Abstract
Objective(s):
Infection with high-risk human papillomavirus is required to develop cervical cancer. Some viruses modulate the Fas/FasL signaling to evade the immune response; the role of these molecules in cervical cancer is not clear. In this study, we measured the expression levels of Fas and FasL mRNA, soluble proteins, and cell surface proteins in peripheral blood mononuclear cells from patients with low- and high-grade squamous intraepithelial lesions and cervical cancer in relation to healthy women, to gain new insights into the role of Fas/FasL in cervical cancer development.
Materials and Methods:
Fas/FasL mRNA expression was measured in cervical tissues and peripheral blood mononuclear cells from patients and healthy subjects; serum soluble proteins Fas/FasL were measured by ELISA, and cell-surface protein expression was detected by flow cytometry.
Results:
Varying expression levels were found for both molecules. Cervical Fas and FasL mRNA expression was decreased in low- and high-grade lesions, but it was increased in cervical cancer cases. While, systemic Fas mRNA expression increased as malignity progressed; systemic FasL mRNA expression was increased in low- and high-grade lesions, but it was decreased in cancer patients. Soluble FasL levels decreased as lesions progressed, while soluble Fas levels increased. Finally, overexpression of Fas/FasL on the surface of peripheral blood mononuclear cells was found in patients with low-grade lesion with respect to healthy donors.
Conclusion:
Fas and FasL act as negative modulators of the immune response, probably by removing specific cytotoxic T lymphocytes against papillomavirus -infected cells and tumor cells.
Key Words: Cervical intraepithelial – neoplasia, Fas ligand protein, Fas receptor, Gene expression, Uterine cervical neoplasms
Introduction
Cervical cancer (CC) is a malignancy in the female reproductive tract that seriously threatens women’s health and even life. According to the World Health Organization (WHO), there were approximately 570,000 new CC cases and 311,000 deaths worldwide in 2018, with 90% of cases occurring in developing countries (1, 2). The main risk factor for this cancer type is an infection with high-risk human papillomavirus (HR-HPV). However, about 92% of infected women clear HR-HPV, and only 8% of them develop cervical lesions. Thus, a persistent HR-HPV infection is required to develop lesions that progress into CC, and this process involves an impairment of the immune response (3-5). Several factors have been linked to this impaired immunity, including an immunosuppressive microenvironment resulting from secretion of immunosuppressive cytokines by tumor cells, alterations in Toll-like Receptor (TLR)-9 expression, a reduction of E-cadherin, and a reduced expression of the activator CD3-zeta chain in T lymphocytes, recruitment of regulatory T cells, and presence of death molecules that kill specific cytotoxic T lymphocytes by apoptosis activation through Fas/ Fas Ligand (FasL) signaling (3-14).
Fas is a member of the TNF receptor superfamily; its ligand, FasL, is located on the surface of various cell types; FasL can be cleaved by a metalloproteinase, releasing a soluble ligand (15, 16). The Fas/FasL system regulates peripheral immune homeostasis, the inflammatory response, apoptosis, and non-apoptotic signaling cell death; it is also involved in tumorigenesis (17-19). When FasL binds its receptor on immune cells, it induces apoptosis of malignant or virus-infected cells. Alterations in the expression of Fas and FasL have been found in many cancer types as a mechanism for tumor cells to escape the immune system; thus, Fas/FasL signaling has been explored as a target for anti-cancer therapies (10, 17, 20-25).
To date, the roles of Fas and FasL in CC are not clear; cervical Fas levels have been reported not to change significantly, while FasL overexpression by tumor cells, ranging from 53 to 94%, has been considered as an evasion mechanism through which tumor cells kill cytotoxic T lymphocytes by apoptosis, which has been linked with carcinogenesis development (26-28). In addition, variable levels of Fas and FasL mRNA or protein expression have been found in CC biopsies (29-33). At a systemic level, increased levels of soluble Fas and FasL have been reported in CC patients (34, 35).
Altogether, these findings suggest that expression of Fas/FasL could be a predictor of CC development. To ascertain this, herein we studied mRNA, soluble proteins, and cell surface protein expression of Fas and FasL in peripheral blood mononuclear cells (PBMCs) from low- and high-grade squamous intraepithelial lesion (L-SIL and H-SIL, respectively) and CC patients, and compared them with cells from women with no cervical lesions.
Materials and Methods
Study design and population
A cross-sectional study was conducted with samples from a biological sample bank built as previously described (36). The Bioethics and Research Committees of the National Institute of Public Health (CI:814) and the INCan (INCan/CC/326/10CB/609) approved the baseline study in which the biological sample bank was built. All participants provided written informed consent for the use of their biological samples in further research studies. Each subject was interviewed for lifestyle, sociodemographic, and reproductive factors. Women with autoimmune diseases, having other sexually transmitted diseases, and who had been given treatment were excluded from the study.
