Summary
Background
Pregnant individuals with coronavirus disease 2019 (COVID-19) are at increased risk of severe disease, prematurity, and stillbirth. In March 2021, vaccination for at risk pregnant women was recommended in Switzerland, expanding this to all pregnant women in May 2021. Our aim was to assess the safety of mRNA COVID-19 vaccines in pregnancy.
Methods
This multicentre prospective cohort study describes early adverse events and perinatal outcomes in pregnant women who received at least one dose of mRNA vaccine between March 1st and December 27th, 2021 in Switzerland, using the COVI-PREG registry. Early adverse events were collected at least one month following vaccine administration. Pregnancy and neonatal outcomes were extracted from medical records using the maternity discharge letters providing follow-up information up to 5 days after birth.
Findings
Of 1012 vaccinated women, 894 (88·3%) received both injections during pregnancy, with BNT162b2 (n = 271) or mRNA-1273 (n = 623) vaccines. Local events (mainly local pain) were reported in 81·3% and 80·5% after the first and second doses. Rates of systemic reactions (mainly fatigue and headache) were similar after the first dose and most frequent after the second dose of mRNA-1273. Of the 1012 women, four (0·4%; 95%CI [0·1-1·0]) severe early adverse events occurred: pulmonary embolism, preterm premature rupture of membranes, isolated fever with hospitalisation, and herpes zoster. Of 107 patients vaccinated before 14 weeks, one (0·9%; 95%CI [0·0-5·1]) early spontaneous abortions was reported (8 weeks). Of 228 vaccinated before 20 weeks one (0·4%; 95%CI [0·0-2·4]) late spontaneous abortion was reported (16 weeks). Of 513 women exposed before 37 weeks, 33 (6·4%; 95%CI [4·5-8·9]) delivered preterm. Among 530 patients exposed in pregnancy, no stillbirth was reported and 25 (4·7%; 95%CI [3·0-6·8]) neonates were admitted to intensive care unit.
Interpretation
Frequent local and systemic effects were described after exposure to mRNA COVID-19 vaccines during pregnancy but severe events were rare. Women vaccinated during pregnancy did not experience higher adverse pregnancy or neonatal outcomes when compared to historical data on background risks in the obstetric population.
Funding
This research was funded by a grant from the Swiss Federal Office of Public Health and the CHUV Foundation.
Keywords: SARS-CoV-2, COVID-19, Vaccine, Safety, mRNA, Pregnant women, Pregnancy
Research in context.
Evidence before this study
Pregnant women are at higher risk of severe form of COVID-19, however, have been excluded from COVID-19 mRNA vaccine clinical trials. We searched on PubMed and pre-print platforms for safety observational studies including pregnant women exposed to mRNA COVID-19 vaccines as of March 28th, 2022. Several studies have reported reassuring safety data in pregnant women exposed to COVID-19 vaccination. These studies, however, were either retrospective, or had only a small number of patients with pregnancy outcomes, or focused on a single outcome (e.g., spontaneous abortion), or specific period of exposure (e.g. third trimester exposure). A single prospective study from the United States surveillance system (v-safe pregnancy registry) reported no obvious safety signals among 827 pregnant women exposed to COVID-19 vaccine throughout all pregnancy, including more than 600 pregnant women exposed before 37 weeks of gestation. The study reported a low level of detail on population baseline characteristics and patients were mostly vaccinated in the third trimester, without describing severe early adverse events following vaccination.
Added value of this study
Our study is the first European study that reports Swiss nationwide safety results from more than 1000 pregnant women exposed to mRNA COVID-19 vaccine with high quality details including more than 500 patients with a pregnancy outcome available. We observed that most pregnant women experienced mild local and systemic early adverse events after injection, and more frequently after the second dose of mRNA-1273 (Moderna) vaccine. We reported similar rates of early and late spontaneous abortions after vaccine exposure during pregnancy when compared to historical data on background risks in the obstetric population. We found that pregnant women exposed to mRNA COVID-19 vaccine had low rates of preterm births, small neonates for gestational age, neonatal intensive care unit admission, and no stillbirth were reported. The mRNA COVID-19 vaccine exposure in pregnant women seemed safe.
Implications of all the available evidence
Our study shows that mRNA COVID-9 vaccines seem safe throughout all pregnancy, in terms of early adverse events, pregnancy, and neonatal outcomes. Pregnant women and health care professionals should be aware of this information as vaccination remains an effective solution against COVID-19 in this population at risk. Further studies are needed to assess long term outcomes such as infant developmental outcomes. Efforts must be made to continue to monitor the safety and efficacy of these already marketed mRNA COVID-19 vaccines in a larger sample of pregnant women and appropriate control groups to provide risk estimates, with a particular focus on first trimester exposure, rare adverse events and long-term outcomes (e.g. infant developmental outcomes).
Alt-text: Unlabelled box
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is of particular concern during pregnancy as pregnant women have a higher susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with a severe form of the disease reported in 8 to 10%.1, 2, 3 Pregnant patients that test positive for COVID-19 also have an increased risk of adverse pregnancy and neonatal outcomes, with higher rates of preterm birth, neonatal intensive care unit admission, and stillbirth.2, 3, 4 As of December 2020, this viral infection became preventable through the rollout of COVID-19 vaccinations. A two-dose regimen of messenger RNA (mRNA) vaccination reported 94·1-95·0% efficacy against COVID-19 illness in adults5,6 and a third vaccine dose (booster) sustained the efficacy at 91-93% while the Delta variant was predominant.7,8 With the emergence of the Omicron variant, even with a substantially lower vaccine efficacy for COVID-19 symptoms,7,9 the efficacy against hospitalization remains at 70%.10 As pregnant women, however, were excluded from COVID-19 vaccine trials, efficacy and safety data were initially lacking for this population.11 In April 2021, the V-safe surveillance system did not identify any obvious safety concerns following vaccination in 35,691 pregnant patients who received mRNA COVID-19 vaccines in the United States (US). In the 827 completed pregnancies stemming from the V-safe pregnancy registry, the distribution of perinatal outcomes was similar to pregnant patients not exposed to the vaccine.12 The risk of spontaneous abortion following mRNA COVID-19 vaccination either before conception or during the first trimester of pregnancy was also similar to historical data on background risks in the obstetric population.13 Despite a still low COVID-19 vaccination rate among pregnant women, available studies show that COVID-19 vaccination in pregnancy is effective against SARS-CoV-2 infection and severe disease.14, 15, 16 Pregnant women exposed to the COVID-19 vaccine experienced similar early adverse events than non-pregnant women and no trends was reported regarding adverse perinatal outcomes, in the still limited available literature.17, 18, 19, 20, 21, 22 As of March 2021, the Swiss Society of Gynaecologists and Obstetricians (SGGG) and the Federal Office of Public Health recommended vaccination for pregnant women with additional risk factors for severe COVID-19 disease, and this recommendation was extended to all pregnant women in May 2021.23 Despite the growing evidence for efficacy and safety of COVID-19 vaccines in pregnancy and the risk-benefit balance in favour of COVID-19 vaccination in pregnancy, many pregnant women remain reluctant to receive the COVID-19 vaccine in Europe.24 The core element to address vaccine hesitancy is to provide consistent and fair information to health care providers giving them the tools to best advise pregnant women.
The aim of our study was to augment the current safety information on early adverse events in pregnant women, as well as on perinatal outcomes after exposure to COVID-19 vaccine any time during pregnancy through the COVI-PREG registry in Switzerland.25
Methods
Data source, information, and study time points
Participants were enrolled between March 1, 2021 and December 27, 2021, in the COVI-PREG vaccine registry, a prospective cohort study that aimed to assess the safety of mRNA vaccines against COVID-19 in pregnant women. Informed consent was obtained for all participants. The study was promoted through the SGGG (www.sggg.ch) to all Swiss private practice gynaecologists and public hospitals. A questionnaire regarding vaccine adverse events was distributed at the vaccination visit before or at the time of injection (Figure S1 - supplementary materials). These questionnaires were collected at least one month after injection by primary care gynaecologists who participated in the study by returning the de-identified questionnaires to their reference centre. The updated final questionnaire was returned at the end of the pregnancy. De-identified information about medical history, pregnancy, and neonatal outcomes were collected from the maternity discharge letters sent by primary care gynaecologists to their reference centre providing follow up information up to 5 days after birth. De-identified data were then recorded by the reference centres using the REDCap (Research Electronic Data Capture) secure web application in accordance with the approval of the Swiss Ethical Board (CER-VD-2020-00548) (Figure S2 - supplementary materials). The STROBE guidelines were used to ensure the reporting quality of this observational study.26
Study population
Pregnant women who received at least one injection of a mRNA vaccine against COVID-19 between one week before their last menstrual period (LMP) and the end of pregnancy were included in the study. Patients who were under 18 years of age or not able to consent were not included. Women with no information on the date of injection, the occurrence of early adverse events and their description if any, or no information about the type of vaccine used were excluded.
