Abstract
Background:
Chronic pancreatitis (CP) patients have a high prevalence of osteoporotic fractures. In addition to prevalence of osteoporotic fractures, we evaluated how often bone health is assessed by dual-energy x-ray absorptiometry (DXA) in clinical practice, and the performance of Fracture Risk Assessment Tool (FRAX®) in predicting fracture risk in CP patients.
Methods:
Medical records of CP patients age ≥40 years prospectively enrolled in the North American Pancreatitis Study 2 (NAPS2) from the University of Pittsburgh Medical Center from 2000-2014 were retrospectively reviewed to gather additional relevant data before, at, and after enrollment until 12/2016. We determined if patients underwent DXA, compared their observed prevalence of fractures with published data from two large US studies based on administrative data, and their predicted fracture risk with US population based on FRAX®.
Results:
Only 21% (49/239) patients were evaluated by DXA during their care. The observed cumulative prevalence of fragility fractures in NAPS2 CP patients (9.2%, 95% confidence interval 5.9-13.6) was significantly greater than in controls (1.46% and 2.16%, p≤0.001 for each comparison) and CP patients (4.66%, and 5.13%, p<0.005 for each comparison) in the two US administrative data studies. The FRAX® 10-year probability of major osteoporotic fracture of ≥20% (5.1% vs. 8.3%, p>0.05) and for hip fracture of ≥3% (19.6% vs. 18.9%, p>0.05) in NAPS2 CP patients did not differ from the US population.
Conclusions:
Despite their high risk of fragility fractures, bone health is infrequently assessed in CP patients. FRAX® may not adequately predict fracture risk in CP patients.
Keywords: dual-energy x-ray absorptiometry (DXA), osteoporosis, fracture, chronic pancreatitis, FRAX®
Introduction
Osteopenia and osteoporosis are disorders of reduced bone density that predispose individuals to an increased risk of fracture.1 Osteoporotic fractures are a significant public health concern due to their negative impact on health and economic burden.2,3 Hip fractures are associated with an 8-36% increase in mortality within 1 year, and approximately 20% of patients with hip fracture require long-term nursing care.1 By 2025, in the US, there are expected to be >3 million fractures per year with an annual cost of over $25 billion.1
Dual-energy x-ray absorptiometry (DXA) is the most widely used method for measuring bone mineral density (BMD) (Table 1). The risk of osteoporosis increases with age, especially in women. The United States Preventative Services Task Force recommends screening for osteoporosis in women age ≥65 years and in younger women whose fracture risk is equal to or greater than that of a 65 year old white woman without additional risk factors.4 Although the relative risk for fracture increases with T-score reduction measured by DXA, most fractures occur in people with osteopenia because there are a greater number of individuals that have osteopenia than osteoporosis.5
Table 1:
Terminology used
| Bone mineral density (BMD) | Measure of the amount of minerals contained in bone; categorized as normal, osteopenia, or osteoporosis |
| Dual-energy x-ray absorptiometry (DXA) | Test used to measure BMD |
| T-score | Measure for reporting BMD; compares the patient’s BMD with that of a healthy young adult |
| Normal BMD | T score of ≥1 |
| Osteopenia | T score ≤ −1 but > −2.5 |
| Osteoporosis | T score ≤ −2.5 or history of hip or vertebral fracture not due to excess trauma |
| Fracture Risk Assessment Tool (FRAX®) | Tool commonly used in clinical practice to evaluate patients’ fracture risk |
| FRAX® MOF score | Patient’s 10-year probability of major osteoporotic fracture (MOF) as calculated by FRAX® tool |
| FRAX® HF score | Patient’s 10-year probability of hip fracture (HF) as calculated by FRAX® tool |
| Elevated Fracture risk: Defined as a FRAX® MOF score ≥20% or FRAX® HF score ≥3% | FRAX® score threshold at which pharmacologic treatment is recommended |
The Fracture Risk Assessment Tool (FRAX®) is commonly used in clinical practice to guide pharmacologic treatment of patients with reduced BMD.6 It is available as an online calculator (https://www.sheffield.ac.uk/FRAX/tool.jsp) into which clinicians may enter twelve patient risk factors: age, sex, weight, height, personal history of fracture, parent history of hip fracture, current cigarette smoking, glucocorticoid use, rheumatoid arthritis, secondary cause of osteoporosis, alcohol ≥3 units/day, and femoral neck BMD. The FRAX® algorithm provides the patient’s predicted 10-year probability of a major osteoporotic fracture (MOF) and 10-year probability of hip fracture (HF) as a percentage. Guidelines recommend initiating pharmacologic treatment in patients with an “elevated risk” defined as the FRAX® MOF score of ≥20% or FRAX® HF score of ≥3%.1
The prevalence of osteoporosis is higher in patients with chronic pancreatitis (CP) when compared with the general population.7,8 Recent data suggests that CP patients also have a higher risk of osteoporotic fractures when compared with controls.9 Tignor et al. found the prevalence of low trauma fracture in patients with CP to be similar to or greater than that of other high-risk gastrointestinal illnesses known to increase the risk for osteoporosis such as Crohn’s disease, celiac disease, cirrhosis, and post-gastrectomy status.10 Guidelines for management of these conditions recommend screening for bone health.11,12,13,14 Although guidelines on the management of CP also mention assessment of bone health, 15-19 there are no data on how often bone health is assessed in clinical practice in CP patients.
