Précis:
In children, Baerveldt implants showed 84% success at 1 year, but decreased to 32% at 8 years. Age, race, and glaucoma type were not risk factors for failure. Concurrent intraocular surgery was associated with complications.
Purpose:
Evaluate success and risk factors for failure and complications of Baerveldt glaucoma implants in children.
Methods:
Retrospective case series of children who underwent Baerveldt implant placement (2012-2019 by single surgeon) with ≥1 year follow-up. Ocular examination and surgical details were collected. Failure defined as intraocular pressure (IOP) <5 mm Hg or >21 mm Hg for 2 consecutive visits, need for IOP related surgery, or visually significant complication.
Results:
One hundred-six eyes of 76 patients underwent 110 Baerveldt placement at median 6.4 years. Baerveldt placement was combined with additional procedures in 49% with vitrectomy most common (30%). Success of first Baerveldt (per patient) was 64% at final follow-up (median 4.7 y). One-, 5-, and 8-year survival rates were 84%, 60%, and 32%, respectively. There was no difference (P=0.97) in survival between first Baerveldt and all Baerveldt surgeries. Failure of first Baerveldt was not associated with sex, age, ethnicity, prior IOP-lowering surgery, concurrent intraocular surgery, or glaucoma type. Complications occurred in 14% and were associated with concurrent surgery. Twenty-six percent required additional IOP-lowering surgery. At final follow-up, IOP and glaucoma medications were significantly decreased (P<0.0001). Eyes underwent an average of 3.8±2.3 ocular surgeries and 3.0±2.0 glaucoma surgeries.
Conclusions:
Baerveldt implants showed good success initially, but survival rates declined over time. No risk factors for failure of first implanted Baerveldt were identified. Concurrent surgery was associated with complications. Majority of eyes required multiple surgeries to achieve IOP control and preserve vision.
Key Words: childhood glaucoma, glaucoma drainage device, Baerveldt implant, primary congenital glaucoma, glaucoma following cataract surgery
Childhood glaucomas typically have etiologies that are distinct from their adult counterparts and more frequently require surgical intervention to preserve vision.1,2 Although angle surgeries are generally the first-line treatment, many eyes, especially those with advanced or secondary forms of glaucoma, often require additional procedures to obtain intraocular pressure (IOP) control.3–5 In children, glaucoma drainage device (GDD) implantation has become the predominant trabecular meshwork bypass surgery due to the more predictable postoperative course and decreased life-long risk of infection compared to trabeculectomy.6–9 The reported pediatric success rates of the most common GDDs (Baerveldt and Ahmed devices) are typically 80% to 90% in the first year, but decrease significantly over time.10–18 However, many of these reports are limited by the small number of eyes and short time of follow-up.10–15,17 Many of the larger studies with longer follow-up in children combine multiple types of GDDs, making it more difficult to measure the individual long-term success of each GDD.19–21
Unlike in the adult population, where a randomized trial comparing Baerveldt and Ahmed GDDs has been performed, there is less data measuring the success of specific GDD types in large cohorts of children.22 While valved Ahmed GDDs have the benefits of immediate pressure lowering effect and decreased risk of postoperative hypotony, they are plagued with the hypertensive phase and lower rates of overall success.23–26 In contrast, the nonvalved Baerveldt GDD may have better longevity in adults with the trade off of delayed pressure lowering effect.22,24 In the current study, we present a large cohort of children who underwent Baerveldt implantation for IOP-control and include visual outcomes and an assessment of risk factors for failure and complications.
METHODS
A retrospective review identified patients 21 years of age or younger who underwent placement of a Baerveldt (101-350 or 101-250, Abbott Medical Optics, Santa Ana, CA) GDD between August 1, 2012 and July 31, 2020 at the University of Michigan by a single surgeon (B.L.B.). Exclusion criteria included <1 year of follow-up after surgery or surgery performed by other surgeons. This study was approved by the Institutional Review Board at the University of Michigan.
Childhood glaucomas were classified based on the World Glaucoma Association consensus.27 Glaucoma was defined by at least 2 repeated IOP measurements >21 mm Hg and accompanying signs of buphthalmos, corneal edema, Haabs striae, or optic nerve cupping. The primary outcome measure was Baerveldt GDD success. Failure was defined as IOP <5 mm Hg or >21 mm Hg (with or without glaucoma medications) for 2 consecutive visits, the need for additional IOP lowering or elevating surgery, or a visually significant complication.12,13,16,28
Collected data included age, sex, ocular and systemic diagnoses, intraocular surgical details, and surgical complications. Examination findings included preoperative (examination immediately before Baerveldt placement) and final best-corrected visual acuity (BCVA), IOP, and number of glaucoma medications. Visual acuity was determined using age and developmentally appropriate measures. In nonverbal children, vision was assessed by ability to react to light, fixate on and follow objects, and ocular preference by induced tropia test. Visual acuity as measured by optotypes was converted to LogMar scale. More than 2 lines difference in visual acuity was considered significant when comparing preoperative and final values. IOP was measured by iCare (Revenue, Vantaa, Finland), Tonopen (Reichert, Depew, NY), or Goldmann applanation. The majority of IOP measurements were obtained on awake patients in an outpatient setting. Intraocular pressures recorded during exams under anesthesia were obtained immediately after induction and before intubation in order to minimize pressure changes secondary to anesthetics.
A similar surgical technique for Baerveldt implantation was used in all patients as previously described.29 The Baerveldt 101-350 was the preferred device and was trimmed along the posterior edge of the plate as needed for smaller eyes. The Baerveldt 101-250 was used in eyes in which a trimmed Baerveldt 101-350 was too large or by surgeon preference when placed in the inferonasal quadrant.30 The single implantation procedure was standardly performed, however, staged procedures were done when (A) both eyes required Baerveldt placement or (B) angle surgery or cycloablation was done concurrently with the stage 1 placement. In the first instance, a single implantation procedure was performed in 1 eye at the same time as the stage 1 placement was done in the second eye. Four to 6 weeks later, the ripcord was removed in the first eye (if the tube had not spontaneously opened) and the stage 2 implantation was performed in the second eye. In the second situation, angle surgery or cycloablation, which was anticipated to have a low chance of success, was performed at the same time as the stage 1 placement. This allowed for stage 2 implantation at any point after 4 weeks following plate placement. Both of these strategies minimized the length of time until the Baerveldt GDDs were functional.
For a single implantation procedure, a 25-G cannula was threaded into the distal end of the tube and used to flush the tube with balanced salt solution. A 5-0 polypropylene suture was placed within the proximal tube and the tube was then ligated with a 6-0 polyglactin suture. The tube was attempted to be irrigated with the previously placed 25-G cannula in order to ensure complete ligation. Using a limbus-based approached, the conjunctiva and Tenon capsule were incised 8 to 10 mm posterior to the limbus. The adjacent extraocular muscles were isolated with muscle hooks so that the plate could be placed underneath the muscles and posterior to the insertions. The plate was affixed to the sclera with 8-0 nylon ~8 mm posterior to the limbus. In staged procedures, the tube was then tucked underneath one of the adjacent extraocular muscles, and Tenon capsule followed by conjunctiva were closed in a double layered manner with 8-0 polyglactin. At the time of the second stage, the conjunctiva and Tenon capsule were incised in the same location as the previous incision. Care was taken to not disrupt the capsule surrounding the plate. The tube was identified and retrieved from behind the extraocular muscle. At this point, for both single and staged procedures, Tenon capsule was dissected anteriorly to the limbus and the tube was trimmed to an appropriate length.
