Abstract
Pseudopapillary solid tumour of the pancreas is a rare neoplasm that mainly affects young women in the second and third decade of life and less frequently children; originates from the exocrine component of the pancreas; and is characterized by slow growth, low potential for malignancy, and excellent prognosis following complete surgical resection. The tumour often presents as an asymptomatic abdominal mass that is accidentally detected during radiological investigations performed for other reasons. In this article, we report the clinical case of a 10-year-old girl who came to our observation for pain in the left hypochondrium, which had arisen for a week following a trauma; the imaging methods revealed a voluminous expansive pancreatic formation in the abdomen; on histological examination, the mass was a solid pseudopapillary tumour. Furthermore, we present a review of the literature aimed at highlighting the salient features of this neoplasm in paediatric age.
Keywords: Paediatric, Pseudopapillary solid tumour of the pancreas, Abdominal mass, Ultrasound, CT, MRI
Introduction
Pseudopapillary solid tumour (SPT) of the pancreas is a rare low-potential malignancy, first described by Virginia Frantz in 1959 as papillary cystic tumour in a 2-year-old boy [1]; the current name “solid-pseudopapillary” was adopted in 1996 by the World Health Organization (WHO), which includes this tumour in the international histological classification of exocrine pancreatic neoplasms [2, 3]. The SPT represents 2–3% of pancreatic neoplasms and 0.9–2.7% of exocrine tumours of the pancreas, affecting almost exclusively young women (over 82% of cases) with an average age of 30 [4, 5]. Although SPT is very rare, it represents the most common pancreatic neoplasm in paediatric age and is frequently localized to the body–tail [6]. The clinical presentation is variable. Children may have a palpable mass and/or abdominal pain, or be totally asymptomatic with occasional evidence of pancreatic expansion during radiological examinations performed for another cause; acute abdominal tumour rupture rarely occurs [7]. In most paediatric patients, SPT of the pancreas has a benign clinical course in the absence of metastases at the time of diagnosis, and the prognosis is favourable with excellent long-term results after complete surgical resection. In this article, we present the case of a 10-year-old girl with SPT of the pancreas, and a review of the literature that highlights the clinical, diagnostic, and therapeutic characteristics of this neoplasm in paediatric age.
Case report
In November 2020, a 10-year-old girl came to our attention sent by the treating paediatrician for persistent pain in the left hypochondrium, which arose a week earlier due to a punch received in the abdomen while playing with her little brother. The medical history revealed that the girl had previously been assisted in another hospital and had undergone abdominal ultrasound which revealed a voluminous expansive formation in the left upper quadrant. Subsequently, a magnetic resonance (MRI) of the abdomen and pelvis was performed without intravenous (IV) administration of contrast medium (contrast medium), which revealed a mass occupying the back cavity of the epiploons and showed no cleavage plane with the body and the tail of the pancreas. The lesion showed clear margins and a complex internal structure with inhomogeneous signal in both T1- and T2-weighted images due to the presence of a conspicuous blood component. The mass showed no diffusion restriction and appeared well delimited by the adjacent anatomical structures (Fig. 1). In the light of these findings, worthy of diagnostic study, the child was admitted to our hospital and subjected to laboratory and instrumental investigations. The blood count and blood chemistry tests were normal, and in particular, the values of haemoglobin and pancreatic enzymes were not altered; furthermore, tumour markers AFP, CA19-9, and CEA were negative. The ultrasound of the abdomen confirmed the presence of a voluminous rounded expansive formation, located medial to the spleen and cranial to the left kidney, inseparable from the body and tail of the pancreas; the mass showed solid, inhomogeneous echo structure, with necrotic/cystic hypo-anechoic areas, and central vascularization on colour Doppler; no other abdominal lesions and no free fluid in the peritoneum were evident (Fig. 2). Based on the diagnostic suspicion of pancreatic neoplasia, total body CT with contrast medium was performed for staging, and subsequently an ultrasound-guided biopsy with tru-cut, to establish a correct therapeutic approach. CT revealed no thoracic lesions, and highlighted the voluminous neoformation of the body–tail of the pancreas, with well-defined margins and inhomogeneous structure due to the presence of vascularized solid components, necrotic-hemorrhagic colliquative areas, and peripheral calcifications. In addition, the mass caused compression on the splenic vein, duodenal loops, and posterior gastric wall, while maintaining a cleavage plane. The spleen, kidney, and left adrenal gland were also well dissociable. There were no appreciable secondary lesions, nor free effusion (Fig. 3). The histological examination of the biopsy samples suggested the presence of an SPT of the pancreas and the girl was undergoing surgery. The laparotomy showed a voluminous expansive formation, originating from the body and tail of the pancreas, which displaced and did not infiltrate the surrounding anatomical structures. The mass was completely removed, preserving as much pancreatic parenchyma as possible. Surgical exploration revealed no metastases. Macroscopic examination of the surgical piece showed a neoformation, 8.5 cm in maximum diameter, apparently capsulated, and with a granular surface, partly hemorrhagic, with a soft-elastic consistency (Fig. 4). The microscopic investigation revealed a neoplasm with a solid, pseudopapillary and cystic pattern, typical mitosis, hemorrhagic areas, necrosis, calcifications, and foci of xanthogranulomatous reaction with giant cells around cholesterol needles. The immunophenotype was positive for vimentin, CKAE1/AE3, nuclear beta-catenin, cyclin D1, CD56, CD99, and synaptophysin; the Ki67 proliferation index was 10% (Fig. 5). The definitive histological examination therefore confirmed the diagnosis of SPT, which focal infiltrated the pseudo-capsule, but not the rim of the adhered pancreatic tissue. On the twelfth postoperative day, the little patient developed a low-flow pancreatic fistula treated conservatively, and she was discharged 30 days after surgery in good general condition, with normal laboratory tests and negative abdominal ultrasound. The girl was placed in a follow-up program that includes MRI abdomen with contrast medium alternating with ultrasound every 3 months for the first year and only MRI every 6 months for the next 2 years, then annually up to the fifth year.
