Abstract
Aspiration pneumonia is a common cause of morbidity and mortality in both adults and children that, however, is difficult to accurately diagnose. In current literature, there are no reports or clinical research study focused on the possible use of lung ultrasound (LUS) in the diagnosis and follow-up of aspiration pneumonia in children. In this case series, we describe clinical, laboratory, radiological results as well as detailed lung ultrasound findings of three children with severe disability and diagnosed with aspiration pneumonia. In these three cases, albeit at different times, LUS played an important role in both the initial diagnostic process and follow-up.
Electronic supplementary material
The online version of this article (10.1007/s40477-020-00520-4) contains supplementary material, which is available to authorized users.
Keywords: Children, Aspiration pneumonia, Lung ultrasound, Pulmonary, Personalized medicine, Radiomics
Introduction
Aspiration pneumonia is a common cause of morbidity and mortality in both adults and children that, however, is difficult to accurately diagnose. Robust diagnostic criteria for aspiration pneumonia are lacking and, as a result, studies of this disorder include heterogeneous patient populations [1].
The diagnosis should be considered in the appropriate clinical settings in patients with known risk factors for aspiration and characteristic clinical and radiographic findings.
In recent years, lung ultrasound (LUS) has been increasingly used for the diagnosis of respiratory diseases in both adult and paediatric patients [2–4].
In current literature there are no reports or clinical research study focused on the possible use of LUS in the diagnosis and follow-up of aspiration pneumonia in children.
For this reason, in the following case series, we describe clinical, laboratory, radiological results as well as detailed lung ultrasound findings of three children with severe disability and diagnosed with aspiration pneumonia. In these three cases, albeit at different times, LUS played an important role in both the initial diagnostic process and follow-up.
Written informed consent was obtained from a parent or guardian before data collection. The study was approved by the Institutional Review Board and Ethic Committee (prot.36173/19 ID2729). All patients’ data were analysed anonymously. The main settings were represented by the paediatric emergency department and paediatric ward. Ultrasound examinations were performed using a MyLab linear transducer at 12 MHz and the small parts preset (EsaoteSpA, Genoa, Italy).
Case descriptions
Case 1
Case 1 was a 4-year-old patient with a past medical history that includes severe intellectual and motor disability, progressive epileptic encephalopathy undergoing diagnostic definition and multi-drug treatment, chronic undernutrition. The child has always been fed orally and postprandial regurgitation episodes were reported by the mother. The child was admitted to our paediatric ward because of drowsiness, loss of appetite, dehydration, acute malnutrition, fever, oxygen requirement. Chest auscultation revealed inspiratory crackles at the left posterior lower lobe and reduced air penetration on the left fields. Blood tests revealed raised inflammatory markers.
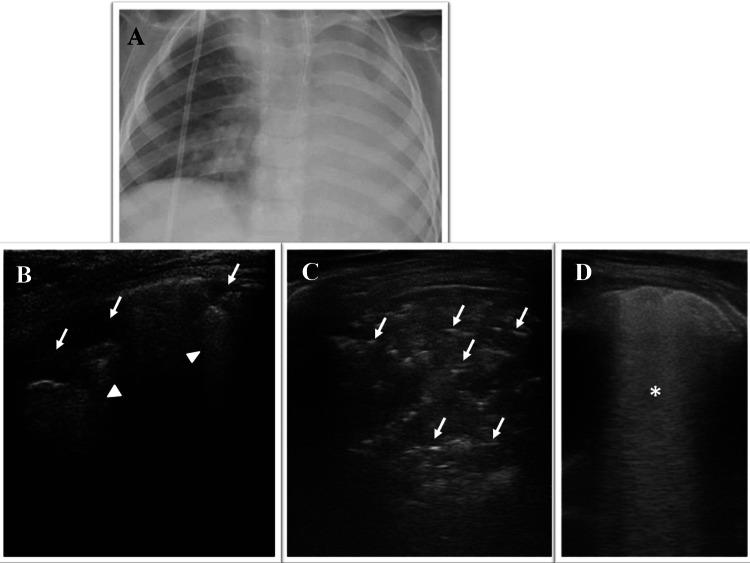
Chest X-ray showed, on the left lung fields, non-specific areas of reduced transparency.
