Abstract
A single-center retrospective study reviewed the following sonographic features of 18 confirmed cases of localized cutaneous leishmaniasis to identify shared presentation patterns: echotexture, lesion borders, hypodermal involvement, soft-tissue changes, and vascular pattern. A second objective was to correlate these patterns with clinical characteristics, including sex, age, anatomical location, nodule vs. plaque presentation, raised borders, granulation tissue, swelling, hyperkeratotic crusting, disease onset, and healing time. Two main patterns were identified with high-frequency ultrasonography. The first pattern was characterized by a high level of inflammation and deep hypodermal involvement, while the second variant showed involvement limited to the dermis, with minimal inflammation. The "inflammatory pattern" showed ill-defined borders, mixed echotexture, prominent vascularity with central distribution, and was correlated with clinical signs of ulceration, granulation tissue, raised borders, and longer healing time (p < 0.05). The "pauci-inflammatory pattern" presented a well-defined structure with decreased echogenicity, reduced or absent vascularity with minimal soft-tissue changes, and was associated with a shorter healing time (p < 0.05).
Keywords: Ultrasound, Localized cutaneous leishmaniasis, Dermatology, Leishmaniasis
Introduction
Localized cutaneous leishmaniasis (LCL) encompasses a wide spectrum of vector-borne human diseases caused by protozoan parasites of the genus Leishmania [1]. The complex interplay between parasite virulence (Leishmania species and strains) and local immune response (variable recruitment of neutrophils and monocytes, and different T cell priming in the early phase) determines its clinical presentation [2]. LCL’s classical clinical presentation consists of an erythematous papule whose surface becomes hyperkeratotic, and finally ulcers in the center developing raised, indurated borders. However, some LCL variants do not ulcerate, while others disseminate in a sporotrichoid pattern and cause nodular lymphangitis.
Both the clinical and histological presentations of LCL are highly proteiform. In the early stage, the parasites are phagocytized by macrophages, which accumulate within the dermis. As the disease progresses, a variable degree of granulomatous dermal inflammation consisting of lymphocytes, epithelioid and multinucleated cells occurs, and changes according to the clinical picture. Dry lesions are characterized by epithelioid granuloma, whereas a diffuse lymphohistiocytic infiltrate, often extended to the septa and lobules of the hypodermis, has been found in ulcerative lesions [3]. Even though LCL dermal granulomata often show an ill-defined coalescent shape with prominent plasma cells [4], a high percentage of granulomatous lesions of unknown origin has proven to be hidden in patients with LCL [5].
Recently, LCL features have been described using high-frequency ultrasonography (US), as a thickened, irregular, hypoechoic dermal lesion with deep hypodermal involvement, showing signs of panniculitis [6]. Another study reported an increased dermal thickness in two cases of LCL presenting a non-ulcerated plaque/nodule, highlighting the loss of vascularization on Doppler Imaging and the restoration of normal dermal thickness as a response to treatment [7].
Materials and methods
A retrospective single-center study was performed to retrieve clinical and sonographic data from confirmed LCL cases treated from January 1, 2016 to April 1, 2020. Each patient recruited in the study had a single LCL lesion. All examinations were conducted in accordance with the Helsinki principles of medical ethics.
The US examination before treatment initiation was set as an inclusion criterion. The primary objective was to identify the sonographic patterns among our series. The secondary objective was to correlate each sonographic pattern with its corresponding clinical presentation and subsequent response to therapy.
The following US parameters were considered: echotexture, echogenicity, lesion borders, hypodermal involvement, soft-tissue changes, and vascular pattern.
The US exams were performed by a single examiner (AS) using a US machine (MyLab One/Touch US System Esaote) equipped with an 8–18 MHz linear array transducer. A slow-flow setting (frequency: 8 MHz, gain 35–48%, depth 20 mm and width 25 mm for color box dimensions, low-flow filter, single focus at the subungual posterior border, Pulse Repetition Frequency: 750–1000 Hz, gain 45–60%) was used for Power Doppler Imaging (PDUS). A subjective assessment of the vascular signal (rich, low, or absent flow) was performed on PDUS scans. Grayscale and PDUS images were obtained in the transverse and longitudinal planes.
