Abstract
Spinal muscular atrophy with respiratory distress type 1 (SMARD1, OMIM #604,320), is a rare autosomal recessive disease resulting from degeneration of motor neurons in the anterior horns, which leads irreversible diaphragmatic palsy and progressive distal symmetrical muscular weakness. Respiratory distress is the main symptom and is severe, rapidly progressive, and frequently requiring invasive ventilation. Despite diaphragm being one of the target organ of the disease, no specific study has been done using ultrasound.We report diaphragm and lung ultrasound findings of a 13-month-old girl affected by SMARD1 (homozygosis c.1540G > A mutation in IGHMPB2 gene) with respiratory failure requiring permanent mechanical ventilation since birth and we discuss the role of diaphragmatic and lung ultrasound in this category of patients and its clinical implications.
Keywords: SMARD1; Neuromuscular disorders; Diaphragm function; Diaphragm ultrasound; Lung ultrasound; Personalized medicine; Precision medicine; JUSD-D-21–00,057; Spinal muscular atrophy; Diaphragm; Ultrasound
Introduction
Spinal muscular atrophy with respiratory distress type 1 (SMARD1, OMIM#604,320), is a rare autosomal recessive disease resulting from degeneration of motor neurons in the anterior horns. The actual prevalence of SMARD1 is unknown, but diaphragmatic paralysis is observed in approximately 1% of patients with an early onset of the clinical features of spinal muscle atrophy and an estimated incidence of 1/100,000 [1, 2].
The main clinical features of this disease include neonatal onset (within the year of life), diaphragmatic paralysis and the wasting of distal limb muscles, which leads affected individuals to be completely dependent on ventilatory support (between 6 weeks and 6 months of age) and the daily supportive care of parents or caregivers [1, 2].
The clinical symptoms rapidly progress in the first years of life, with distal limb muscular atrophy extending to proximal regions. The overall prognosis is poor, and progressive autonomic nervous system dysfunction also develops in association with the progressive worsening of motor functions in affected children. No effective treatment is available yet, but novel therapeutic approaches, such as gene therapy, have shown encouraging results in preclinical settings and thus represent possible methods for treating SMARD1. Significant advancements in the understanding of both the SMARD1 clinical spectrum and its molecular mechanisms have allowed the rapid translation of preclinical therapeutic strategies to human patients to improve the poor prognosis of this devastating disease [1].
Despite diaphragm being one of the target organ of the disease, no specific study has been done using ultrasound. In addition, very few rare studies have described the lung ultrasound findings in patients with neuromuscular disease [3].
Case report
We report diaphragm and lung ultrasound findings of a 13-month-old girl affected by SMARD1 (homozygosis c.1540G > A mutation in IGHMPB2gene) with hypotonia, symmetrical distal muscular weakness, areflexia and respiratory failure requiring permanent mechanical ventilation (APCV mode, IPAP 23 cmH2O, PEEP 5 cmH2O with 2 L/min oxygen support) since birth.
The patient was evaluated from the radiographic and ultrasound point of view in a phase of clinical and respiratory stability during one of the scheduled follow-up checks.
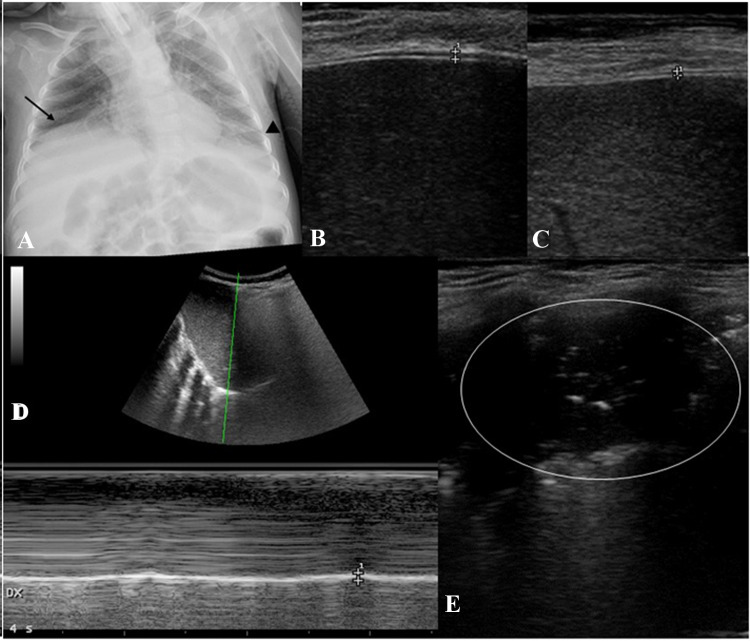
Chest X-ray showed eventration of right diaphragm dome (Fig. 1a).
