Background:
Chronic pain is the leading cause of disability worldwide and is strongly associated with the epidemic of opioid overdosing events. However, the causal links between chronic pain, opioid prescriptions, and mortality remain unclear.
Methods:
This study included 13,884 US adults aged ≥20 years who provided data on chronic pain in the National Health and Nutrition Examination Survey 1999–2004 with linkage to mortality databases through 2015. We employed the generalized form of the front-door formula within the structural causal model framework to investigate the causal effect of chronic pain on all-cause mortality mediated by opioid prescriptions.
Results:
We identified a total of 718 participants at 3 years of follow-up and 1260 participants at 5 years as having died from all causes. Opioid prescriptions increased the risk of all-cause mortality with an estimated odds ratio (OR) (95% confidence interval) = 1.5 (1.1, 1.9) at 3 years and 1.3 (1.1, 1.6) at 5 years. The front-door formula revealed that chronic pain increased the risk of all-cause mortality through opioid prescriptions; OR = 1.06 (1.01, 1.11) at 3 years and 1.03 (1.01, 1.06) at 5 years. Our bias analysis showed that our findings based on the front-door formula were likely robust to plausible sources of bias from uncontrolled exposure–mediator or mediator–outcome confounding.
Conclusions:
Chronic pain increased the risk of all-cause mortality through opioid prescriptions. Our findings highlight the importance of careful guideline-based chronic pain management to prevent death from possibly inappropriate opioid prescriptions driven by chronic pain.
Keywords: Bias analysis, Chronic pain, Front-door formula, G-computation, Mortality, NHANES, Opioids
Chronic pain is a common health problem and the leading cause of disability worldwide.1,2 In 2019, an estimated 20.4% of US adults reported suffering from chronic pain.3 In addition to the health burden of chronic pain itself, an emerging dangerous aspect is its association with the epidemic of opioid overdosing events causing approximately 49,860 deaths in the US in 2019.4 Due to challenges in chronic pain management, the effectiveness of opioids in relieving many types of chronic pain, and the limited availability of therapeutic alternatives, opioids have been one of the commonly prescribed pain medications in the US.5
Access to prescription opioids through physicians is one of the major risk factors for opioid abuse, dependence, and overdose.6 While the use of heroin and illicitly manufactured fentanyl has largely contributed to this crisis, prescription opioids are involved in approximately 40% of all opioid overdoses and are common triggers for illicit opioid use.7,8 In 2016, the Centers for Disease Control and Prevention (CDC) published chronic pain management guidelines that recommended a preference for nonopioid treatment of chronic pain and using opioids only when the benefits are expected to outweigh the risks.9 Despite efforts aimed at controlling the misuse of opioids,10 the opioid overdose epidemic is still ongoing and even worsening, thus, requiring additional attention and investigation.11,12 Although the magnitude of the effect of chronic pain on mortality mediated through physicians’ opioid prescription is critical information to support the current guideline and policies limiting opioid prescriptions, inferring causality from observational data remains difficult and may contribute to a reluctance of the medical profession to act more forcefully. This is partly due to the possibility that observational studies are biased by uncontrolled (and unmeasured) confounding that affects the chronic pain and death associations. Such confounding factors include social, physiologic, and psychologic factors13 resulting in a biased estimate when using traditional statistical methods; that is, it is hard to measure and control for all of these risk factors for both chronic pain and death in the observational study.
Pearl introduced the front-door formula to estimate the effect of an exposure or treatment on an outcome by combining the effect of the exposure on the mediator (that fully mediates the exposure-outcome effect) and the effect of the mediator on the outcome when there is no direct effect of the exposure on the outcome. Pearl’s formulation only allows for identification and estimation of the fully mediated total effect of the exposure on the outcome.14 One of the most important aspects of the front-door formula is that we can estimate the effect of interest even in the presence of unmeasured confounders for the association of exposure and outcome.14–18 Another feature of this method is that it can be used to estimate the path-specific effect—the effect of the exposure on the outcome through some specific mediator(s)—even in the presence of the direct effect of the exposure on the outcome. Fulcher et al.18 laid out a form of the front-door formula as ‘the generalized front-door formula’. Given that chronic pain may have direct effects on death not mediated through opioids (e.g., through affecting mental health and physical activity levels), such a generalized form of the front-door formula (rather than the original form of the front-door formula) can be applied to answer the important public health question: “is there an effect of chronic pain on death mediated through physicians’ opioid prescriptions?”
Using the front-door formula within the structural causal model framework, we aimed to investigate the effect of chronic pain on death mediated by opioid prescriptions in the general population of the US. This involved estimating the effect of chronic pain on opioid prescriptions and the effect of opioid prescriptions on mortality.
METHODS
Data Sources and Study Population
We used data from the US National Health and Nutrition Examination Survey (NHANES) that has been linked to a national mortality database through 2015 for passive follow-up. The NHANES is conducted by the National Center for Health and Statistics (NCHS) at the CDC using a stratified, multistage probability sampling design that enables the representation of the noninstitutionalized US civilian population. Participants complete structured interviews and a physical examination, and some provide urine and/or blood samples.19 We rely on data from three cycles of 2 years (1999–2000, 2001–2002, and 2003–2004) which included a section on “Miscellaneous Pain” in the questionnaires. Among adults enrolled in NHANES, the unweighted response rates for the household interview in 1999–2000 was 82%, in 2001–2002 84%, and in 2003–2004 79%.20 The analytic sample included all NHANES respondents aged 20 years or older who completed the household interview with the Miscellaneous Pain Questionnaire (n = 13,903). After excluding participants without death records (n = 19), the final analytical sample includes 13,884 participants.