For implantation in the anterior chamber or sulcus, a paracentesis was created temporally through which viscoelastic was injected. A 23-G needle was used to create a properly oriented tract ~1 mm posterior to the limbus through the sclera and into the anterior chamber or sulcus. The tube was secured to the sclera with 9-0 nylon suture. In some cases of single implantation procedure, venting slits in the tube were created with the 9-0 nylon suture needle. The tube was covered with donor sclera using 8-0 polyglactin suture. The Tenon capsule and conjunctiva were then closed in a double layered fashion with 8-0 polyglactin. Balanced salt solution and filtered air were injected through the paracentesis and used to remove the viscoelastic. The paracentesis was then closed with a 10-0 polyglactin suture.
For implantation in the pars plana, the pars plana vitrectomy was performed through 25- or 23-G trocars placed inferotemporally, superotemporally and superonasally 0.5 to 2 mm posterior to the limbus. Trocar size and placement were adjusted based on age of patient, size of eye, and underlying eye disease. Surgical procedure using the Constellation Vitreoretinal Surgical System (Alcon Laboratories Inc., Fort Worth, TX) varied depending on ocular pathology. Lensectomy was performed as indicated. A core vitrectomy was performed and posterior hyaloid membrane and cortical vitreous were dissected from the macula using aspiration and/or manual membrane peeling. Peripheral vitreous was removed with particular care taken in the quadrant of tube placement. The superotemporal trocar was removed and the tube was placed through the sclerostomy. The tube was secured using 9-0 nylon and covered with donor sclera that was affixed with 8-0 polyglactin sutures. Tenon capsule and conjunctiva were closed in a double layered fashion with 8-0 polyglactin. The remaining trocars were removed and the sclerostomy sites were closed with 8-0 polyglactin. The conjunctiva was reapproximated at the limbus with 10-0 polyglactin.
GraphPad Prism 9.0 (GraphPad, La Jolla, CA) was used for statistical analyses including Kaplan-Meier survival curves with 95% confidence intervals with Log-Rank test (Manual Cox and for trend),Wilcoxon matched pairs signed rank test for preoperative and postoperative comparisons, Mann-Whitney test for first surgery and all surgery comparisons. The reported P-values were 2-tailed. P-values <0.05 were considered statistically significant.
RESULTS
One hundred-ten Baerveldt GDDs were implanted in 106 eyes of 76 patients at an average age of 7.7±5.7 years (median 6.4 y, range 0.1-20.9 y). The majority of patients were Caucasian (68%) and female (54%), and there was no significant difference in age at the time of surgery or length of follow-up between races (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/IJG/A589). Types of glaucoma included both primary and secondary forms, as detailed in Table 1. Before Baerveldt placement, 75% of eyes (80 eyes of 63 patients) had undergone previous ocular surgery (Table 2) with an average of 2.1±1.1 surgeries per eye (median: 2, range: 1 to 5). Furthermore, 61% of eyes (65 eyes of 49 patients) had prior glaucoma surgery with an average of 1.9±1.0 surgeries per eye (median: 2, range: 1 to 5).
TABLE 1.
Types of Glaucoma
| Type of Glaucoma | # Eyes of # Patients | Age at Surgery (Y) | Follow-up (Y) |
|---|---|---|---|
| Aniridia | 6 eyes of 3 patients | 3.8±2.7 | 4.9±2.5 |
| Anterior segment dysgenesis | 3 eyes of 2 patients | 9.9±0.7 | 5.4±0.6 |
| Axenfeld-Rieger dyndrome | 8 eyes of 5 patients | 9.0±4.3 | 2.8±1.4 |
| Congenital ectropion uvea | 4 eyes of 3 patients | 9.7±6.5 | 4.8±1.9 |
| Glaucoma following cataract surgery | 18 eyes of 15 patients | 7.6±5.9 | 4.5±2.3 |
| Persistent fetal vasculature | 8 eyes of 7 patients | ||
| Hurler syndrome | 2 eyes of 1 patient | 17.2 | 4.0 |
| Juvenile open angle | 7 eyes of 4 patients | 10.9±7.7 | 3.0±1.8 |
| Microspherophakia | 4 eyes of 2 patients | 9.3±3.0 | 4.2±1.8 |
| Peters anomaly | 8 eyes of 6 patients | 3.4±1.2 | 4.2±1.6 |
| Primary congenital | 26 eyes of 17 patients | 4.5±4.9 | 5.6±2.3 |
| Retinopathy of prematurity | 4 eyes of 4 patients | 14.1±7.2 | 3.7±2.6 |
| Sturge Weber | 7 eyes of 6 patients | 8.1±6.4 | 5.6±2.5 |
| Traumatic | 4 eyes of 4 patients | 9.2±4.6 | 4.0±2.2 |
| Uveitic | 5 eyes of 4 patients | 14.3±4.1 | 5.2±2.9 |
TABLE 2.
Prior Intraocular Surgeries
| # of Eyes | |
|---|---|
| Prior nonglaucoma surgeries | |
| Lensectomy | 20 |
| Superficial keratectomy | 4 |
| Retinopathy of prematurity laser | 4 |
| Penetrating keratoplasty | 3 |
| Corneal laceration repair | 3 |
| Optical iridectomy | 2 |
| Prior glaucoma surgeries | |
| Trabeculotomy | 30 |
| Goniotomy | 16 |
| Ahmed GDD placement | 8 |
| Contact transcleral cycloablation | 7 |
| Trabeculectomy with MMC | 3 |
| Endoscopic cycloablation | 1 |
| Iridectomy for angle closure | 1 |
GDD indicates glaucoma drainage device; MMC, mitomycin C.
Three eyes of 3 patients initially had a Baerveldt 101-250 device implanted inferonasally and a second 101-350 device subsequently placed superotemporally. One eye of 1 patient had a Baerveldt 101-350 device placed superotemporally, but due to hypotony, this was exchanged for a 101-250 device. The remaining 102 eyes underwent implantation of a Baerveldt 101-350 device in the superotemporal quadrant.
Twenty-one percent (23 of 110 surgeries) were staged, with tube insertion taking place an average of 58±10 days after the initial plate placement (median: 29 d, range: 21 to 460 d). The remaining 79% (87 of 110 surgeries) were performed as a single procedure with temporary ligation of the tube. Baerveldt placement was combined with additional ocular procedures in 49% of surgeries (Table 3). Pars plana vitrectomy was the most common concurrent surgery (30% of surgeries) and was performed for posterior placement of the tube via pars plana or pars plicata entry.
TABLE 3.
Concurrent Surgeries and Complications
| Concurrent Ocular Surgeries | # Eyes of # Patients |
|---|---|
| Pars plana vitrectomy | 33 eyes of 28 patients |
| Lensectomy without IOL | 8 eyes |
| Lensectomy with IOL | 2 eyes |
| Removal of prior tube | 8 eyes of 6 patients |
| Removal of Ahmed | 7 eyes of 5 patients |
| Removal of Baerveldt | 1 eye of 1 patient |
| Goniotomy | 7 eyes of 5 patients |
| Ipsilateral lateral rectus recession | 6 eyes of 4 patients |
| Keratoprosthesis | 1 eye of 1 patient |
| Complications | # of eyes |
| Retinal detachment | 7 eyes of 7 patients |
| Hypotony requiring surgery* | 4 eyes of 4 patients |
| Suprachoroidal hemorrhage | 1 eye of 1 patient |
| Keratoprosthesis extrusion | 1 eye of 1 patient |
| Vitreous hemorrhage† | 1 eye of 1 patient |
One eye had 2 Baerveldt failures due to hypotony. A Baerveldt 101-350 was initially implanted which was exchanged for a 101-250 device. The 101-250 device was then subsequently exchanged for an Ahmed FP8 implant.
