Abstract
Background:
Aortic pulse wave velocity is a non-invasive measure of aortic stiffness and arterial aging. Its current value in cardiovascular risk estimation practice is unknown. We aimed to establish whether aortic pulse wave velocity identified individuals with higher risk of incident major adverse cardiovascular events and improved performance of the American Heart Association/American College of Cardiology atherosclerotic cardiovascular disease risk score.
Methods:
This prospective analysis included 3837 Whitehall II cohort participants screened in 2008–2009, and followed for 11.7 years (Mean=10.3, SD=1.81), without history of stroke, myocardial infarction, or coronary heart disease.
Results:
Mean age of the sample was 65.0 years (SD=5.6), 2831 participants (73.8%) were male and mean atherosclerotic cardiovascular disease risk score was 13.8%. At the end of follow-up, 411 individuals (10.7%) had suffered a major cardiovascular event. Those in the highest aortic pulse wave velocity quartile were at high risk (hazard ratio 2.99 (95% CI, 2.25, 3.97)) and reached the threshold for statin medication (7.5% risk) after 5 years whereas others reached it after 10 years (difference p<0.001). The addition of aortic pulse wave velocity to the risk score improved the C-statistic (0.68 vs. 0.67, p=0.03) and Net Reclassification Index (4.6%, p=0.04 and 11.3%, p=0.02).
Conclusions:
Our results show that aortic stiffness predicted major adverse cardiovascular events in a cohort of elderly individuals, improving the performance of a widely used cardiovascular disease risk estimator. Aortic pulse wave velocity measurement is scalable, radiation-free, and easy to perform. Further studies on its applicability in cardiovascular disease risk assessment in primary care settings are needed.
Keywords: Epidemiology, aging, population science, arterial stiffness, pulse wave velocity
Background
American Heart Association/American College of Cardiology guidelines recommend the Atherosclerotic Cardiovascular Disease (ASCVD) Score to guide preventive interventions. The ASCVD score takes age, sex, systolic blood pressure, antihypertensive medications, smoking, total cholesterol, high-density lipoprotein cholesterol, and diabetes status as input, providing an individual 10-year risk estimate for major adverse cardiovascular events (MACE) as output. 1 Within the ASCVD score, chronological age is important, however with differing risk factor trajectories 2 and genetic risk categories, 3 individuals of the same age may have a very different atherosclerosis burden. 4
Carotid-femoral (aortic) Pulse Wave Velocity (aPWV) is surrogate measure of arterial stiffness and an established marker of vascular aging and atherosclerosis. 5,6 There is a dose-response increase in risk of MACE and related mortality as aPWV increases. 7,8 Consistently, aPWV measurement may also be clinically useful for estimating the risk of incident hypertension. 9
It is thus possible that inclusion of an aPWV measurement in risk assessment could improve on performance of the ASCVD score in elderly individuals. Current American Heart Association guidelines for primary prevention recommend measurement of coronary artery calcium (CAC) to support shared decision making on initiation of statin therapy. CAC assessment involves ionizing radiation and is often not available in primary care clinics, a common setting for initiation of preventive medications. Since aPWV measurement using applanation tonometry is quick, affordable, and potentially available in primary care settings, it is reasonable to ask whether it improves the ASCVD score and could be used to improve CVD risk assessment in primary care.
This study aimed to assess whether adjunct measurement of aPWV on a single occasion could improve risk stratification over and above the ASCVD score alone in elderly individuals, and thus improve effectiveness of primary prevention.
Methods
Data, analytic methods, and study materials of this study are available from Prof. Eric J. Brunner on reasonable request (data sharing policy: https://www.ucl.ac.uk/epidemiology-health-care/research/epidemiology-and-public-health/research/whitehall-ii/).
Study population
The Whitehall II cohort study recruited 10,308 British Civil servants (67% male) between 1985 and 1988, with a response rate of 73%. Age at baseline ranged from 35 to 55 with a mean age of 44. Participants were asked to attend clinical assessments every 4–5 years. 10 aPWV was assessed on 4,342 participants at the 2007–09 clinical examination, which was the baseline of this analysis. Participants were followed-up for MACE until March 31st, 2019, for a mean period of 11.7 years. 505 participants were excluded of the analysis due to prevalent CVD or lack of data required to calculate the ASCVD score. The final sample comprised 3,837 participants with 411 incident MACE.
Aortic PWV
Aortic PWV is defined as the travel time of the pulse wave along the aorta. It was measured using the SphygmoCor® (Atcor Medical, Australia) applanation tonometer device with the participant in the supine position following standard procedures. The device detects the difference between the peak of the R-wave on ECG and the foot of the pulse waveform at the measurement site. When placed at the carotid artery, the device detects the blood transmission time between the heart and the carotid pulse. Afterwards, the process is repeated at the femoral site. The time of transit was defined as the difference between the heart-carotid and heart-femoral times.
