Abstract
INTRODUCTION:
Ileocolic resection for Crohn’s disease traditionally does not include a high ligation of the ileocolic pedicle, and most commonly is performed with a stapled side-to-side ileocolic anastomosis. The mesentery has recently been implicated in the pathophysiology of Crohn’s disease. Two techniques have been developed and are associated with reduced postoperative recurrence: the Kono-S anastomosis that excludes diseased mesentery and extended mesenteric excision that resects diseased mesentery. We aimed to assess the technical feasibility and safety of a novel combination of techniques: mesenteric excision and exclusion.
TECHNIQUES:
This initial report is a single-center descriptive study of consecutive adults who underwent mesenteric excision and exclusion for primary or recurrent ileocolic Crohn’s disease from September 2020 to June 2021. Medication exposure and endoscopic balloon dilation before surgery were recorded. Phenotype was classified using the Montreal Classification. Thirty-day outcomes were reported. A video of the mesenteric excision and exclusion including the Kono-S anastomosis is presented.
RESULTS:
Twenty-two patients with ileocolic Crohn’s disease underwent mesenteric excision and exclusion: 100% had strictures, 59% had fistulas, 81% were on biologics, and 27% had previous ileocolic resection(s). Seventy-two percent underwent laparoscopic procedures, a mesenteric defect was closed in 86%, omental flaps were fashioned in 77%, and 3 patients were diverted. Median operative time was 175 minutes. Median postoperative stay was 4 days. At 30 days, there were 2 readmissions for reintervention: 1 seton placement and 1 percutaneous drainage of a sterile collection. There were no cases of intra-abdominal sepsis or anastomotic leak.
CONCLUSIONS:
Mesenteric excision and exclusion represents an innovative, progressive, and promising approach that appears to be highly feasible and safe. Further study is warranted to determine if mesenteric excision and exclusion is associated with reduced postoperative recurrence of ileocolic Crohn’s disease.
Keywords: Anastomosis, Crohn’s disease, ileocolic resection, Kono-S, Mesentery, Recurrence
Despite advances in diagnosis and treatment of Crohn’s disease (CD), the pathogenesis and pathophysiology remain enigmatic.1 Endoscopic postoperative recurrence (POR) occurs in up to 70% after ileocolic resection (ICR). It remains controversial whether surgical technique can reduce rates of POR requiring reoperation. The CAST randomized trial, and several meta-analyses, showed no difference in endoscopic or symptomatic POR between ileocolic anastomosis (ICA) techniques.2,3
Recently, the mesentery has been recognized as an active immune organ that may play a role in the pathophysiology of CD.4 Two techniques were developed aimed at reducing the influence of the mesentery on luminal CD. The Kono-S anastomosis (KSA), as described by Kono et al in 2011, is a handsewn antimesenteric side-to-side anastomosis with a wide lumen.5,6 A recent level 1 randomized trial (CD-SuPREMe) showed that KSA compared with stapled side-to-side anastomosis was associated with reduced risk of endoscopic POR, with severe recurrence (Rutgeerts ≥i2) occurring in 18% vs 30% after 2 years.7 Subsequently, extended mesenteric excision (EME) was described by Coffey et al.8 In a level 3 prospective case series, compared with historic controls, they found that surgical POR was lower after EME (2.9% vs 40%).8,9
Given mounting evidence implicating the mesentery in the development of CD, we sought to develop an evidence-based approach to surgical prophylaxis of POR of CD. Herein, we report a descriptive case series assessing the technical feasibility and safety of combining 2 techniques, EME and KSA, into one: mesenteric excision and exclusion (MEE), for ileocolic CD. See Video at http://links.lww.com/DCR/B710.
METHODS
This is a single-center institutional review board-approved retrospective descriptive case series of consecutive adults who underwent MEE (EME combined with KSA) for primary or recurrent ileocolic CD from August 2020 to June 2021 by a single surgeon; patients who underwent EME with end-ileostomy without Kono-S at the same operation, or EME with other anastomotic types, were excluded. We defined previous medication exposure as within 8 weeks before surgery, and endoscopic balloon dilation as occurring at any time since prior ICA. Disease phenotype was stratified by the Montreal Classification. Bowel length wasdetermined from operative and pathological reports. Data are presented as frequency (proportion) or median (interquartile range).
TECHNIQUE OF MESENTERIC EXCISION AND EXCLUSION
Extended Mesenteric Excision
Lymphadenectomy via EME, which includes a high ligation of the ileocolic pedicle, within 1 to 2 cm of the origin of the ileocolics, is a safe and straightforward technique and is the standard of care for right-sided colon adenocarcinoma. Of note, EME also involves excision of mesentery associated with the diseased segment of ileum, as opposed to transecting the mesentery close to the bowel wall and is accomplished by transecting the ileal mesentery from the cut edge of the ileum in a straight line toward the ileocolic pedicle. This results in a trapezoidal-shaped specimen (Figs. 1 and 2).
FIGURE 1.