The postoperative vitreous hemorrhage resolved spontaneously and thus was not considered a failure.
IOL indicates intraocular pressure.
Success rates and survival curves were calculated for first Baerveldt surgery for each patient and for all Baerveldt implantation surgeries. At final follow-up (4.8±2.3 y, median: 4.7 y, range: 1.0 to 8.7 y) for first surgeries, the success rate was 64% (49 of 76 eyes). Twenty-seven eyes failed at an average time to failure of 2.0±2.2 years (median: 1.1, range: 0.1 to 7.8 y). Kaplan-Meier analysis (Fig. 1A, Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/IJG/A589) showed 1-, 5-, and 8-year survival rates of 84% with 95% confidence interval (CI) [74, 90], 60% with 95% CI [46, 72], and 32% with 95% CI [8, 59], respectively. At final follow-up for all Baerveldt surgeries (4.8±2.2 y, median: 4.1 y, range: 1 to 8.7 y) the success rate was 64% (70 of 110 surgeries) with an average time to failure of 1.8±2.1 years (median: 0.8 y, range: 0.1 to 7.8 y). The survival curve of all surgeries (Fig. 1B, Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/IJG/A589) was not significantly different (P=0.97) compared with the first eye analysis. In addition, survival curves based on location of tube placement (anterior chamber or sulcus vs. pars plana placement) were not significantly different (P=0.33, Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/IJG/A589).
FIGURE 1.
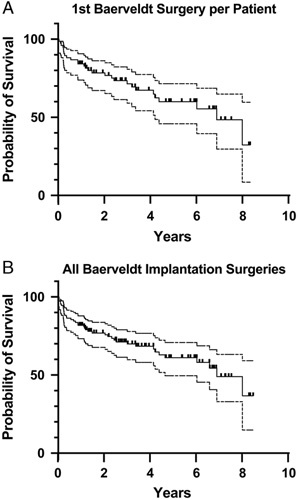
Survival analysis of Baerveldt glaucoma drainage device s in Children. A, Kaplan-Meier analysis of first Baerveldt per patient (n=76) showed 1-, 5-, and 8-year survival rates of 84% with 95% confidence interval (CI) [74, 90], 60% with 95% CI [46, 72], and 32% with 95% CI [8, 59], respectively. B, For all surgeries (n=110), survival rates were 82% with 95% CI [73, 88] at 1 year, 61% with 95% CI [49, 70] at 5 years, and 37% with 95% CI [15, 59] at 8 years. There was no significant difference between first Baerveldt and all surgeries (P=0.97).
Univariate analysis of risk factors showed that failure of the first Baerveldt implanted per patient (n=76) was not associated with gender, age at time of surgery, ethnicity, number of prior glaucoma surgeries, preoperative IOP, number of preoperative glaucoma medications, surgical procedure (single vs. staged) or concurrent surgery. Further, type of glaucoma, either grouped as primary versus secondary (Table 4) or classified based on the World Glaucoma Association (Supplemental Table 3, Supplemental Digital Content 1, http://links.lww.com/IJG/A589) was not a significant risk factor for failure. For all Baerveldt surgeries (n=110), younger (0 to 5 y) and older ages (11+ y) and prior glaucoma surgery were associated with increased failure (Table 4).
TABLE 4.
Univariate Analysis for Failure
| 1st Baerveldt Surgery (n=76) | All Baerveldt Surgeries (n=110) | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk Factor | N (%) (n=76) | 5-Year Cumulative Probability Failure (%) | P * | HR; 95% CI | N (%) (n=110) | 5-Year Cumulative Probability Failure (%) | P * | HR; 95% CI |
| Sex | 0.39 | 0.55 | ||||||
| Male | 41 (54) | 39 | 0.73; 0.34–1.54 | 52 (47) | 37 | 0.83; 0.45–1.56 | ||
| Female | 35 (46) | 37 | 58 (53) | 41 | ||||
| Age (y) | 0.17 | 0.009 | ||||||
| 0-5 | 37 (49) | 43 | 0.38; 0.15–0.99† | 53 (48) | 45 | 0.25; 0.11–0.55† | ||
| 6-10 | 16 (21) | 20 | 2.80; 0.90–8.70‡ | 25 (23) | 13 | 5.05; 1.95–13.08‡ | ||
| 11+ | 23 (30) | 49 | 1.23; 0.54–2.97§ | 32 (29) | 56 | 1.48; 0.73–3.01§ | ||
| Ethnicity | 0.20 | 0.14 | ||||||
| Caucasian | 52 (68) | 31 | 1.62; 0.76–3.76 | 74 (67) | 35 | 1.59; 0.82–3.09 | ||
| Non-Caucasian | 24 (32) | 47 | 36 (33) | 47 | ||||
| Prior glaucoma surgeries | 0.18 | 0.01 | ||||||
| None | 31 (41) | 28 | 1.69; 0.80–3.55 | 46 (42) | 25 | 2.41; 1.29–4.49 | ||
| 1 or more | 45 (59) | 45 | 64 (58) | 49 | ||||
| Preoperative IOP (mm Hg) | 0.14 | 0.53 | ||||||
| 0-20 | 24 (32) | 55 | 0.42; 0.18–1.01∥ | 29 (26) | 52 | 0.70; 0.33–1.46∥ | ||
| 21-30 | 29 (38) | 31 | 0.78; 0.28–2.18¶ | 48 (44) | 36 | 0.92; 0.42–2.04¶ | ||
| 31+ | 23 (30) | 28 | 0.57; 0.24–1.37# | 33 (30) | 29 | 0.66; 0.29–1.47# | ||
| Preoperative medications | 0.44 | 0.55 | ||||||
| 0-2 | 22 (29) | 47 | 0.71; 0.30–1.68 | 31 (28) | 49 | 0.82; 0.40–1.66 | ||
| 3+ | 54 (71) | 35 | 79 (72) | 36 | ||||
| Procedure | 0.45 | 0.79 | ||||||
| Single procedure | 62 (82) | 39 | 1.57; 0.57–4.33 | 88 (79) | 39 | 1.12; 0.51–2.46 | ||
| Staged procedure | 14 (18) | 43 | 23 (21) | 40 | ||||
| Concurrent procedure | 0.45 | 0.76 | ||||||
| Yes | 37 (49) | 41 | 0.75; 0.36–1.58 | 54 (49) | 39 | 0.91; 0.49–1.69 | ||
| No | 39 (51) | 33 | 56 (51) | 38 | ||||
| Type of glaucoma | 0.32 | 0.32 | ||||||
| Primary | 21 (28) | 30 | 1.48; 0.64–3.40 | 34 (31) | 34 | 1.38; 0.70–2.70 | ||
| Secondary | 55 (72) | 41 | 76 (69) | 41 | ||||
Statistically significant P<0.05 values are in bold.
P-value for Log-rank (Mantel Cox) test for 2 variables or for Log-rank for trend test for 3 variables.
Age 0 to 5 versus 6 to 10.
Age 6 to 10 versus 11+.
Age 0 to 5 versus 11+.
IOP 0 to 20 versus 21 to 30.
