Abstract
Background
The prevalence of cardiovascular disease (CVD) is rapidly increasing in the world. The present study aimed to assess the prevalence and Predictors factors of CVD based on the data of Kherameh cohort study.
Methods
The present cross-sectional, analytical study was done based on the data of Kherameh cohort study, as a branch of the Prospective Epidemiological Studies in Iran (PERSIAN). The participants consisted of 10,663 people aged 40–70 years. CVD was defined as suffering from ischemic heart diseases including heart failure, angina, and myocardial infarction. Logistic regression was used to model and predict the factors related to CVD. Additionally, the age-standardized prevalence rate (ASPR) of CVD was determined using the standard Asian population.
Results
The ASPR of CVD was 10.39% in males (95% CI 10.2–10.6%) and 10.21% in females (95% CI 9.9–10.4%). The prevalence of CVD was higher among the individuals with high blood pressure (58.3%, p < 0.001) as well as among those who smoked (28.3%, p = 0.018), used opium (18.2%, p = 0.039), had high triglyceride levels (31.6%, p = 0.011), were overweight and obese (66.2%, p < 0.001), were unmarried (83.9%, p < 0.001), were illiterate (64.2%, p < 0.001), were unemployed (60.9%, p < 0.001), and suffered from diabetes mellitus (28.1%, p < 0.001). The results of multivariable logistic regression analysis showed that the odds of having CVD was 2.25 times higher among the individuals aged 50–60 years compared to those aged 40–50 years, 1.66 folds higher in opium users than in non-opium users, 1.37 times higher in smokers compared to non-smokers, 2.03 folds higher in regular users of sleeping pills than in non-consumers, and 4.02 times higher in hypertensive individuals than in normotensive ones.
Conclusion
The prevalence of CVD was found to be relatively higher in Kherameh (southern Iran) compared to other places. Moreover, old age, obesity, taking sleeping pills, hypertension, drug use, and chronic obstructive pulmonary disease had the highest odds ratios of CVD.
Keywords: Cardiovascular disease, Predictors, Prevalence, Kherameh cohort
Introduction
In recent decades, the rapid growth of Non-Communicable Diseases (NCDs) has become a serious health challenge threatening the health and economic development of communities [1]. Chronic NCDs such as Cardiovascular Disease (CVD), cancer, respiratory diseases, and Diabetes Mellitus (DM) are the leading causes of death worldwide [2]. The incidence of CVD is rapidly increasing in the world and, consequently, it is currently considered the leading cause of death in both developing and developed countries [3, 4]. CVD was responsible for the death of 17.9 million people worldwide in 2016, accounting for 31% of all global deaths [5]. Additionally, the prevalence of CVD increased from 257 million in 1990 to 550 million in 2019. The number of associated deaths showed a steady increase, as well [6]. Evidence has also indicated the global prevalence of angina to be 0.73–14.4% in females and 0.76–15.1% amongst males [7]. Nonetheless, burden, mortality, and prevalence of CVD vary in different parts of the world [8]. Over the past 20 years, the incidence of CVD has witnessed a significant decrease in industrialized countries and an increase in developing countries including the Eastern Mediterranean region [9, 10]. As an Eastern Mediterranean country, Iran has adopted a Western lifestyle. Such changes, along with improved health services, have led to the improvement of life expectancy as well as an increase in the prevalence of NCDs including CVD [11, 12]. In Iran, as in other parts of the world, CVD is the leading cause of death and its prevalence has been estimated as 9.2% in Sari located in north of the country [13, 14]. Nevertheless, contradictory results have been obtained regarding the prevalence of CVD based on gender in different studies. Some studies have reported a higher prevalence in males, while some others have found it to be higher among females [15–18]. According to what was mentioned above, CVD is the most important cause of death in the world as well as in Iran, and the prevalence of this disease and its risk factors is varied in different regions. In recent decades, population-based cohort studies have provided the ground for the performance of accurate cross-sectional studies in this field. The present study aims to determine the prevalence and prognostic factors of CVD based on the data of Kherameh cohort study to provide a more comprehensive image of the disease burden, which will in turn result in the provision of more efficient and targeted health services.
Methods
Study design and population
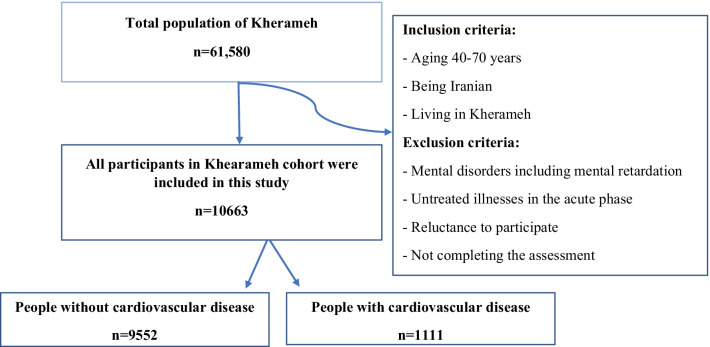
The present cross-sectional, analytical study was done based on the data of Kherameh cohort study, as a branch of Prospective Epidemiological Studies in Iran (PERSIAN). The Persian Cohort Study was launched in 18 separate geographical areas in Iran in 2014 and included all major Iranian ethnic groups. It is one of the largest cohort studies in the region and its rationale, goals, and design have already been published [19]. Kherameh is one of the southern cities of Fars province with a population of 61,580 people. Kherameh cohort is one of the branches of Fars cohort that was started with a population of 10,663 people in the 40–70 age group in 2014 to determine the prevalence and risk factors of NCDs at baseline and during the follow-up period. All participants of Kherameh cohort study were entered into the present research through census (Fig. 1).
Fig. 1.