MEASUREMENT OF VARIABLES
Exposure: Chronic Pain
This household-administered interview collects information on the duration and location of self-reported pain from respondents 20 years of age or older. Participants are asked: “During the past month, have you had a problem with pain that lasted more than 24 hours?”. Those who answered affirmatively were subsequently asked: “For how long have you experienced this pain?”. We defined chronic pain as having reported experiencing pain for at least 3 months according to the International Association for the Study of Pain criteria.21 Then, participants reporting pain were handed cards that listed 32 bodily regions and were asked to identify all regions where they experienced pain. Finally, we organized these regions into the following seven pain locations: (1) back pain (lower back, upper back, spine, shoulder, and neck), (2) legs/feet pain, (3) arms/hands pain, (4) headache/migraine pain, (5) abdominal pain, (6) face/teeth pain, and (7) chest pain according to the previous studies.22,23 In our analyses, we combined locations 5–7 into one category as “other” due to the small sample size.
Mediator: Opioid Prescriptions
Data on prescription medications used in the past 30 days were collected in the household interview. Participants were asked the following question: “In the past 30 days, have you used or taken medication for which a prescription is needed?”. Those who answered affirmatively were asked to show their prescription medication containers to the interviewer and report details related to their use. The interviewer examined the containers and recorded the exact product names from their labels. If containers were not available, the participants verbally reported this information. The collection methodology was consistent throughout each NHANES cycle.24 The NCHS classified the prescription medications based on the therapeutic classification scheme of Cerner Multum’s Lexicon Plus propriety database.25
Based on previous reports,26,27 we identified the following opioids using generic drug codes; codeine, meperidine, pentazocine, propoxyphene, tramadol, hydrocodone, morphine, tapentadol, fentanyl, hydromorphone, methadone, oxycodone, and oxymorphone. We also defined the last eight opioids (i.e., hydrocodone, morphine, tapentadol, fentanyl, hydromorphone, methadone, oxycodone, and oxymorphone) as opioids equivalent to or stronger than morphine.26,27 We did not include dihydrocodeine in the present study because it is a narcotic cough suppressant and may not be related to pain. Respondents who reported using two or more opioid analgesics in the past 30 days (about 6% of opioid users) were categorized into user groups according to the strongest opioid they reported.
Outcome: Mortality at 3 and 5 Years
The outcome of the present study was all-cause mortality during 3-year and 5-year follow-ups after the NHANES household interview. We estimated the short follow-up periods given that opioid overdose can cause death in such a short duration. To evaluate the effect of chronic pain and opioid prescriptions each on mortality for these two different follow-up periods, we used binary outcomes throughout the analysis. Mortality data were ascertained by the NCHS from death certificate information provided by the National Death Index after record matching by social security number, name, date of birth, race/ethnicity, sex, state of birth, and state of residence.
Covariates
Demographic variables included respondents’ age, sex (male and female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican-American, or others), and education status (less than high school, high school or General Education Degree, or more than high school). We calculated the poverty–income ratio (the ratio of household income to the poverty threshold) by dividing family income by the poverty guidelines specific to the reporting year and the participants’ state. Health insurance coverage, marital status, active smoking status, alcohol consumption, coronary heart diseases, cancer, and arthritis were self-reported. Illicit drug use during the past 6 months was also self-reported but only participants aged 20–59 years were asked this question. Antidepressant use was defined as taking at least one prescribed antidepressant medication in the past 30 days, using the same approach as for opioids.
Causal Effect of Interest
Our causal effect of interest is the path-specific front-door effect, that is, the effect of chronic pain on mortality mediated through opioid prescription. In our generalized form of Pearl’s front-door formula, the path-specific front-door effect is estimated by calculating the change in potential outcomes that follows a change in the mediator (opioid) which was caused by changing the exposure (chronic pain). Similar to mediation analysis, our approach requires the assumption that there is no uncontrolled confounding of the effect of the exposure on the mediator and of the effect of the mediator on the outcome. However, our approach does not require another key assumption for mediation analysis that there be no uncontrolled confounding of the effect of the exposure on the outcome; for example, the severity of underlying diseases that cause both chronic pain and death (and opioid prescriptions only through chronic pain) cannot be fully captured in observational studies. More details on this approach and the required assumptions are described in eText 1; http://links.lww.com/EDE/B916.
Statistical Analyses
Descriptive and missing data analyses preceded multivariable data analyses aimed at addressing our core research objective. We handled missing data for education (n = 56), health insurance coverage (n = 222), smoking (n = 29), marital status (n = 505), and poverty (n = 1393) by multiple imputations by chained equations (logistic regression models) that included all variables in the final analytic models using “mi impute chained” command in Stata.28,29
We employed the g-computation algorithm, a generalization of standardization,30,31 to estimate the path-specific front-door effect for 3-year and 5-year mortality, implementing a substitution estimator for the front-door formula (Table 1). We employed logistic regression models adjusting for potential confounders based on our causal diagram (Figure). In our main analysis, we adjusted for age (continuous including squared term), gender, race/ethnicity, education status, poverty-income ratio, health insurance coverage, marital status, active smoking, alcohol intake, and antidepressant medication prescription. We first fit two logistic regression models; (1) a model for the effect of the exposure (chronic pain) on the mediator (opioid prescriptions) adjusting for the above-mentioned covariates, and (2) a model for the effect of the mediator (opioid prescriptions) on the outcome (mortality) adjusting for the abovementioned covariates and the exposure (chronic pain). We then used the regression coefficients obtained from these models to predict the values of the potential mediators and subsequently of the potential outcomes under a hypothetical intervention on the exposure. Robust 95% confidence intervals (CIs) were estimated by repeating these analyses on 1000 bootstrapped samples. We also estimated risk differences using the same g-computation algorithm to interpret the effect on the additive scale.