IOP 21 to 30 versus 31+.
IOP 0 to 20 versus 31+.
CI indicates confidence interval; HR, hazard ratio; IOP, intraocular pressure.
Of the 110 procedures, 14% (14 of 110 surgeries) had postoperative complications (Table 3). Rate of complications was associated with concurrent surgery at time of Baerveldt placement (Table 5). Seven eyes developed postoperative retinal detachment of which 5 underwent vitrectomy surgery for repair. The retinal detachment occurred within 6 months in 3 eyes that had all undergone combined Baerveldt and vitrectomy surgery. Three additional eyes, which had severe microphthalmia and persistent fetal vasculature, developed retinal detachments more than a year after Baerveldt-vitrectomy surgery. Due to poor prognosis, 2 of these eyes did not undergo surgery for the retinal detachment. One eye with a history of primary congenital glaucoma, had a retinal detachment 8 years after Baerveldt placement.
TABLE 5.
Univariate Analysis for Complications
| Risk Factor | N (%) (n=110) | 5-Year Cumulative Probability of Complication (%) | P * | HR; 95% CI |
|---|---|---|---|---|
| Sex | 0.89 | |||
| Male | 52 (47) | 13 | 0.92; 0.27–3.17 | |
| Female | 58 (53) | 13 | ||
| Age (y) | 0.14 | |||
| 0-5 | 53 (48) | 21 | 0.63; 0.15–2.59† | |
| 6-10 | 25 (23) | 7 | 0.58; 0.06–5.57‡ | |
| 11+ | 32 (29) | 4 | 0.24; 0.06–1.01§ | |
| Ethnicity | 0.25 | |||
| Caucasian | 74 (67) | 11 | 2.04; 0.55–7.64 | |
| Non-Caucasian | 36 (33) | 17 | ||
| Prior glaucoma surgeries | 0.66 | |||
| None | 46 (42) | 11 | 1.33; 0.38–4.61 | |
| 1 or more | 64 (58) | 15 | ||
| Preoperative IOP (mm Hg) | 0.84 | |||
| 0-20 | 29 (26) | 20 | 0.62; 0.15–2.57∥ | |
| 21-30 | 48 (44) | 13 | 0.74; 0.15–3.80¶ | |
| 31+ | 33 (30) | 7 | 0.48; 0.10–2.39# | |
| Preoperative medications | 0.20 | |||
| 0-2 | 31 (28) | 25 | 0.39; 0.10–1.55 | |
| 3+ | 79 (72) | 9 | ||
| Procedure | 0.3 | |||
| Single procedure | 88 (79) | 12 | 1.02; 0.21–4.81 | |
| Staged procedure | 23 (21) | 23 | ||
| Concurrent procedure | 0.04 | |||
| No | 56 (51) | 9 | 3.10; 0.89–10.85 | |
| Yes | 54 (49) | 17 | ||
| Type of glaucoma | 0.19 | |||
| Primary | 34 (31) | 13 | 3.78; 0.97–14.71 | |
| Secondary | 76 (69) | 17 |
P-value for Log-rank (Mantel Cox) test for 2 variables or for Log-rank for trend test for 3 variables.
Age 0 to 5 versus 6 to 10.
Age 6 to 10 versus 11+.
Age 0 to 5 versus 11+.
IOP 0 to 20 versus 21 to 30.
IOP 21 to 30 versus 31+.
IOP 0 to 20 versus 31+.
CI indicates confidence interval; HR, hazard ratio; IOP, intraocular pressure.
Four eyes of 4 patients had hypotony after Baerveldt 101-350 implantation that required surgery. In 1 eye with juvenile open angle glaucoma, the ligature suture was found to be loose on postoperative day 1 and was retied within the first postoperative week. The ripcord was removed 3 weeks later and IOP has since been well controlled. One eye became hypotonous following dissolution of the ligature suture, and 3 months later underwent removal of the Baerveldt due to minimal capsule formation over the plate. One eye, which had undergone multiple treatments of transcleral cycloablation before Baerveldt placement, had hypotony immediately after stage 2 implantation and after 2 weeks had placement of a vicryl suture. After the vicryl dissolved, there was no recurrence of hypotony. The last eye also had a history of multiple transcleral cycloablation treatments prior to Baerveldt placement and became hypotonous after the ligature suture dissolved. The eye underwent first an exchange of a Baerveldt 101-350 for a 101-250 device after 3 months, and then a subsequent exchange for an Ahmed FP8 implant after another 1 month.
One eye with microspherophakia had an early postoperative suprachoroidal hemorrhage after stage 2 placement. In the 1 eye, which underwent concurrent keratoprosthesis placement after multiple failed corneal transplants in the setting of Peters Anomaly, the keratoprothesis spontaneously extruded 2 months after surgery. One eye with a history of retinopathy of prematurity had a postoperative vitreous hemorrhage that cleared spontaneously.
Thirty-seventy percent (40 of 110 surgeries) were considered failures at final follow-up. This included 14 procedures in 13 eyes which suffered complications (retinal detachment, hypotony requiring surgery, suprachoroidal hemorrhage, and keratoprosthesis extrusion) as detailed above and in Table 3. Twenty-six Baerveldt procedures were designated as failures due to need for at least 1 additional surgery for increased IOP (Table 6). Six eyes underwent tube revision for elevated IOP which included tube retraction (2), tube blockage (1), and bleb revision for encapsulation (3). Twenty-five eyes of 20 patients underwent at least 1 additional non-Baerveldt related surgery for elevated IOP including cycloablation, trabeculectomy, Ahmed GDD placement, goniotomy, and scleral windows (Table 6). At final follow-up, the average number of glaucoma surgeries was 3.0+2.0 per eye (median: 2, range: 1 to 9). In addition, 24 eyes of 20 patients underwent non–glaucoma-related ocular surgery following Baerveldt implantation. The total number of ocular surgeries at final follow-up was 3.8±2.3 per eye (median: 3, range: 1 to 13).
TABLE 6.
Failures and Additional Surgeries
| At least 1 post-Baerveldt glaucoma related surgery | 39 eyes of 21 patients |
| Baerveldt-related surgery | |
| Tube revision for increased IOP | 6 eyes of 6 patients |
| Tube ligation for hypotony or retinal detachment | 8 eyes of 8 patients |
| Tube shortening* | 9 eyes of 7 patients |
| Cycloablation | |
| Contact transscleral | 15 eyes of 14 patients |
| Endoscopic | 5 eyes of 5 patients |
| Trabeculectomy with mitomycin C | 1 eye of 1 patient |
| Ologen-augmented Ahmed FP7 placement | 1 eye of 1 patient |
| Goniotomy | 1 Eeye of 1 patient |
| Scleral windows | 1 eye of 1 patient |
| Number of post-Baerveldt glaucoma-related surgeries | |
| 1 glaucoma or tube-related surgery | 19 eyes of 18 patients |
| 2 glaucoma or tube-related surgeries | 11 eyes of 10 patients |
| 3 glaucoma or tube-related surgeries | 6 eyes of 6 patients |
| ≥4 glaucoma or tube-related surgeries | 3 eyes of 3 patients |
| At least 1 post-Baerveldt non–glaucoma-related surgery | 24 eyes of 20 patients |
| Lensectomy | 8 eyes of 8 patients |
| Capsulotomy | 5 eyes of 4 patients |
| Pars plana vitrectomy | 5 eyes of 5 patients |
| Keratoplasty (penetrating or partial thickness) | 4 eyes of 3 patients |
| Superficial keratectomy | 3 eyes of 2 patients |
| Secondary intraocular lens placement | 3 eyes of 2 patients |
Tube shortening not considered failure as surgery not performed to control intraocular pressure (IOP).