Flow chart of the participants’ enrolment into the research
Measurement
Demographic and lifestyle information
The present findings were extracted from a research project approved by the Ethics Committee of Shiraz University of Medical Sciences (code: IR.SUMS.REC.1400.612). In this study, the participants were first asked to complete a written consent form. Then, a standardized questionnaire was used by trained experts in order to gather information about the participants’ demographic characteristics (age, sex, Body Mass Index (BMI), marital status, level of education, place of residence, occupational status, social and economic status, and family history of chronic diseases), sleep pattern, problems and underlying diseases (diabetes, cancer, Chronic Obstructive Pulmonary Disease (COPD), hypertension, blood levels of cholesterol, triglycerides, and High Density Lipoprotein (HDL), and other chronic diseases), and behavioral factors (smoking, alcohol consumption, hookah use, drug use, and physical activity) through interviews, laboratory experiments, and physical examination. Since Kherameh cohort was a part of the main project of the PERSIAN cohort, the validity and reliability of the questionnaire had been previously checked by the Persian cohort national team [19]. For the laboratory experiments, the individuals were requested to refrain from eating food and liquids, smoking, and consuming alcohol for 12 h prior to blood sampling. Weight was measured with light clothing and without shoes using a SECA scale (made in Germany), and height was measured using a standard measuring tape. BMI was also calculated by dividing body weight (kg) by height squared (m). Accordingly, the participants were divided into three categories; i.e., less than 25, 25–29, and > 30 kg/m2 [20]. Furthermore, blood levels of glucose, triglycerides, HDL, and cholesterol were measured using the MINDRAY brand tool (made in Japan) and Pars test kit. Dyslipidemia was defined as cholesterol levels above 200 mg/dL or triglyceride serum levels above 150 mg/dL. Additionally, diabetes was defined as a history of diabetes or fasting blood sugar above 126 mg/dL [21, 22]. The participants’ average physical activity was also evaluated during the past year. Moreover, their socioeconomic status was determined by considering such characteristics as home ownership, house size, number of indoor bathrooms, having a car, car price, domestic and international travels, and having a mobile phone, TV, vacuum cleaner, washing machine, refrigerator, microwave oven, and computer. Blood pressure was measured on the participants’ right arms after a five-minute rest in sitting position using a standard calibrated sphygmomanometer (Reister Model, Germany). Blood pressure was measured twice with a minimum interval of ten minutes and the mean was recorded. According to the European Hypertension Management Guidelines, hypertension was defined as systolic blood pressure greater than 140 mmHg and diastolic blood pressure greater than 90 mmHg [23].
Inclusion criteria
The inclusion criteria of this study were the same as those considered in Kherameh cohort study (one of the eighteen cohorts under the name of Persian cohort in Iran). The first inclusion criterion was aging 40–70 years, because people's behaviors and lifestyles are largely established in this age range. Besides, these people are usually in the active period of their lives and have the ability to participate in the study. Finally, the events under investigation can more probably be seen in this age group. The second inclusion criterion of the study was living in Kherameh. The participants had to live in the study area for at least nine months, so that they were somewhat adapted to the environmental and cultural conditions.
Exclusion criteria
The individuals with mental disorders including mental retardation and other untreated acute illnesses, those who were unwilling to participate in the study, and those who did not attend the designated clinics for examinations were excluded from the research.
Patients with cardiovascular disease
The individuals with ischemic heart disease including heart failure, angina, and myocardial infarction who were previously diagnosed in Kherameh cohort were included in the present study.
Statistical analysis
Normally distributed continuous variables were presented as mean ± Standard Deviation (SD), while non-normally distributed ones were presented as median (Q1-Q3). Additionally, qualitative variables were presented as number (percentage). The relationships between the categorical variables were assessed by chi-square test and the means were compared via independent t-test. Besides, logistic regression was used to model and predict the factors related to CVD. Moreover, the Age-Standardized Prevalence Rate (ASPR) of CVD was determined using the standard Asian population [24]. All statistical analyses were conducted by the SPSS 23 software and Stata 12 software. All p-values were two-tailed and the significance level was set at 0.05.
Results
This study was performed on 10,663 individuals whose baseline information has been shown in Table 1. Among the participants, 5944 (55.7%) were female with the mean age of 52.2 ± 8.2 years and 4719 (44.3%) were male with the mean age of 52.5 ± 7.8 years. Among the patients with CVD, 83.9% were unmarried (widowed or divorced), 64.2% were illiterate, 62.4% lived in rural areas, and 60.9% were unemployed. Moreover, 58.3% of the patients had high blood pressure and 28.1% had DM. The results revealed a higher prevalence of CVD in the individuals who were overweight and obese (66.2%, p < 0.001), unmarried (83.9%, p < 0.001), illiterate (64.2%, p < 0.001), and unemployed (60.9%, p < 0.001) as well as in those with a high economic status (49.9%, p = 0.049).
Table 1.