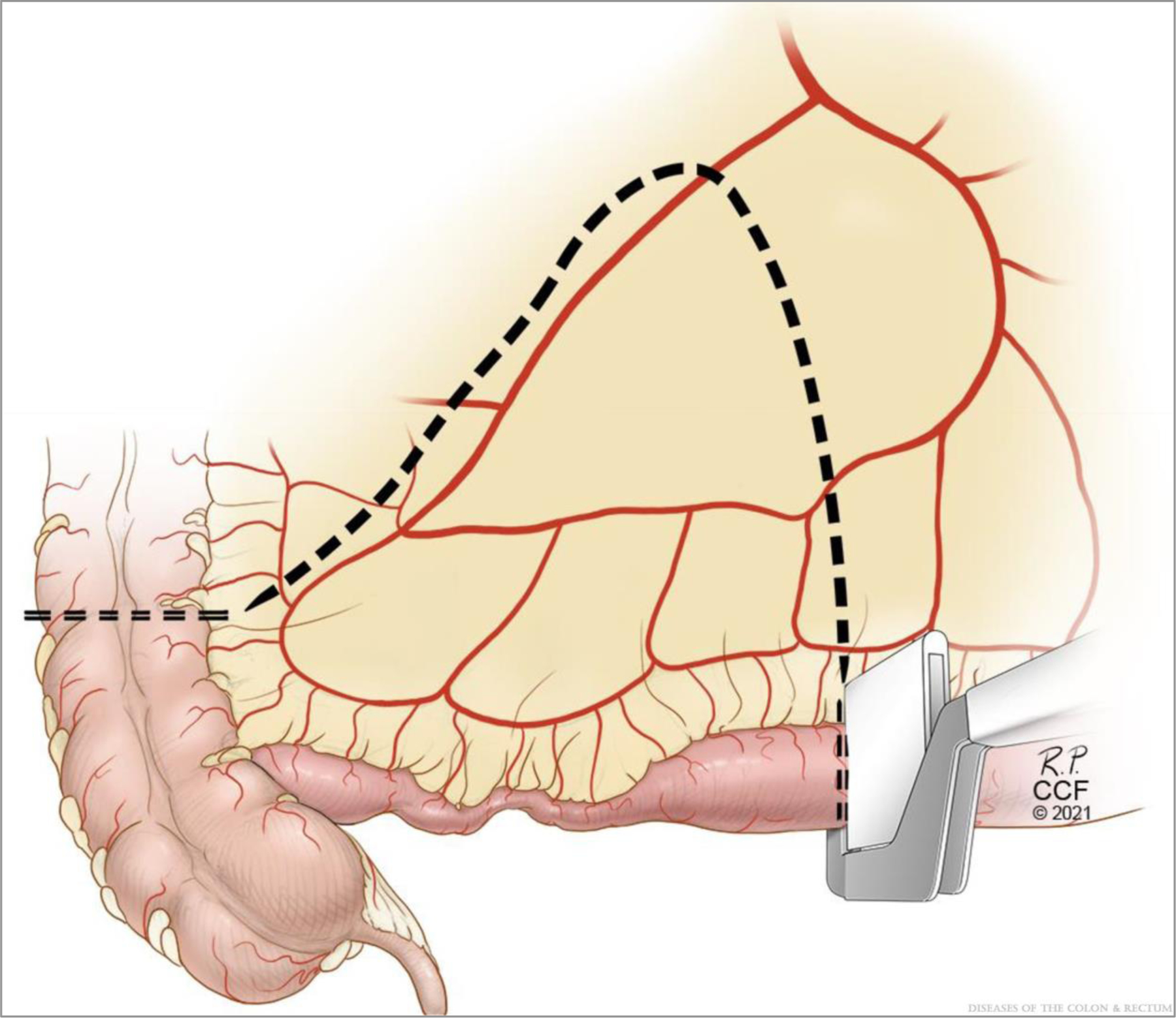
Extended mesenteric excision. ©Cleveland Clinic Foundation, 2021.
FIGURE 2.
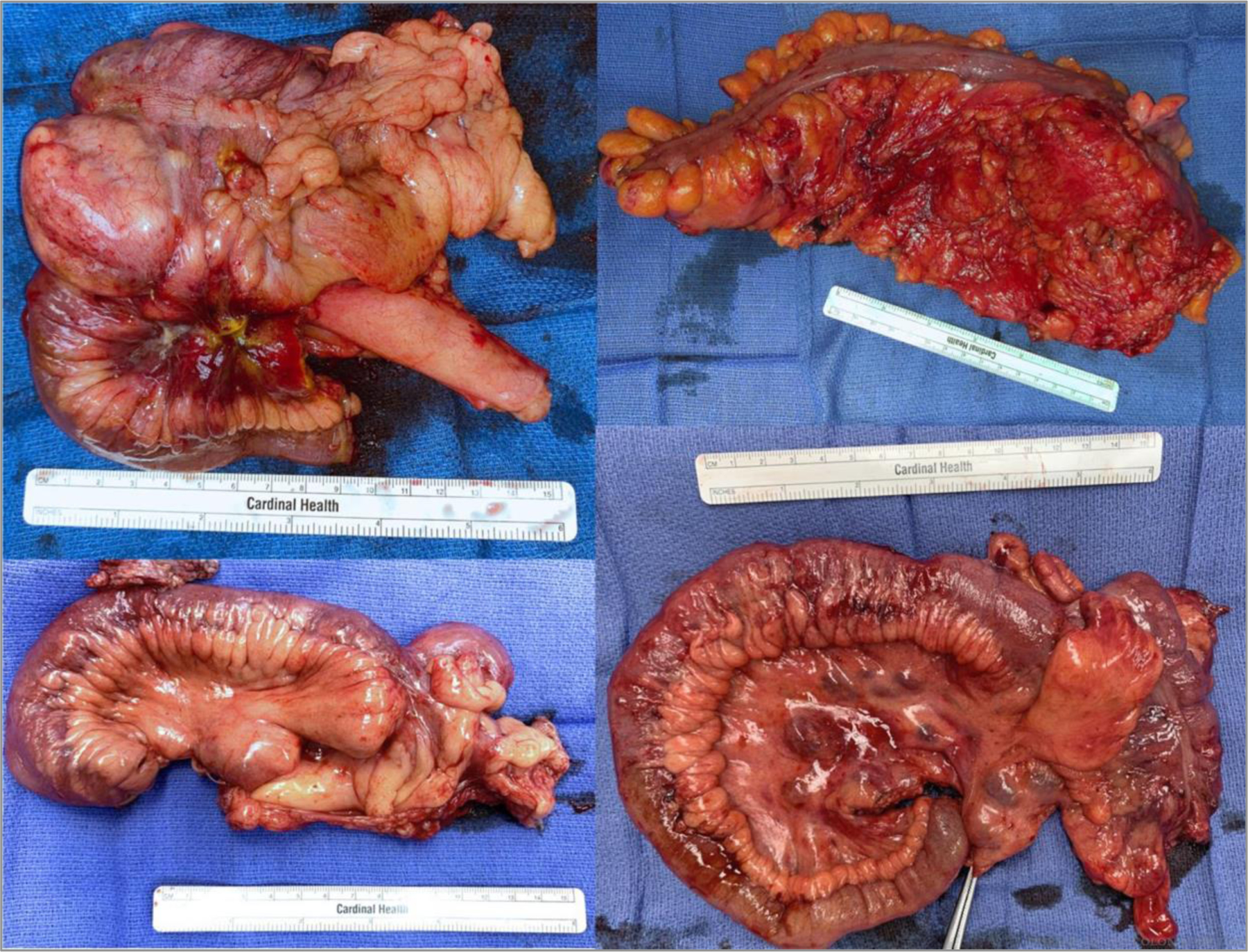
Examples of extended mesenteric excision specimens. ©Cleveland Clinic Foundation, 2021.