Fig. 1.
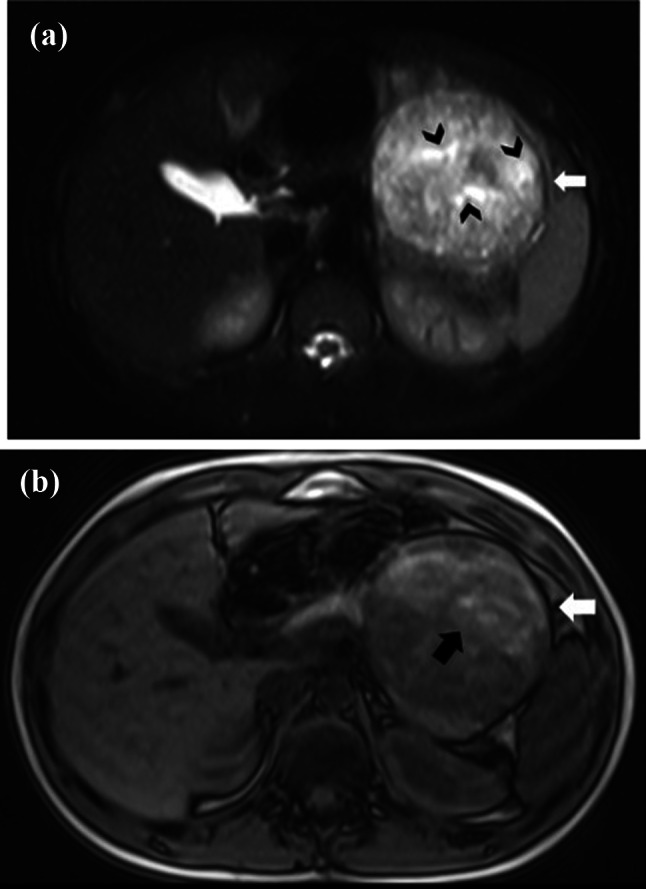
Axial fat-suppressed T2-weighted MR image a shows a well-defined lesion, heterogeneously hyperintense, in the body/tail of the pancreas, with multiple cystic foci (black arrowheads) and a fibrous pseudo-capsule seen as a hypointense rim (white arrow). On axial out-of-phase T1-weighted MR image (b), the fibrous pseudo-capsule is also hypointense (white arrow) and there is a large area of high signal intensity (black arrow), compatible with hemorrhage
Fig. 2.