Demographic and socioeconomic variables of the participants according to the CVD status in Kherameh cohort study
| Variable | CVD | No (n = 9552) n (%) | p value | |
|---|---|---|---|---|
| All participants n (%) n = 10,663 | Yes (n = 1111) n (%) | |||
| Age (years) | ||||
| 40–49 | 4639 (43.7) | 312 (27.9) | 4330 (45.6) | < 0.001 |
| 50–59 | 3759 (35.4) | 442 (39.9) | 3317 (34.9) | |
| 60–70 | 2218 (20.9) | 357 (32.2) | 1861 (19.6) | |
| Sex | ||||
| Male | 4716 (44.2) | 481 (43.3) | 4238 (44.4) | 0.49 |
| Female | 5942 (55.7) | 630 (56.7) | 5314 (55.6) | |
| BMI (kg/m2) | ||||
| < 18.5 | 414 (3.9) | 29 (2.6) | 385 (4) | < 0.001 |
| 18.5–25 | 3883 (36.4) | 347 (31.3) | 3536 (37) | |
| 25–29 | 4449 (41.7) | 489 (44.1) | 3960 (41.5) | |
| > 30 | 1913 (17.9) | 245 (22.1) | 1668 (17.5) | |
| Marital status | ||||
| Married | 9492 (89) | 179 (16.1) | 8560 (89.6) | < 0.001 |
| Unmarried | 1171 (11) | 932 (83.9) | 992 (10.4) | |
| Education level | ||||
| Illiterate | 5587 (52.4) | 713 (64.2) | 4874 (51) | < 0.001 |
| Diploma and below | 4514 (42.3) | 373 (33.6) | 4141 (43.4) | |
| Academic | 562 (5.3) | 25 (2.3) | 537 (5.6) | |
| Living place | ||||
| Urban | 4416 (41.4) | 481 (43.3) | 3935 (41.2) | 0.179 |
| Rural | 6247 (58.6) | 630 (56.7) | 5617 (58.8) | |
| Employment | ||||
| No | 5147 (48.3) | 677 (60.9) | 4470 (46.8) | < 0.001 |
| Yes | 5516 (51.7) | 434 (39.1) | 5082 (53.2) | |
| Physical activity | ||||
| Light | 2670 (25) | 426 (38.3) | 2244 (23.5) | < 0.001 |
| Moderate | 2666 (25) | 276 (24.8) | 2390 (25) | |
| High | 2664 (25) | 224 (20.2) | 2440 (25.5) | |
| Severe | 2663 (25) | 185 (16.7) | 2478 (25.9) | |
| Socioeconomic status | ||||
| Low | 2667 (25) | 258 (23.2) | 2409 (25.2) | 0.049 |
| Moderate | 2977 (27.9) | 299 (26.9) | 2678 (28) | |
| High | 5019 (47.1) | 554 (49.9) | 4465 (46.7) | |
| Unintentional naps | ||||
| No | 5178 (48.6) | 464 (41.8) | 4714 (49.4) | < 0.001 |
| Yes | 5485 (51.4) | 647 (58.2) | 4838 (50.6) | |
| Use of sleeping pills | ||||
| No | 9735 (91.3) | 916 (82.4) | 8819 (92.3) | < 0.001 |
| Yes | 928 (8.7) | 195 (17.6) | 733 (7.7) | |
BMI, body mass index
The prevalence of different clinical and behavioral factors has been presented in Table 2. Among these factors, smoking (28.3%, p = 0.018), opium use (18.2%, p = 0.039), and high triglyceride levels (31.6%, p = 0.011) were more prevalent among the people with CVD. The prevalence of CVD was also higher among the individuals with high blood pressure (58.3%, p < 0.001) and DM (28.1%, p < 0.001).
Table 2.
The clinical and behavioral variables of the participants according to the CVD status in Kherameh cohort
| Variable | CVD | No (n = 9552) n (%) | p value | |
|---|---|---|---|---|
| All participants n (%) n = 10,663 | Yes (n = 1111) n (%) |
|||
| Diabetes | ||||
| No | 9070 (85.1) | 799 (71.9) | 8271 (86.6) | < 0.001 |
| Yes | 1593 (14.9) | 312 (28.1) | 1281 (13.4) | |
| Cancer | ||||
| No | 10,609 (99.5) | 1100 (99) | 9509 (99.5) | < 0.001 |
| Yes | 54 (0.5) | 11 (1) | 43 (0.5) | |
| COPD | ||||
| No | 10,369 (97.2) | 1055 (95) | 9314 (97.5) | < 0.001 |
| Yes | 294 (2.8) | 56 (5) | 238 (2.5) | |
| High blood pressure | ||||
| No | 8153 (76.5) | 463 (41.7) | 7690 (80.5) | < 0.001 |
| Yes | 2510 (23.5) | 648 (58.3) | 1862 (19.5) | |
| High cholesterol level | ||||
| No | 7014 (65.8) | 772 (69.5) | 6242 (65.3) | 0.006 |
| Yes | 3649 (34.2) | 339 (30.5) | 3310 (34.7) | |
| High triglyceride level | ||||
| No | 7637 (71.6) | 760 (68.4) | 6877 (72.1) | 0.011 |
| Yes | 3018 (28.3) | 351 (31.6) | 2667 (27.9) | |
| HDL (mg/dl) | ||||
| < 40 for males and < 50 for females | 4798 (45) | 515 (46.4) | 4283 (44.9) | 0.329 |
| ≥ 40 for males and ≥ 50 for females | 5860 (55) | 595 (53.6) | 5265 (55.1) | |
| White blood cell count (× 1000 cells/mm3) | ||||
| < 5.8 | 4189 (39.3) | 390 (35.1) | 3799 (39.8) | 0.006 |
| 5.8–7 | 3129 (29.3) | 337 (30.3) | 2792 (29.3) | |
| > 7 | 3337 (31.3) | 384 (34.6) | 2953 (30.9) | |
| Opium use | ||||
| No | 8949 (83.9) | 908 (81.8) | 8041 (84.2) | 0.039 |
| Yes | 1710 (16) | 202 (18.2) | 1508 (15.8) | |
| Hookah use | ||||
| No | 10,115 (94.9) | 1062 (95.6) | 9053 (94.8) | 0.28 |
| Yes | 548 (5.1) | 49 (4.4) | 499 (5.2) | |
| Smoking | ||||
| No | 7956 (74.6) | 797 (71.7) | 7163 (75) | 0.018 |
| Yes | 2704 (25.4) | 314 (28.3) | 2389 (25) | |
| Alcohol consumption | ||||
| No | 10,095 (94.7) | 1066 (95.9) | 9029 (94.5) | 0.045 |
| Median serum creatinine (mmol/l) | 0.9 (0.9, 1) | < 0.001 | ||
COPD, chronic obstructive pulmonary disease; HDL, high density lipoprotein
Based on the results presented in Table 3, the crude prevalence and ASPR of CVD were 10.4% (95% CI 9.8–11) and 10.3% (95% CI 10–10.6), respectively. Besides, the ASPR was 10.39 in males (95% CI 10.2–10.6%) and 10.21 in females (95% CI 9.9–10.4%). The ASPRs according to the types of CVD have been presented in Fig. 2.
Table 3.
Crude and age-standardized prevalence rate of CVD according to sex in the adults aged 40–60 years in Kherameh cohort
| 95% CI | 95% CI | |||||
|---|---|---|---|---|---|---|
| Crude | Upper | Lower | ASPR | Lower | Upper | |
| Both sexes | 10.41 | 9.8 | 11 | 10.3 | 10 | 10.6 |
| Male | 10.19 | 9.3 | 11 | 10.39 | 10.2 | 10.6 |
| Female | 10.6 | 9.8 | 11 | 10.21 | 9.9 | 10.4 |
ASPR, age-standardized prevalence rate
Fig. 2.