The path length, defined as the distanced travelled by the pulse wave was measured with a tape measure subtracting the carotid-sternal notch distance from the femoral-sternal notch distance, 11 with the final calculation of aPWV in m/s calculated by dividing the path length over the transit time. Aortic PWV was measured twice, and if the difference in velocity between the two measurements was > 0.5 m/s, a third measurement was taken. The average of the measurements was used in the analysis. aPWV measurements were repeated in 125 study participants within 60 days to assess the short-term reproducibility. The median intraindividual difference in aPWV was 0.83 m/s (SD= 1.27; interquartile range = 0.43–1.40).
Major Adverse Cardiovascular Events (MACE)
MACE is a composite endpoint consisting of nonfatal and fatal stroke, myocardial infarction, definite angina, and cardiovascular death (Table S1). Information about clinical events in the participants of the cohort was extracted from the Hospital Episode Statistics database in the National Health Service (NHS) Central Registry, where all in- and out-patient admissions to hospitals in the United Kingdom are recorded and clinical events are coded according to the ICD-10. 12 The events were retrieved using the unique NHS identification number from the participants. To reduce potential inaccuracies, the endpoints used in this study were matched to specific ICD-codes. 13
Variables used in the equation of the ASCVD score
Baseline ASCVD score was derived using the variables and the equation suggested in American Heart Association guidelines. 14 The participants were categorized according to their ASCVD score in low risk (<5%), borderline risk (5%−7.5%), intermediate risk (≥7.5%−20%) and high risk (≥20%). Data on sociodemographic characteristics, antihypertensive medication, other prescription medications and health behaviors as smoking status (smoker, ex-smoker, non-smoker) were obtained from the self-administered questionnaire. To ascertain that a given drug was prescribed as antihypertensive, hypertension had to be stated as cause.
Blood pressure was measured with the patient in a supine position, after resting for 10 minutes and with the upper right arm exposed, systolic blood pressure and diastolic blood pressure readings in millimeters of mercury were obtained using the oscillometric sphygmomanometer Omron HEM 907. Different cuffs were used according to the size needed by the patient. Two readings were taken with an interval of 1 minute. In the case of a difference greater than 10 mm Hg between the two measurements, a third measurement was obtained. Height, weight and other anthropometric measurements were taken with the participants in a standing position. Waist circumference was obtained using a tape measure with participants in mid-expiration and muscles relaxed. Diabetes status was assessed by an oral glucose tolerance test at clinical screening or a self-reported doctor diagnosis, or the use of diabetes medication. Serum total and high-density lipoprotein cholesterol were measured in a blood sample after fasting either overnight or 4 hours after a fat-free breakfast.
Statistical analysis
Sociodemographic characteristics were presented as proportions for categorical variables and means with standard deviations for continuous variables. Chi-squared tests or t-tests were performed to assess differences between participants above and below the intermediate (7.5%) and high (20%) ASCVD thresholds. Cox proportional hazards models were fitted to estimate the association between aPWV, in quartiles or as a continuous variable, and the risk of MACE. Non-linear effects of aPWV on the risk of MACE were assessed by adding a quadratic term for continuous aPWV, and an interaction term of aPWV by age was added to test whether the effect of aPWV on risk of MACE differed by age. The proportional-hazards assumption was tested using the Schoenfeld method of analysis of residuals. For analysis of predictive performance, all variables included in the Pooled Chained Equations for the calculation of the ASCVD score were included in a Cox proportional hazards model. A further model including also aPWV at baseline, was additionally fitted.
Discrimination ability of the models including and not including aPWV was estimated using Harrell’s C-statistic. The C-statistic is a rank parameter equivalent to the area under the receiver operating characteristic curve that measures the concordance between the predicted and experienced outcomes. 15 The difference between C-statistic scores was calculated using the Somers’ D program. 16 Net Reclassification Index (NRI) analysis after the addition of aPWV to the components of the ASCVD pooled equation was performed using the ‘NRI’ program in Stata. 17 The thresholds set up for the calculation of NRI were 7.5%, which is the lowest risk limit for recommending the use of lipid lowering drugs in the presence of a risk enhancer favoring that decision and 20%, which is defined as high ASCVD risk indicating lipid lowering therapy. Calibration was measured using the Hosmer-Lemeshow test All statistical analysis was performed using Stata 13. 18
Results
There were 3,837 participants with mean age 65.0 years free of MACE at baseline with data on all ASCVD components and aPWV. The mean ASCVD score was 13.8%. At baseline, 28.0% of the participants were in the low and borderline (<7.5%) ASCVD risk threshold (Table 1), 51.2% were in the intermediate ( ≥7.5%−20%), and 20.7% in the high risk (≥20%) groups. Compared with the intermediate and high ASCVD score risk groups, the group low and borderline group tended to be younger and comprised a higher proportion of female participants. Heart rate and frequency of alcohol consumption were similar across groups. Total cholesterol was marginally lower and use of lipid-lowering medication more frequent in the highest ASCVD score group compared to the lowest.