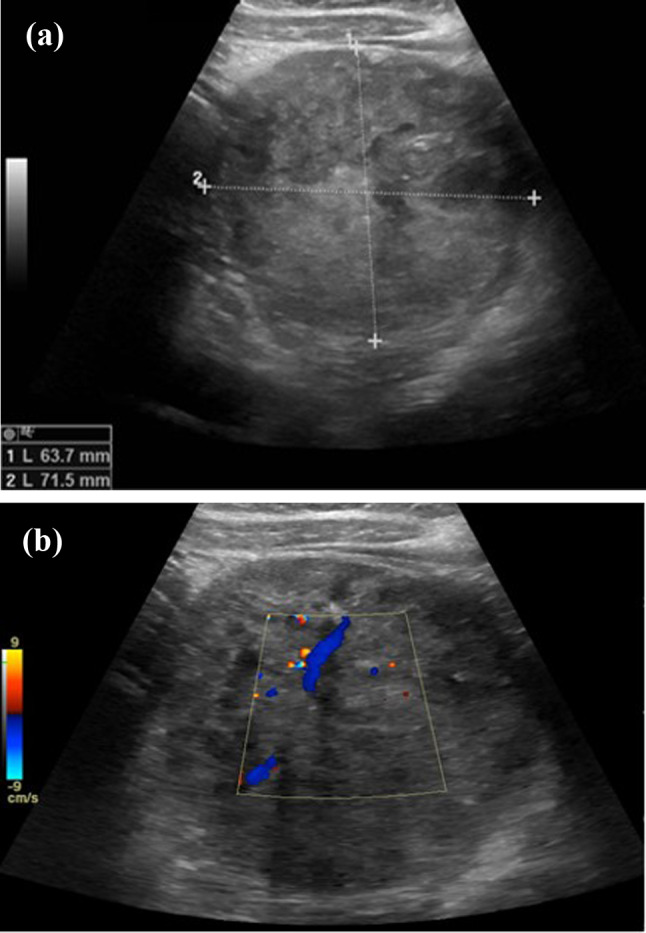
Upper abdomen ultrasound. The transversal scans detect a well-circumscribed heterogeneous hypoechoic expansive formation in the left hypochondrium, arising exophytically from the body and tail of the pancreas (a, b). Presence of intralesional flow on colour Doppler (b)
Fig. 3.
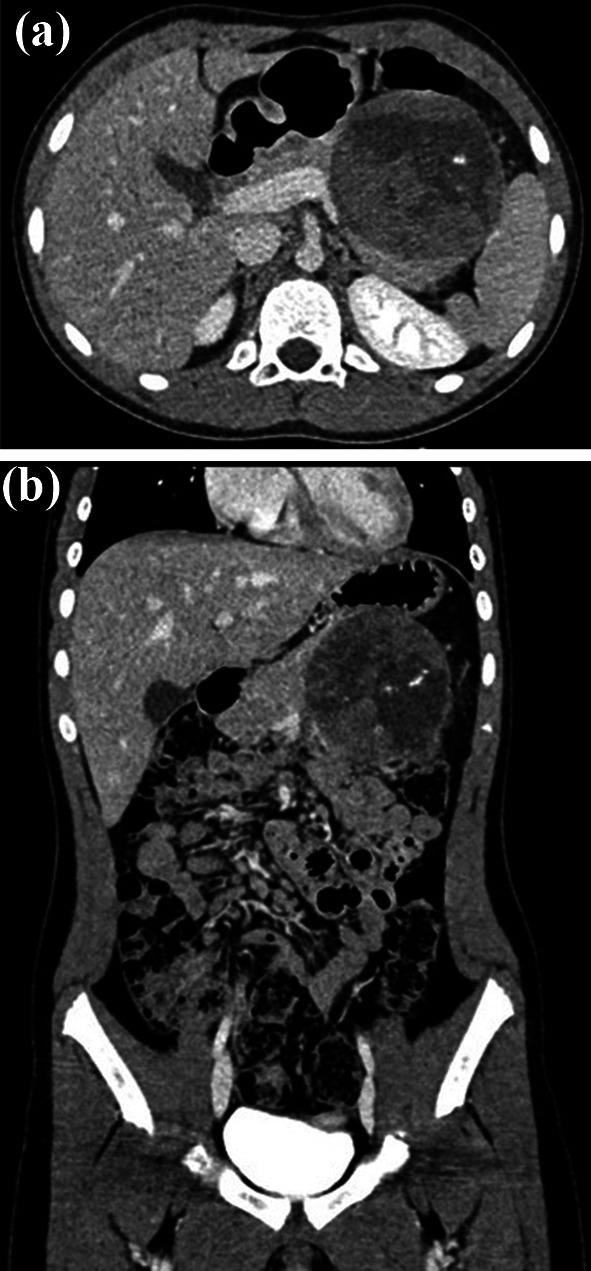
Axial (a) and coronal (b) post-contrast CT images, acquired in equilibrium phase, highlight a large solid mass of pancreatic body and tail, which shows a thin regular peripheral enhancement and inhomogeneous structure due to the presence of calcifications and hypodense areas consistent with necrosis, hemorrhage, and cystic components
Fig. 4.
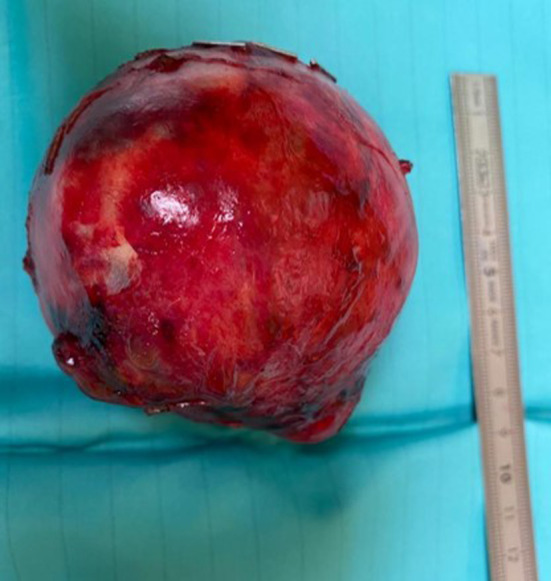
Macroscopic appearance of the mass
Fig. 5.