Therefore, the primary aim of this study was to determine how often DXA is performed during clinical care of patients with CP. Secondary aims were to determine the observed prevalence of fragility fractures and evaluate the performance of FRAX® score in predicting fracture risk in these patients.
Materials and Methods
North American Pancreatitis Studies (NAPS2)
NAPS2 prospectively enrolled patients with CP from 26 US centers from 2000-2014 using a previously published methodology.20,21,22 CP was defined by strict criteria on abdominal imaging studies or histology. Written informed consent was obtained from all participants. The study was approved by institutional review boards for each center. Detailed information was collected from patients and the enrolling physician investigator using structured case report forms.
Cohort for the Current Study
Patients aged ≥40 years enrolled from the University of Pittsburgh Medical Center (UPMC) were included for this analysis. Patients <40 years at the time of enrollment were excluded because the FRAX® model limits input to patients aged ≥40 years, and the fracture risk is low in patients <40 years.
Data Collection
Data available from NAPS2 enrollment questionnaires was retrieved for age at enrollment, gender, ethnicity, body mass index (BMI), cigarette smoking, alcohol use, physician-defined etiology, endocrine and exocrine insufficiency.
Electronic medical records were retrospectively reviewed by a single reviewer (AK) for all available inpatient and outpatient data relevant for this study through December 2016. Information collected included duration of patient contact, oral pancreatic enzyme replacement therapy (PERT), steroid use, secondary causes of osteoporosis other than CP, and history of fracture. Glucocorticoid use was considered significant if the patient was exposed to oral glucocorticoids >3 months at a dose of prednisolone ≥5 mg daily or equivalent doses of other glucocorticoids. Other medications that can potentially be linked to osteoporosis, such as antiepileptics, progestin-based contraceptives and diuretics were not evaluated. Secondary causes of osteoporosis assessed included type I diabetes mellitus, chronic malabsorption, chronic liver disease, untreated long-standing hyperthyroidism, hypogonadism, and chronic malnutrition. Fractures were further investigated for classification as a fragility fracture or traumatic fracture. Fragility fracture was defined as a fracture that resulted from a fall from standing height or less.6
FRAX® scores were calculated using the University of Sheffield FRAX® tool. When information for a risk factor was not available, the patient was assumed not to have that risk factor as per FRAX® guidelines. Other than parent history of hip fracture, information on all other risk factors was available for our patients. Femoral neck BMD is an optional item on the FRAX® questionnaire. Previous reports have shown that FRAX® score is similar in patients whether or not BMD measurement is included.23 In CP patients who had DXA performed, the patient’s FRAX® score was calculated both with and without femoral neck BMD as measured by T-score.
We compared the FRAX® scores of our patients with those of general population in the 2017 National Health Statistics Report.24 This report used data from the National Health and Nutrition Examination Survey (NHANES) 2013-2014, which was conducted to assess the health of a representative sample of the US population, to describe the distribution of FRAX® scores among US adults aged ≥50 years. Scores in this report were then age-adjusted to the 2000 census to provide nationally representative estimates of FRAX® scores. FRAX® scores in the report were defined as elevated if the 10-year probability of MOF was ≥20% or if the 10-year probability of HF was ≥3%, since these are the thresholds at which pharmacologic treatment is indicated in clinical practice. We used a similar definition to define an ‘elevated risk’ of fractures in our cohort.
Since we did not have a control group in NAPS2 for this analysis, we compared the cumulative prevalence of fractures in NAPS2 CP patients with controls from two large US cohorts from Brigham & Women’s Hospital and The US Veterans.9,10 The cumulative prevalence of fractures in NAPS2 CP patients was also compared with CP patients in these studies. Comparisons were stratified by sex and age groups 45-65 and >65 years.