Exposure to mRNA vaccine against COVID-19
Exposure to mRNA vaccine against COVID-19 was defined as at least one injection of vaccine between one week before the date of LMP, or calculated LMP from first trimester ultrasound examination, and the end of pregnancy. Both mRNA vaccines authorized and recommended during pregnancy in Switzerland were assessed: BNT162b2 (Comirnaty®, Pfizer–BioNTech) and mRNA-1273 (SpikeVax®, Moderna) vaccine. Pregnancy exposure periods were stratified into peri-conceptional period (PCP), trimester 1, trimester 2, and trimester 3. The PCP was defined as an injection between one week before LMP and two weeks after LMP. Trimester 1 was defined as the period from two weeks after LMP to 11 weeks of gestation (wks) and 6 days to match the Swiss recommendations to prescribe the vaccine preferentially after 12 wks.23 Trimester 2 was defined as the period from 12 wks to 27 wks and 6 days. Trimester 3 was defined as the period starting from 28 wks to the end of pregnancy. If the pregnancy due date related to the LMP differed by more than five days from the due date obtained by first trimester ultrasound, the due date was set by ultrasound.
Outcomes
Based on the outcomes of interest, the following additional inclusion and exclusion criteria were added to the study population.
Primary outcomes - early adverse events outcomes
Definition of outcomes
Early adverse events following vaccination were divided into three categories: local adverse events, systemic adverse events, and severe adverse events, observed within one month following an injection of mRNA vaccine against COVID-19. Local adverse events were defined as at least one of the following reactions at the injection site: redness, pain, swelling, warmth, itch, haematoma, induration, or other local findings. Systemic adverse events were defined as at least one of the following events: fever, fatigue, headache, chills, nausea, vomiting, diarrhoea, muscle pain, joint pain, malaise, or other events except those defined in the pregnancy and neonatal outcomes. Severe adverse events were defined as at least one of the following events occurring during the pregnancy: hospitalization potentially related to the vaccine, intensive care unit admission following vaccination, confirmed anaphylactic shock, or other potentially severe reaction related to the vaccine according to the patient and investigator interpretation.
Population 1a
For local and systemic adverse events, pregnant women with two mRNA vaccine injections between one week before LMP and the end of pregnancy were included in the analysis to compare the adverse events related to multiple doses. Patients who received only one injection were excluded.
Population 1b
For severe adverse event outcomes, pregnant women with at least one injection during pregnancy or the PCP were included.
Secondary outcomes – pregnancy and neonatal outcomes
Early spontaneous abortion
Definition of the outcome
Early spontaneous abortion was defined as a spontaneous pregnancy loss before 14 wks including spontaneous abortion, blighted ovum, or spontaneously arrested pregnancy. Elective terminations of pregnancy were excluded from this definition.
Population 2a
Pregnant women with at least one injection between one week before LMP and less than 14 wks were considered. Pregnant women with no pregnancy outcome data available at the time of analysis were excluded unless they received their second vaccine dose after 14 wks suggesting an ongoing pregnancy at the obstetrical visit following vaccination as no complications were reported in the questionnaire.
Late spontaneous abortion
Definition of the outcome
Late spontaneous abortion was defined as a spontaneous pregnancy loss between 14 wks and 19 wks and 6 days. Elective terminations of pregnancy were excluded by this definition.
Population 2b
Pregnant women with at least one injection between one week before LMP to 19 wks and 6 days were considered if pregnancy was ongoing after 14 wks. Pregnant women with no pregnancy outcome data available at the time of the analysis were excluded unless their second vaccine dose occurred after 20 wks, supporting ongoing pregnancy at the obstetrical visit following vaccination, as no complications were reported on the questionnaire.
Sensitivity analysis
To estimate the impact of the inclusion or exclusion of pregnant women lost to follow-up after 14 or 20 wks on the rate of early and late spontaneous abortion respectively, we conducted a “strict outcome scenario” sensitivity analysis.
We redefined our study population to include pregnant women exposed to at least one dose of vaccine between one week before LMP and 13 wks and 6 days for early spontaneous abortion and 19 wks and 6 days for late spontaneous abortion but restricted to pregnant women with a pregnancy outcome available at the time of the analysis. This analysis estimates the rate of spontaneous abortion with the hypothesis that patients with no pregnancy outcome available could have had a non-recorded spontaneous abortion following vaccination.
Preterm birth
Definition of the outcome – preterm birth
Preterm birth was defined as a live born infant between 24 wks and 36 wks and 6 days, and was classified as either spontaneous, defined as a delivery after spontaneous labour (assisted or non-assisted vaginal birth or caesarean section following spontaneous labour) or iatrogenic defined as an induction of labour or a caesarean delivery in the absence of spontaneous labour.
Population 2c
Pregnant women with at least one injection between one week before LMP to 36 wks and 6 days were included. Pregnant women with no pregnancy outcome data available at the time of the analysis were excluded as well as patients who terminated their pregnancy before 24 wks. Ongoing pregnancies that had not reached full term (37 wks) at the time of analysis were excluded, to avoid overestimation of an earlier adverse outcome such as preterm birth.
Delivery, livebirth, stillbirth, pre-viable fetus, gestational age at delivery, small for gestational age, neonatal intensive care unit, and neonatal death
Delivery was defined as vaginal birth, either spontaneous or assisted (i.e., by forceps or vacuum) or caesarean section. Livebirth was defined as a liveborn infant born at or after 24 wks. Stillbirth was defined as a fetal demise from 20 wks onwards. A pre-viable fetus was defined as a fetus born extremely preterm, between 20 to 23 wks and 6 days, without neonatal resuscitation. Gestational age (GA) at delivery was defined as the GA in wks at delivery. Small for gestational age (SGA) was defined as a birthweight below the 10th percentile for gestational age according to the INTERGROWTH 21 scale.27 Neonatal intensive care unit (NICU) admission referred to the admission of the neonate into the NICU and was divided into four categories according to the cause of admission: prematurity, respiratory distress syndrome, sepsis, and any other cause. Neonatal death referred to the death within 28 days after birth of a liveborn infant born at 24 wks or more.
Population 2d
For the analysis of pregnancy and neonatal outcomes, pregnant women with at least one injection between one week before LMP and the end of pregnancy were included. Pregnant women with no pregnancy outcome data available at the time of analysis were excluded as well as patients who terminated their pregnancy before 20 wks.
Co-variates
Maternal age was divided into categories: <25 years (y), 25-29 y, 30-34 y, 35-39, and ≥40 y. For each injection, information on the type of vaccine (BNT162b2 or mRNA-1273), place of vaccination (i.e., vaccination centre/health authority, gynaecologist/midwife consultation, general practitioner, pharmacist), site of vaccine injection (i.e., right arm, left arm), and antipyretic intake around the time of injection was collected. Linguistic areas were defined according to the official language of each Swiss county: German, French, or Italian.
For analysis of pregnancy and preterm birth outcomes, maternal comorbidities (pulmonary, cardiac, hypertension, pregestational diabetes, obesity defined as a body mass index >30kg/m2, immunosuppression, auto-immune diseases, hematologic, neurological, digestive, renal, urologic, oncologic, thyroid dysfunction, psychiatric disorders, and other comorbidities) and obstetric characteristics (nulliparity/multiparity, multiple pregnancy, and previous caesarean section status) were collected. Pregnancy due date was defined as 40 weeks after the LMP (LMP was reported by the patient or if unknown, calculated from a first trimester ultrasound examination).
Statistical analysis
Descriptive statistics were used to evaluate baseline demographics and characteristics, as well as the recorded prevalence of early adverse events by type of mRNA vaccine and by first or second doses and all pregnancy and neonatal outcomes overall. The 95% confidence intervals (95% CI) were calculated for each reported prevalence. Tests for normality were done for continuous variables. Statistical analyses were performed using Stata 16 (StataCorp. 2015. Stata Statistical Software: Release 16. College Station, TX: StataCorp LP).
Role of the funding source
This research was supported by a grant from the Swiss Federal Office of Public Health and the CHUV Foundation. The funders had no role in study design, data collection, data analysis, interpretation and writing of the paper.
Results
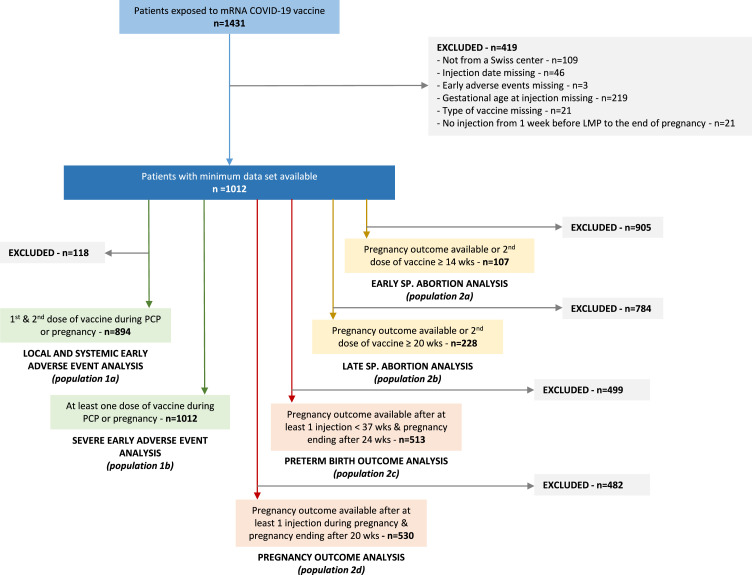
Between March 1- December 27, 2021, a total of 1431 women were enrolled in the registry among which 1012 patients met the inclusion criteria (Figure 1). Results are reported according to the different outcomes of interest. Vaccination patterns represented in our cohort are described in figure S3 (supplementary materials).