Fig. 1.
Chest X-ray and point-of-care ultrasound findings. X-ray shows right diaphragm dome eventration (black arrow) and suspected left pleural effusion (black arrow-head) (a). B-mode diaphragm ultrasound shows diaphragm thickening on inspiration (b) and expiration (c) is showed between the crosses. M-mode imaging from the right subcostal area shows the functioning diaphragm represented as an echogenic line (d). Inspiration is identified on the sonographic tracing as upward flexion; expiration is identified as downward flexion. Estimation of diaphragmatic excursion was conducted by measuring the vertical distance between the upper border of the liver at the end of expiration to the upper border of the liver at the end of inspiration. This vertical distance represents right diaphragmatic excursion (d, white crosses). Grayscale lung ultrasound examination shows, on the left basal field, a subpleural consolidation with static air broncograms and parallel to each other of an atelectasis nature (e, white circle). In the same area no pleural effusion was detected
Diaphragm ultrasound was performed by an expert pediatrician using an Esaote (MyLAb 40) portable system with a 12‐MHz linear probe. The patient was placed in the supine position and ultrasound examination was performed during quiet spontaneous breathing, excluding moments of crying or coughing. The evaluation of right diaphragm thickness at ultrasound examination was performed as previously reported [4, 5].
The standard B‐mode image of the diaphragm was acquired placing the linear probe in the ninth or tenth intercostal space (therefore, following an oblique plane along the intercostal space, to see the longitudinal view of the diaphragm), between the anterior axillary and midaxillaryline [4, 5], at 0.5–2 cm below the costophrenic sinus. In the B‐mode image, the diaphragm thickness at end expiration (TEE) and at end inspiration (TEI) was measured.
Subsequently, the diaphragmatic functionality was also studied in time‐motion mode (M-mode)—with a convex probe—where the normal diaphragm is represented as a hyperechogenic line that moves during the acts of breath (Fig. 1d). In this imaging, during inspiration, the diaphragm moves towards probe and this is recorded as an upward motion of hyperechogenic line corresponding to the pleural and peritoneal membranes (Fig. 1d); during expiration, the diaphragm moves away from transducer resulting in a downward inflexion. The amplitude of excursion of the hemidiaphragm is measured on the vertical line drawn from the baseline to the point of the maximum height of the inspiration [4, 5].
This vertical distance represents right/diaphragmatic excursion [4, 5].
Our patient's point-of-care diaphragmultrasound evaluation showed thinning of both diaphragms with a diaphragm TEI of 0.06 cm (Fig. 1b) and TEE of 0.04 cm (Fig. 1c). These values are 2–3 times lower than the pediatric values reported in the literature [4–6].Diaphragm excursion was of 0.41 cm (Fig. 1d). Point-of-care ultrasound (POCUS) confirmed a stable diaphragmatic function despite changing ventilation parameters (APCV mode, IPAP 18 cmH2O, PEEP 5 cmH2O, no oxygen support): TEI 0.07 cm, TEE 0.05 cm, diaphragm excursion 0.44 cm.
Discussion
The diaphragm acts as the main respiratory muscle during inspiration and accounts for 70% of the inspired air volume during regular breathing [7].
In the framework of SMARD1, the degeneration of motor neurons in the anterior-horns leads irreversible diaphragmatic palsy and progressive distal symmetrical muscular weakness [1, 2].