Table 1.
Sample characteristics at baseline by ASCVD score categories (N=3837)
| Characteristic | ASCVD score <7.5% (N =1076) |
ASCVD score 7.5–19.9% (N= 1966) |
ASCVD score ≥20% (N= 795) |
|---|---|---|---|
|
| |||
| Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | |
| Age | 60.7 (3.2) | 64.9 (4.7)* | 71.2 (4.7)* |
| Heart rate | 62.7 (9.8) | 63.7 (10.8)* | 65.9 (11.9)* |
| Sex (male) | 438 (40.7) | 1678 (85.4)* | 715(89.9)* |
| Ethnicity (non-white) | 69 (6.4) | 117 (5.9)* | 86 (10.8)* |
| aPWV (m/s) | 7.4 (1.3) | 8.4 (1.8)* | 9.9 (2.2)* |
| Individuals in the highest PWV quartile (%) | 80 (7.4) | 427 (21.7)* | 421(52.9)* |
| Body mass index (kg/m 2 ) | 25.4 (4.4) | 26.3 (3.7)* | 26.7 (3.5)* |
| Waist circumference (cm) | 86.5 (11.1) | 93.6 (10.6)* | 96.5 (10.1)* |
| Smoker (%) | 25 (2.3) | 145 (7.4)* | 65 (8.2)* |
| Diabetes mellitus (%) | 23 (2.1) | 114 (5.8)* | 251 (31.6)* |
| Total cholesterol (mg/dl) | 206.8 (38.8) | 206.6 (38.9) | 198.5 (42.9)* |
| HDL cholesterol (mg/dl) | 71.1 (17.6) | 60.1 (16.0)* | 57.1 (16.3)* |
| Systolic blood pressure (mmHg) | 119.2 (12.7) | 129.3 (13.8) | 137.4 (15.4)* |
| Diastolic blood pressure (mmHg) | 67.6 (8.6) | 71.3 (9.3)* | 73.0 (9.7)* |
| Antihypertensive medication (%) | 163 (15.2) | 520 (26.5)* | 390 (49.1)* |
| Lipid lowering drugs (%) | 183 (17.0) | 508(25.8)* | 292(36.7)* |
Chi-square/t-test for equality of means compared to the reference group (ASCVD <7.5%) <0.05 ASCVD: Atherosclerotic Cardiovascular Disease.
After a follow-up of 11.7 years, 10.7% of participants suffered MACE. 52.8% of events were nonfatal coronary heart disease, 18.5% nonfatal stroke, 17.8% definite MI, 5.4% other CVD-related mortality (Table S1). The risk of MACE increased across the quartiles of aPWV (Figure 1), with the highest quartile (aPWV >9.45 m/s) having an unadjusted hazard ratio (HR) of 2.99 (95% CI: 2.25 to 3.97) compared to the lowest. These associations remained after adjustment for the ASCVD score and endured stratification to intermediate risk group and exclusion of those on lipid-lowering and antihypertensive medications (Table S2). After adjustment for the ASCVD score the HR for the highest quartile was 1.93 (95% CI: 1.41 to 2.64). The high-risk group reached the threshold for statin treatment in 5 years, while the other quartiles reached it in 10 years and these results remained the same after adjustment to ASCVD score (Table S2 and Figure S1). Using aPWV as a continuous measure, the unadjusted HR was 1.22 (95% CI: 1.17 to 1.26) for each 1 m/s higher aPWV. After adjustment for ASCVD risk factors, the association attenuated slightly to HR 1.12 (95% CI: 1.07 to 1.18) (Table 2). There was no evidence of non-linearity (p=0.33) or any interaction of aPWV with age (p=0.21). The association between aPWV and MACE remained when analyses were adjusted for systolic blood pressure, and when high-risk participants or those using lipid-lowering or antihypertensive medication were excluded (Table S2).
Figure 1.