The women included in this study attended the “Centro de Atención para la Salud de la Mujer” (CAPASAM) in Morelos, Mexico, from June 2008 to December 2011, and the “Servicio de Ginecología del Instituto Nacional de Cancerología (INCan)” in Mexico City from September 2010 to December 2011, and from February to May 2015. CC cases (aged 22–86) were diagnosed with either invasive squamous cell carcinoma or invasive adenocarcinoma in the cervix (n = 30). Cases of squamous intraepithelial lesions (aged 18–72) were classified as either L-SIL (n = 30) or H-SIL (n = 30). Patient classification was based on the diagnosis by the corresponding Pathology Department of CAPASAM and INCan. Subjects with no cervical lesions (NCL) and a negative PCR result for HPV (aged 17–68) were included as negative controls (n = 30). Cervical epithelial scraping specimens were analyzed to detect HPV DNA. HPV was typified by PCR amplification using consensus primers MY09/11: 5´CGT CCM ARR GCA WAC TGA TC 3´ and 5´ GCM CAG GGW CAT AAY AAT GG 3´ annealing temperature 57 °C, fragment size 450 bp (37), L1C1/L1C2: 5´CGT AAA CGT TTT CCC TAT TTT TTT 3´ and 5´ TAC CCT AAA TAC TAC TCT GTA TTG-3´ annealing temperature 50 °C, fragment size 250 bp (38) and GP5/GP6: 5´ TTT GTT ACT GTG GTA GAT ACT AC-3´ and 5´ GAA AAA TAA ACT GTA AAT CAT ATT C 3´ annealing temperature 40 °C, fragment size 150 bp (39), flanking the HPV L1 gene. PCR products were resolved in 2% agarose and visualized with ethidium bromide staining.
Characteristics of the biological sample bank
The biological sample bank used in this study included single-strand complementary DNA (cDNA) samples extracted from cervical epithelial scraping cells (in NCL subjects), from fresh cell biopsies (in SIL and CC cases), and peripheral blood samples collected from the subjects. Peripheral blood samples were collected in serum-separating vacutainer tubes and centrifuged to obtain serum. Total RNA was isolated from PBMCs obtained by density gradient centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway), and from cervical epithelial scrapings and biopsies using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA was synthesized according to a previously described protocol (36). Primers for the human housekeeping glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH, 450 pb) Fwd 5´ ACCACAGTCCATGCCATCAC 3´ Rev 5´ CCACCACCCTGTTGCTGTA 3´ (40) was used to verify cDNA integrity.
Real-time quantitative PCR for Fas and FasL mRNA
To assess Fas and FasL expression, a TaqMan real-time quantitative PCR assay was performed using a StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s directions. Gene expression was normalized against the reference gene HPRT1. All primers and the probe were purchased commercially (TaqMan Inventoried Gene Expression Assay FasL: Hs00181225_m1; Fas Hs00531110_m1; and HPRT1, Hs01003267_m1; Applied Biosystems, Waltham, MA, USA). Amplification was carried out in duplicate in a final volume of 10 μl, in 96-well reaction plates, as previously described (36). mRNA expression levels for the genes under study were determined by relative quantification with the comparative Ct method (2-ΔCt) and plotted as relative units of expression (RUE) for each gene relative to the endogenous HPRT1 gene and to the control group. All samples were analyzed in duplicate. The values are reported as median ± SE. P-values<0.05 are marked with an asterisk.
sFas and sFasL detection in serum by ELISA
Serum soluble protein levels were measured with the Quantikine kit (Human Fas Ligand/TNFSF6 and Human sFas/TNFRSF6 immunoassay; R&D Systems, Minneapolis, MN, USA), following the manufacturer’s instructions. All assays were performed in duplicate. Soluble protein levels (pg/ml) are reported as mean ± SD. P-values<0.05 are marked with an asterisk.
Cell-surface antigen staining and flow cytometry
EDTA-treated blood samples either from healthy subjects or L-SIL patients (n = 10 for each group) were analyzed. Whole blood (50 µl) was stained directly for surface antigens with the following monoclonal antibodies: anti-human CD95 Alexa Fluor® 488 (Fas, catalog number 305616, Biolegend, San Diego, CA, USA), anti-human CD178 PE (Fas-L, catalog number 306407, Biolegend) and anti-human TBNK BD-Multitest 6-color (CD3 FITC/CD16 PE+CD56 PE/CD45 PerCP-Cy™5.5/CD4 PE-Cy™7/CD19 APC/CD8 APC-Cy™7, catalog number 337166, BD Multitest, Becton Dickinson, NJ, USA), following the manufacturer’s instructions. The tubes were gently mixed and incubated at room temperature in the dark for 30 min. Then, the cells were resuspended in 2 ml of FACS lysing solution (catalog number 349202, Becton Dickinson, NJ, USA) and then gently mixed and incubated at room temperature in the dark for 10 min. After that, they were centrifuged at 540 × g for 5 min. Supernatants were discarded, and the pellets were resuspended in FACS buffer (PBS + 1% bovine serum albumin). Data were acquired in a FACSAria II flow cytometer (Becton Dickinson) and analyzed with the FACS Diva software. Debris and doublets were removed through side (SSC) and forward scatter (FSC) gating (41). Lymphocyte, monocyte/macrophage, and granulocyte populations positive from each marker were determined by acquiring 10,000 events.
Statistical analysis
Median values of Fas and FasL mRNA systemic and cervical expression levels were compared between NCL controls and L-SIL, H-SIL, and CC cases with a Wilcoxon Mann-Whitney test. Mean serum sFas and sFasL concentrations were compared among groups by one-way ANOVA; a Tukey post-hoc test was used to compare frequencies among experimental groups. Fas and FasL mRNA systemic expression was correlated with serum protein concentrations in NCL, L-SIL, H-SIL, and CC cases with a Spearman rank correlation analysis. P<0.05 was regarded as statistically significant. All statistical analyses were performed with the software STATA v.14.0 (StataCorp, College Station, TX, USA). All experiments were performed in duplicate.
