Abstract
Objective:
We sought to compare gastroesophageal junction (GEJ) cancer and gastric cancer (GC) and identify clinicopathological and oncological differences.
Summary Background Data:
GEJ cancer and GC are frequently studied together. Although the treatment approach for each often differs, clinicopathological and oncological differences between the two have not been fully evaluated.
Methods:
We retrospectively identified patients with GEJ cancer or GC who underwent R0 resection at our center between January 2000 and December 2016. Clinicopathological characteristics, disease-specific survival (DSS), and site of first recurrence were compared.
Results:
In total, 2194 patients were analyzed: 1060 (48.3%) with GEJ cancer and 1134 (51.7%) with GC. Patients with GEJ cancer were younger (64 vs. 66 years; p<0.001), more often received neoadjuvant treatment (70.9% vs. 30.2%; p<0.001), and had lower pathological T and N status. Five-year DSS was 62.2% in patients with GEJ cancer and 74.6% in patients with GC (p<0.001). After adjustment for clinicopathological factors, DSS remained worse in patients with GEJ cancer (hazard ratio, 1.78; 95% confidence interval, 1.40–2.26; p<0.001). The cumulative incidence of recurrence was approximately 10% higher in patients with GEJ cancer (p<0.001). The site of first recurrence was more likely to be hematogenous in patients with GEJ cancer (60.1% vs. 31.4%; p<0.001) and peritoneal in patients with GC (52.9% vs. 12.5%; p<0.001).
Conclusions:
GEJ adenocarcinoma is more aggressive, with a higher incidence of recurrence and worse DSS, compared with gastric adenocarcinoma. Distinct differences between GEJ cancer and GC, especially in patterns of recurrence, may affect evaluation of optimal treatment strategies.
Mini-Abstract
Patients with gastroesophageal junction cancer are distinct and have worse disease-specific survival and approximately 10% higher cumulative incidence of recurrence, compared with patients with gastric cancer. The site of first recurrence is more likely to be hematogenous in gastroesophageal junction cancer (60.1% vs. 31.4%) and peritoneal in gastric cancer (52.9% vs. 12.5%).
Introduction
Esophageal cancer and gastric cancer (GC) are two of the most common cancers in the world, with an estimated combined annual incidence of 1.6 million cases and 1.3 million deaths in 2018 alone.1 The incidence of gastroesophageal junction (GEJ) cancer has been increasing, and a United States population–based analysis found a 2.5-fold increase in incidence during the last two decades2 as well as increasing incidence of non-cardia gastric adenocarcinoma in individuals aged <40 years.3 Although surgery remains the mainstay of curative treatment for GEJ cancer and GC, perioperative treatment for advanced disease has proven to be an important component of treatment.4–8 For locally advanced GEJ cancer and GC, perioperative treatment that includes neoadjuvant chemotherapy or chemoradiation combined with curative surgery is the preferred approach, and several randomized controlled trials (RCTs) have proven the oncological benefits of this strategy.4, 6, 7 In these studies, 25% to 74% of patients had GC.4, 6, 7
GEJ cancers are located between the distal esophagus and the proximal stomach and have traditionally been classified as Siewert type I, II, and III on the basis of the distance of the tumor’s epicenter from the GEJ.9 However, estimating the tumor’s epicenter and, thus, distance, from the GEJ by endoscopy is challenging, and therefore GEJ adenocarcinomas have often been lumped together with either esophageal cancer or GC in large studies, especially those from Western countries. The latest National Comprehensive Cancer Network guidelines recommend preoperative chemoradiotherapy or chemotherapy followed by surgery for advanced GEJ adenocarcinoma on the basis of the results of several RCTs.6–8, 10 In addition to patients with GC, these studies included patients with GEJ cancer, totaling between 24% and 64% of the reported cohorts.6–8 On the other hand, RCTs investigating perioperative chemotherapy for gastric adenocarcinoma from Eastern countries, which have a much higher incidence of gastric cancer and a lower incidence of GEJ cancer, included mostly patients with GC, with only 0%−3% of patients with GEJ cancer.11, 12 Furthermore, neoadjuvant treatment followed by surgery for patients with advanced GC is not commonly used in Eastern countries.13
The only RCT to investigate neoadjuvant chemoradiotherapy with perioperative chemotherapy alone for GEJ adenocarcinoma failed to prove a survival benefit for additional preoperative radiotherapy.5 As there are few reports in which patients with GEJ cancer were studied separately from those with GC or esophageal cancer, the optimal perioperative treatment for advanced GEJ adenocarcinoma remains unclear. The identification of clinical and oncological differences between patients with GEJ and gastric adenocarcinoma can enhance patient selection for clinical trials and inform the design of perioperative treatment strategies. The aim of this study was to identify important differences in clinicopathological features and oncological outcomes between patients with GEJ and gastric adenocarcinoma.
