Abstract
Objective:
To explore the combined effect of pediatric sickle cell disease (SCD) and preterm birth on cognitive functioning.
Method:
Cognitive functioning was examined in children ages 6 to 8 with high risk SCD genotypes born preterm (n=20) and full-term (n=59) and lower risk SCD genotypes/no SCD born preterm (n=11) and full-term (n=99) using tests previously shown to be sensitive to SCD-related neurocognitive deficits. Factorial ANOVAs and log linear analyses were conducted to examine the relationship between SCD risk, preterm birth status, and cognitive outcomes. Continuous scores were examined for specific tests. Children were categorized as having an abnormal screening outcome if at least one cognitive score was ≥1.5 standard deviations below the population mean.
Results:
Children with elevated risk due to high risk SCD and preterm birth performed worse than other groups on a test of expressive language but not on tests that emphasize processing speed and working memory. There was a three-way interaction between preterm status, SCD risk, and abnormal screening outcome, which was largely driven by the increased likelihood of abnormal cognitive scores for children with high risk SCD born preterm.
Conclusions:
The combination of SCD and preterm birth may confer increased risk for language deficits and elevated rates of abnormal cognitive screenings. This suggests that neurodevelopmental risk imparted by comorbid SCD and preterm birth may manifest as heterogenous, rather than specific, patterns of cognitive deficits. Future studies are needed to clarify the domains of cognitive functioning most susceptible to disease-related effects of comorbid SCD and preterm birth.
Keywords: premature birth, cognitive functioning, double hit, chronic illness, brain development, neurocognitive risk
Pediatric sickle cell disease (SCD) and preterm birth are medical conditions associated with increased risk for poor neurodevelopmental outcomes due to their impact on early brain development. Infants with SCD are at increased risk for prematurity, with a 29% elevated risk for low birthweight and a 79–92% elevated risk for very low birthweight and very preterm birth (Whiteman et al., 2013). Both conditions contribute to mild to moderate cognitive deficits in some affected children and share common areas of brain involvement; however, despite this comorbidity, little is known about the combined impact of SCD and preterm birth on neurodevelopmental outcomes. SCD is a complex genetic condition that significantly impacts physical and psychological functioning in approximately 1 of every 365 children of African descent born in the United States (Barbarin & Christian, 1999; Edwards et al., 2005). SCD is marked by the production of abnormal hemoglobin and its effects on functioning are widespread, including both physical impairments as well as impairments in cognitive and behavioral functioning (Bakri, Ismail, Elsedfy, Amr, & Ibrahim, 2014; Hijmans et al., 2010). Higher-risk genotypes of SCD (e.g., HbSS, HbSβ0) experience more severe complications, while lower risk genotypes (e.g., HbSC, HbSβ+) are generally associated with milder symptoms (Brosseau, Owens, Mosso, Panepinto, & Steiner, 2010). Children with SCD are at increased risk for heterogenous medical complications, including medical comorbidities such as preterm birth and pain as well as life-threatening complications such as acute chest syndrome and cerebral infarction (Piel, Steinberg, & Rees, 2017; Whiteman et al., 2013).
Cerebral infarction, also referred to as stroke, represents one of the most concerning complications of SCD due to its long-term impacts on neurocognitive and behavioral functioning. There are two primary presentations of cerebral infarction in the SCD population which include overt stroke and silent cerebral infarction. Children with SCD are at significantly increased risk for overt stroke compared to the general population, with approximately 10% of youth with more severe SCD genotypes experiencing an overt stroke before the age of 20 if no preventative treatment is available (Bernaudin et al., 2011; Powars, Wilson, Imbus, Pegelow, & Allen, 1978). Overt strokes are most commonly identified by accompanying physical symptoms (e.g., hemiparesis) and are typically associated with the narrowing or occlusion of larger blood vessels in the brain (Sundd, Gladwin, & Novelli, 2019).
Silent cerebral infarction, marked by the absence of observable symptoms, is the most common form of neurologic complication for children with SCD and is estimated to occur in 37% of children before the age of 14 (Bernaudin et al., 2011; Wang et al., 2008). Silent cerebral infarction is typically the result of occlusion in smaller blood vessels in areas of the brain with decreased blood flow (Bernaudin et al., 2011; Pegelow et al., 2001). Risk factors for silent cerebral infarction include decreased oxygen delivery to the brain due to lower hemoglobin levels and injury to cerebral vasculature (e.g., narrowing of blood vessels), although the precise mechanisms underlying silent cerebral infarction are unclear (Bernaudin et al., 2015; DeBaun et al., 2012) Silent cerebral infarction is a risk factor for the occurrence of later overt strokes, with studies finding a 14-fold increase in risk for overt stroke among children with a prior SCI (Miller et al., 2001). The rates of overt stroke and silent cerebral infarction are notably higher for children diagnosed with high-risk genotypes of SCD compared to lower risk genotypes (Manara et al., 2017; Prengler, Pavlakis, Prohovnik, & Adams, 2002; Prussien, Jordan, DeBaun, & Compas, 2019). Chronic transfusion therapy and hydroxyurea are the current standards of care for primary and secondary stroke prevention (Verduzco & Nathan, 2009).
Both overt stoke and silent cerebral infarction can result in significant tissue damage. Brain imaging on children with SCD who have experienced cerebral infarction reveals the highest density of cerebral infarctions within the white matter of the frontal and parietal lobes (Brown et al., 2000; Ford et al., 2018; Pegelow et al., 2002). However, widespread decreases in white matter density, which are thought to reflect axonal loss, demyelination, and cortical thinning, have been identified among children with SCD with no history of cerebral infarction (Choi et al., 2019; Kawadler et al., 2013; Manara, et al., 2021; Schatz, Finke, Kellett, & Kramer, 2002). In addition to structural changes, there is evidence of disruptions in brain connectivity in areas of the brain associated with higher order cognitive functions (Colombatti et al., 2016). Indeed, there is research indicating that more indirect pathophysiological mechanisms such as widespread inflammation and oxygen deprivation that are associated with SCD as well as non-medical factors may play a role (Brown et al., 1993; Kawadler et al., 2013; Prussien et al., 2020).
