Abstract
Preclinical data indicate that selective kappa opioid receptor antagonists reduce nicotine self-administration and withdrawal symptoms. The aim of the current study was to determine whether treatment with CERC-501, an orally available, potent, and selective kappa opioid receptor antagonist, could alleviate nicotine withdrawal and craving and mitigate mood alterations associated with nicotine withdrawal in humans. Healthy, adult cigarette smokers were enrolled into this randomized, multisite, double-blind, placebo-controlled, crossover study. Participants completed two 8-day treatment phases during which they received either CERC-501 (15 mg, p.o., once daily) or placebo. On the seventh day of each dosing phase, participants were admitted as inpatients for an 18-hour cigarette abstinence period followed by experimental testing. The primary outcome measures were (a) performance on the McKee Smoking Lapse test (ie, latency to smoke in exchange for money) and (b) number of cigarettes self-administered during a 60-minute ad lib smoking period. Other outcomes included measures of craving, mood, anxiety, nicotine withdrawal, and subjective effects of cigarette smoking. A total of 71 participants who smoked an average of approximately 23 cigarettes per day were enrolled, and 56 subjects completed the study. CERC-501 was well tolerated, but it did not significantly alter the latency to start smoking (CERC-501: 16.5 min vs placebo: 17.7 min) or the number of cigarettes smoked (CERC-501: 3.3 cigarettes vs placebo: 3.1 cigarettes). Compared with placebo, CERC-501 also did not affect cigarette craving, mood, anxiety, nicotine withdrawal, or subjective effects of smoking. These findings do not support a role for CERC-501 in the treatment of nicotine use disorder.
Keywords: CERC-501, kappa opioid receptor, nicotine withdrawal, smoking cessation
1 |. INTRODUCTION
Approximately 40 million people are active tobacco cigarette smokers in the United States, and 1.1 billion people smoke cigarettes worldwide (World Health Organization, 2016).1,2 Despite five forms of nicotine replacement therapy and two additional FDA-approved pharmacotherapies, the likelihood of long-term abstinence from smoking remains low.3,4 In clinical trials, continuous abstinence rates at 9 to 12 weeks for the most efficacious pharmacotherapy, varenicline, are between 44% and 56%.5 Long-term abstinence rates for smoking cessation treatments are even poorer; therefore, there is a clear need for more efficacious pharmacological treatments for nicotine dependence.6
Stress and negative mood contribute to relapse to nicotine use and drug use in general.7 Cigarette smoking often provides relief from stress, as well as from anxiety and mood lability during cigarette withdrawal, thus leading to relapse during abstinence.8 A pharmacological therapy that blocks this cycle could aid in prevention of relapse to nicotine-seeking behavior. Kappa opioid receptors (KORs) and the endogenous kappa ligand, dynorphin, are localized in areas of the brain that affect stress and are believed to play a key role in mood, reward, and substance use disorders.9–11 Chronic stress, substance use, and acute withdrawal lead to increased dynorphin expression, activating kappa receptors and downstream signaling pathways, which inhibit tonic dopamine tone, leading to a negative affective state.12–15 Selective antagonism of KORs may provide therapeutic benefit in the treatment of substance use disorders by blocking aversive signaling cascades in the brain triggered by chronic psychological or physical stress.
Preclinical data indicate that selective KOR antagonists produce anxiolytic and antidepressant effects in rodent models.16–18 Additionally, KOR antagonists have been shown to decrease the self-administration of and withdrawal symptoms from nicotine, alcohol, and cocaine.19–23 CERC-501 (previously known as LY2456302) is an orally bioavailable, high-affinity, selective KOR antagonist.24 CERC-501 was studied in two preclinical studies to determine whether it could reduce signs of (a) spontaneous withdrawal from nicotine and (b) nicotine withdrawal induced pharmacologically by the nicotine antagonist mecamylamine. CERC-501 (10 mg/kg) significantly reduced signs of spontaneous nicotine withdrawal.25 In a model of precipitated nicotine withdrawal, CERC-501 (1, 3, and 10 mg/kg) decreased anxiety-like behavior and somatic signs of withdrawal.25
Prior to the current study, 73 healthy human volunteers had been exposed to single or multiple doses of CERC-501 to assess safety and tolerability. In these studies, there were no serious adverse events (AEs) (CERC-501/LY2456302 Investigator’s Brochure). In order to follow up on the promising preclinical findings and assess the treatment potential of CERC-501 for nicotine dependence in humans, a proof-of-concept, phase 2 clinical laboratory study is the logical progression to follow the safety and pharmacokinetic assays. The current study is the first clinical laboratory assessment of the ability of CERC-501 to affect nicotine withdrawal and smoking behavior. In this randomized, multisite, cross-over, double-blind, placebo-controlled trial, the effects of CERC-501 on nicotine self-administration after 18 hours of nicotine abstinence, as well as craving, positive and negative mood, and withdrawal symptoms were evaluated.