Methods
Patient Characteristics and Clinicopathological Data
After approval from the Memorial Sloan Kettering Institutional Review Board, patients were identified from a prospectively maintained institutional database. Inclusion criteria included curative resection for histologically confirmed primary GEJ or gastric adenocarcinoma. Exclusion criteria included noncurative resection, pathological stage IV disease, surgery for remnant GEJ cancer or GC, palliative resection, and synchronous GEJ cancer and GC. Tumor location was classified as GEJ or GC in the final pathological report by a dedicated gastrointestinal pathologist in accordance with the eighth edition of the AJCC staging system.14 For each case in which tumor location was classified as cardia or upper stomach, the detailed description of the surgical specimen was reviewed by a gastrointestinal specialist in pathology. Tumors with their epicenter ≤2 cm below the anatomical GEJ were classified as GEJ cancers. GEJ cancers with >75% of the tumor located above or below the anatomical GEJ were classified as upper or lower GEJ tumors, respectively. All other tumors were classified as middle GEJ tumors. Of the 2359 consecutive patients who underwent surgical treatment with curative intent at our center between January 2000 and December 2016, 2194 had R0 resection for primary GEJ (n=1060) or gastric adenocarcinoma (n=1134) (Supplemental Figure 1). Demographic and clinicopathological characteristics and treatment information were collected from the database and medical records. Tumor depth (T stage) and lymph node status (N status) were classified in accordance with the eighth edition of the AJCC staging system.14 Tumor size and vascular invasion were collected from the final pathologic report. CT scans of the chest, abdomen, and pelvis and, for many cases, endoscopic ultrasonography and PET scans were used for clinical staging. To rule out occult metastatic disease (determined by peritoneal nodules or a positive peritoneal cytologic result), staging laparoscopy was performed during surgery or before the initiation of neoadjuvant treatment for patients with clinical stage ≥II disease. In general, patients with clinical T stage ≥3 and/or positive N status were offered neoadjuvant treatment. The regimen of neoadjuvant treatment was selected on the basis of guideline recommendations or trial regimens. For patients with GEJ cancer, a platinum-based doublet regimen (e.g. carboplatin/paclitaxel, cisplatin/paclitaxel, and cisplatin/irinotecan) with or without concurrent radiotherapy of 41.4–50.4Gy or epirubicin-based triplet regimen (e.g., epirubicin/cisplatin/5-fluorouracil, epirubicin/cisplatin/capecitabine, epirubicin/oxaliplatin/capecitabine) was administered. For patients with GC, an epirubicin-based triplet regimen or FOLFOX (5-fluorouracil/oxaliplatin/leucovorin) was predominantly administered. Pathological tumor regression rates were assessed by an experienced pathologist on a scale between 0% and 100%.
A transthoracic approach including Ivor Lewis esophagectomy with 2-field lymphadenectomy was commonly performed for patients with GEJ cancer. A transabdominal approach with 1-field lymphadenectomy was performed for patients with GEJ cancer mainly located at the abdominal esophagus or distally. In terms of extent of abdominal lymphadenectomy, D2 dissection included perigastric nodes and nodes along the celiac trunk, left gastric artery, common hepatic artery, splenic artery, proper hepatic artery, and portal vein. D1 dissection included perigastric nodes. D2 dissection was commonly indicated to patients with GC, and D1 or D1+ dissection was indicated to patients with early GC. The extent of abdominal lymphadenectomy in surgery for GEJ cancer was the same as that in surgery for GC (i.e., perigastric nodes at the proximal stomach and nodes along the celiac trunk, left gastric artery, common hepatic artery, splenic artery, proper hepatic artery, and portal vein). Mediastinal lymphadenectomy included periesophageal, infracarinal, and hilar nodes when a transthoracic approach was used (with upper paratracheal and/or cervical lymphadenectomy if the tumor extended proximally to the mid esophagus); lower mediastinal nodes up to the level of the proximal margin and pericardial nodes were included with a transabdominal approach. Operative time was measured from skin incision to closing. Postoperative major complication was defined as those grade III or higher using the Clavien-Dindo classification.15
Follow-up and Recurrence Sites
Follow-up after resection consisted of clinical assessment, blood tests (complete blood count, chemistry panel, and, in many cases of GC, carcinoembryonic antigen level and carbohydrate antigen 19–9 level), and a CT scan of the chest, abdomen, and pelvis every 3 to 6 months for the first 2 years and every 6 to 12 months for years 3 to 5. The date of recurrence was determined by the date of the pathological diagnosis or the date of the CT scan if a pathologic specimen was not obtained. The site of first recurrence was categorized as peritoneal metastasis (including ovarian metastasis), hematogenous metastasis, distant lymph node metastasis (including nodes outside of the extent of a D2 resection), regional lymph node involvement (including the gastric bed and those within the extent of a D2 and/or mediastinal resection), and local sites of metastasis (including the anastomosis and gastric bed), as determined by confirmatory radiographic imaging or biopsy. Recurrence in the mediastinal lymph nodes was categorized as regional lymph node metastasis in GEJ cancer and distant lymph node metastasis in GC. Survival was calculated from the date of surgery, and disease-specific survival (DSS) was estimated from the date of surgery to the date of death from recurrent disease or the date of last follow-up, whichever occurred first. Death from recurrence was considered an event in the estimate of DSS.