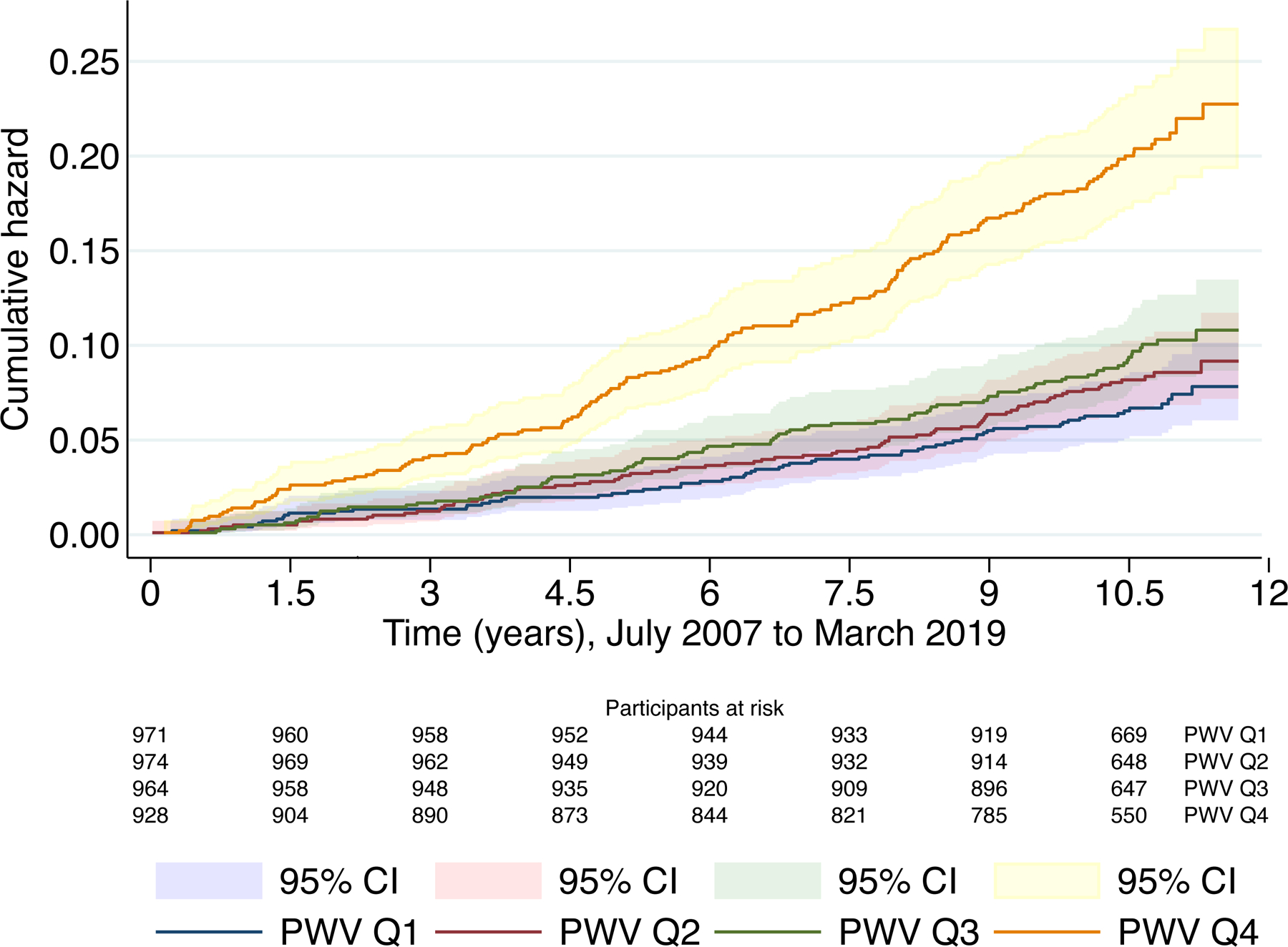
Cumulative hazard for MACE according to quartiles of aPWV with 95% CI. (411 events in 3837 participants)
Figure shows Kaplan-Meier curves depicting the unadjusted cumulative hazard for Major Adverse Cardiovascular Events in different quartiles of aPWV during the follow-up time, individuals in the highest quartile of aPWV showed higher risk of MACE.
Table 2.
Association of MACE with ASCVD risk factors and aPWV and the predictive ability of two models excluding and including PWV (N=3837, Events= 411)
| HR | 95%CI | p-value | C-statistic | 95%CI | p-value | |
|---|---|---|---|---|---|---|
|
Model 1 (ASCVD components)
| ||||||
| Age | 1.08 | 1.06, 1.09 | <0.001 | 0.6680 | (0.6423, 0.6938) | <0.001 |
| Total cholesterol (per SD) | 1.02 | 0.92, 1.13 | 0.71 | |||
| HDL-C (per SD) | 0.89 | 0.79, 0.99 | <0.05 | |||
| Antihypertensive use | 1.61 | 1.30, 1.98 | <0.001 | |||
| SBP (per SD) | 1.15 | 1.04, 1.26 | <0.01 | |||
| Smoker | 1.67 | 1.18, 2.35 | <0.001 | |||
| Incident diabetes | 1.14 | 0.88, 1.48 | 0.319 | |||
| Female | 0.74 | 0.57, 0.96 | <0.05 | |||
|
Model 2 (ASCVD components + aPWV) | ||||||
| Model 1 + aPWV (m/s) | 1.12 | 1.07, 1.18 | <0.001 | 0.6784 | (0.6525, 0.7044) | <0.001 |
| aPWV (SD) | 1.26 | 1.14, 1.38 | <0.001 | |||
| aPWV (SD-log unit) | 1.29 | 1.16, 1.44 | <0.001 | |||
|
| ||||||
| Model 2-Model 1 | 0.0104 | (0.0012, 0.0195) | <0.05 | |||
Participants with history of MACE at baseline are excluded. ASCVD: Atherosclerotic Cardiovascular Disease; aPWV (m/s): aortic Pulse Wave Velocity at baseline (continuous measure in meters per second). SD: Standard Deviation. HDL-C: High-Density Lipoprotein cholesterol. SBP: Systolic blood pressure.
