Abstract
The effective suppression of adaptive immune responses is essential for the success of allogeneic cell therapies. In islet transplantation for Type 1 Diabetes, pre-existing autoimmunity provides an additional hurdle, as memory autoimmune T cells mediate both an autoantigen-specific attack on the donor beta cells and an alloantigen-specific attack on the donor graft cells. Immunosuppressive agents used for islet transplantation are generally successful in suppressing alloimmune responses, but dramatically hinder the widespread adoption of this therapeutic approach and fail to control memory T cell populations, which leaves the graft vulnerable to destruction. In this review, we highlight the capacity of biomaterials to provide local and nuanced instruction to suppress or alter immune pathways activated in response to an allogeneic islet transplant. Biomaterial immunoisolation is a common approach employed to block direct antigen recognition and downstream cell-mediated graft destruction; however, immunoisolation alone still permits shed donor antigens to escape into the host environment, resulting in indirect antigen recognition, immune cell activation, and the creation of a toxic graft site. Designing materials to decrease antigen escape, improve cell viability, and increase material compatibility are all approaches that can decrease the local release of antigen and danger signals into the implant microenvironment. Implant materials can be further enhanced through the local delivery of anti-inflammatory, suppressive, chemotactic, and/or tolerogenic agents, which serve to control both the innate and adaptive immune responses to the implant with a benefit of reduced systemic effects. Lessons learned from understanding how to manipulate allogeneic and autogenic immune responses to pancreatic islets can also be applied to other cell therapies to improve their efficacy and duration.
Keywords: allograft, autoimmunity, islet transplantation, immunomodulation, drug delivery, encapsulation
1. Introduction
1.1. Type 1 Diabetes
Type 1 Diabetes (T1D) is an autoimmune disease caused by aberrant, but targeted, T-cell mediated destruction of insulin-producing beta cells in the pancreas [1]. The resulting loss of blood glucose regulation is associated with increased risks of vascular and neuropathic comorbidities [2]. Despite the fact that T1D is one of the most studied organ-specific autoimmune diseases, the various strategies aimed at intervention, prevention, or reversal of this disease have failed to match animal model predictions of success [3, 4]. Moreover, the global incidence of T1D has increased by 3–4% in the past thirty years, while the factors precipitating this rise remain uncertain [2, 5].
The field of biomedical engineering has made great strides in glucose sensor technologies, making exogenous insulin therapy easier for patients by creating minimally invasive closed-loop systems [6]. However, an artificial pancreas approach, comprised of glucose sensors, control algorithms, and insulin infusion devices, cannot provide physiologic blood glucose control due to delays in glucose sensing from interstitial fluid and insulin action in peripheral tissues [7].
1.2. Clinical Islet Transplantation (CIT)
The clinical transplantation of pancreatic islets of Langerhans has the potential to provide a curative therapy where full physiological glucose-responsive insulin control is restored [8, 9]. Clinical islet transplantation involves the procurement of the allogeneic cadaveric donor organ, mechanical disruption and enzymatic digestion of the pancreas, short-term culture of the resulting islet spheroids, and the infusion of the allogeneic pancreatic islets into the portal vein [10]. The donor islets travel within the bloodstream of the recipient’s liver until they become lodged in the microcapillaries, resulting in loss of blood perfusion in the liver tissue distal to the islet [11]. Based on the most recent clinical trial results, most patients (71.6%) receive multiple distinct islet infusions (n ≥ 2), with some receiving up to six separate islet preparations. It is also important to note that 12.5% of these infusions were procured from multiple pancreatic donors (2–3), meaning islets were pooled from different deceased donors in an attempt to deliver a therapeutic cell dose [12].
With the tremendous advances in donor islet procurement, islet culture, and transplantation techniques, this approach has resulted in several positive clinical trials, with substantial improvements in patient quality of life, restoration of glucose hypo-awareness, and enhanced glycemic control [9, 13–15]. However, the long-term outcomes of this cell therapy have been less than satisfactory, with a general precipitous decline of cell transplant mass that results in the majority of the patients requiring exogenous insulin supplementation after one year [16, 17]. The limited duration of this therapy, as well as the limitations in organ procurement and high cell demand, typically restrict this clinical procedure to a subset of patients with hypoglycemia unawareness and frequent severe hypoglycemic events [18]. Post hoc analysis of these extensive CIT trials has identified early innate immune events and potent adaptive immune responses as key contributors to the rapid decline in the transplanted cell function [19].
Following the implantation of islets into the portal vein, cells are in direct contact with blood. This contact results in the instigation of multiple host responses, including the activation of complement and coagulation cascades, collectively termed the non-specific instant blood-mediated inflammatory reaction (IBMIR) [20, 21]. These early events lead to a significant loss of islet mass (up to ~60%) within two weeks post-implantation [22, 23]. Systemic approaches used to regulate IBMIR have shown promise in pre-clinical and clinical studies; however, their use in standard practice remains challenging due to increased risk of bleeding [24]. Downstream of IBMIR, multiple innate and adaptive immune cells respond to the infusion of the foreign islet graft (Figure 1). In addition to the expected immune pathways activated in response to interactions with the allogenic cell source, Type 1 diabetic recipients also experience the re-initiation of autoimmune responses to the transplanted beta cells. These aggressive immune cell reactions must be fully characterized and potently suppressed to retain the efficacy of the foreign islet graft.
Figure 1: Summary of immune pathways of recognition and rejection following implantation of allogenic pancreatic islets.
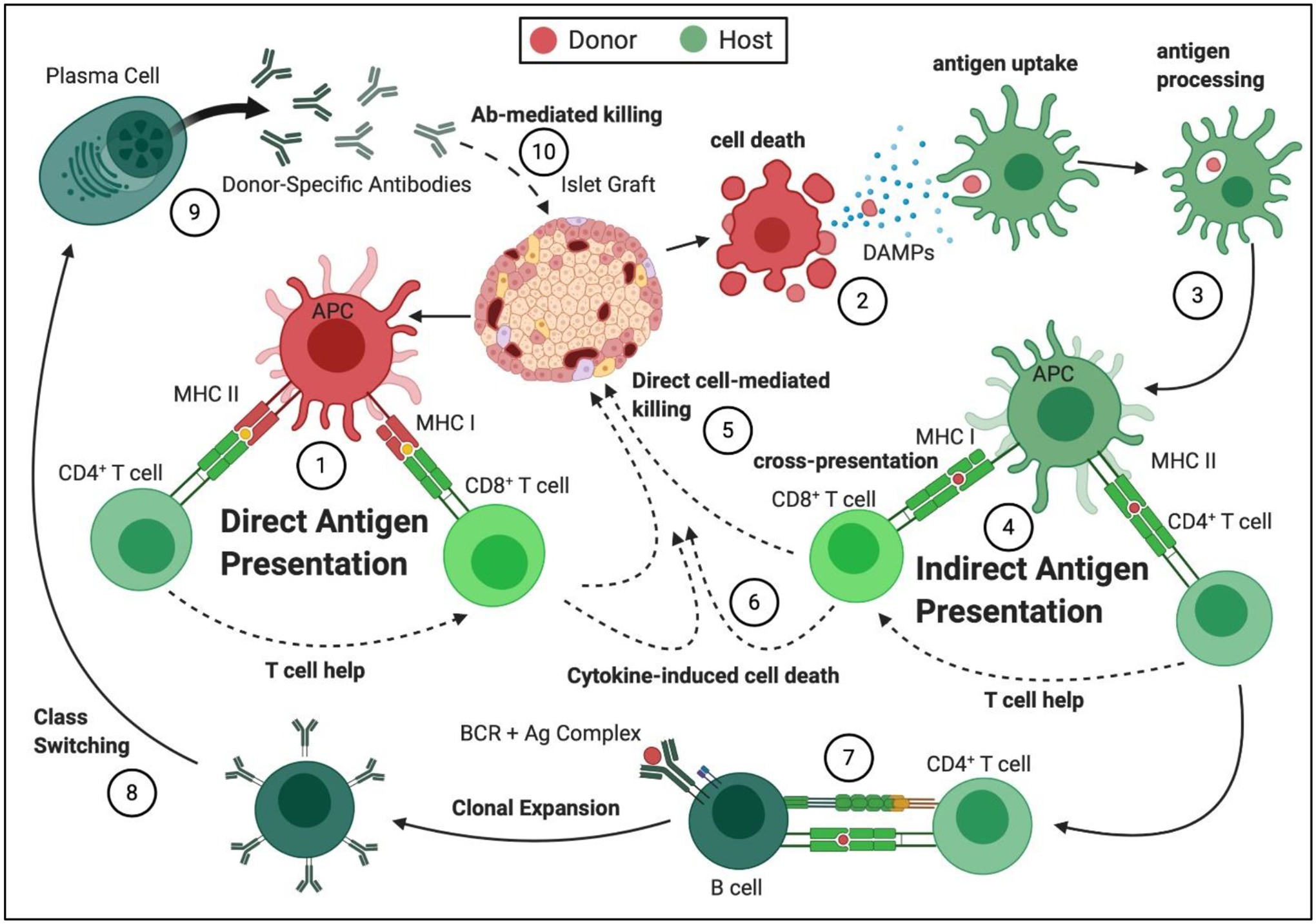
(1) Direct antigen presentation: Donor islet antigen presenting cells (APCs) present antigens to the host T lymphocytes through MHC Class I for CD8+ T cells and MHC Class II for CD4+ T cells. CD4+ T cells become activated and provide help to the CD8+ T cells, resulting in their clonal expansion and activation. Auto-antigens can also be recognized in this manner, when donor and host MHC match. (2) Donor cell death: Dying transplanted cells shed antigen and release danger-associated molecular patterns (DAMPs). (3) Antigen uptake and processing: The presence of DAMPs during antigen uptake and processing by APCs initiate and perpetuate effector signals in APCs (e.g. elevated MHCII expression). (4) Indirect Antigen Presentation: Processed host allo- or autoantigens present to host T cells in context of the self-MHC on the host APCs, resulting in T cell clonal expansion and activation. (5) Direct Cell-Mediated Killing: Cytotoxic T lymphocytes (CTLs) migrate to the implant site and initiate cell-cell interactions with the donor cells, resulting in destruction of the donor cell graft. (6) Cytokine-Induced Cell Death: Activated T cells and innate immune cells produce pro-inflammatory cytokines, which damage cells within the graft site. (7) CD4+ T cell- Mediated B cell Activation: CD4+ T cells, activated through the indirect pathway, interact with antigen-specific B cells through CD40-CD40L and provide Signal 2 for B cell activation, initiating their proliferation and differentiation. (8) Antibody Class Switching: CD4+ T cells activated through the indirect pathway allow for class switching of the produced antibodies to isotype IgG, which is associated with antibody-mediated rejection (AMR). (9) Release of Donor-Specific Antibodies (DSA): Production of DSAs by plasma cells results in acute and chronic (10) Antibody-Mediated Graft Destruction, which involves antibody binding to donor cell surface antigens, complement activation, and graft destruction.
1.3. General Pathways of Allorecognition to Transplanted Cells
Alloimmunity describes the reaction of the host’s immune system to a genetically disparate donor graft from the same species. To reject the foreign cellular graft, three main stages of immune responses must occur: recognition, clonal expansion and effector cell maturation, and graft destruction. The recognition of transplanted donor cells by the adaptive immune system occurs through two major pathways: direct and indirect antigen presentation (Figure 1) [25–28]. For direct allorecognition, host immune cells recognize alloantigens presented by major histocompatibility complex (MHC) ligands expressed by the donor allogeneic cells [29]. For indirect allorecognition, host immune cells recognize alloantigens processed and presented by host antigen presenting cells (APC) [29]. T cells (both CD4+ and CD8+) and B cells express the key receptors involved in allorecognition, which are the T cell and B cell receptors (TCR and BCR, respectively).
For transplanted organs and tissues, historical dogma has implicated direct antigen presentation as the dominant allorecognition pathway in early graft recognition [28, 29]. This is due to the route of allorecognition, which is more direct than the requirement of antigen processing and presentation in the context of self-MHC by host APCs in indirect recognition. However, recent examination of CD4+ and CD8+ T cell allorecognition and activation events have revealed challenges in clearly delineating direct from indirect, as there is significant interplay [30]. What is known about early adaptive immune cell recognition is that the presence of host professional APC (e.g., dendritic cells (DCs)) elevates acute rejection, likely by promoting both direct and indirect antigen recognition [31]. In addition, host T cell recognition of foreign MHC alloantigens is exceptionally high, with up to 10% of the recipient’s T cells recognizing just a single MHC alloantigen [30, 32, 33]. This results in high direct T cell recognition rates following cellular transplantation, with this frequency elevated with each MHC variant. This high recognition rate further facilitates T cell activation, as the co-binding of CD8+ T cells to MHC I and CD4+ T cells to MHC II in close proximity results in co-stimulation of their effector pathways [34, 35]. Given the key role of direct antigen recognition in islet graft rejection and the high propensity of MHC mismatches due to multiple islet infusions, approaches that mask these antigens or suppress co-activation processes would prove to be highly beneficial.
While the indirect pathway may appear arduous and complex, the importance of the indirect pathway in acute and chronic graft rejection has been validated in several animal model studies [28, 36–39]. For example, a murine TCR transgenic system capable of tracking the CD8+ direct, as well as CD4+ direct and indirect pathways, observed a significantly higher expansion and effector cell maturation in the CD4+ indirect T cell population following allograft implantation, compared to either direct T cell populations [36]. Other murine models have also implicated the indirect recognition pathway as a key contributor to islet allograft rejection [40]. As indirect antigen recognition can be easily initiated at or distal to the graft site, immunomodulatory approaches to mitigate indirect antigen recognition are more focused on blocking the co-stimulation of CD4+ T cells.
Although only more recently demonstrated in vivo, the semi-direct pathway also provides a possible explanation to some phenomena not easily explained by direct and indirect antigen recognition pathways [31, 41]. The semi-direct pathway occurs when cell membrane components containing intact allogeneic donor MHC-peptide complexes are transferred to host dendritic cells and presented to the host T cells through direct contact with donor cells or through the internalization or attachment of donor exosomes [25, 30, 42]. With host DCs shown to present the intact MHC-peptide complexes from donor cells, the observed phenomenon of epitope linkage between the CD4+ T cells activated in this manner and the CD8+ T cells activated in the direct pathway finally has an explanation [43]. With this new recognition emerging, additional targets for more effective suppression of immune recognition can be identified [44].