2 |. MATERIALS AND METHODS
2.1 |. Participant recruitment and selection
Cigarette smokers who were not seeking smoking cessation treatment were recruited using advertisements. After the initial telephone screening, those meeting preliminary study criteria were scheduled for in-person screening at one of the study’s three sites: New York State Psychiatric Institute (NYSPI), University of Kentucky, or Vince and Associates. Throughout the trial, a third party was contracted to provide data monitoring for all sites. Prior to any study procedures being performed, informed consent was obtained and documented on site-specific Institutional Review Board (IRB) approved forms. Screening consisted of clinical assessments of mental health and drug use, clinical laboratory tests (blood chemistry, hematology, urinalysis, H. Pylori, HIV test, and electrocardiogram [ECG]), and a physical examination. Inclusion criteria included that males and females aged 18 to 60 years inclusively who were either not of child bearing potential or agreed to use double-barrier methods of contraception, smoking greater than or equal to 15 cigarettes per day on average for the past 6 months, smoking within approximately 5 minutes of waking (on average), positive urine dip test for cotinine, and a Fagerstrom Scale score ≥ 5,26 mentally and physically healthy, and no regular use of drugs other than nicotine or caffeine. Key exclusions for enrollment included recent use of smokeless tobacco or other nicotine-containing products other than cigarettes (eg, e-cigarettes/vaping devices), any substance use disorder other than nicotine or caffeine, recent active or history of gastric disease, active comorbid disease that could interfere with study conduct, or use of psychoactive medications, proton pump inhibitors, or histamine 2 blockers. Study procedures were approved by the Institutional Review Board (IRB) of each respective site, and all study volunteers gave written informed consent (ClinicalTrials.gov Identifier: NCT02641028). The study adhered to the 1964 Declaration of Helsinki and its later amendments.
2.2 |. CERC-501 treatment
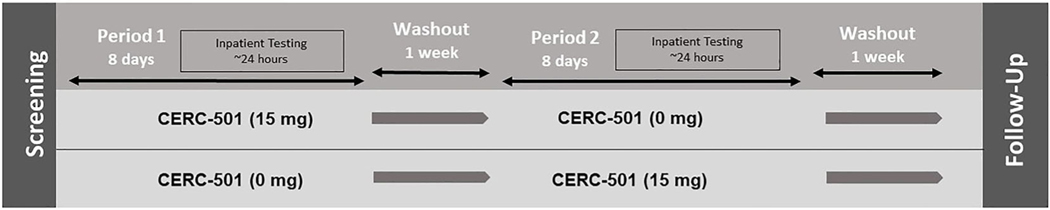
Eligible participants were randomized to one of two maintenance conditions: CERC-501 (15 mg) → Placebo or Placebo → CERC-501 (15 mg). A block randomization based on sex/gender ensured equal allocation of men and women for each condition order. There was an intervening washout period of 7(+3) days between the active and placebo dosing conditions; greater than five half-lives of CERC-501 (30 hours; Figure 1).
FIGURE 1.
Schema of the study design
The active dose of CERC-501 was chosen based on results from a human positron emission tomography study demonstrating that a single oral dose of 10 mg CERC-501 almost completely saturated KORs at 2.5 hours postdose with sustained target engagement (greater than 72%) observed for at least 22.5 hours.27 On week days, participants received their doses under supervision of the study site to enhance adherence. On weekends, holidays, or when necessary, participants were required to provide a “selfie” showing the tablets in their mouth and record the time of dosing in an electronic diary. After the trial was initiated, participants could rely more heavily on providing selfies as proof of medication adherence if they had scheduling conflicts on weekdays. Blinded study medication (active and matched placebo) was provided by Cerecor Inc. Concomitant medications and AEs were recorded throughout the trial. A treatment-emergent AE (TEAE) was defined as any new AE or existing AE worsening in severity after the study drug administration. A serious AE (SAE) was defined as any AE that resulted in death, was considered life-threatening, and/or required in-patient hospitalization.