After adding aPWV to the ASCVD score, there was a modest, but meaningful improvement in the C-statistic of the model from 0.67 to 0.68 (Table 2). In an analysis including only the intermediate ASCVD group (7.5%−20%) or participants not on lipid-lowering or antihypertensive medications, the improvement in C-statistic was similar in magnitude although with a p-value = 0.09 (95%CI = −0.004, 0.048). (Table 3). Internal calibration of the risk prediction model was good (Figure S2). Table 4 shows that including aPWV as additional predictor led 11 (2.7%) extra MACE cases to be reclassified into a higher risk category (improving sensitivity) and 66 (1.9%) extra non-MACE cases to be classified into a lower risk category (improving specificity). The overall net reclassification improvement was 4.6% in the total sample (p= 0.04). The NRI increased to 11.3% when those on medication were excluded (Table 5).
Table 3.
Association between aPWV and MACE and discriminant ability in subgroups
| Participants with intermediate ASCVD risk score (7.5–20%) (208 events among 1966 participants)* | |||||||
|---|---|---|---|---|---|---|---|
| Model 1 (ASCVD components) | |||||||
| Adjustment | HR | 95%CI | p-value | C-statistic | 95%CI | p-value | |
| Age | 1.05 | 1.00, 1.09 | <0.05 | 0.5808 | (0.5402, 0.6211) | <0.001 | |
| Total cholesterol (per SD) | 0.99 | 0.84, 1.17 | 0.92 | ||||
| HDL (per SD) | 0.93 | 0.78, 1.09 | 0.38 | ||||
| Antihypertensive use | 1.56 | 1.15, 2.11 | <0.01 | ||||
| SBP (per SD) | 1.02 | 0.86, 1.20 | 0.83 | ||||
| Smoker | 1.18 | 0.69, 2.00 | 0.53 | ||||
| Incident diabetes | 1.09 | 0.63, 1.89 | 0.76 | ||||
| Female | 1.02 | 0.66, 1.56 | 0.94 | ||||
|
Model 2 (ASCVD components + aPWV) | |||||||
| Model 1 + aPWV (m/s) | 1.13 | 1.06, 1.20 | <0.001 | 0.6032 | (0.5633, 0.6432) | <0.001 | |
| aPWV (SD) | 1.28 | 1.13, 1.45 | <0.001 | ||||
| aPWV (SD-log unit) | 1.32 | 1.14, 1.54 | <0.001 | ||||
|
| |||||||
| Model 2-Model 1 | 0.0225 | (−0.004, 0.048) | 0.09 | ||||
|
Participants without lipid-lowering or antihypertensive medication (185 events among 2301 participants) ^ | |||||||
|
Model 3
(ASCVD components) |
0.6463 | (0.6077, 0.6849) | <0.001 | ||||
|
Model 4
(Model 3 + aPWV) |
0.6588 | (0.6188, 0.6987) | <0.001 | ||||
|
| |||||||
| Model 4-Model 3 | 0.0125 | (−0.0074, 0.0324) | 0.22 | ||||
Participants with history of MACE at baseline and participants with ASCVD <7.5% or >20% are excluded.
Participant with current use of lipid lowering or antihypertensive medication are excluded. ASCVD: Atherosclerotic Cardiovascular Disease; aPWV (m/s): aortic Pulse Wave Velocity at baseline (continuous measure in meters per second). SD: Standard Deviation. HDL-C: High-Density Lipoprotein cholesterol. SBP: Systolic blood pressure.
Table 4.
Net reclassification improvement for MACE after the addition of aPWV to ASCVD risk factors*
| ASCVD risk factors at baseline | ASCVD risk factors + aPWV | ||||
|---|---|---|---|---|---|
| Subpopulation | |||||
|
| |||||
| Incident MACE cases | <7.5% | 7.5–20% | >20% | Total | NRI (95% CI) |
| <7.5% | 67 | 13 | 0 | 80 | 0.027 (−0.016, 0.070) |
| 7.5–20% | 21 | 183 | 33 | 237 | |
| >20% | 0 | 14 | 80 | 94 | |
| Total | 88 | 210 | 113 | 411 | |
| No MACE during follow-up | <7.5% | 7.5–20% | >20% | Total | |
|
| |||||
| <7.5% | 1322 | 109 | 0 | 1431 | 0.019 (0.007, 0.031) |
| 7.5–20% | 176 | 1456 | 77 | 1709 | |
| >20% | 0 | 76 | 210 | 286 | |
| Total | 1498 | 1641 | 287 | 3426 | |
|
| |||||
| Total NRI | 0.046 (0.002,0.090) p=0.04 |
||||
Participants with history of MACE at baseline are excluded. ASCVD: Atherosclerotic Cardiovascular Disease.