Figure 1.
Flow chart of the study. The numbers of patients eligible for each analysis are displayed (Study populations 1a to 2d). Abbreviation: LMP: last menstrual period; PCP: peri-conceptional period; SP.: spontaneous; wks: weeks of gestation.
Early adverse events outcomes
Among 1012 patients, 894 (88·3%) pregnant women had both injections between one week before LMP and the end of pregnancy and were included in this analysis (Figure 1).
Characteristics of the participants (population 1a) according to the type of vaccine received are presented in Table 1.
Table 1.
Population 1a - Baseline characteristics of pregnant women exposed to 2 doses of COVID-19 mRNA vaccine.
| Pfizer/BioNTech | Moderna | ||||||
|---|---|---|---|---|---|---|---|
| BNT162b2 | mRNA-1273 | ||||||
| n = 271 | n = 623 | ||||||
| N | % | n | % | ||||
| Maternal age at first dose (years) | |||||||
| <25 | 5 | 1.9 | % | 5 | 0.8 | % | |
| 25-29 | 34 | 12.6 | % | 72 | 11.6 | % | |
| 30-34 | 107 | 39.5 | % | 283 | 45.4 | % | |
| 35-39 | 99 | 36.5 | % | 199 | 31.9 | % | |
| ≥40 | 15 | 5.5 | % | 37 | 5.9 | % | |
| Missing | 11 | 4.1 | % | 27 | 4.3 | % | |
| Swiss linguistic area | |||||||
| German | 175 | 64.6 | % | 452 | 72.6 | % | |
| French | 89 | 32.8 | % | 152 | 24.4 | % | |
| Italian | 7 | 2.6 | % | 19 | 3.1 | % | |
| EXPOSURE | |||||||
| 1st dose of vaccine | |||||||
| Trimester of injection | |||||||
| Peri-conception (7 days before LMP to 13 days after LMP ) | 7 | 2.6 | % | 25 | 4.0 | % | |
| T1 - 14 days after LMP and <12 wks | 12 | 4.4 | % | 28 | 4.5 | % | |
| T2 - ≥12 and <28 wks | 182 | 67.2 | % | 441 | 70.8 | % | |
| T3 - ≥28 wks | 70 | 25.8 | % | 129 | 20.7 | % | |
| Place of vaccination | |||||||
| Vaccination centre / Health authority | 118 | 43.5 | % | 248 | 39.8 | % | |
| Gynaecologist / Midwife consultation (PRIVATE PRACTICE) | 0 | - | 4 | 0.6 | |||
| Gynaecologist / Midwife consultation (HOSPITAL) | 6 | 2.2 | % | 4 | 0.6 | % | |
| GP (General practitioner) | 2 | 0.7 | % | 6 | 1.0 | % | |
| Occupational health service (at work) | 5 | 1.9 | % | 9 | 1.4 | % | |
| Pharmacist | 2 | 0.7 | % | 16 | 2.6 | % | |
| Unknown | 2 | 0.7 | % | 1 | 0.2 | % | |
| Missing | 136 | 50.2 | % | 335 | 53.8 | % | |
| Injection site | |||||||
| Left arm | 106 | 39.1 | % | 247 | 39.7 | % | |
| Right arm | 24 | 8.9 | % | 40 | 6.4 | % | |
| Missing | 141 | 52.0 | % | 336 | 53.9 | % | |
| Antipyretic intake around injection | |||||||
| Yes | 15 | 5.5 | % | 24 | 3.9 | % | |
| No | 116 | 42.8 | % | 260 | 41.7 | % | |
| Unknown | 6 | 2.2 | % | 0 | - | ||
| Missing | 134 | 49.5 | % | 339 | 54.4 | % | |
| 2nd dose of vaccine | |||||||
| Trimester of injection | |||||||
| Peri-conception (7 days before LMP to 13 days after LMP ) | 1 | 0.4 | % | 1 | 0.2 | % | |
| T1 - 14 days after LMP and <12 wks | 2 | 0.7 | % | 3 | 0.5 | % | |
| T2 - ≥12 and <28 wks | 156 | 57.6 | % | 376 | 60.4 | % | |
| T3 - ≥28 wks | 112 | 41.3 | % | 243 | 39.0 | % | |
| Place of vaccination | |||||||
| Vaccination centre / Health authority | 118 | 43.5 | % | 254 | 40.8 | % | |
| Gynaecologist / Midwife consultation (PRIVATE PRACTICE) | 0 | - | 3 | 0.5 | % | ||
| Gynaecologist / Midwife consultation (HOSPITAL) | 6 | 2.2 | % | 2 | 0.3 | % | |
| GP (General practitioner) | 2 | 0.7 | % | 6 | 1.0 | % | |
| Occupational health service (at work) | 6 | 2.2 | % | 2 | 0.3 | % | |
| Pharmacist | 2 | 0.7 | % | 19 | 3.1 | % | |
| Unknown | 2 | 0.7 | % | 1 | 0.2 | % | |
| Missing | 135 | 49.8 | % | 336 | 53.9 | % | |
| Injection site | |||||||
| Left arm | 107 | 39.5 | % | 234 | 37.6 | % | |
| Right arm | 20 | 7.4 | % | 52 | 8.4 | % | |
| Missing | 144 | 53.1 | % | 337 | 54.1 | % | |
| Antipyretic intake around injection | |||||||
| Yes | 14 | 5.2 | % | 54 | 8.7 | % | |
| No | 110 | 40.6 | % | 227 | 36.4 | % | |
| Unknown | 8 | 3.0 | % | 1 | 0.2 | % | |
| Missing | 139 | 51.3 | % | 341 | 54.7 | % |
T1-3: trimester 1-3.
LMP: last menstrual period.
wks: weeks of gestation.
The number of patients who received two doses of Pfizer–BioNTech BNT162b2 or Moderna mRNA-1273 were 271 (30·3%) and 623 (69·7%), respectively. Most pregnant women were in the age category 30 to 34 y, with 107 (39·5%) and 283 (45·4%), followed by the category 35 to 39 y, with 99 (36·5%) and 199 (31·9%) for Pfizer–BioNTech BNT162b2 and Moderna mRNA-1273, respectively. Independent of vaccine type, 727 (81·3%) reported at least one local adverse event for the first dose and 720 (80·5%) for the second dose. At least one systemic adverse event was reported in 316 (35·4%) and 602 (67·3%), respectively for the first and second dose. Timing of the first vaccine dose was in the PCP for 32 (3·6%), first trimester for 10 (4·5%), second trimester for 623 (69·7%), and third trimester for 199 (22·3%). Timing of the second vaccine dose was in the PCP for 2 (0·2%), first trimester for 5 (0·6%), second trimester for 532 (59·5%), and third trimester for 355 (39·7%). Details by vaccine type are shown in Table 1.
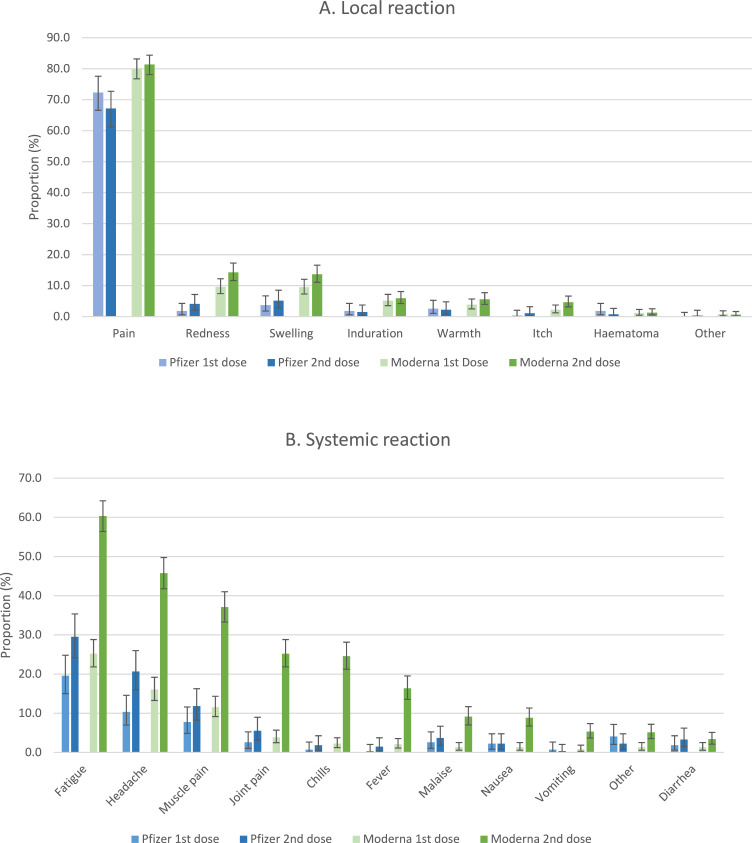
Local and systemic adverse events after the first and the second dose of vaccine, according to the type of vaccine received, are shown in Table 2 and represented in Figure 2. Local reactions were similar between the two vaccine types and the two doses, with pain representing two thirds of complains. The three most common systemic adverse events after the first dose were fatigue in 19·6% (95%CI [15·0-24·8]) and 25·2% (95%CI [21·8-28·8]), headache in 10·3% (95%CI [7·0-14·6]) and 16·1% (95%CI [13·3-19·2]), and muscle pain in 7·7% (95%CI [4·9-11·6]) and 11·6% (95%CI [9·2-14·3]) respectively for Pfizer–BioNTech BNT162b2 and Moderna mRNA-1273 vaccine. Systemic reactions, however, were higher after the second dose of Moderna mRNA-1273 vaccine, compared to the first dose of Moderna mRNA-1273 vaccine or compared to the first and second dose of Pfizer–BioNTech BNT162b2 vaccine. A total of 78·3% (95%CI [74·9-81·5]) of patients experienced at least one adverse systemic reaction after the second dose of Moderna mRNA-1273 vaccine with the 60·4% (95%CI [56·4-64·2]) reporting fatigue, 45·7% (95%CI [41·8-49·8]) reporting headache, 37·1% (95%CI [33·3-41·0]) reporting muscle pain, 25·2% (95%CI [21·8-28·8]) reporting joint pain, 24·6% (95%CI [21·2-28·1]) reporting chills, and 16·4% (95%CI [13·6-19·5]) reporting fever.