The periodic assessment of diaphragmatic function, as the main inspiratory muscle, may prove to be important to evaluate the evolution of the disease of each patient affected by SMARD1.
The techniques traditionally employed to assess diaphragmatic weakness or paralysis, such as transdiaphragmatic pressure, electromyography, fluoroscopy, and plethysmography are, however, either highly invasive or very complex and are not easily applicable in a routine follow‐ up [1, 2].
Chest X‐ ray, which can show the characteristic eventration (the abnormal elevation) of the right or, less frequently, both hemidiaphragms, which is considered a highly suggestive sign of SMARD1 (Fig. 1a), plays a core role in the diagnostic pathway [1, 2]. However, it does not allow to specifically and directly evaluate the morphology and diaphragmatic function, as well as not being able to be performed routinely due to the ionizing radiation associated with it.
Ultrasonography (US) has proven to be useful as a possible alternative to study both diaphragmatic structure and function, in particular diaphragmatic thickness [8], thickening fraction in adults [9]and children [4, 5] and excursion[10].
So far, its role in SMARD patients has never been investigated and despite diaphragm being one of the target organ of the disease no specific study has been done using ultrasound.
Our results report for the first time US finding in a SMARD1 patient. Despite presenting an extremely thinned diaphragm, POCUS allowed us to define baseline personalized data useful to monitor the patient during follow-up.
POCUS technique can be used not only to detect the thinning of the diaphragm and to evaluate its function, but also to assess the morphology of the lung parenchyma in the different clinical conditions of the same patient. Lung ultrasound allows to differentiate the areas of opacity detected on chest radiography (pleural effusion, consolidations and atelectasis) with a sensitivity and specificity that exceeds 90% [14]. As demonstrated by several studies [11–16], LUS is able to differentiate between a consolidation (associated or not with pleural effusion) of an inflammatory/infective nature (which normally presents air dymanic and/or fluid broncograms) from a consolidation of an atelectasis nature (which normally presents itself as a subpleruic consolidation with static and parallel bronchograms) [15, 16] (Fig. 1e).
In particular, these ultrasound findings have an important clinical implication in this context.
If on the one hand the fragile condition of this category of patients results in an use of continuous radiological exams involving a frequent exposure to ionizing radiation and it is, therefore, essential to exploit the advantages of lung ultrasound as a noninvasive, reproducible, radiation‐free diagnostic tool; on the other hand these patients with SMARD1(as well as other neuromuscular patients)undergo various respiratory complications [1–3], in particular of an infectious and/or disventilatory type which, if studied and followed only with chest X-ray, risk always being treated with antibiotic therapy even when not necessary with the risk of developing antibiotic resistance, an undesirable condition in such fragile patients.
In our case, in particular, the chest X-ray (Fig. 1a) highlighted the presence of an area of reduced transparency in the left basal area identified as an area of suspected pleural effusion. Lung ultrasound instead characterized the aforementioned lesion in the left basal area as an air of atelectasis without detecting any pleural effusion (Fig. 1e).We were therefore able to personalize the treatment for our patient without administering useless antibiotic therapy having excluded the presence of a lung infectious process but by optimizing the treatments to resolve the lung disventilation (modification of the ventilatory setting and strengthening of respiratory physiotherapy).
Considering the growing incidence and survival of neuromuscular patients, further studies are needed to standardize the use lung and diaphragm bed-side un-invasive diagnostic/functional techniques need even in these categories of patients who require more and more personalized therapies and follow-ups.
Funding
Nothing to declare.
Declarations
Conflict of interests
Nothing to declare.
Ethical statement
The study was approved by the Institutional Review Board and Ethic Committee (prot.36173/19 ID2729).
Informed consent
Written informed consent was obtained from a parent or guardian before data collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Beatrice Berti and Danilo Buonsenso are first authors.