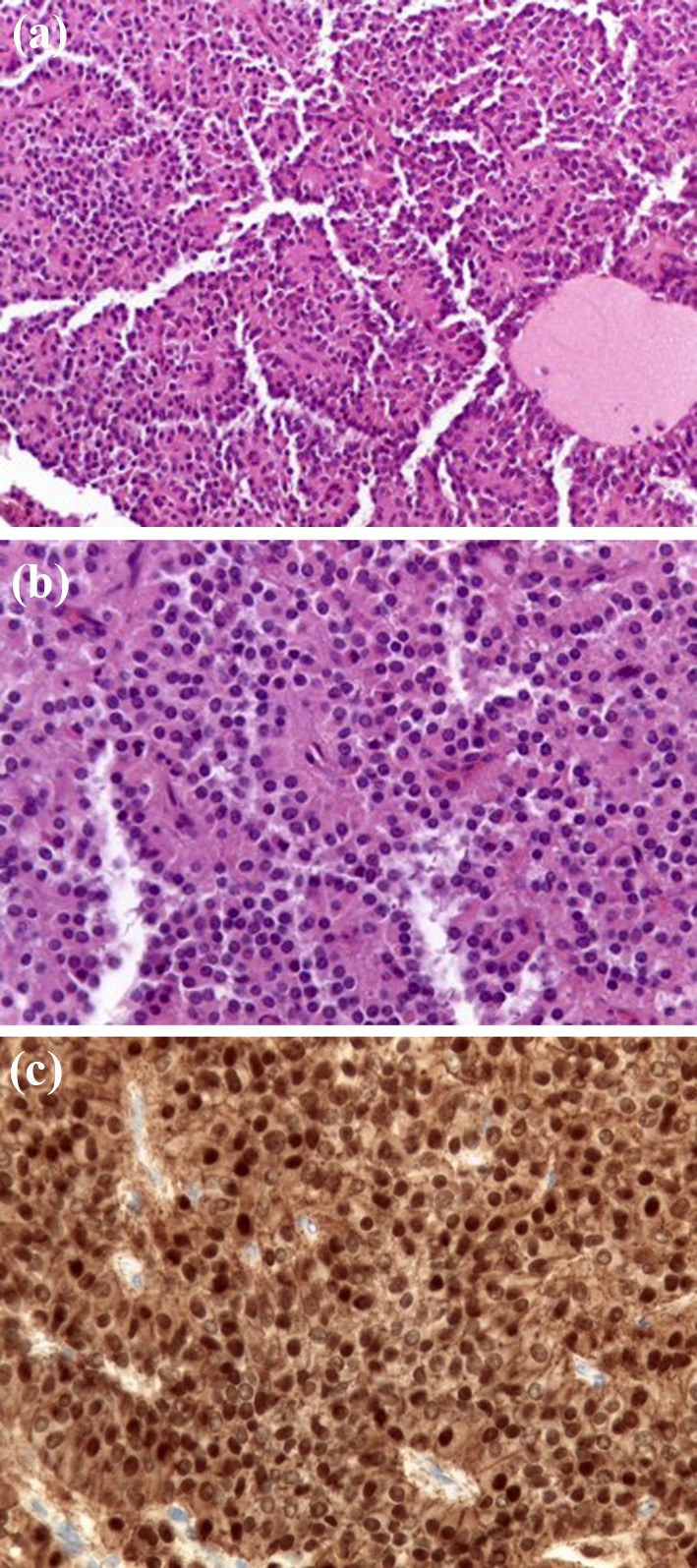
Microscopic histological examination. a The neoplasm shows delicate vascular network and degenerative pseudopapillary pattern. EE 100×. b Sheet of uniform, small cells, with eosinophilic cytoplasm and round to oval nuclei, finely dispersed chromatin and inconspicuous nucleoli. EE 400×. c The cells show nuclear immunostaining for β-catenin. 400×
Discussion
SPT is the most common pancreatic cancer in children with an incidence of about 8–16.6%. It mainly affects the female sex and occurs mainly in the second decade of life, while it is rare before the age of ten [8]. In a review of 523 paediatric patients with SPT, 433 (83%) were female and the mean age at diagnosis was 13.6 years (range 5–23 years) [9]. A recent work by Yang et al. included 20 children with SPT and all were over 5 years old (mean age 10.1 years, range 7–13 years) with a female-to-male ratio of 14: 6 [10]. Consistent with what has been reported in the literature, we have described the case of a 10-year-old female patient. Regarding the localization of the SPT in the pancreas, the results reported in the various studies are discordant. For some authors, the head was the most frequent site of cancer [10]. In contrast, in the retrospective study by Hwang et al., out of a total of 45 paediatric patients, 29 (64.4%) had a body-tail SPT, like our child [11]. Clinical manifestations are nonspecific. In the work of Lee et al., palpable mass was the most common presentation (60%), followed by abdominal pain (33.3%) [12]. In the review by Bender et al., however, the most frequent symptom of onset was abdominal pain (78%), and, moreover, in a small percentage of cases (8.1%), the tumour was found occasionally [9]. In fact, the PTS of the pancreas is a slow-growing neoplasm, which may give no sign of itself and be discovered only accidentally during instrumental investigations of the abdomen carried out for other reasons [11]. In our case, the pain referred to the left hypochondrium, which the little patient attributed to a minor abdominal trauma that occurred a week earlier, depended on a voluminous pancreatic expansive formation detected unexpectedly by the ultrasound examination. Less frequent initial symptoms include nausea, vomiting, weight loss, anorexia, and jaundice, and presentation of the disease with haemoperitoneum secondary to tumour rupture is rare [13]. Laboratory data relating to pancreatic and hepatic function, and oncological markers are almost always normal [1, 2, 4, 6, 10]. Generally, the neoplasm does not show local aggression and vascular invasion; moreover, a few cases with distant metastases have been reported at the time of diagnosis [12]. In the work of Hwang et al., only three children (6.6%) had metastases at onset, which were identified in the liver (two patients) and omentum (one patient) [11]. The diagnosis of PTS of the pancreas can be made on the basis of clinical findings and the results of instrumental examinations. Imaging usually shows a large, circumscribed, well delimited neoplasm, provided with a pseudo-capsule and with a complex structure due to the presence of solid components, haemorrhage, necrosis, and areas of cystic degeneration. The radiological characteristics of our lesion were consistent with what has been reported in numerous studies, including the presence of calcifications, which are observed in up to one-third of patients, mainly thin and peripheral [14]. Ultrasound is the first step in evaluating a pancreatic mass. In paediatric age, it has the advantage of being a non-invasive diagnostic method, of not using ionizing radiation, and of carefully exploring the pancreas thanks to the scarce presence of subcutaneous adipose tissue and to the rather large left hepatic lobe that provides an optimal acoustic window [15–17]. Ultrasound, in addition to identifying the pancreatic origin of the abdominal mass, perfectly recognizes the solid and cystic components of the neoformation, especially with the use of high-frequency linear transducers, and demonstrates intra-tumoral vascularization by colour Doppler [16–18]. Furthermore, under ultrasound guidance, it is possible to perform percutaneous