Results
Fas and FasL mRNA expression in cervical lesions and CC
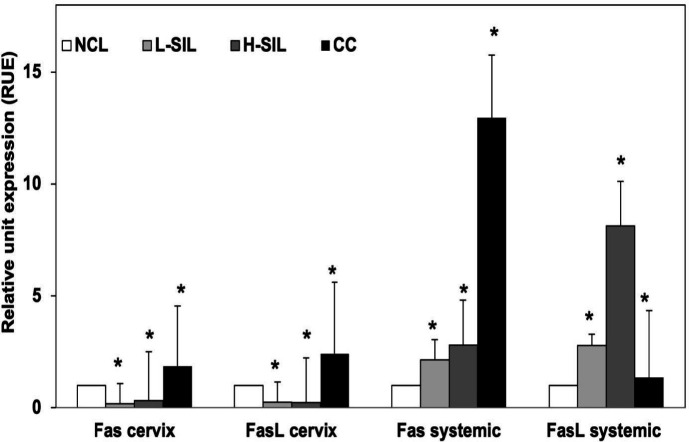
Cervical Fas and FasL expression levels in NCL, L-SIL, H-SIL, and CC patients, normalized with respect to HPRT1 mRNA, are shown in Figure 1. Median cervical expression levels of Fas and FasL mRNA were significantly different in SIL and CC cases with respect to NCL subjects. The median cervical levels of Fas and FasL mRNA relative to HPRT1 were higher in CC patients (1.9 RUE and 2.4 RUE, respectively, P=0.00004) than in NCL subjects. Also, median Fas and FasL expression levels were lower in L-SIL (0.18 RUE and 0.25 RUE, respectively, P=0.00001) and H-SIL patients (0.31 RUE and 0.23 RUE, respectively, P=0.009) than in NCL subjects.
Figure 1.
Fas and FasL mRNA local (cervix) and systemic expression in patients with differing lesion grades and cervical cancer. Medians ± Standard error (*: P<0.05). NCL: Non-cervical lesions. L-SIL: Low Squamous Intraepithelial Lesion. H-SIL: High Squamous Intraepithelial Lesion. CC: Cervical cancer
Systemic Fas and FasL mRNA expression
Statistically significant differences were observed in the median systemic expression levels of Fas and FasL mRNA between SIL and CC cases and the NCL group (Figure 1). A significant and progressive increase in the systemic levels of Fas expression was observed in L-SIL, H-SIL, and CC patients with respect to the NCL group (2.1 RUE, P=0.001; 2.8 RUE, P=0.00001; and 12.9 RUE, P=0.000001, respectively). Interestingly, while increased FasL expression levels were observed in L-SIL and H-SIL cases with respect to the NCL group (2.8 RUE, P=0.008, and 8.1 RUE, P = 000001), they were substantially decreased in CC cases (1.35 RUE, P=0.00001). The highest median systemic expression levels of Fas and FasL mRNA with respect to NCL subjects were found in CC and H-SIL patients, respectively.
Systemic sFas and sFasL protein levels
Serum sFas levels increased as the cervix malignancy progressed. Conversely, sFasL serum concentration decreased as the cervix malignancy progressed (Figure 2). Mean serum sFas levels were significantly higher in CC patients (7,449 pg/ml) than in NCL subjects (5,066 pg/ml, P=0.001). Statistically significant differences were observed in mean serum sFasL levels between NCL subjects and H-SIL (94.202 pg/ml and 74.152 pg/ml, respectively, P=0.01) and CC (68.011 pg/ml, P=0.047), except for NCL subjects and L-SIL cases (P = 0.61).
Figure 2.