The areas of cognition impacted by these neurological complications are widespread. Children with SCD show deficits in both general intellectual functioning and specific domains of functioning (Berkelhammer et al., 2007; Hogan et al., 2006). Higher order processes such as executive functioning as well as attentional processes appear to be particularly vulnerable to sickle cell related disease mechanisms (Berkelhammer et al., 2007; Hijmans et al., 2010), while findings on impairments in areas such as memory, language, and visuomotor functioning are more mixed (Berkelhammer et al., 2007). Research on cognitive functioning in pediatric SCD has largely focused on domain specific deficits; however, there is some evidence for more heterogeneous patterns of deficits across cognitive domains (Schatz, Finke, & Roberts, 2004; Schatz, Puffer, Sanchez, Stancil, & Roberts, 2009). Academic performance is also impacted, with youth with SCD showing higher rates of school absences, grade retention, and receipt of special education services than their peers (Berkelhammer et al., 2007; Epping et al., 2013; Karkoska, Haber, Elam, Strong, & McGann, 2021; Schatz, Brown, Pascual, Hsu, & DeBaun, 2001). For both cognitive and academic functioning, the level of impairment correlates with the severity/type of cerebral infarction, with children with overt stroke evidencing the greatest impairment, followed by silent cerebral infarction and, lastly, those with no history of cerebral infarction (DeBaun et al., 2012).
Preterm birth, broadly defined as birth before 37 weeks of gestation, occurs in approximately 10% of births in the general population and is the leading cause of neonatal death worldwide (Blencowe et al., 2013). There are multiple risk factors for the occurrence of preterm birth that include genetics, maternal factors (e.g., advanced maternal age), multiple pregnancies, lifestyle factors (e.g., stress, substance use), and socioeconomic status (Blencowe et al., 2013). The long-term medical complications of survivors of preterm birth are varied and include increased risk for vision and hearing impairments, chronic lung disease, and cardiovascular disease (Blencowe et al., 2013). In addition to these physical complications, infants born preterm are at increased risk for adverse neurodevelopmental outcomes, with studies showing lower scores on broad measures of intelligence (i.e., IQ quotients) as well as more specific domains (Allotey et al., 2018). The most commonly reported deficits are in executive functioning (e.g., working memory) and attentional processes (e.g., processing speed; Allotey et al., 2018; Brydges et al., 2018; Böhm, Smedler, & Forssberg, 2004; Lundequist, Böhm, & Smedler, 2013). However, research has also noted marked heterogeneity in cognitive outcomes and developmental trajectories following preterm birth, which has been attributed to the variable impact of preterm birth on brain development (Dimitrova et al., 2020; Stålnacke, Lundequist, Böhm, Forssberg, & Smedler, 2015) Academic achievement and behavioral functioning are also impacted, with children born preterm scoring lower on tests of reading, spelling, and mathematics and evidencing increased symptoms of externalizing behavioral disorders such as ADHD (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Breeman, Jaekel, Baumann, Bartmann, & Wolke, 2016). These deficits often persist from infancy through adulthood (Baron & Rey-Casserly, 2010; Breeman, Jaekel, Baumann, Bartmann, & Wolke, 2015)
The elevated risk for poor neurodevelopmental outcomes is thought to be linked to alterations in brain development. Preterm birth occurs during the third trimester, a critical period for brain development, which can increase susceptibility for brain insults during infancy and childhood (Vandewouw et al., 2019). Although many brain structures are implicated, disruptions in white matter development are most common and have been linked to specific impairments in neurodevelopmental functioning among preterm children (Counsell et al., 2008; Volpe, 2019). Longitudinal brain imaging studies have found that preterm children evidence decreased total brain volume, as well as slower maturation of specific brain regions beginning in early childhood (Vandewouw et al., 2019; Batalle et al., 2017). Dysconnectivity in neural networks linked to cognitive and behavioral functioning has also been identified among children born preterm (Boardman & Counsell, 2019).
SCD and preterm birth are independently associated with alterations in brain development; however, the combined impact of both conditions on neurodevelopment outcomes is unknown. The presence of co-occurring conditions that disrupt white matter development and are associated with cognitive impairments are suggestive of an additive effect on cognitive abilities. Indeed, preterm birth has been shown to be a specific risk factor for cognitive impairments for children with neurodevelopmental disorders such as autism (Brayette et al., 2019). Furthermore, studies have identified greater cognitive impairment for preterm infants that sustain early brain insults (e.g., periventricular hemorrhage) than those without such early insults (Sherlock, Anderson, Doyle, & Victorian Infant Collaborative Study Group, 2005; Vohr et al., 2014). There are parallel findings of greater cognitive impairment following repetitive brain insults in the adult traumatic brain injury (TBI) literature (Belanger, Spiegel, & D Vanderploeg, 2010; Vynorius, Paquin, & Seichepine, 2016). Although less is known on the impact of repetitive TBI on the developing brain, animal models support a link between repetitive brain injury in the immature brain and greater cognitive difficulties (Fidan et al., 2016). These findings are in line with a ‘double hit’ model, with the presence of one insult predisposing the individual to increased vulnerability when a second insult occurs, ultimately resulting in additive or synergistic effects of sequential brain insults on functioning.
The mechanisms by which co-occurring disorders with early neurological impacts may impact cognitive functioning in children is not well known. Young children can show remarkable resilience to early brain insults, which has been attributed to brain plasticity processes (Anderson, Spencer-Smith, & Wood, 2011). The presence of multiple brain insults early in development may interfere with protective processes, such as brain plasticity, that buffer against the deleterious impact of brain injury. Cognitive reserve and brain reserve, defined as the resiliency afforded by functional brain networks and the structural morphology of the brain (e.g., brain volume, cortical thickness), are thought to mediate these resiliency processes by serving as a compensatory buffer against the functional impact of brain insult and pathology (Fay et al., 2010; Stern, 2017). Within the SCD literature, there is evidence that these compensatory processes, such as cerebrovascular reserve, are reduced which is linked to increased risk for cerebral injury (Kim, Leung, Lerch, & Kassner, 2016; Nur et al., 2009). Additionally, within the pediatric TBI literature, cognitive reserve has been identified as a moderator of cognitive functioning following brain injury (Donders & Kim, 2019).
Given the impact of pediatric SCD and preterm birth on early brain development as well as the increased prevalence of preterm birth in pediatric SCD, it is important to understand the combined impact of these two conditions on cognitive development. As such, the current study explores the additive effect of pediatric SCD and a history of preterm birth on neurodevelopmental functioning during early childhood. The relative impact of preterm birth and SCD on cognitive functioning was examined by retrospectively comparing children with higher risk SCD born preterm to comparison groups. Both domain-specific and heterogenous effects on cognition were examined given prior research indicating the presence of both patterns of cognitive deficits in the preterm population. Domain specific effects on cognitive functioning were examined using specific cognitive outcomes and heterogenous effects were measured using rates of abnormal cognitive screening results. Given that attentional processes and executive functioning are the most commonly observed cognitive deficits in both pediatric SCD and preterm birth, we hypothesized that there would be greater impairment on tests tapping into working memory and processing speed in children with SCD born preterm than in the comparison groups. Additionally, we hypothesized that children with SCD born preterm would evidence elevated rates of abnormal screenings.