Summary of Technical Steps of KSA
The key concept is mesenteric exclusion by construction of a supporting column so the lumen of the KSA is antimesenteric and thus relatively removed from the mesentery. The supporting column is fashioned by sewing the transverse staple lines together. The blind ends are typically 1 cm in length, so the KSA is effectively an isoperistaltic side-to-side, functional end-to-end anastomosis. The anastomosis is constructed using monofilament or braided 3–0 sutures (Table 1).
TABLE 1.
Summary of technical steps of extended mesenteric excision with Kono-S anastomosis
| 1. | Bowel transection: bowels transected transversely with a stapler perpendicular to mesentery |
| 2. | Extended mesenteric excision: high ligation of the ileocolic pedicle; pedicle tagged with a suture (Figs. 1 & 2) |
| 3. | Mesenteric defect closure: facilitated by tagged suture at apex of pedicle; closed with running 2–0 suture |
| 4. | Supporting columns: Transverse staple lines reapproximated with 3–0 suture in a blind “end-to-end” manner; stays at corners (Fig. 3) |
| 5. | Enterotomies: 5–7 cm on the colon and ileum using electrocautery to allow a final transverse luminal diameter of 7 cm (Fig. 4), starts 1 cm from staple lines; stays at midpoints (x4) and lumens assume diamond shape (Fig. 5) |
| 6. | Outer layer, back wall: seromuscular interrupted or running 3–0 sutures; stays at corners, prior stays removed, new stays placed at corners of backwall. |
| 7. | Inner layer: using full-length sutures, full thickness running 3–0 sutures starting at the middle of the backwall and finishing in the middle of the front wall (Fig. 5) |
| 8. | Outer layer, front wall: interrupted 3–0 sutures (Figs. 6 & 7) |
| 9. | Patency pinch test: widely patent, accommodating the pad of surgeon’s thumb (Fig. 7) |
| 10. | Omental flap: interrupted 3–0 sutures (Fig. 7). |
Preparing the Anastomosis
After mobilizing the terminal ileum through hepatic flexure, the bowel is delivered. Proximally, soft pliable bowel without mesenteric thickening is chosen, and a mesenteric window is made; and the bowels are transected with a stapler perpendicular to the mesentery (Fig. 3); the distal margin is typically the mid-ascending colon (primary cases) or hepatic flexure (redo cases). Robust blood supply to both cut ends is confirmed. The intervening mesentery is then ligated as mentioned above, and the specimen removed. The mesenteric defect is typically closed with running a 2–0 suture. This adds additional support to the anastomosis, reduces the rare risk of reoperations for internal hernia with obstruction, and ensures that the mesentery of the ileum or colon are not inadvertently malrotated as may rarely occur with small extraction incisions and/or obese patients.
FIGURE 3.
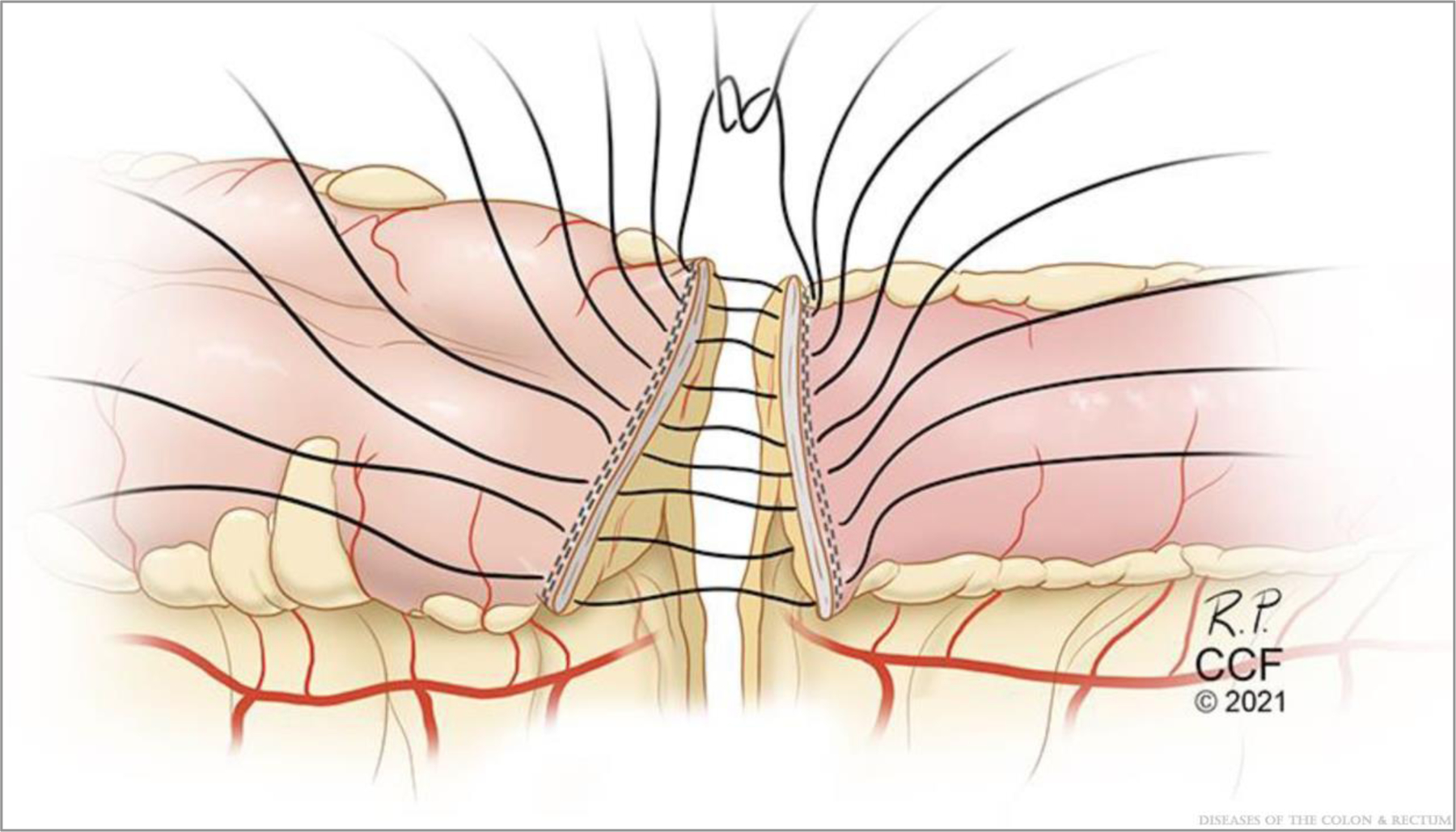
Supporting column construction via reapproximation of the transverse staple lines that are perpendicular to the mesentery. ©Cleveland Clinic Foundation, 2021.