The clinical diagnosis of LCL was confirmed by molecular analyses. Real-time Polymerase chain reactions were performed on fresh tissue specimens obtained from 4 mm punch biopsies. DNA was extracted using the DNAeasy Blood and Tissue kit (Qiagen, Hilden, Germany).
Results
Overall, 18 LCL zoonotic cases caused by Leishmania were identified.
Patients had a mean age of 17 ± 16.1 (range 4–61 years) with a male: female ratio of 3:1. The most common LCL anatomic locations were the face (9/18, 50%), the upper extremities (6/18, 33.3%), and the lower extremities (3/18, 16.7%). Patients were treated either with 1 mL intralesional meglumine antimoniate (Glucantime®) weekly injections (2/18, 11.1%), topical paromomycin 15% cream q.d. (1/18, 5.6%) or a combination of both (15/18, 83.3). Demographic data are summarized in Table 1.
Table 1.
Summary of the assessed clinical and sonographic features and relative frequencies
| Factors | Inflammatory pattern: 7 cases (%) | Pauci-inflammatory pattern: 11 cases (%) |
|---|---|---|
| Age of patients |
Mean: 18.14 SD: 14.5 |
Mean 17.18 SD: 17.2 |
| Disease onset before consultation (weeks) |
Mean: 4.57 SD: 1.5 |
Mean: 3.27 SD: 2.4 |
| Healing time (weeks) |
Mean: 19.57 SD: 3.1 |
Mean: 6.55 p<0.01 SD: 2.9 |
|
Therapy Paramomycin 15% cream alone Intralesional Glucantime® alone Combination |
0 1 (14.3) 6 (85.7) |
1 (9.1) 1 (9.1) 9 (81.8) |
| F: M ratio | 1:6 | 5:6 |
|
Localization: Face Upper extremities Lower extremities |
2 (28.6) 3 (42.9) 2 (28.6) |
7 (63.6) 3 (27.3) 1 (9.1) |
|
Clinical presentation: Nodule/papule Plaque |
1 (14.3) 6 (85.7) |
5 (45.5) 6 (54.5) |
| Ulceration | 6 (85.7) p<0.01 | 2 (18.2) |
| Granulation tissue | 6 (85.7) p<0.01 | 1 (9.1) |
| Erythematous swelling | 7 (100) p<0.01 | 1 (9.1) |
| Hyperkeratotic crusting | 1 (14.3) | 3 (27.3) |
| Raised borders | 7 (100) p<0.05 | 5 (45.5) |
|
Vascular signal: - High - Low - Absent |
7 (100) p<0.01 0 (0) 0 (0) |
0 (0) 6 (54.5) p<0.05 5 (45.5) p<0.05 |
|
Vascular pattern: - Mainly Central - Mainly Peripheral |
6 (85.7) p<0.01 1 (14.3) |
0 (0) 6 (100) p<0.01 |
|
Lesion borders: - Ill-defined - Well-defined |
6 (85.7) p<0.01 1 (14.3) |
0 (0) 11 (100) p<0.01 |
|
Echotexture -Hypoechoic -Hyperechoic -Mixed |
1 (14.3) 0 (0) 6 (85.7) p<0.01 |
11 (100) p<0.01 0 (0) 0 (0) |
| Hypodermal involvement | 7 (100) p<0.01 | 2 (18.2) |
|
Soft tissue changes: - Severe - Mild - Absent |
5 (71.4) p<0.01 2 (28.6) 0 (0) |
0 (0) 4 (36.4) 7 (63.6) p<0.01 |
Statistical analyses of nominal variables were performed by χ2 Test, whereas the T-student test for independent means was used for ordinal data. Significant features are set in bold. Percentages are reported in round brackets. Abbreviations: SD = Standard Deviation
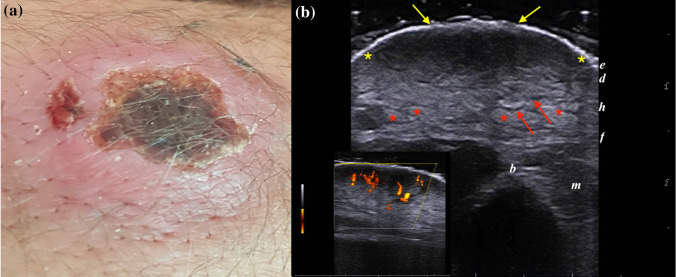
US examination in 7/18 (38.9%) cases showed an ill-defined hypoechoic lesion characterized by deep hypodermal involvement, peripheral and internal vascularity. The dermal layer was thickened, irregular, and hypoechoic, whereas the hypodermis showed increased echogenicity of fatty lobules and the dilation of interlobular spaces due to edema and inflammation. This pattern matched with those described by Saavedra et al. [6] and was consistent with subclinical panniculitis (Fig. 1).