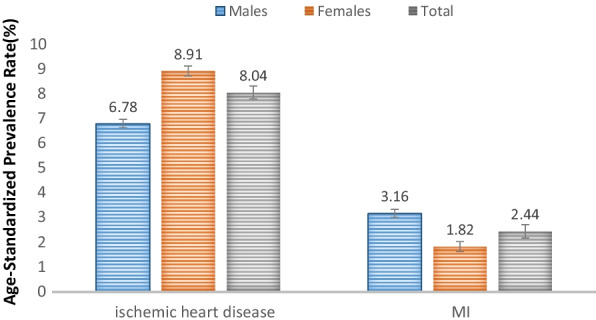
Age-standardized prevalence rate based on different types of CVD, adjusted to the standard Asian population. Note: The error bars represent the 95% CI
According to the results presented in Table 4, multivariate logistic regression analysis showed that the odds of having CVD was 2.25 times higher among the individuals aged 50–60 years compared to those aged 40–50 years, 1.66 times higher in opium users than in non-opium users, 1.37 times higher in smokers compared to non-smokers, 1.62 times higher amongst obese individuals (BMI > 30 kg/m2) in comparison to lean ones (BMI < 18.5 kg/m2), and 2.03 times higher in regular users of sleeping pills than in non-consumers. Considering the clinical variables, the odds of having CVD was 4.02 times higher in hypertensive individuals than in normotensive ones, 1.24 times higher in diabetics than in non-diabetics, and 1.71 times higher among the individuals with COPD compared to those without this disease.
Table 4.
Predictive factors of CVD in Kherameh population according to multivariate logistic regression analysis
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age (years) | ||
| 40–50 | 1 | 1 |
| 50–60 | 1.86 (1.60, 2.17)* | 1.63 (1.39, 1.91)* |
| 60–70 | 2.68 (2.28, 3.16)* | 2.02 (1.70, 2.40)* |
| Sex | ||
| Male | 1 | |
| Female | 1.04 (0.92,1.18) | |
| Body mass index (kg/m2) | ||
| < 18.5 | 1 | 1 |
| 18.5–24.9 | 1.30 (0.87, 1.93) | 1.25 (0.82, 1.90) |
| 25–29.9 | 1.63 (1.11, 2.41)* | 1.47 (0.96, 2.25) |
| ≥ 30 | 1.95 (1.30, 2.91)* | 1.62 (1.04, 2.52)* |
| Marital status | ||
| Married | 1 | |
| Unmarried | 1.65 (1.39, 1.97)* | |
| Education level | ||
| Illiterate | 1 | 1 |
| Diploma and below | 0.61 (0.54, 0.70)* | 1.01 (0.86, 1.20) |
| Academic | 0.31 (0.21, 0.47)* | 0.56 (0.35, 0.88)* |
| Living place | ||
| Urban | 1 | |
| Rural | 0.92 (0.81,1.04) | |
| Employment | ||
| No | 1 | |
| Yes | 0.56 (0.49, 0.64)* | |
| Physical activity | ||
| Light | 1 | 1 |
| Moderate | 0.60 (0.51, 0.71)* | 0.89 (0.74, 1.06) |
| High | 0.48 (0.40, 0.57)* | 0.8 (0.66, 0.97)* |
| Severe | 0.39 (0.32, 0.47)* | 0.73 (0.59, 0.90)* |
| Socioeconomic status | ||
| Low | 1 | 1 |
| Moderate | 1.04 (0.87, 1.24) | 1.02 (0.84, 1.23) |
| High | 1.15 (0.99, 1.35) | 1.21 (1.01, 1.44)* |
| Unintentional naps | ||
| No | 1 | 1 |
| Yes | 1.35 (1.19,1.54)* | 1.15 (1.005, 1.33)* |
| Use of sleeping pills | ||
| No | 1 | 1 |
| Yes | 2.56 (2.15, 3.04)* | 2.03 (1.68, 2.46)* |
| Diabetes | ||
| No | 1 | 1 |
| Yes | 2.52 (2.18,2.91)* | 1.24 (1.06, 1.46)* |
| Cancer | ||
| No | 1 | |
| Yes | 2.21 (1.13, 4.30)* | |
| Chronic obstructive pulmonary disease | ||
| No | 1 | 1 |
| Yes | 2.07 (1.54, 2.79)* | 1.71 (1.23, 2.38)* |
| High blood pressure | ||
| No | 1 | 1 |
| Yes | 5.78 (5.07, 6.58)* | 4.02 (3.46, 4.67)* |
| High triglyceride | ||
| No | 1 | 1 |
| Yes | 1.19 (1.04, 1.36)* | 1.30 (1.11, 1.52)* |
| High cholesterol | ||
| No | 1 | 1 |
| Yes | 0.83 (0.72, 0.94)* | 0.82 (0.71, 0.94)* |
| White blood cell count (× 1000 cells/mm3) | ||
| < 5.8 | 1 | |
| 5.8–7 | 1.17 (1.08, 1.37)* | |
| > 7 | 1.26 (1.09, 1.47)* | |
| Median serum creatinine (mmol/l) | 3.20 (2.33, 4.40)* | 1.62 (1.14, 2.29)* |
| Opium use | ||
| No | 1 | 1 |
| Yes | 1.18 (1.09, 1.39)* | 1.66 (1.35, 2.05)* |
| Hookah use | ||
| No | 1 | |
| Yes | 0.83 (0.62, 1.31) | |
| Alcohol use | ||
| No | 1 | |
| Yes | 0.72 (0.53, 0.99)* | |
| Smoking | ||
| No | 1 | 1 |
| Yes | 1.18 (1.02, 1.35)* | 1.37 (1.13, 1.65)* |
* Values represent p < 0.05
Discussion
The present study aimed to estimate the prevalence and predictors of CVD among the people aged 40–70 years in Kherameh, southern Iran. The findings demonstrated that the prevalence of CVD was 10.3%. Among the demographic and socioeconomic variables, old age, high BMI (> 30 kg/m2), low level of education, low physical activity, high socioeconomic status, unintentional naps, and sleeping pill use were the risk factors for CVD. Among the clinical factors, history of diabetes, cancer, COPD, high blood pressure, and high creatinine level were the risk factors for CVD. Finally, among the behavioral variables, opium use and smoking were found to be risk factors, while hookah use and alcohol consumption had a protective role. However, only drug use and smoking were significant in multivariate analysis.