Method
Participants
Participants included 189 children from four different groups: 20 children with high risk SCD born preterm (high risk SCD + preterm), 59 children with high risk SCD born at term (high risk SCD), 11 children with lower risk SCD genotypes/no SCD born preterm (lower risk SCD + preterm) and 99 children with lower risk SCD genotypes/no SCD born at term (lower risk SCD). Chronological age was used for all participants. All participants self-identified as African-American and approximately one-fifth (20.37%) of children with SCD (both high and lower risk genotypes) were born preterm. Participants with lower risk SCD genotypes (i.e., HbSC, HbS/beta+ thalassemia) and no SCD were combined to form the lower risk SCD group as preliminary analyses revealed no significant differences in neurodevelopmental outcomes between the two groups on any cognitive measure. Preterm birth status and genotype severity was determined through medical chart review.
Participants diagnosed with SCD (both high and lower risk genotypes) were drawn from a larger longitudinal study monitoring and screening for the influence of SCD on neurodevelopment across childhood (Schatz et al, 2009). Participants without SCD were children recruited from after school programs and summer care programs with predominantly African American children from Richland and Sumter counties in South Carolina. Participants without SCD were excluded if parents reported any major neurodevelopmental disorders or health conditions that could significantly impact cognitive functioning. Based on the aforementioned criteria, three participants were excluded for the following diagnoses: hemispherectomy, epilepsy, and traumatic brain injury. No participants were excluded for attention deficit/hyperactivity disorder or learning disabilities. Participant characteristics and relevant medical factors are included in Table 1. All data included in this manuscript were obtained in compliance with the Helsinki Declaration.
Table 1.
Clinical characteristics of participants in the high risk SCD born preterm, high risk SCD born at term, lower risk SCD born preterm, and lower risk SCD born at term groups.
| Variable | Group | |||||
|---|---|---|---|---|---|---|
| High-risk SCD +preterm (n = 20) | High-risk SCD +term (n = 60) | Lower-risk SCD +preterm (n = 11) | Lower-risk SCD +term (n= 98) | p-value | Test statistic | |
| Age in years (M (SD)) | 6.11 (0.79) | 6.36 (0.80) | 6.80 (1.11) | 6.63 (1.04) | p = .053 | F (3,185) = 2.61 |
| Gender (n (% Female)) | 10 (50.0%) | 29 (48.3%) | 4 (36.4%) | 48 (49.0%) | p = .881 | X2 = 0.67 |
| Gestational Age (M (SD)) | 32.33 (3.90)* | -- | 33.73 (3.13) | -- | p = .325 | t = 1.00 |
| Late/moderate preterm | 12 (60.0%) | -- | 8 (72.7%) | -- | ||
| Very/extremely preterm | 6 (30.0%) | -- | 3 (27.3%) | -- | ||
| Household Income | p = .955 | X2 = 3.22 | ||||
| <$10,000 | 4 (20.0%) | 13 (21.7%) | 1 (9.1%) | 20 (20.4%) | ||
| $10,000–29,999 | 7 (35.0%) | 21 (35.0%) | 6 (54.5%) | 39 (39.8%) | ||
| $30,000–49,999 | 7 (35.0%) | 22 (36.7%) | 4 (36.4%) | 34 (34.7%) | ||
| > $50,0000 | 2 (10.0%) | 4 (6.7%) | 0 (0%) | 5 (5.1%) | ||
| Height (M (SD)) | 113.38 (10.46) | 117.20 (6.82) | 122.88 (10.95) | 123.08 (9.25) | p < .001 | F (3,185) = 10.16 |
| Weight (M (SD)) | 19.79 (5.46) | 22.31 (6.38) | 30.83 (16.33) | 28.56 (10.55) | p < .001 | F (3,185) = 9.30 |
| Hematocrit (M (SD)) | 24.28 (3.05) | 24.16 (3.68) | -- | 31.14 (2.74) b | ||
| WBC count (M (SD)) | 12.82 (3.21) | 13.26 (3.85) | -- | 8.08 (3.11) b | ||
| Platelet (M (SD)) | 472.63 (118.98) | 461.19 (178.57) | -- | 313.16 (139.30) b | ||
| WBC count (M (SD)) | 12.82 (3.21) | 13.26 (3.85) | -- | 8.08 (3.11) b | ||
| History of abnormal TCD | 1(5.0%) | 9 (15.0%) | 0 a | 0 b | ||
| Overt stroke | 0 | 1 (1.7%) | 0 a | 0 b | ||
| Chronic transfusion therapy | 1 (5.0%) | 13 (21.7%) | 0 a | 0 b | ||
| Hydroxyurea | 8 (40.0%) | 13 (21.7%) | 0 a | 1 (1.0%) b | ||
| OSA diagnosis | 3 (15.0%) | 16 (26.7%) | 0 a | 4 (4.1%) a |
Note.
DVTMI = Beery Developmental Test of Visual-Motor Integration; OSA= Obstructive sleep apnea; TCD= Transcranial doppler; WBC= White blood count
gestational age missing for two participants
clinical characteristics reported for subset of participants diagnosed with SCD in the high risk SCD + term group (n=2).
clinical characteristics reported for subset of participants diagnosed with SCD in the lower risk SCD + term group (n=25)
Procedure
Participants with SCD were administered a cognitive screening battery at the hematology/oncology clinic during the same visit as their bi-annual clinic appointment. Healthy control participants without SCD were assessed at school or program sites. A parent of all study participants completed informed consent as approved by an Institutional Review Board. Children and their parents were screened for English language proficiency to ensure they could adequately engage with all screening and assessment materials. The cognitive test battery was administered by licensed psychologists or trained psychology doctoral students and was completed in approximately 60 to 90 minutes. Testing results were disseminated to families via a feedback phone call and in a written report which included recommendations for psychoeducational services and follow-up testing, if indicated. Children also received books as compensation for their participation. Information on household income was collected at the time of testing as a proxy for socioeconomic status. Electronic medical records of children with SCD were reviewed following testing to collect information on relevant medical and demographic characteristics.
Measures
Tests sensitive to working memory and attentional processes.
Select subtests from cognitive, achievement, and language batteries that task working memory and attentional processes were used to assess for domains sensitive to disease-related effects of SCD and preterm birth. Processing speed was measured through the Decision Speed subtest of the Woodcock-Johnson Test of Cognitive Abilities, 3rd Edition (WJ-III COG; Woodcock, McGrew, & Mather, 2001a). On this task, pictures are organized in rows with each row containing two related pictures. Children are asked to mark the two related pictures in each row as quickly and accurately as possible within three minutes.