TABLE 1.
Steps in the G-Computation Under the Generalized Form of the Front-Door Formula
| Generalized Form of the Front-Door Formula |
|---|
| Notation: X = Exposure (binary; x and x*), M = Mediator (binary), Y = Outcome (binary), C = Measured confounders, X’ = using X for confounding adjustment at the Y-stage of the front-door formula, U = uncontrolled confounders between X and Y. |
| E(YMx) = E(YX’Mx) = Σm, cP(M = m X = x, C = c)Σx’E (Y M = m, X’ = x’, C = c)P(X’ = x’, C = c) |
| Path-specific frontdoor effect (PSFDE): the contrast between E(YMx) and E(YMx*), |
| with E(YMx) = E(YX’Mx) = Σx’E(Yx’Mx)P(x’) = E(YxMx)P(x) + E(Yx*Mx)P(x*) |
| where the equality hold if no U-M interaction. |
| Step 1: Obtain Empirical Parameters |
| Step 1a. Model for the mediator given the exposure and the confounders: |
| P(M = m X = x, C = c) |
| Step 1b. Model for the outcome given the exposure, the mediator, and the confounders: |
| E(Y M = m, X’ = x’, C = c) |
| Step 2: Simulate the Potential Mediator and the Potential Outcome |
| Step 2a. Create two copies of the original sample |
| Step 2b. Simulate the exposure variable that is marginally independent of the confounders |
| Step 2c. Simulate the mediator variable as a function of its parents (the simulated exposure in step 2b and the confounders) using empirical parameters obtained in step 1a. |
| Step 2d. Simulate the outcome variable as a function of its parents (the original exposure [not the simulated exposure in step 2b]), the simulated mediator in step 2c, and the confounders) using empirical parameters obtained in step 1b. |
| Step 3: Fit the Final Marginal Structural Model |
| Regress the simulated outcome in step 2d on the simulated exposure in step 2b to obtain point estimates of marginal effect using the pooled data with two copies of the original sample. Bootstrap can be used to obtain 95% confidence intervals. |
Details in the distinction between Pearl’s original formula, Fulcher et al.’s generalization, and our approach are described in eText 1; http://links.lww.com/EDE/B916.
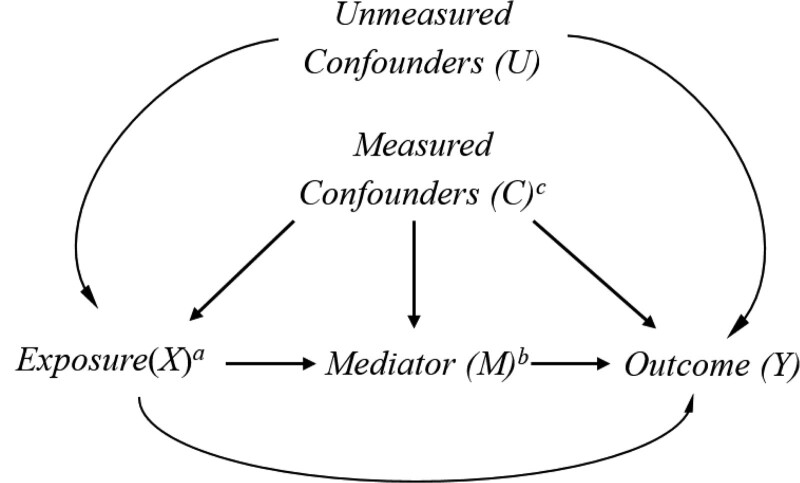
FIGURE.
Causal diagram for the plausible relations between chronic pain, opioid prescriptions, and mortality in the presence of measured and unmeasured confounders. aChronic pain (X) was self-reported and defined as reporting to have experienced pain for at least three months. bOpioids (M) include codeine, dihydrocodeine, meperidine, pentazocine, propoxyphene, tramadol, hydrocodone, morphine, tapentadol, fentanyl, hydromorphone, methadone, oxycodone, and oxymorphone. cMeasured covariates (C): Age, sex, race, education levels, poverty-income ratio, health insurance coverage, marital status, smoking, alcohol intake, and anti-depressant medication prescription. Illicit drug use was additionally adjusted for in a sensitivity analysis because the information was only available for participants aged 20-59 years. In the sensitivity analysis, we additionally adjusted for comorbidities such as cardiovascular diseases, cancer, and arthritis.
We conducted four additional analyses. First, to investigate whether the observed associations differ by the strength of the opioid medication or the pain location, we performed the same analysis for the opioids equivalent to or stronger than morphine as the mediator of interest (reference: non-use of opioid prescriptions) or each pain location as the exposure of interest (reference: without chronic pain). Second, given the potential heterogeneity of the effect of opioid prescriptions on death between participants with and without chronic pain, we also included the exposure-mediator interaction in our g-computation algorithm. Third, we applied survey weights in the above two logistic regression models to account for unequal probabilities of selecting NHANES participants as well as nonresponse of those eligible and approached.32 Fourth, we compared the estimated path-specific front-door effect using our front-door formula with the estimated natural indirect effect using mediation analysis under the additional assumption that there are no uncontrolled confounders between the exposure and the outcome.