Statistical Analysis
Patient demographic and clinicopathological characteristics, recurrence date and site, and survival were examined. Categorical variables were compared using the chi-square test or Fisher’s exact test, and continuous variables were compared using the Mann-Whitney U test. Survival analyses were conducted using the Kaplan-Meier method, with the log-rank test and Cox regression analysis. Multivariate Cox regression analysis was used to adjust for confounding factors. Factors with a significance level of p<0.1 in the univariate analysis were included in the multivariate analysis. Data were expressed as median (interquartile range [IQR]), odds ratio (OR; 95% confidence interval [CI]), or hazard ratio (HR; 95% CI), unless otherwise stated. p<0.05 (two-tailed) was considered to indicate statistical significance. Statistical calculations were conducted using SPSS software (version 25, IBM, Armonk, New York, USA). Competing risk analysis was performed to compare the cumulative incidence of recurrence between patients with GEJ cancer and patients with GC using R software (version 3.6.3, http://www.r-project.org).16
Results
Patient and Pathological Characteristics
In total, 2194 patients were included in the study: 1060 with GEJ cancer and 1134 with GC. Patient and pathological characteristics are shown in Supplemental Table 1. Compared with patients with GC, patients with GEJ cancer were younger (64 vs. 66 years; p<0.001) and were more frequently male (82.2% vs. 53.8%; p<0.001). Neoadjuvant chemotherapy was administered to 10.8% of patients with GEJ cancer versus 29.5% of patients with GC, and neoadjuvant chemoradiotherapy was administered to 60.1% of patients with GEJ cancer versus 0.7% of patients with GC (p<0.001). Regarding the regimen of neoadjuvant chemotherapy, a platinum-based doublet regimen was predominantly administered in patients with GEJ cancer (87.2%), whereas an epirubicin-based triplet regimen was predominantly administered in patients with GC (60.1%). Patients with GEJ cancer had a higher pathological tumor regression rate at the primary site (80% vs. 40%; p<0.001) and smaller tumors (2.5 vs. 3.5 cm; p<0.001). Postoperative pT stage and pN status were lower in patients with GEJ cancer (p<0.001). More patients with GC had poorly differentiated tumors (56.8% vs. 31.8%; p<0.001) and vascular invasion (45.0% vs. 33.1%; p<0.001) (Supplemental Table 1).
DSS
After a median follow-up of 43.0 months (IQR, 21.1–74.5), unadjusted 5-year DSS was 62.2% in patients with GEJ cancer and 74.6% in patients with GC (p<0.001) (Figure 1A). After adjustment for clinicopathological factors, patients with GEJ cancer had significantly worse DSS, compared with patients with GC (HR, 1.78; 95% CI, 1.40–2.26; p<0.001) (Table 1). Among patients who did not receive neoadjuvant treatment, unadjusted 5-year DSS was 75.9% in patients with GEJ cancer and 78.5% in patients with GC (p=0.473) (Figure 1B); however; after adjustment for clinicopathologic factors, DSS was significantly worse in patients with GEJ cancer (HR, 1.71; 95% CI, 1.23–2.36; p=0.001) (Table 2). Among patients who received neoadjuvant chemotherapy, unadjusted 5-year DSS was 56.5% in patients with GEJ cancer and 65.7% in patients with GC (p=0.005) (Figure 1C); this difference persisted after adjustment (HR, 1.78; 95% CI, 1.24–2.55; p=0.002) (Table 2). Of the 1095 patients who received neoadjuvant treatment, 147 with GEJ cancer and 33 with GC had a pathological complete regression at the primary site (Supplemental Table 1). In this subgroup, 5-year DSS was significantly worse in patients with GEJ cancer than in patients with GC (69.7% vs. 92.2%; p=0.026).
Figure 1.