Prevalence of CVD
The present study results showed that the prevalence of CVD was higher in south of Iran compared to other regions and countries. In this study, the prevalence of CVD was 10.3%, representing approximately 10,300 cases per 100,000 population. A study on the global burden of CVD also indicated that the standardized prevalence of CVD was more than 9,000 cases per 100,000 population in Iran as well as in Morocco, Oman, Zambia, and West Africa. In contrast, the standard prevalence of CVD was less than 5,000 per 100,000 population in Singapore, Japan, South Korea, Italy, Western Europe, and the United States, which was lower compared to the estimated prevalence in the current investigation [25]. This discrepancy might be due to the complex interaction of various environmental and genetic factors that play an important role in the development of CVD as well as to the differences in the prevalence of cardio metabolic risk factors (e.g., hyperglycemia, hypertension, dyslipidemia, and central obesity) [26]. The prevalence of these risk factors has been found to increase in Iran in recent years [27]. However, the present study results revealed no significant difference between males and females concerning the prevalence of CVD. In contrast, other studies reported that males were at a higher risk of CVD development [13, 18]. On the other hand, Aapelman et al. reported a higher prevalence of CVD in females than in males [28]. In another study, Zeidan et al. disclosed that significant differences between the two sexes disappeared with age [17]. These contradictory results might be associated with different age groups and different types of CVD. In addition, some studies have attributed the differences between males and females in terms of CVD to pregnancy, menopausal age, and hormonal changes [28–32].The lack of a significant difference between the two sexes regarding the prevalence of CVD in the present study might be associated with the fact that the participants were within the age range of 40–70 years. In fact, the females were at the menopause age and, consequently, had less supportive physiological factors (estrogen and progesterone). Evidence has shown that the incidence of CVD is higher in postmenopausal women than in premenopausal ones and that estrogen supplementation does not reduce the risk of CVD in postmenopausal women [33–35].
The association between the demographic and socioeconomic variables and CVD
Age is a known risk factor for CVD. The present study findings revealed an increase in the odds of CVD development in older adults. Previous studies also showed that age played a vital role in the deterioration of cardiovascular function, thereby enhancing the risk of CVD in the elderly population [36, 37].
In the current research, a positive association was found between CVD and higher BMI, which was consistent with the findings of other studies [38]. Obesity has been found to exert numerous adverse effects on cardiovascular function and structure, increase the risk of CVD, and increase the risk factors associated with CVD such as hypertension, diabetes, insulin resistance, and sleep apnea syndrome [39–41].
In the present investigation, the individuals with low education levels seemed to be more likely to develop CVD. Other studies have also suggested an inverse association between CVD and education level [42–44].
In the current study, socioeconomic status remained in the multivariate analysis after adjusting for other risk factors. Based on the results, the people with a high socioeconomic status were more likely to develop CVD. However, conflicting results have been obtained in other studies regarding the relationship between the socioeconomic status and CVD [45]. Some studies performed in developed countries revealed an inverse relationship between the socioeconomic status and CVD, while those carried out in developing countries showed a direct relationship between the two variables [42, 46, 47]. The direct correlation may be attributed to the fact that rich people in developing countries such as Iran have higher behavioral risk factors such as poor diet and high rates of smoking compared to poor people, as other studies have noted [48, 49].Previous studies demonstrated that short and long sleep durations were associated with an increased risk of CVD [50–53]. Consistently, the present study findings indicated that unintentional nap was a risk factor for CVD and taking sleeping pills increased the odds of CVD development. Some physicians attribute insomnia to the need for sleeping pills for underlying medical disorders such as CVD, high blood pressure, diabetes, and cancer [52, 54]. Therefore, the present study participants might suffer from insomnia due to CVD and, as a result, they were more likely to turn to sleeping pills. Yet, the relationship observed in cross-sectional studies should be interpreted with caution due to the weakness in showing the temporality [55].
The association between the clinical and behavioral variables and CVD
Up to now, many studies have emphasized that high blood pressure, diabetes, and COPD played an important role in the development of CVD [56–58]. In the same line, the current study showed a strong association between these variables and CVD. In this regard, blood pressure, diabetes, and COPD were the most important risk factors, which was in agreement with the findings of other studies. Several prospective cohort studies have demonstrated hypertension as a strong risk factor for CVD [57, 59–61]. Additionally, Coronary Heart Disease (CHD) has been mentioned as the most common early manifestation of CVD in patients with DM [56]. Moreover, the results of a meta-analysis on observational studies indicated that COPD increased the risk of CVD by 2.5 times, which was consistent with the results of the present investigation [58]. However, some other observational studies showed that COPD was more common in the people presented with various forms of CVD [62, 63]. Despite these contradictory results, evidence based on studies, especially longitudinal investigations and meta-analyses, has been in favor of the increased risk of CVD in patients with COPD. Curkendall et al., for example, estimated the age-adjusted risk ratio of heart failure as 4.5 among the individuals with COPD in a Canadian cohort [64–67].
The findings of the present research showed an inverse relationship between the total cholesterol level and CVD, which was on the contrary to the findings of the study carried out by Ghaemian and Bangalore [13, 68]. The observed inverse relationship might result from the practitioners’ recommendations for diet change and use of medications.
In the present study, creatinine and triglyceride levels were significantly associated with CVD, which was consistent with the results of other studies conducted on the issue. For instance, a meta-analysis demonstrated that triglyceride level was an independent risk factor for CHD, even after adjustment for HDL level [69]. Interpretation of the association between creatinine level and CVD should be done with caution, because creatinine levels increase in patients with kidney disease and CVD decreases renal function [70]. Although a number of recent epidemiological studies have revealed chronic kidney disease as an independent risk factor for CVD, this relationship may be due to the presence of kidney disease. However, the information of patients suffering from kidney disease was not available here for adjustment [71, 72].