Point-of-care LUS, showed:—on all posterior fields of the left lung, an extensive consolidation with dynamic branching (tree-like) superficial air bronchogram and static fluid deep bronchogram, suggestive for pneumonia (Fig. 1; Electronic Supplemental Video Clips 1–2);—on all posterior fields of the right lung, irregular pleural line and multiple blurred, irregular B-lines suggestive of initial interstial syndrome.
Fig. 1.

Grayscale lung ultrasound examination shows, on all posterior fields of the left lung, an extensive consolidation with dynamic branching (tree-like) superficial air bronchogram (arrow) and static fluid deep bronchogram (arrowhead), suggestive for pneumonia (Electronic Supplemental Video Clips 1–2)
Due to the suspicion of pneumonia of bacterial origin, intravenous antibiotic therapy with Ceftriaxone was begun. After 3 days of therapy, the child appeared afebrile. Blood tests showed progressive reduction of the inflammation markers until their complete normalization. Chest auscultation showed a significant improvement in bilateral air penetration. His symptoms gradually disappeared. Oxygen therapy was stopped. Furthermore, he was more alert and reactive and began oral eating with milk and baby food. As the days passed, the mother and health workers again reported regurgitations of gastric material. However, about a week after discontinuation of IV antibiotics, high oxygen flow requirement appeared again and postprandial desaturations were reported. In addition, blood tests again showed an increase in inflammation markers. Chest auscultation changed again with a sharp reduction of air penetration bilaterally.
LUS was performed and showed an almost complete resolution of the previously described consolidation on the left posterior fields (Fig. 1; Electronic Supplemental Video Clips 1–2). New ultrasound findings appeared:—on the left posterior and lateral fields mainly in the mid-basal area, subpleural consolidation with static air broncograms and with atelectasis component, associated with coalescent B-lines and large “white lung” areas (Fig. 2a);—on the posterior fields on the right in the apical site, subpleural consolidation of about 2.5 cm with static air broncograms associated with coalescent B-lines and “white lung” areas (Fig. 2b; Electronic Supplementary Video Clip 3).
Fig. 2.
a, b Grayscale lung ultrasound examination shows new lesions: a on the left posterior and lateral fields mainly in the mid-basal area, subpleural consolidation with static air brocograms (puncatate) (arrow) and with atelectasis component associated with coalescent B-lines (asterisk) and large “white lung” areas (arrowhead). b on the posterior fields on the right in the apical site, subpleural consolidation of about 2.5 cm with static air brocograms (puncatate) (arrow), associated with coalescent B-lines and “white lung” areas (Electronic Supplementary video clip 3). c Axial CT image shows: centrolobular micronodules with “tree in budd” appearance in the posterior segments of the lungs (gravity dependent) (arrow) and ground glass opacities with partial consolidation in the apical segment of the right superior lobe and in the postero-basal segment of the left inferior lobe (arrowhead), findings suggestive for aspiration bronchiolitis and aspiration pneumonia. d Grayscale lung ultrasound examination shows bilaterally: coalescent B-lines and a few areas of “white lung” (arrow)
Based on the patient’s clinical history, the appearance of respiratory symptoms related to oral nutrition and the new ultrasound findings (Fig. 2a, b), the patient was diagnosed with aspiration pneumonia. An anti-anaerobic spectrum antibiotic (Piperacillin-Tazobactam) was begun, oral feeding was stopped and enteral feeding with nasogastric tube was started.
Since our patient had neurological impairment, the diagnosis of aspiration pneumonia—suspected because of clinical and ultrasound data—would have been essential to provide an appropriate therapeutic indication for gastrostomy placement [5].