At final follow-up, IOP and number of glaucoma medications were significantly decreased (P<0.0001) compared with the preoperative examination (Table 7). There was no significant difference between preoperative and final LogMar visual acuities in the 59 eyes of 45 patients which had documented visual acuities at both timepoints. Since measurement of optotype visual acuity was limited by age and cooperation in many of the patients, the change in visual acuity between preoperative and final examinations was determined (Table 7). For 71% of all Baerveldt surgeries, vision was stable (30%) or improved (41%), while 28% showed worsening of vision. At final follow-up, over half of the eyes (54%, 57 of 106) had optotype visual acuity of ≥20/150 while 22% (24 of 106) showed visual acuity of ≤20/200 (Supplemental Table 4, Supplemental Digital Content 1, http://links.lww.com/IJG/A589). Six eyes of 6 patients were phthisical due to retinal detachment (4 eyes), keratoprosthesis extrusion (1 eye), and no aqueous humor production due to cycloablation (1 eye).
TABLE 7.
IOP and Vision Outcomes
| 1st Baerveldt Surgery Per Patient (n=76) | All Baerveldt Implantation Surgeries (n=110) | P † | |
|---|---|---|---|
| IOP | |||
| Preoperative | 26±9 mm Hg (median: 24, range: 7–53) | 26±8 mm Hg (median: 25, range: 3–53) | 0.60 |
| Final | 16±6 mm Hg (median: 15, range: 5–36) | 11±6 mm Hg (median: 14, range: 4–36) | 0.01 |
| P * | <0.0001 | <0.0001 | |
| Glaucoma medications | |||
| Preoperative | 3.0±1.0 (median: 3.0, range: 0–5) | 3.0±1.0 (median: 3.0, range: 0–5) | 0.93 |
| Final | 2.0±1.0 (median: 2.0, range: 0–4) | 2.0±1.0 (median: 2.0, range: 0–4) | 0.90 |
| P * | <0.001 | <0.001 | |
| LogMAR visual acuity‡ | |||
| Preoperative | 0.61±0.50 (median: 0.54, range: 0.00–1.40) | 0.52±0.40 (median: 0.48, range: 0.00–1.40) | 0.40 |
| Final | 0.61±0.54 (median: 0.4, range: 0.0–1.90) | 0.56±0.50 (median: 0.4, range: 0.0–1.90) | 0.78 |
| P * | 0.99 | 0.43 | |
| Vision change (preoperative vs. final) | |||
| Improved | 29 surgeries (38%) | 46 surgeries (41%) | |
| Stable | 27 surgeries (36%) | 33 surgeries (30%) | |
| Worse | 20 surgeries (26%) | 31 surgeries (28%) | |
Statistically significant P<0.05 values are in bold.
P-value for Wilcoxon matched pairs signed rank test comparing respective preoperative and final values.
P-value for Mann-Whitney comparing respective first surgery of first eye and all surgeries of all eyes.
Eyes in which both preoperative and final optotype visual acuities were obtained (40 eyes of 40 patients for first surgery of first eye, 59 eyes of 45 patients for all surgeries of all eyes).
DISCUSSION
The management of childhood glaucomas remains a challenge due to the heterogeneity of the diseases, increased inflammatory and thus scarring response to surgery, and a much greater anticipated lifetime to maintain IOP control.1,2 Other additional pediatric-specific issues include difficulties in monitoring disease progression due to cooperation and the need to simultaneously address amblyopia for optimal visual development.31,32 Although the mainstay of pediatric glaucoma management is typically angle surgery, many children, especially those with advanced and secondary forms of glaucoma, require trabecular meshwork bypass surgery.3–5 While trabeculectomy with or without antifibrotics has long been utilized in both pediatric and adult patients, the decreased risk of bleb-related infections and the more predictable early postoperative course have made GDDs a popular choice for children, especially in the United States.6–9
To the best of our knowledge, the current study presents one of the largest cohorts of children (106 eyes of 76 patients) with a long length of follow-up (4.8±2.3 y, median: 4.7 y) who underwent Baerveldt implantation. Importantly we did not detect differences in success or survival rates between first Baerveldt surgery for each patient (76 surgeries for 76 patients) compared with all Baerveldt surgeries (110 surgeries for 106 eyes). Overall, our success and survival rates were comparable to previously published studies which had shorter follow-up, fewer patients, or contained a combination of GDD types. Fellenbaum et al10 was an early study of Baerveldt GDDs with limited follow-up (average: 15.0 mo) and showed a success rate of 86% at 1 year. Additional studies of Baerveldt implants report 2 to 3 year success rates of 61% to 87% in children.11,12,16 In our larger cohort, we found comparable success rates for first Baerveldt per patient of 77% at 2 years and 71% at 3 years. Four additional studies reported longer survival rates.13,14,19,20 Roulim de Moura et al13 and van Overdam et al14 showed 4 to 5 years success rates of 44% to 58%, which were lower than our study. In Autrata et al19 and Mandalos et al20 6 and 8 year success rates were higher than ours at 65% and 40%, respectively, however, both of these studies reported on a combination of nonvalved GDDs (Baerveldts and Moltenos). Taken together, our study which had larger number of eyes and longer follow-up than most reports, was consistent with previous studies showing that success rates of nonvalved GDDs and specifically Baerveldts decrease over time with rates ranging from 80% to 95% at 1 year and declining to as low as 30% at 8 years.
As with many of the other pediatric studies, our study included a heterogenous group of patients with many different types of childhood glaucomas. We did not find significant risk factors for failure of first Baerveldt implanted per patient (n=76), although analysis of all Baerveldt surgeries (n=110), age and prior glaucoma surgery became specific risk factors for failure. However, the interdependence between the eyes as well as the second Baerveldt placement in 4 eyes, may confound these statistically significant effects. In Budenz et al12 and Mandalos et al20 the number of prior glaucoma surgeries, preoperative IOP, or number of preoperative glaucoma medications were not associated with increased failure of Baerveldt GDDs. Type of glaucoma (primary vs. secondary or based on the World Glaucoma Association Classification) also did not influence failure, although in our study the relatively low numbers in each specific form of glaucoma precluded more specific analysis. This is in contrast to the recent study by Medert et al,21 which found that glaucomas associated with congenital anomalies (nonacquired ocular or nonacquired systemic diseases) and primary congenital glaucoma were at higher risk of failure compared with acquired forms of glaucoma. Importantly that study included 112 Baerveldt and 37 Ahmed implants performed in 119 patients, and although the authors comment that there was no significant difference in success rate between the GDD types, they did not report Ahmed and Baerveldt specific rates. In addition, the study did not account for interdependence of the eyes if GDDs were placed in both eyes of a patient.21 In contrast, in adults there are well known risk factors for surgical failure including younger age, black race, and neovascular and uveitic forms of glaucoma.33,34 However, the Ahmed versus Baerveldt trial reported 60% success rate at 5 years in the 114 adults who had Baerveldt placement, which is comparable to our pediatric cohort.22 While the difficulty in identifying consistent risk factors for Baerveldt failure in children may be due to the fewer number of patients in these studies, this could also suggest a higher degree of uniformity in wound and healing responses to eye surgery across the pediatric population. Multi-center studies would be required to fully evaluate GDD success especially in more rare forms of childhood glaucomas.