Table 5.
Net reclassification improvement for MACE after the addition of PWV to ASCVD risk factors* (185 events among 2301 participants).
| ASCVD risk factors at baseline Subpopulation |
ASCVD risk factors + PWV |
||||
|---|---|---|---|---|---|
|
| |||||
| Incident MACE cases | <7.5% | 7.5–20% | ≥20% | Total | NRI (95% CI) |
| <7.5% | 54 | 15 | 0 | 69 | 0.081 (0.009, 0.152) |
| 7.5–20% | 14 | 80 | 16 | 110 | |
| ≥20% | 0 | 2 | 4 | 6 | |
| Total | 68 | 97 | 20 | 185 | |
| No MACE during follow-up | <7.5% | 7.5–20% | ≥20% | Total | |
|
| |||||
| <7.5% | 1076 | 89 | 0 | 1165 | 0.032 (0.015, 0.048) |
| 7.5–20% | 179 | 709 | 33 | 921 | |
| ≥20% | 0 | 11 | 19 | 30 | |
| Total | 1255 | 809 | 52 | 2116 | |
|
| |||||
| Total NRI | 0.113 (0.039, 0.187) p =0.02 |
||||
Participants with history of MACE at baseline and participants with lipid lowering and antihypertensive drugs are excluded. ASCVD: Atherosclerotic Cardiovascular Disease.
Conclusions
Among healthy elderly men and women, participants with aPWV in the top quartile (>9.45 m/s) reached the high CVD risk threshold within five years. This was twice as fast as those in other quartiles, who reached the 7.5% risk level after approximately 10 years. Aortic PWV was associated with major adverse cardiovascular events after adjustment for all ASCVD components and when stratified to intermediate risk group or to those not using lipid-lowering of antihypertensive medications. It also improved performance of the ASCVD score in predicting major adverse cardiovascular events. The net reclassification improvement after adding aPVW to ASCVD score, was 5% in the whole cohort and 11% in those not on lipid-lowering or antihypertensive medications.
Previous studies have shown that aPWV is independently associated with incident CVD events after adjusting for systolic blood pressure and other cardiovascular risk factors. Our study confirms the additional predictive value of the PWV measurement over and above the information provided by systolic blood pressure8,19 Building on pioneer studies showing risk reclassification using aPWV,20 our study explores the clinical relevance of this reclassification within groups of individuals with intermediate risk. Our study is the first to suggest that aPVW can identify older individuals with accelerated risk progression in a similar way to that achieved with measurement of coronary artery calcium, 21 and to show that adding aPWV to ASCVD score improves NRI. The improvement in NRI of 5 to 11% is comparable to other strong risk enhancers and suggests that health benefit could be achieved at population as well as clinical level. Earlier studies on improvement of the ASCVD score have shown that adding CAC and family history increased NRI by 12% and 5%, respectively, 22 whereas ankle-brachial index and C-reactive protein did not show improvements. Recently, a polygenic risk score for coronary heart disease was observed to improve NRI in detection of early onset MACE by 4%. 23 Compared to these findings, our results suggest aPWV improves ASCVD score with similar magnitude as other major risk enhancers. This makes it an interesting tool that could be used in primary prevention to detect subclinical atherosclerosis rather than elevated CVD risk alone. These findings hold promise in improving risk stratification in a growing number of elderly individuals with a high ASCVD risk score driven by age and not by underlying atherosclerosis.
Scalability and applicability of applanation tonometry-based aPWV measurement is feasible in primary health care centers where preventive medication is commonly prescribed. Currently, determination of CAC score is the gold standard recommendation when the treatment decision of lipid-lowering medication is uncertain. 24 However, CAC measurement is dependent on ionizing radiation and computed tomography (CT) scanners are often not available in primary care. The cost per gained quality adjusted life-year (QALY) using CAC measurements (USD $35,000-$48,000) exceeds the cost-effectiveness recommendations in the UK, 25 making its use in primary care less feasible. In comparison, aPWV is less expensive, does not rely on ionizing radiation, and can be performed by trained technicians. This stresses the need of further studies on applicability of aPWV in risk stratification in primary care settings.
The magnitude of risk of MACE associated with aPWV in the present study was similar to that reported in the scientific literature. A meta-analysis of studies assessing risk of MACE according to aPWV reported a summary hazard ratio of 1.4 per m/s similar to that in the present study (HR=1.2 per m/s). 8 The Health ABC study obtained an unadjusted HR for CHD of 1.5 in the highest quartile of aPWV compared to the lowest. 26 In overview, our results appear broadly representative of white ethnic background populations. The magnitude and strength of the association between aPWV and MACE was consistent among low and intermediate risk individuals and non-statin users. In these subgroups, the performance of aPWV as an ASCVD predictor was at least as good as in the main analyses.