Among the behavioral factors in the current study, opium use and smoking were found to be risk factors, while hookah use and alcohol consumption had a protective role. However, the results of multivariate analysis only showed drug use and cigarette smoking to be significant. In the same vein, Khalili et al. reported that based on dose–response and duration of use, opium was directly associated with an increased risk of CVD [73]. Nonetheless, conflicting results have been obtained regarding the relationship between opium use and CVD. Niaki et al. disclosed that opium use increased the odds of CVD by 24.5 times [74]. Sadeghian et al. also stated that opium use was a major risk factor for ischemic heart disease. However, Sabzi et al. and Marmor et al. maintained that opium use was a protective factor for atrial fibrillation (adjusted odds ratio = 0.37) and coronary artery disease (adjusted odds ratio = 0.43) [75, 76]. On the other hand, in the case–control study performed by Azimzadeh et al. and the cross-sectional study done by Hosseini et al., no significant association was observed between opium use and CVD [77, 78]. The differences among the results of the abovementioned studies might be attributed to the following reasons. Firstly, the possible confounding role of smoking might have not been considered in all the studies [79]. Secondly, the dose of opium consumption, method of use, and duration of use might vary in different studies [80]. Thirdly, in some areas, especially in Iran, opium is not a pure substance and contains impurities such as lead, which may increase the risk of CVD amongst opium users [44–46]. Fourthly, considering the social stigma associated with addiction, people might have hidden their addiction, which could have led to information bias [81, 82].
Similar to other studies, the present research revealed smoking as a risk factor for CVD. Hackshaw et al. also conducted a meta-analysis and reported an association between smoking and CHD [83]. In the same line, previous studies demonstrated a relationship between tobacco smoking and the physiological, pathological, and metabolic factors contributing to the atherosclerotic process and other mechanisms resulting in CVD-related morbidity and mortality [84–86].
Limitation
This study had a few limitations. Firstly, the cross-sectional design of the study made it difficult to rule out the inverse causation of variables, especially for cholesterol level, hookah use, and alcohol consumption. Secondly, due to the stigma associated with drug use and alcohol consumption in Iran, the participants might have concealed these behaviors, which could have biased the observed relationships, Thirdly, information regarding genetic, racial, and ethnic backgrounds was not available to determine their roles. Despite these limitations, a strong point of the study was its large sample size of males and females, which was the representative of the population of Kherameh. In addition, highly accurate cohort study data were used in this study.
Conclusion
The prevalence of CVD was found to be relatively higher in Kherameh (southern Iran) compared to other places. In addition, old age, obesity, taking sleeping pills, hypertension, drug use, and COPD had the highest odds ratios. Therefore, plans are recommended to be made in order to reduce the modifiable risk factors. Furthermore, contrary to the existing misconceptions about the positive effects of opium on heart diseases, opium was found to be a risk factor in this research. Thus, the public should be informed about the dangerous effects of opium use [79]. Moreover, further longitudinal cohort studies are recommended to be performed focusing on the incidence data of the related factors, especially sleeping pill use. More attention should also be paid to the individuals who have risk factors related to CVD, especially those who use opium and sleeping pills. Future studies are also needed to improve our understanding of the complex risk factors underlying the development of CVD in females.
Acknowledgements
The authors would like to thank Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for her invaluable assistance in editing the manuscript.
Abbreviations
- CVD
Cardiovascular disease
- ASPR
Age-standardized prevalence rate
- FBS
Fasting blood sugar
- TC
Total cholesterol
- HDL-C
High-density lipoprotein-cholesterol
- LDL-C
Low-density lipoprotein-cholesterol
- TG
Triglyceride
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
Author contributions
NB and MGH did the research, wrote the manuscript, and contributed to data collection. AR and VH critically reviewed the manuscript and approved the final version. RR and LM did the research, analyzed the data, and critically reviewed and edited the manuscript. All authors have read and approved the manuscript.
Funding
This research received no external funding.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to data are not public, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
PERSIAN Cohort Study is being performed in 18 geographical regions of Iran. PERSIAN Cohort Study was approved by the Ethics Committee of the Ministry of Health and Medical Education. This study was in agreement with the Helsinki Declaration and Iranian national guidelines for ethics in research (Reference No. IR.SUMS.REC.1400.612). Besides, written informed consent was obtained from all the participants.
Consent for publication
Written informed consent for publication was obtained from each participant.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zinat Motlagh SF, Chaman R, Ghafari SR, Parisay Z, Golabi MR, Eslami AA, et al. Knowledge, treatment, control, and risk factors for hypertension among adults in Southern Iran. Int J Hypertens. 2015;2015. [DOI] [PMC free article] [PubMed]
- 2.Noncommunicable diseases Fact Sheet. november 2021.World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- 3.Mittal BV, Singh AK. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis. 2010;55(3):590–598. doi: 10.1053/j.ajkd.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low-and middle-income countries. Curr Probl Cardiol. 2010;35(2):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buettner R, Schunter M, editors. Efficient machine learning based detection of heart disease. In: 2019 IEEE international conference on E-health networking, application & services (HealthCom); 2019: IEEE.
- 6.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117(12):1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 8.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardiovascular disease mortality in the developing countries. World Health Statist Quart 1993; 46: 89-150.
- 10.Mohammadi M, Mirzaei M, Karami M. Potential impact fraction of ischemic heart disease associated with diabetes mellitus in Yazd-Iran. 2018.
- 11.Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obesity Targets Ther. 2019;12:2221. doi: 10.2147/DMSO.S216791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalkhen H, Mash R. Multimorbidity in non-communicable diseases in South African primary healthcare. S Afr Med J. 2015;105(2):134–138. doi: 10.7196/SAMJ.8696. [DOI] [PubMed] [Google Scholar]
- 13.Ghaemian A, Nabati M, Saeedi M, Kheradmand M, Moosazadeh M. Prevalence of self-reported coronary heart disease and its associated risk factors in Tabari cohort population. BMC Cardiovasc Disord. 2020;20:1–10. doi: 10.1186/s12872-020-01526-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasanlo M, Zeinalzadeh AH, Rabie Siahkali S, Rashtchi V. Frequency of the cardiovascular risk factors and their relationship with nurse’s gender in intensive Care unit and emergency department, Kermanshah, Iran. Iran J Emergency Care. 2017;1(1):22–31. [Google Scholar]
- 15.Agheli N, Assefzadeh S, Rajabi M. The prevalence of cardiovascular risk factors among population aged over 30 years in Rasht and Qazvin. J Inflamm Dis. 2005;9(2):59–65. [Google Scholar]
- 16.Kooshki A, Mohajeri N, Movahhedi A. Prevalence of Cvd risk factors related to diet in patients referring to Modarres hospital in Tehran in 1379 (1999). 2003.