We also performed four sensitivity analyses to assess the robustness of our findings from the front-door formula: (1) we performed a complete case analysis (i.e., excluding all subjects with missing values instead of using multiple imputations), (2) we performed the main analysis excluding subjects with a history of cancer, (3) we additionally adjusted for illicit drug use and comorbidities which may cause chronic pain, such as coronary heart diseases, cancer, and arthritis restricting participants to those aged 20–59 years (as illicit drug use was reported only by participants in this age group), and (4) we performed the main analysis assuming that antidepressant use was affected by chronic pain (i.e., only adjusted for antidepressant use to estimate the effect of opioid prescriptions on death).
Last, we investigated the potential impact of uncontrolled confounding by unmeasured confounders of the following two effects: (1) the effect of chronic pain (exposure) on opioid prescriptions (mediator), and (2) the effect of opioid prescriptions on all-cause mortality at 3 years (outcome) (eFigure 1; http://links.lww.com/EDE/B916). Based on the associations of smoking and antidepressant use with chronic pain, opioid prescriptions, and mortality calculated using our data (as these two variables are major confounders), we assigned several plausible bias values. Using these assigned values, we computed the adjusted odds ratio (OR) and 95% CI, externally adjusting for the unmeasured confounders (eTable 1; http://links.lww.com/EDE/B916). More details about the bias analysis methods for uncontrolled confounding are given elsewhere.33 We also investigated the potential impact of misclassification (5% or 10%) of the mediator (opioid prescriptions) among participants with chronic pain given the possibility that some of them might have used illicit opioids and therefore did not report the use of opioid prescriptions.
We conducted statistical analyses using STATA version 15 and R version 4.1.0. R code examples with each step are described in eAppendix 1; http://links.lww.com/EDE/B916. The NCHS Research Ethics Review Board approved NHANES study protocols with all participants providing informed written consent.34
RESULTS
The mean age ± standard deviation (SD) of participants was 50.9 ± 19.6 years and 47% were males (Table 2). Of the participants reporting chronic pain, 18% reported opioid prescriptions, compared with 3% of the participants who did not report chronic pain. Among participants who reported chronic pain, the most frequently reported pain location was the back (58%) followed by pain in the legs or feet (45%), headache or migraine (41%), arm or hand pain (39%), abdominal pain (9%), pain in the face or teeth (5%), and chest pain (5%).
TABLE 2.
Baseline Clinical Characteristics in NHANES 1999–2004 With Mortality Followed Through 2015
| Variable | Total | Participants Who Reported Chronic Pain | Participants Who Did Not Report Chronic Pain |
|---|---|---|---|
| N | 13884 | 2168 | 11716 |
| Age, mean (sd) | 50.9 (19.6) | 53.3 (17.7) | 50.4 (20.0) |
| Male, N (%) | 6579 (47) | 922 (43) | 5657 (48) |
| Race/ethnicity, N (%) | |||
| Non-Hispanic White | 6951 (50) | 1274 (59) | 5677 (48) |
| Non-Hispanic Black | 2654 (19) | 393 (18) | 2261 (19) |
| Mexican American | 3112 (22) | 333 (15) | 2779 (24) |
| Other Race | 1167 (8) | 168 (8) | 999 (9) |
| Education years, N (%) | |||
| <9th grade | 2287 (17) | 316 (15) | 1971 (17) |
| 9–11 grade | 2356 (17) | 401 (19) | 1955 (17) |
| High school graduate | 3296 (24) | 561 (26) | 2735 (23) |
| College degree or above | 5889 (43) | 882 (41) | 5007 (43) |
| Poverty income ratio, mean (sd) | 2.6 (1.6) | 2.4 (1.6) | 2.6 (1.6) |
| Health insurance coverage, N (%) | 11038 (81) | 1774 (83) | 9264 (80) |
| Marital status (married), N (%) | 7425 (56) | 1142 (54) | 6283 (56) |
| Smoking, N (%) | 6655 (48) | 1270 (59) | 5385 (46) |
| Alcohol, N (%) | 7937 (57) | 1303 (60) | 6634 (57) |
| Anti-depressant medication prescription, N (%) | 1038 (7) | 372 (17) | 666 (6) |
| Illicit drug usea, N (%) | 1419 (16) | 299 (23) | 1120 (15) |
| Comorbidities, N (%) | |||
| Coronary heart diseases | 639 (5) | 184 (9) | 455 (4) |
| Cancer | 1247 (9) | 283 (13) | 964 (8) |
| Arthritis | 2375 (17) | 735 (34) | 1640 (14) |
| Opioid prescriptions, N(%) | |||
| Total opioidsb | 683 (5) | 382 (18) | 301 (3) |
| Opioids equivalent to or stronger than morphinec | 360 (3) | 223 (10) | 137 (1) |
Illicit drug use was only asked for participants aged 20–59 years (N = 8630).
Total opioids included codeine, dihydrocodeine, meperidine, pentazocine, propoxyphene, tramadol, hydrocodone, morphine, tapentadol, fentanyl, hydromorphone, methadone, oxycodone, and oxymorphone.
Opioids equivalent to or stronger than morphine included hydrocodone, morphine, tapentadol, fentanyl, hydromorphone, methadone, oxycodone, and oxymorphone.
Chronic Pain and Opioids Prescriptions
Self-reported chronic pain was strongly associated with opioid prescriptions [OR (95% CI) = 6.1 (5.1, 7.5)] (Table 3). We observed stronger associations of chronic pain with prescription opioid use equivalent to or stronger than morphine than with any opioid prescriptions [OR (95% CI) = 8.0 (6.1, 11)]. Results did not substantially change in models using survey weights (eTable 2; http://links.lww.com/EDE/B916).
TABLE 3.