Table 2.
Local and systemic early adverse events among pregnant women receiving 2 injections of COVID-19 mRNA vaccine.
| Comirnaty Pfizer/BioNTech - BNT162b2 n = 271 |
Moderna - mRNA-1273 n = 623 |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st dose | 2nd dose | 1st dose | 2nd dose | ||||||||||||||||||||||||||
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | ||||||||||||||||||
| No reaction | 21 | 7.7 | % | 4.9 | - | 11.6 | 25 | 9.2 | % | 6.1 | - | 13.3 | 53 | 8.5 | % | 6.4 | - | 11.0 | 13 | 2.1 | % | 1.1 | - | 3.5 | |||||
| Local reaction | 201 | 74.2 | % | 68.5 | - | 79.3 | 191 | 70.5 | % | 64.7 | - | 75.8 | 526 | 84.4 | % | 81.3 | - | 87.2 | 529 | 84.9 | % | 81.9 | - | 87.6 | |||||
| Pain | 196 | 72.3 | % | 66.6 | - | 77.6 | 182 | 67.2 | % | 61.2 | - | 72.7 | 499 | 80.1 | % | 76.7 | - | 83.2 | 507 | 81.4 | % | 78.1 | - | 84.4 | |||||
| Redness | 5 | 1.8 | % | 0.6 | - | 4.3 | 11 | 4.1 | % | 2.0 | - | 7.1 | 60 | 9.6 | % | 7.4 | - | 12.2 | 89 | 14.3 | % | 11.6 | - | 17.3 | |||||
| Swelling | 10 | 3.7 | % | 1.8 | - | 6.7 | 14 | 5.2 | % | 2.9 | - | 8.5 | 59 | 9.5 | % | 7.3 | - | 12.0 | 85 | 13.6 | % | 11.0 | - | 16.6 | |||||
| Induration | 5 | 1.8 | % | 0.6 | - | 4.3 | 4 | 1.5 | % | 0.4 | - | 3.7 | 32 | 5.1 | % | 3.5 | - | 7.2 | 37 | 5.9 | % | 4.2 | - | 8.1 | |||||
| Warmth | 7 | 2.6 | % | 1.0 | - | 5.2 | 6 | 2.2 | % | 0.8 | - | 4.8 | 24 | 3.9 | % | 2.5 | - | 5.7 | 35 | 5.6 | % | 3.9 | - | 7.7 | |||||
| Itch | 1 | 0.4 | % | 0.0 | - | 2.0 | 3 | 1.1 | % | 0.2 | - | 3.2 | 14 | 2.2 | % | 1.2 | - | 3.7 | 29 | 4.7 | % | 3.1 | - | 6.6 | |||||
| Haematoma | 5 | 1.8 | % | 0.6 | - | 4.3 | 2 | 0.7 | % | 0.1 | - | 2.6 | 7 | 1.1 | % | 0.5 | - | 2.3 | 8 | 1.3 | % | 0.6 | - | 2.5 | |||||
| Other | 0 | 0.0 | % | 0.0 | - | 1.4 | 1 | 0.4 | % | 0.0 | - | 2.0 | 5 | 0.8 | % | 0.3 | - | 1.9 | 4 | 0.6 | % | 0.2 | - | 1.6 | |||||
| Systemic reaction | 82 | 30.3 | % | 24.8 | - | 36.1 | 114 | 42.1 | % | 36.1 | - | 48.2 | 234 | 37.6 | % | 33.7 | - | 41.5 | 488 | 78.3 | % | 74.9 | - | 81.5 | |||||
| Fatigue | 53 | 19.6 | % | 15.0 | - | 24.8 | 80 | 29.5 | % | 24.2 | - | 35.3 | 157 | 25.2 | % | 21.8 | - | 28.8 | 376 | 60.4 | % | 56.4 | - | 64.2 | |||||
| Headache | 28 | 10.3 | % | 7.0 | - | 14.6 | 56 | 20.7 | % | 16.0 | - | 26.0 | 100 | 16.1 | % | 13.3 | - | 19.2 | 285 | 45.7 | % | 41.8 | - | 49.8 | |||||
| Muscle pain | 21 | 7.7 | % | 4.9 | - | 11.6 | 32 | 11.8 | % | 8.2 | - | 16.3 | 72 | 11.6 | % | 9.2 | - | 14.3 | 231 | 37.1 | % | 33.3 | - | 41.0 | |||||
| Joint pain | 7 | 2.6 | % | 1.0 | - | 5.2 | 15 | 5.5 | % | 3.1 | - | 9.0 | 24 | 3.9 | % | 2.5 | - | 5.7 | 157 | 25.2 | % | 21.8 | - | 28.8 | |||||
| Chills | 2 | 0.7 | % | 0.1 | - | 2.6 | 5 | 1.8 | % | 0.6 | - | 4.3 | 14 | 2.2 | % | 1.2 | - | 3.7 | 153 | 24.6 | % | 21.2 | - | 28.1 | |||||
| Fever | 1 | 0.4 | % | 0.0 | - | 2.0 | 4 | 1.5 | % | 0.4 | - | 3.7 | 13 | 2.1 | % | 1.1 | - | 3.5 | 102 | 16.4 | % | 13.6 | - | 19.5 | |||||
| Malaise | 7 | 2.6 | % | 1.0 | - | 5.2 | 10 | 3.7 | % | 1.8 | - | 6.7 | 8 | 1.3 | % | 0.6 | - | 2.5 | 57 | 9.1 | % | 7.0 | - | 11.7 | |||||
| Nausea | 6 | 2.2 | % | 0.8 | - | 4.8 | 6 | 2.2 | % | 0.8 | - | 4.8 | 8 | 1.3 | % | 0.6 | - | 2.5 | 55 | 8.8 | % | 6.7 | - | 11.3 | |||||
| Vomiting | 2 | 0.7 | % | 0.1 | - | 2.6 | 1 | 0.4 | % | 0.0 | - | 2.0 | 5 | 0.8 | % | 0.3 | - | 1.9 | 33 | 5.3 | % | 3.7 | - | 7.4 | |||||
| Other | 11 | 4.1 | % | 2.0 | - | 7.1 | 6 | 2.2 | % | 0.8 | - | 4.8 | 8 | 1.3 | % | 0.6 | - | 2.5 | 32 | 5.1 | % | 3.5 | - | 7.2 | |||||
| Diarrhea | 5 | 1.8 | % | 0.6 | - | 4.3 | 9 | 3.3 | % | 1.5 | - | 6.2 | 8 | 1.3 | % | 0.6 | - | 2.5 | 21 | 3.4 | % | 2.1 | - | 5.1 | |||||
Figure 2.
Local and systemic reactions reported within one month after each injection of mRNA COVID-19 vaccines in pregnancy. Proportions (%) are displayed and I bars represent 95% confidence intervals.
Baseline characteristics of the 1012 pregnant patients who had at least one injection (population 1b) are presented in table S1. A total of four (0·4%; 95%CI [0·1-1·0]) severe early adverse events were reported and are presented in Table 3: deep vein thrombosis associated with pulmonary embolism at 21 wks resolved with adapted treatment; preterm premature rupture of membranes (PPROM) with vaginal bleeding in the context of a partial placental abruption leading to emergency caesarean section at 31 wks; thoracic herpes zoster more than three weeks after the second injection performed at 17 wks; hospitalization for surveillance of fever at 32 wks following the second dose of vaccine. All patients of population 2d (n = 530) delivered liveborn infants, including a preterm neonate born at 31 wks.
Table 3.