Syntactic processing, which has been shown to be sensitive to working memory processes, was measured through the Grammatical Understanding subtest from the Test of Language Development- Primary Version, Third Edition (TOLD-P:3; Newcomer & Hammill, 1997). The TOLD-P:3 has shown reliability at the subtest and domain level, strong validity, and a lack of cultural bias for African American children (Newcomer & Hammill, 1997). Children are instructed to select the picture that best demonstrates the meaning of sentences that have increasingly complex syntax. Completion of syntactic processing tasks draws on language specific knowledge and tasks memory systems that facilitate the storage and mental retrieval of verbally provided information. Prior studies on youth with SCD have found syntactical ability to be particularly sensitive to early cerebrovascular dysfunction (Sanchez, Schatz, & Roberts, 2010). Furthermore, syntactic processing tasks have been linked to frontal brain regions that are also implicated in executive functioning abilities.
The Applied Problems subtest from the Woodcock-Johnson Tests of Achievement, 3rd edition (WJ-III ACH; Woodcock, McGrew, & Mather, 2000) measures quantitative knowledge and requires children to analyze and solve relatively simple math story problems. Math story problems are presented verbally, requiring children to mentally retain and manipulate information to perform increasingly difficult math calculations, thereby heavily taxing working memory systems.
Language and reading abilities.
Expressive language ability was measured using the Oral Vocabulary subtest from the TOLD-P:3, which requires the child to provide definitions for orally presented words. Phonological processing was assessed using the Word Discrimination subtest from the TOLD-P:3, which requires the child to determine whether two similarly sounding words are the same word or different words (e.g., pig – pig versus big – pig). Early reading skills were measured through the Letter-Word Identification subtest from the WJ-III ACH. The Letter-Word Identification subtest requires the child to identify letters and words without having to know the meaning of a word.
Visual- motor integration.
Visual-motor ability was assessed using the Beery Developmental Test of Visual-Motor Integration, 5th edition (DVTMI; Beery & Beery, 2004). The DVTMI instructs children to copy increasingly complex geometric figures using a pencil and paper. Scaled scores were used for the TOLD-P:3 (M=10, SD = 3) and standard scores were used for the WJ-III ACH and DVTMI (M=100, SD=15).
Medical chart review.
A medical chart review was conducted for all participants with SCD. Details of the participants’ demographic characteristics, routine blood lab data, history of disease complications, and treatment history were obtained through medical chart review using a medical chart coding sheet to provide a structured form for the review of electronic and paper medical charts. Specific sickle cell genotypes were recorded and then dichotomized as either high risk or lower risk. Birth history (preterm vs term) was recorded along with gestational age when available. Information on specific SCD genotype and birth history was available for all participants. Hemoglobin, white blood cell counts, and platelet counts were recorded from routine blood lab data collected during the health maintenance visit at which the screening took place. History of overt stroke or abnormal transcranial doppler (TCD) and prior treatment with therapeutic levels of hydroxyurea or chronic blood transfusion therapy were recorded from medical records as indicators of increased risk for cerebrovascular disease. Magnetic resonance imaging (MRI) was not recorded as it was unavailable for the majority of participants.
Statistical Analyses
All statistical analyses were conducted using the Statistical Package for the Social Sciences, 25th Edition (SPSS). Descriptive statistics provide information on demographic and medical characteristics of the sample. Factorial ANOVAs were conducted to examine the relationship between SCD risk (high risk; lower risk SCD), preterm birth status (preterm; full term), and neurodevelopmental outcomes. Neurodevelopmental outcomes included scores on specific tests of cognitive functioning. In order to evaluate the presence of any cognitive weakness, regardless of domain, scores on each cognitive test were categorized as abnormal if they fell ≥1.5 standard deviations below the population mean. This cut off point was selected based on data from previous studies on developing cognitive screening algorithms for pediatric SCD (DeBaun et al., 1998; Schatz et al., 2009). Log linear analyses were then conducted to examine the likelihood of at least one abnormal cognitive screening score by SCD risk and preterm birth status.
Results
Results from descriptive analyses of key demographic and medical characteristics for the four groups are shown in Table 1. Chi squared tests and one-way ANOVAs revealed no significant differences between the four groups on age, gender, or household income. As expected, the high risk SCD groups had significantly lower height and weight compared to the lower risk SCD groups. An independent samples t-test revealed no significant difference in mean gestational age between the high risk SCD+preterm and lower risk SCD+preterm groups. There was similarly no significant difference in the proportion of preterm participants by preterm birth classification (late/moderate preterm, very/extremely preterm) across the two groups (Χ 2 = .117, p =.732, φ = .064). As such, gestational age was not included as a covariate in analyses.
Factorial ANOVAs were run to examine the focused effects of SCD risk and preterm birth status on cognitive outcomes that are most commonly affected by the two conditions (i.e., processing speed, working memory; see Table 2). There was a significant main effect for SCD risk on the WJ-III Decision Speed test, WJ-III Applied Problems test, and TOLD-P:3 Grammatical Understanding test, with the high risk SCD group performing more poorly than the lower risk SCD group. The interaction effect on the TOLD-P:3 Grammatical Understanding test was approaching statistical significance (p = .08) in the hypothesized direction of comorbid high risk SCD and preterm birth associated with poorer performance. However, there were no statistically significant interaction effects between SCD risk and preterm birth status on these measures tasking attentional processes and working memory. Exploratory factorial ANOVAs were run for cognitive tasks assessing language and visual-motor skills. Exploratory analyses revealed a significant interaction effect between SCD risk and preterm birth status on the TOLD-P:3 Oral Vocabulary test, F(1,189) = 4.16, p = .043, ηp2= .022, with the high risk SCD + preterm group evidencing the poorest performance.
Table 2.
Results from factorial ANOVAs examining SCD risk (high risk; lower risk SCD), preterm birth status (preterm; term), and neurodevelopmental outcomes.
| Group | |||||||
|---|---|---|---|---|---|---|---|
| Cognitive tests (M (SD)) | High-risk SCD +preterm (n = 20) | High-risk SCD +term (n = 60) | Lower-risk SCD +preterm (n = 11) | Lower-risk SCD +term (n= 98) | p-value | Test statistic | Effect size |
| Decision Speed | 82.53 (4.01) | 91.52 (2.34) | 100.46 (5.27) | 99.58 (1.79) | p = 0.175 | F (1,178) = 1.85 | ηp2 = .010 |
| Applied Problems | 93.56 (2.86) | 93.17 (1.62) | 97.82 (3.76) | 101.52 (1.27) | p = 0.426 | F (1,181) = 0.64 | ηp2 = .004 |
| Grammatical Understanding | 7.30 (0.60) | 8.58 (0.35) | 9.91 (0.81) | 9.25 (0.27) | p = 0.080 | F (1,185) = 3.11 | ηp2 = .017 |
| Oral Vocabulary | 7.40 (0.53) | 8.78 (0.31) | 10.00 (0.72) | 9.40 (0.24) | p = 0.043 | F (1,185) = 4.16 | ηp2 =.022 |
| Word Discrimination | 7.72 (0.78) | 8.33 (0.44) | 8.36 (1.00) | 8.67 (0.34) | p = 0.826 | F (1,179) = 0.05 | ηp2 = .000 |
| Letter-Word Identification | 86.85 (3.19) | 98.63 (1.86) | 102.09 (4.31) | 105.66 (1.44) | p = 0.163 | F (1,184) = 1.97 | ηp2 = .011 |
| DVTMI | 86.26 (3.02) | 87.12 (1.70) | 93.82 (3.97) | 93.72 (1.33) | p = 0.862 | F (1,184) = 0.03 | ηp2 = .000 |
Note.