Fig. 1.
a) Clinical and sonographic presentations of an 18-year-old male presenting an LCL plaque with a crusted center and raised margins, located on the right elbow. b) Greyscale ultrasound scan at 18 MHz detected an ill-defined dermo-hypodermal structure with mixed echogenicity (between yellow markers) extending up to the fascial plane. Note the loss of epidermal layer (yellow arrows), the increased echogenicity of fatty lobules (red markers), and the dilation of interlobular spaces (red arrows). PDUS Doppler in the bottom left box shows a prominent vascular signal in the center of the lesion, consistent with the inflammatory pattern
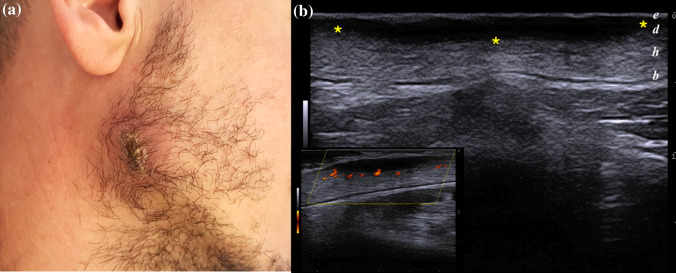
The remaining 11/18 (61.1%) cases presented a dermal or, rarely dermo-hypodermal, well-circumscribed homogeneous hypoechoic structure, with minimal reactive changes in the circumscribing soft tissue, including edema, thickening of interlobular spaces, destruction of adipose tissue, and a less intense vascular signal on PDUS, detected on lesion borders.
In contrast to the first pattern described, this variant was characterized by a low degree of inflammation, and matched the findings provided by Rojas Mora et al. [7], since the dermal thickening was the main sonographic finding, in absence of signs of panniculitis and deep subcutaneous involvement (Fig. 2).
Fig. 2.
a Thick crusted LCL plaque arising on the right mandibular angle of 28 years-old male. b) Well-defined hypoechoic homogeneous dermal structure (between markers), with minimal reactive changes of the underlying hypodermis. A mild peripheral vascular signal was identified on PDUS doppler in the bottom left corner
Statistical analysis showed a significant association between the first inflammatory pattern (IP) and a high vascular signal, central vascularity, ill-defined borders, mixed echotexture, hypodermal involvement, and severe tissue changes (p < 0.05). Conversely, the pauci-inflammatory pattern (PP) correlated with a low or absent vascular signal on PDUS, predominantly peripheral vascularity, well-defined borders, hypoechoic structure, and absent soft-tissue changes (p > 0.05).
As a secondary outcome, we attempted to correlate these two sonographic variants with the demographic (sex and age), anatomical, clinical (nodule vs. plaque, raised borders, granulation tissue, swelling, and hyperkeratotic crusting) and temporal (onset and healing times) characteristics.
