Abstract
Respiratory failure caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is associated with mortality. Patients unresponsive to conventional therapy may benefit from temporary venovenous extracorporeal membrane oxygenation (VV-ECMO). We investigated clinical and echocardiographic characteristics, particularly, right ventricular dysfunction, with survival in patients with respiratory failure caused by SARS-CoV-2. We performed a single-center retrospective cohort study of patients requiring VV-ECMO for respiratory failure from COVID-19 infection between January 2020 and December 2020. Demographics, comorbidities, laboratory parameters, and echocardiographic features of left and right ventricular (LV/RV) function were compared between patients who survived and those who could not be weaned from VV-ECMO. In addition, we evaluated outcomes in a separate population managed with venoarterial extracorporeal membrane oxygenation (VA-ECMO). In total, 10/17 patients failed to wean from VV-ECMO and died in the hospital on average 41.5 ± 10.9 days post admission. Seven were decannulated (41%) and survived to hospital discharge. There were no significant differences in demographics, comorbidities, and laboratory parameters between groups. Moderate to severe RV dysfunction was significantly more in those who died (8/10, 80%) compared to survivors (0/7, 0%) (p = 0.002). Patients supported with VA-ECMO had superior survival with 5/9 patients (56%) decannulated and discharged. Moderate to severe RV dysfunction is associated with increased mortality in patients with respiratory failure requiring VV-ECMO for COVID-19.
Keywords: COVID-19, ECMO, ARDS, right ventricular failure, shock
Coronavirus disease-19 (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was declared a global health emergency by the World Health Organization on March 11, 2020.1 The COVID-19 clinical syndrome is frequently characterized by respiratory failure manifesting as severe hypoxemia and diffuse bilateral infiltrates on chest imaging consistent with noncardiac pulmonary edema in the setting of SARS-CoV-2 infection.2 Early initiation of venovenous extracorporeal membrane oxygenation (VV-ECMO) may improve survival in patients with the most severe disease, and its use as a support strategy has been promoted by the Extracorporeal Life Support Organization.3 However, the mortality rate of COVID-19-associated respiratory failure remains high, approaching 45%.4 Therefore, there is a critical need to define clinical features, imaging findings, and biomarker signatures that may be associated with adverse outcomes in this population.
Right ventricular (RV) dysfunction has been identified in 22–50% of patients with moderate to severe acute respiratory distress syndrome (ARDS) and it is associated with increased mortality.5 Multiple factors contribute to RV dysfunction in this population, including significantly increased RV afterload owing to the underlying lung pathology, hypoxia-induced pulmonary vasoconstriction, hypercapnia, acidemia, and hemodynamic consequences of mechanical ventilation using high driving pressures.5,6 Although the underlying pathophysiology may be different from typical ARDS, severe respiratory failure in the setting of COVID-19 infection prompts similar hemodynamic changes in the pulmonary circulation. Thus, the development of RV dysfunction may represent an important prognostic metric in this population. However, the association remains largely unexplored, particularly in the subset of patients requiring temporary VV-ECMO support.
We performed a single-center retrospective study in a cohort with severe COVID-19 infection with the following aims: 1) define the clinical and biomarker characteristics of patients who required VV-ECMO cannulation for the management of severe respiratory failure; 2) compare clinical, biomarker, echocardiographic, and disease-specific treatment characteristics between patients who expired despite VV-ECMO support and those who survived the index admission; and 3) evaluate outcomes in a separate population managed with venoarterial extracorporeal membrane oxygenation (VA-ECMO). Although this represents a different group of patients, all of them had severe RV dysfunction and VA-ECMO was used as a means of unloading the RV and to provide full circulatory support.