However, the team of paediatric nutritionists and paediatric surgeons who had to provide the definitive indication for gastrostomy, having no official data in the literature regarding the use of LUS for the diagnosis of aspiration pneumonia, required a chest Computed Tomography (CT) scan, currently the gold standard for this condition in that cases with suggestive symptomatology and medical history but with negative and/or not-specific chest X-ray [1, 6]. The radiological findings of chest CT scan (Fig. 2c) were consistent with the LUS results.
After about 7 days of antibiotic therapy and enteral nutrition by nasogastric tube, his symptoms gradually disappeared: postprandial regurgitations were not reported anymore and there was a progressive weaning from respiratory assistance until suspension, with normalization of inflammatory markers.
A follow-up examination was conducted by chest auscultation and LUS, which showed the disappearance of subpleural consolidation bilaterally; some B-lines and a few areas of “white lung” still remained (Fig. 2d).
There was no subsequent recurrence of aspiration when enteral nutrition was performed first by nasogastric tube and subsequently by gastrostomy.
Case 2
Case 2 was a 5-year-old boy with a medical history of a brain stem tumor. Despite severe intellectual and motor disability, the child was fed orally. He was taken to our paediatric emergency department for acute onset of dyspnoea. He had been on day 3 of treatment with intramuscular Ceftriaxone for suspicion of bacterial pneumonia. The mother also reported the presence of postprandial regurgitation and vomiting.
At our observation the patient presented himself in poor general conditions: febrile, dehydrated, dyspnoeic with need high oxygen flow. With the aspiration of airways, some sputum with food residue was aspirated.
In addition, blood tests showed a moderate increase in the markers of inflammation. Chest auscultation changed again with a sharp reduction of air penetration bilaterally.
Chest X-ray showed, on all left lung fields, non-specific areas of reduced transparency (Fig. 3a). Point-of-care LUS, showed:—on the posterior fields on the right in the mid-apical site, small subpleural consolidations associated to coalescent B-lines and “white lung” areas (Fig. 3b);—on the left posterior fields mainly in the mid-basal area, subpleural consolidation of about 4 cm with static air broncograms and with atelectasis component (Fig. 3c).
Fig. 3.
a Chest digital radiography shows diffuse HyperLucency in the left lung consisting with consolidation. b, c Grayscale lung ultrasound examination shows: b—on the posterior fields on the right in the mid-apical site, small subpleural consolidations (arrow) associated to coalescent B-lines and “white lung” areas (arrowhead); c—on the left posterior fields mainly in the mid-basal area, subpleural consolidation of about 4 cm with static air broncograms (punctate) (arrow) and with atelectasis component. d Grayscale lung ultrasound examination shows disappearance of subpleural consolidation on the left posterior fields and shows bilaterally: coalescent B-lines and a few areas of “white lung” (asterisk)
Based on the patient’s clinical history, the appearance of respiratory symptoms related to oral nutrition and the ultrasound findings (Fig. 3b, c), the patient was diagnosed with aspiration pneumonia. Therefore, antibiotic therapy was changed and anti-anaerobic spectrum antibiotic (Piperacillin-Tazobactam) was begun; oral feeding was stopped and enteral feeding with nasogastric tube was started.
After an initial worsening phase for which the patient has needed invasive mechanical ventilation and after about 10 days of antibiotic therapy and enteral nutrition by nasogastric tube, his symptoms gradually improved until progressive weaning from invasive ventilation.
A follow-up examination was conducted by chest auscultation and LUS, which showed the disappearance of subpleural consolidation on the left posterior fields; some B-lines and a few areas of “white lung” still remained bilaterally (Fig. 3d).
Case 3
Case 3 was a 1 year, 8-month-old child, born preterm at 25 weeks of gestational age, who had neuromotor disability and a history of cough and respiratory distress after oral feeding.