2.3 |. Laboratory testing
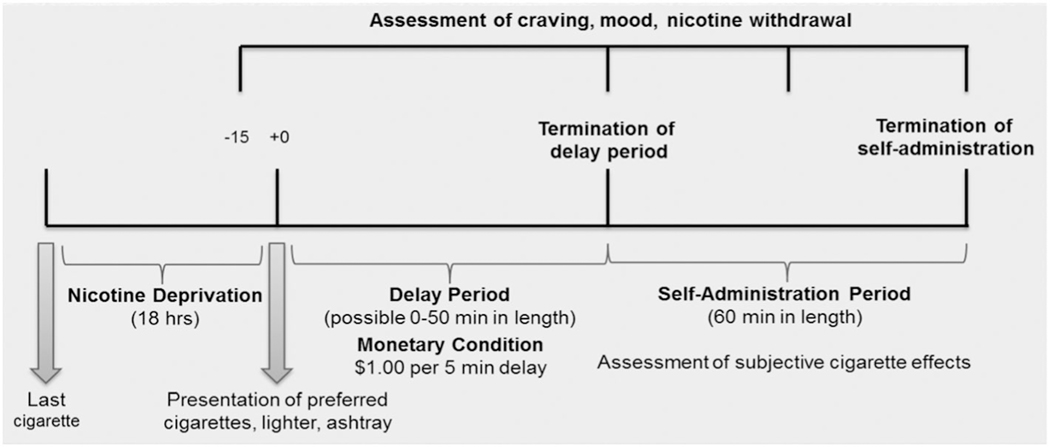
On Day 7, participants were admitted into a clinical unit on the afternoon or evening for an overnight stay. Participants could smoke ad libitum (ad lib) until 22:00 hours after which smoking was not allowed. Overnight smoking abstinence was confirmed by a reduction in CO concentration of greater than or equal to 50%. Participants received their assigned study medication at 10:00 hours in the following day, and at 16:00 hours (nicotine abstinence approximately 18 h) participants completed the McKee Smoking Lapse Test.28
The McKee test consisted of a delay period and a smoking self-administration period (Figure 2). During the delay period (up to 50 min), participants were presented with a tray with eight cigarettes of their preferred brand, a lighter, and ashtray. They were instructed that they could begin smoking at any time, but they would earn $1.00 (U.S.) for each 5-minute period that they delayed smoking ($10 total). Mood, tobacco craving, anxiety, and nicotine withdrawal were assessed when participants decided to terminate the delay period but before they began smoking. Outcome measures from the delay period included latency to initiate smoking (0-50 min), Minnesota Nicotine Withdrawal Scale (MNWS: 0-3229;), Tiffany Questionnaire of Smoking Urges-Brief (QSU-Brief: 0-7030;), modified Circumplex Scale: negative affective states (0-40031;), and the clinically useful depression outcome scale-anxious distress specifier subscale (CUDOS-A-SR: 0-2032;). After participants decided to smoke or after the delay period ended (if they were able to abstain the full 50 minutes), they could smoke their preferred brand of cigarettes ad lib for the next 60 minutes. Outcome measures from the cigarette self-administration period included the total number of cigarettes smoked (0-8) and the modified Cigarette Evaluation Questionnaire (mCEQ33;).
FIGURE 2.
Design and timeline of laboratory sessions
2.4 |. CERC-501 plasma concentration
CERC-501 plasma concentrations were assessed from samples drawn once on the final day of each treatment period (2 h after the last dose). Following storage at −60 to −80°C, plasma samples were analyzed by Covance Bioanalytical Services LLC using liquid chromatography tandem mass spectrometry (LC-MS/MS) using a validated method in accordance with Covance standard operating procedures. The calibration standard data, quality control sample data, incurred sample reproducibility data, and chromatograms indicated that the method performed acceptably.
2.5 |. Discharge, follow-up, and compensation
Participants were discharged at approximately 18:00 hours. Following discharge from their second testing period, participants returned to the study site within 7 and 10 days for a safety follow-up visit. The follow-up visit included: AE assessment, physical examination, ECG, vital signs, fasted clinical laboratory testing, suicidality assessment, and drug and pregnancy testing. Participants received compensation for all study visits and a bonus payment for completing all study procedures.
2.6 |. Statistical analyses
Power analyses were conducted using estimates of the magnitude of the medication effect from McKee et al.28 This study employed the McKee Smoking Lapse Test to examine the effects of varenicline and bupropion (vs placebo) on smoking behavior. Based on these data, a Cohen’s D effect size of 0.68 was calculated for latency to start smoking. A total sample size of 60 completers provided >90% power to detect a mean difference of 19 minutes in latency to begin smoking (0-50 min) and one cigarette smoked (0-8 cigarettes), between the CERC-501 and placebo treatment conditions (α of.05).