Methods
Study Population
Consecutive adult patients who were treated with VV-ECMO for the management of severe respiratory failure between January 2020 and December 2020 were included. Our center uses Avalon (Getinge, Gothenburg, Sweden) and Crescent (Medtronic, Minneapolis, MN) dual lumen jugular catheters that extract blood from the venae cavae and return it to the right atrium after gas exchange. These cannulas do not provide hemodynamic support and transpulmonary flow remains dependent on the function of the RV. Only patients with a positive molecular test for SARS-CoV-2 and severe hypoxemia refractory to conventional interventions were included in the final study cohort. Exclusion criteria included a documented history of left or right ventricular dysfunction, known preexisting moderate to severe pulmonary arterial hypertension, presence of a durable left ventricular assist device, and history of heart or lung transplantation. Over the same time period, we also reviewed outcomes in a separate population requiring VA-ECMO support in the setting of SARS-CoV-2 infection. This group was included as we sought to examine the effects of RV decompression in this critically ill population. As opposed to VV-ECMO that only provides gas exchange, VA-ECMO drains venous blood from the venae cavae and the right atrium, effectively decompressing the RV. Gas exchange is performed by a membrane lung and blood is subsequently returned to the systemic circulation. Therefore, we postulated that the detrimental hemodynamic effects of severe RV dysfunction may potentially be minimized when VA-ECMO is used. It is important to note that all patients in the VA-ECMO group had severe COVID-19 infection but also had significant LV dysfunction caused by out-of-hospital cardiac arrest, refractory cardiogenic shock, or refractory hypoxemia with concomitant undifferentiated shock. The institutional review board of the University of Minnesota approved this study (STUDY00002818).
Study Design and Aim
A retrospective cohort design was used to obtain demographic, clinical, imaging, laboratory, and outcome data for patients that required VV-ECMO for the management of severe respiratory failure associated with COVID-19. Information was collected from electronic health records and from analysis of relevant echocardiograms. Subsequently, the abovementioned characteristics were compared between patients who were successfully weaned and decannulated from VV-ECMO and those who died during the index admission. We aimed to identify features linked to increased mortality among patients requiring VV-ECMO support for the management of severe respiratory failure caused by COVID-19 infection. To further evaluate the potential association between RV dysfunction and increased mortality, we also reviewed the outcome data of patients managed with VA-ECMO for COVID-19 infection.
Data Collection
The clinical variables of interest were chosen a priori due to their relationship with critical respiratory illness and outcomes in COVID-19. Baseline characteristics, including demographics, past medical history, and prescription medications, were obtained for all patients. Laboratory parameters reflecting hepatic and renal function collected at the time of VV-ECMO cannulation and at their peak were evaluated to determine the degree of multiorgan injury. The highest measured values for troponin-I, C-reactive protein, and interleukin-6 were recorded for each patient. In addition, the use of targeted COVID-19 treatments, including dexamethasone, convalescent plasma, antiviral medications (remdesivir), and tocilizumab was recorded.
The following agents were considered when determining the duration of vasopressor medication use: epinephrine, norepinephrine, dopamine, phenylephrine, vasopressin, and angiotensin-II. Dobutamine and milrinone were considered separately, although after chart review, no patients received these agents. Pulmonary vasodilators included inhaled prostacyclin and nitric oxide.
Echocardiographic Evaluation
All patients underwent multiple transthoracic (TTE) or transesophageal (TEE) echocardiograms at our institution after VV-ECMO cannulation. Tomograms from the earliest, most complete study of diagnostic quality were used for further analysis. Aside from one patient, all echocardiograms were performed within 24 hours of cannulation (mean delay: 16 hours and 58 minutes with a standard deviation of 54 minutes), with the majority completed during VV-ECMO initiation. Echocardiography is routinely used in these patients to verify adequate cannula positioning and that the outflow jet is directed toward the tricuspid valve. Echocardiograms were independently interpreted by two expert, board-certified echocardiographers who were blinded to all clinical information. Quantitative measurements were performed at least twice with the average value reported and used in subsequent analyses. Left ventricular (LV) ejection fraction was estimated visually and using biplane tracing when technically possible. LV end-diastolic diameter (LVEDD) was measured in the parasternal long-axis view. Global RV function was assessed visually in all available views and was qualitatively described as normal, mildly, moderately, or severely reduced. The RV function was also quantitatively assessed using RV fractional area change (FAC). The RV area was traced in the apical four chamber and RV-focused views, both in systole (Asys) and diastole (Adias), and RV FAC was calculated according to the following formula: (Adias − Asys)/Adias) normal range: ≥35%.7–9
Statistical Analyses
Normally distributed continuous variables are reported as means ± standard deviation and non-normally distributed data were reported as medians with interquartile ranges. Normally distributed continuous and categorical data were compared using paired t-tests and the chi-square test, respectively. Non-normally distributed continuous data were compared using the paired Wilcoxon rank-sum test. A p value less than 0.05 was considered statistically significant for all analyses.
Results
Study Population and Clinical Outcomes in the Venovenous Extracorporeal Membrane Oxygenation Cohort
A total of 17 patients were treated with VV-ECMO for severe respiratory failure secondary to COVID-19 infection at our institution between January and December 2020. Seven of the 17 patients (41%) were decannulated successfully and 10 (59%) died while on VV-ECMO support.