Severe early adverse events following vaccination.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
|---|---|---|---|---|---|
| Maternal age at first dose | 36 | 25 | 32 | Unknown | |
| Swiss linguistic area | German | German | German | French | |
| Gravidity (G) Parity (P) | G2P1 | G1P0 | G1P0 | G3P1 | |
| Obstetrical history | - Previous C-section (2017) | - Previous vaginal delivery (2007) | |||
| Medical condition | None | - Asthma treated by Salmeterol / Fluticason - 50/500 mcg - 1/day | None | - BMI = 34 kg/m2 - Allergic asthma without treatment - Psoriasis without treatment |
|
| Obstetrical condition | - | - | - Gestational diabetes requirering insuline | ||
| EXPOSURE | |||||
| Type of vaccine | BNT162b2 | mRNA-1273 | mRNA-1273 | mRNA-1273 | |
| 1st DOSE of vaccine | |||||
| Timing of injection (weeks from LMP) | 13 | 15 | 26 | 28 | |
| Local reaction | Pain | Pain | Pain, Induration | Pain, Redness, Swelling, Warmth, Itch | |
| Systemic reaction | Fatigue | Headache | Fatigue | Headache | |
| Severe reaction | No | No | No | No | |
| 2nd DOSE of vaccine | |||||
| Timing of injection (weeks from LMP) | 17 | 20 | 30 | 32 | |
| Local reaction | Pain | Pain | Pain, Induration | Pain, Warmth | |
| Systemic reaction | Fatigue | Headache | - | Fever, Headache, Fatigue, Chills, Nausea, Muscle pain, Joint pain | |
| Severe reaction | YES | YES | YES | YES | |
| Timing | 3 weeks after injection | Within 7 days after injection | Within 7 days after injection | Within 7 days after injection | |
| Details | 1st episode of herpes zoster on the right anterior thoracic wall - Spontaneously resolved with symptomatic treatment (paracetamol) | Deep venous thromboembolism and pulmonary embolism diagnosed 6 days after injection. | PPROM one day after vaccination - Hospital admission: complete fetal lung maturation. Active vaginal bleeding leading to emergency C-section five days after vaccination | Fever 38°C, one day after vaccination leading to hospital admission for clinical surveillance during 24 hours and discharged. | |
| PREGNANCY OUTCOME | Livebirth | Livebirth | Livebirth | Livebirth | |
| Gestational age at delivery (weeks) | 38 | 40 | 31 | 38 |
LMP: last menstrual period.
BNT162b2: Pfizer–BioNTech mRNA vaccine.
mRNA-1273: Moderna mRNA vaccine.
C-section: caesarean section.
PPROM: preterm premature rupture of membranes.
Early spontaneous abortion outcome
A total of 135 patients out of 1012 were exposed to the vaccine before 14 wks and 28 women were excluded because they did not complete the follow-up questionnaire regarding the second dose of vaccine after 14 wks or did not have an available pregnancy outcome at the time of analysis. Patient characteristics (population 2a) are presented in table S2 (supplementary materials). Among 107 patients included, 97 (90·7%; 95%CI [83·5-95·4]) had an ongoing pregnancy at the time of questionnaire completion after the second dose and only 10 patients’ pregnancy outcome data were available at the time of the analysis. One patient (0·9%; 95%CI [0·0-5·1]) had an early spontaneous abortion at 8 wks, five weeks after a single dose of vaccine (Table 4).
Table 4.
Early and late spontaneous abortion outcomes analysis and sensitivity analysis following COVID-19 mRNA vaccination during PCP or pregnancy.
| Early sp. abortion outcome |
“Strict outcome scenario” sensitivity analysis |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 107 | n = 10 | ||||||||||||
| EARLY SP. ABORTION ANALYSIS | n | % | 95% CI | n | % | 95% CI | |||||||
| Sp. abortion <14 wks | 1 | 0.9 | % | 0.0 | - | 5.1 | 1 | 10.0 | % | 0.3 | - | 44.5 | |
| Sp. abortion ≥ 14 wks | 1 | 0.9 | % | 0.0 | - | 5.1 | 1 | 10.0 | % | 0.3 | - | 44.5 | |
| Livebirth | 8 | 7.5 | % | 3.3 | - | 14.2 | 8 | 80.0 | % | 44.4 | - | 97.5 | |
| 2nd vaccine's dose ≥ 14 wks | 97 | 90.7 | % | 83.5 | - | 95.4 | - | ||||||
| Late sp. abortion outcome | "Strict outcome scenario" sensitivity analysis | ||||||||||||
| n = 228 | n = 96 | ||||||||||||
| LATE SP. ABORTION ANALYSIS | n | % | 95% CI | n | % | 95% CI | |||||||
| Sp. abortion ≥ 14 wks | 1 | 0.4 | % | 0.0 | - | 2.4 | 1 | 1.0 | % | 0.0 | - | 5.7 | |
| Livebirth | 95 | 41.7 | % | 35.2 | - | 48.4 | 95 | 99.0 | % | 94.3 | - | 100.0 | |
| 2nd vaccine's dose ≥ 20 wks | 132 | 57.9 | % | 51.2 | - | 64.4 | - | ||||||
wks: weeks of gestation.
sp: spontaneous.
Late spontaneous abortion analysis
Of 1012 pregnant women, a total of 399 were exposed to the vaccine before 20 wks. One case was excluded due to early spontaneous abortion, and 170 patients were excluded because they did not complete the questionnaire regarding the second dose of vaccine after 20 wks or pregnancy outcome data were not available. Patient characteristics (population 2b) are presented in Table S3. Among 228 patients included, 132 (57·9%; 95%CI [51·2-64·4]) patients had an ongoing pregnancy at the time completion of the questionnaire after the second dose and 96 patients had a pregnancy outcome available at the time of the analysis. A total of 95 (41·7%; 95%CI [35·2-48·4]) patients had a liveborn infant and one patient (0·4%; 95%CI [0·0-2·4]) had a late spontaneous abortion at 16 wks, related to chorioamnionitis three weeks after a first dose of vaccine (Table 4). The patient had no reported obstetric risk factors. Placenta pathology examination revealed placental inflammation compatible with incipient chorioamnionitis. Bacterial cultures and bacterial polymerase chain reaction testing were negative.
“Strict outcome scenario” sensitivity analysis
Patient baseline characteristics are presented in Tables S4 and S5 (supplementary materials).
Early spontaneous abortion - Among 135 patients exposed to the vaccine before 14 wks, 10 patients had a pregnancy outcome available, including one patient (1/10, 10·0%; 95%CI [0·3-44·5]) who had an early spontaneous abortion (Table 4).
Late spontaneous abortion - Among 398 patients exposed to the vaccine before 20 wks, after the exclusion of one case for early spontaneous abortion, 96 patients had a pregnancy outcome available. One (1/96, 1·0%; 95%CI [0·0-5·7]) patient had a late spontaneous abortion and 95 women had a livebirth (Table 4).
Preterm birth outcome
Of 1012 patients, 513 patients were included in this analysis because they were exposed to the vaccine before 37 wks, had a pregnancy outcome available, and delivered after 24 wks (Figure 1). Patient baseline characteristics (population 2c) were very similar to the pregnancy outcome population (population 2d) (Tables 5 and 7). All patients gave birth to liveborn infants including five twin pregnancies. Preterm birth before 37 wks was reported in 33 (6·4%; 95%CI [4·5-8·9]) patients, with 19 (3·7%; 95%CI [2·2-5·7]) iatrogenic preterm births and 12 spontaneous preterm births (2·3%; 95%CI [1·2-4·1]) (Table 6).
Table 5.
Patient baseline characteristics for pregnant women exposed to mRNA COVID-19 vaccine before 37 wks and ending their pregnancy after 24 wks (population 2c).
