 |
Substrate specificity and kinetic parameters of methylglyoxal synthase.
Methylglyoxal formation with purified methylglyoxal synthase was examined by using DHAP, glyceraldehyde, and glyceraldehyde-3-phosphate as substrates. None of these compounds except DHAP could be converted to methylglyoxal, although glyceraldehyde and glyceraldehyde-3-phosphate have been reported to be possible precursors of methylglyoxal (20).
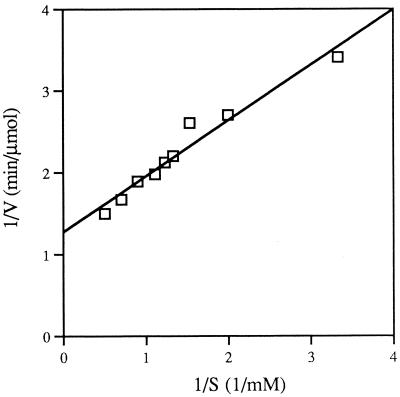
The Michaelis constant for methylglyoxal synthase was determined at pH 7.5 with imidazole-HCl buffer. The substrate saturation curve followed typical Michaelis-Menten kinetics. The Km as determined from a Lineweaver-Burk plot was 0.53 mM, and the Vmax was 1.56 mmol min−1 μg−1 (Fig. 4). The Km value is similar to the value recently reported for the E. coli recombinant methylglyoxal synthase (0.20 ± 0.03 mM) (22) and the value originally reported for the methylglyoxal synthase isolated from E. coli (0.47 mM) (11).
FIG. 4.
Determination of the apparent Michaelis constant for DHAP. The assay mixtures (total volume, 100 μl) contained 50 mM imidazole buffer (pH 7.5), different amounts of purified enzyme, and different amounts of DHAP. After 5 min of incubation at 30°C, the amount of methylglyoxal formed was measured colorimetrically (8).
Methylglyoxal synthase activity in C. acetobutylicum.
Methylglyoxal synthase activity was detected in cells of C. acetobutylicum that produced acetate, butyrate, and butanol as major products before they were harvested; considerable activity was found in solvent-producing cells (36-h culture) (1.8 U/mg), and a lower level of activity was detected in acid-producing cells (18-h culture) (0.6 U/mg). The specific activity of methylglyoxal synthase from C. acetobutylicum was higher than the specific activities of E. coli methylglyoxal synthase (0.3 U/mg) (11) and Clostridium sphenoides methylglyoxal synthase (0.1 U/mg) (28).
Metabolic engineering of 1,2-propanediol production by coexpression of E. coli glycerol dehydrogenase and methylglyoxal synthase in E. coli BL21(DE3).
We improved solvent production by C. acetobutylicum ATCC 824 by using genetic engineering strategies (10, 16). These results motivated us to investigate metabolic engineering approaches for production of other useful solvents, such as 1,2-propanediol. Since E. coli naturally produces some methylglyoxal and overexpression of C. acetobutylicum methylglyoxal synthase resulted in an increase in the production of methylglyoxal, we thought that 1,2-propanediol might be produced if the appropriate reductase was provided. E. coli contains a glycerol dehydrogenase, which is an NADH-linked reductase that is known to have broad substrate specificity and is able to reduce hydroxyacetone (14). Thus, methylglyoxal synthase and glycerol dehydrogenase were coexpressed to see if 1,2-propanediol could be produced.
The gene for E. coli glycerol dehydrogenase (gldA) has been cloned and mapped (29). We amplified this gene by PCR by using two primers (forward primer 5′-GCGGAATTCAGGAGGAATTTAAAATGCCGCATTTGGCACTACTCATCT CTAAAGG-3′ and reverse primer 5′-CGCGGATCCTTATTCCCACTCTTGCAGGAAAGCCTG-3′; E. coli wild-type genomic DNA was the template. A 1,143-bp PCR fragment was cut with EcoRI and BamHI and inserted into the corresponding sites in pEXT (9), a vector containing an R100 origin and a tac promoter, which resulted in plasmid pGLDH. Plasmid pGLDH was transformed into E. coli BL21(DE3) and was selected on Luria broth (23) supplemented with kanamycin (50 mg/ml). Adding 0.4 mM IPTG to liquid cultures resulted in an intense protein band in crude cell extracts that migrated at a molecular mass of 39 kDa (29) on 12.5% acrylamide SDS-PAGE gels (12) which were stained with Coomassie blue R-250 (Fig. 3). Thus, the construct pGLDH resulted in overexpression of the glycerol dehydrogenase gene in E. coli. Glycerol dehydrogenase activity was assayed by using a previously described procedure (29). The extracts obtained from BL21(DE3)/pGLDH exhibited a 17-fold increase in activity compared with the activity of the host with a vector lacking the insert [BL21(DE3)/pEXT] (data not shown).