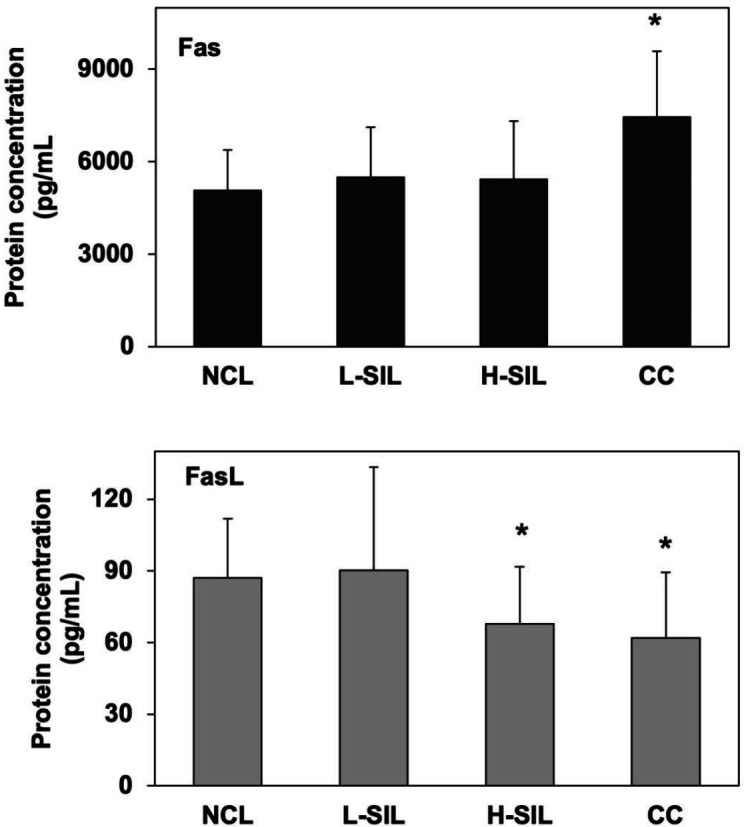
Serum sFas and sFasL levels in patients with differing lesion grades and cervical cancer. Means ± Standard deviation (*: P<0.05). Non-cervical lesions. L-SIL: Low Squamous Intraepithelial Lesion. H-SIL: High Squamous Intraepithelial Lesion. CC: Cervical cancer; NCL: Non-cervical lesions
Correlation between systemic Fas and FasL mRNA expression and serum sFas and sFasL concentration
To identify a potential correlation between systemic Fas and FasL mRNA expression and serum sFas and sFasL concentration we carried out a Spearman rank correlation analysis. Only the systemic expression of Fas mRNA showed a significant correlation with circulating sFas levels in NCL, L-SIL, H-SIL, and CC patients (r = 0.24, P=0.03).
Fas and FasL protein expression in PBMCs from L-SIL patients
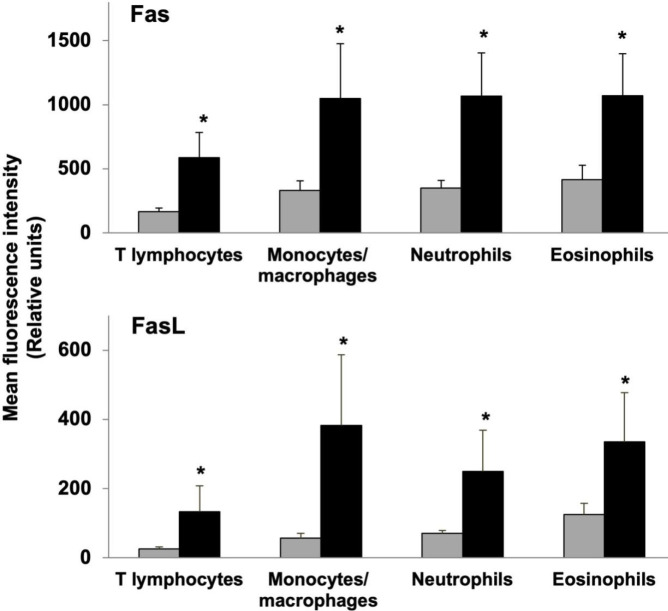
The protein expression of Fas and FasL on the cell surfaces of T lymphocytes, monocytes/macrophages, and granulocytes (neutrophils and eosinophils) was evaluated by flow cytometry. In all cell types analyzed, Fas and FasL expressions were higher in L-SIL patients with respect to healthy donors (Figure 3). A 3.5-fold increase in Fas protein expression was detected in T lymphocytes while a 3-fold increase was shown in monocytes/macrophages and neutrophils, followed by eosinophils (2.6-fold). Similarly, FasL protein was increased on monocytes/macrophages and T lymphocytes (6.8- and 5.1-fold, respectively), followed by neutrophils and eosinophils (3.5- and 2.7-fold, respectively) with respect to healthy individuals.
Figure 3.
Fas and FasL expression in PBMCs from patients with Low Squamous Intraepithelial Lesion (L-SIL) analyzed by flow cytometry. Means ± Standard deviation (*: P<0.05). L-SIL patients (black bars), healthy women (grey bars)
Discussion
Our understanding of the role of Fas and FasL in the development of CC is still limited. In this study, we compared the expression of Fas and FasL mRNA in cervical tissues and PBMCs from patients with cervical lesions in different stages and CC. We found varying levels of expression for both molecules. The cervical expression of Fas and FasL mRNA followed a similar trend, being decreased in L-SIL and H-SIL but increased in CC cases. Meanwhile, in PBMCs, Fas and FasL expression increased as malignity progressed, but FasL levels decreased in CC patients. The lower cervical expression of Fas and FasL mRNA observed at early stages of the disease could indicate that Fas and FasL mRNA had been partially traduced to the respective protein. This suggests an early activation of the immune response to cervical transformed cells, which is maintained throughout the carcinogenesis process. Fas and FasL mRNA expression has been poorly studied; in contrast with our results, a down-regulation of Fas mRNA expression was observed in tissues from CC patients, while FasL mRNA expression levels showed no significant differences with the NCL group (33). Similar results were reported in endometrial carcinoma and ovarian cancer patients (33).