Odds Ratio (95% Confidence Interval) for the Estimated Effects of Chronic Pain on Opioid Prescriptions
| N of Opioids Use/N of Participants | Adjusted OR (95% CI)a | |||
|---|---|---|---|---|
| Pain (+) | Pain (-) | Age + Sex Adjusted | Main Modelb | |
| Total opioids | 382/2168 | 301/11716 | 7.5 (6.3–9.1) | 6.1 (5.1–7.5) |
| Opioids equivalent to or stronger than morphinec | 223/2009 | 137/11552 | 9.8 (7.6–13) | 8.0 (6.1–11) |
1000 iterations were performed for bootstrapping to estimate 95% confidence interval.
Adjusted for age, sex, race, education levels, poverty-income ratio, health insurance coverage, marital status, smoking, alcohol intake, and anti-depressant medication prescriptions. (Logit (Opioid pain, covariates) = β0 + βpain*Pain + βage*Age + βagesq*Age2 + βsex*Sex + βrace*Race + βedu*education + βpir*PIR + βins*Insurance + βmarital*Marital + βsmoke*Smoke + βalc*Alcohol + βantidep*Antidepressant)
Total N is different from total opioids because we excluded opioids weaker than morphine from this analysis.
CI indicates confidence interval; OR, odds ratio.
Opioid Prescriptions and Mortality
During each follow-up period (i.e., 3-year, and 5-year), deaths from all causes were identified in 718 (5%) and 1260 (9%) participants, respectively. Adjusted all-cause mortality odds were higher for participants who reported opioid prescriptions compared to those who did not [OR (95% CI) = 1.5 (1.1, 1.9) at 3 years and 1.3 (1.1, 1.6) at 5 years; Table 4]. We found similar results for participants who reported opioids equivalent to or stronger than morphine prescription compared to those who either did not report using opioid prescriptions or reported having opioid prescriptions weaker than morphine. The estimated ORs were larger for the participants with stronger opioid prescriptions compared to those with any opioid prescriptions over any follow-up period. When stratified by pain status, we estimated larger ORs for participants without chronic pain than for those with chronic pain (eTable 3; http://links.lww.com/EDE/B916). Results did not substantially change in the models using survey weights (eTable 4; http://links.lww.com/EDE/B916).
TABLE 4.
Odds Ratio (95% Confidence Interval) for the Estimated Effects of Opioid Prescriptions on All-Cause Mortality at 3 and 5 Years
| N of Death/N of Participants | Adjusted OR (95% CI)a | |||
|---|---|---|---|---|
| Opioids (+) | Opioids (−) | Age + Sex Adjusted | Main Modelb | |
| A) Total opioids | ||||
| 3-year mortality | 77/683 | 641/13201 | 1.7 (1.3–2.1) | 1.5 (1.1–1.9) |
| 5-year mortality | 117/683 | 1143/13195 | 1.5 (1.2–1.8) | 1.3 (1.1–1.6) |
| B) Opioids equivalent to or stronger than morphinec | ||||
| 3-year mortality | 36/360 | 641/13201 | 1.8 (1.2–2.4) | 1.6 (1.1–2.2) |
| 5-year mortality | 56/360 | 1143/13195 | 1.6 (1.2–2.1) | 1.4 (1.1–1.8) |
1000 iterations were performed for bootstrapping to estimate 95% confidence interval.
Adjusted for age, sex, race, education levels, poverty-income ratio, health insurance coverage, marital status, smoking, alcohol intake, anti-depressant medication prescription and chronic pain. (Logit (Mortality opioids, covariates) = β0 + βopi*Opioids + βpain*Pain + βage*Age + βagesq*Age2 + βsex*Sex + βrace*Race + βedu*education + βpir*PIR + βins*Insurance + βmarital*Marital + βsmoke*Smoke + βalc*Alcohol + βantidep*Antidepressant)
Total N is different from total opioids because we excluded opioids weaker than morphine from this analysis.
CI indicates confidence interval; OR, odds ratio.
From Chronic Pain to Mortality Through Opioids
We estimated that chronic pain appeared to increase the risk of all-cause mortality mediated by opioid prescriptions [path-specific front-door effect; 3-year, OR (95% CI) = 1.06 (1.01, 1.11); and 5-year, OR (95% CI) = 1.03 (1.01, 1.06); Table 5]. We observed almost the same magnitude of the effect for participants who reported having opioid medications equivalent to or stronger than morphine prescriptions. Results were also consistent on the risk difference scale (eTable 5; http://links.lww.com/EDE/B916), across pain locations (eTable 6; http://links.lww.com/EDE/B916), and in the model including the exposure-mediator interaction in our model (eTable 7; http://links.lww.com/EDE/B916). The estimated natural indirect effects using the mediation analysis were larger than the estimated path-specific front-door effect using the front-door formula (eTable 8; http://links.lww.com/EDE/B916).
TABLE 5.
Odds Ratios (95% Confidence Interval) for the Estimated Path-Specific Front-Door Effects of Chronic Pain on All-Cause Mortality Through Physicians’ Opioid Prescription at 3 and 5 Years Using the Front-Door Adjustment
| N of Death/N of Participants | Adjusted OR (95% CI)a,b | |||
|---|---|---|---|---|
| Pain (+) | Pain (–) | Through Total Opioids | Through Opioids Equivalent to or Stronger Than Morphine | |
| 3-year mortality | 157/2168 | 561/11716 | 1.06 (1.01–1.11) | 1.05 (1.01–1.09) |
| 5-year mortality | 261/2168 | 999/11710 | 1.03 (1.01–1.06) | 1.03 (1.00–1.06) |
Adjusted for age, sex, race, education levels, poverty-income ratio, health insurance coverage, marital status, smoking, alcohol intake, and anti-depressant medication prescription.