- 17.Zeidan RK, Farah R, Chahine MN, Asmar R, Hosseini H, Salameh P, et al. Prevalence and correlates of coronary heart disease: first population-based study in Lebanon. Vasc Health Risk Manag. 2016;12:75. doi: 10.2147/VHRM.S97252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li Y, Liu X, Zhang H, Abdulai T, Tu R, et al. Prevalence and influencing factors of coronary heart disease and stroke in Chinese rural adults: the Henan rural cohort study. Front Public Health. 2020;7:411. doi: 10.3389/fpubh.2019.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–655. doi: 10.1093/aje/kwx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organization WH. Global database on Body Mass Index: BMI Classification. 2006. World Health Organization: Geneva, Switzerland. 2015.
- 21.Rezaianzadeh A, Jafari F, Sadeghi SE, Rahimikazerooni S. The prevalence and predictors of pre-hypertension and hypertension in Kherameh cohort study: a population based study on 10,663 persons in south of Iran. J Hum Hypertens. 2021;35(3):257–264. doi: 10.1038/s41371-020-0330-8. [DOI] [PubMed] [Google Scholar]
- 22.Gupta R, Deedwania PC, Achari V, Bhansali A, Gupta BK, Gupta A, et al. Normotension, prehypertension, and hypertension in urban middle-class subjects in India: prevalence, awareness, treatment, and control. Am J Hypertens. 2013;26(1):83–94. doi: 10.1093/ajh/hps013. [DOI] [PubMed] [Google Scholar]
- 23.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 24.Sankoh O, Sharrow D, Herbst K, Whiteson Kabudula C, Alam N, Kant S, et al. The INDEPTH standard population for low-and middle-income countries, 2013. Glob Health Action. 2014;7(1):23286. doi: 10.3402/gha.v7.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 27.Abbasi M, Neishaboury M, Koohpayehzadeh J, Etemad K, Meysamie A, Asgari F, et al. National prevalence of self-reported coronary heart disease and chronic stable angina pectoris: Factor analysis of the underlying cardiometabolic risk factors in the SuRFNCD-2011. Global Heart. 2018;13(2):73–82. e1. [DOI] [PubMed]
- 28.Appelman Y, van Rijn BB, Monique E, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–218. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age-related cardiovascular mortality. PLoS ONE. 2013;8(5):e63347. doi: 10.1371/journal.pone.0063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crimmins EM, Kim JK, Solé-Auró A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Pub Health. 2011;21(1):81–91. doi: 10.1093/eurpub/ckq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z, Chen Z, Sun A, Deng X. Gender differences in cardiovascular disease. Med Novel Technol Devices. 2019;4:100025. doi: 10.1016/j.medntd.2019.100025. [DOI] [Google Scholar]
- 32.Gorodeski GI. Update on cardiovascular disease in post-menopausal women. Best Pract Res Clin Obstet Gynaecol. 2002;16(3):329–355. doi: 10.1053/beog.2002.0282. [DOI] [PubMed] [Google Scholar]
- 33.Investigators WGftWsHI, Investigators WGftWsHI. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. [DOI] [PubMed]
- 34.Peters SA, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139(8):1025–1035. doi: 10.1161/CIRCULATIONAHA.118.035550. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, Hjortland MC, McNAMARA PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 36.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25(4):563. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinton W, McGovern A, Coyle R, Han TS, Sharma P, Correa A, et al. Incidence and prevalence of cardiovascular disease in English primary care: a cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) BMJ Open. 2018;8(8):e020282. doi: 10.1136/bmjopen-2017-020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, et al. The impact of obesity on the cardiovascular system. Journal of diabetes research. 2018;2018. [DOI] [PMC free article] [PubMed]
- 40.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 41.Akil L, Ahmad HA. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J Health Care Poor Underserved. 2011;22(4 Suppl):61. doi: 10.1353/hpu.2011.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy KS, Prabhakaran D, Jeemon P, Thankappan K, Joshi P, Chaturvedi V, et al. Educational status and cardiovascular risk profile in Indians. Proc Natl Acad Sci. 2007;104(41):16263–16268. doi: 10.1073/pnas.0700933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Z, Nissinen A, Vartiainen E, Song G, Guo Z, Zheng G, et al. Associations between socioeconomic status and cardiovascular risk factors in an urban population in China. Bull World Health Organ. 2000;78:1296–1305. [PMC free article] [PubMed] [Google Scholar]
- 44.Pednekar MS, Gupta R, Gupta PC. Illiteracy, low educational status, and cardiovascular mortality in India. BMC Public Health. 2011;11(1):1–12. doi: 10.1186/1471-2458-11-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marmot M. Health in an unequal world: social circumstances, biology and disease. Clin Med. 2006;6(6):559. doi: 10.7861/clinmedicine.6-6-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97(6):596–601. doi: 10.1161/01.CIR.97.6.596. [DOI] [PubMed] [Google Scholar]
- 47.Pearson TA, Siscovick D, Vinicor F, Wilson PF, Fortmann SP, Ford E, et al., editors. Cardiovascular disease. In Disease Control Priorities in Developing Countries; 1993: Citeseer.