Construction of the Supporting Columns and Anastomotic Construction
The staple lines are sewn together in an end-to-end manner with four to six 3–0 sutures (Fig. 3) to complete the supporting columns as originally described by Kono et al.5 Next, antimesenteric lengthwise matching enterotomies of 5 to 7 cm (or more) in length to allow for a final transverse anastomotic luminal diameter of 7 cm, as described by Kono et al,5 are made with electrocautery starting 1 cm away from the reapproximated staple lines (Fig. 4). Stay sutures are placed half-way along each enterotomy, forming diamond shapes (Fig. 5). The back outer wall of the anastomosis is placed with interrupted or running seromuscular sutures.
FIGURE 4.
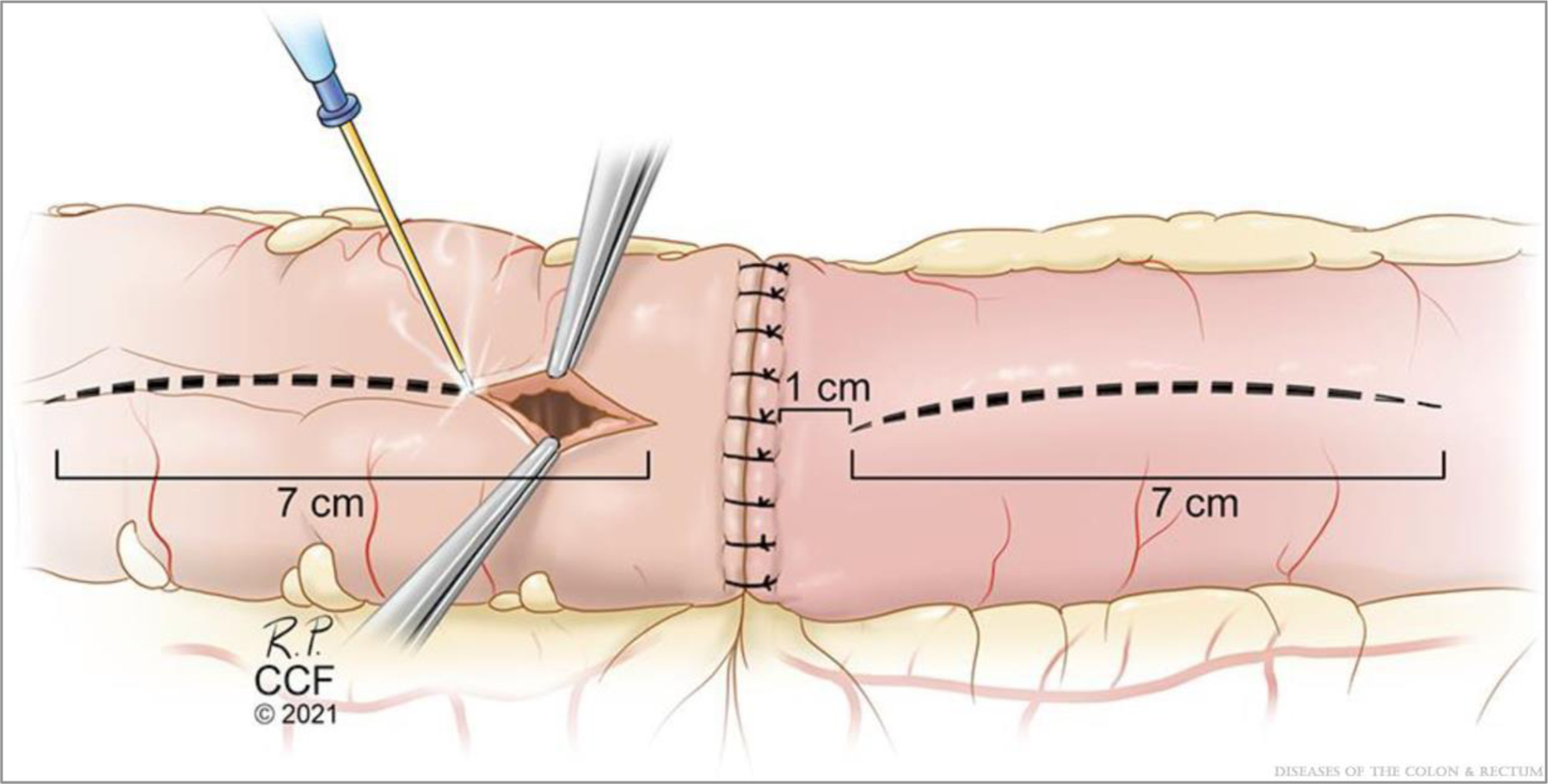
Enterotomy construction. Note the illustration shows an enterotomy of 7 cm but should be tailored (ie, 5–7 cm or larger) to allow for a final transverse luminal diameter of 7 cm as originally proposed by Kono et al.5 ©Cleveland Clinic Foundation, 2021.