Following allorecognition via direct, indirect, and/or semidirect pathways, the key immune cell players involved in the direct destruction of the foreign cells are the CD8+ cytotoxic T lymphocytes (CTLs), which are provided help by the CD4+ T cells (Figure 1) [30]. Growing evidence for the semidirect pathway suggests that this route of antigen presentation extends the period during which the direct CD8+ T cells can receive T cell help, allowing direct cytotoxic T cells to persist longer [30]. The CD4+ T cells, although typically noncytotoxic on their own, also have been shown to mediate the allograft rejection independently, conceivably through the Fas pathway, if activated through the direct route [45, 46].
The allograft immune response is believed to be majorly T cell-dependent, as indicated by the allograft tolerance in transplant studies in animal models with TCR α or β subunit deficiency [44]. However, the involvement of innate immunity should not be overlooked, as recent studies have underscored the role of monocytes and macrophages in the recognition of non-self in the transplantation setting, as well as the establishment of allospecific innate memory [47–49]. Additionally, the cells of the innate immune system contribute to non-specific graft injury and facilitate the B cell-dependent antibody-mediated graft destruction pathway [44, 50]. Animal transplantation studies have shown that antibody production to the epitopes of the direct pathway is dependent on the help of T cells activated by host APCs [51]. Most importantly, only the indirect pathway results in antibody isotype switching in B cells, which is associated with graft antibody-mediated rejection (AMR) [30, 51] (Figure 1). As such, efforts focused on preventing graft rejection should expand to the modulation of APCs and B cells.
1.4. Autoimmunity, a Special Concern for Islet Transplantation
In Type 1 diabetes, smoldering autoimmune responses to the newly transplanted allogeneic beta cells is an additional facet that contributes to graft recognition and rejection. This is due to the presence of memory T cells specific to type 1 diabetes antigens. Memory T cells naturally arise following the activation and resolution of an immune response to a cognate antigen exposure [52]. Following resolution, a small fraction of T cells persists and converts into long-lived memory T cells, which serve to provide future protection in cases of antigen re-exposure [52, 53]. Importantly, memory T cells react to the re-exposure to the cognate antigen more rapidly and robustly than their naïve counterparts [52].
In most clinical islet transplantation cases, the donor and host are not HLA (human leukocyte antigen) matched, however, in the cases of HLA matching, such as identical twin and HLA-identical sibling transplants, the host autoreactive T cells restricted to the shared MHC molecules can contribute to the rejection of the islet allograft via direct recognition pathway [54–56]. In cases with disparate MHC genes, the self-reactive T cells can still mediate the allograft rejection through the indirect antigen recognition pathway. For example, the implantation of islet allografts into autoimmune diabetic NOD mice resulted in the infiltration of autoreactive T cells that exhibited both allo- and auto-reactivity [54]. This finding supports the hypothesis of “heterologous alloimmunity”, which postulates that memory phenotype T cells specific for one cognate antigen presented by self MHC molecule can also facilitate immune reactions against other peptides presented by non-self MHC, conceivably resulting from peptide molecular mimicry [57–59].
Additionally, compared to naïve T cells, memory T cells are less susceptible to standard immunosuppressive regimens [59–63], indicating that islets infused within T1D patients are insufficiently protected. This concept was validated in retrospective analyses of clinical pancreas and simultaneous pancreas-kidney (SPK) transplant recipients [64–67], which observed the selected destruction of the allogeneic beta cells, but not other allogeneic tissues, and the elevation of T1D autoantibodies, in spite of HLA matching and continuous immunosuppression [65, 67–69]. These results indicate that suppressing adaptive immune responses to allogeneic cellular transplants in patients with pre-existing autoimmunity is extremely difficult, as the self-reactive T-cell effector memory populations are, in fact, dual-reactive and mediate allograft rejection [70].
2. CIT Immunosuppression
To prevent the prompt rejection of the islet transplants, diabetic recipients must receive a continuous and systemic cocktail of immunosuppressive drugs. Most anti-rejection regimens for organ transplantation are two-phased: an aggressive depletion of immune cells in the induction phase, which significantly reduces the frequency of acute graft rejection; and continuation of graft protection in the maintenance phase [71, 72]. The use of immunosuppressive drugs for islet transplantation is typically focused on curbing alloimmune responses, with the induction therapeutics administered immediately prior to transplantation and the maintenance phase immunosuppressive agents administered throughout the lifetime of the graft (see Table 1 for summary of drugs used in clinical islet transplant regimens).
Table 1.
Summary of key immunosuppressive drugs for islet transplantation
| Immunosuppression Phase | Category | Immunosuppressive Agent | Target | Naïve T cell Effect | Memory T cell Effect | References |
|---|---|---|---|---|---|---|
| Induction | T cell depletion | Alemtuzumab | CD52 | depletion | sparing | [61, 62] |
| Teplizumab | CD3 | depletion | expansion | [81, 80] | ||
| Antithymocyte Globulin | polyclonal | depletion | sparing | [78, 61, 62] | ||
| T cell activation inhibition | Basiliximab | IL-2R | inhibition | sparing | [74, 76] | |
| Daclizumab | IL-2R | inhibition | sparing | [74, 76] | ||
| Maintenance | Antiproliferative | Mycophenolate Mofetil | IMPDH | inhibition | inhibition | [93] |
| Sirolimus | mTOR | inhibition | expansion | [97] | ||
| Co-stimulation inhibition | Belatacept | CD28-B7 | inhibition | sparing | [64, 97] | |
| Calcineurin inhibition | Tacrolimus | Calcineurin | inhibition | inhibition | [97, 60] | |
| Cyclosporine A | Calcineurin | inhibition | inhibition | [97, 60] | ||
| Anti-inflammatory | Glucocorticoid | Glucocorticoid receptor | inhibition | expansion | [86] | |
| Infliximab | TNF | inhibition | mixed reports | [84] | ||
| Lymphocyte migration inhibition | Alefacept | CD2-LFA-3 | sparing | depletion | [102, 97] | |
| Efalizumab | LFA-1 | sparing | depletion | [99, 97] | ||
| Natalizumab* | VLA-4 | sparing | inhibition | [60, 103] |
Agent of interest that has not yet been applied for use in CIT
Early agents used in the induction phase were interleukin-2 receptor antagonists (IL-2RAs), such as basiliximab and daclizumab, as they offered a rapid control of T cell populations through inhibition of clonal expansion without T cell depletion [73–75]. These agents have mostly been replaced with antibody-based antithymocyte globulin (ATG), teplizumab, and alemtuzumab, which induce robust T cell depletion, as this approach is more effective in preventing acute rejection [59, 76–80]. Studies have shown, however, that antibody-mediated T cell depletion spares effector memory lymphocytes, which contribute to allo- and auto-reactivity in graft rejection [60, 61]. Thus, recent approaches combining T cell depletion with TNFα inhibition via infliximab or etanercept, with or without IL-2 inhibitors, have observed improved duration of efficacy with reduced side effects [81, 82].
Agents used in the maintenance phase for islet transplantation differ from standard organ transplant approaches due to the sensitivity of islets to steroids [83]. The replacement of standard corticosteroids with calcineurin inhibitors (CNIs) and the mechanistic target of rapamycin (mTOR) inhibitor, sirolimus, resulted in a dramatic improvement in the efficacy of islet grafts [14, 15]. CNIs, cyclosporin A and tacrolimus, are effective in the maintenance phase of islet transplantation as they effectively curb both naïve and memory T cell populations [59, 62]; however, this attribute comes at the cost of decreased general immunity for patients [62]. CNIs have also been associated with beta cell toxicity and post-transplant diabetes mellitus (PTDM) in solid-organ transplants [84, 85]. Contrarily, mTOR inhibitors, like sirolimus, suppress naïve T cell activation and clonal expansion while promoting regulatory T cells, which makes it an attractive agent in the quest to induce graft tolerance [86]. Sirolimus, however, has been associated with increased morbidity and islet toxicity [87–89]. Thus, recent protocols have broadly replaced sirolimus with mycophenolate mofetil (MMF), which inhibits T cell proliferation and downregulates the expression of lymphocyte adhesion molecules required for graft infiltration [12, 90]. Another major advantage of MMF is its ability to reduce the need and/or dosage of CNIs [9, 81].
In the quest to reduce the use of both CNIs and mTOR, other immunosuppressive targets have been developed. Belatacept, a fusion protein with an inhibitory action dependent on a T cell’s maturation state, paired with sirolimus, has been shown to prolong islet allograft survival in a non-human primate study and allow for the achievement of insulin independence in 5 patients for over 500 days post transplantation when additionally combined with MMF [91, 92]. As belatacept does not affect memory T cell populations, its suppressive effect is limited in comparison to the CNIs, conceivably rendering belatacept suboptimal in cell therapy for patients with pre-existing autoimmunity [59, 63]. Other approaches have targeted effector memory cells via blocking of adhesion molecules, as these become upregulated on T cells as they mature [59, 93]. This approach, although partially unverified in clinical islet transplantation, includes the use of MMF in the induction phase, as well as blockades of adhesion markers leukocyte function-associated antigen-1 (LFA-1) via efalizumab [92, 94, 95], CD2 via alefacept [96–98], very late antigen-4 (VLA-4) via natalizumab [99], or lymphocyte Peyer’s patch adhesion molecule-1 (LPAM-1) via vedolizumab [100]. Although these therapeutics require further validation of safety and long-term efficacy, they lay the foundation for targeting key immune pathways to allogeneic islet transplantation within autoimmune patients.
While pharmacotherapy approaches are potent, they come with the requirement of lifelong and stringent medication adherence by the patient and the associated high risk of systemic side effects [101–103]. With these elevated risk and compliance needs, islet transplantation will remain relegated to a selected T1D cohort experiencing extreme challenges in glycemic control. As such, translation of systemic to targeted and localized immunosuppression, as well as the incorporation of more immunoregulatory methods, is needed to treat a broader population.
3. Effects of biomaterial approaches on immune pathways in islet transplantation
3.1. Biomaterial Immunoisolation
With direct antigen recognition as a key pathway that initiates adaptive immune responses, approaches that mask the islet cell surface could substantially suppress immune reactivity to the implant (Figure 2). Contrary to solid-organ transplantation, islet clusters permit ease in the incorporation of biomaterial-based approaches. Cellular encapsulation involves masking of the cell surface from the immune system by imparting a physical, semipermeable, polymer barrier [104–106]. The barrier must balance blocking non-specific IBMIR, as well as allo- and auto- direct recognition and rejection, while also allowing the effective exchange of nutrients, oxygen, metabolites, and hormones, such as insulin. To achieve this balance of protection and exchange, the pore size of the encapsulating material must be highly controlled. The most desirable porosity would be one that allows the efficient permeation of glucose (0.18 kDa; 0.4 nm) and insulin (5.8 kDa, 1.35 nm), but prevents immune cell (~8–15μm), immunoglobulin G (150 kDa; 5.9 nm), immunoglobulin A (300–400 kDa), complement C1q (410 kDa), and immunoglobulin M (950 kDa) infiltration [107, 108]. This tight pore range is challenging to impose, except in highly controlled porous membranes [109]. Furthermore, based on experimental evidence, molecular weight and Stokes radius are not the only aspects governing the diffusion of the molecules into the hydrogel [108].
Figure 2. Potential impacts of cell encapsulation on immune pathways in pancreatic islet transplantation.
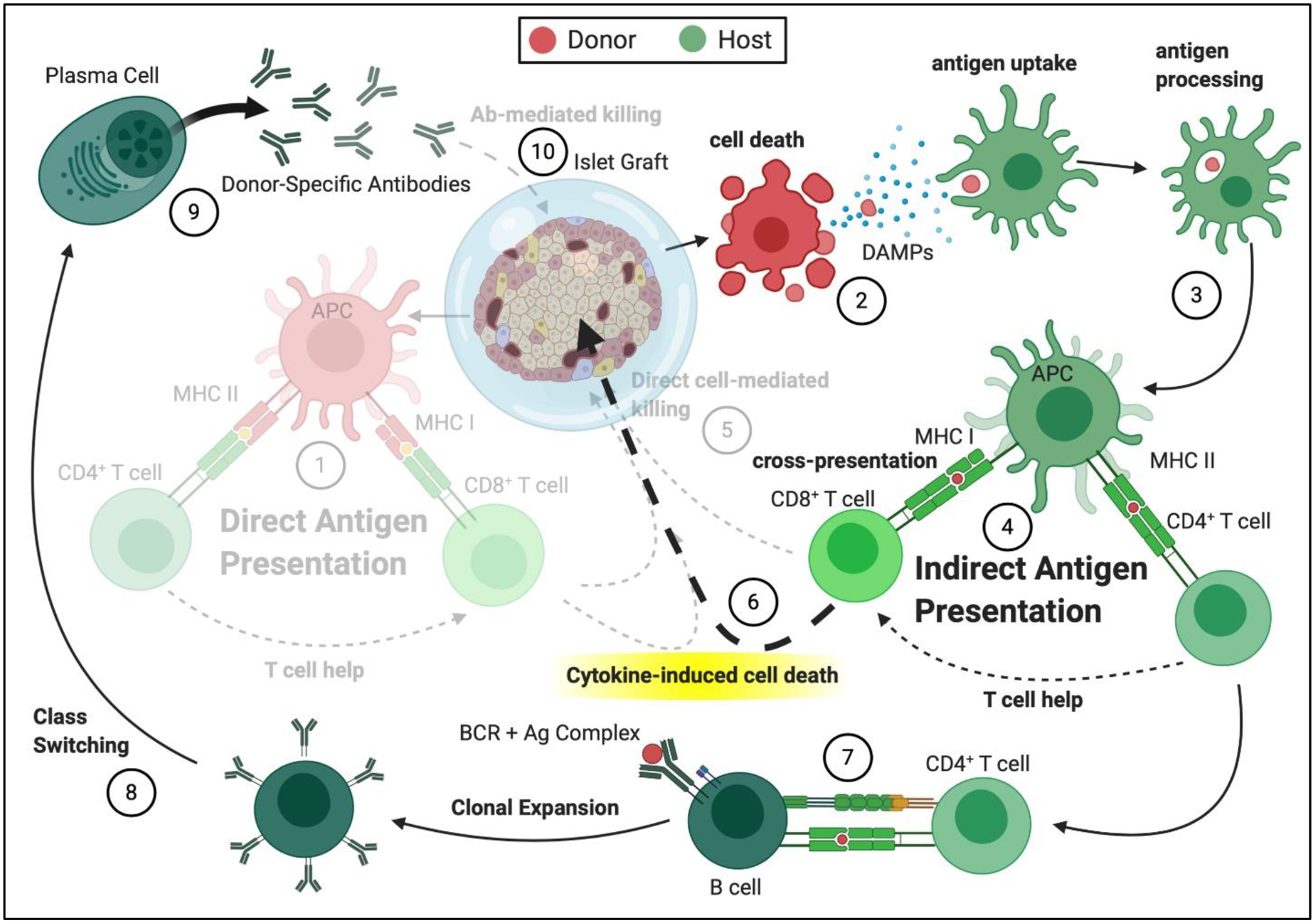
(1) Direct antigen presentation is blocked through use of immunoisolatory biomaterials. (2) Donor cell death persists and can be exacerbated due to deficiency in oxygen availability, elevating antigen shedding, which can diffuse through the semipermeable membrane. This supports (3) antigen uptake and processing and (4) retention of indirect antigen presentation. (5) Direct cell-mediated killing is blocked by the immunoisolatory material, which suppresses direct host immune cell infiltration. But, (6) cytokine-induced cell death can still occur, as cytokines can permeate the porous biomaterial. (7) CD4+ T cell-mediated B cell activation, (8) antibody class switching, and (9) production of donor-specific antibodies continues uninterrupted, although, (10) antibody-mediated graft destruction can be blocked by some encapsulating materials.