Multiple types of GDDs have been introduced into the market, with the Baerveldt and Ahmed implants being most popular. Preference for the Baerveldt by many surgeons is based on the higher success rate with longer duration of IOP control, while Ahmed GDDs are favored by others due to the ease of implantation, more straightforward postoperative course, and lower risk of hypotony.23–26 The Ahmed versus Baerveldt trial was specifically performed in adult onset glaucomas, and due to the rarity of childhood glaucomas there has not been a comparable study in the pediatric population.22 Like Baerveldts, Ahmed GDDs in children have a >80% success rate at 1 year, but by 5 years, success is often <50% which is lower than our cohort.17,35,36 Furthermore, while both Baerveldt and Ahmed GDDs have been shown to decrease IOP and the number of glaucoma medications, Ahmed implants often have a hypertensive phase between 1 and 2 months postoperatively that requires reinitiation of IOP-lowering medications.17,35,36 Due to these factors, we implanted Baerveldt GDDs in >80% of patients undergoing tube surgery during the time encompassed by the study (2012-2020).
Since all surgeries were performed by a single surgeon, this suggests more uniform criteria for performing surgery and the choice of type and location of the implant as well as greater consistency of the surgical procedure and postoperative management. However, within the last year, we have employed our recently published technique for Ologen-augmentation of Ahmed GDDs as our primary glaucoma tube surgery.28 Since in our hands, Ologen augmentation significantly improves the 1- and 2-year success rates of the Ahmed implant, we have changed our practice pattern such that Baerveldt GDDs are primarily used in select forms of glaucoma (eg, Sturge Weber) and secondarily after a failed, encapsulated Ahmed GDD. To this point, concurrent explantation of a superotemporal Ahmed with placement of a Baerveldt in the same quadrant was performed in 7 of the surgeries in our cohort with a similar technique that has been shown in adults.37 Although to date, there are no similar published studies in children, 6 of the 7 eyes in our cohort, which had concurrent Ahmed removal, still remained successful at final follow-up. Additional studies are required to not only compare the different types of GDDs, but also assess tube exchange in childhood glaucomas.
The rate of complications associated with Baerveldt GDDs in children is reported to be between 5% to 44%; our rate was on the lower end of that range at 14%.10–14,19,21 Medert et al21 reported a 25% complication rate and found that younger age and higher preoperative IOP were associated with increased risk for complications. The only significant risk factor in our study was concurrent surgery at the time of Baerveldt placement. Half of our complications were retinal detachments, and all 7 eyes in our cohort that developed retinal detachments had complex congenital eye anomalies (eg, microphthalmia, Peters anomaly, anterior persistent fetal vasculature) which in themselves predispose patients to retinal problems. Furthermore, 6 of these eyes underwent vitrectomy at the time of Baerveldt placement. While vitrectomy surgery has a known risk of retinal detachment, it is difficult to determine whether the retinal detachments in these eyes were directly caused by the surgery or were more related to the abnormal anatomy. This has not dissuaded our use of combined vitrectomy and GDD placement in aphakic or pseudophakic eyes due to the benefit of posterior placement of the tube. Additional studies of pars plana tube insertion in children have shown similar results where there is less risk of corneal decompensation, but potentially a greater incidence of posterior segment complications.29,38,39
In children, increased scleral elasticity commonly causes anterior rotation of the tube resulting in corneal touch. In addition, lowering the pressure in buphthalmic eyes can lead to elongation of the intraocular portion of the tube.11,40,41 Together these factors increase the risk of corneal decompensation with GDDs that are placed in the anterior chamber. To that end, we did not have any tube-related causes of corneal decompensation, however, 9 eyes, which were not deemed failures, underwent ab interno tube shortening in the anterior chamber to prophylactically prevent corneal damage. Hence, it is important to consider both scenarios when placing tubes, either within the anterior segment or in the pars plana, and anticipate potential complications.
Hypotony after tube opening is the most commonly reported postoperative complication associated with Baerveldt GDDs, occurring in up to 40% of cases.20,42 In Mandalos and colleagues, 39% of the eyes were hypotonus in the first 6 postoperative months and underwent intracameral viscoelastic injection to deepen the anterior chamber and/or religation of the tube. While the hypotony resolved in the majority of these eyes, 1/3 of the eyes continued to have low pressure after 6 months postoperatively.20 In our cohort, less than 4% of eyes had hypotony which required additional surgery—a rate more consistent with the Ahmed versus Baerveldt trial.22 The high rate of hypotony in Mandalos et al20 may be related to the adjunct use of mitomycin C with the tubes in that study. Other studies have found that antifibrotics cause a higher incidence of bleb leaks, but do not significantly improve long-term IOP control success of GDDs.43–45 Thus, we do not routinely use antifibrotics in conjunction with Baerveldt placement, which may account for our lower rate of hypotony. In our study, it is important to note that 2 of the eyes with hypotony underwent multiple sessions of transcleral cycloablation prior to tube placement, highlighting that there is a fine line between hypotony and glaucoma in eyes with minimal to no aqueous outflow. While one of these eyes required re-ligation of the tube with a polyglactin suture to provide more time for capsule formation, the other eye eventually went phthisical, even after exchange of the Baerveldt GDD for an Ahmed FP8 GDD. Thus, despite the anatomic challenges, we advocate for earlier GDD placement rather than cycloablation in eyes with minimal aqueous outflow.
Additional complications in our cohort included postoperative suprachoroidal and vitreous hemorrhages. While suprachoroidal hemorrhage is always a concern with intraocular surgery, buphthalmic eyes are at higher risk due to collapse of the stretched out eye wall with pressure reduction and subsequent shearing of choroidal and retinal blood vessels.46 Furthermore, since surgery in children is almost always performed under general anesthesia, there is a risk for emergence coughing and bucking during extubation that may trigger hemorrhage. In our study, both hemorrhages occurred in buphthalmic eyes in which the eye pressure was immediately decreased with surgery. The suprachoroidal hemorrhage followed a combined vitrectomy and stage 2 procedure and the vitreous hemorrhage occurred after a combined vitrectomy and single-staged Baerveldt with venting slits. Interestingly, only a few other pediatric studies reported suprachoroidal hemorrhage as a complication, while choroidal effusions were more commonly noted.14,20,21
The majority of failures in Baerveldt GDD studies are related to increased IOP and the need for additional surgery.10–14,19,21 Increased IOP following Baerveldt placement can be attributed to the tube, such as in tube retraction or blockage. In our cohort, this occurred in about 5% of eyes, but others report rates that range from 0 to 40%.10–14,19,21 These problems can be resolved by a adding a tube extender or by removing the occlusions from the tube tip. However, increased IOP due to encapsulation is a more challenging problem. Previous studies show rates of Baerveldt encapsulation with failure due to increased IOP between 17% and 26% of pediatric eyes.12–14,19,20 In our study, 23% of eyes failed due to bleb encapsulation and required at least 1 non-tube related glaucoma surgery to lower IOP.