Statistical Analyses
Descriptive statistics were computed for all study variables. Categorical data are reported as frequency and percentage, and continuous data as mean ± standard deviation or median (interquartile range, IQR). Due to skewed distribution for the risk of MOF and HF on FRAX® scores, the probability of fracture scores was transformed using Box-Cox analyses and the data were then back-transformed for presentation. Z score was used to compare the study population with cohorts in the 2017 National Health Statistics Report. We used T-test or Mann-Whitney-U test for comparing continuous variables and Fisher’s-exact or chi-squared tests for categorical data as appropriate. P values <0.05 were considered statistically significant.
Results
Study Cohort
A total of 239 patients with CP enrolled into NAPS2 from the University of Pittsburgh Medical Center who were ≥40 years at the time of enrollment formed the study cohort. Select characteristics at the time of study enrollment and duration of contact are summarized in Table 2. The median age was 55 (IQR 45, 67) years, just over half were male (54%), and over three quarters were Caucasian (84%). The median BMI was 24 kg/m2 (IQR 17, 31), and over half were current smokers (55%). The enrolling physician identified alcohol as the primary etiology in 54% and exocrine or endocrine insufficiency each in about one third of patients. The median duration of contact from the time of study enrollment until the end of observation was 4.9 years.
Table 2:
Select Characteristics of NAPS2 CP Patients from UPMC
| All (n=239) | DXA performed (n=49) |
DXA not performed (n=190) |
P value | |
|---|---|---|---|---|
| Characteristics included in FRAX® score | ||||
| Age at enrollment, years, median (IQR) | 55 (47, 63) | 56 (53, 59) | 54 (46, 62) | 0.11 |
| White ethnicity, n (%) | 200 (84) | 43 (88) | 157 (83) | 0.5 |
| Male sex, n (%) | 130 (54) | 12 (24) | 118 (62) | < 0.001 |
| BMI, kg/m2, median (IQR) | 24 (21, 28) | 23 (8) | 24 (7) | 0.73 |
| Current cigarette smoking, n (%) | 132 (55) | 24 (49) | 108 (57) | 0.4 |
| Current alcohol use (≥3 drinks/day), n (%) | 20 (8) | 2 (4) | 18 (9) | 0.38 |
| Fragility fracture at enrollment | 7 (2.9) | 4 (8.2) | 3 (1.6) | 0.01 |
| Steroid use, n (%) | 18 (7.5) | 7 (14) | 11 (6) | 0.06 |
| Rheumatoid arthritis, n (%) | 3 (1.2) | 0 | 3 (2) | 1 |
| Secondary osteoporosis, n (%) | 37 (15) | 14 (29) | 23 (12) | 0.0074 |
| FRAX® MOF score, median (IQR) | 4.3 (2.2, 6.9) | 5.7 (2.5, 8.9) | 3.8 (1.6, 9.7) | < 0.001 |
| FRAX® HF score, median (IQR) | 0.4 (0.1, 1.2) | 0.8 (0.1, 1.5) | 0.4 (0.1, 0.8) | 0.02 |
| Characteristics not included in FRAX® score | ||||
| Alcohol etiology, n (%) | 130 (54) | 19 (39) | 111 (58) | 0.02 |
| Exocrine insufficiency, n (%) | 81 (34) | 19 (39) | 62 (33) | 0.5 |
| Endocrine insufficiency, n (%) | 79 (33) | 11 (22) | 68 (36) | 0.09 |
| Fragility fracture at end of study, n (%) | 22 (9) | 12 (24) | 10 (5) | < 0.001 |
| Duration of contact in years*, median (IQR) | 4.9 (1.6, 8.1) | 7.6 (2.1, 13.1) | 4.2 (1.1, 7.25) | < 0.001 |
from time of enrollment into NAPS2 to end of study
On review of records, a history of fragility fracture either prior to or after enrollment until the last contact was noted in 22/239 patients (9.2%, 95% confidence interval 5.9-13.6). The median FRAX® MOF score was 4.3% and FRAX® HF score was 0.4%. An ‘elevated risk’ of fracture i.e. FRAX® MOF score of ≥20% and FRAX® HF score of ≥3% was noted in 3.3% and 13.3% patients, respectively.
Bone Health Assessment
A DXA scan was performed in 49/239 (21%) of patients (Figure 1). In the large majority of patients who did not undergo a DXA scan, no obvious reason was determined on review of records (169/190, 89%). Among patients in whom a reason could be identified, in 14 the test was deferred to the primary care physician or a subspecialist, 4 patients were unwilling to complete the test after recommendation by the physician, and in 3 patients osteoporosis screening was discussed but the reason it was not completed was unclear.