| PRE-TERM BIRTH OUTCOME ANALYSIS |
||||
|---|---|---|---|---|
| n = 513 | ||||
| n | % | |||
| Maternal age at first dose (years | ||||
| <25 | 2 | 0.4 | % | |
| 25-29 | 53 | 10.3 | % | |
| 30-34 | 219 | 42.7 | % | |
| 35-39 | 170 | 33.1 | % | |
| ≥40 | 31 | 6.0 | % | |
| Missing | 38 | 7.4 | ||
| Swiss linguistic area | ||||
| German | 334 | 65.1 | % | |
| French | 172 | 33.5 | % | |
| Italian | 7 | 1.4 | % | |
| Maternal medical condition | 107 | 20.9 | % | |
| Pulmonary comorbidities | 10 | 1.9 | % | |
| Cardiac comorbidities | 20 | 3.9 | % | |
| Hypertension | 7 | 1.4 | % | |
| Pregestational diabetes | 4 | 0.8 | % | |
| Obesity (BMI >30kg/m2) | 28 | 5.5 | % | |
| Immunosuppression | 3 | 0.6 | % | |
| Auto-immune diseases | 7 | 1.4 | % | |
| Hematologic comorbidities | 3 | 0.6 | % | |
| Neurological comorbidities | 1 | 0.2 | % | |
| Digestive comorbidities | 3 | 0.6 | % | |
| Renal comorbidities | 4 | 0.8 | % | |
| Urological comorbidities | 1 | 0.2 | % | |
| Oncological comorbidities | 1 | 0.2 | % | |
| Thyroid dysfunction | 33 | 6.4 | % | |
| Psychiatric disorders | 6 | 1.2 | % | |
| Other | 9 | 1.8 | % | |
| Pregnancy | ||||
| Nulliparous | 215 | 41.9 | % | |
| Twin pregnancy | 5 | 1.0 | % | |
| Previous caesarean section | 74 | 14.4 | % | |
| EXPOSURE | ||||
| Type of vaccine | ||||
| Pfizer/BioNTech - BNT162b2 | 144 | 28.1 | % | |
| Moderna - mRNA-1273 | 369 | 71.9 | % | |
| 1st dose of vaccine | ||||
| Trimester of injection | ||||
| Peri-conception (7 days before LMP to 13 days after LMP) | 0 | - | ||
| T1 - 14 days after LMP and <12 wks | 2 | 0.4 | % | |
| T2 - ≥12 and <28 wks | 303 | 59.1 | % | |
| T3 - ≥28 wks | 208 | 40.6 | % | |
| Place of vaccination | ||||
| Vaccination centre / Health authority | 171 | 33.3 | % | |
| Gynaecologist / Midwife consultation (PRIVATE PRACTICE) | 1 | 0.2 | ||
| Gynaecologist / Midwife consultation (HOSPITAL) | 11 | 2.1 | % | |
| GP (General practitioner) | 5 | 1.0 | % | |
| Occupational health service (at work) | 8 | 1.6 | % | |
| Pharmacist | 8 | 1.6 | % | |
| Unknown | 4 | 0.8 | % | |
| Missing | 305 | 59.5 | % | |
| Injection site | ||||
| Left arm | 165 | 32.2 | % | |
| Right arm | 32 | 6.2 | % | |
| Missing | 316 | 61.6 | % | |
| Antipyretic intake around injection | ||||
| Yes | 22 | 4.3 | % | |
| No | 174 | 33.9 | % | |
| Unknown | 7 | 1.4 | % | |
| Missing | 310 | 60.4 | % | |
| 2nd dose of vaccine | ||||
| - During pregnancy | 456 | 88.9 | % | |
| Trimester of injection | ||||
| Peri-conception (7 days before LMP to 13 days after LMP) | 0 | - | ||
| T1 - 14 days after LMP and <12 wks | 0 | - | ||
| T2 - ≥12 and <28 wks | 174 | 33.9 | % | |
| T3 - ≥28 wks | 282 | 55.0 | % | |
| Place of vaccination | ||||
| Vaccination centre / Health authority | 154 | 30.0 | % | |
| Gynaecologist / Midwife consultation (PRIVATE PRACTICE) | 1 | 0.2 | % | |
| Gynaecologist / Midwife consultation (HOSPITAL) | 8 | 1.6 | % | |
| GP (General practitioner) | 4 | 0.8 | % | |
| Occupational health service (at work) | 6 | 1.2 | % | |
| Pharmacist | 7 | 1.4 | % | |
| Unknown | 3 | 0.6 | % | |
| Missing | 273 | 53.2 | % | |
| Injection site | ||||
| Left arm | 141 | 27.5 | % | |
| Right arm | 30 | 5.8 | % | |
| Missing | 285 | 55.6 | % | |
| Antipyretic intake around injection | ||||
| Yes | 31 | 6.0 | % | |
| No | 140 | 27.3 | % | |
| Unknown | 6 | 1.2 | % | |
| Missing | 279 | 54.4 | % | |
T1-3: trimester 1-3.
LMP: last menstrual period.
wks: weeks of gestation.
Table 7.
Patient baseline characteristics for pregnant women exposed to mRNA COVID-19 vaccine and ending their pregnancy during pregnancy after 20 wks (population 2d).
| PREGNANCY OUTCOME ANALYSIS |
|||||
|---|---|---|---|---|---|
| n = 530 | |||||
| n | % | ||||
| Maternal age at first dose (years) | |||||
| <25 | 2 | 0.4 | % | ||
| 25-29 | 53 | 10.0 | % | ||
| 30-34 | 224 | 42.3 | % | ||
| 35-39 | 178 | 33.6 | % | ||
| ≥40 | 32 | 6.0 | % | ||
| Missing | 41 | 7.7 | |||
| Swiss linguistic area | |||||
| German | 344 | 64.9 | % | ||
| French | 179 | 33.8 | % | ||
| Italian | 7 | 1.3 | % | ||
| Maternal medical condition | 108 | 20.4 | % | ||
| Pulmonary comorbidities | 11 | 2.1 | % | ||
| Cardiac comorbidities | 20 | 3.8 | % | ||
| Hypertension | 7 | 1.3 | % | ||
| Pre-gestational diabetes | 4 | 0.8 | % | ||
| Obesity (BMI >30kg/m2) | 28 | 5.3 | % | ||
| Immunosuppression | 3 | 0.6 | % | ||
| Auto-immune diseases | 7 | 1.3 | % | ||
| Hematologic comorbidities | 3 | 0.6 | % | ||
| Neurological comorbidities | 1 | 0.2 | % | ||
| Digestive comorbidities | 3 | 0.6 | % | ||
| Renal comorbidities | 4 | 0.8 | % | ||
| Urological comorbidities | 1 | 0.2 | % | ||
| Oncological comorbidities | 1 | 0.2 | % | ||
| Thyroid dysfunction | 33 | 6.2 | % | ||
| Psychiatric disorders | 6 | 1.1 | % | ||
| Other | 9 | 1.7 | % | ||
| Pregnancy | |||||
| Nulliparous | 220 | 41.5 | % | ||
| Twin pregnancy | 5 | 0.9 | % | ||
| Previous caesarean section | 77 | 14.5 | % | ||
| EXPOSURE | |||||
| Type of vaccine | |||||
| Pfizer/BioNTech - BNT162b2 | 150 | 28.3 | % | ||
| Moderna - mRNA-1273 | 380 | 71.7 | % | ||
| 1st dose of vaccine | |||||
| Trimester of injection | |||||
| Peri-conception (7 days before LMP to 13 days after LMP) | 0 | - | |||
| T1 - 14 days after LMP and <12 wks | 2 | 0.4 | % | ||
| T2 - ≥12 and <28 wks | 303 | 57.2 | % | ||
| T3 - ≥28 wks | 225 | 42.5 | % | ||
| Place of vaccination | |||||
| Vaccination centre / Health authority | 177 | 33.4 | % | ||
| Gynaecologist / Midwife consultation (PRIVATE PRACTICE) | 1 | 0.2 | |||
| Gynaecologist / Midwife consultation (HOSPITAL) | 11 | 2.1 | % | ||
| GP (General practitioner) | 5 | 0.9 | % | ||
| Occupational health service (at work) | 8 | 1.5 | % | ||
| Pharmacist | 8 | 1.5 | % | ||
| Unknown | 4 | 0.8 | % | ||
| Missing | 316 | 59.6 | % | ||
| Injection site | |||||
| Left arm | 171 | 32.3 | % | ||
| Right arm | 32 | 6.0 | % | ||
| Missing | 327 | 61.7 | % | ||
| Antipyretic intake around injection | |||||
| Yes | 22 | 4.2 | % | ||
| No | 180 | 34.0 | % | ||
| Unknown | 7 | 1.3 | % | ||
| Missing | 321 | 60.6 | % | ||
| 2nd dose of vaccine | |||||
| - During pregnancy | 456 | 86.0 | % | ||
| Trimester of injection | |||||
| Peri-conception (7 days before LMP to 13 days after LMP) | 0 | - | |||
| T1 - 14 days after LMP and <12 wks | 0 | - | |||
| T2 - ≥12 and <28 wks | 174 | 32.8 | % | ||
| T3 - ≥28 wks | 282 | 53.2 | % | ||
| Place of vaccination | |||||
| Vaccination centre / Health authority | 154 | 29.1 | % | ||
| Gynaecologist / Midwife consultation (PRIVATE PRACTICE) | 1 | 0.2 | |||
| Gynaecologist / Midwife consultation (HOSPITAL) | 8 | 1.5 | % | ||
| GP (General practitioner) | 4 | 0.8 | % | ||
| Occupational health service (at work) | 6 | 1.1 | % | ||
| Pharmacist | 7 | 1.3 | % | ||
| Unknown | 3 | 0.6 | % | ||
| Missing | 273 | 51.5 | % | ||
| Injection site | |||||
| Left arm | 153 | 28.9 | % | ||
| Right arm | 33 | 6.2 | % | ||
| Missing | 298 | 56.2 | % | ||
| Antipyretic intake around injection | |||||
| Yes | 31 | 5.8 | % | ||
| No | 168 | 31.7 | % | ||
| Unknown | 6 | 1.1 | % | ||
| Missing | 279 | 52.6 | % | ||
T1-3: trimester 1-3.
LMP: last menstrual period.
wks: weeks of gestation.
Table 6.