References
- 1.Saladini M, Nizzardo M, Govoni A, et al. Spinal muscular atrophy with respiratory distress type 1: clinical phenotypes, molecular pathogenesis and therapeutic insights. J Cell Mol Med. 2020;24(2):1169–1178. doi: 10.1111/jcmm.14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanoli F, Rinchetti P, Porro F, Parente V, Corti S. Clinical and molecular features and therapeutic perspectives of spinal muscular atrophy with respiratory distress type 1. J Cell Mol Med. 2015;19:2058–2066. doi: 10.1111/jcmm.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullmann N, D'Andrea ML, Gioachin A, et al. Lung ultrasound: a useful additional tool in clinician’s hands to identify pulmonary atelectasis in children with neuromuscular disease. PediatrPulmonol. 2020;55(6):1490–1494. doi: 10.1002/ppul.24760. [DOI] [PubMed] [Google Scholar]
- 4.Buonsenso D, Supino MC, Giglioni E, et al. Point of care diaphragm ultrasound in infants with bronchiolitis: A prospective study. PediatrPulmonol. 2018;53:778–786. doi: 10.1002/ppul.23993. [DOI] [PubMed] [Google Scholar]
- 5.Buonsenso D, Berti B, Palermo C, et al (2020) Ultrasound assessment of diaphragmatic function in type spinal muscular atrophy. PediatrPulmonol 55(7):1781-1788 [DOI] [PubMed]
- 6.Harlaar L, Ciet P, van der Ploeg AT, et al. Imaging of respiratory muscles in neuromuscular disease: a review. NeuromusculDisord. 2018;28:246–256. doi: 10.1016/j.nmd.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Fayssoil A, Behin A, Ogna A, et al. Diaphragm: pathophysiology and ultrasound imaging in neuromuscular disorders. J Neuromuscul Dis. 2018;5(1):1–10. doi: 10.3233/JND-170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin CE, Paratz JD, Bersten AD. Diaphragm and peripheral muscle thickness on ultrasound: intra-rater reliability and variability of a methodology using non-standard recumbent positions. Respirology. 2011;16(7):1136–1143. doi: 10.1111/j.1440-1843.2011.02005.x. [DOI] [PubMed] [Google Scholar]
- 9.Dubé BP, Dres M, Mayaux J, Demiri S, Similowski T, Demoule A. Ultrasound evaluation of diaphragm function in mechanically ventilated patients: comparison to phrenic stimulation and prognostic implications. Thorax. 2017;72(9):811–818. doi: 10.1136/thoraxjnl-2016-209459. [DOI] [PubMed] [Google Scholar]
- 10.Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, GentiloniSilveri N. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol. 2011;37(1):44–52. doi: 10.1016/j.ultrasmedbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Musolino AM, Tomà P, Supino MC, et al. Lung ultrasound features of children with complicated and non-complicated community acquired pneumonia: a prospective study. PediatrPulmonol. 2020;54:1479–1486. doi: 10.1002/ppul.24426. [DOI] [PubMed] [Google Scholar]
- 12.Tomà P. Lung ultrasound in pediatric radiology - cons. PediatrRadiol. 2020;50:314–320. doi: 10.1007/s00247-019-04524-z. [DOI] [PubMed] [Google Scholar]
- 13.Buonsenso D, De Rose C, Morello R, Lazzareschi I, Valentini P. Aspiration pneumonia in children with neurological disorders: a new indication for lung ultrasound? A case series. J Ultra. 2020 doi: 10.1007/s40477-020-00520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berce V, Tomazin M, Gorenjak M, Berce T, Lovrenčič B. The usefulness of lung ultrasound for the aetiological diagnosis of community-acquired pneumonia in children. Sci Rep. 2019;9(1):17957. doi: 10.1038/s41598-019-54499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi P, Vasishta A, Gupta M. Ultrasound of the pediatric chest. Br J Radiol. 2019;92(1100):20190058. doi: 10.1259/bjr.20190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovrenski J. Pediatric lung ultrasound - pros and potentials. PediatrRadiol. 2020;50(3):306–313. doi: 10.1007/s00247-019-04525-y. [DOI] [PubMed] [Google Scholar]