Figure 1:
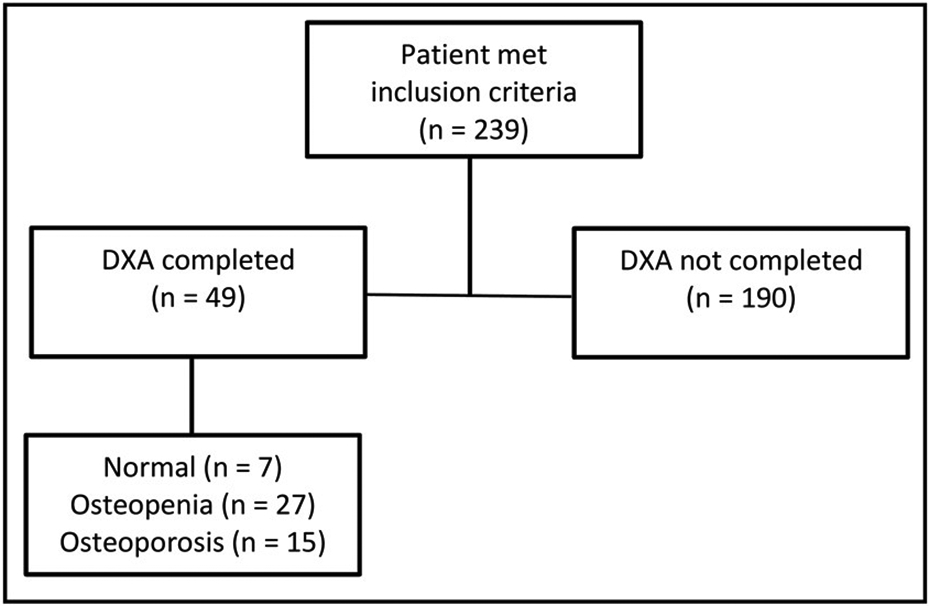
Performance of DXA scan in NAPS2 patients from UPMC.
Patients in whom a DXA scan was performed were significantly more likely to be female (76% vs. 38%, p<0.001) and have a non-alcohol etiology (61% vs. 42%, p=0.02), prior history of fragility fracture (24% vs. 5%, p<0.001), and longer duration of contact (7.6 years vs. 4.2 years, p<0.001) (Table 2). Patients who underwent a DXA scan had a significantly higher median FRAX® MOF score (5.7% vs. 3.8%, p<0.001) and FRAX® HF score (0.8% vs. 0.4%, p=0.02) when compared with those who did not have DXA performed. There was no significant difference in age, ethnicity, BMI, smoking status, steroid use and exocrine or endocrine insufficiency between patients who did and did not undergo a DXA scan.
FRAX® Score as a Predictor of Elevated Fracture Risk
The ‘elevated risk’ of fracture, i.e. FRAX® MOF score ≥20% or FRAX® HF score ≥3% among those ≥50 years old was not significantly different in CP patients in our study (n=158) when compared with the general US population as estimated in the 2017 National Health Statistics Report (MOF 5.1% vs. 8.3%; HF 19.6% vs. 18.9%, p >0.05 for both).
The elevated fracture risk was also similar when stratified by age groups (Table 3). Malabsorption is one of the variables (i.e., risk factor for secondary osteoporosis) in FRAX® calculator. As sensitivity analysis, we calculated FRAX® scores by assigning ‘yes’ to malabsorption for CP patients on PERT and all CP patients. In this analysis the elevated fracture risk was similar in the controls when compared with CP patients with PERT (MOF- 6.3% vs. 8.3%, p=0.2 and HF- 21.5 % vs. 18.9%, p=1.0) or all CP patients (MOF- 10% vs. 8.3%, p=0.6 and HF- 26.2 % vs. 18.9%, p=0.1).