As in the adult population, there is a lack of consensus in regards to subsequent surgical approaches after bleb encapsulation.47–49 Revision of the Baerveldt with removal of the capsule risks hypotony and rarely yields long-term IOP due to re-encapsulation. Placement of a second GDD or cycloablation are common options for additional IOP-lowering surgeries. In adult patients, Murakami et al48 showed similar IOP-reduction with endoscopic cyclophotocoagulation compared with implantation of a second GDD at 6 and 12 months postoperatively. However, additional studies in adults with longer follow-up reported earlier failure of cycloablation while second GDDs had late failures and higher rates of complications.47,49 In contrast to adult studies, there is less data regarding outcomes of secondary procedures following GDD placement in children. Medert and colleagues found a lower success rate of second (n=22) compared with first GDDs (n=150), with increased risk of failure associated with younger age and concurrent procedures. Due to the history of bleb encapsulation, we predominantly employed cycloablation (transcleral and endoscopic) for high IOP after Baerveldt placement and have previously shown that pairing GDD with cycloablation is highly effective in obtaining IOP control in glaucoma associated with Axenfeld-Rieger syndrome and Peters Anomaly.50,51 Five eyes underwent additional GDD placement, of which 4 were second Baerveldt devices and 1 was an Ologen-augmented Ahmed FP7 placed in the superonasal quadrant adjacent to the initial superotemporal Baerveldt. As discussed above, the Ologen-augmented Ahmed has more recently become our primary GDD, and this secondary tube placement was performed after our initial successes.
In 1 eye of a patient with glaucoma secondary to Hurlers syndrome, the sclera was extremely thickened due to dermatan and heparan sulfate deposition, thus mimicking uveal effusion syndrome. Creation of 75% depth scleral windows effectively decreased IOP, although the procedure needed to be repeated as the windows refilled in over time.52 Thus, while we mainly utilize cycloablation, changes in practice patterns as well as differences in types of glaucoma have influenced our choices for secondary procedures. Additional studies in children are required to further investigate the best management practices in these often refractory forms of glaucoma.
Regardless of age, controlling eye pressure is critical for maintaining vision. However, in children, there is the added complexity of promoting both monocular and binocular visual development as well as coping with poor cooperation with in-clinic testing. While we found no significant difference between preoperative and final LogMar visual acuities in the 56% of eyes which had documented visual acuities for both eyes, ~71% showed stable or improved vision. This is consistent with Budenz et al12 and Tai and Song16 which reported that 62% and 77% stability or improvement of vision in eyes that underwent visual acuity testing. However, in our cohort, 54% of eyes had best corrected visual acuity better than 20/150, which was greater than Budenz et al12 (27%) and Tai and Song (15%).16 This discrepancy may be due to the longer time of follow-up and thus the greater number of patients who were able to cooperate with optotype visual acuity testing at final follow-up.
While our study showed a significant decrease in IOP and number of glaucoma medications at final follow-up, there are limitations that are important to recognize. This study is retrospective without a control group and has a heterogeneous cohort in regard to type of glaucoma and length of follow-up. In addition, while all of the implants were Baerveldt GDDs, there was not uniformity of plate size or placement nor tube location. However, strengths of our study include the relatively large number of surgeries that were all performed by the same surgeon.
In summary, we present one of the largest cohorts of pediatric patients who underwent Baerveldt GDD placement performed by a single surgeon. Overall, our success and survival rates mirrored both pediatric and adult reports. Unlike in adults, there are inconsistencies as to specific risk factors for Baerveldt failure and complications in children. For first Baerveldt placement per patient we did not find any significant risk factors for failure. However, complications were associated with concurrent surgery. Ultimately, children with glaucoma often require multiple surgeries, but IOP control with preservation or improvement in visual acuity is achieved in the majority of patients.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.glaucomajournal.com.
Footnotes
Disclosure: The authors declare no conflict of interest.
Contributor Information
Adam Jacobson, Email: adajac@med.umich.edu.
Cagri G. Besirli, Email: cbesirli@med.umich.edu.
Brenda L. Bohnsack, Email: bbohnsack@luriechildrens.org.
REFERENCES
- 1. Chang I, Caprioli J, Ou Y. Surgical management of pediatric glaucoma. Dev Ophthalmol. 2017;59:165–178. [DOI] [PubMed] [Google Scholar]
- 2. Malik R, AlDarrab A, Edward DP. Contemporary management of refractory pediatric glaucoma. Curr Opin Ophthalmol. 2020;31:123–131. [DOI] [PubMed] [Google Scholar]
- 3. Anderson DR. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology. 1983;90:805–806. [DOI] [PubMed] [Google Scholar]
- 4. Akimoto M, Tanihara H, Negi A, et al. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol. 1994;112:1540–1544. [DOI] [PubMed] [Google Scholar]
- 5. Ikeda H, Ishigooka H, Muto T, et al. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch Ophthalmol. 2004;122:1122–1128. [DOI] [PubMed] [Google Scholar]
- 6. Waheed S, Ritterband DC, Greenfield DS, et al. Bleb-related ocular infection in children after trabeculectomy with mitomycin C. Ophthalmology. 1997;104:2117–2120. [DOI] [PubMed] [Google Scholar]
- 7. Beck AD, Freedman SF, Kammer J, et al. Aqueous shunt devices compared with trabeculectomy with mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003;136:994–1000. [DOI] [PubMed] [Google Scholar]
- 8. Wallin Ö, Al-ahramy AM, Lundström M, et al. Endophthalmitis and severe blebitis following trabeculectomy. Epidemiology and risk factors; a single-centre retrospective study. Acta Ophthalmol. 2014;92:426–431. [DOI] [PubMed] [Google Scholar]
- 9. Razeghinejad MR, Havens SJ, Katz LJ. Trabeculectomy bleb-associated infections. Surv Ophthalmol. 2017;62:591–610. [DOI] [PubMed] [Google Scholar]
- 10. Fellenbaum PS, Sidoti PA, Heuer DK, et al. Experience with the Baerveldt implant in young patients with complicated glaucomas. J Glaucoma. 1995;4:91–97. [PubMed] [Google Scholar]
- 11. Donahue SP, Keech RV, Munden P, et al. Baerveldt implant surgery in the treatment of advanced childhood glaucoma. J AAPOS. 1997;1:41–45. [DOI] [PubMed] [Google Scholar]
- 12. Budenz DL, Gedde SJ, Brandt JD, et al. Baerveldt glaucoma implant in the management of refractory childhod glaucomas. Ophthalmology. 2004;111:2204–2210. [DOI] [PubMed] [Google Scholar]
- 13. Rolim de Moura C, Fraser-Bell S, Stout A, et al. Experience with the Baerveldt glaucoma implant in the management of pediatric glaucoma. Am J Ophthalmol. 2005;139:847–854. [DOI] [PubMed] [Google Scholar]