DVTMI = Beery Developmental Test of Visual-Motor Integration
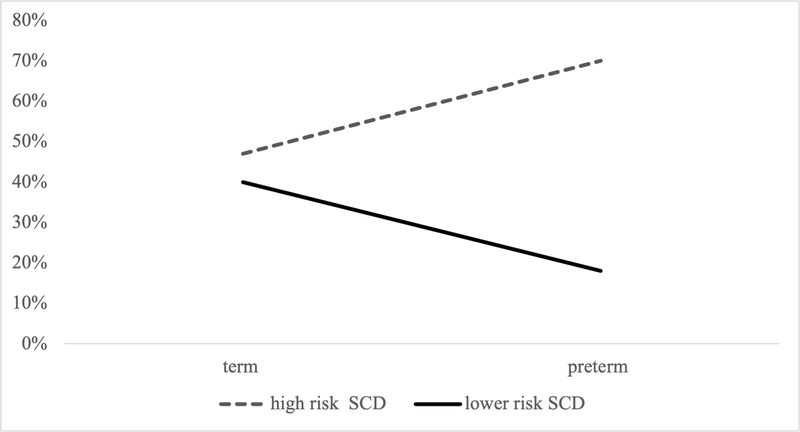
Log linear analyses were conducted to examine the impact of SCD risk and preterm birth status on rates of abnormal screening results. The high risk SCD + preterm group had the highest rate of abnormal screening results (70%), followed by high risk SCD (47%), lower risk SCD (40%), and lower risk SCD + preterm groups (18%). See Supplemental Table 1 for rates of abnormal screenings for each group by neurodevelopmental outcome. The three-way loglinear analysis for SCD risk (high, lower), preterm status (preterm, term) and screening outcome (normal, abnormal) indicated that the three-way interaction was statistically significant, G2(4)=5.22, p = .027 (see Figure 1). To break down this effect, chi-squared tests were performed separately for the preterm and term groups. There was a significant association between SCD risk status and screening outcome (χ2(1) = 7.63, p = .006, φ = .496) for participants born preterm, but not for participants born at term (χ2(1) = 7.19, p = .396, φ = .067).
Figure 1.
Graph showing the interaction between SCD risk, preterm birth status, and abnormal screening outcome.
Discussion
SCD and preterm birth are commonly co-occurring conditions that are independently associated with elevated risk for poor neurodevelopmental outcomes. However, there has been no research to date exploring the combined effects of SCD and preterm birth on cognitive functioning. The current study compared cognitive functioning in children with high risk SCD born preterm to comparison groups to examine the additive effects of SCD and preterm birth on specific cognitive outcomes and increased risk for abnormal cognitive screening results.
Significant differences in working memory and processing speed were observed between the high risk SCD group and lower risk SCD group, which is in line with well-documented research on the negative impact of pediatric SCD on neurocognitive outcomes (Hijmans et al., 2010; Schatz et al., 2002; Schatz, et al., 2009). However, results indicate that the combination of SCD and preterm birth did not confer greater risk for impairments on cognitive tests that assess working memory and processing speed, domains previously found to be sensitive to the individual disease-related impact of SCD and preterm birth. As such, the hypothesis of greater cognitive impairment on tests tapping into working memory and attentional processes in children with high risk SCD born preterm was not supported.
Exploratory analyses did reveal an association between comorbid SCD and preterm birth and deficits in expressive vocabulary, suggesting that language skills may be susceptible to the additive effect of the two conditions. There is some support for language deficits in young school age children with pediatric SCD, with deficits primarily identified for children at high neurologic risk (Schatz et al., 2009). Language deficits have similarly been identified among children at varying degrees of prematurity (Guarini et al., 2019; Rabie, Bird, Magann, Hall, & McKelvey, 2015; Taylor et al., 2011). Research in both SCD and preterm populations has found that such language deficits can present independent of general cognitive deficits, indicating a need for domain specific investigations of functioning (Aylward, 2002; Schatz et al., 2009). However, given the exploratory nature of these analyses, further research is needed to substantiate this finding.
The combination of high risk SCD and history of preterm birth was significantly associated with increased rates of abnormal cognitive screenings. This suggests that the neurodevelopmental risk imparted by comorbid SCD and preterm birth may manifest as heterogenous, rather than specific, patterns of cognitive deficits. There are many factors that may contribute to this heterogeneity in neurodevelopmental risk. Both high risk SCD genotypes and preterm birth are associated with injury to white matter tracts caused by acute neurologic injury (e.g., silent cerebral infarction) or diffuse neurologic processes such as delays in white matter development. Small variations in the location of white matter injuries can result in markedly different impacts on cognitive functioning due to the organization of the white matter tracts implicated in cognitive abilities in the brain. As such, heterogeneity in the affected domains of neurodevelopmental functioning may reflect heterogeneity in injury location. Future research may benefit from the inclusion of screening tools, such as functional MRI or cognitive evoked potentials, in addition to cognitive testing to assist in detecting abnormalities in brain functioning (Colombatti et al., 2015; Colombatti et al., 2016)
Heterogeneity in patterns of neurodevelopmental risk may also reflect developmental processes. Within the preterm literature there is evidence to suggest that heterogeneity in patterns of cognitive deficits may be more characteristic of younger age groups, with more selective patterns of cognitive deficits emerging later in life (Sansavini, Guarini, & Caselli, 2011). Specifically, the development of more selective deficits in higher order cognitive functions (e.g., executive functioning) in older youth is thought to be influenced by “catching up” in more basic cognitive competencies and increased challenges with complex competencies due to elevated environmental demands (Sansavini et al., 2011). Longitudinal research is needed to examine the development of cognitive abilities into middle/late childhood and adolescence for youth with comorbid SCD and preterm birth to explore how developmental processes impact neurodevelopment.
Finally, prior studies have demonstrated that neurodevelopmental outcomes for youth with SCD and preterm birth are impacted by social and environmental processes in addition to medical risk (Bills, Johnston, Shi, & Bradshaw, 2020; Prussien et al., 2020; Yarboi et al., 2017; Wong & Edwards, 2013). This dynamic interplay between medical and environmental risk factors may lead to diverging developmental trajectories which can manifest as impairments in a variety of cognitive domains. There is a need for future research examining the interaction between other risk factors, such parent and family functioning, and the elevated medical risk imparted by multiple early brain insults.