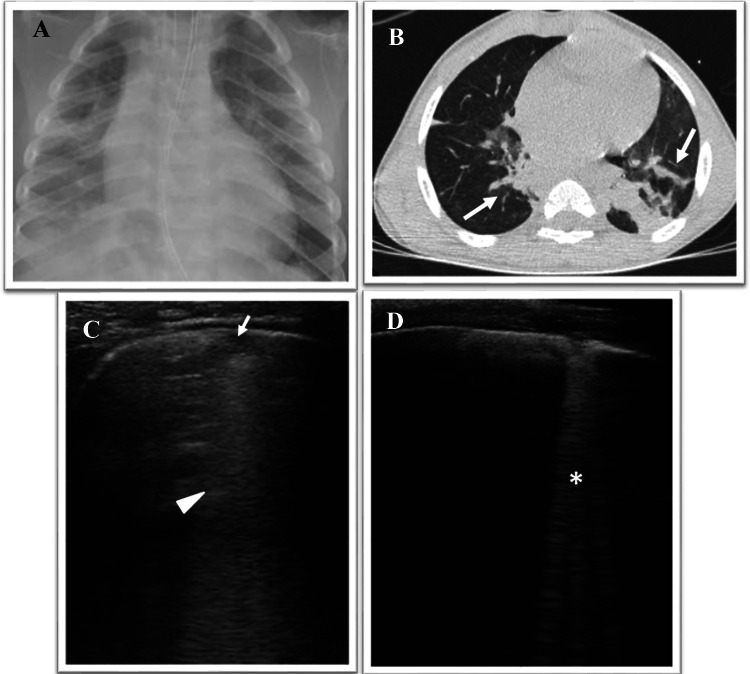
He came to our attention at the paediatric emergency room for acute onset of severe respiratory distress, and noisy breathing for which ingestion of a foreign body was suspected. Therefore, after performing chest X-ray (Fig. 4a), fibro-bronchoscopy was carried out which excluded the presence of a foreign body but documented the presence of a ductal cyst of the left vocal cord, which partially was occluding the airways. Due to the rapidly worsening respiratory distress, a CT scan was performed and showed atelectasis of the right upper lobe, which overlapped a picture of suspected aspiration pneumonia (Fig. 4b).
Fig. 4.
a Chest digital radiography shows areas of HyperLucency in the middle and inferior right lung fields and in the lower left lung fields consisting with consolidation. b Axial CT image shows bilateral subsegmental ground glass opacities with partial consolidation in posterior segments of the lungs (gravity dependent) (arrow), consisting with aspiration pneumonia. c, d Grayscale lung ultrasound examination during the follow-up, shows: disappearance of subpleural consolidations that give way to ultrasound interstitial syndrome characterized by the bilaterally presence of—at first c—areas of subpleural micro consolidations (arrow) associated with “white lung” and coalescent B-lines (arrowhead) and—subsequently, in the resolution phase d—areas of single and non-coalescent B-lines (asterisk)
He was admitted in the paediatric intensive care unit requiring invasive mechanical ventilation. Anti-anaerobic spectrum antibiotic (Piperacillin-Tazobactam) and enteral nutrition with nasogastric tube were begun.
The patient’s general and respiratory conditions progressively improved until the suspension of respiratory assistance and transfer to the paediatric ward.
During the hospitalization, the evolution of atelectasis and aspiration pneumonia was documented by LUS which showed progressive resolution of the consolidations (Fig. 4c, d) after about 10 days of antibiotic therapy and enteral nutrition with nasogastric tube.
Discussion
Aspiration pneumonia is a common cause of morbidity and mortality in both adults and children that, however, is difficult to accurately diagnose and to distinguish from other aspiration syndromes and community- and hospital-acquired pneumonias [1].
Robust diagnostic criteria for aspiration pneumonia are lacking and, as a result, studies of this disorder include heterogeneous patient populations [1].
Variables affecting patient presentation and disease management include bacterial virulence, the risk of repeated events and the site of acquisition. According to this spectrum, patients labeled as having aspiration pneumonia usually represent a clinical phenotype with risk factors for macro–micro aspiration and involvement of characteristic anatomical pulmonary locations [7]. Aspiration syndromes may involve the airways or pulmonary parenchyma, resulting in a variety of clinical presentations, often presenting a diagnostic dilemma for clinicians [1, 7].