Hydrogels are widely used in cell encapsulation, as their mechanical properties, along with the high hydration degree, mimic soft tissues [110]. They can be synthesized in the micro and macro scale, which typically imposes a volume increase that prevents intrahepatic infusion [111–113]. To limit cytotoxicity, the process of hydrogel gelation must occur at physiological conditions with mild cross-linkers, which lead the field to the frequent use of alginate, agarose, and polyethylene glycol (PEG), as well as chitosan, collagen, and cellulose in cell encapsulation [110, 114–119].
Alginate is the most extensively used and studied microencapsulation material, reaching the stages of non-human primate and clinical trials [111, 114, 120–123]. Although the mechanical properties, pore size, and permeability of the alginate microcapsules cannot be independently altered, the monomer ratios, molecular weight, and utilization of different divalent cations for cross-linking can be utilized to adjust these parameters [124, 125]. Furthermore, the permselectivity of the alginate can be modified by coating the hydrogel with poly-L-lysine (PLL), PEG, and other polycations, resulting in improved immunoisolation in selected animal studies [126–130].
Agarose macrogels and microbeads have also been explored in animal allograft and autoimmune diabetes models, with one study showing protection of islet allografts in nonobese diabetic (NOD) mice for 100+ days [131–133]. The advantages of agarose include its ease of fabrication, temperature-controlled gelation, inert properties, and enhanced in vivo stability, when compared to alginate. The immunoisolatory properties of agarose can also be modulated via gel concentration, which allows for some degree of customization [134]. However, agarose exhibits some instability over time in the presence of cells, which limits it durability in vivo [135].
An alternative to encapsulating hydrogels sourced from natural materials is poly(ethylene glycol) (PEG). The synthetic and modular nature of PEG results in a high degree of control over permselectivity, cross-linking density, stability, and reactivity [136–138]. Unlike alginate, which swells over time under physiological conditions, PEG withstands osmotic stress [125, 139]. Its chemical structure also allows for relatively easy functionalization with extracellular matrix (ECM) or peptides such as arginylglycylaspartic acid (RGD), rendering it a highly versatile polymer for cell encapsulation [140–143]. Macro- and micro-scale PEG-based hydrogels have been used for islet encapsulation, with promising survival and immunoprotection observed in animal models [144, 145]. Leveraging cross-linking methods and fluidic technology, conformal coatings, on the scale of tens of microns, have also been generated around islet and stem-cell derived beta cell clusters, resulting in promising protection in several diabetic murine models [146, 147]. Further studies are needed, however, to translate a PEG-based hydrogel to larger animal models and to validate the long-term durability of the material.
Another method for masking the cellular surface is via polymer cell grafting, where polymers are bound to the cell or spheroid surface to mask cell surface proteins and antigens. This approach imparts a negligible increase in cell volume, which allows for the preservation of the current clinical transplant site; however, it is challenged by limited control over permeability and the need for highly cytocompatible polymers and cell surface conjugation methods. Long-chain PEG polymers are the most popular material used in this approach, as the terminal ends of the polymer can be easily controlled for selected cell surface ligation and the polymeric properties of PEG are inherently anti-fouling [148–150]. Islet surface modification using PEG has shown significant promise in both rodent and non-human primate diabetic models when combined with a reduced immunosuppressive regimen [112, 148, 151–154]. It is suspected that polymeric cell surface grafting reduces early inflammatory and innate immune responses to the implanted islets through the generation of a zone of hydration on the islet surface by the PEG chain [155]. However, the long-term immunosuppressive effects of PEG grafting remain unclear as the anti-fouling effects are likely short-term and intra-islet host cell infiltration have been observed. In addition to long-chain PEG grafting, other ultrathin layer-by-layer approaches have shown promise in masking the islet surface within theoretically more durable coatings that still provide the benefits of intraportal infusion [156–160].
While highly promising in murine models, the success of biomaterial encapsulation approaches in larger animal models and clinical trials have been limited. Of concern is that the efficacy of immunoisolation devices relies on the semipermeability of encapsulating materials, which are designed to ideally allow diffusion of nutrients, oxygen, and metabolites, while blocking host cells, antibodies, and complement to protect the graft [108, 114, 121]. Hydrogels with a porosity sufficient to block direct cell contact can effectively block the direct antigen recognition pathway [161]. Additionally, such hydrogels can block the contact-dependent CD8+ T cell-mediated killing resulting from the indirect pathway, which remains unaffected by encapsulation, as the antigens shed by donor cells can still escape the material barrier, become processed, and be presented by the host APCs to the host T cells [161]. Furthermore, the permeability of the barrier typically supports the diffusion of small pro-inflammatory cytokines (e.g., TNF (17–51 kDa), IL-1 (20 kDa), IFNγ (17kDa) released by indirectly activated T cells into the capsule [108]), meaning the cellular graft could still experience cytokine-mediated cell death (Figure 2) [161].
In addition to T cell activation, humoral immune pathways can also play a role, as accelerated graft failure is associated with the presence and elevation of islet autoantibodies pre- and post-transplantation of islet allografts into T1D recipients [69, 162, 163]. Therefore, the biomaterial permeability should be tailored to block antibody-mediated graft destruction [23, 108]. In addition, in vivo animal studies show that biomaterial encapsulation is insufficient in preventing the activation of humoral immunity through the indirect pathway, resulting in the generation and accumulation of donor-specific antibodies at the graft site, albeit with mixed results in terms of infiltration through the material [123, 164–167]. While antibodies may be blocked from directly interacting with the cellular graft, the de novo generation of alloantibodies has deleterious consequences in supporting other immune responses, as well as in patient sensitization [50].
3.2. Reducing encapsulated-cell death and material immunogenicity
Biomaterials initiate a foreign body reaction (FBR) depending on their chemical composition, shape, size, and geometry [168, 169]. Directly following implantation, the biomaterial surface becomes coated with a range of host proteins, where the biomaterial surface properties modulate the type and conformation of the adsorbed proteins [168, 170]. This process occurs even before the host immune cells have a chance to interact with the biomaterial surface, which means that the innate immune cell adhesion molecules interact with these surface-adsorbed proteins and direct the resultant immune response [168, 171, 172]. Following inflammatory cell infiltration and interaction with the biomaterial surface and adsorbed proteins, monocytes differentiate into macrophages and mediate the wound healing process at the site of implantation [168]. In most cases, the response to the material persists, resulting in a shift toward chronic inflammation, which leads to macrophage fusion, the generation of foreign body giant cells (FBGCs), and granulation tissue formation, leading to eventual foreign body response (FBR) via fibrous capsule formation around the implant [168]. When cells are placed within these biomaterial implants, host responses are further exacerbated, as shed antigens and danger signals released by the cells activate the adaptive immune arm [123, 164]. Globally, these responses detrimentally impact graft efficacy. For example, two recent clinical trials of islet and beta-cell transplants exhibited substantial declines in cellular function as the host responses to the implant transitioned from acute to chronic inflammation, and the FBR fully encapsulated the graft [173, 174].
A primary approach to modulate FBR is via the manipulation of the material properties, such as chemical structure, material charge, surface topography, mechanical properties, and crosslinking features. Improvement of material purity can improve biocompatibility and reduce immunogenicity [175, 176]. In addition, incorporating materials with biocompatible features, such as PEG or zwitterionic hydrogels, has shown decreased FBR to the implanted materials [177–180]. Alternatively, utilizing natural degradation processes of the material and/or modulating the material geometry can direct a more “healing” host cell response to the implant [181–183]. The careful selection and tailoring of material properties and features to minimize foreign body responses to the implant also imparts additional benefits in adaptive immune responses. Specifically, minimizing material immunogenicity can skew the innate APC population towards a more tolerogenic phenotype, which can reduce activation of the adaptive effector cells and potentially improve implant efficacy and/or graft survival [179, 184] (Figure 3).
Figure 3. Potential impacts of reduced material immunogenicity and improved cell implant viability on immune pathways in pancreatic islet transplantation.
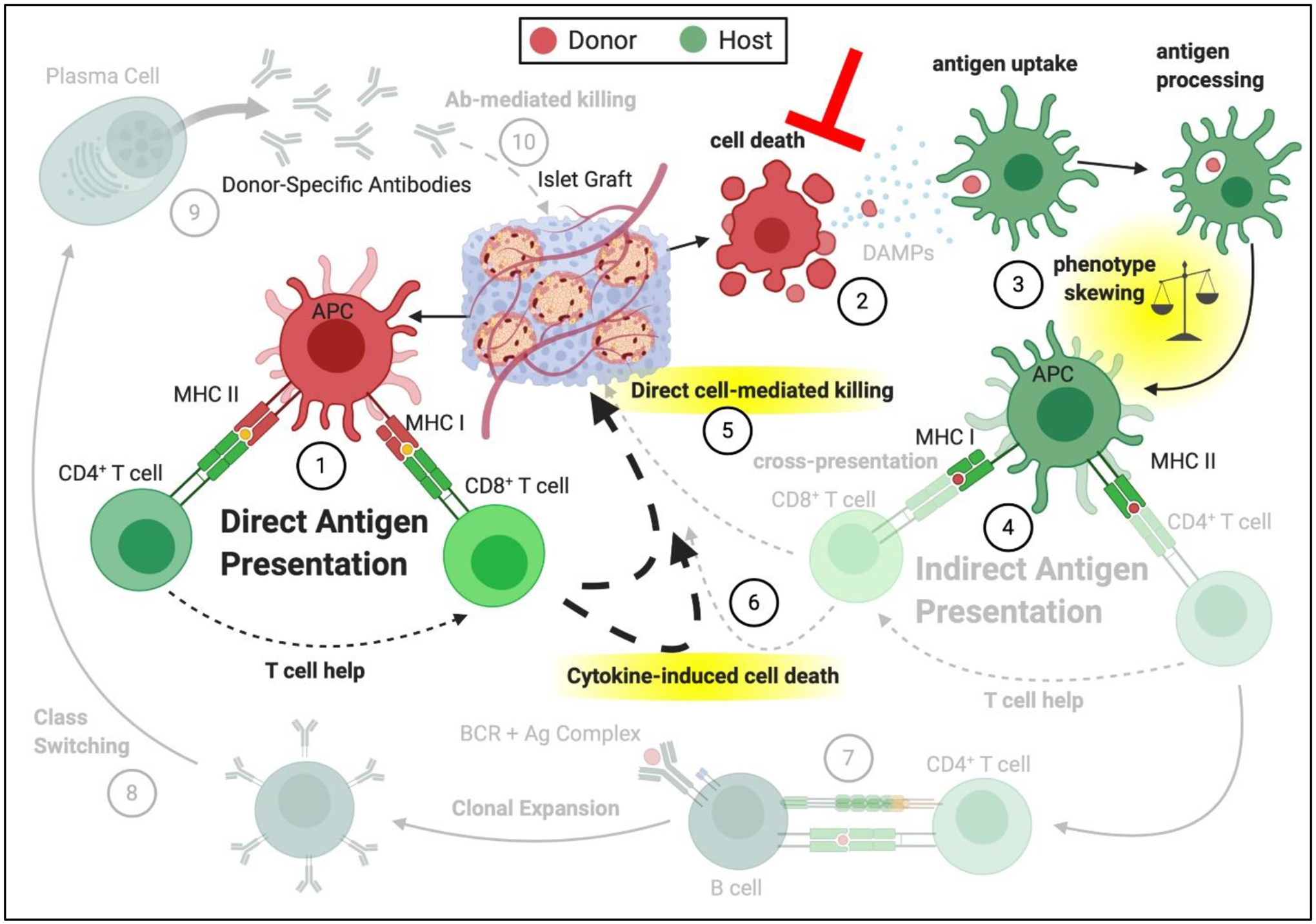
(1) Direct antigen presentation is retained for non-immunoisolatory, vascularized scaffolds. Approaches focused on improving donor cell viability post-transplantation, through vascularization, optimized 3-D material implants, and/or material-driven oxygen supplementation can result in reduced (2) donor cell death or change in the cell death pathway, which reduces DAMPs and/or shed antigen. (3) Antigen uptake and processing can be modified in the absence of DAMPs, leading to skewing of the phenotype of the innate APCs involved in the (4) indirect antigen presentation. APC phenotype shift can alter interactions with T lymphocytes and abrogate their activation. (5) Direct cell-mediated killing and the (6) cytokine-induced cell death can be mediated through the direct pathway only. Due to decreased T cell activation in the indirect pathway, (7) CD4+ T cell-mediated B cell activation, (8) antibody class switching, (9) production of donor-specific antibodies, and (10) antibody-mediated graft destruction can also be minimized.