These findings have important clinical implications for screening and intervention efforts. Screenings that solely target attention and executive functioning abilities may miss youth at increased risk for poor neurodevelopmental outcomes. As such, screening programs assessing a wider range of neurodevelopmental domains may be needed to identify early deficits in young children with high risk SCD born preterm. It is notable that the current study directly assessed for early cognitive delays, rather than solely relying on caregiver or provider reports, which may be important for detecting early deficits. Furthermore, the increased rates of abnormal screenings are suggestive of the need for increased preventative and intervention resources for this subset of children with SCD. The exploratory finding of a specific impairment in expressive language is concerning as early language skills play a vital role in the development of later academic abilities and skills among children entering elementary school.
There are a number of limitations associated with the current study. First, there is an unbalanced sample size across the four groups, with two groups notably smaller in sample size, thereby limiting statistical power. However, as SCD is a relatively rare disease population and this is the first study to date to examine cognitive functioning among children with comorbid SCD and preterm birth, these analyses make an important contribution to the literature. Another limitation is the lack of information on gestational age for all participants in the study. Specific gestational age was unavailable for two participants in the high risk SCD group born preterm and for children born at term. The cross-sectional nature of the current study limits conclusions related to changes in profiles of cognitive deficits across time for children with SCD born preterm. Future research is needed to determine when and if more specific patterns of cognitive deficits emerge. Finally, the current test battery was developed to be sensitive to screening for SCD-related neurocognitive effects, but its degree of sensitivity to the neurocognitive risk imparted by prematurity is not established.
In summary, this study is the first to examine the relationship between preterm birth and neurodevelopmental concerns in children with SCD. Findings suggest that preterm birth is associated with increased risk for abnormal developmental screenings and deficits in expressive language skills. Although further studies are needed to confirm and expand on these findings, this suggests a more diffuse pattern of cognitive risk for children with high risk SCD born preterm as opposed to the more focused effects found in the SCD literature. This more nuanced understanding of risk for children with comorbid SCD and preterm birth has the potential to aid in the early identification of cognitive deficits. For instance, neurodevelopmental concerns may not be evident on focused screeners that measure a limited number of cognitive domains. Current findings highlight the need for further investigation of the impact of preterm birth on cognitive functioning in pediatric SCD to aid in the development of more nuanced treatment approaches for this particularly vulnerable population.
Supplementary Material
Acknowledgements
Funding
This publication was made possible in part by Grant Number T32-GM081740 from NIH-NIGMS and R21DC017252 from NIH-NIDCD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Footnotes
Disclosure statement
No potential conflict of interest
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, & Oosterlaan J (2009). Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124(2), 717–728. [DOI] [PubMed] [Google Scholar]
- Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E.., & Birtles D (2018). Cognitive, motor, behavioural and academic performances of children born preterm: A meta-analysis and systematic review involving 64 061 children. BJOG: An International Journal of Obstetrics & Gynaecology, 125(1), 16–25. [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, & Wood A (2011). Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain, 134(8), 2197–2221. [DOI] [PubMed] [Google Scholar]
- Aylward GP (2002). Cognitive and neuropsychological outcomes: More than IQ scores. Mental Retardation and Developmental Disabilities Research Reviews, 8(4), 234–240. [DOI] [PubMed] [Google Scholar]
- Bakri MH, Ismail EA, Elsedfy GO, Amr MA, & Ibrahim A (2014). Behavioral impact of sickle cell disease in young children with repeated hospitalization. Saudi Journal of Anaesthesia, 8(4), 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin OA, & Christian M (1999). The social and cultural context of coping with sickle cell disease: I. A review of biomedical and psychosocial issues. Journal of Black Psychology, 25(3), 277–293. [Google Scholar]
- Baron IS, & Rey-Casserly C (2010). Extremely preterm birth outcome: A review of four decades of cognitive research. Neuropsychology Review, 20(4), 430–452. [DOI] [PubMed] [Google Scholar]
- Batalle D, Hughes EJ, Zhang H, Tournier J-D, Tusor N, Aljabar P…, & Nosarti C (2017). Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage, 149, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE (2004). The Beery-Buktenica developmental test of visual-motor integration: Beery VMI, with supplemental developmental tests of visual perception and motor coordination, and stepping stones age norms from birth to age six. NCS Pearson Minneapolis, MN. [Google Scholar]
- Belanger HG, Spiegel E, & D Vanderploeg R (2010). Neuropsychological performance following a history of multiple self-reported concussions: A meta-analysis. Journal of the International Neuropsychological Society: JINS, 16(2), 262. [DOI] [PubMed] [Google Scholar]
- Berkelhammer LD, Williamson AL, Sanford SD, Dirksen CL, Sharp WG, Margulies AS, & Prengler RA (2007). Neurocognitive sequelae of pediatric sickle cell disease: A review of the literature. Child Neuropsychology, 13(2), 120–131. [DOI] [PubMed] [Google Scholar]
- Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Chevret S, Hau,.., & Lesprit E (2011). Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood, 117(4), 1130–1140. [DOI] [PubMed] [Google Scholar]
- Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Vasile M, Kasbi F, ...Pondarré C (2015). Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood, 125(10), 1653–1661. [DOI] [PubMed] [Google Scholar]
- Bills SE, Johnston JD, Shi D, & Bradshaw J (2020). Social-environmental moderators of neurodevelopmental outcomes in youth born preterm: A systematic review. Child Neuropsychology, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B…, & the Born Too Soon Preterm Birth Action Group (see acknowledgement for full list). (2013). Born Too Soon: The global epidemiology of 15 million preterm births. Reproductive Health, 10(1), S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J, & Counsell S (2020). Invited review: Factors associated with atypical brain development in preterm infants: Insights from magnetic resonance imaging. Neuropathology and Applied Neurobiology, 46(5), 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm B, Smedler A, & Forssberg H (2004). Impulse control, working memory and other executive functions in preterm children when starting school. Acta Paediatrica, 93(10), 1363–1371. [DOI] [PubMed] [Google Scholar]