There are three main strengths of our study. First is the quality of the data, using aPWV, the gold-standard for measuring arterial stiffness. 27,28 Second, although Whitehall II is an occupational cohort, risk factor estimates are similar to those derived from community-based cohorts, 29 suggesting our results may be extrapolated to the general elderly population. Third, ascertainment of fatal and nonfatal MACE through the use of NHS Hospital Episode Statistics means that neither outcomes nor participants were lost over the follow-up. 12 The validity of cardiovascular disease ascertainment using linkage to the UK Hospital Episode Statistics database records is supported by agreement with high resolution disease data collected in the cohort. 30 Also, the quality of the NHS Hospital Episode Statistics data is high enough to be used in studies of clinical outcomes. 31 Taking all these factors into account, consideration of aPWV as a risk enhancer to the ASCVD score is an option that could be developed for the improvement of treatment decision making.
Our study has some limitations. We studied only the ASCVD score, although many other risk estimation tools exist. 32 ASCVD score was chosen because earlier studies on other potential risk enhancers for ASCVD score are numerous, which eased the comparison of aPWV to other risk enhancers. In addition, risk algorithms tend to exhibit similar performance after calibration. 32 As an occupational cohort recruiting London-based civil servants in the 1980s, Whitehall II has largely male participants of white ethnicity. Our results would benefit therefore from replication in cohorts with diverse ethnic composition and younger baseline age range. However, the results were consistent in subgroup analyses including younger individuals with lower CVD risk and individuals with no statin medications.
Supplementary Material
Pathophysiologic Novelty and relevance.
What is new?
This research shows that the measurement of aortic stiffness with pulse wave velocity improves the performance of cardiovascular risk stratification tools, especially in risk groups where the decision of initiation of primary prevention therapies might not be clear.
What is relevant?
The results suggest aortic stiffness measurement holds potential to improve cost-effectiveness of cardiovascular disease prevention in primary care.
What are the pathophysiological implications?
The improved performance of the combined model predicting cardiovascular events when aortic pulse wave velocity is added to ASCVD risk score may be explained by better capture of the degree of arterial ageing.
Perspectives.
Among older men and women, adjunct measurement of aPWV on a single occasion modestly improved the performance of the ASCVD score. Due to scalability and cost-effectiveness of aPWV, there is potential gain in cardiovascular prevention in primary care where patient volumes are high. The clinical benefits of aPWV should be evaluated in future research.
Acknowledgements
Thanks to the participants of the Whitehall II study, researchers and support staff who make our research possible.
Sources of funding:
The Whitehall II Study has been supported by grants from the British Medical Research Council; British Economic and Social Research Council; British Heart Foundation (RG/16/11/32334); United Kingdom Health and Safety Executive; United Kingdom Department of Health; National Heart Lung and Blood Institute (HL36310), National Institutes of Health; National Institute on Aging ((R01AG056477, RF1AG062553), National Institutes of Health; Agency for Health Care Policy Research (HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. United Kingdom Stroke Association; United Kingdom Health and Safety Executive.). EJB is supported by UKRI grant ES/T014377/1, UK-China Health And Social Challenges Ageing Project (UKCHASCAP)
IBW and CMM acknowledge funding support from the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the NIHR or the Dept Health and Social Care. CVH is supported by the doctoral fellowship 728–2015 of Colciencias and the “Study Abroad” loan from Colfuturo. JVL was supported by Academy of Finland (339568).
Footnotes
Disclosures
None
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):E1082–E1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindbohm JV, Sipilä PN, Mars NJ, et al. 5-year versus risk-category-specific screening intervals for cardiovascular disease prevention: a cohort study. The Lancet Public Health. 2019;4(4):e189–e199. doi: 10.1016/S2468-2667(19)30023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis CM, Vassos E. Polygenic risk scores: From research tools to clinical instruments. Genome Medicine. 2020;12(1). doi: 10.1186/s13073-020-00742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131(24):2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310 [DOI] [PubMed] [Google Scholar]
- 5.Brunner EJ, Shipley MJ, Witte DR, et al. Arterial stiffness, physical function, and functional limitation: The whitehall II study. Hypertension. 2011;57(5):1003–1009. doi: 10.1161/HYPERTENSIONAHA.110.168864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamoto M, Shipley MJ, Wilkinson IB, et al. Does Poorer Pulmonary Function Accelerate Arterial Stiffening?: A Cohort Study with Repeated Measurements of Carotid-Femoral Pulse Wave Velocity. Hypertension. 2019;74(4):929–935. doi: 10.1161/HYPERTENSIONAHA.119.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covic A, Siriopol D. Pulse wave velocity ratio: the new “gold standard” for measuring arterial stiffness. Hypertension (Dallas, Tex : 1979). 2015;65(2):289–290. doi: 10.1161/HYPERTENSIONAHA.114.04678 [DOI] [PubMed] [Google Scholar]