The mean age of the study population was 48 ± 10 years (Table 1). Eleven were male (64.7%) and the average body mass index (BMI) was 33.2 ± 6.3. Comorbid conditions, including diabetes mellitus type 2, hypertension, and chronic obstructive pulmonary disease were present in 65%, 53%, and 12% of the cohort, respectively. Five patients (29.4%) were cannulated at our institution whereas 12 (70.6%) were transferred in from a referring hospital (Table 1). The average P/F ratio was 65 ± 10 before cannulation.
Table 1.
Characteristics of the VV-ECMO and VA-ECMO study populations
| VV-ECMO cohort | VV-ECMO patients survived | VV-ECMO deceased patients | p value | VA-ECMO cohort | |
|---|---|---|---|---|---|
| Number of patients, n | 17 | 7 | 10 | - | 9 |
| Age, years (mean ± SD) | 48 ± 10 | 44 ± 10 | 51 ± 9 | 0.15 | 45 ± 20 |
| Men, n | 11 (65%) | 5 (71%) | 6 (60%) | 0.65 | 7 (78%) |
| Women, n | 6 (35%) | 2 (29%) | 4 (40%) | 2 (22%) | |
| BMI, kg/m2 (mean ± SD) | 33.2 ± 6.3 | 35.6 ± 4.5 | 31.5 ± 7.0 | 0.19 | 32 ± 7 |
| Diabetes mellitus, n | 11 (65%) | 3 (43%) | 8 (80%) | 0.13 | 4 (44%) |
| Hypertension, n | 9 (53%) | 4 (57%) | 5 (50%) | 0.79 | 3 (33) |
| COPD, n | 2 (12%) | 0 (0%) | 2 (20%) | 0.23 | 0 (0%) |
| Active tobacco use, n | 1 (6%) | 0 (0%) | 1 (10%) | 0.42 | 3 (33%) |
| Home ACEi/ARB/ARNI use, n | 6 (35%) | 2 (26%) | 4 (40%) | 0.65 | 1 (11%) |
| Hospital days before ECMO cannulation (median, IQR) | 7.8 (4.8–11.1) | 4.8 (2.7–8.6) | 9.3 (6.6–13.3) | 0.09 | 2.1 (0.7–1.4) |
| Prone positioning, n | 13 (76%) | 6 (86%) | 7 (70%) | 0.48 | 0 (0%) |
| P/F ratio before cannulation (median, IQR) | 65 (59–72) | 65 (59–75) | 65 (59–70) | 0.92 | 306 (163–408) |
| Days on ECMO (median, IQR) | 23 (18–37) | 18 (12–26) | 29 (21–42) | 0.20 | 7 (3–8) |
| Days of vasoactive agent use (median, IQR) | 15 (8–20) | 7 (4–8) | 19 (15–33) | 0.001 | 7 (4–8) |
| Number of RBC transfusions per days on VV-ECMO (mean ± SD) | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.84 | 0.9 ± 0.8 |
The p values listed compare differences between VV-ECMO patients.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; COPD, chronic obstructive pulmonary disease; P/F ratio (Horowitz index), ratio of arterial oxygen partial pressure (PaO2) to fraction of inspired oxygen (FiO2); RBC, red blood cell; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VV-ECMO, venovenous extracorporeal membrane oxygenation.
Two of the transferred patients were initially started on veno-arterial-venous (VAV)-ECMO but were transitioned to a VV configuration shortly after their arrival at our institution. Cardiohelp (Getinge, Gothenburg, Sweden) and Centrimag (Abbott Laboratories, Abbott Park, IL, US) systems were used to support 15 and 2 patients, respectively. Eleven patients were cannulated using a percutaneous right internal jugular dual lumen catheter (Avalon or Crescent), ranging in size from 27 to 31Fr. The femoral and right internal jugular veins were cannulated for six patients with no person requiring surgical intervention. Intravenous heparin was administered to achieve an initial activated clotting time of 180–200 seconds, though this was adjusted at the care team’s discretion considering individual patient characteristics and comorbidities. Mean VV-ECMO circuit flow upon initiation was 3.6 L/minute in the group that died, and 4.2 L/minute in patients who were successfully decannulated (p = 0.125).
The median hospital length of stay preceding VV-ECMO initiation was 7.8 (4.8, 11.1) days and the median time to decannulation was 23 (18, 37) days. We did not experience any device failures in our cohort.