1000 iterations were performed for bootstrapping to estimate 95% confidence interval.
CI indicates confidence interval; OR, odds ratio.
Results of Sensitivity Analyses
The results did not substantially change in the complete case analysis (eTable 9; http://links.lww.com/EDE/B916), analysis excluding participants with a history of cancer (eTable 10; http://links.lww.com/EDE/B916), additional adjustment for comorbidities related to other types of diseases or pain, such as arthritis, diabetes, cardiovascular diseases, and cancer, and illicit drug use among participants aged 20–59 years (eTable 11; http://links.lww.com/EDE/B916), and analysis assuming that antidepressant use was affected by chronic pain (eTable 12; http://links.lww.com/EDE/B916). The bias analysis for uncontrolled confounding indicated that some, but not all, of the observed effect of chronic pain on all-cause mortality at 3-years mediated through opioid prescriptions could be explained by uncontrolled confounding if the controlled confounders have similar associations in size and direction with our study exposure, mediator, and outcome as smoking or antidepressant use have (eTable 13; http://links.lww.com/EDE/B916). The estimated effect assuming misclassification of opioid prescriptions among participants with chronic pain was slightly larger than the estimated effect in the original analysis (eTable 14; http://links.lww.com/EDE/B916).
DISCUSSION
In this cohort study of a nationally representative database in the US, we found that chronic pain increased the risk of all-cause mortality through opioid prescriptions using the front-door formula. The estimated causal effects did not differ by chronic pain location. Moreover, the estimated causal effects of opioid prescriptions on all-cause mortality were slightly higher for the shorter follow-up periods and participants using stronger opioid (equivalent to or stronger than morphine) prescriptions.
To the best of our knowledge, this is the first study to estimate the causal effect of chronic pain on all-cause mortality specifically mediated by opioid prescriptions among noninstitutionalized US adults. Chronic pain is one of the most common symptoms related to poor physical and mental health.35–37 However, the relationship between chronic pain and long-term adverse outcomes is not yet well understood and the results from previous studies are inconsistent, partially due to uncontrolled confounders.13,38–40 For example, a previous cohort study conducted in the United Kingdom reported a moderate size crude association between chronic pain and all-cause mortality (Hazard Ratio [HR] = 1.3, 99% CI = 1.1, 1.5) but the observed association disappeared after adjusting for socio-demographic factors and comorbidities (HR = 0.90, 99% CI = 0.74, 1.1) suggesting the presence of strong confounding bias due to these factors.40 Moreover, we focused on the path-specific effect (rather than total effect) of chronic pain on death “mediated by opioid prescriptions” in the era of the opioid crisis, a question that is challenging to answer with traditional statistical approaches. The estimated effect size in our study may have non-ignorable policy implications because one-fifth of US adults suffer from chronic pain3 and prescriptions of drugs by physicians for this common symptom should not increase mortality risk.
This study is also one of few empirical applications of Pearl’s front-door formula. In 1993, Pearl developed the front-door formula, which provides unbiased estimates under conditions where there are uncontrolled confounders for the association of the exposure and the outcome.14 Despite the difficulty of finding appropriate mediating variables, the front-door formula has two major strengths: (1) by using mediating variables that lie on the path from the exposure to the outcome of interest, it focuses on a specified mechanism of the exposure effect under study without necessarily requiring us to identify and estimate the average total effect of the exposure, and (2) it is a general method that applies to both parametric and nonparametric settings.14,15,18 Fulcher et al.18 introduced the concept of the generalized front-door formula to estimate the population indirect intervention effect by taking the contrast of observed population prevalence, E[Y], and the outcome E(YMx*), where YMx* denotes the potential outcome if the exposure had taken its observed value and M had been set to Mx* (the potential mediator if the exposure had taken value X = x*; notation can be found in Table 1 and eText 1; http://links.lww.com/EDE/B916).18 Here, we similarly applied the generalized form of the front-door formula but to estimate the path-specific front-door effect by taking the contrast of two potential outcomes; that is, YMx and YMx* where Mx denotes the potential mediator if the exposure had taken value X = x, and YMx is the potential outcome if M had been set to Mx (eText 1; http://links.lww.com/EDE/B916). These two different approaches can be used when we are interested in path-specific effects in the presence of a direct effect of the exposure on the outcome. Of note, as shown in our study and the previous study,41 the pure natural indirect effect can be identified even when there are uncontrolled confounders between the exposure and the outcome if there is no interaction between uncontrolled confounders and the mediator, although mediation analysis does not work in such a scenario due to the violation of the conditional exchangeability assumption.
Our bias analysis revealed that our findings based on the front-door formula were likely robust to uncontrolled confounding. Given the inter-physician variation in opioid prescribing behavior for pain management across geographical areas and physician characteristics including their relationship with the pharmaceutical industry,42–44 the lack of this information might have induced confounding bias. However, it is less likely that such bias explains our findings away even if these factors were confounders as strong as smoking or antidepressant use. The positivity assumption also holds in our analysis because participants could have received opioid prescriptions even without chronic pain due to a lack of adherence to the clinical guidelines.