FIGURE 5.
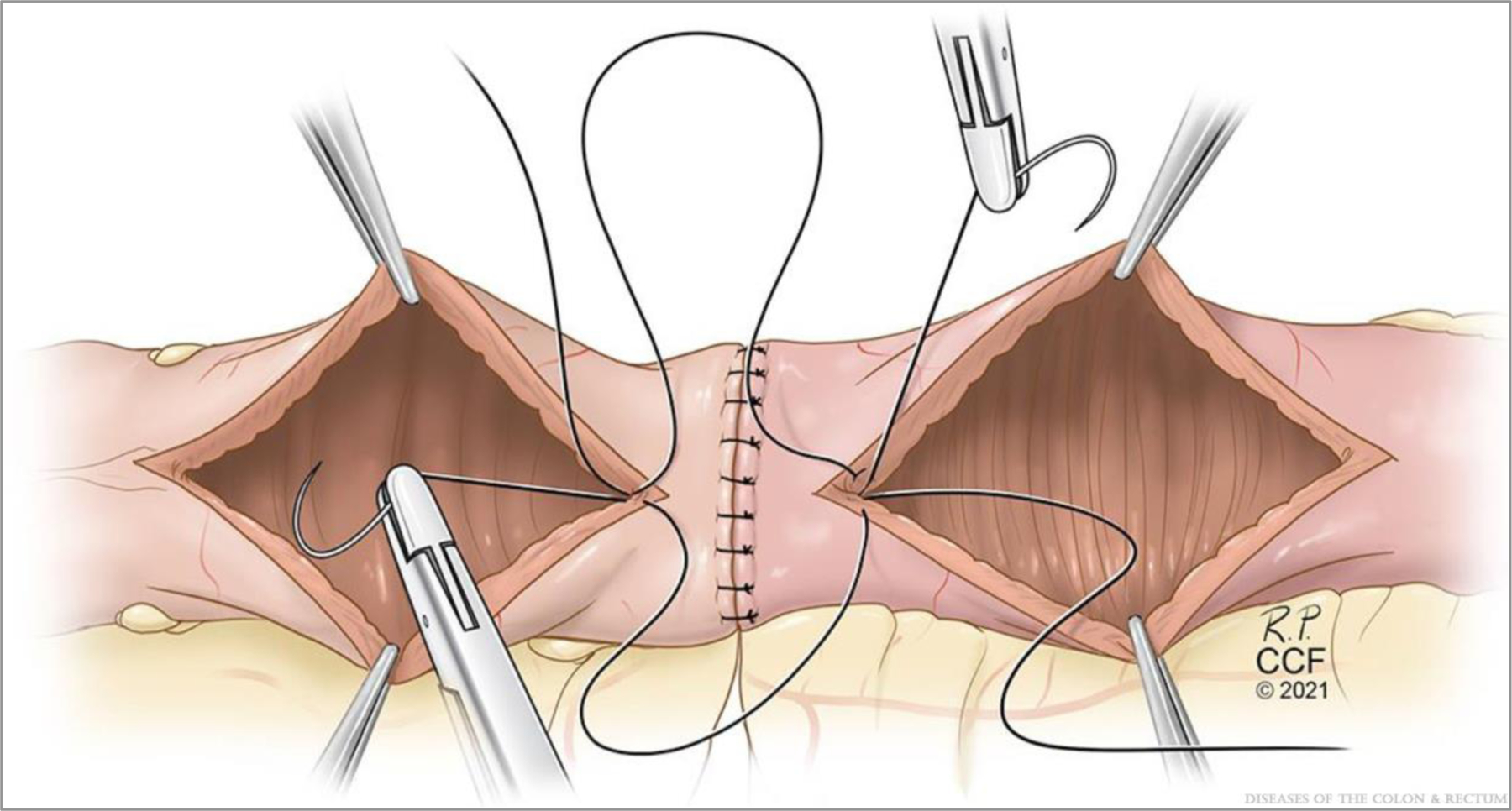
Inner layer construction. ©Cleveland Clinic Foundation, 2021.
Next, the inner wall is constructed using 2 full-length sutures with full-thickness bites. The first suture is at the center of the backwall and tied to itself. The assisting surgeon then places a similar suture adjacent to the first (Fig. 5). The gap between these is closed with the first pass of one of the sutures, and then each surgeon runs the suture line toward themselves and transitions to the front wall at the corners. One surgeon places a reversing stitch to facilitate sewing toward oneself. Care must be taken to avoid “back-walling” and occluding the anastomosis. It is important to maintain tension on the tails to construct a secure inner layer. The inner layer is then finished at the center of the front wall. Finally, the front-wall outer layer is constructed with interrupted Lembert sutures, completing the anastomosis (Fig. 6). A pinch test is then performed to confirm patency, and an omental flap may be constructed (Fig. 7).
FIGURE 6.