Table 3:
10-year predicted risk of fracture based on FRAX® in NAPS2 CP patients from UPMC compared to US population
| Major osteoporotic fracture ≥ 20% | Hip fracture ≥ 3% | |||
|---|---|---|---|---|
| CP patients | US population | CP patients | US population | |
| All ≥ age 50 | 8/158 (5.1%) | 185/2230 (8.3%) | 31/158 (19.6%) | 421/2230 (18.9%) |
| 50-59 | 0/78 (0%) | 23/784 (2.9%) | 1/78 (1.2%) | 53/784 (6.7%) |
| 60-69 | 3/49 (6.1%) | 50/788 (6.4%) | 8/49 (16.3%) | 89/788 (11.3%) |
| 70-79 | 1/22 (4.5%) | 69/428 (16.1%) | 13/22 (59.1%) | 165/428 (38.6%) |
| 80 and over | 4/9 (44.4%) | 63/230 (27.4%) | 7/9 (77.8%) | 165/230 (71.6%) |
Age in years; all p values >0.05
Femoral neck BMD (measured by DXA) is an optional factor that can be entered into the FRAX® calculator, but most of our patients did not have BMD measurements since they did not have DXA performed. In order to account for possible differences in FRAX® score with and without BMD measurement, FRAX® score was calculated both using and omitting femoral neck BMD in patients who had that measurement available in 43 out of 49 patients who underwent DXA scan. There was no significant difference between the median FRAX® scores calculated with and without femoral neck BMD measurement (FRAX® MOF scores of 5.6% vs. 8.2%, p=0.19; FRAX® HF scores of 2.1% vs. 3.7%, p=0.11, respectively). This finding is consistent with previous reports that inclusion of BMD measurement does not impact FRAX® score.20
Observed Cumulative Risk of Fragility Fracture
The cumulative risk of fragility fractures in NAPS2 CP patients was 9.2% (22/239). To compare our data with the two published US studies, we limited the analysis to the risk of fractures in NAPS2 CP patients who were ≥ 45 years at last contact (n=227). In these patients, the risk of fractures was 4.49-6.62 folds greater when compared to control subjects and 1.89-2.07 folds greater when compared with CP patients in the published US studies.9,10
Discussion
In this large study of well-phenotyped patients with CP, we found that less than 25% underwent evaluation for BMD with a DXA scan during clinical care. Among those who did undergo this test, there was a high prevalence of osteoporosis and osteopenia. Ours is the first study to evaluate the utility of FRAX® score in this patient population. The 10-year risk of fractures based on FRAX® scores in NAPS2 patients with CP did not differ significantly from population controls, suggesting that factors contributing to osteopathy in CP are not captured by variables used to calculate FRAX® score.
While previous studies have reported on the prevalence of osteoporosis, osteopenia and of fractures in patients with CP, how often bone health is assessed in these patients during clinical practice has not been known. We found that less than 1 in 4 patients undergo a DXA scan to assess BMD, and not surprisingly, DXA was more likely to be performed in patients with other known risk factors for fractures. Osteoporosis screening is suboptimal in the general population as well. Studies have estimated that BMD screening with DXA in females ≥65 years, which is the population recommended for screening by the USPSTF, ranges from 11 to 70%.25,26 The reason BMD is not routinely tested is likely multifactorial, and several specific factors may contribute towards this in CP patients. First, although guidelines suggest assessment of bone mineral density, there is a lack of consensus on initiation and intervals for screening in this patient population.15-19 Second, there may be a lack of provider and patient awareness of the association between CP and reduced BMD.27 Third, the complexity of care of CP patients is such that other complications of the disease may take precedence over osteoporosis screening.28 Fourth, expectations as to whether screening should be managed by the primary care physician or subspecialist may be unclear. Lastly, cost of the study may be a barrier, especially if not covered by insurance. We suggest that physicians -- primary care, gastroenterologists or pancreatologists, providing care to patients with CP discuss bone health with patients and coordinate performance of bone health assessment in individual patients. Our group has more recently adopted a discussion of bone health assessment and performance of DXA in the routine clinical care of patients with CP.
The FRAX® score is a commonly used tool in clinical practice to guide management of patients with reduced BMD. While we noted that patients with CP in our study had significantly higher rates of fragility fractures, their FRAX® scores did not differ from age- and sex-matched general population. This suggests that FRAX® score may not be an adequate indicator of fracture risk in CP patients, and factor(s) contributing to osteopathy in these patients differ from the general population. This observation appears to be similar to inflammatory bowel disease, where a meta-analysis found that FRAX® 10-year risk of fracture was only modestly increased in these patients despite a known increased prevalence of osteoporosis.29 Future studies, hopefully with larger sample sizes and longitudinal follow-up will confirm these findings.
The mechanisms underlying reduced BMD in patients with CP although studied remain unclear. Chronic malabsorption and malnutrition are likely major contributing factors and may in part be reversible with adequate supplementation with calcium, vitamin D, and PERT. However, studies addressing markers of pancreatic exocrine function have shown variable results, and a recent meta-analysis concluded that there was no significant difference in the prevalence of vitamin D deficiency in patients with CP when compared with healthy controls.30,31,32 Limitation of prior studies include small size, the lack of details on the temporal relationship between assessment of vitamin D status, administration and duration of vitamin D use and PERT use, and assessment of confounding effects of patient and disease-related factors, such as age, sex, race, smoking, duration of disease, morphologic features, and pancreatic function.