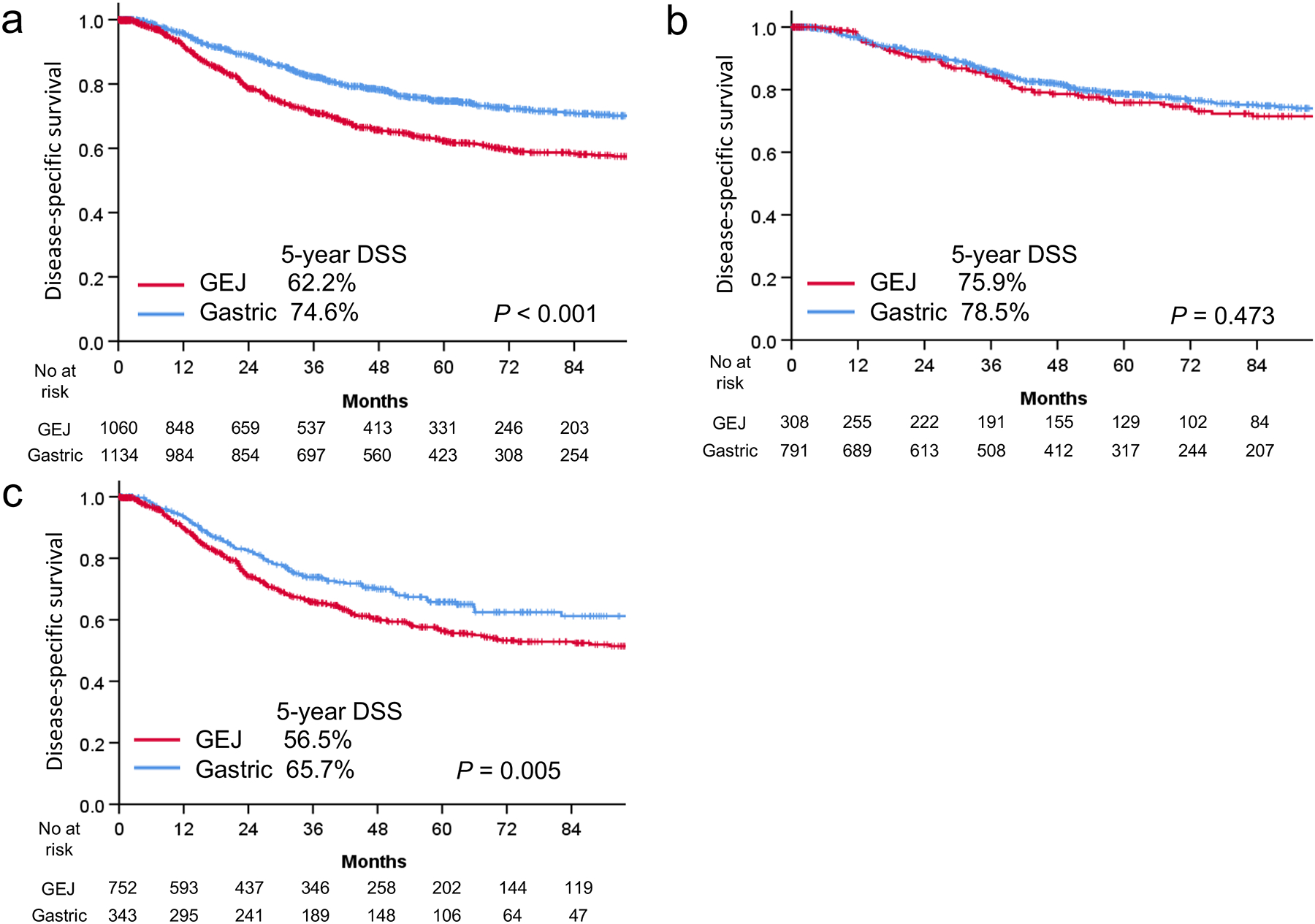
Disease-specific survival (DSS) in patients with gastroesophageal junction (GEJ) cancer and gastric cancer for the entire cohort (A), patients who did not receive neoadjuvant treatment (B), and patients who received neoadjuvant treatment (C).
Table 1.
Adjusted comparison of disease-specific survival between patients with gastroesophageal junction cancer and gastric cancer
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Location | ||||||
| Gastric | 1 | 1 | ||||
| GEJ | 1.64 | 1.39–1.93 | <0.001 | 1.78 | 1.40–2.26 | <0.001 |
| Year of surgery 2010–2016 | 0.82 | 0.70–0.98 | 0.025 | 0.80 | 0.67–0.95 | 0.013 |
| Age | 1 | 1.00–1.01 | 0.704 | |||
| Sex female | 0.76 | 0.63–0.91 | 0.002 | 0.86 | 0.71–1.05 | 0.133 |
| Neoadjuvant treatment | ||||||
| None | 1 | 1 | ||||
| Chemotherapy only | 1.85 | 1.50–2.28 | <0.001 | 1.53 | 1.22–1.92 | <0.001 |
| Chemoradiotherapy | 2.38 | 1.98–2.87 | <0.001 | 2.58 | 1.97–3.39 | <0.001 |
| pT | ||||||
| 1 | 1 | 1 | ||||
| 2 | 2.2 | 1.58–3.04 | <0.001 | 1.07 | 0.76–1.52 | 0.696 |
| 3 | 4.23 | 3.29–5.44 | <0.001 | 1.53 | 1.14–2.07 | 0.005 |
| 4 | 8.34 | 6.41–10.85 | <0.001 | 3.42 | 2.43–4.79 | <0.001 |
| Complete regression | 2.45 | 1.68–3.57 | <0.001 | 1.22 | 0.77–1.94 | 0.403 |
| pN | ||||||
| 0 | 1 | 1 | ||||
| 1 | 2.37 | 1.89–2.98 | <0.001 | 1.77 | 1.37–2.27 | <0.001 |
| 2 | 4.32 | 3.45–5.40 | <0.001 | 2.68 | 2.05–3.50 | 0.001 |
| 3 | 7.51 | 6.08–9.34 | <0.001 | 4.62 | 3.47–6.16 | <0.001 |
| Differentiation | ||||||
| Poorly | 1 | 1 | ||||
| Moderately | 0.75 | 0.63–0.89 | 0.001 | 0.85 | 0.71–1.02 | 0.084 |
| Well | 0.22 | 0.12–0.39 | <0.001 | 0.68 | 0.38–1.24 | 0.208 |
| Dissected nodes | 1.00 | 0.99–1.01 | 0.575 | |||
| Tumor size | 1.14 | 1.12–1.17 | <0.001 | 1.02 | 0.99–1.06 | 0.185 |
| Vascular invasion | 2.78 | 2.36–3.28 | <0.001 | 1.51 | 1.22–1.86 | <0.001 |
| Major complications | 1.06 | 0.84–1.33 | 0.615 | |||
| Adjuvant chemotherapy | 1.38 | 1.16–1.65 | <0.001 | 0.81 | 0.66–0.99 | 0.039 |
CI, confidence interval; GEJ, gastroesophageal junction; HR, hazard ratio. Major complications were defined as those with a Clavien-Dindo classification grade III or higher within 30 days of the operation.