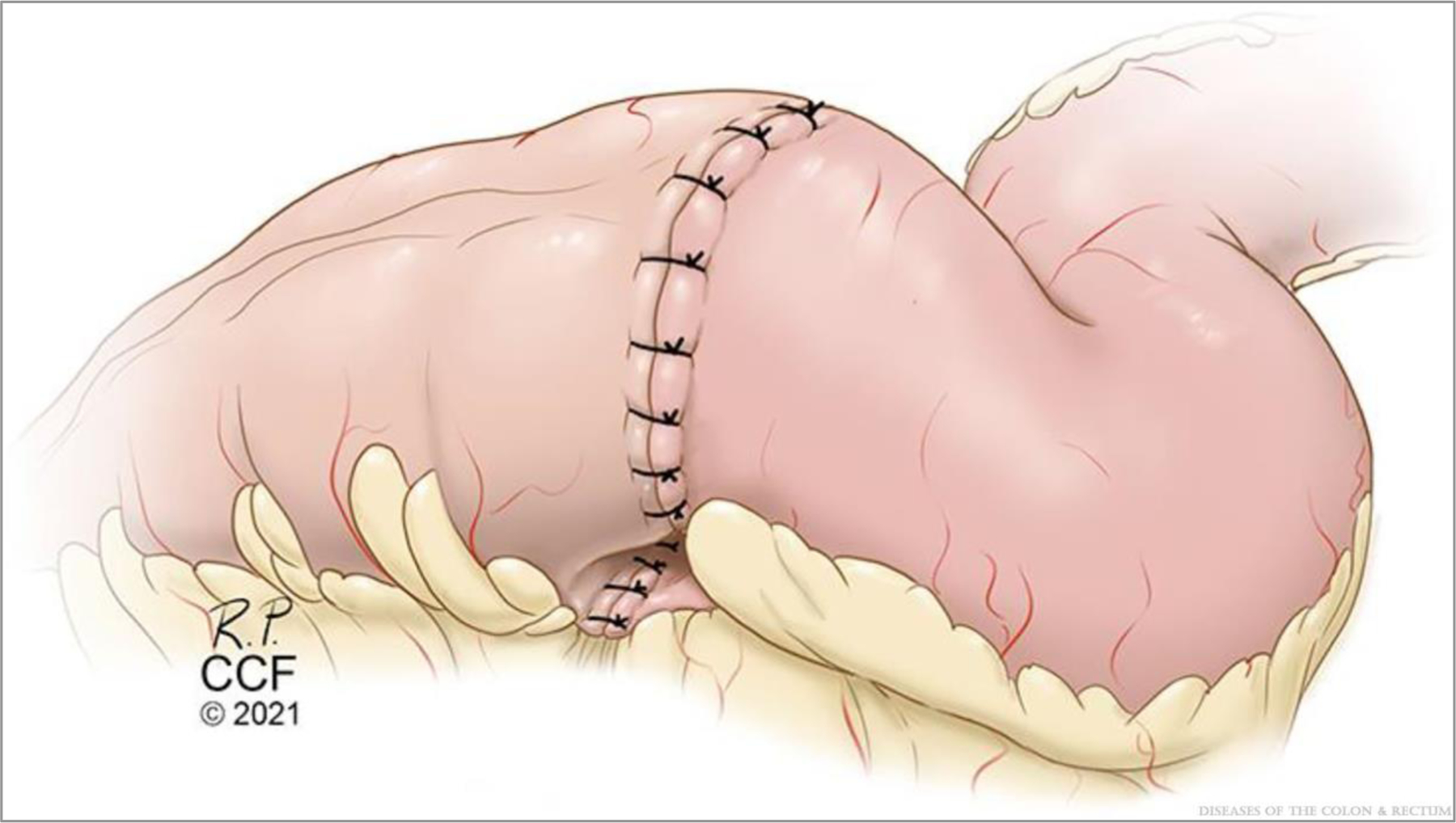
Kono-S ileocolic anastomosis, final appearance. ©Cleveland Clinic Foundation, 2021.
FIGURE 7.
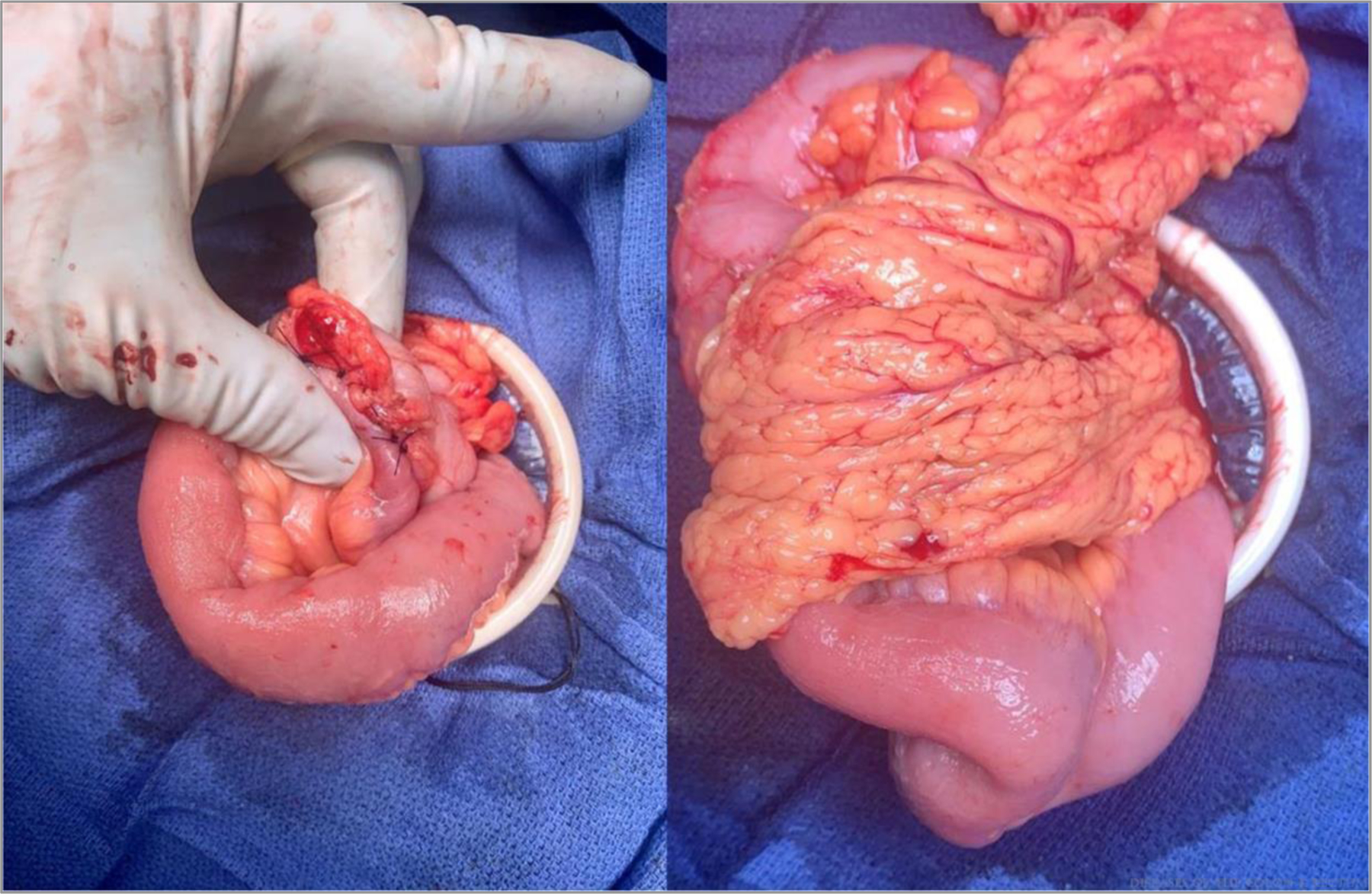
Pinch (patency) test and omental flap. ©Cleveland Clinic Foundation, 2021.