pancreatic biopsy with a tru-cut needle, which allows the histological diagnosis of PTS and indicates the surgical intervention to remove the mass, as was the case in our case [4, 6]. In the study by Wang et al., which included 105 paediatric patients with abdominal-pelvic masses, 96.5% of children with malignancy had a complete histological diagnosis via ultrasound-guided biopsy and complications occurred in only 5 cases and mild entities such as pain (2 patients), bleeding from the biopsy site with spontaneous haemostasis (2 patients) and wound infection (1 patient). Therefore, ultrasound-guided percutaneous needle biopsy is an effective, accurate, safe, and minimally invasive procedure, which quickly identifies the type of cancer and positively affects the management of paediatric patients with abdominal or pelvic mass [19]. In the ultrasound suspicion of pancreatic neoplasia, MRI of the abdomen represents the most important diagnostic investigation, as it does not expose children to ionizing radiation and exploits the properties of magnetic fields to obtain images with high spatial resolution and contrast graphics [15]. The intrinsic multiparametricity and multiplanariness of this method allow to analyze in detail the morphology of the neoformation, its content, and its relationship with the adjacent anatomical structures; therefore, it provides information on the resection of the lesion and an accurate local staging of the tumour [20]. In our case, the MRI of the abdomen, carried out in another health facility and exhibited in vision, was performed without contrast medium, and highlighted an inhomogeneous neoformation of the pancreas with the characteristics of the SPT, which in most cases shows: pseudo-capsule with low intensity in both T1- and T2-weighted images, T1 hyperintense hemorrhagic areas and low or heterogeneous T2 signal, iso-hypointense solid components in T1 and slightly hyperintense in T2, and cystic foci with high signal intensity in T2 and low signal in T1 [4, 7, 10, 20]. The pseudo-capsule and the intralesional bleeding are considered typical findings of the PTS, and useful for the purpose of diagnosis, since they are rarely appreciable in other pancreatic neoplasms [7, 10, 14, 20]. The pseudo-capsule, composed of compressed normal pancreatic tissue and reactive fibrosis, after i.v. of contrast medium often shows early enhancement in the arterial phase both with respect to the healthy pancreatic parenchyma and to the solid components of the lesion, showing the latter gradual and heterogeneous filling in the late phases with less enhancement than the adjacent normal pancreas [7, 20–22]. Therefore, the dynamic post-contrast MRI study frequently detects a poorly vascularized lesion, and intralesional haemorrhage, found in more than 70% of patients, is certainly due to the inevitable growth process of the neoplasm supported by a slender vascular structure; however, the absence of bleeding does not exclude the diagnosis [4, 7, 10, 20]. Diffusion-weighted imaging (DWI) does not seem to be useful in evaluating the SPT of the pancreas, since sometimes the prevalence of areas of cystic degeneration does not hinder the movement of water molecules with consequent non-reduction of ADC (apparent diffusion coefficient) and absence of diffusion restriction, as in our case [10, 21, 22]. MRI usually shows a growth that compresses adjacent structures without invading them, and dilation of the main pancreatic duct is rare even in the presence of a large mass of the pancreatic head. As reported by Lee et al., pancreatic duct dilation and vascular invasion are strongly predictors of PTS malignancy, both in the presence and absence of metastases [4, 6, 7, 20, 23]. However, pancreatic duct dilation may also be associated with a benign SPT, suggesting that this finding should be interpreted with caution [22]. CT is a diagnostic technique that uses ionizing radiation and does not allow for an optimal assessment of a complex abdominal mass. The pancreatic lesion of our little patient was first studied with ultrasound and MRI, and subsequently with chest-abdomen CT examination with i.v. contrast medium performed for staging. Compared