The diagnosis of aspiration pneumonia depends on (1) a characteristic clinical history (witnessed macro-aspiration), (2) risk factors (oesophageal disease, neurologic diseases, impaired consciousness, degenerative neurologic disease, increased chance of gastric contents reaching the lung as in the case of reflux, tube feeding, impaired cough reflex), (3) clinical respiratory manifestations often related to feeding and (4) compatible findings on chest X-ray [1]. These radiographic findings include infiltrates in gravity-dependent lung segments (superior lower lobe or posterior upper-lobe segments, if the patient is in a supine position during the event, or basal segments of the lower lobe, if the patient is upright during the event). However, chest X-ray may be negative in the early course of aspiration pneumonia [6]. Chest X-ray or computed tomography (CT) scan—in that cases with suggestive symptomatology and medical history but with negative and/or not-specific chest X-ray—are currently the gold standards for the radiologic diagnosis of aspiration pneumonia [1, 6].
In recent years, lung ultrasound (LUS) has been increasingly used for the diagnosis of respiratory diseases in both adult and paediatric patients [2–4].
Except for a report [8] on the role of portable ultrasound as an initial diagnosis and subsequent monitoring tool for aspiration pneumonia in the geriatric population, in current literature there are no reports or clinical research study focused on the possible use of LUS in the diagnosis and follow-up of aspiration pneumonia in children.
In the first and the second case, LUS has played an important role enabling us to make a real-time diagnosis of aspiration pneumonia—described non-specifically on the chest X-ray- without waiting to perform second-level instrumental examinations. In fact, in the first case, the chest CT scan, which had been performed only for legal aspects, just confirmed the diagnosis that we already made based on the clinical and the ultrasound results.
In the third case, LUS was not performed at the time of diagnosis because the child, having also a massive right lung atelectasis due to the ductal cyst of the vocal cord, was immediately admitted to the paediatric intensive care unit. Nonetheless, LUS was helpful in monitoring the evolution of atelectasis and aspiration pneumonia in response to therapeutic care, without performing other chest X-rays.
Although chest X-ray is a routine tool to diagnose and monitor aspiration pneumonia, it lacks sensitivity and has relatively low accuracy [9, 10], as our first two cases also demonstrate. On the other hand, the chest CT scan bears disadvantages particularly in children, because of its costs, needing for sedation and high radiation exposure [11].
Conversely, LUS is a non-invasive, non-ionizing radiation tool and a rapid, affordable, point-of-care imaging modality that allow both real-time diagnosis and follow-up [2, 3, 10, 11]; LUS results are immediately available to the clinician, who must decide about the initial empirical treatment.
Furthermore, LUS seems to be a sufficiently accurate technique for diagnosing community acquired pneumonia (CAP) in the paediatric population with high sensitivity and specificity. It represents an alternative diagnostic tool to chest X-ray for various childhood respiratory diseases [3, 10, 12–15].
In this contest, another advantage of LUS is that it can help to establish the aetiology of pneumonia in children with CAP, as reported by the study by Vojko Berce et al. [12] in which they show how the etiologically different types of CAP (bacterial, viral, atypical bacterial) differ in their LUS characteristics. In particular, LUS is especially useful in differentiating between the viral and bacterial CAP when used in combination with epidemiological, clinical and laboratory data [12]. In this perspective, going back to our cases, LUS characteristics at the time of diagnosis of aspiration pneumonia in the first two cases are similar both for lung localization (bilaterally gravity-dependent lung segments) and for ultrasound patterns (Figs. 2a, b and 3a, b), while they are different from the ultrasound pattern of a bacterial pneumonia (Fig. 1—consolidation with a greater number of dynamic branching (tree-like) air bronchogram). Hence, even in the diagnostic process of aspiration pneumonia in children, LUS could help in the differential aetiology diagnosis, taking into account the patient’s clinical, medical and laboratory characteristics.
We suggest to include LUS in the diagnostic and monitoring pathway of suspected aspiration pneumonia in children. However, further studies are needed to standardize this possible new indication for LUS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
we are grateful to Dr Emiliano Visconti, radiologist at Ospedale “M.