Beyond responses to the material alone, the cellular cargo contained within the implant also dramatically contributes to the nature of the host response. Thus, minimizing the stress and death of the transplanted cells is crucial for curtailing the immune response to the graft. Intracellular factors, such as damage-associated molecular patterns (DAMPs), are released during cell necrosis due to tissue injury related to the donor organ procurement process [185]. Clinical studies in solid-organ transplants, where living-donor grafts are possible, such as kidney or liver, showed lower levels of immune cell infiltration and cytokine concentrations compared to cadaveric donor grafts [186, 187]. Studies show that both DAMPs and autoantigens, which are released during secondary necrosis, can activate APCs and induce an adaptive immune response, promoting chronic inflammation, fibrosis formation, and eventual graft rejection [188–190]. Thus, in the selection of the implantation site, awareness of vascular accessibility to ensure adequate nutrient delivery should be a critical parameter [191, 192]. While inflammation at the implant site can have deleterious effects for the transplanted cells, certain pro-inflammatory cytokines and immune cells have been shown to be in fact pro-angiogenic [193, 194]. This in turn indicates that low levels of inflammation at the implant site might be beneficial to the long-term survival of transplanted cells by promoting device vascularization. Alternatively, biomaterial and/or cellular approaches that enhance vascular infiltration into the graft site should result in improved cell viability and function [195–197] (Figure 3). For encapsulation devices on the macroscale, ensuring high cellular viability post-implantation is particularly challenging, as their large scale barriers introduce inefficient nutrient delivery [198]. Promoting more efficient accessibility of critical nutrients, particularly oxygen, is a challenge that several groups [199–202], including our own [203], have aimed to resolve. Enhancement of encapsulated beta cell viability has also been pursued through the use of agents acting on the encapsulated cells directly and provides an additional route of curtailing the indirect pathway, where donor cell death plays a critical role in delivering antigens to the host APCs [204–206]. Overall, supporting the viability of encapsulated islets results in two-fold benefits: it reduces the release of immunogenic intracellular contents; and preserves the cell load required for the achievement of glycemic control.
3.3. Designing materials for active and local immunomodulation
In addition to the careful selection of material properties and the implantation site, the biomaterials used for cell transplantation can be further enhanced by leveraging them for controlled drug release. These local drug delivery platforms can act as a sparing factor for systemic immunosuppression, resulting in decreased systemic side effects and enhanced general immune equilibrium, as well as a means to locally deliver novel agents that may not be suitable for systemic delivery. These local agents can target specific or multiple immune pathways that are activated following the implantation of either an unencapsulated or encapsulated cell platform (Figure 4).
Figure 4. Potential impacts of local immunosuppression and tolerogenic drug delivery on immune pathways in pancreatic islet transplantation.
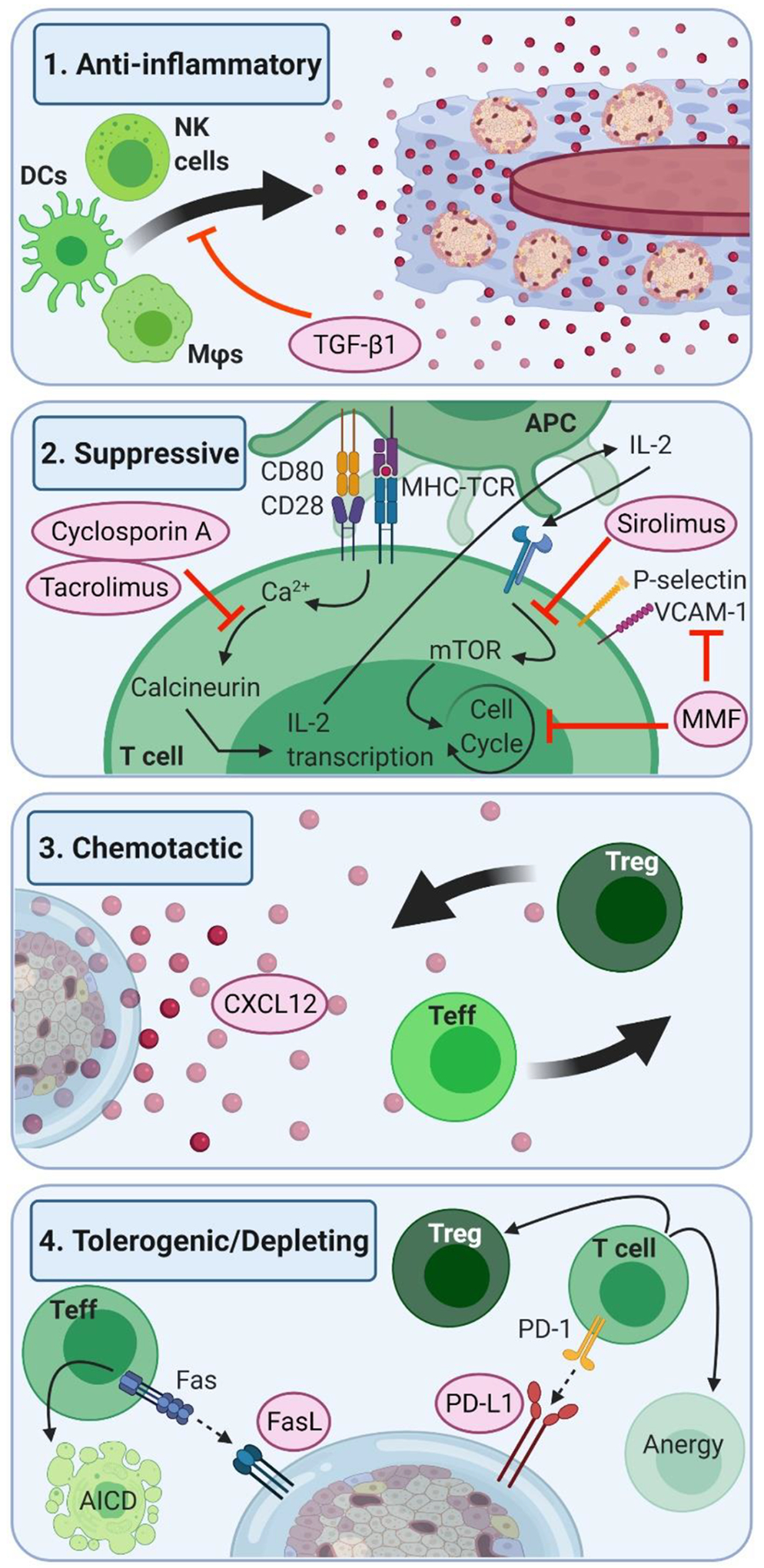
(1) Anti-Inflammatory biomaterial approaches can reduce inflammation in the immediate graft area, through the use of locally delivered anti-inflammatory drugs or cytokines, such as TGF-β1, which results in decreased infiltration of the innate cell populations responsible for aggravating adaptive responses to the graft. (2) Suppressive drug approaches utilize the local delivery of known systemic immunosuppressants, such as CNIs, mTOR inhibitors, or MMF which allow for the achievement of local suppression of the effector T cells, with the added benefit of decreased systemic side effects. (3) Chemotactic agents used in local delivery aim to stop the infiltration of effector immune cells, resulting in lowered immune response to the graft, without imparting a systemic lowered response to pathogenic infections. Agents such as CXCL12 have the additional benefit of increasing regulatory T cell infiltration, which aids in promoting graft tolerance. (4) Tolerogenic/Depleting strategies, such as local presentation of FasL or PD-L1 on biomaterial surface utilize potent native immune checkpoints and activation-induced cell death (AICD) pathways for the depletion of effector T cells, and induction of regulatory T cells in the graft environment.
The transplantation procedure, as well as cell death within tissue graft, initiates local tissue inflammation, which plays a key instructive role in both innate and adaptive immune responses, such as cell maturation and pro-inflammatory cytokine production [207]. This is further exacerbated in cases of cadaveric organ transplantation, such as clinical islet transplantation, due to a series of inflammatory changes occurring at the time of death of the donor that lead to inferior graft outcomes when compared to living donor transplants [187, 207]. The local delivery of anti-inflammatory agents, cytokines, and antioxidants provide an approach for reducing inflammation and downstream immune activation pathways, while potentially promoting a “healing” microenvironment at the graft site [208–211] (Figure 4.1). However, further studies are required, especially in T1D autoimmune models, to validate the potential efficacy of these approaches.
Utilization of the implant material to locally deliver agents is a classic approach of controlled drug delivery. Novel approaches incorporating immunosuppressive drugs, such as mTOR inhibitors, CNIs, and MMT, for local delivery provide a potential solution to the comorbidity problem associated with the systemic use of these agents [212–215] (Figure 4.2). In a complementary approach, a recent promising study adapted dibenzocyclooctyne (DBCO)-azide click chemistry to create re-fillable alginate drug depots capable of binding freshly injected intravenous functionalized drugs, allowing for long-term localized immunosuppression [212]. A challenge of translating effective systemic agents to local approaches, however, is that the delivery method can impart differences in immune cell effect or impair implant engraftment/survival. For instance, while systemic delivery of fingolimod has been found to reduce islet allograft rejection in animal model studies, its local delivery was detrimental to islet viability and function [216].
Local immunomodulation can also be achieved by looking beyond traditional immunosuppressive agents and instead harnessing the potential of local chemokine delivery to selectively limit the infiltration of effector T cells (Figure 4.3). In addition to impairment of recruitment, selective agents can also preserve or even recruit regulatory T cells to the implant site. For example, the chemokine CXCL12 has been shown to impart such effects on host T cell populations, while delivering survival signals for beta-cells and curbing inflammation [217–219]. Linking this chemokine onto the islet surface showed prolonged allograft survival but did not translate to the autoimmune diabetes mouse model [220]. When CXCL12 was combined with alginate encapsulation, graft efficacy and durability was substantially increased in both standard diabetic allograft and NOD models, when compared to alginate encapsulation alone [220]. In addition, further tests indicated that CXCL12 plus encapsulation protects grafts from memory T cells. In this study, recipient NOD mice were pretreated with skin transplantation from the donor mice strain, which subsequently rejected, and then received CXCL12-alginate encapsulated allograft islets [220]. Despite the presence of memory T cells created through the allograft skin transplantation and rejection process, the transplanted CXCL12-alginate encapsulated allograft islets survived significantly longer than alginate alone implants [220]. Given that both the CXCL12 and alginate encapsulation approach was needed to impart the desired effect, this approach implicates that the immune pathways blocked by encapsulation are distinct to those impaired by CXCL12.
Another approach to control immune responses seeks to mimic natural inhibitory and/or tolerogenic immune pathways through the modulation of specific immune cell interactions, such as Fas- Fas ligand (FasL) and programmed death ligand (PD-L1) (Figure 4.4). The immune checkpoint receptor Fas is a particularly desirable target, as nearly all cells of the immune system express this receptor and binding initiates activation-induced cell death (AICD), a native pathway crucial for the maintenance of self-tolerance [221]. While the Fas- Fas ligand (FasL) apoptotic pathway has long been implicated in the immunopathogenesis of autoimmune T1D, it is emerging as a target of interest in islet transplantation to trigger apoptosis in target cells after direct cell surface contact [221–224]. Recent approaches had used materials to locally present Fas ligand within the transplant site, where a shift in graft survival of allogeneic islets was observed when the FasL material was present [222–224]. The efficacy of this approach was further elevated when combined with a systemic short course of rapamycin, implicating a synergy between the local and systemic agents.
Cytotoxic T lymphocyte populations are more susceptible to FasL than the regulatory T cell populations, which adds to the attractiveness of this approach in inducing graft tolerance [221]. However, animal studies indicate that primed memory T cells were less susceptible to Fas-mediated AICD when compared to their naïve counterparts, potentially implicating that this approach might not be suitable for CIT in T1D patients where pre-existing autoimmunity results in a large population of memory T cells [54, 70, 225].
Of interest, the matter of delivery and presentation of FasL may be a key modulator of the downstream immune response, as FasL exists in two forms leading to duality in mediating apoptosis: a pro-apoptotic membrane-bound form expressed on the cell surface; and a usually anti-apoptotic soluble form created by proteolysis of the membrane-bound form that can become pro-apoptotic if bound to the surrounding matrix proteins [221, 226, 227]. This polarization of action is important to consider when designing biomaterials aimed at local immunomodulation, as presenting this protein in a soluble, rather than surface grafted, manner could switch downstream pathways from apoptotic to effector [227]. This phenomenon might not be isolated to the function of FasL, thus requiring factoring in the manner of delivery and the kinetics of therapeutic dose release in the design of immunomodulatory biomaterials.
The local presentation of another immune checkpoint, programmed cell death-ligand 1 (PD-L1), has also recently showed improved survival of allogeneic islet allografts in rodent models, when combined with short-course rapamycin treatment [228, 229]. For this approach, two methods were examined: local surface presentation onto the islet itself; and local surface presentation on a microbead co-transplanted within the islet graft. While the streptavidin (SA) mediated grafting of PD-L1 directly on islet surface supported lasting allograft survival and function in 90% of hosts, the presentation of PD-L1 on the surface of PEG-4MAL hydrogel microspheres resulted in the long-term function of ~58% of allografts [228, 229]. Both approaches yielded increases in local populations of regulatory T cells, implicating the benefits of leveraging this checkpoint in promoting more regulatory phenotypes. While the direct islet grafting approach resulted in improved allograft protection, the off-the-shelf accessibility of the material-only approach may lead to ease in clinical translation. Future studies should examine the efficacy of this approach in autoimmune models.