In order to select transformants with different antibiotics, two plasmids, pGLDH and pMGS2, were cotransformed into E. coli BL21(DE3), and colonies were selected by using ampicillin and kanamycin. Fermentation by this recombinant E. coli strain was carried out in a 5-ml anaerobic culture tube at 37°C on M9 medium supplemented with 5 g of yeast extract per liter, 5 g of fructose per liter, 2 mM MgSO4, 0.1 M CaCl2, 100 μg of ampicillin per ml, and 50 μg of kanamycin per ml. After 24 h of growth, 0.4 mM IPTG was added. The amount of 1,2-propanediol in the medium was measured by HPLC 72 h after induction. Some 1,2-propanediol was produced in the uninduced cultures (due to the strength of T7 and the tac promoter, we detected expression of some methylglyoxal synthase and glycerol dehydrogenase even without induction [data not shown]). 1,2-Propanediol (3.9 mM, 0.3 g/liter) was produced by induced cultures containing pMGS2 and pGLDH. A lower concentration, 2.7 mM 1,2-propanediol, was produced when the host culture contained either pMGS2 or pGLDH. We did not detect 1,2-propanediol production in a control experiment in which we used a host containing a plasmid(s) without the insert. The 1,2-propanediol concentrations were low, but they were higher than the concentrations observed in recombinant E. coli cultures that reportedly fermented sugars to 1,2-propanediol (0.2 g/liter) (4) and recombinant organisms that reportedly fermented sugars to 1,3-propanediol (less than 0.1 g/liter) (13). Although methylglyoxal synthase and glycerol dehydrogenase were expressed very well, the level of production of 1,2-propanediol was not as high as desired. The possible limitations include the possibility that the formation of methylglyoxal is metabolically regulated because this compound is toxic and the possibility that the substrate specificity and efficiency of the glycerol dehydrogenase may not be optimal. Other reductases, such as human aldose reductases, which have been reported to exhibit high substrate specificity for methylglyoxal (5, 25, 30), may be useful. We also plan to investigate the possibility of 1,2-propanediol production by C. acetobutylicum ATCC 824 by using constructs that express methylglyoxal synthase and an appropriate reductase.
Acknowledgments
This research was supported by grant MCB 9604562 from the National Science Foundation and by grant 3604-051 from the Texas Advanced Technology Program.
We thank T. Linn (University of Western Ontario, London, Ontario, Canada) for providing plasmid pEXT and Yea-Tyng Yang (Department of Bioengineering, Rice University) for performing the HPLC analysis.
REFERENCES
- 1.Babel W, Hofmann K H. The conversion of triosephosphate via methylglyoxal, a bypass to the glycolytic sequence in methylotrophic yeasts? FEMS Microbiol Lett. 1981;10:133–136. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M, Cerami A. The biochemistry of the complication of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- 4.Cameron D C, Altaras N E, Hoffman M L, Shaw A J. Metabolic engineering of propanediol pathways. Biotechnol Prog. 1998;14:116–125. doi: 10.1021/bp9701325. [DOI] [PubMed] [Google Scholar]
- 5.Cao D, Fan S T, Chung S S M. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273:11429–11435. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- 6.Cooper R A. Methylglyoxal formation during glucose catabolism by Pseudomonas saccharophila: identification of methylglyoxal synthase. Eur J Biochem. 1974;44:81–86. doi: 10.1111/j.1432-1033.1974.tb03459.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper R A. Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol. 1984;38:49–68. doi: 10.1146/annurev.mi.38.100184.000405. [DOI] [PubMed] [Google Scholar]
- 8.Cooper R A. Methylglyoxal synthase. Methods Enzymol. 1975;41:502–508. doi: 10.1016/s0076-6879(75)41106-5. [DOI] [PubMed] [Google Scholar]
- 9.Dykxhoorn D M, Pierre R S, Linn T. A set of compatible tac promoter expression vectors. Gene. 1996;177:133–136. doi: 10.1016/0378-1119(96)00289-2. [DOI] [PubMed] [Google Scholar]
- 9a.Genome Therapeutics Corp. 3 May 1999, revision date. http://www.genomecorp.com/genesequences/clostridium/clospage.html. [26 May 1999, last date accessed.]