Outcome measures were analyzed using an analysis of covariance (ANCOVA) model with treatment (placebo, CERC-501) and sequence (placebo or CERC-501 first) as fixed factors, and baseline characteristics (eg, # cigarettes/day, gender) as covariates. Measurements collected at multiple times within each treatment period (eg, mood scales) were analyzed in the same manner, with study period (time) added to the model. In some cases, paired t tests were used for planned comparisons. Analyses were conducted on all subjects who had at least one postbaseline efficacy evaluation from the testing session. All hypothesis tests were two-sided, and the significance level was set at P < .05.
3 |. RESULTS
3.1 |. Participants and CERC-501 tolerability
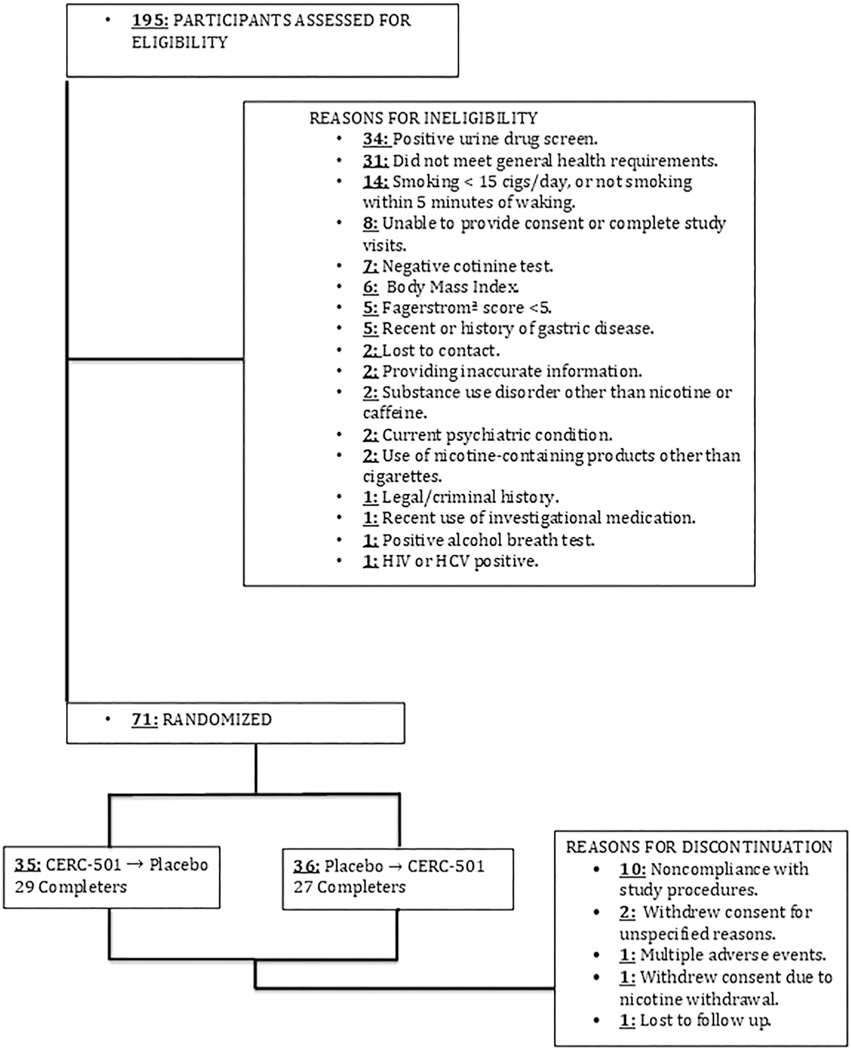
A total of 195 potential subjects were screened across the three study sites. Participants (N = 71) were randomized with 35 subjects being assigned to CERC-501 → Placebo (29 completers), and 36 assigned to Placebo → CERC-501 (27 completers) for a completion rate of 79% (Table 1). Of the 71 participants randomized, 15 were discontinued by the investigators, or withdrew consent (refer to Figure 3 for a subject disposition flow chart).
TABLE 1.
Participant demographics (N = 71)
| Randomization sequence (N) | CERC-501 → Placebo 35 | Placebo → CERC-501 36 |
|---|---|---|
|
| ||
| Age years (SD) | 45 (10) | 39 (12) |
|
| ||
| Female/male (N) |
19/16 | 19/17 |
|
| ||
| Race (N) |
24 White or Caucasian 9 Black or African-American |
27 White or Caucasian 8 Black or African-American |
| 1 Native-American or Alaska Native 1 not disclosed |
1 Native Hawaiian or other Pacific Islander | |
|
| ||
| Ethnicity (N) |
33 Not Hispanic/Latino 2 Hispanic/Latino |
35 Not Hispanic/Latino 1 Hispanic/Latino |
|
| ||
| Cigarettes smoked/day mean # (SD) | 22 (6) | 24 (10) |
|
| ||
| Baseline Fagerstrom score (SD) | 7.2 (1.5) | 7.1 (1.1) |
FIGURE 3.