- Brayette M, Saliba E, Malvy J, Blanc R, Ponson L, Tripi G…, & Bonnet-Brilhault F (2019). Incomplete Gestation has an Impact on Cognitive Abilities in Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 49(10), 4339–4345. [DOI] [PubMed] [Google Scholar]
- Breeman LD, Jaekel J, Baumann N, Bartmann P, & Wolke D (2016). Attention problems in very preterm children from childhood to adulthood: The Bavarian Longitudinal Study. Journal of Child Psychology and Psychiatry, 57(2), 132–140. [DOI] [PubMed] [Google Scholar]
- Breeman LD, Jaekel J, Baumann N, Bartmann P, & Wolke D (2015). Preterm cognitive function into adulthood. Pediatrics, 136(3), 415–423 [DOI] [PubMed] [Google Scholar]
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, & Steiner CA (2010). Acute care utilization and rehospitalizations for sickle cell disease. Journal of the American Medical Association, 303(13), 1288–1294. [DOI] [PubMed] [Google Scholar]
- Brown RT, Buchanan I, Doepke K, Eckman JR, Baldwin K, Goonan B, & Schoenherr S (1993). Cognitive and academic functioning in children with sickle-cell disease. Journal of Clinical Child Psychology, 22(2), 207–218. [Google Scholar]
- Brown RT, Davis PC, Lambert R, Hsu L, Hopkins K, & Eckman J (2000). Neurocognitive functioning and magnetic resonance imaging in children with sickle cell disease. Journal of Pediatric Psychology, 25(7), 503–513. [DOI] [PubMed] [Google Scholar]
- Brydges CR, Landes JK, Reid CL, Campbell C, French N, & Anderson M (2018). Cognitive outcomes in children and adolescents born very preterm: A meta-analysis. Developmental Medicine & Child Neurology, 60(5), 452–468. [DOI] [PubMed] [Google Scholar]
- Choi S, O’Neil SH, Joshi AA, Li J, Bush AM, Coates TD, … & Wood JC (2019). Anemia predicts lower white matter volume and cognitive performance in sickle and non-sickle cell anemia syndrome. American Journal of Hematology, 94(10), 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombatti R, Ermani M, Rampazzo P, Manara R, Montanaro M, Basso G, … & Sainati L (2015). Cognitive evoked potentials and neural networks are abnormal in children with sickle cell disease and not related to the degree of anaemia, pain and silent infarcts. British Journal of Haematology, 169(4), 597–600. [DOI] [PubMed] [Google Scholar]
- Colombatti R, Lucchetta M, Montanaro M, Rampazzo P, Ermani M, Talenti G, … & Sainati L (2016). Cognition and the default mode network in children with sickle cell disease: a resting state functional MRI study. PloS one, 11(6), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L…, & Rutherford MA (2008). Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain, 131(12), 3201–3208. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, & Kirkham FJ (2012). Silent cerebral infarcts: A review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood, 119(20), 4587–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Schatz J, Siegel M, Koby M, Craft S, Resar L…, & Lee R (1998). Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology, 50(6), 1678–1682. [DOI] [PubMed] [Google Scholar]
- Dimitrova R, Pietsch M, Christiaens D, Ciarrusta J, Wolfers T, Batalle D.., & Price AN (2020). Heterogeneity in brain microstructural development following preterm birth. Cerebral Cortex, 30(9), 4800–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders J, & Kim E (2019). Effect of cognitive reserve on children with traumatic brain injury. Journal of the International Neuropsychological Society: JINS, 25(4), 355–361. [DOI] [PubMed] [Google Scholar]
- Edwards CL, Scales MT, Loughlin C, Bennett GG, Harris-Peterson S, De Castro LM.., & Johnson S (2005). A brief review of the pathophysiology, associated pain, and psychosocial issues in sickle cell disease. International Journal of Behavioral Medicine, 12(3), 171–179. [DOI] [PubMed] [Google Scholar]
- Epping AS, Myrvik MP, Newby RF, Panepinto JA, Brandow AM, & Scott JP (2013). Academic attainment findings in children with sickle cell disease. Journal of School Health, 83(8), 548–553. [DOI] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Taylor HG, Bangert B, Dietrich A, Nuss KE…, & Wright M (2010). Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. Journal of the International Neuropsychological Society: JINS, 16(1), 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidan E, Lewis J, Kline AE, Garman RH, Alexander H, Cheng JP…, & Kochanek PM (2016). Repetitive mild traumatic brain injury in the developing brain: Effects on long-term functional outcome and neuropathology. Journal of Neurotrauma, 33(7), 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Ragan DK, Fellah S, Binkley MM, Fields ME, Guilliams…, & Lee J-M (2018). Silent infarcts in sickle cell disease occur in the border zone region and are associated with low cerebral blood flow. Blood, 132(16), 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarini A, Bonifacci P, Tobia V, Alessandroni R, Faldella G, & Sansavini A (2019). The profile of very preterm children on academic achievement. A cross-population comparison with children with specific learning disorders. Research in Developmental Disabilities, 87(8709782, rid), 54–63. [DOI] [PubMed] [Google Scholar]
- Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters M, & Oosterlaan J (2010). Neurocognitive deficits in children with sickle cell disease: A comprehensive profile. Pediatric Blood & Cancer, 56(5), 783–788. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Kirkham FJ, Prengler M, Telfer P, Lane R, Vargha-Khadem F, & de Haan M (2006). An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. British Journal of Haematology, 132(1), 99–107. [DOI] [PubMed] [Google Scholar]
- Karkoska KA, Haber K, Elam M, Strong S, & McGann PT (2021). Academic Challenges and School Service Utilization in Children with Sickle Cell Disease. The Journal of Pediatrics, 230, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawadler JM, Clayden JD, Kirkham FJ, Cox TC, Saunders DE, & Clark CA (2013). Subcortical and cerebellar volumetric deficits in paediatric sickle cell anaemia. British Journal of Haematology, 163(3), 373–376. [DOI] [PubMed] [Google Scholar]
- Kim JA, Leung J, Lerch JP, & Kassner A (2016). Reduced cerebrovascular reserve is regionally associated with cortical thickness reductions in children with sickle cell disease. Brain Research, 1642, 263–269. [DOI] [PubMed] [Google Scholar]
- Lundequist A, Böhm B, & Smedler A-C (2013). Individual neuropsychological profiles at age 5½ years in children born preterm in relation to medical risk factors. Child Neuropsychology, 19(3), 313–331. [DOI] [PubMed] [Google Scholar]
- Manara R, Dalla Torre A, Lucchetta M, Ermani M, Favaro A, Baracchini C, … & Colombatti R (2021). Visual cortex changes in children with sickle cell disease and normal visual acuity: a multimodal magnetic resonance imaging study. British Journal of Haematology, 192(1), 151–157. [DOI] [PubMed] [Google Scholar]