There were no significant differences in the demographics, incidence of comorbidities, angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitor (ARNI) use, precannulation mechanical ventilator settings, prone positioning, and the number of packed red blood cell units transfused per VV-ECMO days between patients who died and those who survived (Table 1). The median hospital length of stay before VV-ECMO initiation and the duration of VV-ECMO support was also similar. Vasoactive agent and inhaled pulmonary vasodilator use were significantly longer in the group who died: 19 (15, 33) vs. 7 (4, 8) days (p < 0.001). All survivors had a Cerebral Performance Category (CPC) score of 1–2 at the time of discharge. Two patients required tracheostomy and were transferred to a long-term acute care facility for prolonged ventilator weaning. Laboratory values obtained at the time of VV-ECMO initiation were not statistically different between the groups (Table 2).
Table 2.
Laboratory Parameters at the Time of VV-ECMO or VA-ECMO Cannulation and at Their Peak
| VV-ECMO, initiation | p value | VV-ECMO, peak values | p value | VA-ECMO, initiation | |||
|---|---|---|---|---|---|---|---|
| Survivors | Deceased | Survivors | Deceased | ||||
| Creatinine (mg/dl) | 0.66 (0.58–0.92) | 0.84 (0.56–1.24) | 0.70 | 1.07 (0.8–2.8) | 2.24 (1.25–3.24) | 0.93 | 1.36 (1.04–2.10) |
| Hemoglobin (g/dl) | 10.30 (10.00–11.70) | 11.40 (10.40–12.3) | 0.57 | 11.20 (10.80–11.60) | 11.60 (10.80–12.40) | 0.41 | 12.20 (10.20–12.40) |
| D-dimer (µg/ml) | 2.90 (2.10–3.90) | 6.60 (3.30–11.20) | 0.06 | >20 | >20 | 0.70 | 4.90 (1.00–12.20) |
| Albumin (g/dl) | 2.80 (2.10–3.00) | 2.10 (1.80–2.40) | 0.13 | 3.40 (3.15–3.95) | 3.30 (2.70–3.70) | 0.49 | 2.60 (2.20–2.60) |
| AST (U/L) | 47 (39–81) | 64 (44–83) | 0.87 | 128 (102–463) | 134 (115–199) | 0.42 | 187 (83–584) |
| ALT (U/L) | 51 (36–55) | 60 (42–80) | 0.20 | 212 (156–300) | 112 (94–182) | 0.30 | 81 (47–163) |
| Bilirubin (mg/dl) | 0.6 (0.55–0.66) | 0.8 (0.53–1.85) | 0.07 | 2.60 (0.85–5.56) | 2.20 (1.60–14.50) | 0.24 | 0.60 (0.40–0.90) |
| INR | 1.24 (1.08–1.36) | 1.37 (1.20–1.55) | 0.22 | 1.41 (1.36–2.00) | 1.90 (1.46–2.20) | 0.40 | 1.40 (1.10–1.80) |
| CRP (mg/L) | 180 (142–220) | 160 (82–220) | 0.84 | 270 (180–300) | 275 (213–305) | 0.97 | 400 (9.60–170) |
| IL-6 (pg/ml) | - | - | - | 1,878 (1,270–2,810) | 2,679 (548–14,319) | 0.36 | - |
| Troponin-I (µg/L) | - | - | - | 0.98 (0.67–4.50) | 0.05 (0.02–0.08) | 0.32 | - |
Data are shown as median (IQR).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; IL-6, interleukin-6; INR, international normalized ratio; IQR, interquartile range; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VV-ECMO, venovenous extracorporeal membrane oxygenation.
The use of COVID-19-directed therapies, including convalescent plasma (n = 8), remdesivir (n = 11), and tocilizumab (n = 12) was similar between deceased patients and survivors (Table 3). Dexamethasone was administered less frequently to people who were successfully decannulated (43% vs. 100%, p = 0.004).
Table 3.