Overall, the examination of adaptive immune responses to allogeneic islets indicates the need for a combinatory approach that targets multiple activation pathways. For example, combining cellular encapsulation, which blocks the direct recognition pathway, with localized immunomodulatory agents that target both the indirect pathway and memory T cells would result in optimal transplant protection. As local immunomodulation may be imperfect, there are other complementary approaches that may provide further benefits, such as the integration of agents capable of scavenging diffusible pro-inflammatory cytokines before they can impart cellular damage [230]. In addition, a deeper understanding of knowledge gaps in the field regarding the role of indirect allorecognition in the autoimmune setting, and the resulting potential drug targets is needed to provide more targeted immunomodulatory approaches. Recent research in the development of humanized mouse models and benchtop diabetes screening platforms generated using human cells can facilitate the discovery of these key immune pathways [231, 232].
4. Conclusions and Future Recommendations
Beta cell replacement therapy has tremendous potential in providing durable and physiological glycemic control in people with T1D. Restricting its success, however, are the aggressive allogeneic and autoimmune responses following cadaveric islet infusion. While current immunosuppressive regimens are generally successful in curbing allogeneic immune responses, they fail to control the autoreactivity of the host immune system and restrict this therapy to the most at-risk T1D cohort.
The careful and targeted utilization of biomaterial-based approaches has the potential to both improve immunosuppression and decrease systemic impacts. While cellular encapsulation has been an appealing and well investigated approach to block direct antigen recognition and cell-mediated effector immune cell attack, it is evident that it is insufficient in blocking indirect immune activation and its downstream pathways, particularly in large scale models and humans. As such, tailored immunomodulatory biomaterial approaches that provide more instructive cues to the responding immune cells are needed, either to suppress their activation or convert these cells towards a more regulatory phenotype. The inherent modular nature of materials provides a unique platform for customizing immunomodulatory materials that inhibit or suppress specific pathways at the graft site. Thus, future work should continue to leverage materials for not only classic drug release, but also for scavenging activity and delivering instructional cues.
Finally, in order to efficiently optimize immunomodulatory biomaterial device design for cell therapy, more clinically predictive in vitro studies are needed to fill the knowledge gaps. Utilizing simplified but robust in vitro platforms for testing of biomaterial approaches conceivably allows for reductions in time from bench to bedside, research cost, and use of animals in in vivo studies. Lessons learned from understanding how to manipulate the allogeneic and autogenic immune responses to pancreatic islets can be applied to other diseases where underlying autoimmunity stands in the way of potential cell therapy, such as autoimmune thyroiditis (Hashimoto’s disease) and autoimmune adrenalitis (Addison’s disease) [233–235]
Statement of significance.
This review explores key immunologic concepts and critical pathways mediating graft rejection in Type 1 Diabetes, which can instruct the future purposeful design of immunomodulatory biomaterials for cell therapy. A summary of immunological pathways initiated following cellular implantation, as well as current systemic immunomodulatory agents used, is provided. We then outline the potential of biomaterials to modulate these responses. The capacity of polymeric encapsulation to block some powerful rejection pathways is covered. We also highlight the role of cellular health and biocompatibility in mitigating immune responses. Finally, we review the use of bioactive materials to proactively modulate local immune responses, focusing on key concepts of anti-inflammatory, suppressive, and tolerogenic agents.
Acknowledgements
The authors are grateful for funding from the US National Institutes of Health grants DK126413 and DK122638, as well as from JDRF grant 3-SRA-2021-1033-S-B. M. M. Samojlik is a predoctoral fellow in the NIH NIDDK T32 Interdisciplinary Graduate Program in Type 1 Diabetes and Biomedical Engineering (DK108736). All figures created with BioRender.com
References
- [1].Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG, Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients, J Exp Med 209(1) (2012) 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Atkinson MA, Eisenbarth GS, Michels AW, Type 1 diabetes, Lancet 383(9911) (2014) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gomez-Tourino I, Arif S, Eichmann M, Peakman M, T cells in type 1 diabetes: Instructors, regulators and effectors: A comprehensive review, J Autoimmun 66 (2016) 7–16. [DOI] [PubMed] [Google Scholar]
- [4].Bluestone JA, Bour-Jordan H, Cheng M, Anderson M, T cells in the control of organ-specific autoimmunity, J Clin Invest 125(6) (2015) 2250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Norris JM, Johnson RK, Stene LC, Type 1 diabetes-early life origins and changing epidemiology, Lancet Diabetes Endocrinol 8(3) (2020) 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cappon G, Vettoretti M, Sparacino G, Facchinetti A, Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications, Diabetes Metab J 43(4) (2019) 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cobelli C, Renard E, Kovatchev B, Artificial pancreas: past, present, future, Diabetes 60(11) (2011) 2672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ, Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges, Diabetes Metab Syndr Obes 7 (2014) 211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shapiro AM, Pokrywczynska M, Ricordi C, Clinical pancreatic islet transplantation, Nat Rev Endocrinol 13(5) (2017) 268–277. [DOI] [PubMed] [Google Scholar]
- [10].Ricordi C, Strom TB, Clinical islet transplantation: advances and immunological challenges, Nat Rev Immunol 4(4) (2004) 259–68. [DOI] [PubMed] [Google Scholar]
- [11].Henriksnäs J, Lau J, Zang G, Berggren PO, Köhler M, Carlsson PO, Markedly decreased blood perfusion of pancreatic islets transplanted intraportally into the liver: disruption of islet integrity necessary for islet revascularization, Diabetes 61(3) (2012) 665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].E. Corporation, 10th Annual Report, Collaborative Islet Transplant Registry, Rockville, MD, 2017. [Google Scholar]
- [13].Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR, International trial of the Edmonton protocol for islet transplantation, N Engl J Med 355(13) (2006) 1318–30. [DOI] [PubMed] [Google Scholar]
- [14].Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM, Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol, Diabetes 50(4) (2001) 710–9. [DOI] [PubMed] [Google Scholar]
- [15].Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV, Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen, N Engl J Med 343(4) (2000) 230–8. [DOI] [PubMed] [Google Scholar]
- [16].Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA, Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia, Diabetes Care 39(7) (2016) 1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roep BO, Improving Clinical Islet Transplantation Outcomes, Diabetes Care 43(4) (2020) 698–700. [DOI] [PubMed] [Google Scholar]
- [18].Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM, Five-year follow-up after clinical islet transplantation, Diabetes 54(7) (2005) 2060–9. [DOI] [PubMed] [Google Scholar]
- [19].Harlan DM, Kenyon NS, Korsgren O, Roep BO, Current advances and travails in islet transplantation, Diabetes 58(10) (2009) 2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O, Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation?, Diabetes 48(10) (1999) 1907–14. [DOI] [PubMed] [Google Scholar]
- [21].Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF, Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation, Am J Transplant 14(2) (2014) 428–37. [DOI] [PubMed] [Google Scholar]
- [22].Eich T, Eriksson O, Lundgren T, Nordic T Network for Clinical Islet, Visualization of early engraftment in clinical islet transplantation by positron-emission tomography, N Engl J Med, United States, 2007, pp. 2754–5. [DOI] [PubMed] [Google Scholar]
- [23].Tjernberg J, Ekdahl KN, Lambris JD, Korsgren O, Nilsson B, Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation, Transplantation 85(8) (2008) 1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nilsson B, Ekdahl KN, Korsgren O, Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment, Curr Opin Organ Transplant 16(6) (2011) 620–6. [DOI] [PubMed] [Google Scholar]
- [25].Safinia N, Afzali B, Atalar K, Lombardi G, Lechler RI, T-cell alloimmunity and chronic allograft dysfunction, Kidney Int Suppl (119) (2010) S2–12. [DOI] [PubMed] [Google Scholar]
- [26].Afzali B, Lombardi G, Lechler RI, Pathways of major histocompatibility complex allorecognition, Curr Opin Organ Transplant 13(4) (2008) 438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gill RG, Antigen presentation pathways for immunity to islet transplants. Relevance to immunoisolation, Ann N Y Acad Sci 875 (1999) 255–60. [DOI] [PubMed] [Google Scholar]
- [28].Gould DS, Auchincloss H Jr., Direct and indirect recognition: the role of MHC antigens in graft rejection, Immunol Today 20(2) (1999) 77–82. [DOI] [PubMed] [Google Scholar]
- [29].Rogers NJ, Lechler RI, Allorecognition, Am J Transplant 1(2) (2001) 97–102. [PubMed] [Google Scholar]
- [30].Siu JHY, Surendrakumar V, Richards JA, Pettigrew GJ, T cell Allorecognition Pathways in Solid Organ Transplantation, Front Immunol 9 (2018) 2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brown K, Sacks SH, Wong W, Coexpression of donor peptide/recipient MHC complex and intact donor MHC: evidence for a link between the direct and indirect pathways, Am J Transplant 11(4) (2011) 826–31. [DOI] [PubMed] [Google Scholar]
- [32].Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA, Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question, J Immunol 166(2) (2001) 973–81. [DOI] [PubMed] [Google Scholar]
- [33].Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Lechler RI, The role of the allograft in the induction of donor-specific T cell hyporesponsiveness, Transplantation 72(3) (2001) 480–5. [DOI] [PubMed] [Google Scholar]
- [34].Taylor AL, Negus SL, Negus M, Bolton EM, Bradley JA, Pettigrew GJ, Pathways of helper CD4 T cell allorecognition in generating alloantibody and CD8 T cell alloimmunity, Transplantation 83(7) (2007) 931–7. [DOI] [PubMed] [Google Scholar]
- [35].Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR, Help for cytotoxic-T-cell responses is mediated by CD40 signalling, Nature 393(6684) (1998) 478–80. [DOI] [PubMed] [Google Scholar]
- [36].Brennan TV, Jaigirdar A, Hoang V, Hayden T, Liu FC, Zaid H, Chang CK, Bucy RP, Tang Q, Kang SM, Preferential priming of alloreactive T cells with indirect reactivity, Am J Transplant 9(4) (2009) 709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shoskes DA, Wood KJ, Indirect presentation of MHC antigens in transplantation, Immunol Today 15(1) (1994) 32–8. [DOI] [PubMed] [Google Scholar]
- [38].Benichou G, Direct and indirect antigen recognition: the pathways to allograft immune rejection, Front Biosci 4 (1999) D476–80. [DOI] [PubMed] [Google Scholar]
- [39].Auchincloss H Jr., Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH, The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice, Proc Natl Acad Sci U S A 90(8) (1993) 3373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burrack AL, Martinov T, Fife BT, T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes, Frontiers in Endocrinology 8(343) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brown K, Sacks SH, Wong W, Extensive and bidirectional transfer of major histocompatibility complex class II molecules between donor and recipient cells in vivo following solid organ transplantation, Faseb j 22(11) (2008) 3776–84. [DOI] [PubMed] [Google Scholar]
- [42].Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE, Donor dendritic cell-derived exosomes promote allograft-targeting immune response, J Clin Invest 126(8) (2016) 2805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sivaganesh S, Harper SJ, Conlon TM, Callaghan CJ, Saeb-Parsy K, Negus MC, Motallebzadeh R, Bolton EM, Bradley JA, Pettigrew GJ, Copresentation of intact and processed MHC alloantigen by recipient dendritic cells enables delivery of linked help to alloreactive CD8 T cells by indirect-pathway CD4 T cells, J Immunol 190(11) (2013) 5829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lin CM, Gill RG, Direct and indirect allograft recognition: pathways dictating graft rejection mechanisms, Curr Opin Organ Transplant 21(1) (2016) 40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG, CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II, J Clin Invest 106(8) (2000) 1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Plenter RJ, Grazia TJ, Nelson DP, Zamora MR, Gill RG, Pietra BA, Ectopic expression of Fas Ligand on cardiomyocytes renders cardiac allografts resistant to CD4(+) T-cell mediated rejection, Cell Immunol 293(1) (2015) 30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, Rothstein DM, Lakkis FG, Non-self recognition by monocytes initiates allograft rejection, J Clin Invest 124(8) (2014) 3579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ochando J, Ordikhani F, Boros P, Jordan S, The innate immune response to allotransplants: mechanisms and therapeutic potentials, Cell Mol Immunol 16(4) (2019) 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhao D, Abou-Daya KI, Dai H, Oberbarnscheidt MH, Li XC, Lakkis FG, Innate Allorecognition and Memory in Transplantation, Front Immunol 11 (2020) 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gill RG, Lin CM, Linking innate immunity and chronic antibody-mediated allograft rejection, Curr Opin Organ Transplant 24(6) (2019) 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sauvé D, Baratin M, Leduc C, Bonin K, Daniel C, Alloantibody production is regulated by CD4+ T cells’ alloreactive pathway, rather than precursor frequency or Th1/Th2 differentiation, Am J Transplant 4(8) (2004) 1237–45. [DOI] [PubMed] [Google Scholar]