We found that opioid prescriptions were associated with an increased risk of all-cause mortality as previously suggested. The estimates were slightly larger in the shorter-term follow-up periods and among participants who reported stronger opioid prescriptions. Ample evidence exists that the duration of an opioid’s action and the prescribed opioid doses are associated with the risk of overdose and related deaths.45–47 Given that the percentage of adults using opioids stronger than morphine is increasing,26 the risk of developing opioid use disorders is greater.9 While it is still unclear whether reducing the inappropriate prescription of opioids alone is an effective strategy for addressing the opioid crisis,48,49 our findings highlight the importance of considering the strength of opioids as well as the duration of opioids’ actions and the prescribed doses for overdose-related death. Moreover, we found an association of larger magnitude between opioid prescriptions and mortality among people without chronic pain rather than those with chronic pain. Because the affinity for the opioid receptor is different for each opioid50,51 and the risk of opioid addiction or dependence may vary by socioeconomic status, underlying medical conditions, and prescribing patterns,45,52,53 further subgroup analyses with a larger sample size are needed to clarify whether these factors act as effect measure modifiers.
Limitations of the Study
The present study has several limitations. First, as chronic pain was self-reported, we might have misclassified the exposure. Particularly, some participants with chronic pain might have not reported opioid prescriptions as they used opioids illicitly. However, assuming that these people may have a high mortality risk, such misclassification induces bias toward the null as shown in our sensitivity analysis. Second, while the front-door formula does not require the assumption of no uncontrolled confounders between the exposure and the outcome, we need to assume that there are no uncontrolled confounders between the exposure and the mediator and between the mediator and the outcome. Third, although we assumed that increased mortality risks related to opioid prescription were mainly due to inappropriate prescription by physicians or nonmedical use of prescription opioids by patients,7,8 our data did not have information on whether physicians’ prescription and patients’ use of opioids were clinically appropriate. Fourth, given the different ways in which chronic pain levels could arise or persist, there is a possibility of violating the consistency assumption for this exposure. The consistency assumption could also be violated for the mediator given that duration and dose of opioid intake drive opioid-related death53 and several alternatives to opioids for chronic pain management exist. Thus, our findings do not suggest the effectiveness of a specific intervention. These should be the subject of further research with more detailed information on chronic pain and opioids. Fifth, we estimated the risk of mortality during the follow-up periods after the household interview; that is we did not have information about how long and how much of the prescribed drugs they had taken before the interview. Therefore, we cannot negate the possibility that, for some respondents, the reported chronic pain might be in response to prior opioid prescriptions or withdrawal from such prescriptions. Nonetheless, one would expect that opioids are not directly inducing chronic pain. Furthermore, prior opioid prescriptions most likely would have reduced the likelihood of chronic pain given its primary role as a painkiller. Finally, it is important to note that the obtained estimate is the path-specific effect of chronic pain on death mediated by opioid prescriptions. Therefore, our findings may not be generalizable to the effect of acute pain or the overall effect of chronic pain through other paths or mechanisms including non-medical use of opioids.
CONCLUSIONS
In conclusion, using a nationally representative database in the US and implementing the front-door formula, we found that self-reported chronic pain increased the risk of all-cause mortality through opioid prescriptions. The opioid prescriptions increased the risk of all-cause mortality and effect sizes are stronger in the shorter-term follow-up periods and for stronger opioids. Our findings support the current CDC guidelines for pain management9 to prevent death from possibly inappropriate opioid prescriptions driven by chronic pain.
ACKNOWLEDGMENTS
We thank the editors and several anonymous reviewers for their helpful comments. We also thank Qi Yan (Department of Epidemiology, UCLA Fielding School of Public Health) for his help with the statistical analyses.
Supplementary Material
Footnotes
K.I. was supported by the Burroughs Wellcome Fund Interschool Training Program in Chronic Diseases (BWF-CHIP), the Japan Society for the Promotion of Science (JSPS; 21K20900), Meiji Yasuda Life Foundation of Health and Welfare, and the Program for the Development of Next-generation Leading Scientists with Global Insight (L-INSIGHT) sponsored by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. O.A. was partly supported by grant number UL1TR001881 from the National Institutes of Health’s National Center for Advancing Translational Sciences (NCATS) to UCLA Clinical Translational Science Institute (CTSI). O.A. also benefited from facilities and resources provided by the California Center for Population Research at UCLA (CCPR), which receives core support (P2C-HD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. Study sponsors were not involved in study design, data interpretation, writing, or the decision to submit the article for publication.
The authors report no conflicts of interest.
The study was exempted from human subjects review by the institutional review board at University of California, Los Angeles.
All data (continuous NHANES 1999–2004, and mortality data) are publicly available and can be accessed from CDC.gov (https://www.cdc.gov/nchs/nhanes/index.htm). Code will be shared on request to the corresponding author.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlhamer J. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Drug Overdose Deaths. 2021. Available at: https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed 16 June 2021.
- 5.Hales CM, Martin CB, Gu Q. Prevalence of prescription pain medication use among adults: United States, 2015–2018. NCHS Data Brief, no 369. Hyattsville, MD: National Center for Health Statistics. 2020. Available at: https://www.cdc.gov/nchs/products/databriefs/db369.htm. Accessed 16 June 2021. [PubMed] [Google Scholar]
- 6.Volkow ND, McLellan AT. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. [DOI] [PubMed] [Google Scholar]
- 7.Seth P, Rudd RA, Noonan RK, Haegerich TM. Quantifying the epidemic of prescription opioid overdose deaths. Am J Public Health. 2018;108:500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–163. [DOI] [PubMed] [Google Scholar]
- 9.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. [DOI] [PubMed] [Google Scholar]
- 11.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths–United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–1382. [DOI] [PubMed] [Google Scholar]
- 12.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith D, Wilkie R, Uthman O, Jordan JL, McBeth J. Chronic pain and mortality: a systematic review. PLoS One. 2014;9:e99048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearl J. Mediating Instrumental Variables. Technical Report R-210, Cognitive Systems Laboratory, UCLA Computer Science Department. 1993. Available at: https://ftp.cs.ucla.edu/pub/stat_ser/r210.pdf. Accessed June 16, 2021. [Google Scholar]
- 15.Pearl J. Causality. Cambridge University Press; 2009;486:78–85. [Google Scholar]
- 16.Glynn A, Kashin K. Front-door versus back-door adjustment with unmeasured confounding: bias formulas for front-door and hybrid adjustments with application to a job training program:S. JASA. 2018;113:1040–1049. [Google Scholar]
- 17.Pearl J, Mackenzie D. The Book of Why: The New Science of Cause and Effect. Basic Books; 2018; 470:219–258. [Google Scholar]
- 18.Fulcher IR, Shpitser I, Marealle S, Tchetgen Tchetgen EJ. Robust inference on population indirect causal effects: the generalized front door criterion. J R Stat Soc Series B Stat Methodol. 2020;82:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2019. Available at: www.cdc.gov/nchs/nhanes.htm. Accessed June 16, 2021.