Pregnancy and neonatal outcomes among pregnant women exposed to COVID-19 mRNA vaccine before 37 wks and ending their pregnancy after 24 wks (population 2c).
| PRE-TERM BIRTH ANALYSIS | n | % |
95% CI |
||||
|---|---|---|---|---|---|---|---|
| Pregnant women | 513 | ||||||
| Twin pregnancies | 5 | ||||||
| Number of foetuses | 518 | ||||||
| DELIVERY | |||||||
| Vaginal delivery | 354 | 69.0 | % | 64.8 | - | 73.0 | |
| Spontaneous | 291 | 56.7 | % | 52.3 | - | 61.1 | |
| Assisted (forceps, vacuum) | 59 | 11.5 | % | 8.9 | - | 14.6 | |
| Unknown | 4 | 0.8 | % | 0.2 | - | 2.0 | |
| Caesarean section | 154 | 30.0 | % | 26.1 | - | 34.2 | |
| Unknown | 5 | 1.0 | % | 0.3 | - | 2.3 | |
| PRE-TERM BIRTH (among pregnant women) | |||||||
| Preterm <37 wks | 33 | 6.4 | % | 4.5 | - | 8.9 | |
| Iatrogenic preterm birth | 19 | 3.7 | % | 2.2 | - | 5.7 | |
| Spontaneous preterm birth | 12 | 2.3 | % | 1.2 | - | 4.1 | |
| Unknown | 2 | 0.4 | % | 0.0 | - | 1.4 | |
| PREGNANCY OUTCOMES | |||||||
| Stillbirth (Fetal demise ≥ 20 wks) | 0 | - | |||||
| Livebirth | 518 | 100 | % | ||||
| GA at delivery (in wks) - median (IQR) | 39 wks | (38-40) | |||||
| NEONATAL OUTCOMES (among livebirth infants) | |||||||
| - Small for gestational age* | 21 | 4.1 | % | 2.5 | - | 6.1 | |
| - NICU admission (any cause)⁎⁎ | 25 | 4.8 | % | 3.1 | - | 7.0 | |
| NICU admission for prematurity | 13 | 2.5 | % | 1.3 | - | 4.3 | |
| NICU admission for respiratory distress | 6 | 1.2 | % | 0.4 | - | 2.5 | |
| NICU admission for sepsis | 1 | 0.2 | % | 0.0 | - | 1.1 | |
| NICU admission for other cause | 6 | 1.2 | % | 0.4 | - | 2.5 | |
| - Neonatal death | 0 | - | |||||
<10th percentile for gestational age according to INTERGROWTH 21.
GA: gestational age.
wks: weeks of gestation.
NICU: neonatal Intensive Care Unit.
reason for NICU admission can be multiple.
Pregnancy and neonatal outcomes
Of 1012 patients, 530 patients had a pregnancy outcome available with a gestational age of at least 20 wks and were included in this analysis (Figure 1). Patient baseline characteristics (population 2d) are presented in Table 7. The majority (42·3% - n = 224) of patients were 30 to 34 y of age, 20·4% (n = 108) had a medical comorbidity, 41·5% (n = 220) were nulliparous, 0·9% (n = 5) had an ongoing twin pregnancy, and 14·5% (n = 77) had at least one previous caesarean section. Pfizer–BioNTech BNT162b2 vaccine was given to 28·3% (n = 150) of pregnant woman and Moderna mRNA-1273 vaccine to 71·7% (n = 380). A total of 46 (8·7%) patients did not receive a second dose, 28 (5·8%) had a second injection within six weeks after the end of the pregnancy, and 456 (86·0%) had a second injection during pregnancy. Most patients had a vaginal birth, including 302 (57·0%) spontaneous and 61 (11·5%) assisted, and 158 (29·8%) patients had a caesarean section. No stillbirths nor non-viable births were reported and all 530 patients delivered liveborn infants including five twin pregnancies. Of these 535 new-borns, 21 (3·9%; 95%CI [2·4-5·9]) were small for gestational age. A total of 25 (4·7%; 95%CI [3·0-6·8]) neonates were admitted to NICU, including 13 (2·4%; 95%CI [1·3-4·1] for prematurity, 6 (1·1% 95%CI [0·4-2·4]) for respiratory distress syndrome, 1 (0·2% 95%CI [0·0-1·0]) for sepsis, and 6 (1·1% 95%CI [0·4-2·4]) for other reasons. No neonatal death was recorded (Table 8).
Table 8.
Pregnancy and neonatal outcomes among pregnant women exposed to COVID-19 mRNA vaccine and ending their pregnancy during pregnancy after 20 wks (population 2d).
| Pregnancy outcomes analysis | n | % | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Pregnant women | 530 | ||||||
| Twin pregnancies | 5 | ||||||
| Number of fetuses | 535 | ||||||
| DELIVERY | |||||||
| Vaginal delivery | 367 | 69.2 | % | 65.1 | - | 73.2 | |
| Spontaneous | 302 | 57.0 | % | 52.6 | - | 61.2 | |
| Assisted (forceps, vacuum) | 61 | 11.5 | % | 8.9 | - | 14.5 | |
| Unknown | 4 | 0.8 | % | 0.2 | - | 1.9 | |
| Caesarean section | 158 | 29.8 | % | 25.9 | - | 33.9 | |
| Unknown | 5 | 0.9 | % | 0.3 | - | 2.2 | |
| PREGNANCY OUTCOMES | |||||||
| Stillbirth (Fetal demise ≥ 20 wks) | 0 | - | |||||
| Pre-viable fetus (≥ 20 and <24 wks) | 0 | - | |||||
| Livebirth | 535 | 100 | % | ||||
| GA at delivery (in wks) - median (IQR) | 39 wks | (38-40) | |||||
| NEONATAL OUTCOMES (among livebirth infants) | |||||||
| - Small for gestational age* | 21 | 3.9 | % | 2.4 | - | 5.9 | |
| - NICU admission (any cause)⁎⁎ | 25 | 4.7 | % | 3.0 | - | 6.8 | |
| NICU admission for prematurity | 13 | 2.4 | % | 1.3 | - | 4.1 | |
| NICU admission for respiratory distress | 6 | 1.1 | % | 0.4 | - | 2.4 | |
| NICU admission for sepsis | 1 | 0.2 | % | 0.0 | - | 1.0 | |
| NICU admission for other cause | 6 | 1.1 | % | 0.4 | - | 2.4 | |
| - Neonatal death | 0 | - |
<10th percentile for gestational age according to INTERGROWTH 21.
GA: gestational age.
wks: weeks of gestation.
NICU: neonatal Intensive Care Unit.
Reason for NICU admission can be multiple.
Discussion
In this prospective cohort of 1012 pregnant patients from the Swiss COVI-PREG registry25, we identified that pregnant women were vaccinated against COVID-19 throughout pregnancy but preferentially after 12 wks as recommended by the Swiss Society of Gynaecology and Obstetrics.23 Most of the women (88·3%) received two doses just before or during pregnancy.
Local and systemic reactions after vaccination were primarily pain at the injection site, fatigue, headache, and muscle pain, with a higher rate of systemic reaction occurring after the second dose of vaccine (67·3% vs 35·3% after the first dose), especially with Moderna mRNA-1273 vaccine, which is consistent with already published data in the general population and during pregnancy.5,6,12,28 Comparisons across vaccine types revealed that receipt of the second dose, Moderna mRNA-1273 vaccine, younger age, female sex, and having had COVID-19 before vaccination were associated with greater odds of adverse effects.29
Severe adverse events were rare, including venous thromboembolism, fever requiring hospitalization, and herpes zoster, which can occur following any vaccine injection. In the general population, COVID-19 mRNA vaccine does not appear to increase the risk of deep vein thrombosis.30 The risk of venous thromboembolism, however, is four times greater during pregnancy than in the non-pregnant population with an estimated incidence of 0·76 to 1·72 per 1000 and thus is expected to occur independently of vaccination.31 Herpes zoster has been mentioned as a possible complication of mRNA COVID-19 vaccination, but a recent study of more than one million patients vaccinated in the US showed a similar prevalence to historical cohorts.32 During pregnancy, herpes zoster remains a rare condition with no risk associated for the mother or her infant.33 The case of preterm birth resulted from PPROM followed by vaginal haemorrhage 48 hours later, leading to an emergency caesarean section, which is likely not secondary to the vaccine.
With respect to spontaneous abortion, our study showed very low rates of early and late spontaneous abortion of 0·9% and 0·4%, respectively. The only identified late spontaneous abortion occurred three weeks after the first vaccine dose at 16 wks in the context of chorioamnionitis. However these findings were constrained by limited outcome data on most of the pregnancies vaccinated before 14 wks (10/135; 7·4%) and before 20 weeks (96/398; 24·1%). Several studies have not shown an increased risk of spontaneous abortion following COVID-19 vaccination.13,34,35 Further studies once outcome data is obtained may confirm findings noted by other authors.