- 48.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148(1):7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Clark AM, DesMeules M, Luo W, Duncan AS, Wielgosz A. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol. 2009;6(11):712–722. doi: 10.1038/nrcardio.2009.163. [DOI] [PubMed] [Google Scholar]
- 50.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 51.Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6(2):102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- 52.Partinen M, Putkonen P, Kaprio J, Koskenvuo M, Hilakivi I. Sleep disorders in relation to coronary heart disease. Acta Med Scand. 1982;211(S660):69–83. doi: 10.1111/j.0954-6820.1982.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 53.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease-a review of the recent literature. Curr Cardiol Rev. 2010;6(1):54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills: is increased mortality associated? Arch Gen Psychiatry. 1979;36(1):103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 55.Carlson MD, Morrison RS. Study design, precision, and validity in observational studies. J Palliat Med. 2009;12(1):77–82. doi: 10.1089/jpm.2008.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: determinants of risk and outcomes in patients with diabetes. Prog Cardiovasc Dis. 2019;62(4):306–314. doi: 10.1016/j.pcad.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 59.Eshak ES, Iso H, Kokubo Y, Saito I, Yamagishi K, Inoue M, et al. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre–based study cohort I. Am J Clin Nutr. 2012;96(6):1390–1397. doi: 10.3945/ajcn.112.037903. [DOI] [PubMed] [Google Scholar]
- 60.Kokubo Y, Matsumoto C. Hypertension is a risk factor for several types of heart disease: review of prospective studies. Hypertension: from basic research to clinical practice. 2016:419–26. [DOI] [PubMed]
- 61.Kjeldsen SE. Hypertension and cardiovascular risk: general aspects. Pharmacol Res. 2018;129:95–99. doi: 10.1016/j.phrs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Franssen FM, Soriano JB, Roche N, Bloomfield PH, Brusselle G, Fabbri LM, et al. Lung function abnormalities in smokers with ischemic heart disease. Am J Respir Crit Care Med. 2016;194(5):568–576. doi: 10.1164/rccm.201512-2480OC. [DOI] [PubMed] [Google Scholar]
- 63.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agusti A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 64.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 65.Rothnie KJ, Yan R, Smeeth L, Quint JK. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. 2015;5(9):e007824. doi: 10.1136/bmjopen-2015-007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Portegies ML, Lahousse L, Joos GF, Hofman A, Koudstaal PJ, Stricker BH, et al. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam Study. Am J Respir Crit Care Med. 2016;193(3):251–258. doi: 10.1164/rccm.201505-0962OC. [DOI] [PubMed] [Google Scholar]
- 67.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, Jr, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada: cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Bangalore S. Cholesterol variability: a marker for increased risk or a risk factor? European heart journal. 2017. [DOI] [PubMed]
- 69.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219. doi: 10.1097/00043798-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167(11):1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 71.Minami J, Ishimitsu T, Sudo Y, Matsuoka H. Chronic kidney disease (CKD) as an independent risk factor for cardiovascular disease (CVD) Nihon rinsho Japanese J Clin Med. 2008;66(9):1657–1663. [PubMed] [Google Scholar]
- 72.Lu J, Mu Y, Su Q, Shi L, Liu C, Zhao J, et al. Reduced kidney function is associated with cardiometabolic risk factors, prevalent and predicted risk of cardiovascular disease in Chinese adults: results from the REACTION study. J Am Heart Assoc. 2016;5(7):e003328. doi: 10.1161/JAHA.116.003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khalili P, Ayoobi F, Mohamadi M, Jamalizadeh A, La Vecchia C, Esmaeili-Nadimi A. Effect of opium consumption on cardiovascular diseases–a cross-sectional study based on data of Rafsanjan cohort study. BMC Cardiovasc Disord. 2021;21(1):1–11. doi: 10.1186/s12872-020-01788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niaki MRK, Hamid M, Farshidi F, Mohammadpour M, Omran MTS. Evaluation of the role of opium addiction in acute myocardial infarction as a risk factor. Caspian J Intern Med. 2013;4(1):585. [PMC free article] [PubMed] [Google Scholar]
- 75.Sabzi F, Zokaei AH, Moloudi AR. Predictors of atrial fibrillation following coronary artery bypass grafting. Clinical Medicine Insights: Cardiology. 2011;5:CMC. S7170. [DOI] [PMC free article] [PubMed]
- 76.Marmor M, Penn A, Widmer K, Levin RI, Maslansky R. Coronary artery disease and opioid use. Am J Cardiol. 2004;93(10):1295–1297. doi: 10.1016/j.amjcard.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 77.AZIMZADE SB, Gholamreza Y, Narooey S. A case-control study of effect of opium addiction on myocardial infarction. 2005.
- 78.Hosseini SA, Abdollahi A, Behnampour N, Salehi A. The relationship between coronary risk factors and coronary artery involvement based on angiogrpahy findings. Koomesh. 2012;14(1).
- 79.Nakhaee S, Ghasemi S, Karimzadeh K, Zamani N, Alinejad-Mofrad S, Mehrpour O. The effects of opium on the cardiovascular system: a review of side effects, uses, and potential mechanisms. Substance Abuse Treat Prevent policy. 2020;15(1):1–13. doi: 10.1186/s13011-019-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hosseini SK, Masoudkabir F, Vasheghani-Farahani A, Alipour-Parsa S, Fathollahi MS, Rahimi-Foroushani A, et al. Opium consumption and coronary atherosclerosis in diabetic patients: a propensity score-matched study. Planta Med. 2011;77(17):1870–1875. doi: 10.1055/s-0031-1280017. [DOI] [PubMed] [Google Scholar]
- 81.Moezi SA, Azdaki N, Kazemi T, Moghaddam HRM, Partovi N, Hamidi F, et al. Effects of opium use on cardiovascular mortality: a critical appraisal of a topic. Iran J Public Health. 2019;48(10):1937. [PMC free article] [PubMed] [Google Scholar]
- 82.Matthews S, Dwyer R, Snoek A. Stigma and self-stigma in addiction. J Bioethical Inquiry. 2017;14(2):275–286. doi: 10.1007/s11673-017-9784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hackshaw A, Morris JK, Boniface S, Tang J-L, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360. [DOI] [PMC free article] [PubMed]
- 84.Roy A, Rawal I, Jabbour S, Prabhakaran D. Tobacco and cardiovascular disease: A summary of evidence. Cardiovascular, Respiratory, and Related Disorders 3rd edition. 2017. [PubMed]
- 85.Borgerding M, Klus H. Analysis of complex mixtures–cigarette smoke. Exp Toxicol Pathol. 2005;57:43–73. doi: 10.1016/j.etp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to data are not public, but are available from the corresponding author on reasonable request.