Use of COVID-19-specific Interventions in the Study Cohorts
| Patients survived (n = 7) |
Deceased patients (n = 10) |
p value | |
|---|---|---|---|
| Remdesivir, n | 5 (71%) | 6 (60%) | 0.65 |
| Convalescent plasma, n | 3 (43%) | 5 (50%) | 0.79 |
| Dexamethasone, n | 3 (43%) | 10 (100%) | 0.004 |
| Tocilizumab, n | 6 (86%) | 6 (60%) | 0.28 |
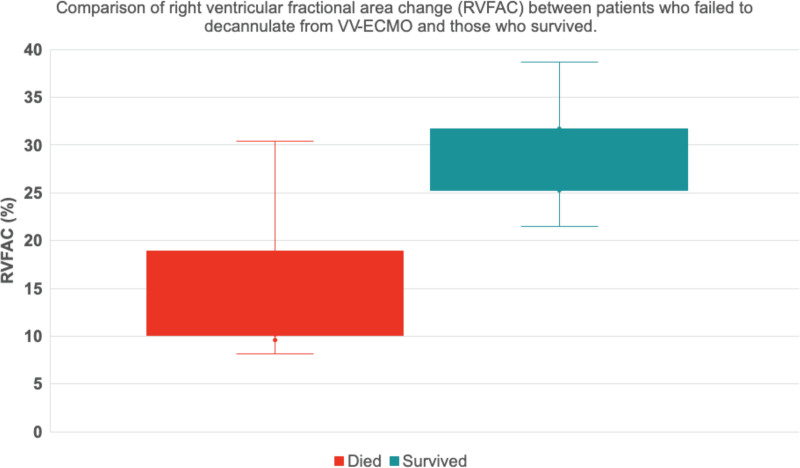
Seven patients had precannulation echocardiogram performed and all had normal LV function. Only one patient had mildly dilated RV with mildly reduced function and this particular individual survived to hospital discharge. Post-cannulation echocardiographic findings are detailed in Table 4. LV function was preserved and LVEDD was within the normal range with no difference between the groups: 4.6 cm (4.4, 4.8) vs. 4.5 cm (4.4, 5.0); p = 0.61. RV dysfunction, based on visual assessment, was more prevalent in patients who died. Quantitative evaluation using RV FAC confirmed these findings with a median value of 11.3% (10.3, 15.0) vs. 30.1% (27.6, 31.4) in the deceased group vs. survivors (p = 0.002) (Figure 1). There was no significant difference in mechanical ventilator settings, plateau pressure, arterial oxygen saturation, and inhaled pulmonary vasodilator agent use at the time of image acquisition between the groups.
Table 4.
Echocardiographic Parameters and Relevant Clinical Variables Collected at the Time of Image Acquisition
| Patients survived (n = 7) | Deceased patients (n = 10) | p value | |
|---|---|---|---|
| LVEF, % (median, IQR) | 65 (65–70) | 63 (60–70) | 0.22 |
| LVEDD, cm (median, IQR) | 4.5 (4.4–5.0) | 4.6 (4.4–4.8) | 0.61 |
| Median RVFAC, % (median, IQR) | 30.1 (27.6–31.4) | 11.3 (10.3–15.0) | 0.002 |
| Prone positioning, n | 6 (86%) | 7 (70%) | 0.48 |
| Arterial oxygen saturation, % (mean ± SD) | 96 ± 4 | 94 ± 4 | 0.27 |
| FiO2, % (mean ± SD) | 74 ± 15 | 72 ± 19 | 0.80 |
| PEEP, cmH2O (mean ± SD) | 10 ± 0 | 9 ± 2 | 0.28 |
| Plateau pressure, cmH2O (mean ± SD) | 21.6 ± 7.6 | 16.5 ± 5.1 | 0.12 |
| Inhaled vasodilator use, n | 3 (43%) | 5 (50%) | 0.10 |
| VV-ECMO flow, L/min (mean ± SD) | 4.2 ± 0.9 | 3.6 ± 0.8 | 0.12 |
All patients represented in the table were receiving VV-ECMO at the time of the study.
FiO2, fraction of inspired oxygen provided through the mechanical ventilator; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; PEEP, positive end-expiratory pressure; RVFAC, right ventricular fractional area change.
Figure 1.
Comparison of right ventricular fractional area change (RVFAC) between patients who failed to decannulate from VV-ECMO and those who survived. Median RVFAC was significantly higher in survivors (30.1% vs. 11.3%; p = 0.002). VV-ECMO, venovenous extracorporeal membrane oxygenation.