Table 2.
Adjusted comparison of disease-specific survival stratified by neoadjuvant therapy and gastric location
| Variable | No. | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Neoadjuvant therapy (−) | |||||||
| Gastric | 791 | 1 | 1 | ||||
| GEJ | 308 | 1.11 | 0.83–1.49 | 0.473 | 1.71 | 1.23–2.36 | 0.001 |
| Neoadjuvant therapy (+) | |||||||
| Gastric | 343 | 1 | |||||
| GEJ | 752 | 1.38 | 1.10–1.73 | 0.006 | 1.78 | 1.24–2.55 | 0.002 |
| Neoadjuvant therapy (−) | |||||||
| GEJ | 308 | 1 | 1 | ||||
| Upper third | 81 | 1.33 | 0.81–2.17 | 0.263 | 0.88 | 0.52–1.50 | 0.638 |
| Middle third | 262 | 0.89 | 0.61–1.28 | 0.524 | 0.47 | 0.31–0.69 | <0.001 |
| Lower third | 401 | 0.81 | 0.58–1.14 | 0.229 | 0.58 | 0.41–0.83 | 0.003 |
| Neoadjuvant therapy (+) | |||||||
| GEJ | 752 | 1 | |||||
| Upper third | 59 | 0.87 | 0.55–1.37 | 0.540 | 0.51 | 0.29–0.91 | 0.022 |
| Middle third | 131 | 0.74 | 0.52–1.04 | 0.079 | 0.56 | 0.36–0.87 | 0.009 |
| Lower third | 147 | 0.62 | 0.45–0.86 | 0.005 | 0.57 | 0.37–0.89 | 0.014 |
CI, confidence interval; GEJ, gastroesophageal junction; HR, hazard ratio.
Comparison by Gastric Location
When patients were stratified by gastric location, among patients who received neoadjuvant treatment, those with tumors located in the middle or lower third of the stomach had significantly better DSS than patients with GEJ cancer, whereas DSS was not statistically different between patients with GEJ cancer and upper GC after adjustment (p=0.638) (Table 2). Among patients who had upfront surgery, DSS was significantly worse in patients with cancer in all gastric locations, compared with patients with GEJ cancer (Table 2).
Timing and Site of Recurrence
Of the 2194 patients in the overall cohort, 705 (32.1%) had a tumor recurrence during follow-up: 393 (37.1%) with GEJ cancer and 312 (27.5%) with GC. Five-year recurrence-free survival was 48.6% for patients with GEJ cancer and 62.1% for patients with GC (p<0.001). Competing risk analysis revealed that patients with GEJ cancer had an approximately 10% higher cumulative incidence of recurrence, compared with patients with GC (p<0.001) (Figure 2A). At the time of first recurrence, 122 patients (17.3%) had recurrences in multiple categories, including 77 (19.6%) with GEJ cancer and 45 (14.4%) with GC (p=0.069). Among all patients with a recurrence, peritoneal metastasis was more commonly observed in patients with GC (52.9% vs. 12.5%; p<0.001), and hematogenous metastasis was more frequently observed in patients with GEJ cancer (60.1% vs. 31.4%; p<0.001). There was no statistically significant difference in the incidence of distant lymph node metastasis (18.6% vs. 13.8%; p=0.088) or local site recurrence (15.8% vs. 11.5%; p=0.106). Regional lymph node metastasis was observed more often in patients with GEJ cancer (17.0% vs. 8.0%; p<0.001) (Figure 2B). Brain metastasis, which was categorized as hematogenous metastasis in this study, was observed in 27 patients with GEJ cancer and 1 patient with GC (all patients, 2.5% vs. 0.1%; p<0.001; patients with recurrence, 6.9% vs. 0.3%; p<0.001).