- [52].Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM, T cell responses: naive to memory and everything in between, Adv Physiol Educ 37(4) (2013) 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rathmell JC, Thompson CB, Pathways of apoptosis in lymphocyte development, homeostasis, and disease, Cell 109 Suppl (2002) S97–107. [DOI] [PubMed] [Google Scholar]
- [54].Burrack AL, Landry LG, Siebert J, Coulombe M, Gill RG, Nakayama M, Simultaneous Recognition of Allogeneic MHC and Cognate Autoantigen by Autoreactive T Cells in Transplant Rejection, J Immunol 200(4) (2018) 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sutherland DE, Goetz FC, Sibley RK, Recurrence of disease in pancreas transplants, Diabetes 38 Suppl 1 (1989) 85–7. [DOI] [PubMed] [Google Scholar]
- [56].Sibley RK, Sutherland DE, Goetz F, Michael AF, Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases, Lab Invest 53(2) (1985) 132–44. [PubMed] [Google Scholar]
- [57].Gras S, Kjer-Nielsen L, Chen Z, Rossjohn J, McCluskey J, The structural bases of direct T-cell allorecognition: implications for T-cell-mediated transplant rejection, Immunol Cell Biol 89(3) (2011) 388–95. [DOI] [PubMed] [Google Scholar]
- [58].Ely LK, Burrows SR, Purcell AW, Rossjohn J, McCluskey J, T-cells behaving badly: structural insights into alloreactivity and autoimmunity, Curr Opin Immunol 20(5) (2008) 575–80. [DOI] [PubMed] [Google Scholar]
- [59].Espinosa JR, Samy KP, Kirk AD, Memory T cells in organ transplantation: progress and challenges, Nat Rev Nephrol 12(6) (2016) 339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD, Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion, Am J Transplant 5(3) (2005) 465–74. [DOI] [PubMed] [Google Scholar]
- [61].Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA, Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it, J Immunol 176(8) (2006) 4632–9. [DOI] [PubMed] [Google Scholar]
- [62].Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD, The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation, Am J Transplant 14(2) (2014) 319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB 3rd, George R, Cheeseman J, Stempora L, Townsend R, Durrbach A, Kirk AD, CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection, Am J Transplant 16(4) (2016) 1102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vendrame F, Hopfner YY, Diamantopoulos S, Virdi SK, Allende G, Snowhite IV, Reijonen HK, Chen L, Ruiz P, Ciancio G, Hutton JC, Messinger S, Burke GW 3rd, Pugliese A, Risk Factors for Type 1 Diabetes Recurrence in Immunosuppressed Recipients of Simultaneous Pancreas-Kidney Transplants, Am J Transplant 16(1) (2016) 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, Diamantopoulos S, Standifer N, Geubtner K, Falk BA, Ichii H, Takahashi H, Snowhite I, Chen Z, Mendez A, Chen L, Sageshima J, Ruiz P, Ciancio G, Ricordi C, Reijonen H, Nepom GT, Burke GW 3rd, Pugliese A, Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells, Diabetes 59(4) (2010) 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Laughlin E, Burke G, Pugliese A, Falk B, Nepom G, Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation, Clin Immunol 128(1) (2008) 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gruessner RW, Pugliese A, Reijonen HK, Gruessner S, Jie T, Desai C, Sutherland DE, Burke GW 3rd, Development of diabetes mellitus in living pancreas donors and recipients, Expert Rev Clin Immunol 7(4) (2011) 543–51. [DOI] [PubMed] [Google Scholar]
- [68].Bosi E, Braghi S, Maffi P, Scirpoli M, Bertuzzi F, Pozza G, Secchi A, Bonifacio E, Autoantibody response to islet transplantation in type 1 diabetes, Diabetes 50(11) (2001) 2464–71. [DOI] [PubMed] [Google Scholar]
- [69].Bosi E, Bottazzo GF, Secchi A, Pozza G, Shattock M, Saunders A, Gelet A, Touraine JL, Traeger J, Dubernard JM, Islet cell autoimmunity in type I diabetic patients after HLA-mismatched pancreas transplantation, Diabetes 38 Suppl 1 (1989) 82–4. [DOI] [PubMed] [Google Scholar]
- [70].Gill RG, Burrack AL, Diverse Routes of Allograft Tolerance Disruption by Memory T Cells, Front Immunol 11 (2020) 580483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gala-Lopez B, Pepper AR, Shapiro AM, Biologic agents in islet transplantation, Curr Diab Rep 13(5) (2013) 713–22. [DOI] [PubMed] [Google Scholar]
- [72].Tejani A, Emmett L, Acute and chronic rejection, Semin Nephrol 21(5) (2001) 498–507. [DOI] [PubMed] [Google Scholar]
- [73].Sun ZJ, Du X, Su LL, Zhang XD, Wang W, Efficacy and Safety of Basiliximab Versus Daclizumab in Kidney Transplantation: A Meta-Analysis, Transplant Proc 47(8) (2015) 2439–45. [DOI] [PubMed] [Google Scholar]
- [74].Zhang Y, Jin W, Cai X, Anti-interleukin-2 receptor antibodies for the prevention of rejection in liver transplant recipients: a systematic review and meta-analysis, Ann Med 49(5) (2017) 365–376. [DOI] [PubMed] [Google Scholar]
- [75].Dai Z, Konieczny BT, Lakkis FG, The dual role of IL-2 in the generation and maintenance of CD8+ memory T cells, J Immunol 165(6) (2000) 3031–6. [DOI] [PubMed] [Google Scholar]
- [76].Parker MJ, Xue S, Alexander JJ, Wasserfall CH, Campbell-Thompson ML, Battaglia M, Gregori S, Mathews CE, Song S, Troutt M, Eisenbeis S, Williams J, Schatz DA, Haller MJ, Atkinson MA, Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice, Diabetes 58(10) (2009) 2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mohty M, Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond, Leukemia 21(7) (2007) 1387–94. [DOI] [PubMed] [Google Scholar]
- [78].Froud T, Baidal DA, Faradji R, Cure P, Mineo D, Selvaggi G, Kenyon NS, Ricordi C, Alejandro R, Islet transplantation with alemtuzumab induction and calcineurin-free maintenance immunosuppression results in improved short- and long-term outcomes, Transplantation 86(12) (2008) 1695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tooley JE, Vudattu N, Choi J, Cotsapas C, Devine L, Raddassi K, Ehlers MR, McNamara JG, Harris KM, Kanaparthi S, Phippard D, Herold KC, Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes, Eur J Immunol 46(1) (2016) 230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Masharani UB, Becker J, Teplizumab therapy for type 1 diabetes, Expert Opin Biol Ther 10(3) (2010) 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rickels MR, Robertson RP, Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions, Endocrine Reviews 40(2) (2018) 631–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DER, Alejandro R, Hering BJ, Potent Induction Immunotherapy Promotes Long-Term Insulin Independence After Islet Transplantation in Type 1 Diabetes, American Journal of Transplantation 12(6) (2012) 1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ngo A, Sutherland DE, Beilman GJ, Bellin MD, Deterioration of glycemic control after corticosteroid administration in islet autotransplant recipients: a cautionary tale, Acta Diabetol 51(1) (2014) 141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fung JJ, Alessiani M, Abu-Elmagd K, Todo S, Shapiro R, Tzakis A, Van Thiel D, Armitage J, Jain A, McCauley J, Selby R, Starzl TE, Adverse effects associated with the use of FK 506, Transplant Proc 23(6) (1991) 3105–8. [PMC free article] [PubMed] [Google Scholar]
- [85].Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N, Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus, Am J Transplant 7(6) (2007) 1506–14. [DOI] [PubMed] [Google Scholar]
- [86].McMahon G, Weir MR, Li XC, Mandelbrot DA, The evolving role of mTOR inhibition in transplantation tolerance, J Am Soc Nephrol 22(3) (2011) 408–15. [DOI] [PubMed] [Google Scholar]
- [87].Berney T, Secchi A, Rapamycin in islet transplantation: friend or foe?, Transpl Int 22(2) (2009) 153–61. [DOI] [PubMed] [Google Scholar]
- [88].Cross SE, Richards SK, Clark A, Benest AV, Bates DO, Mathieson PW, Johnson PR, Harper SJ, Smith RM, Vascular endothelial growth factor as a survival factor for human islets: effect of immunosuppressive drugs, Diabetologia 50(7) (2007) 1423–32. [DOI] [PubMed] [Google Scholar]
- [89].Marcelli-Tourvieille S, Hubert T, Moerman E, Gmyr V, Kerr-Conte J, Nunes B, Dherbomez M, Vandewalle B, Pattou F, Vantyghem MC, In vivo and in vitro effect of sirolimus on insulin secretion, Transplantation 83(5) (2007) 532–8. [DOI] [PubMed] [Google Scholar]
- [90].Mele TS, Halloran PF, The use of mycophenolate mofetil in transplant recipients, Immunopharmacology 47(2–3) (2000) 215–45. [DOI] [PubMed] [Google Scholar]
- [91].Lowe MC, Badell IR, Turner AP, Thompson PW, Leopardi FV, Strobert EA, Larsen CP, Kirk AD, Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy, Am J Transplant 13(2) (2013) 312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, McElroy J, Ramos MD, Kerlan RK, Fong L, Vincenti F, Bluestone JA, Stock PG, Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade, Transplantation 90(12) (2010) 1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Page AJ, Ford ML, Kirk AD, Memory T-cell-specific therapeutics in organ transplantation, Curr Opin Organ Transplant 14(6) (2009) 643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, Avila JG, Cano JA, Johnson BE, Song M, Leopardi FV, Swygert S, Strobert EA, Ford ML, Kirk AD, Larsen CP, LFA-1-specific therapy prolongs allograft survival in rhesus macaques, J Clin Invest 120(12) (2010) 4520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, Bluestone JA, Stock PG, Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab, Am J Transplant 10(8) (2010) 1870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, Kampen RL, Stempora L, Song M, Larsen CP, Kirk AD, Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates, Nat Med 15(7) (2009) 746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jacobsen LM, Bundy BN, Greco MN, Schatz DA, Atkinson MA, Brusko TM, Mathews CE, Herold KC, Gitelman SE, Krischer JP, Haller MJ, Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes, Diabetes Technol Ther (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Patel CM, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Moran A, Russell WE, Pinckney A, Keyes-Elstein L, Howell M, Aggarwal S, Lim N, Phippard D, Nepom GT, McNamara J, Ehlers MR, Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial, Lancet Diabetes Endocrinol 1(4) (2013) 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kekre N, Kim HT, Hofer J, Ho VT, Koreth J, Armand P, Nikiforow S, Gooptu M, Romee R, Alyea EP, Nageshwar P, Glotzbecker B, El-Jawahri A, DeFilipp Z, Soiffer RJ, Antin JH, Chen YB, Cutler C, Phase II trial of natalizumab with corticosteroids as initial treatment of gastrointestinal acute graft-versus-host disease, Bone Marrow Transplant (2020). [DOI] [PubMed] [Google Scholar]
- [100].Fløisand Y, Lazarevic VL, Maertens J, Mattsson J, Shah NN, Zachée P, Taylor A, Akbari M, Quadri S, Parfionovas A, Chen YB, Safety and Effectiveness of Vedolizumab in Patients with Steroid-Refractory Gastrointestinal Acute Graft-versus-Host Disease: A Retrospective Record Review, Biol Blood Marrow Transplant 25(4) (2019) 720–727. [DOI] [PubMed] [Google Scholar]
- [101].Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR, Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression, Am J Transplant 9(11) (2009) 2597–606. [DOI] [PubMed] [Google Scholar]
- [102].Takemoto SK, Pinsky BW, Schnitzler MA, Lentine KL, Willoughby LM, Burroughs TE, Bunnapradist S, A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients, Am J Transplant 7(12) (2007) 2704–11. [DOI] [PubMed] [Google Scholar]
- [103].Jasiak NM, Park JM, Immunosuppression in Solid-Organ Transplantation: Essentials and Practical Tips, Crit Care Nurs Q 39(3) (2016) 227–40. [DOI] [PubMed] [Google Scholar]
- [104].Buchwald P, Cechin SR, Weaver JD, Stabler CL, Experimental evaluation and computational modeling of the effects of encapsulation on the time-profile of glucose-stimulated insulin release of pancreatic islets, Biomed Eng Online 14 (2015) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Buchwald P, Tamayo-Garcia A, Manzoli V, Tomei AA, Stabler CL, Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets, Biotechnol Bioeng 115(1) (2018) 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Weber LM, Lopez CG, Anseth KS, Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function, J Biomed Mater Res A 90(3) (2009) 720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schweicher J, Nyitray C, Desai TA, Membranes to achieve immunoprotection of transplanted islets, Front Biosci (Landmark Ed) 19 (2014) 49–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ríhová B, Immunocompatibility and biocompatibility of cell delivery systems, Adv Drug Deliv Rev 42(1–2) (2000) 65–80. [DOI] [PubMed] [Google Scholar]
- [109].Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, Tang Q, Roy S, Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport, Scientific Reports 6(1) (2016) 23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nicodemus GD, Bryant SJ, Cell encapsulation in biodegradable hydrogels for tissue engineering applications, Tissue Eng Part B Rev 14(2) (2008) 149–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, Khan A, Yeh CC, Xing Y, Isa D, Ghani S, Li J, Landry C, Bader AR, Olejnik K, Chen M, Hollister-Lock J, Wang Y, Greiner DL, Weir GC, Strand BL, Rokstad AMA, Lacik I, Langer R, Anderson DG, Oberholzer J, Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques, Nat Biomed Eng 2(11) (2018) 810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Teramura Y, Asif S, Ekdahl KN, Nilsson B, Cell Surface Engineering for Regulation of Immune Reactions in Cell Therapy, Adv Exp Med Biol 865 (2015) 189–209. [DOI] [PubMed] [Google Scholar]
- [113].Van Der Windt DJ, Echeverri GJ, Ijzermans JNM, Cooper DKC, The Choice of Anatomical Site for Islet Transplantation, Cell Transplant 17(9) (2008) 1005–1014. [DOI] [PubMed] [Google Scholar]