Chronic inflammation may contribute to poor bone health in CP. In a study of 29 CP patients with an equal number of matched controls, Duggan et al found patients with CP to have higher markers of bone turnover and inflammation, suggesting that systemic inflammation may play a role in reduced BMD.33 When compared with controls, level of markers for bone formation (procollagen 1 amino-terminal propeptide [P1NP], osteocalcin [OC]), the number of patients with elevated bone resorption marker (carboxy-terminal telopeptide of type 1 collagen, CTX-1), and the level of high-sensitivity C-reactive protein [hsCRP]) (used as a surrogate for metabolic effect of proinflammatory cytokines) were significantly higher in CP patients. Vitamin D levels were inversely associated with smoking, P1NP and bone health, and correlated with hsCRP. Although this is the most comprehensive study to date on this topic, and these pilot data highlight the potential role of systemic inflammation in CP on bone health, due to small sample sizes, the study could not perform a detailed analysis and the interactive relationship between smoking, vitamin D deficiency, bone turnover markers and inflammation. As pointed out by the authors, systemic inflammation may affect bone metabolism in health individuals, smoking has an impact in vitamin D metabolism, and vitamin D has anti-inflammatory properties.30 Smoking also has effects independent of vitamin D, such as alterations in body weight, hormonal alterations, and direct effect on osteogenesis.34
Future research should focus on clarifying the role of patient (demographics, behavioral factors) and disease-related factors (disease duration, exocrine dysfunction, morphologic changes), vitamin D status on bone health, mechanisms underlying osteopathy in CP, as well as developing strategies for prevention and treatment. These strategies could include therapies specific to CP, such as PERT or calcium and vitamin D supplementation, treatments specific to osteoporosis, such as bisphosphonates, or other novel approaches. Treatment with bisphosphonates has been shown to have variable effects in improving BMD in celiac disease and inflammatory bowel disease.35,36 For example, in patients with celiac disease, gluten free diet can partially recover BMD. However, a systematic review concluded that there was no difference in recovery of BMD in patients on a gluten free diet versus gluten free diet plus bisphosphonate therapy.37
Our study has several strengths. The definition of CP was stringent, and patients were under longitudinal care with subspecialist physicians with specific interest in CP. Fractures were rigorously evaluated by reviewing patients’ medical records for a history of fracture and determining whether it met criteria for fragility fracture in contrast with previous US studies relying solely on administrative data which could lead to underestimation.9,10 Moreover, the positive predictive value of CP diagnosis in administrative data is suboptimal leading to overestimation of CP cases.38 Therefore, our results provide further validity to both the risk of fractures as well as the diagnosis of CP in our study cohort.
The study also had limitations. One such limitation was retrospective data collection of FRAX® variables. Since family history of osteoporotic fracture is often not reliably documented in the electronic medical record, FRAX® may have been underestimated in some patients. However, per FRAX® guidelines, patients with missing data for a risk factor are assumed not to have that risk factor, and family history has a relatively low contribution to FRAX® score compared to other variables. Not all patients underwent a DXA scan to calculate femoral neck BMD. To address this limitation, we performed a sensitivity analysis by calculating FRAX® scores with and without inclusion of femoral neck BMD only in patients who underwent a DXA scan. There was no difference in 10-year risk of major osteoporotic fracture or hip fracture in this analysis, which is consistent with prior studies.23 Another possible limitation is that patients may have had a DXA scan at an outside facility which was not available in our medical record, leading to underestimation of its performance.
In conclusion, patients with CP have a high prevalence of osteopenia, osteoporosis, and fragility fractures. BMD testing in CP patients is suboptimal. FRAX® score does not adequately predict fracture risk in these patients. Assessment of bone health using DXA should be incorporated into the routine clinical care of CP patients. Further studies should evaluate the barriers to screening and methods to improve screening for bone health, mechanisms that underlie osteopathy, and strategies for prevention and treatment to improve bone health in patients with CP.