CONSORT diagram of participants from screening to completion
Of 71 subjects randomized, 62 (87%) received at least one dose of CERC-501 (15 mg) and 67 (94%) received at least one dose of placebo. CERC 501 was well tolerated. While under active CERC-501 maintenance, TEAEs that occurred in greater than 5% of subjects were headache, diarrhea, upper respiratory tract infection, decreased appetite, and leukocytosis. The frequency of TEAEs was similar between CERC-501 (52%) and placebo conditions (54%). All TEAE’s were classified as either mild or moderate in severity. No serious AEs were reported.
3.2 |. Effects of CERC-501 on smoking behavior
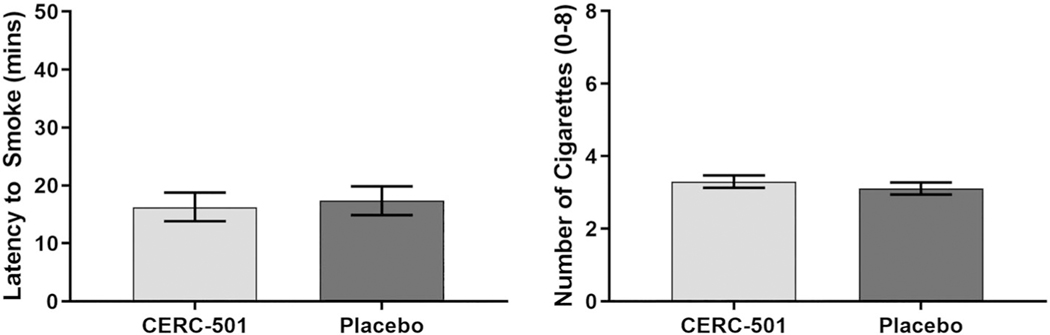
The primary endpoints were latency to smoke after the 18-hour deprivation period and number of cigarettes smoked during the self-administration period (expressed below as least square [LS] means). Treatment with CERC-501 did not produce any changes in either primary endpoint. During active CERC-501 treatment, the mean latency to smoke was 16 minutes and 28 seconds (±2 min 48 s), and during placebo treatment, the mean latency was 17 minutes and 39 seconds (± 2 min 48 s; .64) (Figure 4). The number of cigarettes self-administered during active CERC-501 treatment (3.3 ± 0.17) did not differ from placebo (3.1 ± 0.17; .19). Of the $10 available during the delay period, participants eared $3.30 while on active CERC-501 and $3.54 on placebo. There was no significant effect of sequence (CERC-501 → placebo: mean latency to smoke [17 min 7 s], # cigarettes [3.1] vs placebo → CERC-501: mean latency to smoke [17 min 30 s], # cigarettes [3.3]).
FIGURE 4.
Mean time (minutes + SE) participants (N = 58) chose to delay smoking in exchange for money ($1 for every 5 min) following 18 hours of nicotine deprivation (left panel). Mean number of cigarettes (+SE) participants chose to smoke during the 60-minute self-administration period following 18 hours of nicotine deprivation (right panel)
3.3 |. Effects of CERC-501 on craving, withdrawal, and mood
Secondary efficacy endpoint analyses included cigarette/nicotine craving (QSU), withdrawal (MNWS), anxious/distress (CUDOS-A-SR), and negative mood (Circumplex). These outcomes were assessed upon admission to the inpatient unit (baseline), after the 18-hour nicotine deprivation period, and during the McKee Test, at the end of the cigarette self-administration period. From baseline, negative mood and craving increased during nicotine deprivation. Following the cigarette self-administration period, scores significantly deceased (Table 2). Mean scores at each assessment point did not significantly vary between CERC-501 and placebo conditions. In addition, CERC-501 had no effect on another secondary efficacy endpoint, the mCEQ (CERC-501 = 39.89 [1.70]; Placebo = 38.77 [1.69]), which evaluated the subjective effects of smoking (measured at last cigarette prior to nicotine deprivation, first cigarette following deprivation period, and at the end of the self-administration period). No significant effects of sequence (ie, CERC-501 → placebo vs placebo → CERC-501) or study site were found on any of the aforementioned outcome measures.
TABLE 2.