RESULTS
Over a 9-month period, 22 patients with CD underwent MEE, of which 27% were redo cases and 72% were attempted laparoscopically (Table 2). The mesenteric defect was closed in 86%, omental flaps were used in 77%; 3 patients (17%) required a diverting loop ileostomy due to risk factors for leak (eg, corticosteroid use, suboptimal nutritional, or penetrating disease). Median operative time was 175 minutes. All patients had an intraoperative transversus abdominus plane block, and all received enhanced recovery protocol. Median postoperative length of stay was 4 days. With respect to 30-day outcomes, no patients required blood transfusion, and 2 required readmission and intervention: 1 for seton placement for perianal abscess/fistula and 1 for percutaneous drainage of a sterile peritoneal fluid collection. There were no cases of intra-abdominal septic complications or anastomotic leak. Overall, 81% received biologics postoperatively. All 3 ileostomies were closed by 4 months without complication.
TABLE 2.
Perioperative patient characteristics
| Variable | n = 22 |
|---|---|
| Baseline characteristics | |
| Age | 31.5 (23.75–40.25) |
| BMI, kg/m2 | 24.5 (21.4–26.0) |
| Female, n (%) | 9 (40) |
| Indications, n (%) | |
| Stricture (including 4 anastomotic strictures) | 22 (100) |
| Fistula | 13 (59) |
| Montreal classification, n (%) | |
| A2: 17–40 y | 16 (72) |
| A3: >40 y | 6 (27) |
| L1: ileal | 19 (86.4) |
| L3: ileocolonic | 2 (9.1) |
| L1/L2: ileal and colonic | 1 (4.5) |
| B1: nonpenetrating, nonstricturing | nil |
| B2: structuring | 9 (40) |
| B2/B3: stricturing + penetrating | 13 (59) |
| p: perianal disease | 5 (23) |
| Preoperative therapy, n (%) | |
| Corticosteroids | 12 (54) |
| Weaned off preoperatively | 11 (92) |
| Budesonide | 3 (14) |
| Antibiotics | 7 (31) |
| Immunomodulators (thioguanines or methotrexate) | nil |
| Biologics | 18 (81) |
| TNFi | 6 (27) |
| Ustekinumab | 7 (31) |
| Vedolizumab | 5 (23) |
| Interventional | 9 (41)a |
| Endoscopic balloon dilation | 6 (27) |
| Percutaneous drain | 4 (18) |
| Nutritional | 6 (27) |
| Exclusive enteral nutrition | 4 (18) |
| Total parenteral nutrition | 2 (9) |
| Operative techniques, n (%) | |
| Redo ileocolic resection | 6 (27) |
| Extensive adhesiolysis | 8 (36) |
| Laparoscopic, attempted | 16 (72) |
| Laparoscopy converted | 5 (31) |
| Laparotomy (open) | 6 (27) |
| Laparoscopic, completed | 11 (50) |
| Length of incision, cm | 5.25 (3.9–15.25) |
| Mode | p = 0.0002 |
| Lap-completed, cm | 4 (3.5–4) |
| Lap-converted, cm | 10 (8–16.5) |
| Laparotomy (open), cm | 20 (14.5–22.5) |
| Mesenteric defect closed, n (%) | 19 (86) |
| Omental flap, n (%) | 17 (77) |
| Diverting loop ileostomy, n (%) | 3 (14) |
| Length of bowel | |
| Small bowel, operative note, cm | 22.5 (13.8–30) |
| Small bowel, gross pathology, cm | 21 (11–31.5) |
| Small bowel remaining in situ, cm (missing in 4) | 400 (350–432.5) |
| Colon, gross pathology, cm | 8 (6.7–12.8) |
| Perioperative outcomes | |
| Operative time, min | 174.5 (149.8–236) |
| Estimated blood loss, mL | 87.5 (50–150) |
| Blood transfusions | nil |
| Ileus, n (%) | 2 (9) |
| Length of stay, days | 4 (3–5) |
| Readmission, n (%) | 2 (9)b |
| Reoperation (EUA, seton), n (%) | 1 (4.5) |
| Intra-abdominal septic complication | nil |
| Surgical site infections (superficial), n (%) | 1 (4.5) |
| Anastomotic leaks | nil |
| Postoperative medical therapy, n (%) | |
| Biologicc | 18 (81) |
| TNFi (1 combined with methotrexate) | 7 (31.5) |
| Ustekinumab | 8 (36) |
| Vedolizumab | 3 (13) |
| None | 4 (18) |
| Corticosteroids/antibiotics/TPN | nil |
Figures represent frequency (proportion) or median (interquartile range); p value represents Wilcoxon rank-sum test (length of incision by mode).
EBD = endoscopic balloon dilation; EUA = examination under anesthetic; TNFi = tumor necrosis factor inhibitor; TPN = total parenteral nutrition.
One patient had both EBD and percutaneous drainage.
One for EUA, seton; 1 for percutaneous drainage of sterile fluid collection.
Of the 18 patients on preoperative biologics, 13 continued, 3 were discontinued, and 2 were class switched; 3 biologic-naive patients started a biologic postoperatively.
DISCUSSION
In this initial descriptive study, we report a novel MEE approach to ICR and ICA for ileocolic CD, which combines EME and KSA. We observed that MEE appears to be both highly feasible and safe, and observed no major complications. Ultimately, we will evaluate the outcome of EME based on long-term POR.