- Manara R, Talenti G, Rampazzo P, Ermani M, Montanaro M, Baracchini C, … & Colombatti R (2017). Longitudinal evaluation of cerebral white matter hyperintensities lesion volume in children with sickle cell disease. British Journal of Haematology, 176(3), 485–487. [DOI] [PubMed] [Google Scholar]
- Miller ST, Macklin EA, Pegelow CH, Kinney TR, Sleeper LA, Bello JA, … & Cooperative Study of Sickle Cell Disease. (2001). Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. The Journal of Pediatrics, 139(3), 385–390. [DOI] [PubMed] [Google Scholar]
- Newcomer PL, & Hammill DD (1997). Test of language development-primary. Pro-ed. [DOI] [PubMed] [Google Scholar]
- Nur E, Kim YS, Truijen J, van Beers EJ, Davis SC, Brandjes DP, … & van Lieshout JJ (2009). Cerebrovascular reserve capacity is impaired in patients with sickle cell disease. Blood, The Journal of the American Society of Hematology, 114(16), 3473–3478. [DOI] [PubMed] [Google Scholar]
- Piel FB, Steinberg MH, & Rees DC (2017). Sickle cell disease. New England Journal of Medicine, 376(16), 1561–1573. [DOI] [PubMed] [Google Scholar]
- Pegelow CH, Macklin EA, Moser FG, Wang WC, Bello JA, Miller ST…, & Zimmerman RA (2002). Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood, The Journal of the American Society of Hematology, 99(8), 3014–3018. [DOI] [PubMed] [Google Scholar]
- Pegelow CH, Wang W, Granger S, Hsu LL, Vichinsky E, Moser FG, … & Brambilla D (2001). Silent infarcts in children with sickle cell anemia and abnormal cerebral artery velocity. Archives of Neurology, 58(12), 2017–2021. [DOI] [PubMed] [Google Scholar]
- Powars D, Wilson B, Imbus C, Pegelow C, & Allen J (1978). The natural history of stroke in sickle cell disease. The American Journal of Medicine, 65(3), 461–471. [DOI] [PubMed] [Google Scholar]
- Prengler M, Pavlakis SG, Prohovnik I, & Adams RJ (2002). Sickle cell disease: The neurological complications. Annals of Neurology, 51(5), 543–552. [DOI] [PubMed] [Google Scholar]
- Prussien KV, Jordan LC, DeBaun MR, & Compas BE (2019). Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. Journal of Pediatric Psychology, 44(8), 948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussien KV, Siciliano RE, Ciriegio AE, Anderson AS, Sathanayagam R, DeBaun MR.., & Compas BE (2020). Correlates of Cognitive Function in Sickle Cell Disease: A Meta-Analysis. Journal of Pediatric Psychology, 45(2), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie N, Bird T, Magann E, Hall R, & McKelvey S (2015). ADHD and developmental speech/language disorders in late preterm, early term and term infants. Journal of Perinatology, 35(8), 660–664. [DOI] [PubMed] [Google Scholar]
- Sanchez CE, Schatz J, & Roberts CW (2010). Cerebral blood flow velocity and language functioning in pediatric sickle cell disease. Journal of the International Neuropsychological Society: JINS, 16(2), 326. [DOI] [PubMed] [Google Scholar]
- Sansavini A, Guarini A, & Caselli MC (2011). Preterm birth: Neuropsychological profiles and atypical developmental pathways. Developmental Disabilities Research Reviews, 17(2), 102–113. [DOI] [PubMed] [Google Scholar]
- Schatz J, Brown RT, Pascual J, Hsu L, & DeBaun M (2001). Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology, 56(8), 1109–1111. [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke RL, Kellett JM, & Kramer JH (2002). Cognitive functioning in children with sickle cell disease: A meta-analysis. Journal of Pediatric Psychology, 27(8), 739–748. [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke R, & Roberts CW (2004). Interactions of biomedical and environmental risk factors for cognitive development: A preliminary study of sickle cell disease. Journal of Developmental & Behavioral Pediatrics, 25(5), 303–310. [DOI] [PubMed] [Google Scholar]
- Schatz J, Puffer ES, Sanchez C, Stancil M, & Roberts CW (2009). Language processing deficits in sickle cell disease in young school-age children. Developmental Neuropsychology, 34(1), 122–136. [DOI] [PubMed] [Google Scholar]
- Sherlock RL, Anderson PJ, Doyle LW, & Victorian Infant Collaborative Study Group. (2005). Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Human Development, 81(11), 909–916. [DOI] [PubMed] [Google Scholar]
- Stålnacke J, Lundequist A, Böhm B, Forssberg H, & Smedler A-C (2015). Individual cognitive patterns and developmental trajectories after preterm birth. Child Neuropsychology, 21(5), 648–667. [DOI] [PubMed] [Google Scholar]
- Stern Y (2017). An approach to studying the neural correlates of reserve. Brain Imaging and Behavior, 11(2), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundd P, Gladwin MT, & Novelli EM (2019). Pathophysiology of sickle cell disease. Annual Review of Pathology: Mechanisms of Disease, 14, 263–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Anselmo MG, Minich N, Espy KA, & Hack M (2011). Learning problems in kindergarten students with extremely preterm birth. Archives of Pediatrics & Adolescent Medicine, 165(9), 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewouw MM, Young JM, Mossad SI, Sato J, Whyte HA, Shroff MM, & Taylor MJ (2019). Mapping the neuroanatomical impact of very preterm birth across childhood. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduzco LA, & Nathan DG (2009). Sickle cell disease and stroke. Blood, 114(25), 5117–5125. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Allan W, Katz KH, Schneider K, Tucker R, & Ment LR (2014). Adolescents born prematurely with isolated grade 2 haemorrhage in the early 1990s face increased risks of learning challenges. Acta Paediatrica, 103(10), 1066–1071. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2019). Dysmaturation of premature brain: Importance, cellular mechanisms, and potential interventions. Pediatric Neurology, 95, 42–66. [DOI] [PubMed] [Google Scholar]
- Vynorius KC, Paquin AM, & Seichepine DR (2016). Lifetime multiple mild traumatic brain injuries are associated with cognitive and mood symptoms in young healthy college students. Frontiers in Neurology, 7, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Pavlakis SG, Helton KJ, McKinstry RC, Casella JF, Adams RJ, & Rees RC (2008). MRI abnormalities of the brain in one-year-old children with sickle cell anemia. Pediatric Blood & Cancer, 51(5), 643–646. [DOI] [PubMed] [Google Scholar]
- Whiteman V, Salinas A, Weldeselasse HE, August EM, Mbah AK, Aliyu MH, & Salihu HM (2013). Impact of sickle cell disease and thalassemias in infants on birth outcomes. European Journal of Obstetrics & Gynecology and Reproductive Biology, 170(2), 324–328. [DOI] [PubMed] [Google Scholar]
- Wong HS, & Edwards P (2013). Nature or nurture: A systematic review of the effect of socioeconomic status on the developmental and cognitive outcomes of children born preterm. Maternal and Child Health Journal, 17(9), 1689–1700. [DOI] [PubMed] [Google Scholar]
- Woodcock R, McGrew KS, & Mather N (2001). Technical manual. Woodcock-Johnson III. [Google Scholar]
- Yarboi J, Compas BE, Brody GH, White D, Rees Patterson J, Ziara K, & King A (2017). Association of social-environmental factors with cognitive function in children with sickle cell disease. Child Neuropsychology, 23(3), 343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.