When measuring the serum levels of sFas and sFasL in patients with cervical lesions and healthy controls, sFas levels were increased as cervical malignancy progressed. By contrast, sFasL levels decreased as the malignancy evolved. Correlation analyses between mRNA expression and protein levels for both molecules only showed a relation between systemic Fas mRNA expression levels with sFas serum concentrations. As the malignity progressed, a higher Fas and FasL mRNA expression correlated with higher serum sFas levels, especially in CC patients, while a decreased FasL mRNA expression in CC cases correlated with lower serum FasL levels. The decrease of sFasL in patients with CC indicates that this molecule may be bound to Fas on the surface of T lymphocytes, where we found high expression levels of Fas, which may induce T cell apoptosis. In agreement with our results, other authors have reported increased sFas levels in CC (10, 34, 35); however, increased sFasL levels have been observed in CC and other uterine tumors (34), while no differences were found in patients with oral squamous cell carcinoma (42).
While we did not evaluate the local expression of the proteins Fas and FasL in the cervix, other authors have assessed it by immunohistochemistry and reported a different pattern of Fas expression. In most cases, Fas protein expression values were similar in normal tissue and early intraepithelial lesions, but it was slightly increased in H-SIL and/or down-regulated as carcinogenesis progressed. On the other hand, overexpression of FasL was observed as malignancy progressed into CC, accompanied by a down-regulation of apoptosis and the inflammatory response; this resulted in cervical carcinoma cells escaping the immune system, metastasis, a worse prognosis, and a lower patient survival rate (20, 27, 28, 30, 31). Taken together, these findings suggest that deregulation of Fas, overexpression of FasL, and the inverse correlation between FasL overexpression and the presence of CD45+ tumor-infiltrating lymphocytes in CC tissues as reported previously (20), could indicate that FasL induced apoptosis in local (cervical) lymphocytes to favor the immune evasion of tumor cells.
Our results showed increased levels of both Fas mRNA (cervix and systemic) and sFas (serum) in CC. Various alternatively spliced Fas mRNAs, which produce sFas lacking the single membrane-spanning domain, have been described. Furthermore, each sFas isoform inhibits FasL-induced apoptosis (43). In CC, it is well known that the HPV-derived E6 and E7 oncoproteins alter several cellular actions through a plethora of interactions with cellular proteins, some of them associated with immune escape and apoptosis. E6 interacts with proteins involved in the extrinsic apoptosis pathway, such as FADD, TNF-R1, and pro-caspase-8 (44). However, whether the E6 oncoprotein induces an increase in Fas mRNA expression as an escape mechanism induced by HPV is still unknown.
In this study, both Fas and FasL were found to be overexpressed on the surface of PBMCs from L-SIL patients with respect to healthy, HPV-negative subjects in a similar age range (22–58 years). T lymphocytes and monocytes/macrophages expressed twice as much Fas than FasL. The high amounts of Fas and FasL on the surface of PBMCs were associated with mRNA overexpression for both molecules at the systemic level. On the other hand, only a significant increase was found in the serum level of Fas protein in CC patients. Taken together, our results indicate a clear overexpression in Fas and FasL at a systemic level, both in mRNA and cell surface protein in HPV-positive patients, possibly as a response from activated lymphocytes to the virus. These findings are consistent with those reported previously by Kurmyshkina et al. 2017, who showed in H-SIL and CC patients a significant up-regulation in the levels of circulating CD3+CD95+ (Fas) and CD4+CD95+ in T lymphocytes, that also were positive for the apoptosis marker annexin-V, with respect to healthy donors, suggesting that CD95-dependent pathway could be associated with CC development (45). Furthermore, an altered proportion of CD4+/CD8+ T lymphocytes was reported in PBMCs from L-SIL and CC cases, in relation to healthy donors (8); also in women with CC, the CD8+ T cells were predominant compared with CD4+ (8); this difference may be explained for the high levels of TGF-β in these patients, which induce apoptosis in CD4+ lymphocytes, but not in CD8+ T cells (46). Finally, Sun et al. (2005) evaluated also by flow cytometry the Fas and FasL protein expression in CD3+ T lymphocytes from healthy donors carrying different SNPs (single nucleotide polymorphisms) for these molecules, finding significant differences in Fas/FasL expression according to the genotype, which could be associated with susceptibility to CC (47). To the best of our knowledge, no evidence has been reported on Fas and FasL expression assessed by flow cytometry on the surface of macrophages or granulocytes from patients with cervical lesions.
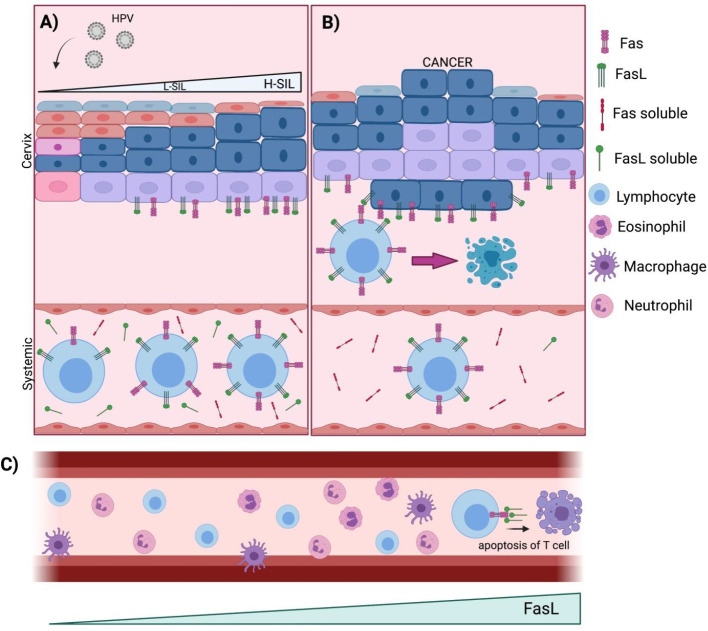
In summary, the local decrease in Fas and FasL mRNA expression suggests that both molecules could be involved in an evasion of the immune system induced by HR-HPV, while the increased levels of Fas and FasL on the surface of circulating PBMCs suggests an activation of the immune system against viral infection. The increase of Fas expression in the cervix and PBMCs from CC patients suggests that HPV-infected cells can evade cell death by cytotoxic T lymphocytes through Fas and FasL. Additionally, the increased expression levels of Fas and FasL at a systemic level and on the surface of T lymphocytes, and the higher cervical levels of FasL in CC patients suggest that HPV-transformed cells could remove tumor-infiltrating lymphocytes (TILs); this is further supported by the lower systemic, but higher cervical FasL levels in CC cases, which may target Fas-expressing T lymphocytes, inducing cell death by apoptosis. A schematic representation of the role of Fas and FasL in CC progression is shown in Figure 4.