Bufalini” Cesena—AUSL Romagna, for his support in reviewing the radiologic studies.
Abbreviations
- CT
Computed tomography
- LUS
Lung ultrasound
- CAP
Community acquired pneumonia
Author contributions
All authors have read and approved the manuscript for submission; have made a substantial contribution to the conception, design, gathering of data and a contribution to the writing and intellectual content of the article. They have exercised due care in ensuring the integrity of the work.
None of the original material contained in the manuscript has been submitted for consideration nor will any of it be published elsewhere except in abstract form in connection with scientific meetings.
Funding
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interests to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mandell LA, Niederman MS. Aspiration Pneumonia. N Engl J Med. 2019;380(7):651–663. doi: 10.1056/NEJMra1714562. [DOI] [PubMed] [Google Scholar]
- 2.Volpicelli G. Lung sonography. J Ultrasound Med. 2013;32:165–171. doi: 10.7863/jum.2013.32.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Musolino AM, Tomà P, Supino MC, et al. Lung ultrasound features of children with complicated and non-complicated community acquired pneumonia: a prospective study. Pediatr Pulmonol. 2019;54:1479–1486. doi: 10.1002/ppul.24426. [DOI] [PubMed] [Google Scholar]
- 4.Tomà P. Lung ultrasound in pediatric radiology—cons. Pediatr Radiol. 2020;50:314–320. doi: 10.1007/s00247-019-04524-z. [DOI] [PubMed] [Google Scholar]
- 5.Romano C, van Wynckel M, Hulst J, et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. 2017;65:242–264. doi: 10.1097/MPG.0000000000001646. [DOI] [PubMed] [Google Scholar]
- 6.Miyashita N, Kawai Y, Tanaka T, et al. Detection failure rate of chest radiography for the identification of nursing and healthcare-associated pneumonia. J Infect Chemother. 2015;21:492–496. doi: 10.1016/j.jiac.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Di Bardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care. 2015;30:40–48. doi: 10.1016/j.jcrc.2014.07.01. [DOI] [PubMed] [Google Scholar]
- 8.Namiki H, Kobayashi T. Lung ultrasound for initial diagnosis and subsequent monitoring of aspiration pneumonia in elderly in home medical care setting. Gerontol Geriatr Med. 2019;5:2333721419858441. doi: 10.1177/2F2333721419858441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ticinesi A, Lauretani F, Nouvenne A, et al. Lung ultrasound and chest x-ray for detecting pneumonia in an acute geriatric ward. Medicine. 2016;95:e4153. doi: 10.1097/md.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonsenso D, Brancato F, Valentini P, Curatola A, Supino M, Musolino AM. The use of lung ultrasound to monitor the antibiotic response of community-acquired pneumonia in children: a preliminary hypothesis. J Ultrasound Med. 2019;39(4):817–826. doi: 10.1002/jum.15147. [DOI] [PubMed] [Google Scholar]
- 11.Cattarossi L, Copetti R, Poskurica B. Radiation exposure early in life can be reduced by lung ultrasound. Chest. 2011;139:730–731. doi: 10.1378/chest.10-2338. [DOI] [PubMed] [Google Scholar]
- 12.Berce V, Tomazin M, Gorenjak M, Berce T, Lovrenčič B. The usefulness of lung ultrasound for the aetiological diagnosis of community-acquired pneumonia in children. Sci Rep. 2019;9(1):17957. doi: 10.1038/s41598-019-54499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitale V, Rossi E, Di Serafino M, et al. Pediatric encephalic ultrasonography: the essentials. J Ultrasound. 2020;23:127–137. doi: 10.1007/s40477-018-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21:183–195. doi: 10.1007/s40477-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botta F, Raimondi S, Rinaldi L, et al. Association of a CT-Based clinical and radiomics score of non-small cell lung cancer (NSCLC) with lymph node status and overall survival. Cancers. 2020;12:1432. doi: 10.3390/cancers12061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.