The two groups presenting, respectively, IP and PP characteristics had a comparable mean age (18.14 ± 14.5 vs. 17.18 ± 17.2 years), and disease onset before consultation (4.57 ± 1.5 vs. 3.27 ± 2.4 weeks), but slightly differed in terms of F: M ratio (1:6 vs. 5:6) and face involvement (28.6% vs. 63.6%). They also showed significant differences in healing time after therapy administration (19.57 ± 3.1 vs. 6.55 ± 2.9 weeks, p < 0.01). Moreover, the IP positively correlated with the presence of ulceration, raised borders, and granulation tissue (p < 0.05).
Discussion
Localized cutaneous leishmaniasis represents an intermediate form between two opposites: mucosal and diffuse cutaneous immunophenotypes [2]. The control of leishmania mainly relies on an effective TH1 response producing high levels of INF-γ, which activates the macrophages to kill the amastigotes [8] and TNF-α to promote granuloma formation [9]. Excessive activation of this pattern leads to mucosal leishmaniasis. However, there is a mismatch between the disease severity and the host response. On the one hand, enhanced CD8 + cytotoxicity was associated with ineffective parasite destruction, increased inflammation, and disease progression towards ulceration, as upregulation of CD8 + cytolytic functions occurs concomitantly to the loss of skin integrity [10]. On the other hand, the development of an IL4-mediated TH2 response is not protective and was associated with a more severe course in a murine experiment [11]. As a consequence, a low level of TH1 polarizing cytokines leads to a high parasite burden, increased antibody production, and weak cell-mediated immunity, which results in diffuse cutaneous leishmaniasis [2]. We believe that the PP is the sonographic counterpart of a granulomatous response and reflects an effective delayed-type hypersensitivity reaction. In this case, the destruction of parasites occurs within well-defined dermo-hypodermal structures, associated with a mild consensual inflammatory reaction. Conversely, a harmful cell-mediated immune host response triggers inflammation and, consequently, ulceration, necrotic granulation tissue, neo-angiogenesis, and intercellular edema, which translates into a deep IP and a slower healing time. Probably, the penetration of topically administered drugs is less efficient in cases with deep soft-tissue involvement. These cases may require a higher number of intralesional administrations or be candidates for systemic therapy. In clinical practice, US is a useful tool not only to assess the degree of soft-tissue involvement in LCL, but also to monitor treatment response and investigate the underlying healing process. In the case of treatment failure or relapse, US evaluation could be an early indicator to shift from a topical to intralesional or oral therapy.
The differential diagnosis of LCL on US is broad. It should take into account a wide range of dermatologic disorders with a nodular/plaque presentation [12] arising on exposed areas of the skin, including abscess, pilomatrixoma, inflamed epidermal cyst, idiopathic facial aseptic granuloma (IFAG), vascular tumor or B-cell lymphoma and pyoderma gangrenosum. US could show a feeder vessel in the vascular tumor, hyperechoic calcium deposits and hypoechoic rim in pilomatrixoma, a connecting epidermal punctum or an onion-like appearance in the epidermal cyst, and a swirling internal content after manual compression in the soft-tissue abscess, thus providing useful clues to guide the diagnosis. The US features of vascular tumors is proteiform: Color-Doppler Imaging often detects high density and distribution of intralesional vessels, with a low resistivity index [13]. In this scenario, it is important to evaluate the degree of compressibility of the lesions, noting that a hard not-compressible mass must evoke the need for differential diagnosis [13]. The suspicion of malignancy should always raise in front of deep-seated lesions > 5 cm in diameter, heterogenous echotexture, rounded or lobulated shape, ill-defined borders and an infiltrating spreading pattern [12]. Color-Doppler interrogation may show anarchic internal vascularity, with multiple vascular poles or necrotic avascular areas. On the other hand, the lack of benignity criteria including a small size, superficial location, homogeneous echotexture and hypovascular pattern, is equally a red flag for concern [12].