Figure 4.
Schematic representation of the role of Fas and FasL in CC progression. The main event in human papillomavirus (HPV)-induced carcinogenesis is the infection of cervical epithelial cells at the basement membrane. Upon infection, an average of 2–3 years are required to develop L-SIL and H-SIL. The immune system plays a key role in HPV-induced carcinogenesis, since more than 90% of high-risk HPV infections regress, as well as most of the low-grade lesions (75%). The long period of time between the viral infection and the development of an invasive disease implies a failed immune response, crucial for cancer progression. A) Upon HPV infection, Fas and FasL are overexpressed at the systemic level, on the surface of immune cells, and in the serum of L-SIL patients, possibly derived from activated lymphocytes in response to HR-HPV infection. B) FasL levels are increased in cervical cancer cells in correspondence with the lesion grade, with the highest concentrations measured in cancer patients. This may lead to release of FasL from cervical cancer cells, which interacts with the Fas receptor on the surface of T lymphocytes, probably inducing apoptosis. C) These events are key for the impairment of immune responses allowing the persistence of HPV and eventually results in cell transformation
Conclusion
The results herein reported strongly support the hypothesis that Fas and FasL play a key role as negative modulators of the local immune response, probably by removing specific cytotoxic T lymphocytes, which in turn could favor the survival and proliferation of tumor cells and CC progression. Further studies are required to ascertain the role of Fas and FasL in different subsets of T lymphocytes from patients with cervical lesions and cancer.
Authors’ Contributions
COCO, MBR, and LYLD Performed experiments; COCO, MBR, and KTP Designed the experiments and analysed and interpreted data; JMM Helped elaborate the graphical model and critically revised the manuscript; CMC Supported FACS analyses; COCO, MBR, ALM, JMM, KTP, and VMM Wrote the manuscript; VMM Conceived and designed the study and helped with funding acquisition.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgment
This study was supported by the Instituto Nacional de Salud Pública, México, and grants from the Consejo Nacional de Ciencia y Tecnología (CONACYT, Mexico) to VMM (FONSEC SSA/IMSS/ISSSTE-2008 grant #87701, and FONSEC SSA/IMSS/ ISSSTE-2014 grant #234191) and Instituto Nacional de Cancerología to JMM (grant (018/037/IBI) CEI/1284/18). The results presented in this paper were part of a LYLD student thesis. The authors would like to express their appreciation to Karina Delgado-Romero (CAPASAM) and Alejandro García Carrancá (INCan) for their valuable support in the patient recruitment included in the sample bank used in this study.
References
- 1.Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:191–203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Smola S. Immunopathogenesis of HPV-Associated Cancers and Prospects for Immunotherapy. Viruses. 2017;9:254–269. doi: 10.3390/v9090254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman SR, Le T, Lockhart A, Sanusi A, Santo LD, Davis M, et al. Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): A systematic review. Int J Cancer. 2017;141:8–23. doi: 10.1002/ijc.30623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westrich JA, Warren CJ, Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017;231:21–33. doi: 10.1016/j.virusres.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres-Poveda K, Bahena-Román M, Madrid-González C, Burguete-García AI, Bermúdez-Morales VH, Peralta-Zaragoza O, et al. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol. 2014;10:753–763. doi: 10.5306/wjco.v5.i4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Campos C, Bahena-Román M, Torres-Poveda K, Burguete-García AL, Madrid-Marina V. TLR9 gene polymorphism-1486T/C (rs187084) is associated with uterine cervical neoplasm in Mexican female population. J Cancer Res Clin Oncol. 2017;143:2437–2445. doi: 10.1007/s00432-017-2495-2. [DOI] [PubMed] [Google Scholar]