Venoarterial Extracorporeal Membrane Oxygenation Group
Nine patients with severe COVID-19 infection were managed with peripheral VA-ECMO at our institution between January 1, 2020, and December 31, 2020. We typically use 17Fr and 25Fr cannulas inserted into the femoral artery and vein, respectively. An 8Fr distal reperfusion catheter is placed routinely into the superficial femoral artery to ensure adequate lower extremity perfusion. The mean age in this group was 45 ± 20 years, seven were male (78%), and the average BMI was 32 ± 7 (Table 1). The prevalence of hypertension was 33% and diabetes mellitus was 44%. The indication for VA-ECMO cannulation was cardiac arrest, refractory cardiogenic shock, and severe hypoxemia with mixed cardiogenic and vasoplegic shock in four, three, and two patients, respectively. Three patients were determined to be COVID-19-positive after VA-ECMO initiation, whereas the infection was known for six before cannulation. Of the nine patients included in this group, we believe that seven had a causal relationship with COVID-19 and the shock developed as a direct sequela of the infection. For these patients, the underlying pathology included COVID-related myocarditis, multisystem inflammatory syndrome in adults, pulmonary emboli, or thromboembolic events leading to cardiogenic shock, all thought to be related to COVID-19 infection. All these patients were seen and followed by our infectious disease colleagues and received therapy for COVID-19 disease. Baseline laboratory parameters collected at the time of VA-ECMO initiation are shown in Table 2. Echocardiographic data were available for six patients and six were found to have at least moderate RV dysfunction. Mean VA-ECMO flow was 4.07 L/minute, which was not significantly different between survivors and those who died before decannulation. It was also similar between patients with normal and reduced RV function. Patients received VA-ECMO support for a median of 5 (3, 8) days. Five patients (56%) were decannulated successfully and their survival to hospital discharge was 100%, all with CPC 1–2 performance status. Two patients were discharged home, one was discharged to an acute rehabilitation facility, one was transferred to a temporary skilled nursing facility, and one to an inpatient psychiatry unit for assistance with substance use disorder. Four of the six patients who had documented RV dysfunction by echocardiography survived to discharge.
Discussion
In this retrospective single-center study, we found that patients with severe respiratory dysfunction requiring VV-ECMO cannulation in the setting of COVID-19 infection have an exceedingly high mortality rate. Deceased patients and survivors of the index admission had relatively similar baseline characteristics, ICU course, and COVID-19-specific therapeutic interventions. However, median RV FAC was significantly lower in patients who could not be weaned from VV-ECMO support and died (30.1% [27.6, 31.4] vs. 11.3% [10.3, 15.0]; p = 0.002). We also identified nine patients out of 162 who were treated with VA-ECMO for severe COVID-19 infection and associated complications. Survival to hospital discharge was 56% in this group, higher than the 41% observed in the VV-ECMO cohort. The strong association between moderate to severe RV dysfunction on echocardiography and failure to decannulate from VV-ECMO, as well as our finding of increased survival with RV unloading using VA-ECMO calls for further studies to evaluate the potential role of early mechanical RV support in this subpopulation.
A combination of factors promotes RV dysfunction in patients with COVID-19. These include profound hypoxia and associated pulmonary vasoconstriction, the negative inotropic effect of inflammatory cytokines, and direct cardiac injury that is mediated by ACE 2.10–15 In addition, the observed decline in RV free wall longitudinal strain, despite preserved lung compliance, suggests an important role for accumulating microthrombi and hemodynamically significant pulmonary emboli.16 Together, these changes lead to an increase in RV afterload, RV wall stress, and a significant rise in right atrial pressure. This prompts a reduction of transpulmonary flow, and, ultimately, a decline in cardiac output. Several investigators have evaluated RV remodeling and dysfunction in patients with COVID-19 disease and found these to be an independent predictor of mortality.12,17,18 However, patients on VV-ECMO have been excluded from these reports.
In view of the significant role of hypoxemia in the development of RV dysfunction, VV-ECMO may be used in patients with refractory respiratory failure, who have failed conventional therapies, as a bridge to recovery. This temporary support system uses a dual lumen jugular cannula to drain blood from the central venous circulation, pumps it through a membrane lung for gas exchange, and then returns the blood into the right atrium rather than the pulmonary artery. This design may have important clinical implications in the setting of moderate to severe RV dysfunction. Although patients receive oxygenated blood through the VV-ECMO cannula, we postulate that the native RV function may be insufficient to propel the blood across the pulmonary circulation. This can lead to multiple unfavorable consequences: 1) left ventricular preload may be compromised prompting a decline in cardiac output, 2) hypoxia-induced vasoconstriction will persist, and 3) lung-protective ventilation strategies aimed at reducing intrathoracic pressure may not be implemented. Accordingly, the use of a mechanical circulatory support system that not only provides gas exchange but also unloads the RV may be more suitable in this population. We believe that the additional hemodynamic support may have been responsible, at least in part, for the improved survival we found in the VA-ECMO group.