In doubtful cases, the combination of US with a thorough clinical examination usually proves successful. For instance, B-cell lymphoma presents as a heterogeneous, mixed hypoechoic dermo-hypodermal nodule with prominent vascularity (high-flow arterial vessels) but lacks the superficial crusting or ulcerations (unpublished data). In children, both IFAG and LCL are often localized on the cheeks or eyelids, and their differential diagnosis can be tricky. In our experience, IAFGs present a well-circumscribed homogeneous hypoechoic dermal structure, devoid of internal vascularity, producing a posterior enhancement. However, other studies report a heterogenous presentation with an ill-defined posterior wall or increased vascularity of IAFG in early stages, which tends to evolve into a well-defined homogenous structure devoid of vascularization on color Doppler [14]. Literature is virtually lacking in US data of Pyoderma Gangrenosum: the sole published study reported a hypodermal involvement with fistula formation [15].
The use of a stand-off pad should be encouraged to increase the detection of peri- and intralesional vessels, which are often missed on PDUS [16]. In our clinical practice, we adopt an easy solution to minimize the external pressure and to enhance stability of handling the probe.[17]. Besides interposing a copious amount of gel, we place the fully extended fifth finger below the probe, in touch with the perilesional skin. In this way, we cautiously avoid any artefact produced by the manual compression and we are able to maintain a fixed distance between the target lesion and the probe.
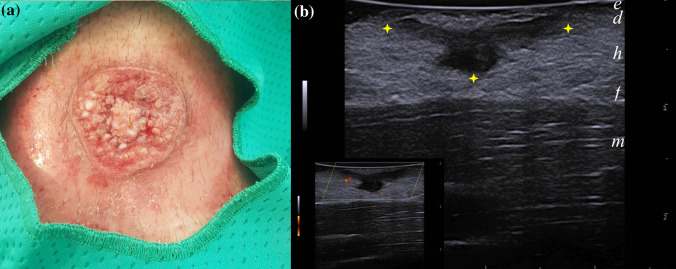
This study has several limitations. First of all, there is a lack of pathological correlation in most cases. This was pursued to avoid additional invasive procedures on sensitive sites, such as the face or in very young patients. Also, due to the retrospective nature of this observation, not all LCLs examined were in the same evolution stage; probably, some of the scanned lesions would later evolve into granulating ulcerative lesions or hyperkeratotic plaques. It is hard to predict whether the clinical evolution may cause a shift from a pauci-inflammatory to an inflammatory presentation on US. In our case study, two cases of fully developed ulcerative plaques still presented an IP (Fig. 3). It would also be interesting to evaluate whether bacterial superinfection, although not clinically present in our cases, could alter the US pattern.
Fig. 3.
a 19 years-old male presenting with an ulcerated plaque with hyperkeratotic bottom and raised borders of his left arm. b) Well circumscribed hypoechoic dermo-hypodermal band-like structure (between markers), devoid of internal vascularity. No reactive soft-tissue changes could be observed either on PDUS or B-mode greyscale scans. Abbreviations: e = epidermis; d = dermis; h = hypodermis; f = superficial fascia; b = bone; m = muscle
Moreover, the sonographic presentation and the degree of inflammation may change according to the anatomical site. For instance, panniculitis is mainly found at the extremities and trunk, where adipose tissue is abundant and well represented. In our series, 9/18 (50%) of LCL arose on the face, whereas in the paper of Saavedra et al. [6] 75% (15/20) of the lesions were located on the extremities. Further studies may assess whether the prevalence of the two US pattern varies according to the different topography. Lastly, a future study could evaluate the dynamic change of US presentation along with treatment administration as well as assess the scarring evolution or the long-term restoration of dermal and hypodermal layers of IP and PP by US.