In the past decade, there has been significant progress in elucidating the role of the mesentery in the pathophysiology of luminal CD and POR. In parallel, major advances in our surgical techniques have been made.1,4,10,11 In 2011, Kono et al5 first reported the results of his innovative KSA, which aimed to attenuate the effect of a diseased mesentery on the development of recurrent CD via mesenteric exclusion by construction of a supporting column, thus allowing the anastomosis to be relatively removed from the mesentery. At the time of his original description, the concept of EME had not yet been refined, and with respect to close bowel resection of the mesentery, they reported leaving the mesentery intact to avoid potential devascularization of the KSA, and not as having a direct role in the supporting column.
Subsequently, in 2018, Coffey et al8 reported the results of EME, importantly with the same rationale as the KSA with respect to CD recurrence. Of note, EME for CD has not yet been as extensively studied. There are ongoing randomized trials, but with respect to safety, it has recently been shown that there does not appear to be any significant difference in functional or surgical outcomes after ICR for CD vs colon cancer operations.12
Critics of the EME approach have properly pointed out that care must be taken to preserve the vascularity, and length, of the proximal small intestine.9 Experienced surgeons are familiar with handling the severely inflamed, friable, thickened mesenteric often seen in CD. The mesentery can be difficult to control surgically and can lead to significant blood loss jeopardizing small-bowel length, unless great care is taken to avoid inadvertent bowel loss. We did not observe this in this case series of mainly primary, fibrostenotic presentations, indicating the potential for selection bias. Given this risk, as well as the potential for encroachment of the superior mesenteric vessels, we do not advocate for an overly aggressive approach to resecting small-bowel mesentery. However, in the case of thickened ileocolic pedicles, we often observed a transition point where the ileocolic pedicle normalized and transecting above the thickened mesentery actually facilitated safe mesenteric excision. Finally, practically speaking, it should be noted that handsewn anastomoses are less expensive (fewer staplers) but do require roughly 30 extra minutes of operative time to construct.
CONCLUSIONS
We report the first step in the development of an innovative approach, MEE, for primary and recurrent ileocolic resections in CD by combining EME with KSA. We found this progressive, promising technique to be highly feasible and safe. A long-term, follow-up study is warranted to determine if MEE is associated with a long-term reduction in the rate of POR of ileocolic CD.
Supplementary Material
ACKOWLEDGMENTS
This article is dedicated to the memory and legacy of Dr Toru Kono. His contribution to the advancement of science regarding the association between surgical technique, mesenteric fat, and patterns of postoperative Crohn’s disease recurrence have helped move the field forward and has resulted in renewed critical assessment of one of the common operations we do, and how we do it. The authors thank Ross Papalardo CMI and Theresa Kline MLIS for their technical expertise. Dr Holubar would like to personally thank the Cleveland Clinic Colorectal Fellows class of 2021 who assisted with these cases: Drs Nicholas Berger, Jamshid Darabnia, Leo Duares, Rebecca Gunter, Arielle Kanters, Ira Leeds, Gal Westrich, and Zhaomin Xu.
Funding/Support:
None reported.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s website (www.dcrjournal.com).
Financial Disclosures: Dr Holubar received consulting fees from Shionogi, Takeda, Guidepoint; funding from Crohn’s and Colitis Foundation. Dr Lightner received funding from Takeda. Dr Click received consulting fees from Takeda, TARGET-RWE, and MedEd. Dr Rieder served as consultant or advisory board member for Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene/BMS, CDISC, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB; received funding from National Institutes of Health, Helmsley Charitable Trust, Crohn’s and Colitis Foundation, UCB, Pliant, BMS, Pfizer, Boehringer Ingelheim, Morphic, and Kenneth Rainin Foundation. Dr Regeuiro received consulting fees from Abbvie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, S.p.A., and Bristol Meyer Squibb (BMS).
REFERENCES
- 1.Mao R, Kurada S, Gordon IO, et al. The mesenteric fat and intestinal muscle interface: creeping fat influencing stricture formation in Crohn’s disease. Inflamm Bowel Dis 2019;25:421–426. [DOI] [PubMed] [Google Scholar]
- 2.Choy PY, Bissett IP, Docherty JG, Parry BR, Merrie A, Fitzgerald A. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev 2011;(9):CD004320. [DOI] [PubMed] [Google Scholar]
- 3.McLeod RS, Wolff BG, Ross S, Parkes R, McKenzie M; Investigators of the CAST Trial. Recurrence of Crohn’s disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum 2009;52:919–927. [DOI] [PubMed] [Google Scholar]
- 4.Coffey JC, O’Leary DP, Kiernan MG, Faul P. The mesentery in Crohn’s disease: friend or foe? Curr Opin Gastroenterol 2016;32:267–273. [DOI] [PubMed] [Google Scholar]
- 5.Kono T, Ashida T, Ebisawa Y, et al. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn’s disease. Dis Colon Rectum 2011;54:586–592. [DOI] [PubMed] [Google Scholar]
- 6.Fichera A, Zoccali M, Kono T. Antimesenteric functional end-to-end handsewn (Kono-S) anastomosis. J Gastrointest Surg 2012;16:1412–1416. [DOI] [PubMed] [Google Scholar]
- 7.Luglio G, Rispo A, Imperatore N, et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: The SuPREMe-CD Study - a randomized clinical trial. Ann Surg 2020;272:210–217. [DOI] [PubMed] [Google Scholar]
- 8.Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis 2018;12:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buskens CJ, Bemelman WA. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence: editorial by Coffey et al. J Crohns Colitis 2018;12:1137–1138. [DOI] [PubMed] [Google Scholar]
- 10.de Groof EJ, van der Meer JHM, Tanis PJ, et al. Persistent mesorectal inflammatory activity is associated with complications after proctectomy in Crohn’s disease. J Crohns Colitis 2019;13:285–293. [DOI] [PubMed] [Google Scholar]
- 11.Ha CWY, Martin A, Sepich-Poore GD, et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 2020;183:666–683.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grass F, Zhu E, Brunel C, et al. Crohn’s versus cancer: comparison of functional and surgical outcomes after right-sided resections. Dig Dis 2021;39:106–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