Figure 2.

A, Cumulative incidence of recurrence among patients with gastroesophageal junction (GEJ) cancer and gastric cancer. B, Comparison of recurrence between patients with GEJ cancer and patients with gastric cancer by site of recurrence. LNs, lymph nodes.
Discussion
In this study analyzing a large cohort of patients with long-term follow-up, we have clearly defined the distinct clinicopathological and survival differences and recurrence patterns between GEJ and gastric adenocarcinoma. Because so many Western trials tend to lump GEJ cancer and GC together, it is of paramount importance to define the unique aspects of these two distinct clinical subtypes of adenocarcinoma, as these differences have important implications for interpretation of clinical trials results and design of optimal treatment strategies, including those from Eastern countries. GEJ cancers and GCs are a heterogenous group of diseases—even within the subgroup of GC, at least four distinct molecular subtypes have been identified in The Cancer Genome Atlas.17 For GEJ cancers, the optimal treatment strategy (chemotherapy versus chemoradiation), surgical approach (Ivor Lewis versus total gastrectomy), and pathological classification (based on Siewert classification) remain to be determined, and debates regarding these topics persist.
The higher prevalence of men in the GEJ group could be explained by the known risk factors for GEJ cancer, including esophageal reflux disease, obesity, diets high in fats and red meat, and smoking, which may be more common in men than women.18–21 The difference in differentiation grade could be the result of differences in tumor heterogeneity between patients with GEJ cancer and patients with GC. GEJ adenocarcinoma generally develops from cardia intestinal metaplasia caused by chronic inflammation from gastroesophageal reflux and/or Helicobacter pylori infection,22 whereas gastric cancer has various histologic characteristics and causes, including infection with Helicobacter pylori and Epstein-Barr virus, and is believed to be more heterogenous.17, 23
In our study, patients with GEJ cancer more frequently received neoadjuvant chemoradiotherapy (60.1% vs. 0.7%; p<0.001), whereas patients with GC more often received neoadjuvant chemotherapy (29.5% vs. 10.8%; p<0.001), and perhaps for this reason patients with GEJ cancer had a better pathological tumor regression rate (80% vs. 40%; p<0.001). As described above, preoperative chemoradiation for locally advanced GEJ adenocarcinoma is recommended as a preferred treatment by the latest National Comprehensive Cancer Network guidelines on the basis of the results of several randomized trials, including the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study trial, which showed better survival after neoadjuvant chemoradiotherapy followed by surgery, compared with surgery alone.8, 10, 24 One RCT, one systematic review, and one review of the national clinical database comparing neoadjuvant chemoradiotherapy with neoadjuvant chemotherapy showed that additional preoperative radiotherapy provided higher rates of pathological complete regression and higher rates of R0 resection but no improvement in overall survival.25–27 In addition, a few RCTs that compared neoadjuvant chemoradiotherapy with surgery alone for GEJ carcinoma showed that additional preoperative radiotherapy failed to provide a survival benefit.8, 28 Therefore, whether additional preoperative radiotherapy confers a survival benefit in patients with GEJ adenocarcinoma remains unclear. At present, three ongoing randomized trials are comparing neoadjuvant chemoradiotherapy with neoadjuvant chemotherapy for esophagogastric adenocarcinomas—the results of these trials will help define the optimal preoperative approach for these patients.29–31
Overall, we found that patients with GEJ cancer had significantly worse DSS, compared with patients with GC (p<0.001), and this difference persisted after adjustment for clinicopathological factors and stratification by neoadjuvant treatment, with the exception of patients who had proximal tumors and patients who did not receive neoadjuvant treatment. The finding of poorer survival in patients with GEJ cancer was interesting, given that patients with GEJ cancer were younger and had smaller tumors, relatively better differentiation, better pathological tumor regression, and lower pT and pN status. To our knowledge, no large-scale studies have specifically compared survival between GEJ and gastric adenocarcinoma. The previously mentioned meta-analysis, which showed that additional radiotherapy results in higher rates of pathological complete regression without reducing the risk of death,26 suggests that downstaging by chemoradiotherapy might not be associated with an oncological benefit. However, among patients who did not receive neoadjuvant treatment and patients who had a complete regression after neoadjuvant treatment, patients with GEJ cancer had worse DSS than patients with GC. With the consideration that the cumulative incidence of recurrence was approximately 10% higher in patients with GEJ cancer and that patients with GEJ cancer had significantly worse DSS after adjustment for clinicopathological factors, our results suggest that GEJ adenocarcinoma is a more aggressive cancer and more likely to recur (and hematogenously), compared with gastric adenocarcinoma.