In conclusion, these sonographic findings could be a starting point not only to understand LCL specific immune responses but also to monitor the clinical evolution and treatment response over time.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hepburn NC. Cutaneous leishmaniasis. Clin Exp Dermatol. 2000;25:363–370. doi: 10.1046/j.1365-2230.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- 2.Scott P, Novais FO. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. 2016;16(9):581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 3.Sharquie KE, Hameed AF, Noaimi AA. Panniculitis is a common unrecognized histopathological feature of cutaneous leishmaniasis. Indian J Pathol Microbiol. 2016;59(1):16–19. doi: 10.4103/0377-4929.178216. [DOI] [PubMed] [Google Scholar]
- 4.Thilakarathne IK, Ratnayake P, Vithanage A, Sugathadasa DP. Role of histopathology in the diagnosis of cutaneous leishmaniasis: a case-control study in Sri Lanka. Am J Dermatopathol. 2019;41(8):566–570. doi: 10.1097/DAD.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 5.Merino-Espinosa G, Corpas-López V, Díaz-Sáez V, et al. Cutaneous leishmaniasis by Leishmania infantum: behind granulomatous lesions of unknown aetiology. J Eur Acad Dermatol Venereol. 2018;32(1):117–124. doi: 10.1111/jdv.14506. [DOI] [PubMed] [Google Scholar]
- 6.Saavedra AC, Valencia BM, Tueros P, Wortsman X, Llanos-Cuentas A, Lavarello RJ. Ultrasonographic characteristics of cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2019;34(4):e193–e195. doi: 10.1111/jdv.16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas Mora E, Garrido Ríos A, Echeverría García B, Borbujo J. An unusual presentation of cutaneous leishmaniasis: the role of skin ultrasound. Actas Dermosifiliogr. 2019;110(2):171–174. doi: 10.1016/j.ad.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Novais FO, Nguyen BT, Beiting DP, et al. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J Infect Dis. 2014;209(8):1288–1296. doi: 10.1093/infdis/jiu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Maté A, Martínez-Sánchez D, Tardío JC, et al. Cutaneous leishmaniasis with histopathologic pattern of non-necrotizing granulomatous dermatitis in patients treated with adalimumab. Dermatol Online J. 2012;18(9):7. doi: 10.5070/D30C96W76K. [DOI] [PubMed] [Google Scholar]
- 10.Faria DR, Souza PEA, Durães FV, et al. Recruitment of CD8+ T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 2009;31(8):432–439. doi: 10.1111/j.1365-3024.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM (1990). Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med 171(1):115–27. [DOI] [PMC free article] [PubMed]
- 12.Catalano O, Varelli C, Sbordone C, et al. A bump: what to do next? Ultrasound imaging of superficial soft-tissue palpable lesions. J Ultrasound. 2020;23:287–300. doi: 10.1007/s40477-019-00415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito S, Ferrara D, Di Serafino M, et al. Classification and ultrasound findings of vascular anomalies in pediatric age: the essential. J Ultrasound. 2019;22(1):13–25. doi: 10.1007/s40477-018-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knöpfel N, Gómez-Zubiaur A, Noguera-Morel L, Torrelo A, Hernandez-Martin A. Ultrasound findings in idiopathic facial aseptic granuloma: case series and literature review. Pediatr Dermatol. 2018;35(3):397–400. doi: 10.1111/pde.13324. [DOI] [PubMed] [Google Scholar]
- 15.Pousa-Martínez M, Sánchez-Aguilar D, Aliste C, Vázquez-Veiga H. Usefulness of ultrasound in the diagnosis and follow-up of pyoderma gangrenosum. Actas Dermosifiliogr. 2017;108(10):962–964. doi: 10.1016/j.ad.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Corvino A, Sandomenico F, Corvino F, et al. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesions of the skin (2020). J Ultrasound. 23(1):45–53. [DOI] [PMC free article] [PubMed]
- 17.Catalano O, Alfageme Roldán F, Varelli C, et al. Skin cancer: findings and role of high-resolution ultrasound. J Ultrasound. 2019;22(4):423–431. doi: 10.1007/s40477-019-00379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]