- 8.Díaz-Benítez CE, Navarro-Fuentes KR, Flores-Sosa JA, Juárez-Díaz j, Uribe-Salas FJ, Román-Basaure E, et al. CD3zeta expression and T cell proliferation are inhibited by TGF-beta1 and IL-10 in cervical cancer patients. J Clin Immunol. 2009;29:532–544. doi: 10.1007/s10875-009-9279-7. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Tomoko S, Ai S, Takao H, Akitoshi N, Osamu T, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar-Lemarroy A, Romero-Ramos JE, Olimon-Andalon V, Hernandez-Flores G, Lerma-Diaz JM, Ortiz-Lazareno PC, et al. Apoptosis induction in Jurkat cells and sCD95 levels in women’s sera are related with the risk of developing cervical cancer. BMC Cancer. 2008;8:1–12. doi: 10.1186/1471-2407-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y, Yuan J-L, Abudula A, Hasimu A, Kadeer N, Guo X. TLR9 expression in uterine cervical lesions of Uyghur women correlate with cervical cancer progression and selective silencing of human papillomavirus 16 E6 and E7 oncoproteins in vitro. Asian Pac J Cancer Prev. 2014;15:5867. doi: 10.7314/apjcp.2014.15.14.5867. [DOI] [PubMed] [Google Scholar]
- 12.Leong C-M, Doorbar J, Nindl I, Yoon H-S, Hibma MH. Deregulation of E-cadherin by human papillomavirus is not confined to high-risk, cancer-causing types. Br J Dermatol. 2010;163:1253–1263. doi: 10.1111/j.1365-2133.2010.09968.x. [DOI] [PubMed] [Google Scholar]
- 13.Contreras DN, Krammer PH, Potkul RK, Bu P, Rossi JL Kaufmann et al. Cervical cancer cells induce apoptosis of cytotoxic T lymphocytes. J Immunother. 2000;23:67–74. doi: 10.1097/00002371-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez-Hoya A, Zerecero-Carreón O, Valle-Mendiola A, Moreno-Lafont M, López-Santiago R, Weiss-Steider B, et al. Cervical Cancer Cells Express Markers Associated with Immunosurveillance. J Immunol Res. 2019;6:1242979. doi: 10.1155/2019/1242979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knox PG, Milner AE, Green NK, Eliopoulos AG, Young LS. Inhibition of metalloproteinase cleavage enhances the cytotoxicity of Fas ligand. J Immunol . 2003;170:677–685. doi: 10.4049/jimmunol.170.2.677. [DOI] [PubMed] [Google Scholar]
- 16.Rossin A, Miloro G, Hueber A-0. TRAIL and FasL functions in cancer and autoimmune diseases: Towards an increasing complexity. cancers (Basel) 2019;11:639–656. doi: 10.3390/cancers11050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada A, Arakaki R, Saito M, Kudo Y, Ishimaru N. Dual role of fas/fasl-mediated signal in peripheral immune tolerance. Front Immunol. 2017;8:403–412. doi: 10.3389/fimmu.2017.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guégan JP, Legembre P. Nonapoptotic functions of Fas/CD95 in the immune response. FEBS J. 2018;285:809–827. doi: 10.1111/febs.14292. [DOI] [PubMed] [Google Scholar]
- 19.Tang R, Xu J, Zhang B, Liu J, Liang Ch, Hua J. et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110–127. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anggraeni TD, Rustamadji P, Aziz MF. Fas ligand (FasL) in association with tumor-infiltrating lymphocytes (TILs) in early stage cervical cancer. Asian Pac J Cancer Prev. 2020;21:831–835. doi: 10.31557/APJCP.2020.21.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villa-Morales M, Fernández-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Targets . 2012;16:85–101. doi: 10.1517/14728222.2011.628937. [DOI] [PubMed] [Google Scholar]
- 22.Wajant H. CD95L/FasL and TRAIL in tumour surveillance and cancer therapy. Cancer Treat Res. 2006;130:141–165. doi: 10.1007/0-387-26283-0_7. [DOI] [PubMed] [Google Scholar]
- 23.Richards DM, Merz Ch, Gieffers Ch, Krendyukov A. CD95L and anti-tumor immune response: Current understanding and new evidence. Cancer Manag Res. 2021;13:2477–2482. doi: 10.2147/CMAR.S297499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koff J L, Ramachandiran S, Bernal-Mizrachi L. A time to kill: Targeting apoptosis in cancer. Int J Mol Sci. 2015;16:2942–2955. doi: 10.3390/ijms16022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadir AS, Stults AM, Murmann AE, Peter ME. The mechanism of how CD95/Fas activates the Type I IFN/STAT1 axis, driving cancer stemness in breast cancer. Sci Rep. 2020;10 doi: 10.1038/s41598-020-58211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jian-Hong Z, Huai-Zeng Ch, Feng Ye, Wei-Guo Lu, Xing X. Fas-mediated pathway and apoptosis in normal cervix, cervical intraepithelial neoplasia and cervical squamous cancer. Oncol Rep. 2006;16:307–311. [PubMed] [Google Scholar]
- 27.Lerma E, Romero M, Gallardo A, Pons C, Muñoz J, Fuentes J, et al. Prognostic significance of the Fas-receptor/Fas-ligand system in cervical squamous cell carcinoma. Virchows Arch. 2008;452:65–74. doi: 10.1007/s00428-007-0535-z. [DOI] [PubMed] [Google Scholar]
- 28.Reesink-Peters N, Hougardy FAJ, van den Heuvel FAJ, Ten Hoor KA, Hollema H, Boezen HM, et al. Death receptors and ligands in cervical carcinogenesis: an immunohistochemical study. Gynecol Oncol. 2005;96:705–713. doi: 10.1016/j.ygyno.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Wu SF, Zhang JW, Qian WY, Yang YB, Liu Y, Dong Y, et al. Altered expression of survivin, Fas and FasL contributed to cervical cancer development and metastasis. Eur Rev Med Pharmacol Sci. 2012;16:2044–2050. [PubMed] [Google Scholar]