- [114].Desai T, Shea LD, Advances in islet encapsulation technologies, Nat Rev Drug Discov 16(5) (2017) 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stabler CL, Li Y, Stewart JM, Keselowsky BG, Engineering immunomodulatory biomaterials for type 1 diabetes, Nat Rev Mater 4(6) (2019) 429–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Harrington S, Williams J, Rawal S, Ramachandran K, Stehno-Bittel L, Hyaluronic Acid/Collagen Hydrogel as an Alternative to Alginate for Long-Term Immunoprotected Islet Transplantation<sup/>, Tissue Eng Part A 23(19–20) (2017) 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Risbud MV, Bhargava S, Bhonde RR, In vivo biocompatibility evaluation of cellulose macrocapsules for islet immunoisolation: Implications of low molecular weight cut-off, J Biomed Mater Res A 66(1) (2003) 86–92. [DOI] [PubMed] [Google Scholar]
- [118].Risbud MV, Bhonde RR, Islet immunoisolation: experience with biopolymers, J Biomater Sci Polym Ed 12(11) (2001) 1243–52. [DOI] [PubMed] [Google Scholar]
- [119].Yang KC, Qi Z, Wu CC, Shirouza Y, Lin FH, Yanai G, Sumi S, The cytoprotection of chitosan based hydrogels in xenogeneic islet transplantation: An in vivo study in streptozotocin-induced diabetic mouse, Biochem Biophys Res Commun 393(4) (2010) 818–23. [DOI] [PubMed] [Google Scholar]
- [120].Duvivier-Kali VF, Omer A, Parent RJ, O’Neil JJ, Weir GC, Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane, Diabetes 50(8) (2001) 1698–705. [DOI] [PubMed] [Google Scholar]
- [121].Scharp DW, Marchetti P, Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution, Adv Drug Deliv Rev 67–68 (2014) 35–73. [DOI] [PubMed] [Google Scholar]
- [122].Weir GC, Islet encapsulation: advances and obstacles, Diabetologia 56(7) (2013) 1458–61. [DOI] [PubMed] [Google Scholar]
- [123].Dufrane D, Goebbels RM, Gianello P, Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression, Transplantation 90(10) (2010) 1054–62. [DOI] [PubMed] [Google Scholar]
- [124].Lee KY, Mooney DJ, Alginate: properties and biomedical applications, Prog Polym Sci 37(1) (2012) 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bhujbal SV, Paredes-Juarez GA, Niclou SP, de Vos P, Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells, J Mech Behav Biomed Mater 37 (2014) 196–208. [DOI] [PubMed] [Google Scholar]
- [126].Soon-Shiong P, Feldman E, Nelson R, Komtebedde J, Smidsrod O, Skjak-Braek G, Espevik T, Heintz R, Lee M, Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets, Transplantation 54(5) (1992) 769–74. [DOI] [PubMed] [Google Scholar]
- [127].Lanza RP, Jackson R, Sullivan A, Ringeling J, McGrath C, Kühtreiber W, Chick WL, Xenotransplantation of cells using biodegradable microcapsules, Transplantation 67(8) (1999) 1105–11. [DOI] [PubMed] [Google Scholar]
- [128].Clayton HA, James RF, London NJ, Islet microencapsulation: a review, Acta Diabetol 30(4) (1993) 181–9. [DOI] [PubMed] [Google Scholar]
- [129].Gattás-Asfura K, Valdes M, Celik E, Stabler C, Covalent layer-by-layer assembly of hyperbranched polymers on alginate microcapsulesto impart stability and permselectivity, J Mater Chem B 2(46) (2014) 8208–8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Verheyen CA, Morales L, Sussman J, Paunovska K, Manzoli V, Ziebarth NM, Tomei AA, Characterization of Polyethylene Glycol-Reinforced Alginate Microcapsules for Mechanically Stable Cell Immunoisolation, Macromol Mater Eng 304(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Iwata H, Takagi T, Amemiya H, Shimizu H, Yamashita K, Kobayashi K, Akutsu T, Agarose for a bioartificial pancreas, J Biomed Mater Res 26(7) (1992) 967–77. [DOI] [PubMed] [Google Scholar]
- [132].Iwata H, Kobayashi K, Takagi T, Oka T, Yang H, Amemiya H, Tsuji T, Ito F, Feasibility of agarose microbeads with xenogeneic islets as a bioartificial pancreas, J Biomed Mater Res 28(9) (1994) 1003–11. [DOI] [PubMed] [Google Scholar]
- [133].Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanaga M, Ko S, Nagao M, Nakajima Y, Indefinite islet protection from autoimmune destruction in nonobese diabetic mice by agarose microencapsulation without immunosuppression, Transplantation 75(5) (2003) 619–25. [DOI] [PubMed] [Google Scholar]
- [134].Hu S, de Vos P, Polymeric Approaches to Reduce Tissue Responses Against Devices Applied for Islet-Cell Encapsulation, Front Bioeng Biotechnol 7 (2019) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shoichet MS, Li RH, White ML, Winn SR, Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose, Biotechnol Bioeng 50(4) (1996) 374–81. [DOI] [PubMed] [Google Scholar]
- [136].Smith LJ, Taimoory SM, Tam RY, Baker AEG, Binth Mohammad N, Trant JF, Shoichet MS, Diels–Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation, Biomacromolecules 19(3) (2018) 926–935. [DOI] [PubMed] [Google Scholar]
- [137].Day JR, David A, Kim J, Farkash EA, Cascalho M, Milašinović N, Shikanov A, The impact of functional groups of poly(ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host, Acta Biomaterialia 67 (2018) 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Weber LM, He J, Bradley B, Haskins K, Anseth KS, PEG-based hydrogels as an in vitro encapsulation platform for testing controlled β-cell microenvironments, Acta Biomaterialia 2(1) (2006) 1–8. [DOI] [PubMed] [Google Scholar]
- [139].Manzoli V, Villa C, Bayer AL, Morales LC, Molano RD, Torrente Y, Ricordi C, Hubbell JA, Tomei AA, Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol, Am J Transplant 18(3) (2018) 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Phelps EA, Headen DM, Taylor WR, Thulé PM, García AJ, Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes, Biomaterials 34(19) (2013) 4602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Weaver JD, Headen DM, Hunckler MD, Coronel MM, Stabler CL, García AJ, Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation, Biomaterials 172 (2018) 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lan T, Guo J, Bai X, Huang Z, Wei Z, Du G, Yan G, Weng L, Yi X, RGD-modified injectable hydrogel maintains islet beta-cell survival and function, J Appl Biomater Funct Mater 18 (2020) 2280800020963473. [DOI] [PubMed] [Google Scholar]
- [143].Lin CC, Anseth KS, Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function, Proc Natl Acad Sci U S A 108(16) (2011) 6380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Weaver JD, Headen DM, Coronel MM, Hunckler MD, Shirwan H, García AJ, Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site, American Journal of Transplantation 19(5) (2019) 1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA, In Vitro and in Vivo Performance of Porcine Islets Encapsulated in Interfacially Photopolymerized Poly(Ethylene Glycol) Diacrylate Membranes, Cell Transplantation 8(3) (1999) 293–306. [DOI] [PubMed] [Google Scholar]
- [146].Stock AA, Manzoli V, De Toni T, Abreu MM, Poh Y-C, Ye L, Roose A, Pagliuca FW, Thanos C, Ricordi C, Tomei AA, Conformal Coating of Stem Cell-Derived Islets for β Cell Replacement in Type 1 Diabetes, Stem Cell Reports 14(1) (2020) 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA, Device design and materials optimization of conformal coating for islets of Langerhans, Proceedings of the National Academy of Sciences 111(29) (2014) 10514–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Giraldo JA, Molano RD, Rengifo HR, Fotino C, Gattás-Asfura KM, Pileggi A, Stabler CL, The impact of cell surface PEGylation and short-course immunotherapy on islet graft survival in an allogeneic murine model, Acta Biomater 49 (2017) 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lee DY, Park SJ, Lee S, Nam JH, Byun Y, Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication, Tissue Eng 13(8) (2007) 2133–41. [DOI] [PubMed] [Google Scholar]
- [150].Lee DY, Park SJ, Nam JH, Byun Y, A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: long-functioning PEGylated islets in vivo, Tissue Eng 12(3) (2006) 615–23. [DOI] [PubMed] [Google Scholar]
- [151].Stabler CL, Giraldo JA, Berman DM, Gattás-Asfura KM, Willman MA, Rabassa A, Geary J, Diaz W, Kenyon NM, Kenyon NS, Transplantation of PEGylated islets enhances therapeutic efficacy in a diabetic nonhuman primate model, Am J Transplant 20(3) (2020) 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, Yu Y, Mundwiler KE, Cole JF, Hubbell JA, Hegre OD, Scharp DW, Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets, Ann N Y Acad Sci 831 (1997) 332–43. [DOI] [PubMed] [Google Scholar]
- [153].Lee DY, Park SJ, Nam JH, Byun Y, A combination therapy of PEGylation and immunosuppressive agent for successful islet transplantation, J Control Release 110(2) (2006) 290–295. [DOI] [PubMed] [Google Scholar]
- [154].Yun Lee D, Hee Nam J, Byun Y, Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year, Biomaterials 28(11) (2007) 1957–66. [DOI] [PubMed] [Google Scholar]
- [155].Meyers SR, Grinstaff MW, Biocompatible and bioactive surface modifications for prolonged in vivo efficacy, Chem Rev 112(3) (2012) 1615–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Rengifo HR, Giraldo JA, Labrada I, Stabler CL, Long-term survival of allograft murine islets coated via covalently stabilized polymers, Adv Healthc Mater 3(7) (2014) 1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Gattás-Asfura KM, Abuid NJ, Labrada I, Stabler CL, Promoting Dendrimer Self-Assembly Enhances Covalent Layer-by-Layer Encapsulation of Pancreatic Islets, ACS Biomater Sci Eng 6(5) (2020) 2641–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Gattás-Asfura KM, Stabler CL, Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers, ACS Appl Mater Interfaces 5(20) (2013) 9964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wilson JT, Cui W, Chaikof EL, Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation, Nano Lett 8(7) (2008) 1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Teramura Y, Kaneda Y, Iwata H, Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane, Biomaterials 28(32) (2007) 4818–25. [DOI] [PubMed] [Google Scholar]
- [161].Li Y, Frei AW, Yang EY, Labrada-Miravet I, Sun C, Rong Y, Samojlik MM, Bayer AL, Stabler CL, In vitro platform establishes antigen-specific CD8(+) T cell cytotoxicity to encapsulated cells via indirect antigen recognition, Biomaterials 256 (2020) 120182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Jaeger C, Brendel MD, Hering BJ, Eckhard M, Bretzel RG, Progressive islet graft failure occurs significantly earlier in autoantibody-positive than in autoantibody-negative IDDM recipients of intrahepatic islet allografts, Diabetes 46(11) (1997) 1907–10. [DOI] [PubMed] [Google Scholar]
- [163].Thivolet C, Abou-Amara S, Martin X, Lefrancois N, Petruzzo P, McGregor B, Bosshard S, Dubernard JM, Serological markers of recurrent beta cell destruction in diabetic patients undergoing pancreatic transplantation, Transplantation 69(1) (2000) 99–103. [DOI] [PubMed] [Google Scholar]
- [164].Safley SA, Cui H, Cauffiel SM, Xu BY, Wright JR Jr., Weber CJ, Encapsulated piscine (tilapia) islets for diabetes therapy: studies in diabetic NOD and NOD-SCID mice, Xenotransplantation 21(2) (2014) 127–39. [DOI] [PubMed] [Google Scholar]
- [165].Duvivier-Kali VF, Omer A, Lopez-Avalos MD, O’Neil JJ, Weir GC, Survival of microencapsulated adult pig islets in mice in spite of an antibody response, Am J Transplant 4(12) (2004) 1991–2000. [DOI] [PubMed] [Google Scholar]
- [166].Jones KS, Sefton MV, Gorczynski RM, In vivo recognition by the host adaptive immune system of microencapsulated xenogeneic cells, Transplantation 78(10) (2004) 1454–62. [DOI] [PubMed] [Google Scholar]
- [167].Siebers U, Horcher A, Brandhorst H, Brandhorst D, Hering B, Federlin K, Bretzel RG, Zekorn T, Analysis of the cellular reaction towards microencapsulated xenogeneic islets after intraperitoneal transplantation, J Mol Med (Berl) 77(1) (1999) 215–8. [DOI] [PubMed] [Google Scholar]
- [168].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin Immunol 20(2) (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates, Nat Mater 14(6) (2015) 643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Roach P, Farrar D, Perry CC, Interpretation of protein adsorption: surface-induced conformational changes, J Am Chem Soc 127(22) (2005) 8168–73. [DOI] [PubMed] [Google Scholar]
- [171].Kushiro K, Lee CH, Takai M, Simultaneous characterization of protein-material and cell-protein interactions using dynamic QCM-D analysis on SAM surfaces, Biomater Sci 4(6) (2016) 989–97. [DOI] [PubMed] [Google Scholar]
- [172].Visalakshan RM, MacGregor MN, Sasidharan S, Ghazaryan A, Mierczynska-Vasilev AM, Morsbach S, Mailänder V, Landfester K, Hayball JD, Vasilev K, Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses, ACS Appl Mater Interfaces 11(31) (2019) 27615–27623. [DOI] [PubMed] [Google Scholar]
- [173].Carlsson PO, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, Goldman T, Barkai U, Avni Y, Westermark GT, Carlbom L, Ahlström H, Eriksson O, Olerud J, Korsgren O, Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus, Am J Transplant 18(7) (2018) 1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Odorico J, Markmann J, Melton D, Greenstein J, Hwa A, Nostro C, Rezania A, Oberholzer J, Pipeleers D, Yang L, Cowan C, Huangfu D, Egli D, Ben-David U, Vallier L, Grey ST, Tang Q, Roep B, Ricordi C, Naji A, Orlando G, Anderson DG, Poznansky M, Ludwig B, Tomei A, Greiner DL, Graham M, Carpenter M, Migliaccio G, D’Amour K, Hering B, Piemonti L, Berney T, Rickels M, Kay T, Adams A, Report of the Key Opinion Leaders Meeting on Stem Cell-derived Beta Cells, Transplantation 102(8) (2018) 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Clayton HA, London NJ, Colloby PS, Bell PR, James RF, The effect of capsule composition on the biocompatibility of alginate-poly-l-lysine capsules, J Microencapsul 8(2) (1991) 221–33. [DOI] [PubMed] [Google Scholar]
- [176].De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R, Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets, Diabetologia 40(3) (1997) 262–70. [DOI] [PubMed] [Google Scholar]