- 8.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 2014;63(7):636–646. doi: 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koivistoinen T, Lyytikäinen LP, Aatola H, et al. Pulse Wave Velocity Predicts the Progression of Blood Pressure and Development of Hypertension in Young Adults. Hypertension. Published online 2018. doi: 10.1161/HYPERTENSIONAHA.117.10368 [DOI] [PubMed] [Google Scholar]
- 10.Marmot M, Brunner E. Cohort profile: The Whitehall II study. International Journal of Epidemiology. Published online 2005. doi: 10.1093/ije/dyh372 [DOI] [PubMed] [Google Scholar]
- 11.Agnoletti D, Millasseau SC, Topouchian J, Zhang Y, Safar ME, Blacher J. Pulse wave analysis with two tonometric devices: a comparison study. Physiological measurement. 2014;35(9):1837–1848. doi: 10.1088/0967-3334/35/9/1837 [DOI] [PubMed] [Google Scholar]
- 12.Britton A, Milne B, Butler T, et al. Validating self-reported strokes in a longitudinal UK cohort study (Whitehall II): Extracting information from hospital medical records versus the Hospital Episode Statistics database. BMC Medical Research Methodology. 2012;12. doi: 10.1186/1471-2288-12-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake SR, Roome C, Shahzad A, et al. A comparison of hospital episode statistics and traditional methods to identify outcomes in a randomized trial; a sub-study of HEAT-PPCI. Journal of Public Health. Published online 2019. doi: 10.1093/pubmed/fdy225 [DOI] [PubMed] [Google Scholar]
- 14.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Journal of the American College of Cardiology. 2014;63(25 PART B):2935–2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. Stata Journal. 2010;10(3):339–358. doi: 10.1177/1536867x1001000303 [DOI] [Google Scholar]
- 16.Newson R Confidence intervals for rank statistics: Somers’ D and extensions. Stata Journal. 2006;6(3):309–334. doi: 10.1177/1536867x0600600302 [DOI] [Google Scholar]
- 17.Sundström J, Byberg L, Gedeborg R, Michaëlsson K, Berglund L. Useful tests of usefulness of new risk factors: Tools for assessing reclassification and discrimination. Scandinavian Journal of Public Health. Published online 2011. doi: 10.1177/1403494810396556 [DOI] [PubMed] [Google Scholar]
- 18.StataCorp. Stata Statistical Software: Release 13. 2013. Published online 2013. doi: 10.2307/2234838 [DOI] [Google Scholar]
- 19.Kullo IJ, Malik AR. Arterial Ultrasonography and Tonometry as Adjuncts to Cardiovascular Risk Stratification. Journal of the American College of Cardiology. 2007;49(13):1413–1426. doi: 10.1016/j.jacc.2006.11.039 [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). European Heart Journal. 2018;39(25):2401b–2408b. doi: 10.1093/eurheartj/ehy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeboah J, Young R, McClelland RL, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. Journal of the American College of Cardiology. 2016;67(2):139–147. doi: 10.1016/j.jacc.2015.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mars N, Koskela JT, Ripatti P, et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nature medicine. 2020;26(4):549–557. doi: 10.1038/s41591-020-0800-0 [DOI] [PubMed] [Google Scholar]
- 24.Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guidestatin therapy a cost-effectiveness analysis. Circulation: Cardiovascular Quality and Outcomes. 2014;7(2):276–284. doi: 10.1161/CIRCOUTCOMES.113.000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence. How NICE Measures Value for Money in Relation to Public Health Interventions. National Institute for Health and Care Excellence; 2013. [Google Scholar]
- 26.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. Published online 2005. doi: 10.1161/CIRCULATIONAHA.104.483628 [DOI] [PubMed] [Google Scholar]
- 27.Kim HL, Kim SH. Pulse Wave Velocity in Atherosclerosis. Frontiers in Cardiovascular Medicine. 2019;6. doi: 10.3389/fcvm.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann B, Riemer M, Erbs C, et al. Carotid to femoral pulse wave velocity reflects the extent of coronary artery disease. Journal of Clinical Hypertension. Published online 2014. doi: 10.1111/jch.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batty GD, Shipley M, Tabák A, et al. Generalizability of occupational cohort study findings. Epidemiology. 2014;25(6):932–933. doi: 10.1097/EDE.0000000000000184 [DOI] [PubMed] [Google Scholar]
- 30.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of Cardiovascular Disease Event Ascertainment Using Linkage to UK Hospital Records. Epidemiology. 2017;28(5):735–739. doi: 10.1097/EDE.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright-Hughes A, Graham E, Cottrell D, Farrin A. Routine hospital data – is it good enough for trials? An example using England’s Hospital Episode Statistics in the SHIFT trial of Family Therapy vs. Treatment as Usual in adolescents following self-harm. Clinical Trials. 2018;15(2):197–206. doi: 10.1177/1740774517751381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennells L, Kaptoge S, Wood A, et al. Equalization of four cardiovascular risk algorithms after systematic recalibration: individual-participant meta-analysis of 86 prospective studies. European heart journal. 2019;40(7):621–631. doi: 10.1093/eurheartj/ehy653 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