to MRI, CT is less accurate in demonstrating the structural characteristics of the tumour such as bleeding, cystic degeneration, and the presence of pseudo-capsule, but clearly identifies calcifications even of small dimensions [2, 14, 20, 22, 24]. The contrast pattern of the SPT in dynamic CT imaging is similar to that found in MRI. The lesion presents peripheral, heterogeneous, and early enhancement in the arterial phase, progressive, and inhomogeneous filling during the portal and equilibrium phases, and lower attenuation compared to the normal pancreas; furthermore, as our images show, slight late enhancement of the pseudo-capsule can be appreciable [4, 7, 20, 23–25]. On CT examination, it is difficult to identify highly indicative findings of PTS aggression such as perineural invasion and deep infiltration of the adjacent pancreas, sometimes detected by histological examination even in the absence of distant metastases [6, 9, 11–13, 23, 25]. However, the finding of a predominantly solid neoplasm in CT has been shown to be associated with a higher risk of malignancy and recurrence [4, 8, 9, 11, 13]. Although the PTS has distinctive features such as pseudo-capsule and intralesional haemorrhage, misdiagnosis is common [4, 7, 10, 14, 20]. In the study by Yu et al., only 24% of patients had a suspected PTS of the pancreas based on imaging findings prior to surgery [26]. In our case, the differential diagnosis was pancreatoblastoma (PB). In the work of Yang et al., the PB differed from the SPT in: high prevalence in children aged ≤ 5 years, elevated serum AFP in more than half of patients, larger size with poorly defined margins, higher incidence of calcifications, haemorrhage almost always absent, intra-tumoral vessels visible in the arterial contrast phase CT, invasion of peripancreatic vascular structures, and more frequent distant metastases, finally lower ADC values in the diffusion MRI study [10]. Despite the evident differences between the two neoplasms, the persistence of the diagnostic doubt made it necessary to subject the child to an ultrasound-guided biopsy to obtain a diagnosis of certainty and plan the correct treatment. On microscopic examination, the SPT typically presents solid and cystic areas, associated with a delicate vascular network. The solid component consists of uniform and polygonal small cells, with eosinophilic cytoplasm and oval nucleus, containing fragments of chromatin and nucleoli. The proliferative activity is usually low, reflecting the attenuated malignancy of the neoplasm. The characteristic histological aspect is the formation of pseudo-papillae with tumour cells that line up in layers to line a thin fibrovascular axis. The cystic spaces, of variable size and devoid of fibrous septae, are a consequence of the hemorrhagic-necrotic phenomena, due to the fragility of the vascular structure of the neoplasm; more or less extensive areas of histiocytes and/or cholesterol needles are frequently observed, occasionally calcifications [4, 7, 8, 24, 26]. The fibrous pseudo-capsule can sometimes be focal infiltrated, but not overcome by the neoplasm, as in our case, or the involvement of the surrounding pancreatic parenchyma is also evident. Deep infiltration of the adjacent pancreas, perineural invasion, angio-invasiveness, as well as marked cell pleomorphism, high-grade nuclear atypia, elevated mitotic index, and prominent necrobiotic cell nests (clusters of cells with nuclei pycnotics and eosinophilic cytoplasm) are indicative of malignant lesion [4, 8, 11–13, 23]. Immunophenotypically, the diagnostic marker is represented by nuclear positivity for beta-catenin. More than 90% of PTS patients have mutations in exon 3 of the beta-catenin CTNNB1 gene, which accumulates in the nucleus of cancer cells, resulting in increased expression of cyclin D1 and other genes that promote tumour progression [2, 6, 8, 9, 13, 26]. The potential malignancy of the SPT, defined only on definitive histological examination, requires the complete surgical removal of the neoplasm, preserving as much pancreatic parenchyma as possible and removing a large healthy margin of surrounding tissue to prevent local recurrence [2, 