Effects on Mood, Craving and Withdrawal
| Upon Admission (Baseline) | 18-hour Abstinent | After Cigarette Self-Administration | ||||
|---|---|---|---|---|---|---|
| CERC-501 mean (SD) | Placebo mean (SD) | CERC-501 mean (SD) | Placebo mean (SD) | CERC-501 mean (SD) | Placebo mean (SD) | |
| Circumplex scale (total negative mood score: 0-400) | 88.1 (53.8) | 82.1 (58.2) | 115* (76.4) | 108* (70.6) | 83.5 (66.1) | 79.7 (56.4) |
| CUDOS-A-SR (anxious/distress subscale: 0-20) | 1.8 (2.7) | 1.9 (2.8) | 2.1 (2.9) | 2.9 (3.7) | 1.3 (2.1) | 1.9 (2.7) |
| Minnesota Nicotine Withdrawal Scale (0-32) | 4.6 (4.0) | 4.5 (4.1) | 8.2* (6.7) | 7.3* (6.5) | 2.9* (4.4) | 2.7* (3.9) |
| Questionnaire of smoking urges (0-70) | 33.0 (14.8) | 31.9 (13.7) | 44.9** (15.5) | 42.8** (17.7) | 17.1** (9.8) | 17.3** (9.3) |
Note. No significant differences between CERC-501 and placebo conditions were found.
Significant at P < .05.
Significant at P < .01.
3.4 |. Pharmacokinetics of CERC-501
Plasma samples were collected on the eighth consecutive day of study drug administration, 2 hours after the final dose of study drug for that sequence (CERC-501 or placebo). Mean plasma concentration of CERC-501 was 45.22 ± 15.87 ng/mL. Plasma levels of CERC-501 with placebo treatment were below the limit of quantitation (LOQ = 0.200 ng/mL) with the exception of four participants. These four participants all received active medication prior to placebo treatment (mean = 0.308 ± 0.076 ng/mL: range: 0.259-0.421). Data from these four subjects were included in the primary outcome analyses.
4 |. DISCUSSION
This is the first clinical study to assess the effects of repeated administration of CERC-501, a KOR antagonist, on nicotine self-administration and withdrawal. CERC-501 failed to significantly alter any smoking-related outcomes. CERC-501 had no effects on mood and craving induced by 18 hours of abstinence from nicotine. During the laboratory sessions that followed this abstinence period, the behavioral outcomes (latency to smoke, cigarette self-administration) did not differ between the CERC-501 and placebo treatment groups. CERC-501 also failed to alter the subjective effects of cigarettes smoked during the testing session. These negative finding do not support the utility of CERC-501 for the treatment of nicotine/tobacco use disorders.
The findings of the current study are inconsistent with preclinical studies that suggest a role for KOR antagonists in the treatment of tobacco use.17,22,25 Why a robust effect was observed in preclinical models, and not here, remains unknown. Negative findings in clinical trials, despite robust preclinical results, is common within drug discovery.34,35 A retrospective futility analysis was conducted to support our lack of a treatment effect. The futility analysis suggested that a significant CERC-501 effect was unlikely to be observed with a larger sample size.36
The testing model employed in the current study is an established human laboratory model of stress-precipitated smoking-lapse behavior that has (a) been used as a validated proof-of-concept design to bridge preclinical studies and clinical trials and (b) is sensitive to medications with known efficacy (eg, bupropion28,37–39;). The current study also employed a design that assessed acute and chronic dosing to maximize the chances of observing a medication effect. Based on previous human studies, at the observed plasma level, the active dose of CERC-501 should have resulted in saturation of KORs.27 The behavioral and reinforcing effects of nicotine may have been too great to be altered among the current sample with moderate nicotine dependence. A medication signal may have been easier to detect had a sample of lighter tobacco users been employed. The use of a nontreatment seeking sample may also have made a medication signal difficult to detect, as there is evidence that intrinsic quit motivation impacts sensitivity and validity of clinical laboratory models.40,41
Medication tolerability or pharmacokinetics were unlikely to be factors in the negative findings. The study was designed to ensure that peak plasma concentrations of CERC-501 would occur during the test session. Additionally, based on previous studies, medication levels should have been near steady state following the sixth day of CERC-501 treatment.42 Interestingly, pharmacokinetic analyses revealed that four subjects, who first completed active CERC-501 maintenance, did not completely washout, prior to the second testing session. This small number of individuals with negligible CERC-501 levels (only slightly above the limit of detection) are unlikely to have altered the study findings. However, the factors responsible for this individual variability in drug metabolism may be important to examine for future clinical investigation.