- 30.Ibrahim R, Frederickson H, Parr A, Ward Y, Moncur J, Khleif S N. Expression of FasL in squamous cell carcinomas of the cervix and cervical intraepithelial neoplasia and its role in tumor escape mechanism. Cancer. 2006;106:1065–1077. doi: 10.1002/cncr.21697. [DOI] [PubMed] [Google Scholar]
- 31.Liang SN, YJ Huang YJ, Liu LL, Liu X. Study on the correlation between the expression of Ki67 and FasL and prognosis of cervical carcinoma. Genet Mol Res . 2015;14:8634–8639. doi: 10.4238/2015.July.31.11. [DOI] [PubMed] [Google Scholar]
- 32.Munakata S, Watanabe O, Ohashi K, Morino H. Expression of Fas ligand and bcl-2 in cervical carcinoma and their prognostic significance. Am J Clin Pathol. 2005;123:879885. doi: 10.1309/0773-N4Q3-GFP3-4J5V. [DOI] [PubMed] [Google Scholar]
- 33.Das H, Koizumi T, Sugimoto T, Chakraborty S, Ichimura T, Hasegawa K, et al. Quantitation of Fas and Fas ligand gene expression in human ovarian, cervical and endometrial carcinomas using real-time quantitative RT-PCR. Br J Cancer. 2000;82:1682–1688. doi: 10.1054/bjoc.2000.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondera-Anasz Z, Mielczarek-Palacz A, J Sikora J. Soluble Fas receptor and soluble Fas ligand in the serum of women with uterine tumors. Apoptosis . 2005;10:1143–1149. doi: 10.1007/s10495-005-1018-9. [DOI] [PubMed] [Google Scholar]
- 35.Konno R, Takano T, Sato S, Yajima A. Serum soluble Fas level as a prognostic factor in patients with gynecological malignancies. Clin Cancer Res . 2000;6:3576–3580. [PubMed] [Google Scholar]
- 36.Torres-Poveda K, Burguete-García AI, Cruz M, Martínez-Nava G, Bahena-Román M, Ortíz-Flores E, et al. The SNP at −592 of human IL-10 gene is associated with serum IL-10 levels and increased risk for human papillomavirus cervical lesion development. Infect Agent Cancer. 2012;7:32:1–9. doi: 10.1186/1750-9378-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernard HU, Chan SY, Manos MM, Ong CK, Villa LL, Delius H, et al. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994;70:1077–1085. doi: 10.1093/infdis/170.5.1077. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa H, Kawana T, Kitagawa K, Mizuno M, Yoshikura H, Iwamoto A. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res. 1991;82:524–531. doi: 10.1111/j.1349-7006.1991.tb01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use general primers GP5 and GP6 elongated at their 3´end with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 40.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 41.Kalina T, Flores-Montero J, van der Velden VHJ, Martin-Ayuso M, Böttcher S, Ritgen M, et al. EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708) EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia . 2012;26:1986–2010. doi: 10.1038/leu.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zepeda-Nuño JS, Guerrero-Velázquez C, Del Toro-Arreola S, Vega-Magaña N, Ángeles-Sánchez J, Haramati J, et al. Expression of ADAM10, Fas, FasL and soluble FasL in patients with oral squamous cell carcinoma (OSCC) and their sssociation with clinical-pathological parameters. Pathol Oncol Res . 2017;23:345–353. doi: 10.1007/s12253-016-0102-5. [DOI] [PubMed] [Google Scholar]
- 43.Papoff G, Cascino I, Eramo A, Starace G, Lynch DH, Ruberti G. An N-terminal domain shared by Fas/Apo-1 (CD95) soluble variants prevents cell death in vitro. J Immunol. 1996;156:4622–4630. [PubMed] [Google Scholar]
- 44.Lagunas-Martínez A, Madrid-Marina V, Gariglio P. Modulation of apoptosis by early human papillomavirus proteins in cervical cancer. Biochim Biophys Acta. 2010;1805:6–16. doi: 10.1016/j.bbcan.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Kurmyshkina OV, Kovchur PI, Schegoleva LV, Volkova TO. T- and NK-cell populations with regulatory phenotype and markers of apoptosis in circulating lymphocytes of patients with CIN3 or microcarcinoma of the cervix: Evidence for potential mechanisms of immune suppression. Infect Agent Cancer . 2017;12:56–73. doi: 10.1186/s13027-017-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Muñoz H, Escobar-Sánchez ML, López-Marure R, Lascurain-Ledesma R, Zenteno E, Hernández-Vazquez JMV, et al. Cervical cancer cells induce apoptosis in TCD4+ lymphocytes through the secretion of TGF-β. Arch Gynecol Obstet. 2013;287:755–763. doi: 10.1007/s00404-012-2621-y. [DOI] [PubMed] [Google Scholar]
- 47.Sun T, Zhou Y, Li H, Han X, Shi Y, Wang L, et al. FASL-844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med . 2005;202:967–974. doi: 10.1084/jem.20050707. [DOI] [PMC free article] [PubMed] [Google Scholar]