4, 7, 8, 11, 13, 14, 24, 25]. Depending on the location and extent of the PTS, different surgical procedures are possible, and patients with liver metastases can undergo resection of the primary tumour and synchronous or metachronous removal of repetitive lesions, with good results. Even if complete surgical resection of metastases or primary neoplasia is not feasible, surgical debulking is able to offer patients long survival after the operation [4, 6–8, 11, 13, 24, 26]. The role of neoadjuvant and/or adjuvant chemo-radiotherapy in the treatment of pancreatic SPT is still poorly defined, also due to the high percentage of resectable neoplasms [4, 8, 9, 12–14, 24, 26]. Most children recover after complete surgical removal of the tumour and overall survival 5 years after surgery exceeds 95%; local recurrence occurs in less than 10% of cases and usually within 4 years of surgical resection. Finally, even patients presenting with metastases or local invasion can survive for more than 10 years after the operation [1, 4, 7–9, 13, 24, 26].
Conclusion
In paediatric age, SPT must be strongly suspected in the presence of a pancreatic neoformation with typical imaging features such as large size, well-defined contours, mixed solid and cystic structure, and intralesional haemorrhage. Given the low potential for malignancy of the tumour and the excellent prognosis obtainable with complete surgical resection, it is essential to formulate a correct pre-operative diagnosis.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975, and its late amendments. Additional informed consented was obtained from all patients for which identifying information is not included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frantz VK (1959) Tumours of the pancreas. In: Frantz VK (ed) Atlas of tumour pathology. Section VII. Fascicles 27 and 28. Armed Forces Institute of Pathology, Washington, DC, pp 32–33. 10.1002/bjs.18004720344
- 2.Ozcan A, Arslanoglu C, Unal E, Patiroglu T, Ozdemir MA, Deniz K, Ozcan SS, Karakukcu M (2018) Evaluation of childhood solid pseudopapillary tumours of the pancreas. North Clin Istanb 5(3):207–210. 10.14744/nci.2017.27443 [DOI] [PMC free article] [PubMed]
- 3.Crucitti A, Grossi U, Giustacchini P, Tomaiuolo PM, Bellantone R. Solid pseudopapillary tumour of the pancreas in children: report of a case and review of the literature. Updates Surg. 2010;62(1):69–72. doi: 10.1007/s13304-010-0006-y. [DOI] [PubMed] [Google Scholar]
- 4.Berrada G, Belaaroussi S, Chbani K, Salam S, Laoudiyi D, Ouzidane L, Kebir AE, Guebessi NB, Benayad S, Mernissi F, Karkouri M, Anis S, Zemmouri MA (2020) Solid pseudopapillary tumour of the pancreas: a rare entity in children. Pan Afr Med J 35:137. 10.11604/pamj.2020.35.137.22404 [DOI] [PMC free article] [PubMed]
- 5.Buerke B, Domagk D, Heindel W, Wessling J. Diagnostic and radiological management of cystic pancreatic lesions: important features for radiologists. Clin Radiol. 2012;67(8):727–737. doi: 10.1016/j.crad.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Hager T, Kônigsrainer A, Gassner I, Klein-FrankeA SC, Hager J. Solid pseudopapillary tumour of the páncreas in a 12-year-old girl—7 years follow-up and histopathological reevaluation: case report and subject review. Eur Surg. 2010;42(2):96–102. doi: 10.1007/s10353-010-0525-2. [DOI] [Google Scholar]
- 7.Chung EM, Travis MD, Conran RM. Pancreatic tumours in children: radiologic-pathologic correlation. Radiographics. 2006;26(4):1211–1238. doi: 10.1148/rg.264065012. [DOI] [PubMed] [Google Scholar]
- 8.Papavramidis T, Papavramidis S. Solid pseudopapillary tumours of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(6):965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Bender AM, Thompson ED, Hackam DJ, Cameron JL, Rhee DS. Solid pseudopapillary neoplasm of the pancreas in a young paediatric patient: a case report and systematic review of the literature. Pancreas. 2018;47(10):1364–1368. doi: 10.1097/MPA.0000000000001183. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Gong Y, Ji M, Yang B, Qiao Z. Differential diagnosis of pancreatoblastoma (PB) and solid pseudopapillary neoplasms (SPNs) in children by CT and MR imaging. Eur Radiol. 2020 doi: 10.1007/s00330-020-07309-3. [DOI] [PubMed] [Google Scholar]