Finally, our lack of significant CERC-501 effects cannot be attributed to divergence from the study procedures. Participants with a protocol deviation that had the potential to affect medication efficacy evaluation were excluded from this analysis. Therefore, given the sound design of this trial and our adherence to the protocol, we must consider the possibility that kappa antagonism alone may not produce an effect sufficiently robust to alter nicotine withdrawal severity or smoking behavior in humans.
The primary limitation of this study is its reliance on a single active dose of CERC-501. The steady state plasma exposure levels we found exceed (3-4×) the Cmax that saturated KORs in previous studies.27 The maintenance of this degree of receptor saturation/blockade may have had unexpected biological effects. Chronic, robust antagonism could have resulted in KOR receptor upregulation that mitigated any putative therapeutic effect of acute CERC-501 antagonism. Testing a range of CERC-501 doses would have allowed the investigators to further assess this possibility. Compliance with study medication is also a minor concern. Doses taken outside of the lab (eg, weekends and holidays) were monitored through the use of photographs of the medication in the subjects’ mouth, but actual ingestion cannot be confirmed. A final limitation of our study is that smoking topography (eg, number of puffs smoked and inter-puff interval) was not measured because participants were required to smoke the entire cigarette within a fixed amount of time. These endpoints were not included because the requirement to smoke within a prespecified time period might have altered participants’ normal smoking patterns.
Concerning the lack of CERC-501 effects on mood, it should be noted that the current design only assesses medication effects upon withdrawal-related stress that may not be predictive of the effects of KOR antagonism on other forms of stress that reinforce smoking. The clinical utility of kappa-acting medications for the treatment of mood and substance use disorders has been supported by so called “virtual kappa antagonists,” produced by combining buprenorphine, a mu partial agonist/kappa antagonist with a potent mu antagonist. Virtual kappa antagonists have shown modest promise for the treatment of depression and modest positive effects on secondary outcomes in a trial of treatment for cocaine use.43–45 However, there is recent in vitro research that casts doubt on the interpretation of these studies of buprenorphine as a kappa antagonist.46
Nonetheless, the data from the current study do not support a potential role for CERC-501 in the treatment of tobacco use disorder. Although CERC-501 failed to alter the behavioral and subjective measures under the current parameters, other human studies have also shown the tolerability of repeated CERC-501 administration in patients with cocaine use disorder.47 Therefore, future clinical studies may have more success replicating the promising preclinical findings with alcohol and cocaine.19,21
ACKNOWLEDGMENTS
The research team would like to thank the study participants, along with the support staff who helped ensure the safe and successful completion of this study.
Funding information
National Institute on Drug Abuse, Grant/Award Number: R01DA040976; Cerecor Inc
FUNDING AND ROLE OF THE STUDY SPONSOR
This study was funded by Cerecor Inc and by National Institute on Drug Abuse (NIDA) grant R01DA040976 to Drs Blake Paterson (PI, former CEO of Cerecor Inc.), Sandra Comer (co-PI), and Sharon Walsh (co-PI). NIDA played no role in the data analysis or publication of this manuscript. Cerecor contributed to the design of this study as well as preparation, review, and approval of the manuscript. Data analyses were performed by a third party (Algorithme Pharma Inc.); however, Drs Comer and Walsh were provided with full access to all study data.
Footnotes
CONFLICT OF INTERESTS
Drs Marcus, Fraser, and Paterson are/were employees of Cerecor Inc. Currently, Dr Marcus is an employee of Supernus Pharmaceuticals and Dr Patterson an employee of NRZ Consulting. Drs Comer, Walsh, Lofwall, Babalonis, Jones, Kelsh, and Vince received research contract support from Cerecor to complete the study. The authors have no other competing financial interests in relation to the work described.
REFERENCES
- 1.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. Morb Mortal Wkly Rep.2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 2.United NationsOffice on Drugs and Crime, World Drug Report. (United Nations publication, Sales No. E.16. XI. 7). 2016.