- [177].Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Graham AC, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nat Biotechnol 34(3) (2016) 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Liu Q, Chiu A, Wang L, An D, Li W, Chen EY, Zhang Y, Pardo Y, McDonough SP, Liu L, Liu WF, Chen J, Ma M, Developing mechanically robust, triazole-zwitterionic hydrogels to mitigate foreign body response (FBR) for islet encapsulation, Biomaterials 230 (2020) 119640. [DOI] [PubMed] [Google Scholar]
- [179].Li B, Yuan Z, Jain P, Hung HC, He Y, Lin X, McMullen P, Jiang S, De novo design of functional zwitterionic biomimetic material for immunomodulation, Sci Adv 6(22) (2020) eaba0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Liu XY, Nothias J-M, Scavone A, Garfinkel M, Millis JM, Biocompatibility Investigation of Polyethylene Glycol and Alginate-Poly-l-Lysine for Islet Encapsulation, ASAIO Journal 56(3) (2010) 241–245. [DOI] [PubMed] [Google Scholar]
- [181].Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF, Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine, Biomaterials 33(15) (2012) 3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Witherel CE, Abebayehu D, Barker TH, Spiller KL, Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis, Advanced Healthcare Materials 8(4) (2019) 1801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF, Macrophage polarization in response to ECM coated polypropylene mesh, Biomaterials 35(25) (2014) 6838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Zhu F.-j., Tong Y.-l., Sheng Z.-y., Yao Y.-m., Role of dendritic cells in the host response to biomaterials and their signaling pathways, Acta Biomaterialia 94 (2019) 132–144. [DOI] [PubMed] [Google Scholar]
- [185].Hébert M-J, Jevnikar AM, The Impact of Regulated Cell Death Pathways on Alloimmune Responses and Graft Injury, Current Transplantation Reports 2(3) (2015) 242–258. [Google Scholar]
- [186].D’Alessandro AM, Pirsch JD, Knechtle SJ, Odorico JS, Van der Werf WJ, Collins BH, Becker YT, Kalayoglu M, Armbrust MJ, Sollinger HW, Living unrelated renal donation: the University of Wisconsin experience, Surgery 124(4) (1998) 604–10; discussion 610–1. [DOI] [PubMed] [Google Scholar]
- [187].Hann A, Osei-Bordom DC, Neil DAH, Ronca V, Warner S, Perera M, The Human Immune Response to Cadaveric and Living Donor Liver Allografts, Front Immunol 11 (2020) 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sachet M, Liang YY, Oehler R, The immune response to secondary necrotic cells, Apoptosis 22(10) (2017) 1189–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J, Kern P, Herrmann M, Clearance deficiency and systemic lupus erythematosus (SLE), J Autoimmun 28(2–3) (2007) 114–21. [DOI] [PubMed] [Google Scholar]
- [190].Balam S, Kesselring R, Eggenhofer E, Blaimer S, Evert K, Evert M, Schlitt HJ, Geissler EK, van Blijswijk J, Lee S, Reis ESC, Brunner SM, Fichtner-Feigl S, Cross-presentation of dead-cell-associated antigens by DNGR-1(+) dendritic cells contributes to chronic allograft rejection in mice, Eur J Immunol 50(12) (2020) 2041–2054. [DOI] [PubMed] [Google Scholar]
- [191].Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM, Revascularization of transplanted pancreatic islets and role of the transplantation site, Clin Dev Immunol 2013 (2013) 352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Coronel MM, Stabler CL, Engineering a local microenvironment for pancreatic islet replacement, Curr Opin Biotechnol 24(5) (2013) 900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Naldini A, Carraro F, Role of inflammatory mediators in angiogenesis, Curr Drug Targets Inflamm Allergy 4(1) (2005) 3–8. [DOI] [PubMed] [Google Scholar]
- [194].Benelli R, Lorusso G, Albini A, Noonan DM, Cytokines and chemokines as regulators of angiogenesis in health and disease, Curr Pharm Des 12(24) (2006) 3101–15. [DOI] [PubMed] [Google Scholar]
- [195].Forbes S, Bond AR, Thirlwell KL, Burgoyne P, Samuel K, Noble J, Borthwick G, Colligan D, McGowan NWA, Lewis PS, Fraser AR, Mountford JC, Carter RN, Morton NM, Turner ML, Graham GJ, Campbell JDM, Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization, Science Translational Medicine 12(526) (2020) eaan5907. [DOI] [PubMed] [Google Scholar]
- [196].Coindre VF, Kinney SM, Sefton MV, Methacrylic acid copolymer coating of polypropylene mesh chamber improves subcutaneous islet engraftment, Biomaterials 259 (2020) 120324. [DOI] [PubMed] [Google Scholar]
- [197].Citro A, Moser PT, Dugnani E, Rajab TK, Ren X, Evangelista-Leite D, Charest JM, Peloso A, Podesser BK, Manenti F, Pellegrini S, Piemonti L, Ott HC, Biofabrication of a vascularized islet organ for type 1 diabetes, Biomaterials 199 (2019) 40–51. [DOI] [PubMed] [Google Scholar]
- [198].Hwa AJ, Weir GC, Transplantation of Macroencapsulated Insulin-Producing Cells, Curr Diab Rep 18(8) (2018) 50. [DOI] [PubMed] [Google Scholar]
- [199].Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, Vardi P, German T, Shabtay N, Rotem A, Evron Y, Neufeld T, Mimon S, Ludwig S, Brendel MD, Bornstein SR, Barkai U, A novel device for islet transplantation providing immune protection and oxygen supply, Horm Metab Res 42(13) (2010) 918–22. [DOI] [PubMed] [Google Scholar]
- [200].Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodström-Tullberg M, Tibell A, The TheraCyte™ Device Protects against Islet Allograft Rejection in Immunized Hosts, Cell Transplantation 22(7) (2013) 1137–1146. [DOI] [PubMed] [Google Scholar]
- [201].Mao D, Zhu M, Zhang X, Ma R, Yang X, Ke T, Wang L, Li Z, Kong D, Li C, A macroporous heparin-releasing silk fibroin scaffold improves islet transplantation outcome by promoting islet revascularisation and survival, Acta Biomater 59 (2017) 210–220. [DOI] [PubMed] [Google Scholar]
- [202].Montazeri L, Hojjati-Emami S, Bonakdar S, Tahamtani Y, Hajizadeh-Saffar E, Noori-Keshtkar M, Najar-Asl M, Ashtiani MK, Baharvand H, Improvement of islet engrafts by enhanced angiogenesis and microparticle-mediated oxygenation, Biomaterials 89 (2016) 157–65. [DOI] [PubMed] [Google Scholar]
- [203].Coronel MM, Liang JP, Li Y, Stabler CL, Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device, Biomaterials 210 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Lee SM, Kim D, Kwak KM, Khin PP, Lim OK, Kim KW, Kim BJ, Jun HS, Comparison of the Effects of Liraglutide on Islet Graft Survival Between Local and Systemic Delivery, Cell Transplant 29 (2020) 963689720971245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Pathak S, Regmi S, Nguyen TT, Gupta B, Gautam M, Yong CS, Kim JO, Son Y, Kim JR, Park MH, Bae YK, Park SY, Jeong D, Yook S, Jeong JH, Polymeric microsphere-facilitated site-specific delivery of quercetin prevents senescence of pancreatic islets in vivo and improves transplantation outcomes in mouse model of diabetes, Acta Biomater 75 (2018) 287–299. [DOI] [PubMed] [Google Scholar]
- [206].Lew B, Kim IY, Choi H, Kim KK, Sustained exenatide delivery via intracapsular microspheres for improved survival and function of microencapsulated porcine islets, Drug Deliv Transl Res 8(3) (2018) 857–862. [DOI] [PubMed] [Google Scholar]
- [207].Mori DN, Kreisel D, Fullerton JN, Gilroy DW, Goldstein DR, Inflammatory triggers of acute rejection of organ allografts, Immunol Rev 258(1) (2014) 132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Kuwabara R, Hamaguchi M, Fukuda T, Sakai H, Inui M, Sakaguchi S, Iwata H, Long-term Functioning of Allogeneic Islets in Subcutaneous Tissue Pretreated With a Novel Cyclic Peptide Without Immunosuppressive Medication, Transplantation 102(3) (2018) 417–425. [DOI] [PubMed] [Google Scholar]
- [209].Barra JM, Kozlovskaya V, Kharlampieva E, Tse HM, Localized Immunosuppression With Tannic Acid Encapsulation Delays Islet Allograft and Autoimmune-Mediated Rejection, Diabetes 69(9) (2020) 1948–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Liu JMH, Zhang J, Zhang X, Hlavaty KA, Ricci CF, Leonard JN, Shea LD, Gower RM, Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets, Biomaterials 80 (2016) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Wofford KL, Singh BS, Cullen DK, Spiller KL, Biomaterial-mediated reprogramming of monocytes via microparticle phagocytosis for sustained modulation of macrophage phenotype, Acta Biomaterialia 101 (2020) 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Wang H, Sobral MC, Snyder T, Brudno Y, Gorantla VS, Mooney DJ, Clickable, acid labile immunosuppressive prodrugs for in vivo targeting, Biomater Sci 8(1) (2020) 266–277. [DOI] [PubMed] [Google Scholar]
- [213].Fan Y, Zheng X, Ali Y, Berggren PO, Loo SCJ, Local release of rapamycin by microparticles delays islet rejection within the anterior chamber of the eye, Sci Rep 9(1) (2019) 3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Pathak S, Acharya S, Regmi S, Shrestha P, You Z, Bae YK, Park MH, Yook S, Kim JR, Park SY, Jeong D, Yong CS, Kim JO, Chang JH, Jeong JH, Particulate-Based Single-Dose Local Immunosuppressive Regimen for Inducing Tolerogenic Dendritic Cells in Xenogeneic Islet Transplantation, Adv Healthc Mater (2020) e2001157. [DOI] [PubMed] [Google Scholar]
- [215].Velluto D, Bojadzic D, De Toni T, Buchwald P, Tomei AA, Drug-Integrating Amphiphilic Nanomaterial Assemblies: 1. Spatiotemporal control of cyclosporine delivery and activity using nanomicelles and nanofibrils, J Control Release (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Frei AW, Li Y, Jiang K, Buchwald P, Stabler CL, Local delivery of fingolimod from three-dimensional scaffolds impacts islet graft efficacy and microenvironment in a murine diabetic model, J Tissue Eng Regen Med 12(2) (2018) 393–404. [DOI] [PubMed] [Google Scholar]
- [217].Papeta N, Chen T, Vianello F, Gererty L, Malik A, Mok YT, Tharp WG, Bagley J, Zhao G, Stevceva L, Yoon V, Sykes M, Sachs D, Iacomini J, Poznansky MC, Long-term survival of transplanted allogeneic cells engineered to express a T cell chemorepellent, Transplantation 83(2) (2007) 174–83. [DOI] [PubMed] [Google Scholar]
- [218].McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS, CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis, J Immunol 177(11) (2006) 8053–64. [DOI] [PubMed] [Google Scholar]
- [219].Liu Z, Habener JF, Stromal cell-derived factor-1 promotes survival of pancreatic beta cells by the stabilisation of beta-catenin and activation of transcription factor 7-like 2 (TCF7L2), Diabetologia 52(8) (2009) 1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Chen T, Yuan J, Duncanson S, Hibert ML, Kodish BC, Mylavaganam G, Maker M, Li H, Sremac M, Santosuosso M, Forbes B, Kashiwagi S, Cao J, Lei J, Thomas M, Hartono C, Sachs D, Markmann J, Sambanis A, Poznansky MC, Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression, Am J Transplant 15(3) (2015) 618–27. [DOI] [PubMed] [Google Scholar]
- [221].Pearl-Yafe M, Yolcu ES, Yaniv I, Stein J, Shirwan H, Askenasy N, The dual role of Fas-ligand as an injury effector and defense strategy in diabetes and islet transplantation, Bioessays 28(2) (2006) 211–22. [DOI] [PubMed] [Google Scholar]
- [222].Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT, Shea L, Yolcu ES, García AJ, Shirwan H, Local immunomodulation Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance, Nat Mater 17(8) (2018) 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Skoumal M, Woodward KB, Zhao H, Wang F, Yolcu ES, Pearson RM, Hughes KR, García AJ, Shea LD, Shirwan H, Localized immune tolerance from FasL-functionalized PLG scaffolds, Biomaterials 192 (2019) 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Woodward KB, Zhao H, Shrestha P, Batra L, Tan M, Grimany-Nuno O, Bandura-Morgan L, Askenasy N, Shirwan H, Yolcu ES, Pancreatic islets engineered with a FasL protein induce systemic tolerance at the induction phase that evolves into long-term graft-localized immune privilege, Am J Transplant 20(5) (2020) 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Inaba M, Kurasawa K, Mamura M, Kumano K, Saito Y, Iwamoto I, Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells, J Immunol 163(3) (1999) 1315–20. [PubMed] [Google Scholar]
- [226].Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG, Nabel GJ, Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity, Nat Immunol 2(4) (2001) 333–7. [DOI] [PubMed] [Google Scholar]
- [227].Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S, Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing, J Exp Med 186(12) (1997) 2045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Coronel MM, Martin KE, Hunckler MD, Barber G, O’Neill EB, Medina JD, Opri E, McClain CA, Batra L, Weaver JD, Lim HS, Qiu P, Botchwey EA, Yolcu ES, Shirwan H, García AJ, Immunotherapy via PD-L1-presenting biomaterials leads to long-term islet graft survival, Sci Adv 6(35) (2020) eaba5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Batra L, Shrestha P, Zhao H, Woodward KB, Togay A, Tan M, Grimany-Nuno O, Malik MT, Coronel MM, García AJ, Shirwan H, Yolcu ES, Localized Immunomodulation with PD-L1 Results in Sustained Survival and Function of Allogeneic Islets without Chronic Immunosuppression, J Immunol 204(10) (2020) 2840–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Su J, Hu BH, Lowe WL Jr., Kaufman DB, Messersmith PB, Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation, Biomaterials 31(2) (2010) 308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Luce S, Guinoiseau S, Gadault A, Letourneur F, Blondeau B, Nitschke P, Pasmant E, Vidaud M, Lemonnier F, Boitard C, Humanized Mouse Model to Study Type 1 Diabetes, Diabetes 67(9) (2018) 1816–1829. [DOI] [PubMed] [Google Scholar]
- [232].Wallet MA, Santostefano KE, Terada N, Brusko TM, Isogenic Cellular Systems Model the Impact of Genetic Risk Variants in the Pathogenesis of Type 1 Diabetes, Frontiers in Endocrinology 8(276) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Sewell W, Lin R-Y, Generation of Thyroid Follicular Cells from Pluripotent Stem Cells: Potential for Regenerative Medicine, Frontiers in Endocrinology 5(96) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Lin R-Y, New Insights into Thyroid Stem Cells, Thyroid 17(10) (2007) 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Ruiz-Babot G, Hadjidemetriou I, King PJ, Guasti L, New Directions for the Treatment of Adrenal Insufficiency, Frontiers in Endocrinology 6(70) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