- 11.Hwang J, Kim DY, Kim SC, Namgoong JM, Hong SM. Solid-pseudopapillary neoplasm of the pancreas in children: can we predict malignancy? J Pediatr Surg. 2014;49(12):1730–1733. doi: 10.1016/j.jpedsurg.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Lee SE, Jang JY, Hwang DW, Park KW, Kim SW. Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch Surg. 2008;143(12):1218–1221. doi: 10.1001/archsurg.143.12.1218. [DOI] [PubMed] [Google Scholar]
- 13.Park JY, Kim SG, Park J. Solid pseudopapillary tumour of the pancreas in children: 15-year experience at a single institution with assays using an immunohistochemical panel. Ann Surg Treat Res. 2014;86(3):130–135. doi: 10.4174/astr.2014.86.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minh Xuan N, Khanh Tuong TT, Quang Huy H (2020) A rare case of large solid pseudopapillary tumour in a child. Am J Case Rep. 21:e923990. 10.12659/AJCR.923990 [DOI] [PMC free article] [PubMed]
- 15.Brillantino C, Rossi E, Baldari D, Minelli R, Bignardi E, Paviglianiti G, Restivo G, Cangemi MA, Zeccolini R, Zeccolini M. Duodenal hematoma in paediatric age: a rare case report. J Ultrasound. 2020 doi: 10.1007/s40477-020-00545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brillantino C, Rossi E, Bifano D, Minelli R, Tamasi S, Mamone R, Bignardi E, Zeccolini R, Zeccolini M, Vallone G (2020) An unusual onset of paediatric acute lymphoblastic leukemia. J Ultrasound. 10.1007/s40477-020-00461-y [DOI] [PMC free article] [PubMed]
- 17.Di Serafino M, Vitale V, Severino R, Barbuto L, Vezzali N, Ferro F, Rossi E, Caprio MG, Raia V, Vallone G. Paediatric ultrasonography of the pancreas: normal and abnormal findings. J Ultrasound. 2019;22(3):261–272. doi: 10.1007/s40477-018-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farina R, Foti PV, Iannace FA, Conti A, Pennisi I, Calcagno MC, Basile A. True congenital multicystic disease of the pancreas in the infant: a very rare case. J Ultrasound. 2020 doi: 10.1007/s40477-020-00472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Li F, Liu J, Zhang S. Ultrasound-guided core needle biopsy in diagnosis of abdominal and pelvic neoplasm in paediatric patients. Pediatr Surg Int. 2014;30(1):31–37. doi: 10.1007/s00383-013-3427-0. [DOI] [PubMed] [Google Scholar]
- 20.Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, Ros PR, Silverman SG. MR imaging features of solid pseudopapillary tumour of the pancreas in adult and paediatric patients. AJR Am J Roentgenol. 2003;181(2):395–401. doi: 10.2214/ajr.181.2.1810395. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues-Duarte H, Torrão H, Coelho P, Noruegas M, Sanches M. Solid pseudopapillary tumour of the pancreas in a child: imaging findings with diffusion-weighted MR imaging. JOP. 2013;14(2):195–198. doi: 10.6092/1590-8577/1185. [DOI] [PubMed] [Google Scholar]
- 22.Guerrache Y, Soyer P, Dohan A, Faraoun SA, Laurent V, Tasu JP, Aubé C, Cazejust J, Boudiaf M, Hoeffel C. Solid-pseudopapillary tumour of the pancreas: MR imaging findings in 21 patient. Clin Imaging. 2014;38(4):475–482. doi: 10.1016/j.clinimag.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Yu JS, Kim H, Kim JK, Kim TH, Kim KW, Park MS, Kim JH, Kim YB, Park C. Solid pseudopapillary carcinoma of the pancreas: differentiation from benign solid pseudopapillary tumour using CT and MRI. Clin Radiol. 2008;63(9):1006–1014. doi: 10.1016/j.crad.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Shet NS, Cole BL, Iyer RS. Imaging of paediatric pancreatic neoplasms with radiologic histopathologic correlation. AJR Am J Roentgenol. 2014;202(6):1337–1348. doi: 10.2214/AJR.13.11513. [DOI] [PubMed] [Google Scholar]
- 25.Hu S, Lin X, Song Q, Chen K. Solid pseudopapillary tumour of the pancreas in children: clinical and computed tomography manifestation. Radiol Med. 2012;117(7):1242–1249. doi: 10.1007/s11547-012-0854-2. [DOI] [PubMed] [Google Scholar]
- 26.Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q. Solid pseudopapillary tumour of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16(10):1209–1214. doi: 10.3748/wjg.v16.i10.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]