Published studies on the use of VV-ECMO in patients with COVID-19 infection are largely observational with relatively small sample sizes.19–24 Patients are of comparable demographics, have a similar rate of comorbidities and precannulation medical management to our group. However, none of these studies evaluated RV echocardiographic parameters and thus the prognostic value of RV dysfunction in this population remains unclear.19–23 Our results suggest that moderate to severe RV dysfunction is associated with decreased survival to decannulation in patients requiring VV-ECMO in the setting of COVID-19 infection. Conversely, an absence of RV dysfunction on the post VV-ECMO echocardiogram was associated with successful decannulation and survival to hospital discharge. Although our results are similar to those reported by Ortiz et al.25 in patients with undifferentiated ARDS, this is the first study in a population with refractory COVID-19-associated respiratory failure.
The failure of RV function to recover may be another important prognostic factor, in addition to the degree of baseline RV dysfunction. In patients with baseline RV dysfunction, serial echocardiograms could be useful to monitor for RV recovery. Thus, patients who do not respond to medical interventions (such as inotropic support and pulmonary vasodilators), or exhibit worsening RV function on VV-ECMO, may need to be considered for additional temporary mechanical circulatory support, such as VA-ECMO. In particular, patients with preexisting biventricular heart failure or severe LV failure may be at high risk of mortality in the setting of cardiogenic shock with worsening RV dysfunction. In these patients, early VA-ECMO cannulation might be reasonable as a bridging strategy. Further studies are needed in these specific patient groups to establish the optimal treatment pathway.
Limitations
There are several limitations to mention. This was a retrospective, single-center study with a relatively low number of patients enrolled. An inherent selection bias may be present. Older patients, those with the most severe hypoxia or advanced comorbid conditions, may not have had the opportunity to proceed with VV-ECMO support due to resource constraints, transportation limitations, or family/medical team decisions. While the difference did not reach statistical significance, there was a trend toward earlier VV-ECMO initiation in patients who survived to decannulation (4.8 days) versus those who died (9.3 days; p = 0.09, see Table 1). Survivors were 7 years younger on average (also not statistically significant), which may have biased clinicians to consider VV-ECMO earlier as a more “aggressive” treatment approach. These differences may have contributed to the improved outcomes in the survivors. Owing to technical constraints and infectious precautions, the timing between VV-ECMO initiation and the first diagnostic quality TTE/TEE study varies between patients. Nevertheless, this variation does not limit our findings that the development of RV failure on VV-ECMO support is associated with patient mortality. We attempted to collect additional echocardiographic surrogate parameters for RV function, such as tricuspid annular plane systolic excursion and peak systolic annular velocity (S′). However, owing to the acute clinical setting, limited tomographic windows, and variation in imaging technique (TTE vs. TEE), these could only be recorded for a small fraction of patients in each group. Therefore, further statistical analysis was not attempted. The difference in selected laboratory parameters between the group of patients who survived and those who failed to decannulate from VV-ECMO did not reach statistical significance in our study. This may be due to the relatively small cohort. Our group of patients managed with VA-ECMO was small with a variable initial indication for mechanical circulatory support. A better comparator group could potentially be patients on VV-ECMO in addition to inotropic agents and pulmonary vasodilators versus those receiving isolated mechanical RV support with Protek Duo or Impella RP. However, the number of such patients was extremely low at our institution in the setting of SARS-CoV-2 infection.
Conclusion
Refractory respiratory failure associated with COVID-19 infection carries an exceptionally high mortality risk despite the use of VV-ECMO and bundled ICU strategies. Echocardiographic findings consistent with moderate to severe RV dysfunction were associated with significantly increased mortality in this population. The temporal sequence of RV function should be evaluated with further studies in this population. It is quite possible that patients may exhibit severe RV dysfunction in the early stages of the illness and that subsequent improvement in RV dysfunction may portend a better clinical prognosis.
Clinical Perspectives
COVID-19 leads to severe morbidity and mortality around the world, with a segment of patients developing refractory respiratory failure requiring venovenous extracorporeal membrane oxygenation (VV-ECMO). Patients who showed echocardiographic evidence of right ventricular dysfunction, despite VV-ECMO support, had increased mortality compared to those with a normal right ventricle. Other forms of temporary mechanical support, such as venoarterial ECMO, may be needed to support people through the disease process.