- 10.Green E M, Boynton Z L, Harris L M, Rudolph F B, Papoutsakis E T, Bennett G N. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology. 1996;142:2070–2086. doi: 10.1099/13500872-142-8-2079. [DOI] [PubMed] [Google Scholar]
- 11.Hopper D J, Cooper R A. The purification and properties of Escherichia coli methylglyoxal synthase. Biochem J. 1972;128:321–329. doi: 10.1042/bj1280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Laffend, L. A., V. Nagarajan, and V. C. E. Nakamura. November 1996. Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism. International patent WO 96/35796.
- 14.Lee L G, Whitesides G M. Preparation of optically active 1,2-diols and α-hydroxy ketones using glycerol dehydrogenase as catalyst: limits to enzyme-catalyzed synthesis due to noncompetitive and mixed inhibition byproduct. J Org Chem. 1986;51:25–36. [Google Scholar]
- 15.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 16.Nair R V, Green E M, Watson D E, Bennett G N, Papoutsakis E T. Regulation of the sol locus genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 by a putative transcriptional repressor. J Bacteriol. 1999;181:319–330. doi: 10.1128/jb.181.1.319-330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakas J P, Schaedle M, Parkinson C M, Coonley C E, Tannenbaum S W. System development for linked-fermentation production of solvents from algal biomass. Appl Environ Microbiol. 1983;46:1017–1023. doi: 10.1128/aem.46.5.1017-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oren A, Gurevich P. Occurrence of the methylglyoxal bypass in halophilic Archae. FEMS Microbiol Lett. 1995;125:83–88. [Google Scholar]
- 19.Ray S, Ray M. Isolation of methylglyoxal synthase from goat liver. J Biol Chem. 1981;256:6230–6233. [PubMed] [Google Scholar]
- 20.Rizza V, Hu A S. Gluconate metabolism in Pseudomonas: a novel pathway of glyceraldehyde-3-phosphate metabolism. Biochem Biophys Res Commun. 1973;54:168–175. doi: 10.1016/0006-291x(73)90904-2. [DOI] [PubMed] [Google Scholar]
- 21.Ruch F E, Lengeler J, Lin E C. Regulation of glycerol catabolism in Klebsiella aerogenes. J Bacteriol. 1974;119:50–56. doi: 10.1128/jb.119.1.50-56.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saadat D, Harrison D H T. Identification of catalytic bases in the active site of Escherichia coli methylglyoxal synthase: cloning, expression, and functional characterization of conserved aspartic acid residues. Biochemistry. 1998;37:10074–10086. doi: 10.1021/bi980409p. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 24.Shutz H, Radler F. Anaerobic reduction of glycerol to propanediol-1,3 by Lactobacillus brevis and Lactobacillus buchneri. Syst Appl Microbiol. 1984;5:169–178. [Google Scholar]
- 25.Srivastava S, Harter T M, Chandra A, Bhatnagar A, Srivastava S K, Petrash J M. Kinetic studies of FR-1, a growth factor-inducible aldo-keto reductase. Biochemistry. 1998;15:12909–12917. doi: 10.1021/bi9804333. [DOI] [PubMed] [Google Scholar]
- 26.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 27.Totemyer S, Booth N A, Nichols W W, Dunbar B, Booth I R. From famine to feast: the role of methylglyoxal production in Escherichia coli. Mol Microbiol. 1998;27:553–562. doi: 10.1046/j.1365-2958.1998.00700.x. [DOI] [PubMed] [Google Scholar]
- 28.Tran-Dinh K, Gottschalk G. Formation of d(−)-1,2-propanediol and d(−)-lactate from glucose by Clostridium sphenoides under phosphate limitation. Arch Microbiol. 1985;142:87–92. [Google Scholar]
- 29.Truniger V, Boos W. Mapping and cloning of gldA, the structural gene of the Escherichia coli glycerol dehydrogenase. J Bacteriol. 1994;176:1796–1800. doi: 10.1128/jb.176.6.1796-1800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandertagt D V, Robinson B, Taylor K K, Hunsaker L A. Reduction of trioses by NADH-dependent aldo-keto reductases. J Biol Chem. 1992;267:4364–4369. [PubMed] [Google Scholar]