In the present study, among patients who had upfront surgery, DSS was not statistically different between patients with GEJ cancer and patients with proximal GC, and this is consistent with a previous report from our center.32 On the contrary, among patients who received neoadjuvant treatment, DSS was better in patients with cancer in all gastric locations, compared with patients with GEJ cancer. Although it is clinically difficult to classify GEJ cancer and proximal GC clearly and although the number of patients with proximal GC was relatively small, migration of the epicenter of the tumor after neoadjuvant treatment may explain this difference in DSS between patients who did and did not receive neoadjuvant treatment.
We also found that peritoneal metastasis was predominant as the first site of recurrence in patients with GC, and this is consistent with previous reports.33–35 However, we observed a clear difference in recurrence patterns between GEJ and gastric adenocarcinoma. We previously reported that Lauren intestinal type was related to distant metastasis and that diffuse-type cancer was related to peritoneal metastasis in patients with GEJ cancer and GC.36
Brain metastases are rarely observed in patients with esophagogastric cancer, with an incidence of 0% to 0.6%.37–40 We previously reported that brain metastases are found more frequently in patients with GEJ cancer and GC who have a pathological complete regression, compared with those without a pathological complete regression (36% vs. 4%; p=0.01),41 and that 2% of patients with resected esophageal cancers (squamous cell carcinoma and adenocarcinoma) have brain metastases.42 In the present study, we found that patients with GEJ cancer had a higher incidence of brain metastasis, compared with patients with GC (all patients, 2.5% vs. 0.1%; patients with recurrence, 6.9% vs. 0.3%), which suggests that GEJ cancer may require unique follow-up strategies, including systemic imaging such as PET-CT or CT of the head as well as the chest and abdomen, to find brain metastasis.
In addition to standard cytotoxic chemotherapy, targeted therapy that includes immune checkpoint inhibitors has emerged as a therapeutic option for advanced or metastatic GEJ cancer and GC following evidence of a survival benefit from several RCTs.43–45 All of these studies enrolled patients with GEJ cancer and patients with GC together.43–45 In the Trastuzumab for Gastric Cancer trial, which demonstrated a survival benefit with trastuzumab in patients with amplified or overexpressed human epidermal growth factor receptor 2, human epidermal growth factor receptor 2 was amplified or overexpressed in 33.2% of patients with GEJ cancer and 20.9% of patients with GC (p<0.001).43 In 2014, the Cancer Genome Atlas research network classified gastric adenocarcinoma (including GEJ adenocarcinoma) into four subtypes: positive for Epstein-Barr virus, microsatellite unstable, genomically stable, and chromosomal instability. This classification has since been used as a guide for patient stratification and design of trials of targeted therapy.17 However, no studies have compared the genomics profiles of GEJ cancer and GC. Further investigation of the differences in genomic profiles between GEJ and gastric adenocarcinoma could provide a more specific roadmap for targeted treatment strategies.
Our study has several limitations, including the potential of selection bias, which is inherent in any retrospective study design. Although curative resection with lymphadenectomy has been the main treatment for GEJ and gastric adenocarcinoma and the extent of resection has not been changed, the neoadjuvant chemoradiotherapy and chemotherapy regimens used in our study population varied during the 16-year study period because of updates to the guideline recommendations, and this might have influenced pathological and oncological outcomes. In addition, clinical follow-up strategies varied by surgeon and from patient to patient, and this might have influenced the precise timing of recurrence-related outcomes. In addition, that the extent of appropriate lymphadenectomy (either D2 abdominal only or 2-field) varies by tumor location could make it difficult to compare the exact location of nodal metastasis; nevertheless, patients who underwent a more aggressive lymphadenectomy (D2 + mediastinal) experienced recurrence more often, suggesting that the disease itself, rather than the lymphadenectomy, is responsible for this pattern.
In conclusion, GEJ and gastric adenocarcinoma differ significantly in recurrence patterns, DSS, and clinicopathological findings. GEJ adenocarcinoma has a more aggressive behavior, with higher recurrence rates, more hematogeneous recurrences, and worse DSS. In contrast, gastric adenocarcinoma has a less aggressive behavior, with lower recurrence rates, more peritoneal recurrences, and better DSS. These two distinct diseases need to be considered separately when treatment strategies and trials are designed and developed.
Supplementary Material
Supplemental Figure 1. CONSORT diagram of the study population. GEJ, gastroesophageal junction.
Acknowledgments
We gratefully acknowledge David B. Sewell of the Memorial Sloan Kettering Department of Surgery for editing this manuscript. We gratefully acknowledge Murray F. Brennan, MD, for providing critical review of the manuscript.