Table 4:
Comparison of cumulative prevalence of fragility fracture in NAPS2 CP patients from UPMC with controls in two published US studies
| NAPS-2 CP patients N (%)# |
Tignor et al. 2010 Control Subjects N (%) |
Munigala et al. 2016 Control Subjects N (%) |
|
|---|---|---|---|
| Total subjects | 22/227 (9.69) | 11486/785556 (1.46) | 7097/328983 (2.16) |
| Males | |||
| 45-65 years | 7/86 (8.13) | 1724/170520 (1.01) | 4064/199745 (2.03) |
| > 65 years | 4/41 (9.75) | 2036/133797 (1.52) | 2528/104453 (2.42) |
| Females | |||
| 45-65 years | 5/62 (8.06) | 2599/303266 (0.86) | 330/20260 (1.63) |
| > 65 years | 6/38 (15.79) | 5127/177973 (2.88) | 175/4525 (3.87) |
Only patients ≥ 45 years at last contact were included in this analysis
All p-values are ≤0.001 for all comparisons between NAPS2 CP patients vs. controls
Table 5:
Comparison of cumulative prevalence of fragility fracture in NAPS2 CP patients from UPMC with CP patients in two published US studies
| NAPS-2 Subjects N (%)# |
Tignor et al. 2010 CP Subjects N (%) |
Munigala et al. 2016 CP Subjects N (%) |
|
|---|---|---|---|
| Total patients | 22/227 (9.69) | 120/2573 (4.66) ** | 137/2672 (5.13) * |
| Males | |||
| 45-65 years | 7/86 (8.13) | 32/737 (4.34) | 97/2038 (4.76) |
| > 65 years | 4/41 (9.75) | 18/561 (3.21)* | 35/525 (6.67) |
| Females | |||
| 45-65 years | 5/62 (8.06) | 18/679 (2.65)* | 5/98 (5.10) |
| > 65 years | 6/38 (15.79) | 60/596 (10.07) | 0/11 (0) |
Only patients ≥ 45 years at last contact were included in this analysis
p-value ≤0.001 and
p-value <0.05 for comparisons between NAPS2 CP patients vs. CP patients in other studies
Acknowledgements:
Grant Support:
This research was partly supported by the NIH DK061451 (DCW), DK077906 (DY), U01 DK108306 (DCW, DY). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Abbreviations:
- BMD
bone mineral density
- BMI
body mass index
- CP
chronic pancreatitis
- DXA
dual energy x-ray absorptiometry
- FRAX®
Fracture Risk Assessment
- HF
hip fracture
- IQR
interquartile range
- MOF
major osteoporotic fracture
- NAPS2
North American Pancreatitis Study
- NHANES
National Health and Nutrition Examination Survey
Footnotes
Disclosures:
DCW serves as a consultant for AbbVie, Regeneron, Ariel Precision Medicine, is a cofounder of Ariel Precision Medicine and may have equity. Other authors report no conflict.
Some data were previously presented as a poster at APA meeting, November 8–11, 2017, San Diego, California, and published as an abstract in Pancreas, November/December 2017, Volume 46, Issue 10, p1386-1448.
References
- 1.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75. [DOI] [PubMed] [Google Scholar]
- 3.Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res 1997; 12: 24–35. [DOI] [PubMed] [Google Scholar]
- 4.Calonge N, Bibbins-Domingo K, Cantu AG, Curry S, Dietrich AJ, Flores G, et al. U.S. Preventative Services Task Force. Screening for osteoporosis: U.S. Preventative Services Task Force recommendation statement. Ann Intern Med 2011;154:356–64. [DOI] [PubMed] [Google Scholar]
- 5.Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, Favus MJ, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014;25:1439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, et al. AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(Suppl):3:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morán CE, Sosa EG, Martinez SM, Geldern P, Messina D, Russo A, et al. Bone mineral density in patients with pancreatic insufficiency and steatorrhea. Am J Gastroenterol 1997;92:867–71. [PubMed] [Google Scholar]
- 8.Duggan SN, Smyth ND, Murphy A, Macnaughton D, O'Keefe SJ, Conlon KC. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:219–28. [DOI] [PubMed] [Google Scholar]
- 9.Munigala S, Agarwal B, Gelrud A, Conwell DL. Chronic Pancreatitis and Fracture: A Retrospective, Population-Based Veterans Administration Study. Pancreas 2016;45:355–61. [DOI] [PubMed] [Google Scholar]
- 10.Tignor AS, Wu BU, Whitlock TL, Lopez R, Repas K, Banks PA,et al. High prevalence of low-trauma fracture in chronic pancreatitis. Am J Gastroenterol 2010;105:2680–86. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein CN1, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:795–841. [DOI] [PubMed] [Google Scholar]
- 12.Leslie WD1, Bernstein CN, Leboff MS. American Gastroenterological Association Clinical Practice Commitee. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology 2003;125:941–66. [DOI] [PubMed] [Google Scholar]
- 13.Jeong HM, Kim DJ. Bone Diseases in Patients with Chronic Liver Disease. Int J Mol Sci 2019;20:4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am J Gastroenterol 2017;112:241–58 [DOI] [PubMed] [Google Scholar]