Translational Outlook
Prospective multicenter studies are needed to validate alternative forms of temporary mechanical support when treatment of refractory hypoxia and right ventricular dysfunction is present in the setting of COVID-19 acute respiratory distress syndrome.
Footnotes
Disclosure: Jason A. Bartos and Demetris Yannopoulos received philanthropic grants for resuscitation and ECMO research from NIH. The remaining authors have no conflicts of interest to report.
Valmiki Maharaj and Tamas Alexy contributed equally to this work.
Contributor Information
Valmiki Maharaj, Email: maha0104@umn.edu.
Tamas Alexy, Email: alexy001@umn.edu.
Arianne C. Agdamag, Email: agdam001@umn.edu.
Rajat Kalra, Email: kalra@umn.edu.
Bellony N. Nzemenoh, Email: nzeme001@umn.edu.
Victoria Charpentier, Email: charp024@umn.edu.
Jason A. Bartos, Email: jabartos@umn.edu.
Melissa E. Brunsvold, Email: mbrunsvo@umn.edu.
References
- 1.World Health Organization: WHO Director-General’s opening remarks at the media briefing on COVID-19. Published 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19. Accessed March 11, 2020.
- 2.Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM, Bahreini E: A comprehensive review of COVID-19 characteristics. Biol Proced Online. 22: 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badulak J, Antonini MV, Stead CM, et al. ; ELSO COVID-19 Working Group Members: Extracorporeal membrane oxygenation for COVID-19: Updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 67: 485–495, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M: Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit Care. 24: 516, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F: The right ventricle in ARDS. Chest. 152: 181–193, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Bunge JJH, Caliskan K, Gommers D, Reis Miranda D: Right ventricular dysfunction during acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation. J Thorac Dis. 10(suppl 5): S674–S682, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang RM, Badano LP, Mor-Avi V, et al. : Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 28: 1–39.e14, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Rudski LG, Lai WW, Afilalo J, et al. : Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 23: 685–713, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD: Two-dimensional assessment of right ventricular function: An echocardiographic-MRI correlative study. Echocardiography. 24: 452–456, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Prins KW, Rose L, Archer SL, et al. : Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic lung disease. J Am Heart Assoc. 8: e011464, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis Miranda D, van Thiel R, Brodie D, Bakker J: Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 191: 346–348, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Volodarskiy A, Sultana R, et al. : Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 76: 1965–1977, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis): High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 46: 1089–1098, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann M, Verleden SE, Kuehnel M, et al. : Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 383: 120–128, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JF, Banerjee S, Umar S: In the eye of the storm: The right ventricle in COVID-19. Pulm Circ. 10: 2045894020936660, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson LE, Fenza RD, Lang M, et al. : Right ventricular strain is common in intubated COVID-19 patients and does not reflect severity of respiratory illness. J Intensive Care Med. 36: 900–909., 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bursi F, Santangelo G, Sansalone D, et al. : Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography. 37: 2029–2039, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wats K, Rodriguez D, Prins KW, et al. : Association of right ventricular dysfunction and pulmonary hypertension with adverse 30-day outcomes in COVID-19 patients. Pulm Circ. 11: 20458940211007040, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Guo Z, Li B, et al. : Extracorporeal membrane oxygenation for Coronavirus Disease 2019 in Shanghai, China. ASAIO J. 66: 475–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giraud R, Legouis D, Assouline B, et al. : Timing of VV-ECMO therapy implementation influences prognosis of COVID-19 patients. Physiol Rep. 9: e14715, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreier E, Malfertheiner MV, Dienemann T, et al. : ECMO in COVID-19-prolonged therapy needed? A retrospective analysis of outcome and prognostic factors. Perfusion. 36: 582–591., 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassen CL, Philipp A, Akyol D, et al. : Out-of-center initiation of venovenous extracorporeal membrane oxygenation in COVID-19 patients. ASAIO J. 67: 4–6, 2021. [DOI] [PubMed] [Google Scholar]
- 23.Garcia B, Cousin N, Bourel C, Jourdain M, Poissy J, Duburcq T; Lille Intensive Care COVID-19 group: Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. 24: 428, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haiduc AA, Alom S, Melamed N, Harky A: Role of extracorporeal membrane oxygenation in COVID-19: A systematic review. J Card Surg. 35: 2679–2687, 2020. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz F, Brunsvold ME, Bartos JA: Right ventricular dysfunction and mortality after cannulation for venovenous extracorporeal membrane oxygenation. Crit Care Explor. 2: e0268, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]