- 14. van Overdam KA, de Faber JTHN, Lemij HG, et al. Baerveldt glaucoma implant in paediatric patients. Br J Ophthalmol. 2006;90:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirwan C, Lanigan B, O’Keefe M. Glaucoma in aphakic and pseudophakic eyes following surgery for congenital cataract in the first year of life. Acta Ophthalmol. 2010;88:53–59. [DOI] [PubMed] [Google Scholar]
- 16. Tai AX, Song JC. Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J AAPOS. 2014;18:550–553. [DOI] [PubMed] [Google Scholar]
- 17. Pakravan M, Esfandiari H, Yazdani S, et al. Clinical outcomes of Ahmed glaucoma valve implantation in pediatric glaucoma. Eur J Ophthalmol. 2019;29:44–51. [DOI] [PubMed] [Google Scholar]
- 18. Mofti A, Ahlharbi A, Alsuhaibani M, et al. Long-term outcomes of the Ahmed glaucoma valve surgery in childhood glaucoma. J AAPOS. 2020;24:346.e1–346.e8. [DOI] [PubMed] [Google Scholar]
- 19. Autrata R, Helmanova I, Oslejskova H, et al. Glaucoma drainage implants in the treatment of refractory glaucoma in pediatric patients. Eur J Ophthalmol. 2007;17:928–937. [DOI] [PubMed] [Google Scholar]
- 20. Mandalos A, Tailor R, Parmar T, et al. The long-term outcomes of glaucoma drainage device in pediatric glaucoma. J Glaucoma. 2016;25:e189–e195. [DOI] [PubMed] [Google Scholar]
- 21. Medert CM, Cavuoto KM, Vanner EA, et al. Risk factors for glaucoma drainage device failure and complication in the pediatric population. Ophthalmol Glaucoma. 2021;4:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christakis PG, Zhang D, Budenz DL, et al. Five-year pooled data analysis of the Ahmed Baerveldt comparison study and the Ahmed versus Baerveldt study. Am J Ophthalmol. 2017;176:118–126. [DOI] [PubMed] [Google Scholar]
- 23. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105:1968–1976. [DOI] [PubMed] [Google Scholar]
- 24. Tsai JC, Johnson CC, Kammer JA, et al. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma II: longer-term outcomes from a single surgeon. Ophthalmology. 2006;113:913–917. [DOI] [PubMed] [Google Scholar]
- 25. Minckler DS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1089–1098. [DOI] [PubMed] [Google Scholar]
- 26. Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789.e2–803e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinreb RN, Grajewski AL, Papadopoulos M, et al. Childhood Glaucoma The 9th Consensus Report of the World Glaucoma Association. The Netherlands: Kugler Publications; 2013. [Google Scholar]
- 28. Jacobson A, Rojas C, Bohnsack BL. Ologen augmentation of Ahmed glaucoma drainage devices in pediatric glaucomas. BMC Ophthalmol. 2021;21:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozgonul C, Besirli CG, Bohnsack BL. Combined vitrectomy and glaucoma drainage device implantation surgical approach for complex pediatric glaucomas. J AAPOS. 2017;21:121–126. [DOI] [PubMed] [Google Scholar]
- 30. Chandramouli SA, Glaser TS, Umunakwe O, et al. Glaucoma drainage devices calculator app: a modern clinidal decision tool. Ophthalmol Glaucoma. 2021;4:550–551. [DOI] [PubMed] [Google Scholar]
- 31. Nilsson J. The burden of amblyopia and strabismus: justification of treatment and screening revisited. Arch Ophthalmol. 2008;126:143–145. [DOI] [PubMed] [Google Scholar]
- 32. DeSantis D. Amblyopia. Pediatr Clin North Am. 2014;61:505–518. [DOI] [PubMed] [Google Scholar]
- 33. Lavin MJ, Franks WA, Wormald RP, et al. Clinical risk factors for failure in glaucoma tube surgery. A comparison of three tube designs. Arch Ophthalmol. 1992;110:480–485. [DOI] [PubMed] [Google Scholar]
- 34. Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10:237–249. [DOI] [PubMed] [Google Scholar]
- 35. Eksioglu U, Yakin M, Sungur G, et al. Short- to long-term results of Ahmed glaucoma valve in the management of elevated intraocular pressure in patients with pediatric uveitis. Can J Ophthalmol. 2017;52:295–301. [DOI] [PubMed] [Google Scholar]
- 36. Spiess K, Calvo JP. Outcomes of Ahmed glaucoma valve in paediatric glaucoma following congenital cataract surgery in persistent foetal vasculature. Eur J Ophthalmol. 2021;31:1070–1078. [DOI] [PubMed] [Google Scholar]
- 37. Zuo W, Lesk MR. Surgical outcome of replacing a failed Ahmed glaucoma valve by a Baerveldt glaucoma implant in the same quadrant in refractory glaucoma. J Glaucoma. 2018;27:421–428. [DOI] [PubMed] [Google Scholar]
- 38. Banitt MR, Sidoti PA, Gentile RC, et al. Pars plana Baerveldt implantation for refractory childhood glaucomas. J Glaucoma. 2009;18:412–417. [DOI] [PubMed] [Google Scholar]
- 39. Vinod K, Panarelli JF, Gentile RC, et al. Long-term outcomes and complications of pars plana Baerveldt implantation in children. J Glaucoma. 2017;26:266–271. [DOI] [PubMed] [Google Scholar]
- 40. Eid TE, Katz LJ, Spaeth GL, et al. Long-term effects of tube shunt procedures on management of refractory childhood glaucoma. Ophthalmology. 1997;104:1011–1016. [DOI] [PubMed] [Google Scholar]
- 41. Djodeyre MR, Peralta Calvo J, Abelairas Gomez J. Clinical evaluation and risk factors of time to failure of Ahmed glaucoma valve implant in pediatric patients. Ophthalmology. 2001;108:614–620. [DOI] [PubMed] [Google Scholar]
- 42. Stein JD, McCoy AN, Asrani S, et al. Surgical management of hypotony owing to overfiltration in eyes receiving glaucoma drainage devices. J Glaucoma. 2009;18:638–641. [DOI] [PubMed] [Google Scholar]
- 43. Al-Mobarak F, Khan AO. Two-year survival of Ahmed valve implantation in the first 2 years of life with and without intraoperative mitomycin-C. Ophthalmology. 2009;116:1862–1865. [DOI] [PubMed] [Google Scholar]
- 44. Razeghinejad MR, Nowroozzadeh MH. Mitomycin-C and pediatric Ahmed vlaves. Ophthalmology. 2010;117:1464–1465. [DOI] [PubMed] [Google Scholar]
- 45. Mahdy RA. Adjunctive use of bevacizumab versus mitomycin C with Ahmed valve implantation in treatment of pediatric glaucoma. J Glaucoma. 2011;20:458–463. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen QH, Budenz DL, Parrish RK. Complications of Baerveldt glaucoma drainage implants. Arch Ophthalmol. 1998;116:571–575. [DOI] [PubMed] [Google Scholar]
- 47. Schaefer JL, Levine MA, Martorana G, et al. Failed glaucoma drainage implant: long-term outcomes of a second drainage device versus cyclophotocoagulation. Br J Ophthalmol. 2015;99:1718–1724. [DOI] [PubMed] [Google Scholar]
- 48. Murakami Y, Akil H, Chahal J, et al. Endoscopic cyclophotocoagulation versus second glaucoma drainage device after prior aqueous tube shunt surgery. Clin Exp Ophthalmol. 2017;45:241–246. [DOI] [PubMed] [Google Scholar]
- 49. Wang MY, Patel K, Blieden LS, et al. Comparison of efficacy and complications of cyclophotocoagulation and second glaucoma drainage device after initial glaucoma drainage device failure. J Glaucoma. 2017;26:1010–1018. [DOI] [PubMed] [Google Scholar]
- 50. Dolezal KA, Besirli CG, Mian SI, et al. Glaucoma and cornea surgery outcomes in Peters Anomaly. Am J Ophthalmol. 2019;208:367–375. [DOI] [PubMed] [Google Scholar]
- 51. Zepeda EM, Branham K, Moroi SE, et al. Surgical outcomes of glaucoma associated with Axenfeld-Rieger syndrome. BMC Ophthalmol. 2020;20:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abell RG, Kerr NM, Vote BJ. Bilateral nanophthalmic uveal effusion syndrome: clinical presentation and surgical management. Retin Cases Brief Rep. 2013;7:386–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.glaucomajournal.com.