- 15.Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J 2017;5:153–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delhaye M, Van Steenbergen W, Cesmeli E, Pelckmans P, Putzeys V, Roeyen G, et al. Belgian consensus on chronic pancreatitis in adults and children: statements on diagnosis and nutritional, medical, and surgical treatment. Acta Gastroenterol Belg 2014;77:47–65. [PubMed] [Google Scholar]
- 17.Martinez J, Abad-Gonzalez A, Aparicio JR, Aparisi L, Boadas J, Boix E, et al. The Spanish Pancreatic Club recommendations for the diagnosis and treatment of chronic pancreatitis: part 1 (diagnosis). Pancreatology 2013;13:8–17. [DOI] [PubMed] [Google Scholar]
- 18.Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol 2020;115:322–39. [DOI] [PubMed] [Google Scholar]
- 19.Arvanitakis M, Ockenga J, Bezmarevic M, Gianotti L, Krznarić Ž, Lobo DN, et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin Nutr 2020;39:612–31. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, et al. North American Pancreatic Study Group. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox CM, Sandhu BS, Singh V, Gelrud A, Abberbock JN, Sherman S, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol 2016;111:1488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox CM, Yadav D, Ye T, Gardner TB, Gelrud A, Sandhu BS, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015;13:552–60; quiz e28-e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadam RK, Schlauch K, Izuora KE. FRAX prediction without BMD for assessment of osteoporotic fracture risk. Endocr Pract 2013;19:780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. FRAX-based Estimates of 10-year Probability of Hip and Major Osteoporotic Fracture Among Adults Aged 40 and Over: United States, 2013 and 2014. Natl Health Stat Report 2017;(103):1–16. [PubMed] [Google Scholar]
- 25.Lafata JE, Kolk D, Peterson EL, McCarthy BD, Weiss TW, Chen YT, et al. Improving osteoporosis screening: results from a randomized cluster trial. J Gen Intern Med 2007;22:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell H, O'Connor K, Greenberg D. Adherence to the U.S. Preventive Services Task Force 2002 osteoporosis screening guidelines in academic primary care settings. J Womens Health (Larchmt) 2012;21:50–53. [DOI] [PubMed] [Google Scholar]
- 27.Nayak S, Roberts M, Chang C, Greenspan SL. Health Beliefs about Osteoporosis and Osteoporosis Screening in Older Women and Men. Health Educ J 2010;69:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon DH, Brookhart MA, Gandhi TK, Karson A, Gharib S, Orav EJ, et al. Adherence with osteoporosis practice guidelines: A multilevel analysis of patient, physician, and practice setting characteristics. Am J Med 2004;117:919–24. [DOI] [PubMed] [Google Scholar]
- 29.Serrano-Montalbán B, Arias Á, Friginal-Ruiz AB, Lucendo AJ. The Use of the Fracture Risk Assessment (FRAX®) Tool in Predicting Risk of Fractures in Patients With Inflammatory Bowel Disease: A Systematic Review. J Clin Densitom 2017;20:180–87. [DOI] [PubMed] [Google Scholar]
- 30.Sudeep K, Chacko A, Thomas N, Selvakumar R, George B, Paul TV, et al. Predictors of osteodystrophy in patients with chronic nonalcoholic pancreatitis with or without diabetes. Endocr Pract 2011;17:897–905. [DOI] [PubMed] [Google Scholar]
- 31.Haas S, Krins S, Knauerhase A, Löhr M. Altered bone metabolism and bone density in patients with chronic pancreatitis and pancreatic exocrine insufficiency. JOP 2015;16:58–62. [DOI] [PubMed] [Google Scholar]
- 32.Hoogenboom SA, Lekkerkerker SJ, Fockens P, Boermeester MA, van Hooft JE. Systematic review and meta-analysis on the prevalence of vitamin D deficiency in patients with chronic pancreatitis. Pancreatology 2016;16:800–6. [DOI] [PubMed] [Google Scholar]
- 33.Duggan SN, Purcell C, Kilbane M, O'Keane M, McKenna M, Gaffney P, et al. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: a case-matched study. Am J Gastroenterol 2015;110:336–45. [DOI] [PubMed] [Google Scholar]
- 34.Al-Bashaireh AM, Haddad LG, Weaver M, Chengguo X, Kelly DL, Yoon S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J Osteoporos 2018;2018:1206235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2014;12:32–44. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Wu R, Gong J, Zhu W, Li Y, Li N, et al. The efficacy and safety of bisphosphonates for osteoporosis or osteopenia in Crohn's disease: a meta-analysis. Dig Dis Sci 2013;58:915–22. [DOI] [PubMed] [Google Scholar]
- 37.Grace-Farfaglia P. Bones of contention: bone mineral density recovery in celiac disease--a systematic review. Nutrients 2015;7:3347–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy NG, Nangia S, DiMagno MJ. The Chronic Pancreatitis International Classification of Diseases, Ninth Revision, Clinical Modification Code 577.1 Is Inaccurate Compared With Criterion-Standard Clinical Diagnostic Scoring Systems. Pancreas 2016;45:1276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