Financial Support:
This research was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosures:
SSY reported partial ownership of Attis Lab outside the submitted work. YYJ has received research funding from Rgenix, Boehringer Ingelheim, Bayer, Genentech/Roche, Bristol-Myers Squibb, Eli Lilly, and Merck and has served on advisory boards for Rgenix, Merck Serono, Bristol-Myers Squibb, Eli Lilly, Pfizer, Bayer, Imugene, Merck, Daiichi-Sankyo, and AstraZeneca, Zymeworks Inc, Seattle Genetics, and stock options from Rgenix. VWR has received clinical trial monies (institutional) from Genelux, Inc. and Genentech, travel reimbursement from Intuitive Surgical, and meeting prep reimbursement as Co-Chair of NCI Thoracic Malignancies Steering Committee. DRJ has served as a consultant for Merck and on the advisory panel for AstraZeneca. DM has served as a consultant for Johnson & Johnson, Boston Scientific, Urogen, and AstraZeneca. No other disclosures were reported.
Footnotes
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013; 23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson WF, Camargo MC, Fraumeni JF Jr., et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010; 303(17):1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 5.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009; 27(6):851–6. [DOI] [PubMed] [Google Scholar]
- 6.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393(10184):1948–1957. [DOI] [PubMed] [Google Scholar]
- 7.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29(13):1715–21. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16(9):1090–1098. [DOI] [PubMed] [Google Scholar]
- 9.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85(11):1457–9. [DOI] [PubMed] [Google Scholar]
- 10.Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019; 17(7):855–883. [DOI] [PubMed] [Google Scholar]
- 11.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007; 357(18):1810–20. [DOI] [PubMed] [Google Scholar]
- 12.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379(9813):315–21. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 15.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 16.Chappell R. Competing risk analyses: how are they different and why should you care? Clin Cancer Res 2012; 18(8):2127–9. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513(7517):202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chak A, Faulx A, Eng C, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer 2006; 107(9):2160–6. [DOI] [PubMed] [Google Scholar]
- 19.Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008; 44(3):465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang G, Li B, Liao X, et al. Poultry and fish intake and risk of esophageal cancer: A meta-analysis of observational studies. Asia Pac J Clin Oncol 2016; 12(1):e82–91. [DOI] [PubMed] [Google Scholar]
- 21.Kabat GC, Ng SK, Wynder EL. Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control 1993; 4(2):123–32. [DOI] [PubMed] [Google Scholar]
- 22.Balaji NS, DeMeester SR, Wickramasinghe KS, et al. Etiology of intestinal metaplasia at the gastroesophageal junction. Surg Endosc 2003; 17(1):43–8. [DOI] [PubMed] [Google Scholar]
- 23.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64:31–49. [DOI] [PubMed] [Google Scholar]
- 24.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366(22):2074–84. [DOI] [PubMed] [Google Scholar]
- 25.von Dobeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus 2019; 32(2). [DOI] [PubMed] [Google Scholar]
- 26.Petrelli F, Ghidini M, Barni S, et al. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis. Gastric Cancer 2019; 22(2):245–254. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sukhni E, Gabriel E, Attwood K, et al. No survival difference with neoadjuvant chemoradiotherapy compared with chemotherapy in resectable esophageal and gastroesophageal junction adenocarcinoma: results from the National Cancer Data Base. J Am Coll Surg 2016; 223(6):784–792 e1. [DOI] [PubMed] [Google Scholar]
- 28.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005; 6(9):659–68. [DOI] [PubMed] [Google Scholar]
- 29.Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016; 16:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 2017; 24(8):2252–2258. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds JV, Preston SR, O’Neill B, et al. ICORG 10–14: NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS). BMC Cancer 2017; 17(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach. Results and comparisons to distal adenocarcinoma of the stomach. Ann Surg 1997; 225(6):678–83; discussion 683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87(2):236–42. [DOI] [PubMed] [Google Scholar]
- 34.Wu CW, Lo SS, Shen KH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg 2003; 27(2):153–8. [DOI] [PubMed] [Google Scholar]
- 35.Barbetta A, Sihag S, Nobel T, et al. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J Thorac Cardiovasc Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Chang KK, Yoon C, et al. Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg 2018; 267(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch G, Ross HJ, Patel NP, et al. Incidence of brain metastasis from esophageal cancer. Dis Esophagus 2017; 30(9):1–6. [DOI] [PubMed] [Google Scholar]
- 38.Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med 2018; 7(8):3662–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esmaeilzadeh M, Majlesara A, Faridar A, et al. Brain metastasis from gastrointestinal cancers: a systematic review. Int J Clin Pract 2014; 68(7):890–9. [DOI] [PubMed] [Google Scholar]
- 40.Kasakura Y, Fujii M, Mochizuki F, et al. Clinicopathological study of brain metastasis in gastric cancer patients. Surg Today 2000; 30(6):485–90. [DOI] [PubMed] [Google Scholar]
- 41.Fields RC, Strong VE, Gonen M, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer 2011; 104(12):1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobel TB, Dave N, Eljalby M, et al. Incidence and risk factors for isolated esophageal cancer recurrence to the brain. Ann Thorac Surg 2020; 109(2):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376(9742):687–97. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383(9911):31–39. [DOI] [PubMed] [Google Scholar]
- 45.Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018; 392(10142):123–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CONSORT diagram of the study population. GEJ, gastroesophageal junction.