- 3.Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer Clin. 2005;55(5):281–299. [DOI] [PubMed] [Google Scholar]
- 4.Jain R, Majumder P, Gupta T. Pharmacological intervention of nicotine dependence. Biomed Res Int. 2013;2013:1–8. 10.1155/2013/278392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur K, Kaushal S, Chopra SC. Varenicline for smoking cessation: A review of the literature. Curr Ther Res. 2009;70(1):35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;363:2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2(1):19–37. [DOI] [PubMed] [Google Scholar]
- 8.Paolini M, De Biasi M. Mechanistic insights into nicotine withdrawal. Biochem Pharmacol. 2011;82(8):996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid systemas a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoll AT, Carlezon WA Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schank JR, Goldstein AL, Rowe KE, et al. The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety.Addict Biol. 2012;17(3):634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivell B, Uzelac Z, Sundaramurthy S, et al. A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology. 2014;86:228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: from addiction to depression, and back. Front Psych. 2014;5:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116(2):306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van’t Veer A, Carlezon WA Jr. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl). 2013;229(3): 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35(3):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mague SD, Pliakas AM, Todtenkopf MS, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305(1):323–330. [DOI] [PubMed] [Google Scholar]
- 18.Valenza M, Butelman ER, Kreek MJ. Effects of the novel relativelyshort-acting kappa opioid receptor antagonist LY2444296 in behaviors observed after chronic extended-access cocaine self-administration in rats. Psychopharmacology (Berl). 2017;234(15): 2219–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects ofthe novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl). 2005;183(1):118–126. [DOI] [PubMed] [Google Scholar]
- 20.Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA.Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62(1):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domi E, Barbier E, Augier E, et al. Preclinical evaluation of the kappa-opioid receptor antagonist CERC-501 as a candidate therapeutic for alcohol use disorders. Neuropsychopharmacology. 2018;43(9): 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rorick-Kehn LM, Witkin JM, Statnick MA, et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014a;77:131–144. [DOI] [PubMed] [Google Scholar]
- 23.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rorick-Kehn LM, Witcher JW, Lowe SL, et al. Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human. Int J Neuropsychopharmacol. 2014b;18(2). 10.1093/ijnp/pyu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson KJ, Jackson A, Carroll FI, Damaj MI. Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology. 2015;97:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 27.Naganawa M, Dickinson GL, Zheng MQ, et al. Receptor occupancy ofthe kappa-opioid antagonist LY2456302 measured with positron emission tomography and the novel radiotracer 11C-LY2795050. J Pharmacol Exp Ther. 2016;356(2):260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14(11):1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 30.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 31.Russell JA. A circumplex model of affect. J Pers Soc Psychol. 1980;39(6):1161–1178. [Google Scholar]
- 32.Zimmerman M, Chelminski I, McGlinchey JB, Posternak MA. A clinically useful depression outcome scale. Compr Psychiatry. 2008; 49(2):131–140. [DOI] [PubMed] [Google Scholar]
- 33.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007; 32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 34.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol.2014;32(1):40–51. [DOI] [PubMed] [Google Scholar]
- 35.Morgan P, Van der Graaf PH, Arrowsmith J, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17(9-10):419–424. [DOI] [PubMed] [Google Scholar]
- 36.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med. 2005;24(18):2747–2764. [DOI] [PubMed] [Google Scholar]
- 37.McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14(1): 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verplaetse TL, Weinberger AH, Ashare RL, et al. Pilot investigation ofthe effect of carvedilol on stress-precipitated smoking-lapse behavior. Psychopharmacol. 2018;32(9):1003–1009. 10.1177/0269881118767647. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verplaetse TL, Weinberger AH, Oberleitner LM, et al. Effect of doxazosin on stress reactivity and the ability to resist smoking. J Psychopharmacol. 2017;31(7):830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins KA, Lerman C, Stitzer ML, et al. Development of procedures for early human screening of smoking cessation medications. Clin Pharmacol Ther. 2008;84(2):216–221. [DOI] [PubMed] [Google Scholar]
- 41.Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl). 2006;184(3-4):628–636. [DOI] [PubMed] [Google Scholar]
- 42.Lowe SL, Wong CJ, Witcher J, et al. Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. J Clin Pharmacol. 2014;54(9): 968–978. [DOI] [PubMed] [Google Scholar]
- 43.Fava M, Memisoglu A, Thase ME, et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo controlled trial. Am J Psychiatry. 2016;173(5):499–508. [DOI] [PubMed] [Google Scholar]
- 44.Ling W, Hillhouse MP, Saxon AJ, et al. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the cocaine use reduction with buprenorphine (CURB) study. Addiction. 2016;111(8):1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragguett RM, Rong C, Rosenblat JD, Ho RC, McIntyre RS. Pharmaco-dynamic and pharmacokinetic evaluation of buprenorphine + samidorphan for the treatment of major depressive disorder. Expert Opin Drug Metab Toxicol. 2018;14(4):475–482. [DOI] [PubMed] [Google Scholar]
- 46.Bidlack JM, Knapp BI, Deaver DR, et al. In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther. 2018;367(2):267–281. [DOI] [PubMed] [Google Scholar]
- 47.Reed B, Butelman ER, Fry RS, Kimani R, Kreek MJ. Repeated Administration of Opra Kappa (LY2456302), a novel, short-acting, selective KOP-r antagonist, in persons with and without cocaine dependence. Neuropsychopharmacology. 2018;3(4):739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]