All infants delivered from mothers exposed to mRNA COVID-19 vaccine in pregnancy were liveborn. A low prevalence (6·4%; 95%CI [4·5-8·9]) of preterm birth before 37 wks was reported in this study. This is similar to the rates for the last four years in Switzerland ranging from 6·4 to 7·0% and in Europe ranging from 5·5 to 11·4%.36,37 A low rate of SGA (3·8%; 95%CI [2·3-5·8]) was reported using the INTERGROWTH 21 scale.27 NICU admission was also low (3·7%; 95%CI [2·9-6·7]) in our cohort, compared to a recently published rate of 6·3% in a cohort of neonates born after 35 wks in the US, which did not include very preterm births.38
These results are consistent with a recent US study stemming from a retrospective cohort of more than 40,000 pregnant women, where COVID-19 vaccine exposure was not associated with increased risk of preterm birth or small for gestational age at birth.39 Similarly, a nationwide Scottish study reported that despite the low prevalence of vaccination among pregnant women, it was safe and reduced maternal and perinatal complications associated with COVID-19.40
The prospective design of the study enabled exhaustive and precise collection of adverse events following vaccination directly from a nation-wide cohort of vaccine recipients. This study is limited, however, by a relatively small number of pregnant women at the time of analysis, making it difficult to assess rare events such as serious adverse events following vaccination or stillbirths, which would require several thousands of patients. Most women were exposed during the second and third trimesters, limiting the assessment of the vaccine impact on early pregnancy and embryogenesis. This could explain the very low rate of early spontaneous abortion. Our study was not designed to specifically target these early pregnancy outcomes as most of the enrolled patients were vaccinated after 12 wks according to national recommendations in Switzerland in 2021. Thus, the available population to estimate the rate of early spontaneous abortion may not appropriately represent the population at risk for spontaneous abortion. Spontaneous abortion incidence rates are sensitive to gestational age at enrollment as the risk decreases over gestation, later enrollees caring a lower risk or no risk of the outcome. Some selection biases may have lowered the spontaneous abortion risk estimates as well. Women may have not known that they were pregnant at the time of vaccination or had an early pregnancy loss and did not consult with a gynaecologist. Furthermore, patients that presented to a gynaecologist for the first time with a diagnosis of early spontaneous abortion, may have simply declined to participate in the study because their psychological state was not conducive to scientific research. Finally, this study does not provide a control group of non-vaccinated pregnant women for comparison, which could have given more strength to our results and allow for evaluation of association between exposure and outcomes. This study was also not designed to test the efficacy of the vaccine during pregnancy.
The results may not be representative of the general pregnant population as the COVID-19 mRNA vaccine was first offered to pregnant patients with comorbidities three months before being extended to all pregnant women in Switzerland. This led to the inclusion of more high-risk pregnancies with maternal comorbidities, as shown in Table 5. Because maternal comorbidities are associated with increased adverse pregnancy outcomes, our results may have overestimated the rate of adverse outcomes. As they were, however, not increased compared to the general population, this reinforces the evidence of safety of the mRNA vaccines observed in our cohort.
In conclusion, this prospective study has provided further evidence that mRNA vaccination against SARS-CoV-2 during pregnancy seems safe in terms of early adverse events, pregnancy, and neonatal outcomes, within the limitation of the information provided by a descriptive non-controlled study design. Exposure to mRNA vaccine anytime in pregnancy seemed not associated with higher adverse pregnancy or neonatal outcomes as compared to historical data. In addition, pregnant patients exposed to the vaccine before 37 wks seemed not at increased risk of preterm birth as compared to the data on neonatal health from the Swiss Federal statistical office. Long term outcomes, however, such as infant developmental outcomes were not within the available time frame of this study and would require further studies. Efforts must be made to continue to monitor the safety and efficacy of these already marketed mRNA COVID-19 vaccines in larger sample of pregnant women and appropriate control groups to provide risk estimates. The focus should be on first trimester exposure, rare adverse events and long-term outcomes (e.g. infant developmental outcomes).
Contributors
GF, EM, DB, and AP conceived and designed the study. GF, DB and AP were in charge of the funding acquisition and of the project administration. GF, EM, LP, and AP analysed and interpreted the data. GF, EM, DB, and AP drafted the manuscript. DB and AP provided supervision of the work. All authors (GF, EM, LP, UW, CD, BMT, DD, SC, MM, MTB, SS, IH, CM, BFT, SK, CB, JM, RZ, APR, DS, DB, AP) contributed to data collection, reviewed and edited the manuscript. All authors made a significant contribution in reviewing the manuscript drafting or revision and accepts accountability for the overall work. All authors approved the final version of the report.
Declaration of interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. Alice Panchaud received grants from the Swiss Federal Office of Public Health and the CHUV Foundation; she also received grants from Vifor, the European Medicine Agency (EMA/2017/09/PE and EMA/2017/09/PE/11), the Fonds Paritaire RBP IV and a H2020 grant (ConcePTION WP 3-4), outside the submitted work. Begoña Martinez de Tejada reported receiving financial support from the General Health Division in Geneva, Switzerland, and being a medical advisor for Effik consulting fees and lectures) and Pierre Fabre (consulting fees), outside the submitted work; she also reported having a research agreement for clinical devices with Pregnolia and having been paid as a legal expert in a malpractice case, outside the submitted work. All other authors declare no conflicts of interest
Acknowledgments
Data sharing statement
www.icmje.org/coi_disclosure.pdf Data are available through joint research agreements from the corresponding authors.
Acknowledgments
We thank every patient who agreed to participate in this study. We are very grateful to our collaborators (listed in Table S6) who recruited patients and collected data for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100410.
Appendix. Supplementary materials
References
- 1.Lokken EM, Taylor GG, Huebner EM, et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol. 2021;225(1) doi: 10.1016/j.ajog.2021.02.011. 75.e1-75.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vouga M, Favre G, Martinez-Perez O, et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep. 2021;11(1):13898. doi: 10.1038/s41598-021-92357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(47):1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchan SA, Chung H, Brown KA, et al. 2022. Effectiveness of COVID-19 Vaccines Against Omicron or Delta infection [Internet]https://www.medrxiv.org/content/10.1101/2021.12.30.21268565v1 [cited 2022 Jan 11]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. The Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study [Internet]. 2021 [cited 2022 Jan 11]. Available from: https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v3
- 10.Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 vaccine against Omicron Variant in South Africa. N Engl J Med. 2021;0(0) doi: 10.1056/NEJMc2119270. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dashraath P, Nielsen-Saines K, Madhi SA, Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020;396(10252):e22. doi: 10.1016/S0140-6736(20)31822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan JA, Biggio JR, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139(1):107–109. doi: 10.1097/AOG.0000000000004621. [DOI] [PubMed] [Google Scholar]
- 15.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 16.Goldshtein I, Nevo D, Steinberg DM, et al. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA. 2021;326(8):728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bookstein Peretz S, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450–456. doi: 10.1002/uog.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6) doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248–255. doi: 10.1111/1471-0528.16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2) doi: 10.1016/j.ajog.2021.08.007. 236.e1-236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. 2021;3(6) doi: 10.1016/j.ajogmf.2021.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SGGG - Recommandation of the Swiss Society of Gynecologists and Obstetricians on Vaccination with COVID-19 mRNA vaccine during pregnancy [Internet]. 2021 [cited 2021 Dec 15];Available from: https://www.sggg.ch/fr/nouvelles/detail/1/infection-a-coronavirus-covid-19-et-grossesse/
- 24.Stuckelberger S, Favre G, Ceulemans M, et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a cross-sectional study in Switzerland. Viruses. 2021;13(7):1199. doi: 10.3390/v13071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panchaud A, Favre G, Pomar L, Vouga M, Aebi-Popp K, Baud D. An international registry for emergent pathogens and pregnancy. Lancet. 2020;395(10235):1483–1484. doi: 10.1016/S0140-6736(20)30981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 27.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. 2018;218(2S):S630–S640. doi: 10.1016/j.ajog.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houghton DE, Padmanabhan A, Ashrani AA, et al. Deep vein thrombosis after COVID-19 vaccinations. Blood. 2021;138:291. [Google Scholar]
- 31.Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359(19):2025–2033. doi: 10.1056/NEJMra0707993. [DOI] [PubMed] [Google Scholar]
- 32.Birabaharan M, Kaelber DC, Karris MY. Risk of herpes zoster reactivation after messenger RNA COVID-19 vaccination: a cohort study. J Am Acad Dermatol. 2021;S0190-9622(21) doi: 10.1016/j.jaad.2021.11.025. 02892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343(8912):1548–1551. doi: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 34.Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326(16):1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Office Fédérale de la Statistique Suisse (Swiss Federal Statistical Office). Santé des nouveau-nés [Internet]. [cited 2022 Feb 25];Available from: https://www.bfs.admin.ch/bfs/fr/home/statistiken/gesundheit/gesundheitszustand/gesundheit-neugeborene.html
- 37.Zeitlin J, Mohangoo A, Cuttini M. The European Perinatal Health Report: comparing the health and care of pregnant women and newborn babies in Europe. J Epidemiol Commun Health. 2009;63(9):681–682. doi: 10.1136/jech.2009.087296. [DOI] [PubMed] [Google Scholar]
- 38.Haidari ES, Lee HC, Illuzzi JL, Phibbs CS, Lin H, Xu X. Hospital variation in admissions to neonatal intensive care units by diagnosis severity and category. J Perinatol. 2021;41(3):468–477. doi: 10.1038/s41372-020-00775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth - eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022 doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.