- 20.National Center for Health Statistics, Centers for Disease Control and Prevention. NHANES response rates and population totals. Available at: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. Accessed June 16, 2021.
- 21.Merskey H, Bogduk N. IASP pain terminology. Classification of Chronic Pain. 1994;209–214. [Google Scholar]
- 22.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–812. [DOI] [PubMed] [Google Scholar]
- 23.Hollingshead NA, Vrany EA, Stewart JC, Hirsh AT. Differences in Mexican Americans’ prevalence of chronic pain and co-occurring analgesic medication and substance use relative to non-hispanic white and black Americans: results from NHANES 1999–2004. Pain Med. 2016;17:1001–1009. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. Dietary Supplements and Prescription Medication Questionnaire. 2011. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/dsq.pdf. Accessed June 16, 2021.
- 25.NCHS. National Health and Nutrition Examination Survey, 1988–2014 data documentation, codebook, and frequencies: Prescription medications—drug information. 2016. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm. Accessed June 16, 2021.
- 26.Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999-2012. NCHS Data Brief. 2015; 189:1–8. [PubMed] [Google Scholar]
- 27.Frenk SM, Lukacs SL, Gu Q. Factors associated with prescription opioid analgesic use in the US population, 2011-2014. Pain Med. 2019;20:1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp LLC. Stata multiple-imputation reference manual release 16. Available at: https://www.stata.com/manuals/mi.pdf. Accessed 16 June 2021.
- 30.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel RM, Cousens SN, De Stavola BL, Kenward MG, Sterne JA. Methods for dealing with time-dependent confounding. Stat Med. 2013;32:1584–1618. [DOI] [PubMed] [Google Scholar]
- 32.National Center for Health Statistics, Centers for Disease Control and Prevention. NHANES - Continuous NHANES Web Tutorial - Specifying Weighting Parameters. Available at: https://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/intro.htm. Accessed 16 June 2021.
- 33.Arah OA. Bias analysis for uncontrolled confounding in the health sciences. Annu Rev Public Health. 2017;38:23–38. [DOI] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics, Centers for Disease Control and Prevention. NCHS research ethics review board (ERB) approval. 2017. Available at: www.cdc.gov/nchs/nhanes/irba98.htm. Accessed 16 June 2021.
- 35.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:403–425. [DOI] [PubMed] [Google Scholar]
- 36.Kamaleri Y, Natvig B, Ihlebaek CM, Bruusgaard D. Localized or widespread musculoskeletal pain: does it matter? Pain. 2008;138:41–46. [DOI] [PubMed] [Google Scholar]
- 37.Bergman S. Psychosocial aspects of chronic widespread pain and fibromyalgia. Disabil Rehabil. 2005;27:675–683. [DOI] [PubMed] [Google Scholar]
- 38.Macfarlane GJ, McBeth J, Silman AJ. Widespread body pain and mortality: prospective population based study. BMJ. 2001;323:662–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macfarlane GJ, Jones GT, Knekt P, et al. Is the report of widespread body pain associated with long-term increased mortality? Data from the Mini-Finland Health Survey. Rheumatology (Oxford). 2007;46:805–807. [DOI] [PubMed] [Google Scholar]
- 40.Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain. 2010;14:380–386. [DOI] [PubMed] [Google Scholar]
- 41.Fulcher IR, Shi X, Tchetgen Tchetgen EJ. Estimation of natural indirect effects robust to unmeasured confounding and mediator measurement error. Epidemiology. 2019;30:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooten WM, Dvorkin J, Warner NS, Pearson AC, Murad MH, Warner DO. Characteristics of physicians who prescribe opioids for chronic pain: a meta-narrative systematic review. J Pain Res. 2019;12:2261–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue K, Figueroa JF, Orav EJ, Tsugawa Y. Association between industry payments for opioid products and physicians’ prescription of opioids: observational study with propensity-score matching. J Epidemiol Community Health. 2020;74:647–654. [DOI] [PubMed] [Google Scholar]
- 45.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. [DOI] [PubMed] [Google Scholar]
- 46.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175:608–615. [DOI] [PubMed] [Google Scholar]
- 47.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. 2019;2:e187621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitt AL, Humphreys K, Brandeau ML. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health. 2018;108:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzschentke TM, Christoph T, Kögel B, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323:265–276. [DOI] [PubMed] [Google Scholar]
- 51.Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37:1105–1121. [DOI] [PubMed] [Google Scholar]
- 52.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174:796–801. [DOI] [PubMed] [Google Scholar]
- 53.WHO. Information sheet on opioid overdose. Available at: http://www.who.int/substance_abuse/information-sheet/en/. Accessed February 18, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.